Application of Steel Slag for Degraded Land Remediation
Abstract
:1. Introduction
- As liming material;
- As amendment nutrient source.
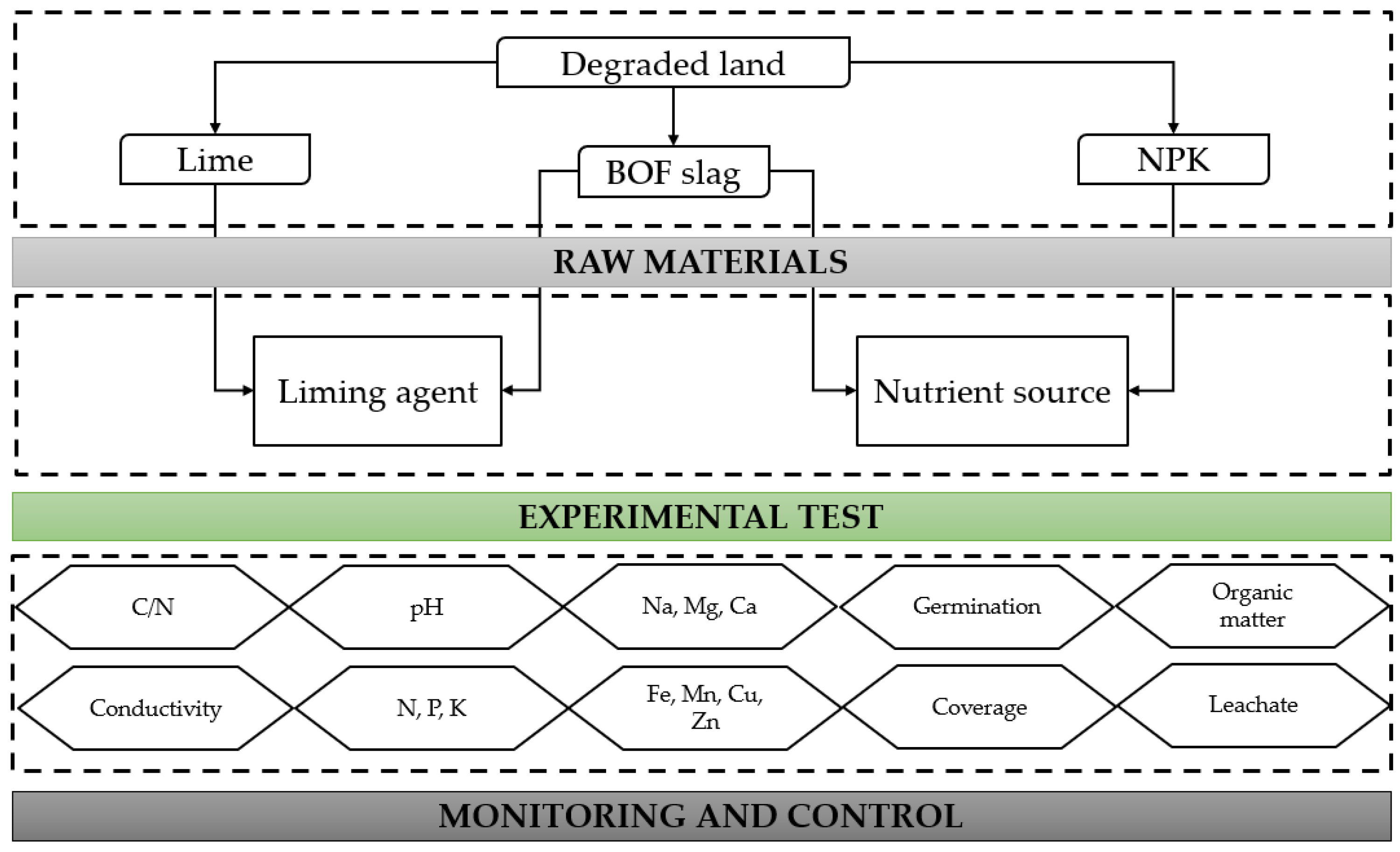
2. Materials and Methods
2.1. Basic Oxygen Furnace Slag
2.2. Characteristics of Degraded Soil
