A First Study of Urginea maritima Rings: A Case Study from Southern Jordan
Abstract
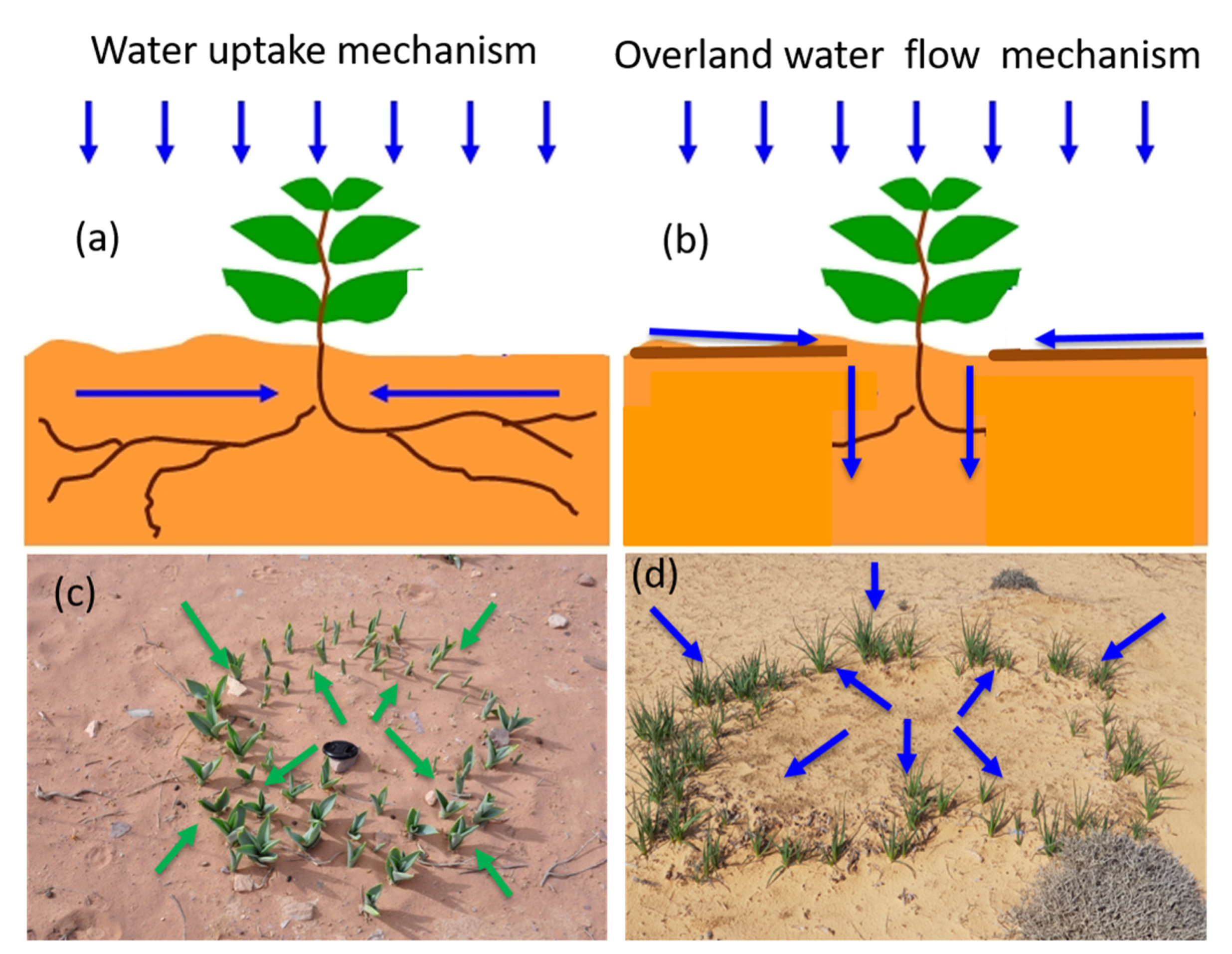
1. Introduction
2. Materials and Methods
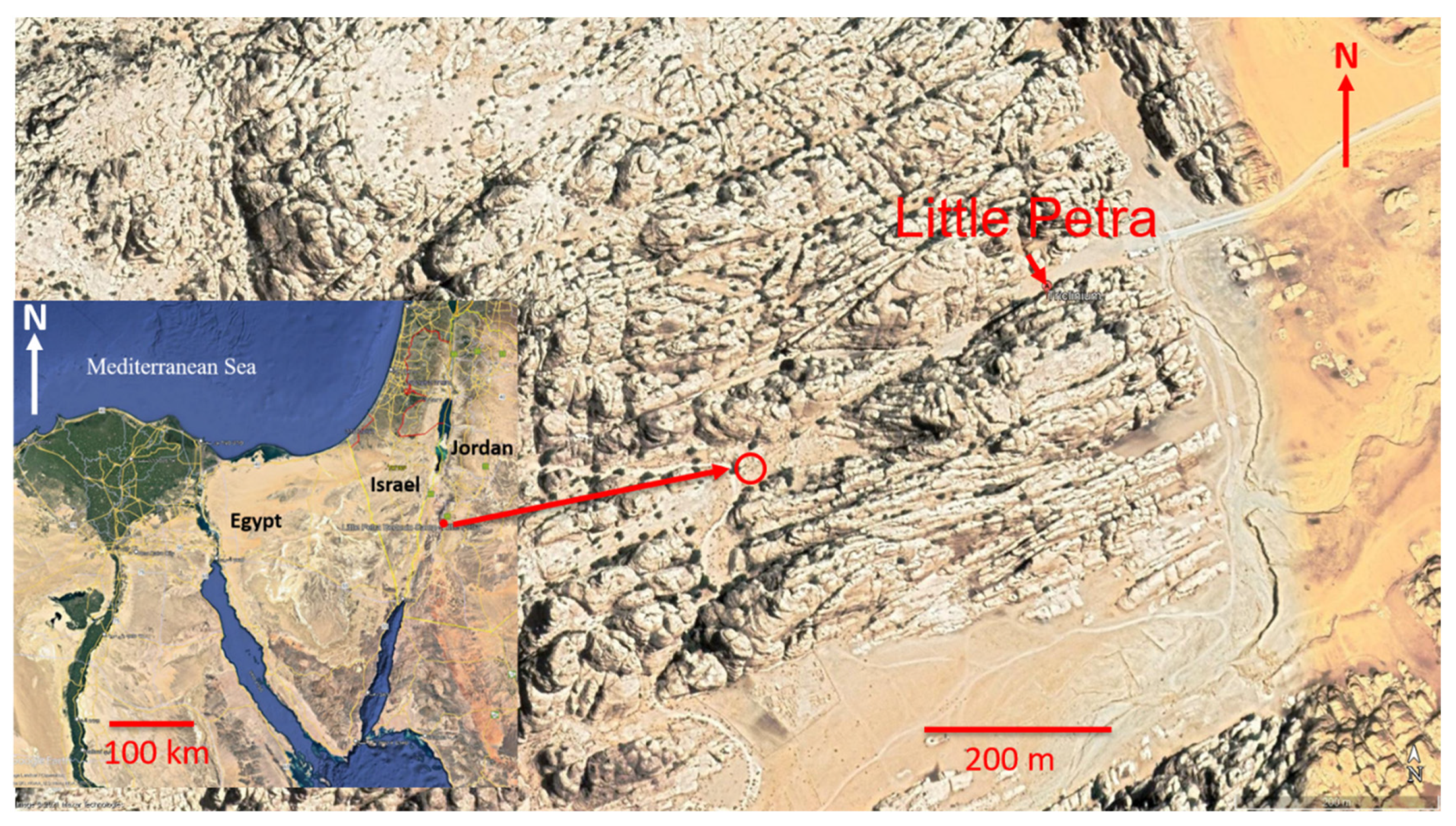
2.1. Regional Settings
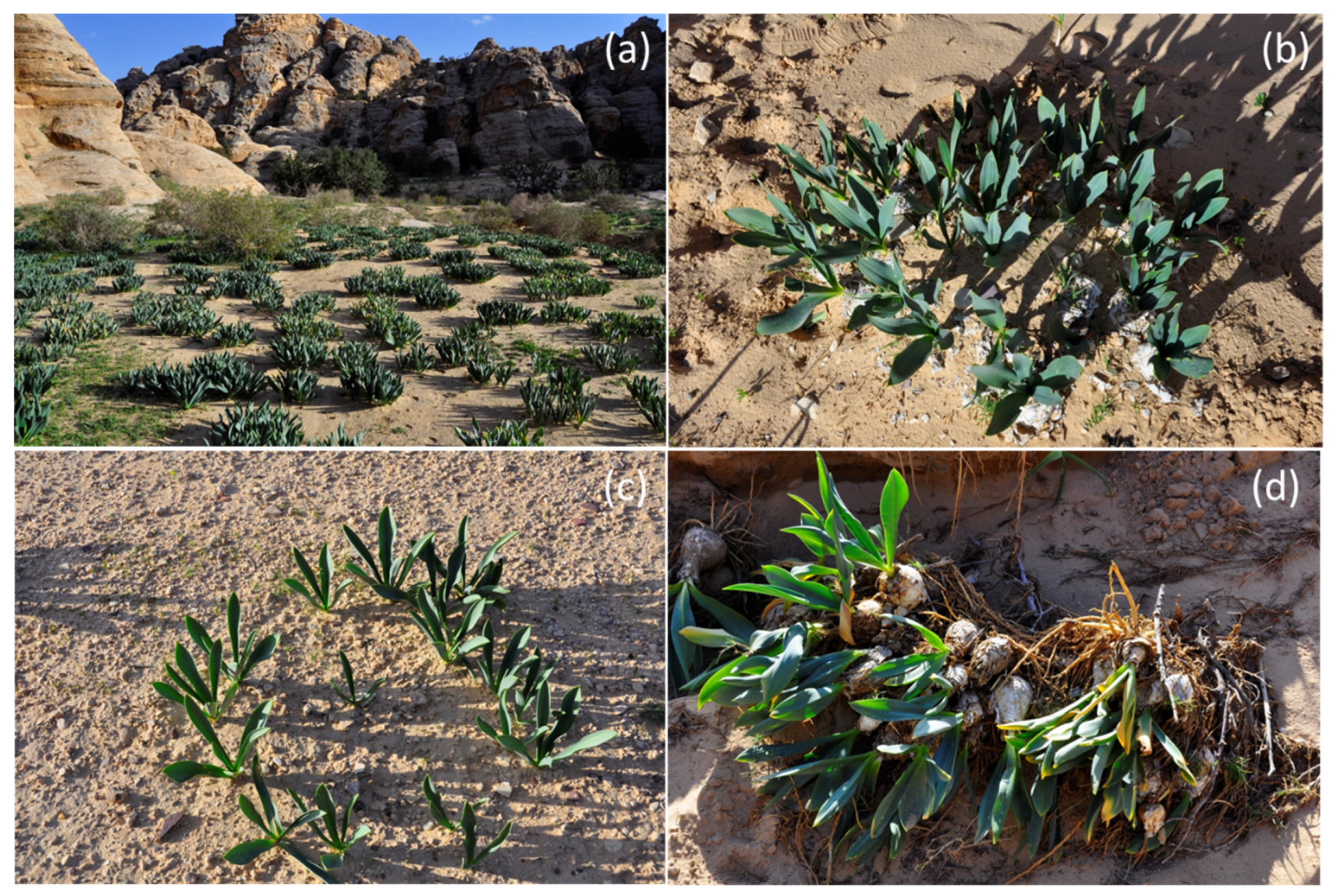
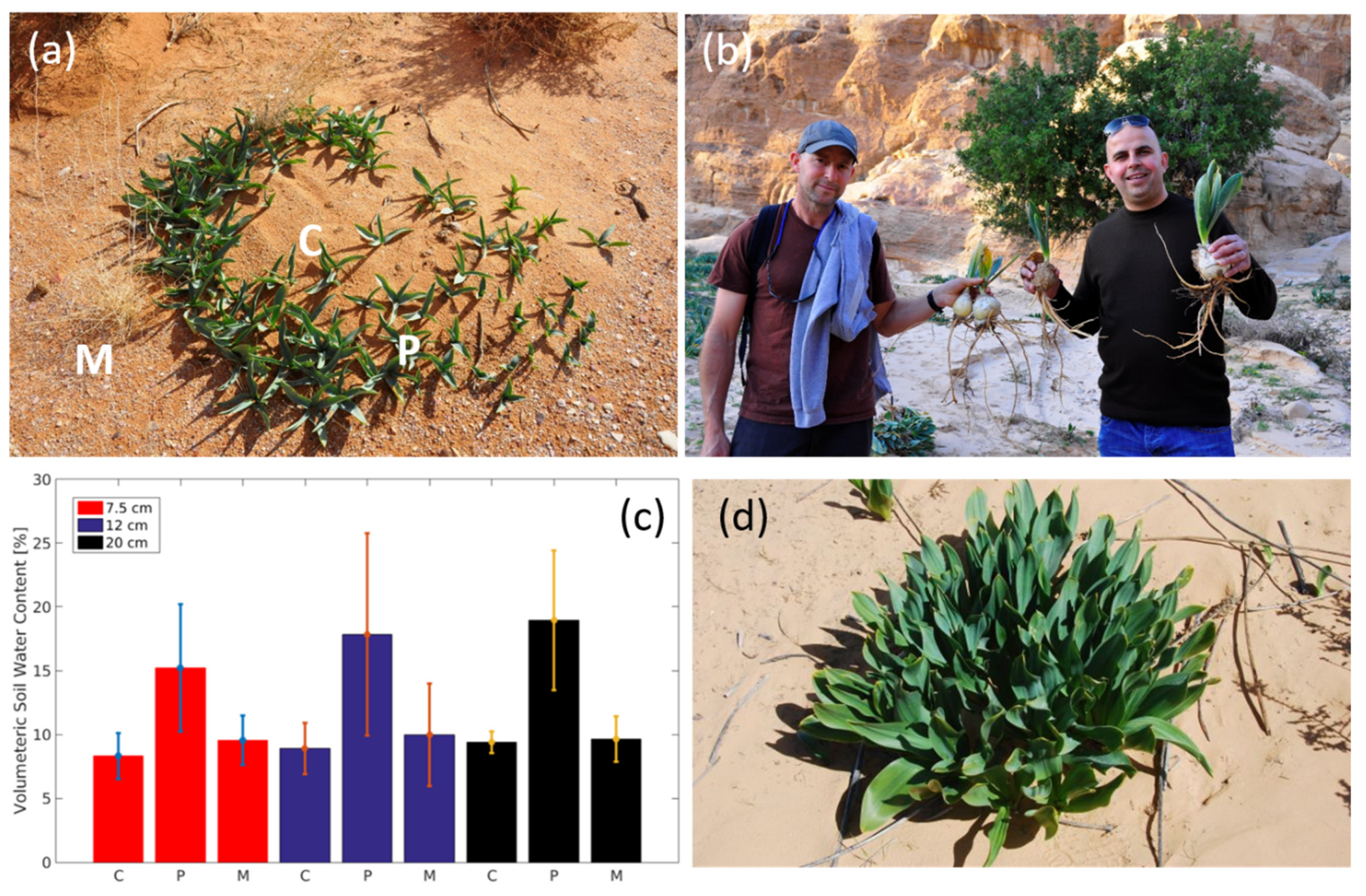
2.2. Field Measurements
2.3. Mathematical Modeling
3. Results and Discussion
3.1. Soil-Water Content and Soil Analyses
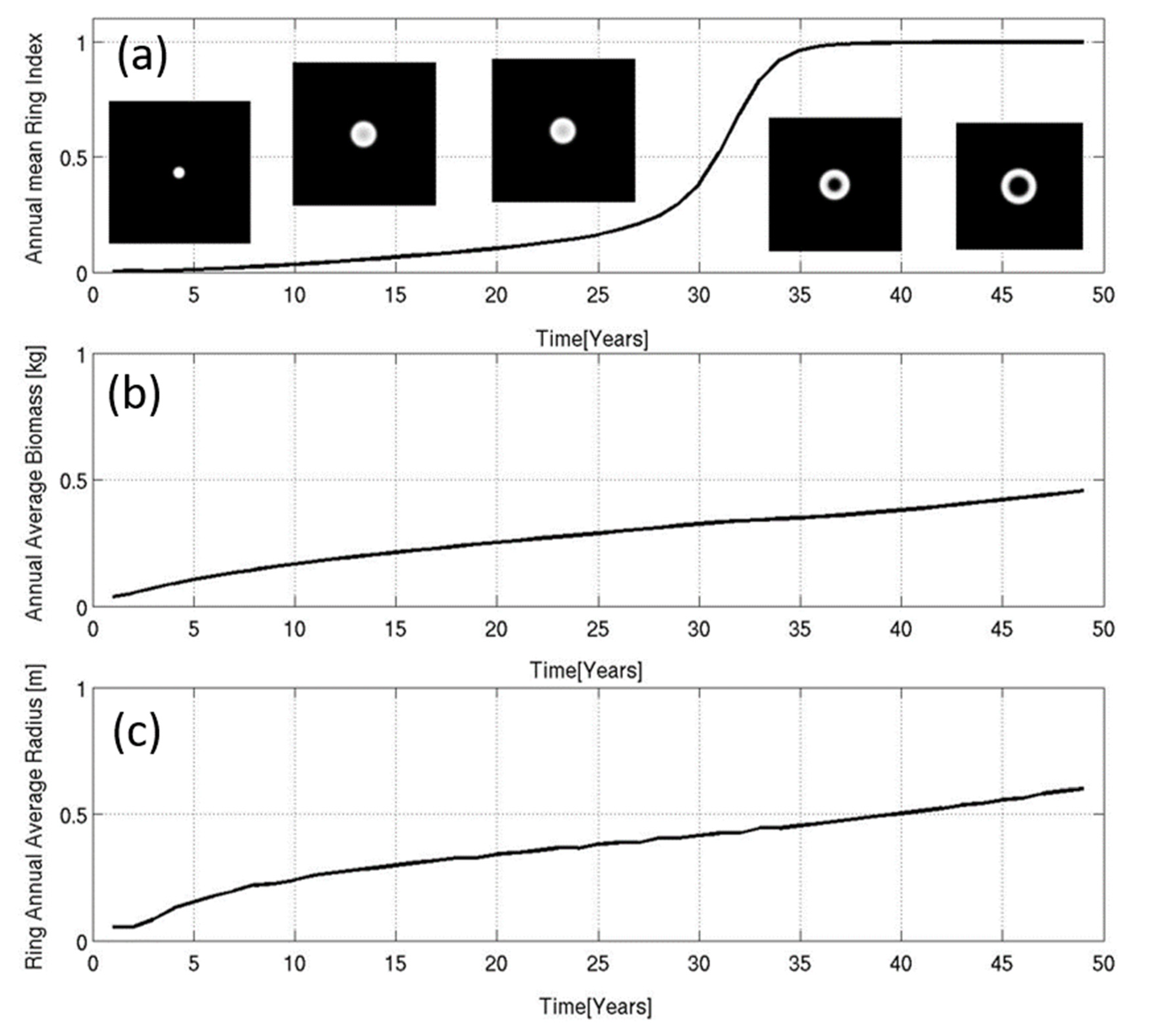
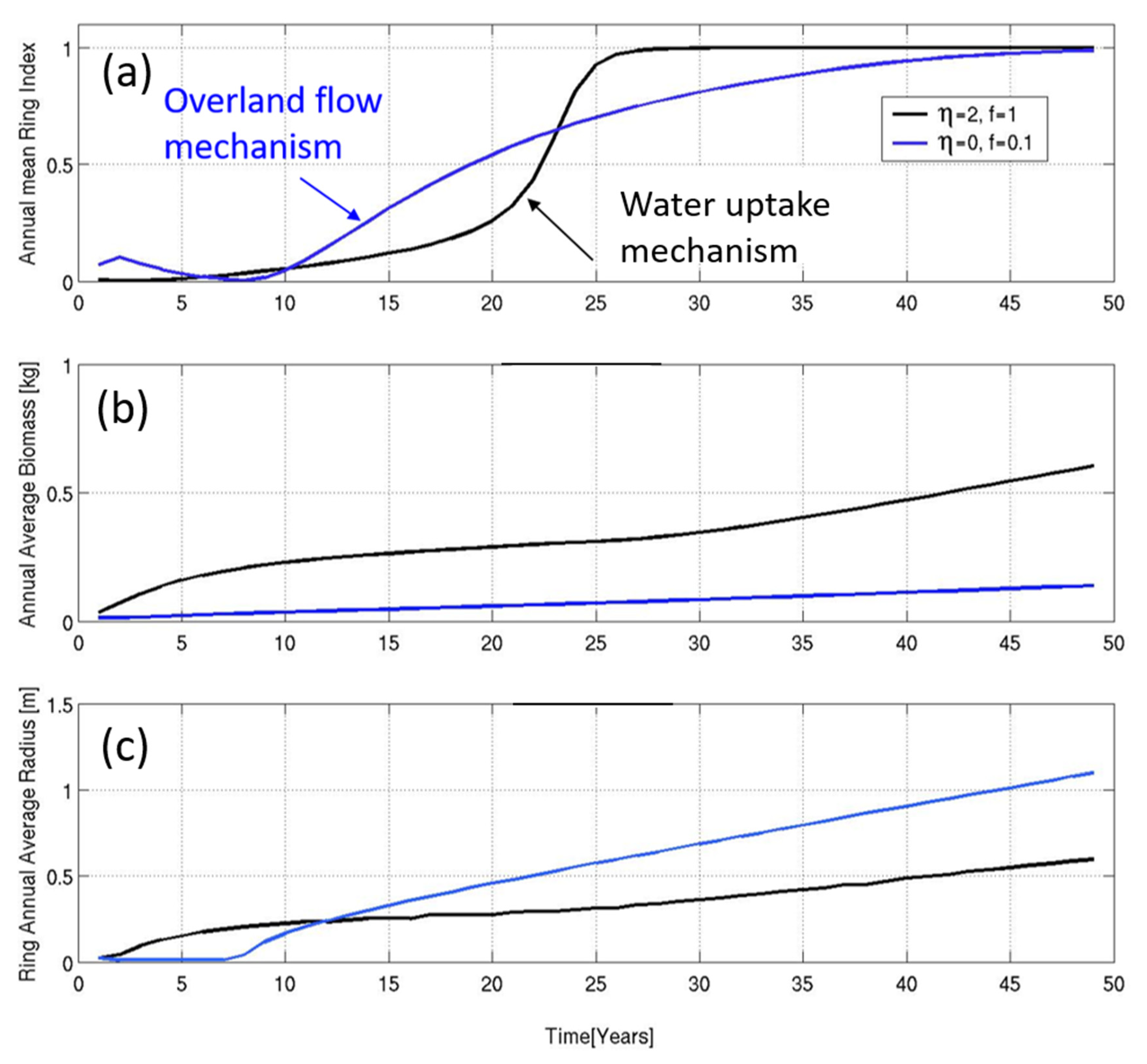
3.2. Numerical Simulations
3.3. Biogeographic Implications
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meron, E.; Yizhaq, H.; Gilad, E. Localized structures in dryland vegetation: Forms and functions. Chaos 2007, 17, 037109.1–037109.9. [Google Scholar] [CrossRef]
- Getzin, S.; Yizhaq, H.; Tschinkel, W. Definition of “fairy circles” and how they differ from other common vegetation gaps and plant rings. J. Veg. Sci. 2021, 32, e13092. [Google Scholar] [CrossRef]
