The Use of biochar in the Remediation of Pb, Cd, and Cu-Contaminated Soils. The Impact of biochar Feedstock and Preparation Conditions on Its Remediation Capacity
Abstract
1. Introduction
1.1. The Soil as a Background for Human Activities
1.2. The Heavy Metals (HMs) Soil Pollution
1.3. The Use of Biochar for Remediation of HMs Polluted Soils
- (a)
- charcoal, which is a material of solid nature where the carbon composition often exceeds 70%, formed by heating up under anoxic conditions, OM coming from plants, wood, or animal tissues or bones [54].
- (b)
- decarbonized OM, which comes from the thermochemical biomass conversion under restricted oxygen conditions [55].
| biochar-HMs interaction mechanisms |  | Electrostatic attraction | Ion exchange | Complexation | Precipitation |
| Physicochemical parameters influence the mechanisms |  | pH | CEC | Inorganic ions | OM |
- -
- Electrostatic attraction: recent research proves that a high degree of biochar electronegativity attracts positively charged groups. The strength of this attraction depends on the surface load of the negatively charged groups [62]. The overall negative charge of biochar grows with the increase in pH [13,63].
- -
- Ion exchange: in general, biochar is characterized by high CEC and, consequently, by the presence of free cations, for example Ca2+ and Mg2+. Between biochar and soil, an exchange of ions may be established [1,14]. The increase in biochar CEC could increase its adsorption capacity for HMs. The oxygen-containing functional groups in biochar, mostly carboxyl groups (-COOH), can also bind metal ions through ion exchange [64].
- -
- Complexation: the functional groups on the surface of the biochar can immobilize HMs, forming stable complexes [58]. The functional groups provide binding points for HMs to form association complexes, increasing the rate of the specific adsorption [41,65]. This is more effective for biochar possessing a low mineral content. The complexation is enhanced by the increase in Fe, Mn, and C content. In this way, insoluble and stable metal complexes are formed. Additionally, inorganic ions containing Si, S, or Cl present in biochar, can form complexes with HMs, further reducing their mobility in the soil.
- -
- Precipitation: biochar contains minerals that can bind HMs, yielding insoluble sediments. Indeed, precipitation has been observed in the case of absorption of inorganic phosphorus by biochar [41]. Nevertheless, after the modification of the soil by addition of biochar, its characteristics, including pH, SOC, sulphate concentrations, and CEC, may change [1,38], affecting the HMs–biochar interactions and, finally, the mobility and bioavailability of HMs in the soil.
- -
- pH: pH has a significant influence on the chemistry of both soil and biochar [13]. More specifically, it affects the speciation and the mobility of HMs in the soil [66]. Generally, biochar is usually alkaline, and its application in the soil increases its pH, especially when the contaminated soil is acidic. In this way, however, the hydrolysis of HMs is increased, leading to an enhanced adsorption by the soil and an accelerated transformation of oxidizable and residual fractions of the pollutants. In addition, the increase in pH enhances the complexation of HMs [10], resulting in a decrease in the HM risks.
- -
- Cation Exchange Capacity (CEC): since the CEC of biochar is particularly high, it enhances the corresponding property of the soil when added to it. It has been suggested that the reduction in HM concentration and solubility in the soils is partly due to the high proportion of cations exchange points on the biochar surface [1,13].
- -
