Composition and Diversity of Soil Microbial Communities in Walnut Orchards at Different Altitudes in Southeastern Tibet
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site Description
2.2. Experiment Design
2.3. Sample Determination
2.4. Statistical Analysis
3. Results
3.1. Walnut Orchard Soil Properties
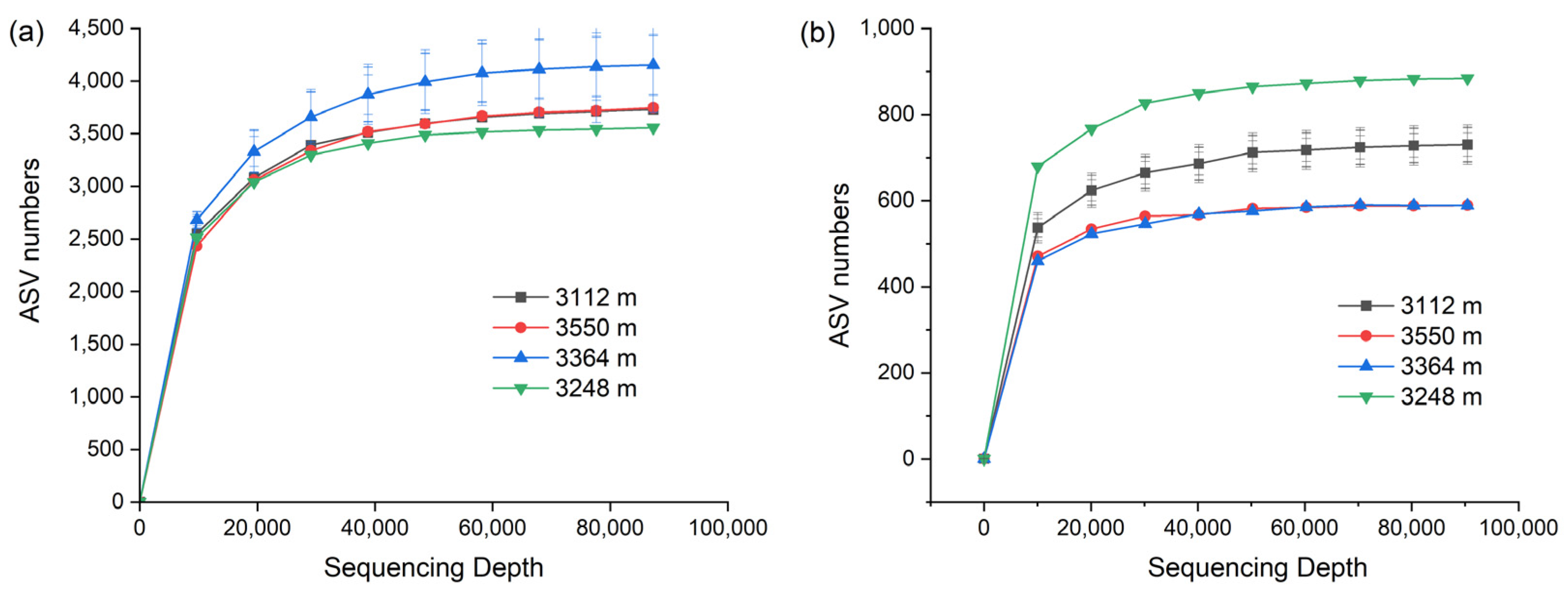
3.2. Alpha Diversity of Soil Bacteria and Fungi in Walnut Orchards
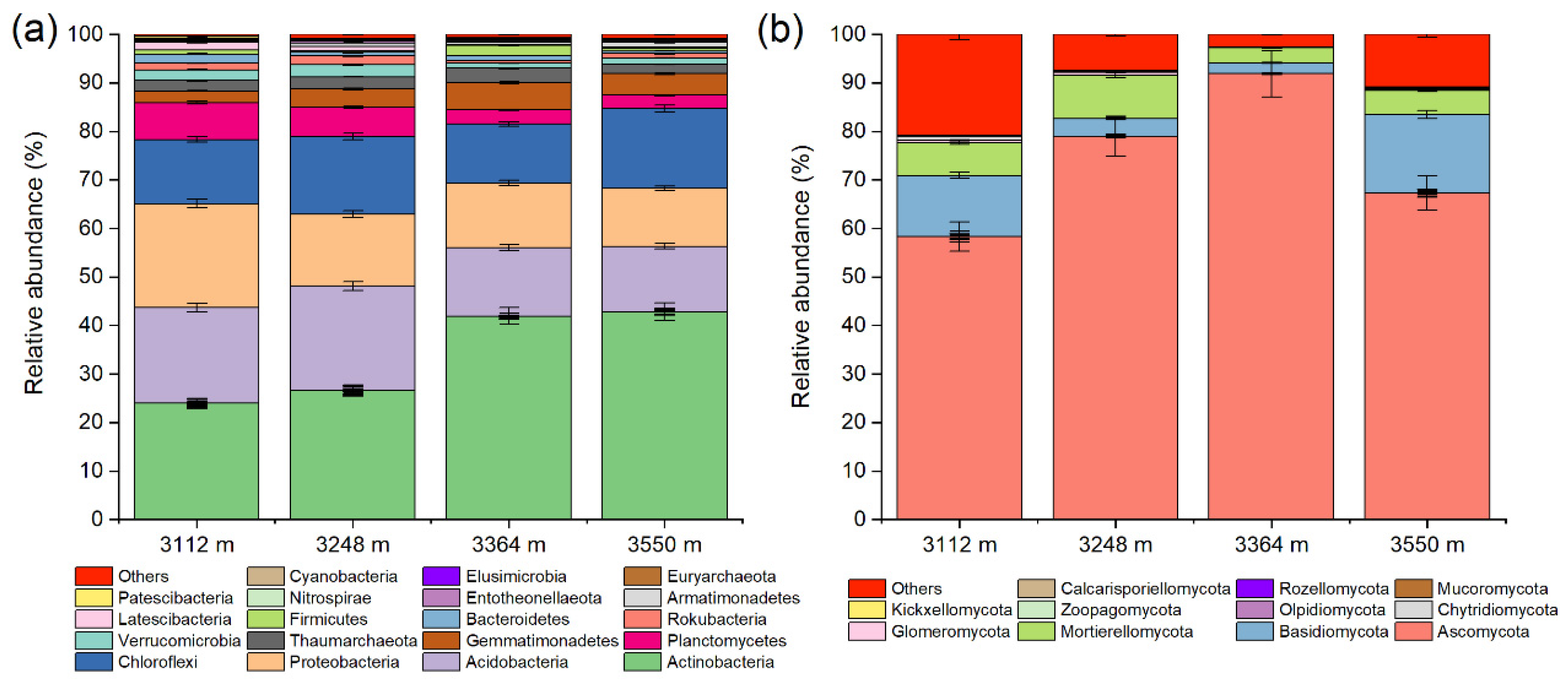
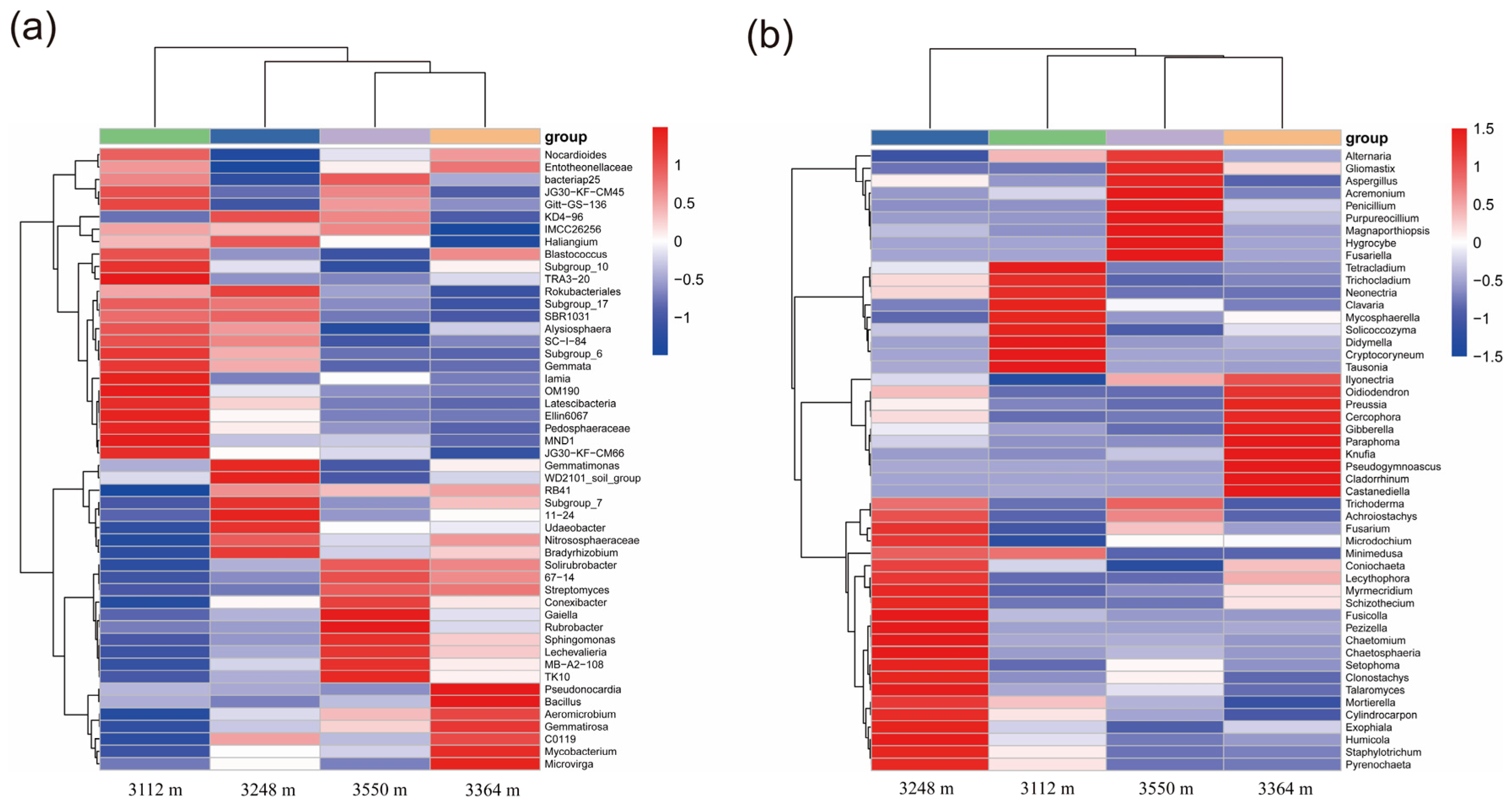
3.3. Composition of Soil Bacteria and Fungi in Walnut Orchards
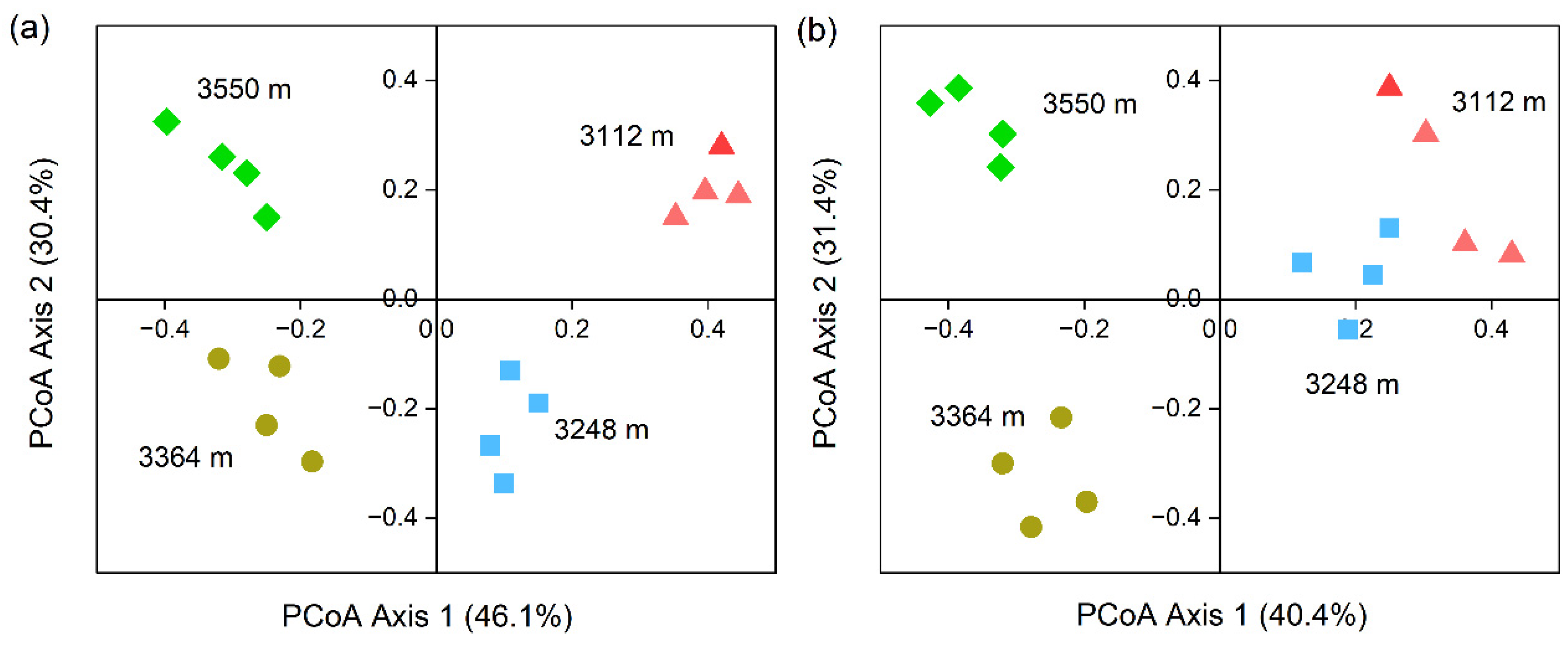
3.4. Beta Diversity of Soil Bacteria and Fungi in Walnut Orchard Soil
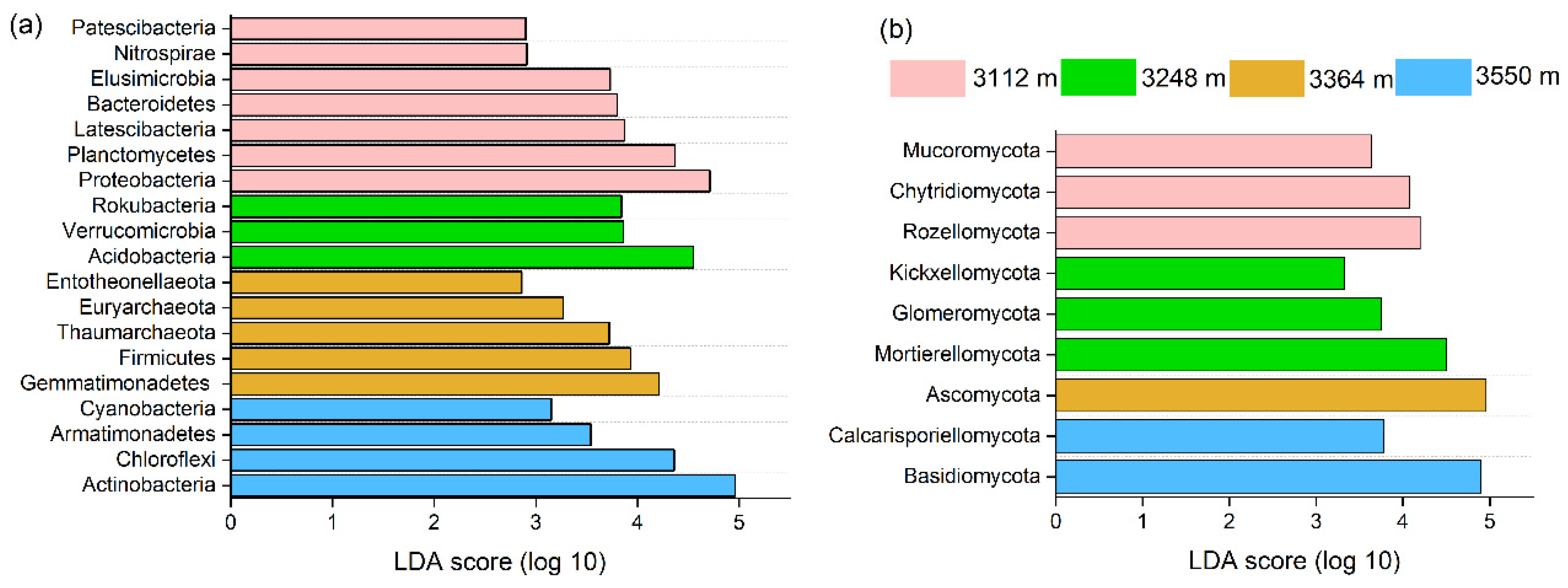
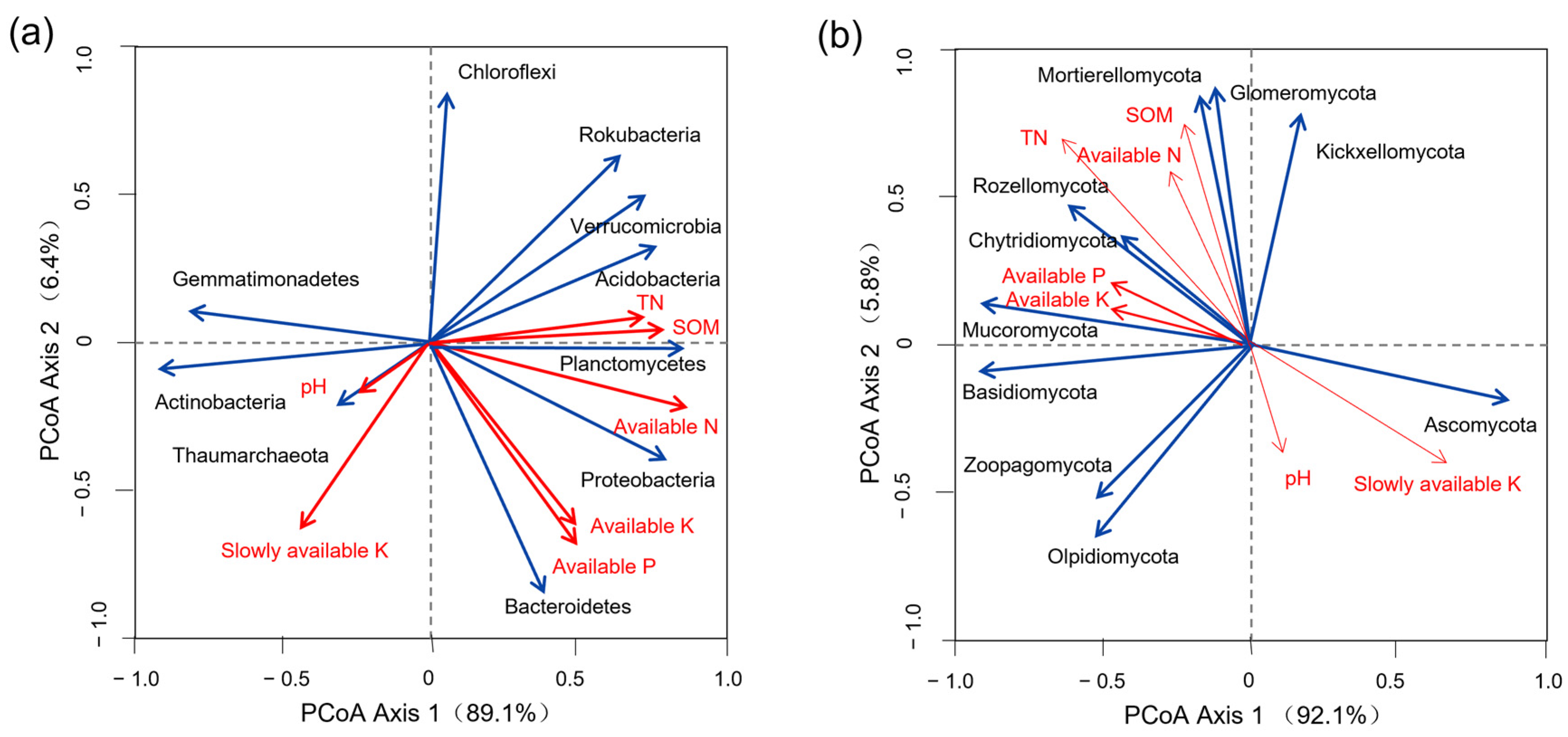
3.5. Correlation of Dominant Microbial Communities with Altitude
3.6. Correlation between Dominant Microbial Communities and Soil Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Tang, Y.; Bao, J.; Wang, H.; Peng, F.; Chen, M.; Tan, P. Pecan plantation age influences the structures, ecological networks, and functions of soil microbial communities. Land Degrad. Dev. 2022, 33, 3294–3309. [Google Scholar] [CrossRef]
- Dong, N.; Hu, G.; Zhang, Y.; Qi, J.; Chen, Y.; Hao, Y. Effects of green-manure and tillage management on soil microbial community composition, nutrients and tree growth in a walnut orchard. Sci. Rep. 2021, 11, 16882. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zu, C.; Wang, C.; Yang, J.; Yu, H.; Wu, H. Different responses of rhizosphere and non-rhizosphere soil microbial communities to consecutive Piper nigrum L. monoculture. Sci. Rep. 2016, 6, 35825. [Google Scholar] [CrossRef] [PubMed]