- Bonanomi, G.; Incerti, G.; Stinca, A.; Cartenì, F.; Giannino, F.; Mazzoleni, S. Ring formation in clonal plants. Community Ecol. 2014, 15, 77–86. [Google Scholar] [CrossRef]
- Danin, A.; Orshan, G. Circular arrangement of Stipagrostis ciliate clumps in the Negev, Israel and near Goakeb, Namibia. J. Arid. Environ. 1995, 30, 301–313. [Google Scholar] [CrossRef]
- Danin, A. Plants of Desert Dunes; Springer: Berlin, Germany, 1996. [Google Scholar]
- Adachi, N.; Terashima, I.; Takahashi, M. Central dieback of monoclonal stands of Reynoutria japónica in an early stage of primary succession on Mount Fuji. Ann. Bot. 1996, 77, 477–486. [Google Scholar] [CrossRef][Green Version]
- Ravi, S.; D’Odorico, P.; Okin, G.S. Hydrologic and aeolian controls on vegetation patterns in arid landscapes. Geophys. Res. Lett. 2007, 34, L24S23. [Google Scholar] [CrossRef]
- Ravi, S.; D’Odorico, P.; Wang, L.; Collins, S. Form and function of grass ring patterns in arid grasslands: The role of abiotic controls. Oecologia 2008, 158, 545–555. [Google Scholar] [CrossRef]
- Yizhaq, H.; Stavi, I.; Swet, N.; Zaady, E.; Katra, I. Vegetation ring formation by water overland flow in water-limited environments: Field measurements and mathematical modelling. Ecohydrology 2019, 12, e2135. [Google Scholar] [CrossRef]
- Sheffer, E.; Yizhaq, H.; Gilad, E.; Shachak, M.; Meron, E. Why do plants in resource deprived environments form rings? Ecol. Complex. 2007, 4, 192–200. [Google Scholar] [CrossRef]
- Sheffer, E.; Yizhaq, H.; Shachak, M.; Meron, E. Mechanisms of vegetation-ring formation in water-limited systems. J. Theor. Biol. 2011, 273, 138–146. [Google Scholar] [CrossRef]
- Herooty, Y.; Bar, P.; Yizhaq, H.; Katz, O. Soil hydraulic properties and water source-sink relations affect plant rings’ formation and sizes under arid conditions. Flora 2020, 270, 151664. [Google Scholar] [CrossRef]