- The presence of inorganic elements: biochar may contain high concentrations of elements, such as Na, K, Mg, Ca, and P [67]. These inorganic elements are free in the contaminated soil and, possibly, after adding biochar, contribute to cation-exchange processes between biochar and soil [47]. Additionally, the oxides of Ca, Si, and Mn that are contained in biochar may function as additional absorption points for metal cations present in the soil.
- -
- Change in the organic carbon content: the addition of biochar to the soil results in a release of OC dissolved. In this way, a reduction of the mobility and bioavailability of HMs could be caused by the enhancement of complexation between biochar functional groups and HMs [58].
2. Materials and Methods
3. Results
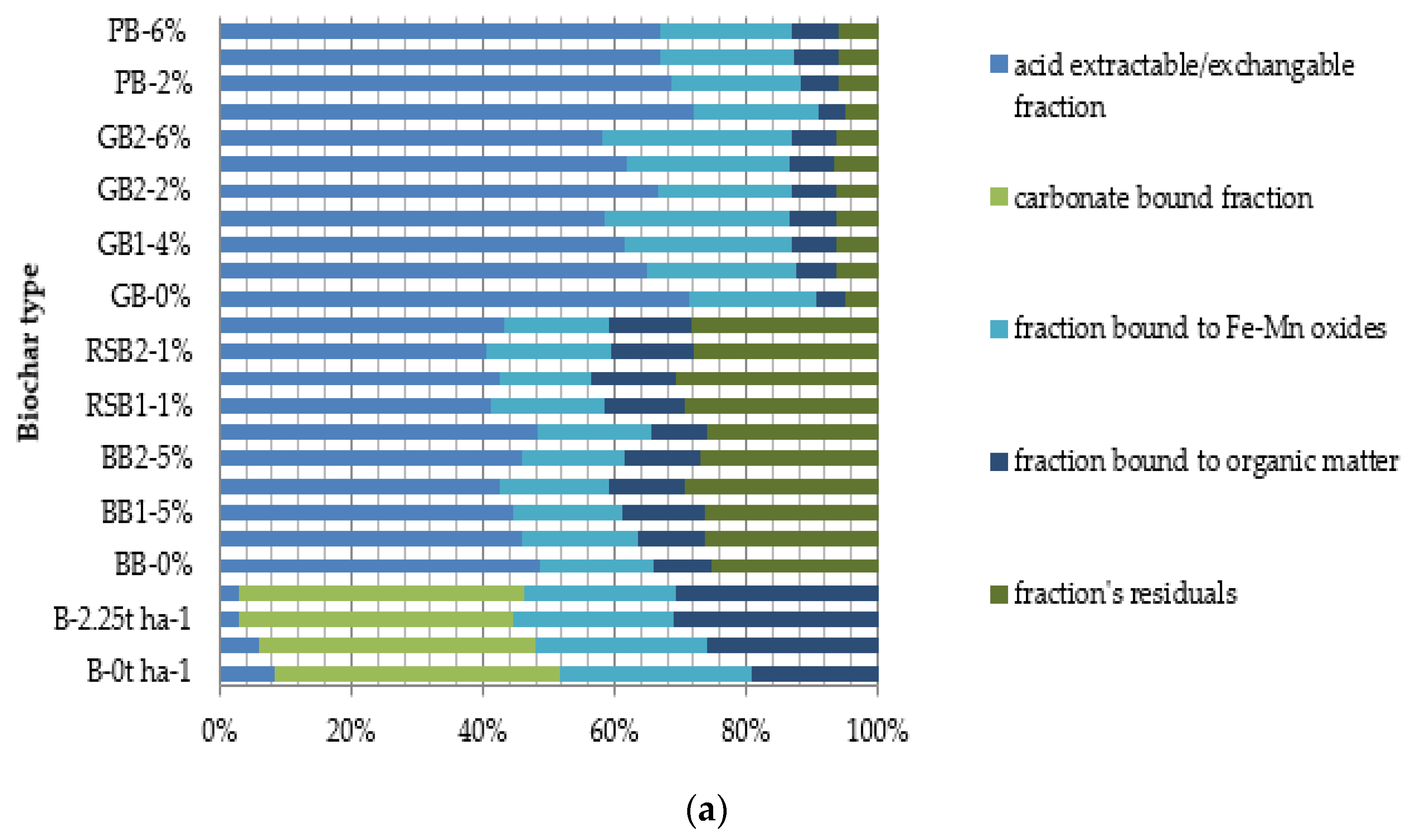
- Lead (Pb)

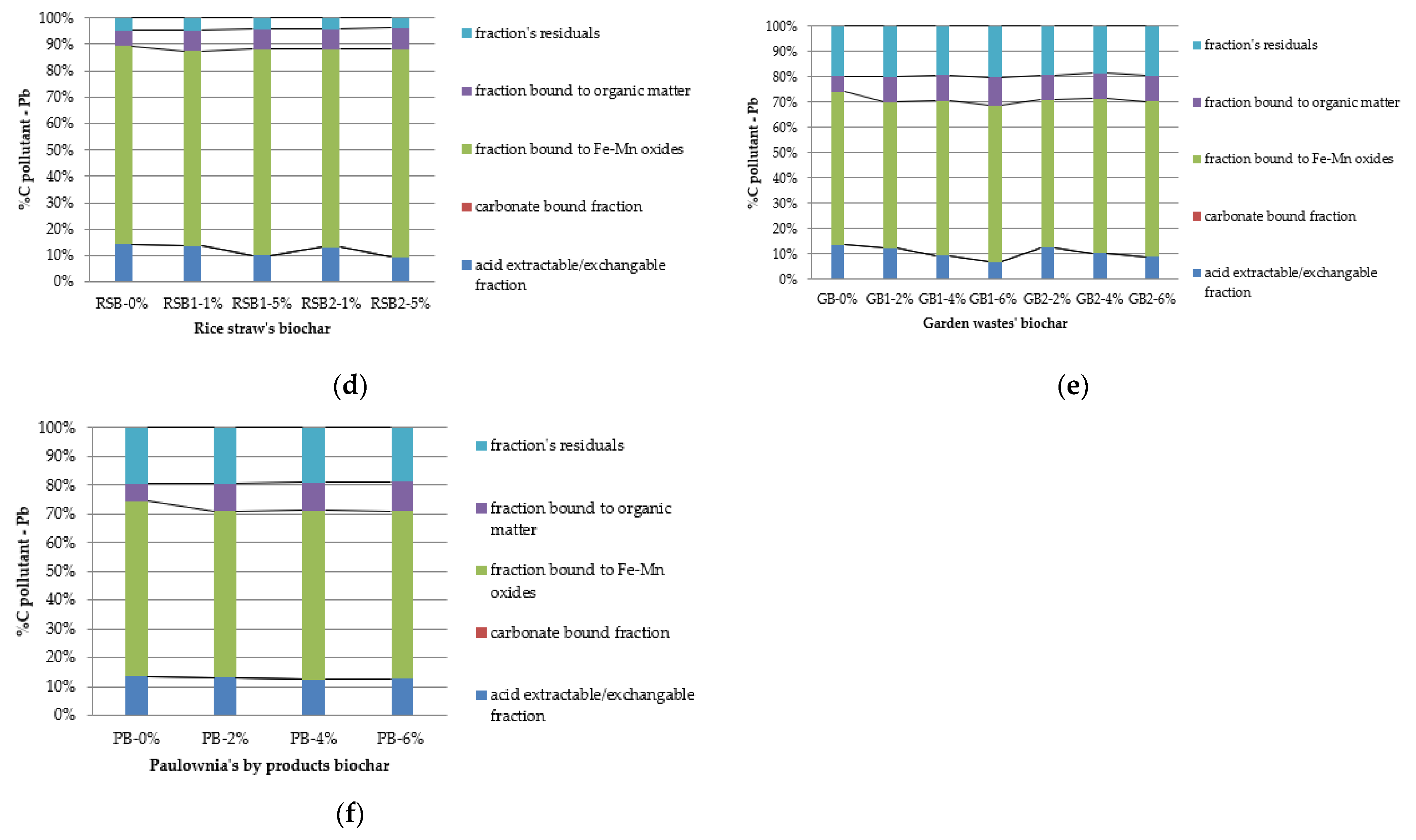
- Cadmium (Cd)

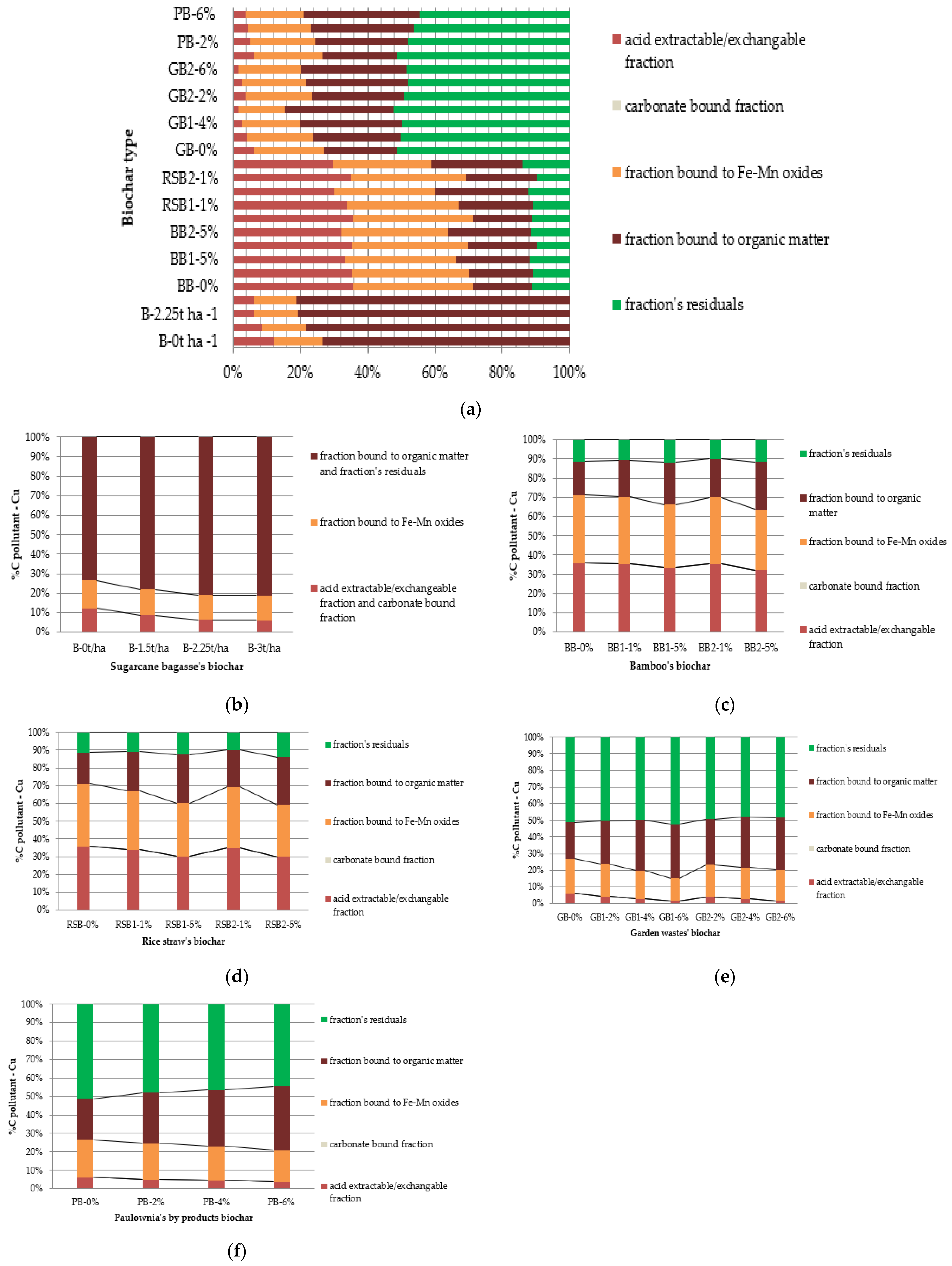
- Copper (Cu)
3.1. Acid Extractable/Exchangeable Fraction
3.2. Carbonate Bound Fraction
3.3. Fe/Mn Oxides Fraction
3.4. Organic Bound Fraction
3.5. Residual Fraction
4. Discussion
4.1. Acid Extractable/Exchangeable Fraction
4.2. Carbonate Bound Fraction
4.3. Fe/Mn Oxides Fraction
4.4. Organic Bound Fraction
4.5. Residual Fraction
4.6. Effects of Pyrolysis Temperatures and Application Percentages of Biochar Used
- -
- the physical adsorption on the biochar surface
- -
- the chemical bonds with the ions on the biochar surface
- -
- the formation of complexes with the active functional groups
- -
- the precipitation on the biochar surface by the phosphate ions
- -
- the precipitation due to the pH increase in the contaminated soil, especially when the soil is acidic
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; Press, C., Ed.; Taylor and Francis Group: Ann Arbor, MI, USA, 2010; ISBN 9781420093704. [Google Scholar]
- Sintim, H.Y.; Shahzad, K.; Yin, X. Editorial: Innovative Agricultural Practices to Improve Soil Health and Sustain Food Production. Front Sustain Food Syst. 2022, 6, 21–23. [Google Scholar] [CrossRef]
- Islam, N.F.; Patowary, R.; Sarma, H. Biosurfactant-Assisted Phytoremediation for a Sustainable Future. In Assisted Phytoremediation; Elsevier: Amsterdam, The Netherlands, 2021; pp. 399–414. [Google Scholar]
- Blum, W.E.H. Soil and Land Resources for Agricultural Production: General Trends and Future Scenarios-A Worldwide Perspective. Int. Soil Water Conserv. Res. 2013, 1, 1–14. [Google Scholar] [CrossRef]
- Dong, M.; Chen, W.; Chen, X.; Xing, X.; Shao, M.; Xiong, X.; Luo, Z. Geochemical Markers of the Anthropocene: Perspectives from Temporal Trends in Pollutants. Sci. Total Environ. 2021, 763, 142987. [Google Scholar] [CrossRef]
- Amoah-Antwi, C.; Kwiatkowska-Malina, J.; Thornton, S.F.; Fenton, O.; Malina, G.; Szara, E. Restoration of Soil Quality Using Biochar and Brown Coal Waste: A Review. Sci. Total Environ. 2020, 722, 137852. [Google Scholar] [CrossRef] [PubMed]
- Dror, I.; Yaron, B.; Berkowitz, B. The Human Impact on All Soil-Forming Factors during the Anthropocene. ACS Environ. Au 2022, 2, 11–19. [Google Scholar] [CrossRef]
- Jansson, J.K.; Wu, R. Soil Viral Diversity, Ecology and Climate Change. Nat. Rev. Microbiol. 2022; in press. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Zia-ur-Rehman, M.; Farooq Qayyum, M.; Abbas, F.; Hannan, F.; Rinklebe, J.; Sik Ok, Y. Effect of Biochar on Cadmium Bioavailability and Uptake in Wheat (Triticum aestivum L.) Grown in a Soil with Aged Contamination. Ecotoxicol. Environ. Saf. 2017, 140, 37–47. [Google Scholar] [CrossRef]
- Devi, U.; Bhattacharyya, K.G. Mobility and Bioavailability of Cd, Co, Cr, Cu, Mn and Zn in Surface Runoff Sediments in the Urban Catchment Area of Guwahati, India. Appl. Water Sci. 2018, 8, 18. [Google Scholar] [CrossRef]