- Manirakiza, E.; Ziadi, N.; Hamel, C.; Levesque, V.; Antoun, H.; Karam, A. Soil microbial community dynamics after co-application of biochar and paper mill biosolids. Appl. Soil Ecol. 2021, 165, 103960. [Google Scholar] [CrossRef]
- Cui, Y.; Bing, H.; Fang, L.; Wu, Y.; Yu, J.; Shen, G.; Jiang, M.; Wang, X.; Zhang, X. Diversity patterns of the rhizosphere and bulk soil microbial communities along an altitudinal gradient in an alpine ecosystem of the eastern Tibetan Plateau. Geoderma 2019, 338, 118–127. [Google Scholar] [CrossRef]
- Grosso, A.; Riveros, C.; Asensio, C.; Grosso, N.; Nepote, V.; Nepote, V. Improving walnuts’ preservation by using walnut phenolic extracts as natural antioxidants through a walnut protein based edible coating. J. Food Sci. 2020, 85, 3043–3051. [Google Scholar] [CrossRef]
- Chen, Z.; Qi, J. Current situation and prospect of walnut industry in China. SHS Web Conf. 2022, 148, 03046. [Google Scholar] [CrossRef]
- Jin, J.; Wang, L.; Müller, K.; Wu, J.; Wang, H.; Zhao, K.; Fu, W. A 10-year monitoring of soil properties dynamics and soil fertility evaluation in Chinese hickory plantation regions of southeastern China. Sci. Rep. 2021, 11, 23531. [Google Scholar] [CrossRef]
- Du, T.; He, H.; Zhang, Q.; Lu, L.; Mao, W.; Zhai, M. Positive effects of organic fertilizers and biofertilizers on soil microbial community composition and walnut yield. Appl. Soil Ecol. 2022, 175, 104457. [Google Scholar] [CrossRef]