- Vasek, F.C. Creosote bush: Long-lived clones in the Mojave Desert. Am. J. Bot. 1980, 67, 246–255. [Google Scholar] [CrossRef]
- Cartení, F.; Marasco, A.; Bonanomi, G.; Mazzoleni, S.; Rietkerk, M.; Giannino, F. Negative plant soil feedback and ring formation in clonal plants. J. Theor. Biol. 2012, 313, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Inderjit; Callaway, R.M.; Meron, E. Belowground feedbacks as drivers of spatial self-organizationand community assembly. Phys. Life Rev. 2021, 38, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Ross, N.D.; Moles, A.T. The contribution of pathogenic soil microbes to ring formation in an iconic Australian arid grass, Triodia basedowii (Poaceae). Aust. J. Bot. 2021, 69, 113–120. [Google Scholar] [CrossRef]
- Meron, E. Nonlinear Physics of Ecosystems; Taylor and Francis: Abingdon, UK, 2015. [Google Scholar]
- Maestre, F.T.; Benito, B.; Berdugo, M.; Concostrina-Zubiri, L.; Delgado-Baquerizo, M.; Eldridge, D.J.; Guirado, E.; Gross, N.; Kéfi, S.; Le Bagousse-Pinguet, Y.; et al. Biogeography of global drylands. New Phytol. 2021, 231, 540–558. [Google Scholar] [CrossRef]
- Mitrakos, K.; Price, L.; Tzanni, H. The growth pattern of the flowering shoot of Urginea Marituma L. (Liliaceae). Am. J. Bot. 1974, 61, 920–924. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analyses of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis (Part 2). Madison; Page, A.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Gilad, E.; von Hardenberg, J.; Provenzale, A.; Shachak, M.; Meron, E. Ecosystem engineers: From pattern formation to habitat creation. Phys. Rev. Lett. 2004, 93, 098105. [Google Scholar] [CrossRef]
- Gilad, E.; von Hardenberg, J.; Provenzale, A.; Shachak, M.; Meron, E. A mathematical model of plants as ecosystem engineers. J. Theor. Biol. 2007, 244, 680–691. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Zaady, E.; Shachak, M. Infiltration through three contrasting biological soil crusts in patterned landscapes in the Negev, Israel. Catena 2000, 40, 323–336. [Google Scholar] [CrossRef]
- Carlton, L.; Duncritts, N.C.; Anny Chung, Y.; Rudgers, J.A. Plant–microbe interactions as a cause of ring formation in Bouteloua gracilis. J. Arid. Environ. 2018, 152, 1–5. [Google Scholar] [CrossRef]
- Olivares, B.O.; Calero, J.; Rey, J.C.; Lobo, D.; Landa, B.B.; Gómez, J.A. Correlation of banana productivity levels and soil morphological properties using regularized optimal scaling regression. Catena 2022, 208, 105718. [Google Scholar] [CrossRef]
- Campos, O.; Araya-Alman, M.; Acevedo-Opazo, C.; Cañete-Salinas, P.; Rey, J.C.; Lobo, D.; Landa, B. Relationship between soil properties and banana productivity in the two main cultivation areas in Venezuela. J. Soil Sci. Plant Nutr. 2020, 20, 2512–2524. [Google Scholar] [CrossRef]
| A | 53–106 µm (%) | 106–250 µm (%) | 250–500 µm (%) | 500–1000 µm (%) | 1000–2000 µm (%) |
| very fine sand | fine sand | medium sand | coarse sand | very coarse sand | |
| p value | 0.2138 | 0.8849 | 0.213 | 0.3004 | 0.6751 |
| Center | 7.8 a (0.6) | 39.2 a (1.2) | 39.1 a (1.5) | 2.2 a (0.1) | 0.38 a (0.05) |
| Periphery | 7.5 a (0.6) | 39.4 a (0.9) | 36.7 a (1.2) | 2.1 a (0.2) | 0.34 a (0.04) |
| Matrix | 8.2 a (0.5) | 38.7 a (1.0) | 36.8 a (1.4) | 1.9 a (0.2) | 0.37 a (0.06) |
| B | 53–106 µm (%) | 106–250 µm (%) | 250–500 µm (%) | 500–1000 µm (%) | 1000–2000 µm (%) |
| p value | 0.0295 | 0.0292 | 0.8345 | 0.3055 | 0.5812 |
| Shallow (3.5 cm) | 8.2 a (0.5) | 40.3 a (1.0) | 37.7 a (1.1) | 2.0 a (0.2) | 0.35 a (0.04) |
| Deep (7.5 cm) | 7.5 b (0.5) | 37.9 b (0.7) | 37.4 a (1.2) | 2.1 a (0.1) | 0.37 a (0.04) |
| C | Gravimetric SM (%) | K (cm s−1) | SOC (g kg−1) | ||
| p value | 0.2142 | 0.7498 | 0.0047 | ||
| Center | 4.86 a (0.20) | 0.00109 a (0.00016) | 11.9 ab (0.6) | ||
| Periphery | 5.16 a (0.26) | 0.00131 a (0.00028) | 13.0 a (0.6) | ||
| Matrix | 4.68 a (0.25) | 0.00110 a (0.00021) | 10.6 b (0.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yizhaq, H.; Al-Tawaha, A.R.M.S.; Stavi, I. A First Study of Urginea maritima Rings: A Case Study from Southern Jordan. Land 2022, 11, 285. https://doi.org/10.3390/land11020285
Yizhaq H, Al-Tawaha ARMS, Stavi I. A First Study of Urginea maritima Rings: A Case Study from Southern Jordan. Land. 2022; 11(2):285. https://doi.org/10.3390/land11020285
Chicago/Turabian StyleYizhaq, Hezi, Abdel Rahman Mohammad Said Al-Tawaha, and Ilan Stavi. 2022. "A First Study of Urginea maritima Rings: A Case Study from Southern Jordan" Land 11, no. 2: 285. https://doi.org/10.3390/land11020285
APA StyleYizhaq, H., Al-Tawaha, A. R. M. S., & Stavi, I. (2022). A First Study of Urginea maritima Rings: A Case Study from Southern Jordan. Land, 11(2), 285. https://doi.org/10.3390/land11020285