- Karpouzas, D.G.; Pantelelis, I.; Menkissoglu-Spiroudi, U.; Golia, E.; Tsiropoulos, N.G. Leaching of the Organophosphorus Nematicide Fosthiazate. Chemosphere 2007, 68, 1359–1364. [Google Scholar] [CrossRef]
- Hancock, T. Health in the Anthropocene: From the Global to the Local. In International Encyclopedia of Human Geography; Elsevier: Amsterdam, The Netherlands, 2020; pp. 323–328. [Google Scholar]
- Alloway, B.J. Heavy Metals and Metalloids as Micronutrients for Plants and Animals BT—Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability. Environ. Pollut. 2013, 22, 195–209. [Google Scholar]
- Khan, S.; Naushad, M.; Lima, E.C.; Zhang, S.; Shaheen, S.M.; Rinklebe, J. Global Soil Pollution by Toxic Elements: Current Status and Future Perspectives on the Risk Assessment and Remediation Strategies—A Review. J. Hazard. Mater. 2021, 417, 126039. [Google Scholar] [CrossRef] [PubMed]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Qin, G.; Niu, Z.; Yu, J.; Li, Z.; Ma, J.; Xiang, P. Soil Heavy Metal Pollution and Food Safety in China: Effects, Sources and Removing Technology. Chemosphere 2021, 267, 129205. [Google Scholar] [CrossRef]
- Golia, E.E.; Dimirkou, A.; Floras, S.A. Spatial Monitoring of Arsenic and Heavy Metals in the Almyros Area, Central Greece. Statistical Approach for Assessing the Sources of Contamination. Environ. Monit. Assess. 2015, 187, 399–412. [Google Scholar] [CrossRef]
- Awasthi, G.; Nagar, V.; Mandzhieva, S.; Minkina, T.; Sankhla, M.S.; Pandit, P.P.; Aseri, V.; Awasthi, K.K.; Rajput, V.D.; Bauer, T. Sustainable Amelioration of Heavy Metals in Soil Ecosystem: Existing Developments to Emerging Trends. Minerals 2022, 12, 85. [Google Scholar] [CrossRef]
- O’Connor, D.; Hou, D.; Ok, Y.S.; Lanphear, B.P. The Effects of Iniquitous Lead Exposure on Health. Nat. Sustain 2020, 3, 77–79. [Google Scholar] [CrossRef]
- Jakubus, M.; Bakinowska, E. The Effect of Immobilizing Agents on Zn and Cu Availability for Plants in Relation to Their Potential Health Risks. Appl. Sci. 2022, 12, 6538. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A. Impact of Heavy Metals on the Environment and Human Health: Novel Therapeutic Insights to Counter the Toxicity. J. King Saud. Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Golia, E.E.; Tsiropoulos, G.N.; Füleky, G.; Floras, S.; Vleioras, S. Pollution Assessment of Potentially Toxic Elements in Soils of Different Taxonomy Orders in Central Greece. Environ. Monit. Assess. 2019, 191, 106. [Google Scholar] [CrossRef]
- Wang, L.; Jin, Y.; Weiss, D.J.; Schleicher, N.J.; Wilcke, W.; Wu, L.; Guo, Q.; Chen, J.; O’Connor, D.; Hou, D. Possible Application of Stable Isotope Compositions for the Identification of Metal Sources in Soil. J. Hazard. Mater. 2021, 407, 124812. [Google Scholar] [PubMed]
- Wang, J.; Shi, L.; Zhai, L.; Zhang, H.; Wang, S.; Zou, J.; Shen, Z.; Lian, C.; Chen, Y. Analysis of the Long-Term Effectiveness of Biochar Immobilization Remediation on Heavy Metal Contaminated Soil and the Potential Environmental Factors Weakening the Remediation Effect: A Review. Ecotoxicol. Environ. Saf. 2021, 207, 111261. [Google Scholar] [CrossRef]
- Wang, H.; Xia, W.; Lu, P. Study on Adsorption Characteristics of Biochar on Heavy Metals in Soil. Korean J. Chem. Eng. 2017, 34, 1867–1873. [Google Scholar] [CrossRef]
- Gong, H.; Chi, J.; Ding, Z.; Zhang, F.; Huang, J. Removal of Lead from Two Polluted Soils by Magnetic Wheat Straw Biochars. Ecotoxicol. Environ. Saf. 2020, 205, 111132. [Google Scholar] [CrossRef]