- Thioye, B.; Legras, M.; Castel, L.; Hirissou, F.; Chaftar, N.; Trinsoutrot-Gattin, I. Understanding arbuscular mycorrhizal colonization in walnut plantations: The contribution of cover crops and soil microbial communities. Agriculture 2022, 12, 1. [Google Scholar] [CrossRef]
- Jing, Z.; Chen, R.; Wei, S.; Feng, Y.; Zhang, J.; Lin, X. Response and feedback of C mineralization to P availability driven by soil microorganisms. Soil Biol. Biochem. 2017, 105, 111–120. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Fierer, N.; Turner, B.; Whitaker, J.; Ostle, N.J.; Mcnamara, N.P. Microbes follow Humboldt: Temperature drives plant and soil microbial diversity patterns from the Amazon to the Andes. Ecology 2018, 99, 2455–2466. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, Y.; Shi, Z.; Rossel, R.A.V.; Liang, Z.Z.; Wang, H.Z.; Zhou, L.Q.; Yu, W. Interactive effects of elevation and land use on soil bacterial communities in the Tibetan Plateau. Pedosphere 2020, 30, 817–831. [Google Scholar] [CrossRef]
- He, Z.S.; Gu, X.G.; Jiang, L.; Xu, D.W.; Liu, J.F.; Li, W.Z.; Chen, W.W. Characteristics and influencing factors of bacterial communities in forest soil dominance at different altitudes on the southern slope of Daiyun Mountain. J. Beijing For. Univ. 2022, 44, 107–116. [Google Scholar] [CrossRef]
- Cao, L.H.; Liu, H.M.; Yang, H.; Lian, Y.Z. Characteristics of soil microbial and fungal community composition at different altitudes in the Sejla Mountains. J. Soil Water Conserv. 2022, 36, 371–378. [Google Scholar] [CrossRef]
- Tianzhu, L.; Guicai, S.; Jian, W.; Gengxin, Z. Microbial communities and associated enzyme activities in alpine wetlands with increasing altitude on the Tibetan Plateau. Wetlands 2017, 37, 401–412. [Google Scholar] [CrossRef]
- Essel, E.; Xie, J.; Deng, C.; Peng, Z.; Wang, J.; Shen, J.; Xie, J.; Coulter, J.; Li, L. Bacterial and fungal diversity in rhizosphere and bulk soil under different long-term tillage and cereal/legume rotation. Soil Tillage Res. 2019, 194, 104302. [Google Scholar] [CrossRef]
- Fisher, K.E.; Roberson, R.W. Hyphal tip cytoplasmic organization in four zygomycetous fungi. Mycologia 2016, 108, 533–542. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, R.; Shi, Y.; Guo, L. Natural and political determinants of ecological vulnerability in the Qinghai–Tibet plateau: A case study of Shannan, China. ISPRS Int. J. Geo-Inf. 2021, 10, 327. [Google Scholar] [CrossRef]
- Tian, H.; Wang, H.; Hui, X.; Wang, Z.; Drijber, R.A.; Liu, J. Changes in soil microbial communities after 10 years of winter wheat cultivation versus fallow in an organic-poor soil in the Loess Plateau of China. PLoS ONE 2017, 12, e0184223. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Ma, T.; Raza, W.; Li, J.; Howland, J.G.; Huang, Q.; Shen, Q. Impacts of inorganic and organic fertilization treatments on bacterial and fungal communities in a paddy soil. Appl. Soil Ecol. 2017, 112, 42–50. [Google Scholar] [CrossRef]
- Yan, T.; Xue, J.; Zhou, Z.; Wu, Y. Biochar-based fertilizer amendments improve the soil microbial community structure in a karst mountainous area. Sci. Total Environ. 2021, 794, 148757. [Google Scholar] [CrossRef] [PubMed]