- AL-Huqail, A.A.; Kumar, P.; Eid, E.M.; Adelodun, B.; Abou Fayssal, S.; Singh, J.; Arya, A.K.; Goala, M.; Kumar, V.; Širić, I. Risk Assessment of Heavy Metals Contamination in Soil and Two Rice (Oryza sativa L.) Varieties Irrigated with Paper Mill Effluent. Agriculture 2022, 12, 1864. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Sharma, R.K.; Chauhan, A.; Ranjan, A.; Rajput, V.D.; Minkina, T.; Mandzhieva, S.S.; Mina, U.; Wadhwa, S.; Bobde, P.; et al. Assessment of Heavy Metal Distribution and Health Risk of Vegetable Crops Grown on Soils Amended with Municipal Solid Waste Compost for Sustainable Urban Agriculture. Water 2023, 15, 228. [Google Scholar] [CrossRef]
- Houri, T.; Khairallah, Y.; Al Zahab, A.; Osta, B.; Romanos, D.; Haddad, G. Heavy Metals Accumulation Effects on The Photosynthetic Performance of Geophytes in Mediterranean Reserve. J. King Saud. Univ. Sci. 2020, 32, 874–880. [Google Scholar] [CrossRef]
- Waheed, A.; Haxim, Y.; Islam, W.; Ahmad, M.; Ali, S.; Wen, X.; Khan, K.A.; Ghramh, H.A.; Zhang, Z.; Zhang, D. Impact of Cadmium Stress on Growth and Physio-Biochemical Attributes of Eruca Sativa Mill. Plants 2022, 11, 2981. [Google Scholar] [CrossRef]
- Giannakoula, A.; Therios, I.; Chatzissavvidis, C. Effect of Lead and Copper on Photosynthetic Apparatus in Citrus (Citrus aurantium L.) Plants. the Role of Antioxidants in Oxidative Damage as a Response to Heavy Metal Stress. Plants 2021, 10, 155. [Google Scholar] [CrossRef]
- Wang, R.Z.; Huang, D.L.; Liu, Y.G.; Zhang, C.; Lai, C.; Zeng, G.M.; Cheng, M.; Gong, X.M.; Wan, J.; Luo, H. Investigating the Adsorption Behavior and the Relative Distribution of Cd2+ Sorption Mechanisms on Biochars by Different Feedstock. Bioresour. Technol. 2018, 261, 265–271. [Google Scholar] [CrossRef]
- Fryda, L.; Visser, R. Biochar for Soil Improvement: Evaluation of Biochar from Gasification and Slow Pyrolysis. Agriculture 2015, 5, 1076–1115. [Google Scholar] [CrossRef]
- Zaheer, M.S.; Ali, H.H.; Soufan, W.; Iqbal, R.; Habib-Ur-rahman, M.; Iqbal, J.; Israr, M.; el Sabagh, A. Potential Effects of Biochar Application for Improving Wheat (Triticum aestivum L.) Growth and Soil Biochemical Properties under Drought Stress Conditions. Land 2021, 10, 1125. [Google Scholar] [CrossRef]
- Medyńska-Juraszek, A.; Rivier, P.A.; Rasse, D.; Joner, E.J. Biochar Affects Heavy Metal Uptake in Plants through Interactions in the Rhizosphere. Appl. Sci. 2020, 10, 5105. [Google Scholar] [CrossRef]
- Golia, E.E.; Aslanidis, P.S.C.; Papadimou, S.G.; Kantzou, O.D.; Chartodiplomenou, M.A.; Lakiotis, K.; Androudi, M.; Tsiropoulos, N.G. Assessment of Remediation of Soils, Moderately Contaminated by Potentially Toxic Metals, Using Different Forms of Carbon (Charcoal, Biochar, Activated Carbon). Impacts on Contamination, Metals Availability and Soil Indices. Sustain. Chem. Pharm. 2022, 28, 100724. [Google Scholar] [CrossRef]
- Amin, M.A.; Haider, G.; Rizwan, M.; Schofield, H.K.; Qayyum, M.F.; Zia-ur-Rehman, M.; Ali, S. Different Feedstocks of Biochar Affected the Bioavailability and Uptake of Heavy Metals by Wheat (Triticum aestivum L.) Plants Grown in Metal Contaminated Soil. Environ. Res. 2023, 217, 114845. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A Review on Heavy Metals Contamination in Soil: Effects, Sources, and Remediation Techniques. Soil Sediment. Contam. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology; Taylor and Francis: London, UK, 2012; pp. 235–282. [Google Scholar]
- Kang, S.; Jung, J.; Choe, J.K.; Ok, Y.S.; Choi, Y. Effect of Biochar Particle Size on Hydrophobic Organic Compound Sorption Kinetics: Applicability of Using Representative Size. Sci. Total Environ. 2018, 634, 619–620. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, M.; Hu, X. Removal of Heavy Metals from Soil with Biochar Composite: A Critical Review of the Mechanism. J. Environ. Chem. Eng. 2021, 9, 105830. [Google Scholar] [CrossRef]