- Mcbain, A.J.; Bartolo, R.G.; Catrenich, C.E.; Charbonneau, D.; Ledder, R.G.; Price, B.B.; Gilbert, P. Exposure of sink drain micro-cosmos to triclosan: Population dynamics and antimicrobial susceptibility. Appl. Environ. Microbiol. 2003, 69, 5433–54442. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, B.; Liu, Y.; Guo, Y.; Shi, P.; Wei, G. Distinct large-scale biogeographic patterns of fungal communities in bulk soil and soybean rhizosphere in China. Sci. Total Environ. 2018, 644, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tang, Y.; Bao, J.; Wang, H.; Peng, F.; Tan, P.; Chu, G.; Liu, S. A stronger rhizosphere impact on the fungal communities compared to the bacterial communities in Pecan plantations. Front. Microbiol. 2022, 13, 899801. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.C.; Li, B.X.; Xu, C.Y.; Raza, M.; Wang, Q.; Wang, Q.Z.; Xu, Y.J. Intercropping walnut and tea: Effects on soil nutrients, enzyme activity, and microbial communities. Front. Microbiol. 2022, 13, 852342. [Google Scholar] [CrossRef]
- Baruch, Z.; Liddicoat, C.; Laws, M.; Marker, L.K.; Morelli, H.; Yan, D.F.; Young, J.M.; Breed, M.F. Characterizing the soil fungal microbiome in metropolitan green spaces across a vegetation biodiversity gradient. Fungal Ecol. 2020, 47, 100939. [Google Scholar] [CrossRef]
- Gu, M.; Xu, W.; Ma, K.; Mahmut, O.; Zhang, Z.; Zhu, J.; Tang, Q.; Chu, M.; Zhang, L. Soil Microbial Ecological Evaluation of Walnut with Different Planting Years Based on Principal Component-Cluster Analysis. Xinjiang Agric. Sci. 2022, 59, 474–484. [Google Scholar]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J. Introducing mothur: Open source, platform independent, community supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, X.; Zi, H.; Hu, L.; Ade, L.; Wang, G.; Lerdau, M. The effect of simulated warming on root dynamics and soil microbial community in an alpine meadow of the Qinghai-Tibet Plateau. Appl. Soil Ecol. 2017, 116, 30–41. [Google Scholar] [CrossRef]
- Wang, J.Y. Prediction of Ecosystem Versatility by the Complexity of Soil Microbial Community Networks at Different Altitudes in the Qinghai-Tibet Alpine Meadow; Northwest A&F University: Xi’an, China, 2022. [Google Scholar]
- Zhang, C.; Liu, G.B.; Xue, S.; Wang, G.L. Soil bacterial community dynamics reflect changes in plant community and soil properties during the secondary succession of abandoned farmland in the Loess Plateau. Soil Biol. Biochem. 2016, 97, 40–49. [Google Scholar] [CrossRef]
- Vanegas-León, M.L.; Sulzbacher, M.A.; Rinaldi, A.C.; Roy, M.; Selosse, M.A.; Neves, M.A. Are trechisporales ectomycorrhizal or non-mycorrhizal root endophytes? Mycol. Prog. 2019, 18, 1231–1240. [Google Scholar] [CrossRef]
- Cahanovitc, R.; Livne-Luzon, S.; Angel, R.; Klein, T. Ectomycorrhizal fungi mediate belowground carbon transfer between pines and oaks. ISME J. 2022, 16, 1420–1429. [Google Scholar] [CrossRef] [PubMed]


| Walnut Orchard | Altitude | Vegetation Type | Parent Material | Agrotype |
|---|---|---|---|---|
| Ciren village, Anrao town | 3112 m | Walnut, rapeseed, highland barley | sediment | Calcareous alluvial soil |