- Wang, S.; Xu, Y.; Norbu, N.; Wang, Z. Remediation of Biochar on Heavy Metal Polluted Soils. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 108. [Google Scholar]
- Ali, A.; Guo, D.; Arockiam Jeyasundar, P.G.S.; Li, Y.; Xiao, R.; Du, J.; Li, R.; Zhang, Z. Application of Wood Biochar in Polluted Soils Stabilized the Toxic Metals and Enhanced Wheat (Triticum aestivum) Growth and Soil Enzymatic Activity. Ecotoxicol. Environ. Saf. 2019, 184, 109635. [Google Scholar] [CrossRef]
- Banik, C.; Koziel, J.A.; Bonds, D.; Singh, A.K.; Licht, M.A. Comparing Biochar-Swine Manure Mixture to Conventional Manure Impact on Soil Nutrient Availability and Plant Uptake-a Greenhouse Study. Land 2021, 10, 372. [Google Scholar] [CrossRef]
- Huang, R.; Li, B.; Chen, Y.; Tao, Q.; Xu, Q.; Wen, D.; Gao, X.; Li, Q.; Tang, X.; Wang, C. Biochar Application Increases Labile Carbon and Inorganic Nitrogen Supply in a Continuous Monocropping Soil. Land 2022, 11, 473. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Neugschwandtner, R.W.; Kopecký, M.; Amirahmadi, E.; Bucur, D.; Walkiewicz, A. Interaction of Biochar with Chemical, Green and Biological Nitrogen Fertilizers on Nitrogen Use Efficiency Indices. Agronomy 2022, 12, 2106. [Google Scholar] [CrossRef]
- Liang, M.; Lu, L.; He, H.; Li, J.; Zhu, Z.; Zhu, Y. Applications of Biochar and Modified Biochar in Heavy Metal Contaminated Soil: A Descriptive Review. Sustainability 2021, 13, 14041. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; Tang, L.; Su, M.; Tian, D.; Zhang, L.; Li, Z.; Hu, S. Enhanced Pb Immobilization via the Combination of Biochar and Phosphate Solubilizing Bacteria. Environ. Int. 2019, 127, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Wang, W.; Liu, L.; Wang, L.; Hu, J.; Li, X.; Qiu, G. Remediation of Cadmium-Polluted Weakly Alkaline Dryland Soils Using Iron and Manganese Oxides for Immobilized Wheat Uptake. J. Clean. Prod. 2022, 365, 132794. [Google Scholar] [CrossRef]
- Verma, R.K.; Sankhla, M.S.; Jadhav, E.B.; Parihar, K.; Awasthi, K.K. Phytoremediation of Heavy Metals Extracted from Soil and Aquatic Environments: Current Advances as Well as Emerging Trends. Biointerface Res. Appl. Chem. 2022, 12, 5486–5509. [Google Scholar]
- Lu, K.; Yang, X.; Gielen, G.; Bolan, N.; Ok, Y.S.; Niazi, N.K.; Xu, S.; Yuan, G.; Chen, X.; Zhang, X. Effect of Bamboo and Rice Straw Biochars on the Mobility and Redistribution of Heavy Metals (Cd, Cu, Pb and Zn) in Contaminated Soil. J. Environ. Manag. 2017, 186, 285–292. [Google Scholar] [CrossRef]
- Guo, M.; Song, W.; Tian, J. Biochar-Facilitated Soil Remediation: Mechanisms and Efficacy Variations. Front Environ. Sci. 2020, 8, 183–206. [Google Scholar] [CrossRef]
- Lin, H.; Liu, C.; Li, B.; Dong, Y. Trifolium repens L. Regulated Phytoremediation of Heavy Metal Contaminated Soil by Promoting Soil Enzyme Activities and Beneficial Rhizosphere Associated Microorganisms. J. Hazard. Mater. 2021, 402, 123829. [Google Scholar] [CrossRef] [PubMed]
- Torabian, S.; Qin, R.; Noulas, C.; Lu, Y.; Wang, G. Biochar: An Organic Amendment to Crops and an Environmental Solution. AIMS Agric. Food 2021, 6, 401–415. [Google Scholar] [CrossRef]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of Heavy Metal Contaminated Soils by Biochar: Mechanisms, Potential Risks and Applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef]
- Meng, J.; Tao, M.; Wang, L.; Liu, X.; Xu, J. Changes in Heavy Metal Bioavailability and Speciation from a Pb-Zn Mining Soil Amended with Biochars from Co-Pyrolysis of Rice Straw and Swine Manure. Sci. Total Environ. 2018, 633, 300–307. [Google Scholar] [CrossRef]
- Patra, J.; Panda, S.; Dhal, N. Biochar as a Low-Cost Adsorbent for Heavy Metal Removal: A Review. Int. J. Res. Stud. Biosci. 2017, 6, 1–7. [Google Scholar]
- Amoah-Antwi, C.; Kwiatkowska-Malina, J.; Fenton, O.; Szara, E.; Thornton, S.F.; Malina, G. Holistic Assessment of Biochar and Brown Coal Waste as Organic Amendments in Sustainable Environmental and Agricultural Applications. Water Air Soil Pollut. 2021, 232, 106. [Google Scholar] [CrossRef]
- Zhu, Y.; Lv, X.; Song, J.; Li, W.; Wang, H. Application of Cotton Straw Biochar and Compound Bacillus Biofertilizer Decrease the Bioavailability of Soil Cd through Impacting Soil Bacteria. BMC Microbiol. 2022, 22, 35. [Google Scholar] [CrossRef]
- Tisserant, A.; Cherubini, F. Potentials, Limitations, Co-Benefits, and Trade-Offs of Biochar Applications to Soils for Climate Change Mitigation. Land 2019, 8, 179. [Google Scholar] [CrossRef]
- Taraqqi-A-Kamal, A.; Atkinson, C.J.; Khan, A.; Zhang, K.; Sun, P.; Akther, S.; Zhang, Y. Biochar Remediation of Soil: Linking Biochar Production with Function in Heavy Metal Contaminated Soils. Plant Soil Environ. 2021, 67, 183–201. [Google Scholar] [CrossRef]
- Liang, Y.; Li, X.; Yang, F.; Liu, S. Tracing the Synergistic Migration of Biochar and Heavy Metals Based on 13C Isotope Signature Technique: Effect of Ionic Strength and Flow Rate. Sci. Total Environ. 2023, 859, 160229. [Google Scholar] [CrossRef]
- Sparks, D.L. Toxic Metals in the Environment: The Role of Surfaces. Elements 2005, 1, 193–197. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Lu, H.; Fu, S.; Méndez, A.; Gascó, G. Use of Phytoremediation and Biochar to Remediate Heavy Metal Polluted Soils: A Review. Solid Earth 2014, 5, 65–75. [Google Scholar] [CrossRef]
- Dimirkou, A.; Ioannou, Z.; Golia, E.E.; Danalatos, N.; Mitsios, I.K. Sorption of Cadmium and Arsenic by Goethite and Clinoptilolite. Commun. Soil Sci. Plant Anal. 2009, 40, 259–272. [Google Scholar] [CrossRef]
- Golia, E.E.; Chartodiplomenou, M.A.; Papadimou, S.G.; Kantzou, O.D.; Tsiropoulos, N.G. Influence of Soil Inorganic Amendments on Heavy Metal Accumulation by Leafy Vegetables. Environ. Sci. Pollut. Res. 2021; in press. [Google Scholar] [CrossRef]
- Alam, I.; Alam, M.; Khan, A.; Haq, S.; Ayaz, A.; Jalal, A.; Bhat, J.A. Biochar Supplementation Regulates Growth and Heavy Metal Accumulation in Tomato Grown in Contaminated Soils. Physiol. Plant. 2021, 173, 13414. [Google Scholar] [CrossRef] [PubMed]
- Manyà, J.J.; Azuara, M.; Manso, J.A. Biochar Production through Slow Pyrolysis of Different Biomass Materials: Seeking the Best Operating Conditions. Biomass Bioenergy 2018, 117, 115–123. [Google Scholar] [CrossRef]
- Nie, C.; Yang, X.; Niazi, N.K.; Xu, X.; Wen, Y.; Rinklebe, J.; Ok, Y.S.; Xu, S.; Wang, H. Impact of Sugarcane Bagasse-Derived Biochar on Heavy Metal Availability and Microbial Activity: A Field Study. Chemosphere 2018, 200, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.; Liu, Z.; Skalicky, M.; Dessoky, E.S.; Brestic, M.; Mbarki, S.; Rastogi, A.; el Sabagh, A. Fractionation of Heavy Metals in Multi-Contaminated Soil Treated with Biochar Using the Sequential Extraction Procedure. Biomolecules 2021, 11, 448. [Google Scholar] [CrossRef]
- Awad, M.; El-Sayed, M.M.; Li, X.; Liu, Z.; Mustafa, S.K.; Ditta, A.; Hessini, K. Diminishing Heavy Metal Hazards of Contaminated Soil via Biochar Supplementation. Sustainability 2021, 13, 12742. [Google Scholar] [CrossRef]
- Gholami, L.; Rahimi, G. Chemical Fractionation of Copper and Zinc after Addition of Carrot Pulp Biochar and Thiourea–Modified Biochar to a Contaminated Soil. Environ. Technol. 2021, 42, 3523–3532. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Golia, E.E.; Tsiropoulos, N.G.; Vleioras, S.; Antoniadis, V. Investigation of Extraction Methods for the Assessment of the Pseudo-Total Concentration of Potentially Toxic Elements in Moderately Contaminated Soils of Central Greece. Water Air Soil Pollut. 2020, 231, 484. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, J.; Liu, M.; Wu, Y.; Yuan, Z. Immobilization of Metals in Contaminated Soil from E-Waste Recycling Site by Dairy-Manure-Derived Biochar. Environ. Technol. 2018, 39, 2801–2809. [Google Scholar] [CrossRef] [PubMed]