| Longba village, Gyaca town | 3248 m | Walnut, grassland, shrub | glutenite | Brush prairie soil |
| Lagang village, Anrao town | 3364 m | Walnut, grassland | deluvium | Plateau prairie soil |
| Sangzhu village, Anrao town | 3550 m | Walnut, grassland | eluvium | Mountain meadow soil |
| Altitude | pH | SOM (g kg−1) | TN (g kg−1) | Available N (mg kg−1) | Available P (mg kg−1) | Available K (mg kg−1) | Slowly Available K (mg kg−1) |
|---|---|---|---|---|---|---|---|
| 3112 m | 7.42 ab | 38.9 a | 0.06 a | 3.99 a | 21.73 a | 194.8 a | 1126.2 b |
| 3246 m | 7.36 b | 32.8 a | 0.05 a | 2.13 b | 1.91 d | 50.8 c | 780.9 c |
| 3364 m | 7.51 a | 16.5 c | 0.02 c | 0.86 d | 3.31 c | 57.4 c | 2539.7 a |
| 3550 m | 7.52 a | 20.7 b | 0.04 b | 1.12 c | 4.03 b | 73.5 b | 579.6 d |
| Microbe Type | Altitudes | Richness Index | Diversity Index | Coverage | ||
|---|---|---|---|---|---|---|
| Chao1 | Species | Simpson | Shannon | |||
| Bacteria | 3112 m | 3750 b | 3725 b | 0.9986 a | 10.79 a | 0.999 a |
| 3248 m | 3561 c | 3556 c | 0.9987 a | 10.76 a | 1.000 a | |
| 3364 m | 4172 a | 4151 a | 0.9987 a | 10.85 a | 0.999 a | |
| 3550 m | 3769 b | 3746 b | 0.9986 a | 10.65 a | 0.999 a | |
| Fungi | 3112 m | 731 b | 730 b | 0.9733 a | 6.54 b | 1.000 a |
| 3248 m | 884 a | 884 a | 0.9854 a | 7.41 a | 1.000 a | |
| 3364 m | 591 c | 589 c | 0.8732 b | 5.54 c | 1.000 a | |
| 3550 m | 589 c | 589 c | 0.9301 a | 6.10 b | 1.000 a | |
| Bacteriophyta | Correlation | Eumycophyta | Correlation |
|---|---|---|---|