| Contaminated Soils | ||||
|---|---|---|---|---|
| Soil pollution source | Metal foundry wastewater effluent | Copper smelter gaseous emissions | Wastes disposal | Mining activities |
| HMs pollutants | Cd, Cu, and Pb | Cd, Cu, and Pb | Cd, Cu, Pb, and Zn | Cd, Cu, Pb, and Zn |
| pH | 5.8 | <6.0 | 5.5 | 5.5 |
| OC (g kg−1) | 14.5 | 10 | 23.4 | 23.4 |
| Physicochemical properties of biochar types | ||||
| Feedstock | sugarcane bagasse | Bamboo rice straw | garden wastes | paulownia by-products |
| Treatment method/°C | pyrolysis/450 °C | pyrolysis/750 °C pyrolysis/500 °C | (1) pyrolysis/400 °C (2) pyrolysis/600 °C | pyrolysis/700–800 °C |
| Particle size | <2 mm | (1) < 0.25 mm (2) < 1 mm | <0.1 mm | <0.1 mm |
| References | [69] | [51] | [70] | [71] |
| Biochar Type | Temperature (°C) | Particle Size (mm) | Biochar Soil Application Amount | Pb | Cd | Cu |
|---|---|---|---|---|---|---|
| Sugarcane bagasse (B) | 450 | 0 t ha−1-control | 74a | 0.12a | 34a | |
| <2 | 1.5 t ha−1 | 55ba | 0.08ab | 24ab | ||
| 2.25 t ha−1 | 49ba | 0.04b | 17b | |||
| 3 t ha−1 | 44b | 0.04b | 17b | |||
| Bamboo (BB) | 700 | 0%-control | 71a | 0.4a | 190a | |
| <0.25 <1 | 1% 5% 1% | 71a 69a 71a | 0.4a 0.4a 0.4a | 175ab 158ab 175ab | ||
| 5% | 65a | 0.4a | 144b | |||
| Rice straw (RSB) | 500 | 0%-control | 71a | 0.4a | 190a | |
| <0.25 <1 | 1% 5% 1% 5% | 68a 51b 67a 49b | 0.3a 0.3a 0.3a 0.4a | 173b 127c 180b 123c | ||
| Garden wastes (GB) | 400 | 0%-control | 138a | 1.8a | 2a | |
| <0.1 | 2% | 120a | 1.5a | 1.3b | ||
| 4% | 95ba | 1.4a | 0.9c | |||
| 6% 2% | 67b 125a | 1.4a 1.6a | 0.5c 1.3b | |||
| 600 | 4% | 104a | 1.4a | 0.9c | ||
| 6% | 86ba | 1.4a | 0.5c | |||
| Paulownia (PG) | 700–800 | 0%-control | 137a | 1.9a | 2a | |
| <0.1 | 2% | 126a | 1.7a | 1.7ab | ||
| 4% | 125a | 1.6a | 1.5ab | |||
| 6% | 123a | 1.6a | 1.3b | |||
| Biochar Type | Temperature (°C) | Particle Size (mm) | Biochar Soil Application Amount | Pb | Cd | Cu |
|---|---|---|---|---|---|---|
| Sugarcane bagasse (B) | 450 | 0 t ha−1-control | 74a | 0.12a | 34a | |
| <2 | 1.5 t ha−1 | 55ba | 0.08b | 24b | ||
| 2.25 t ha−1 | 49b | 0.04c | 17c | |||
| 3 t ha−1 | 44b | 0.04c | 17c | |||
| Bamboo (BB) | 700 | 0%-control | 0 | 0 | 0 | |
| <0.25 <1 | 1% 5% 1% | 0 0 0 | 0 0 0 | 0 0 0 | ||
| 5% | 0 | 0 | 0 | |||
| Rice straw (RSB) | 500 | 0%-control | 0 | 0 | 0 | |
| <0.25 <1 | 1% 5% 1% 5% | 0 0 0 0 | 0 0 0 0 | 0 0 0 0 | ||
| Garden wastes (GB) | 400 | 0%-control | 0 | 0 | 0 | |
| <0.1 | 2% | 0 | 0 | 0 | ||
| 4 % | 0 | 0 | 0 | |||
| 6% 2% | 0 0 | 0 0 | 0 0 | |||
| 600 | 4% | 0 | 0 | 0 | ||
| 6% | 0 | 0 | 0 | |||
| Paulownia (PG) | 700–800 | 0%-control | 0 | 0 | 0 | |
| <0.1 | 2% | 0 | 0 | 0 | ||
| 4% | 0 | 0 | 0 | |||
| 6% | 0 | 0 | 0 | |||
| Biochar Type | Temperature (°C) | Particle Size (mm) | Biochar Soil Application Amount | Pb | Cd | Cu |
|---|---|---|---|---|---|---|
| Sugarcane bagasse (B) | 450 | 0 t ha−1-control | 136a | 0.4a | 40a | |
| <2 | 1.5 t ha−1 | 128a | 0.4a | 36a | ||
| 2.25 t ha−1 | 130a | 0.3b | 35a | |||
| 3 t ha−1 | 128a | 0.3b | 35a | |||
| Bamboo (BB) | 700 | 0%-control | 377a | 0.2a | 187a | |
| <0.25 <1 | 1% 5% 1% | 369a 343a 356a | 0.1b 0.1b 0.1b | 172ab 155ab 172a | ||
| 5% | 321a | 0.1b | 141b | |||
| Rice straw (RSB) | 500 | 0 %-control | 377a | 0.2a | 187a | |
| <0.25 <1 | 1% 5% 1% 5% | 369a 400a 386a 412a | 0.1b 0.1b 0.2a 0.1b | 170ab 125b 177ab 121b | ||
| Garden wastes (GB) | 400 | 0 %-control | 620a | 0.5a | 6.9a | |
| <0.1 | 2% | 561b | 0.5a | 6.3a | ||
| 4% | 614a | 0.6ba | 5.5b | |||
| 6% 2% | 593ab 583ab | 0.7b 0.5a | 4.3b 6.6a | |||
| 600 | 4% | 616a | 0.6ba | 6.4a | ||
| 6% | 593ab | 0.7b | 6.4a | |||
| Paulownia (PG) | 700–800 | 0 %-control | 609a | 0.5a | 6.7a | |
| < 0.1 | 2% | 549b | 0.5a | 6.5a | ||
| 4% | 593ab | 0.5a | 6.3a | |||
| 6% | 558b | 0.5a | 5.9a | |||
| Biochar Type | Temperature (°C) | Particle Size (mm) | Biochar Soil Application Amount | Pb | Cd | Cu |
|---|---|---|---|---|---|---|
| Sugarcane bagasse (B) | 450 | 0 t ha−1-control | 141a | 0.3b | 205a | |
| <2 | 1.5 t ha−1 | 165ba | 0.4a | 218ab | ||
| 2.25 t ha−1 | 168ba | 0.4a | 224ab | |||
| 3 t ha−1 | 176b | 0.4a | 225b | |||
| Bamboo (BB) | 700 | 0%-control | 30a | 0.1a | 60a | |
| <0.25 <1 | 1% 5% 1% | 34a 31a 34a | 0.1a 0.1a 0.1a | 53a 56a 49b | ||
| 5% | 33a | 0.1a | 52a | |||
| Rice straw (RSB) | 500 | 0%-control | 30a | 0.1a | 60a | |
| <0.25 <1 | 1% 5% 1% 5% | 38b 38b 38b 41b | 0.1a 0.1a 0.1a 0.1a | 56b 52b 51b 58a | ||
| Garden wastes (GB) | 400 | 0%-control | 62a | 0.1b | 17a | |
| <0.1 | 2% | 96ba | 0.1b | 16a | ||
| 4% | 105b | 0.2a | 16a | |||
| 6% 2% | 109b 97ba | 0.2a 0.2a | 17a 17a | |||
| 600 | 4 % | 100b | 0.2a | 16a | ||
| 6 % | 100b | 0.2a | 17a | |||
| Paulownia (PG) | 700–800 | 0%-control | 62a | 0.1b | 17a | |
| <0.1 | 2% | 91ba | 0.1b | 16a | ||
| 4% | 99ba | 0.2a | 16a | |||
| 6% | 100b | 0.2a | 15a | |||
| Biochar Type | Temperature (°C) | Particle Size (mm) | Biochar Soil Application Amount | Pb | Cd | Cu |
|---|---|---|---|---|---|---|
| Sugarcane bagasse (B) | 450 | 0 t ha−1-control | 141a | 0 | 205a | |
| <2 | 1.5 t ha−1 | 165ba | 0 | 218a | ||
| 2.25 t ha−1 | 168ba | 0 | 224a | |||
| 3 t ha−1 | 176b | 0 | 225a | |||
| Bamboo (BB) | 700 | 0%-control | 23a | 0.2a | 60 | |
| <0.25 <1 | 1% 5% 1% | 23a 20a 22a | 0.2a 0.2a 0.2a | 53 56 49 | ||
| 5% | 20a | 0.2a | 52 | |||
| Rice straw (RSB) | 500 | 0%-control | 23a | 0.2a | 60 | |
| <0.25 <1 | 1% 5% 1% 5% | 24a 22a 22a 20a | 0.2a 0.3a 0.2a 0.2a | 56 52 51 58 | ||
| Garden wastes (GB) | 400 | 0%-control | 197 | 0.1a | 17a | |
| <0.1 | 2% | 194 | 0.1a | 16a | ||
| 4% | 194 | 0.2b | 16a | |||
| 6% 2% | 194 193 | 0.1a 0.2b | 17a 17a | |||
| 600 | 4% | 187 | 0.2b | 16a | ||
| 6% | 188 | 0.2b | 17a | |||
| Paulownia (PG) | 700–800 | 0%-control | 196a | 0.1a | 17a | |
| <0.1 | 2% | 187ba | 0.1a | 16a | ||
| 4% | 194ba | 0.1a | 16a | |||
| 6% | 181b | 0.1a | 15a | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bousdra, T.; Papadimou, S.G.; Golia, E.E. The Use of biochar in the Remediation of Pb, Cd, and Cu-Contaminated Soils. The Impact of biochar Feedstock and Preparation Conditions on Its Remediation Capacity. Land 2023, 12, 383. https://doi.org/10.3390/land12020383
Bousdra T, Papadimou SG, Golia EE. The Use of biochar in the Remediation of Pb, Cd, and Cu-Contaminated Soils. The Impact of biochar Feedstock and Preparation Conditions on Its Remediation Capacity. Land. 2023; 12(2):383. https://doi.org/10.3390/land12020383
Chicago/Turabian StyleBousdra, Theodora, Sotiria G. Papadimou, and Evangelia E. Golia. 2023. "The Use of biochar in the Remediation of Pb, Cd, and Cu-Contaminated Soils. The Impact of biochar Feedstock and Preparation Conditions on Its Remediation Capacity" Land 12, no. 2: 383. https://doi.org/10.3390/land12020383
APA StyleBousdra, T., Papadimou, S. G., & Golia, E. E. (2023). The Use of biochar in the Remediation of Pb, Cd, and Cu-Contaminated Soils. The Impact of biochar Feedstock and Preparation Conditions on Its Remediation Capacity. Land, 12(2), 383. https://doi.org/10.3390/land12020383