| Actinobacteria | 0.819 ** | Ascomycota | 0.069 |
| Acidobacteria | −0.673 * | Basidiomycota | 0.070 |
| Proteobacteria | −0.809 ** | Mortierellomycota | −0.285 |
| Chloroflexi | 0.193 | Glomeromycota | −0.499 |
| Planctomycetes | −0.837 ** | Chytridiomycota | −0.655 * |
| Gemmatimonadetes | 0.462 | Olpidiomycota | 0.672 * |
| Thaumarchaeota | −0.015 | Zoopagomycota | 0.685 * |
| Verrucomicrobia | −0.527 * | Kickxellomycota | −0.014 |
| Rokubacteria | −0.396 | Mucoromycota | −0.089 |
| Bacteroidetes | −0.605 * | Rozellomycota | −0.645 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, R.; Zhao, X.; Li, P.; Si, Z.; Gao, Y.; Li, J. Composition and Diversity of Soil Microbial Communities in Walnut Orchards at Different Altitudes in Southeastern Tibet. Land 2023, 12, 1419. https://doi.org/10.3390/land12071419
Yan R, Zhao X, Li P, Si Z, Gao Y, Li J. Composition and Diversity of Soil Microbial Communities in Walnut Orchards at Different Altitudes in Southeastern Tibet. Land. 2023; 12(7):1419. https://doi.org/10.3390/land12071419
Chicago/Turabian StyleYan, Ruyu, Ximei Zhao, Penghui Li, Zhuanyun Si, Yang Gao, and Jifu Li. 2023. "Composition and Diversity of Soil Microbial Communities in Walnut Orchards at Different Altitudes in Southeastern Tibet" Land 12, no. 7: 1419. https://doi.org/10.3390/land12071419
APA StyleYan, R., Zhao, X., Li, P., Si, Z., Gao, Y., & Li, J. (2023). Composition and Diversity of Soil Microbial Communities in Walnut Orchards at Different Altitudes in Southeastern Tibet. Land, 12(7), 1419. https://doi.org/10.3390/land12071419