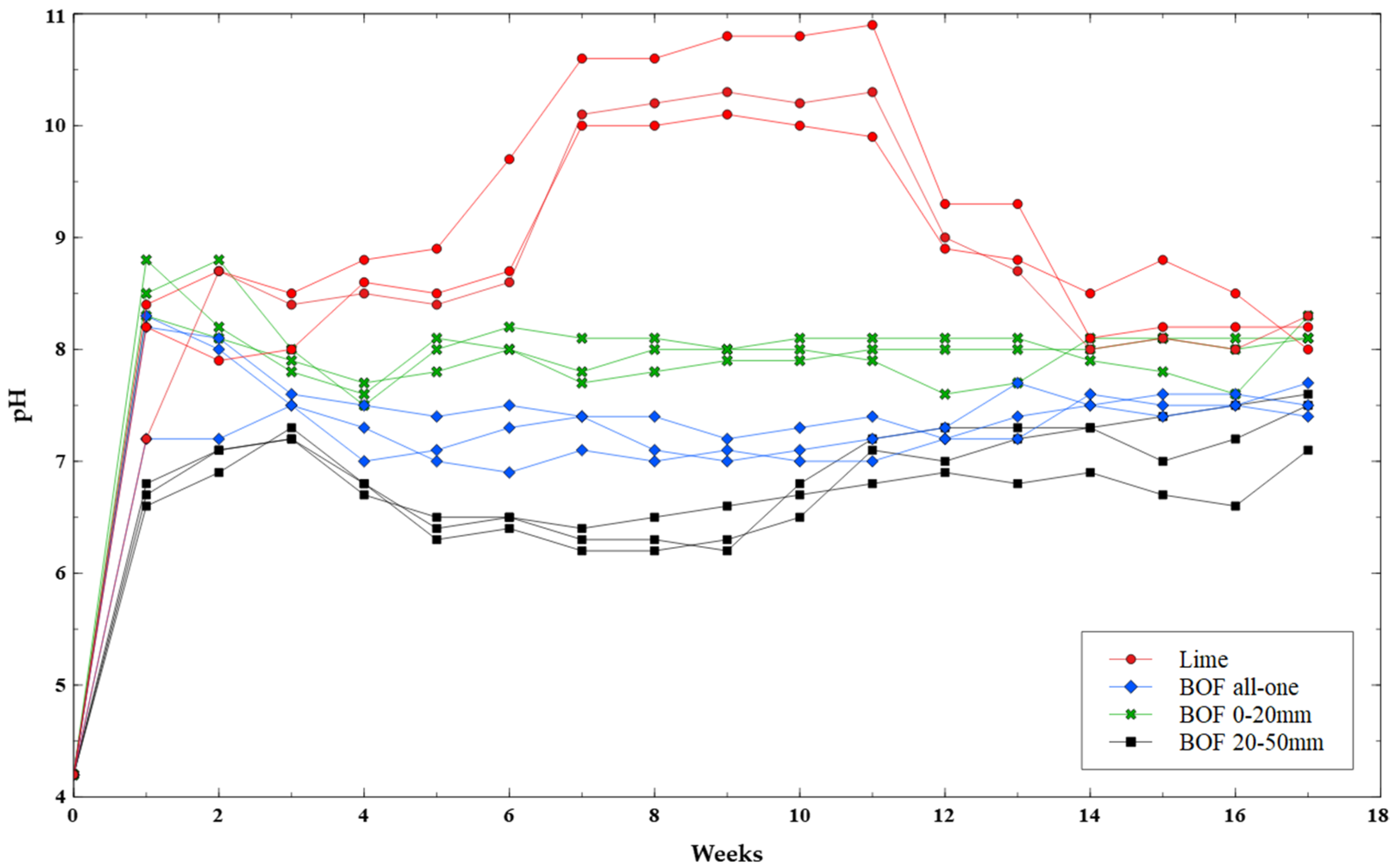
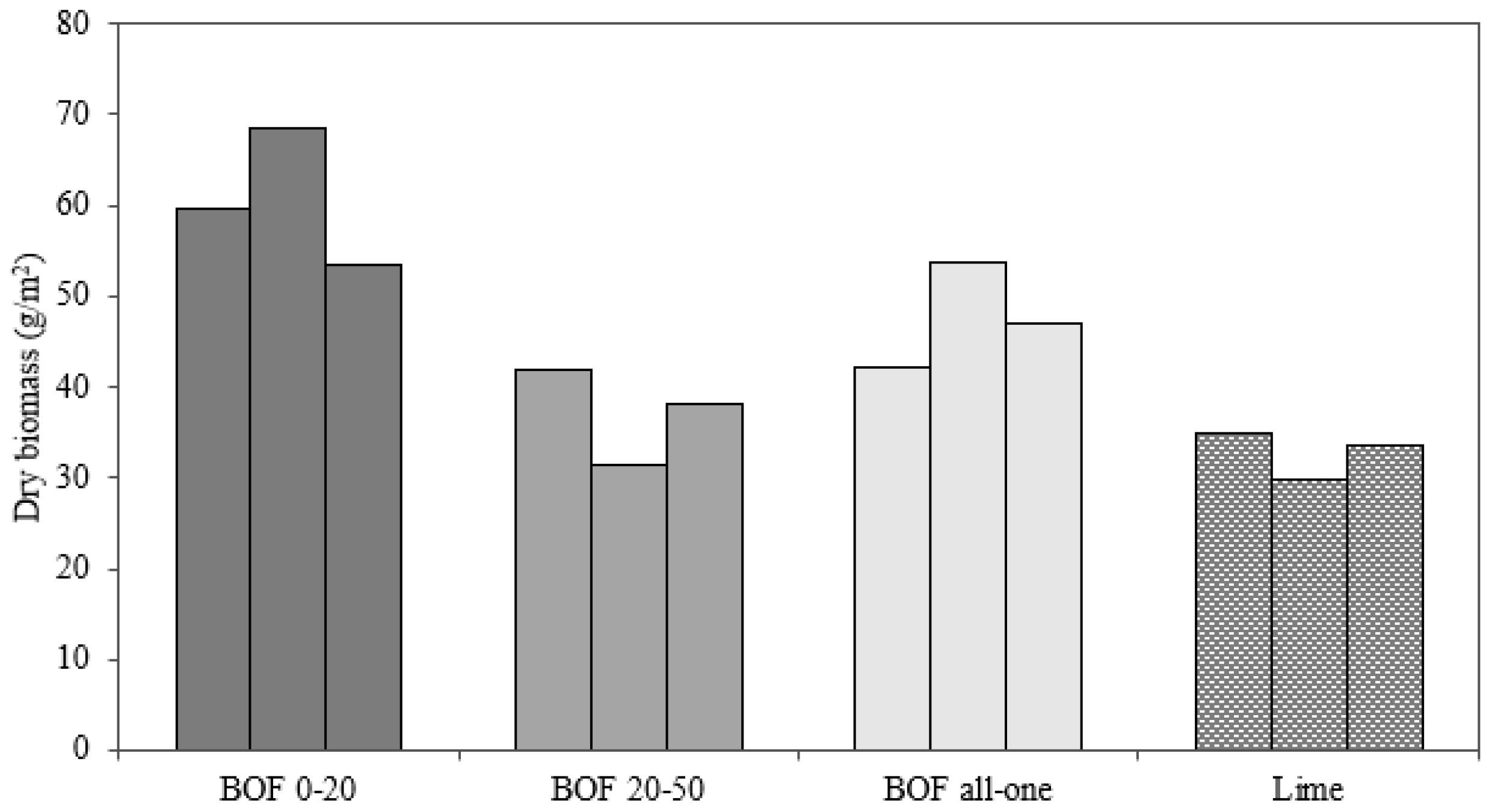
2.3. Test 1: BOF Slag as Liming Agent
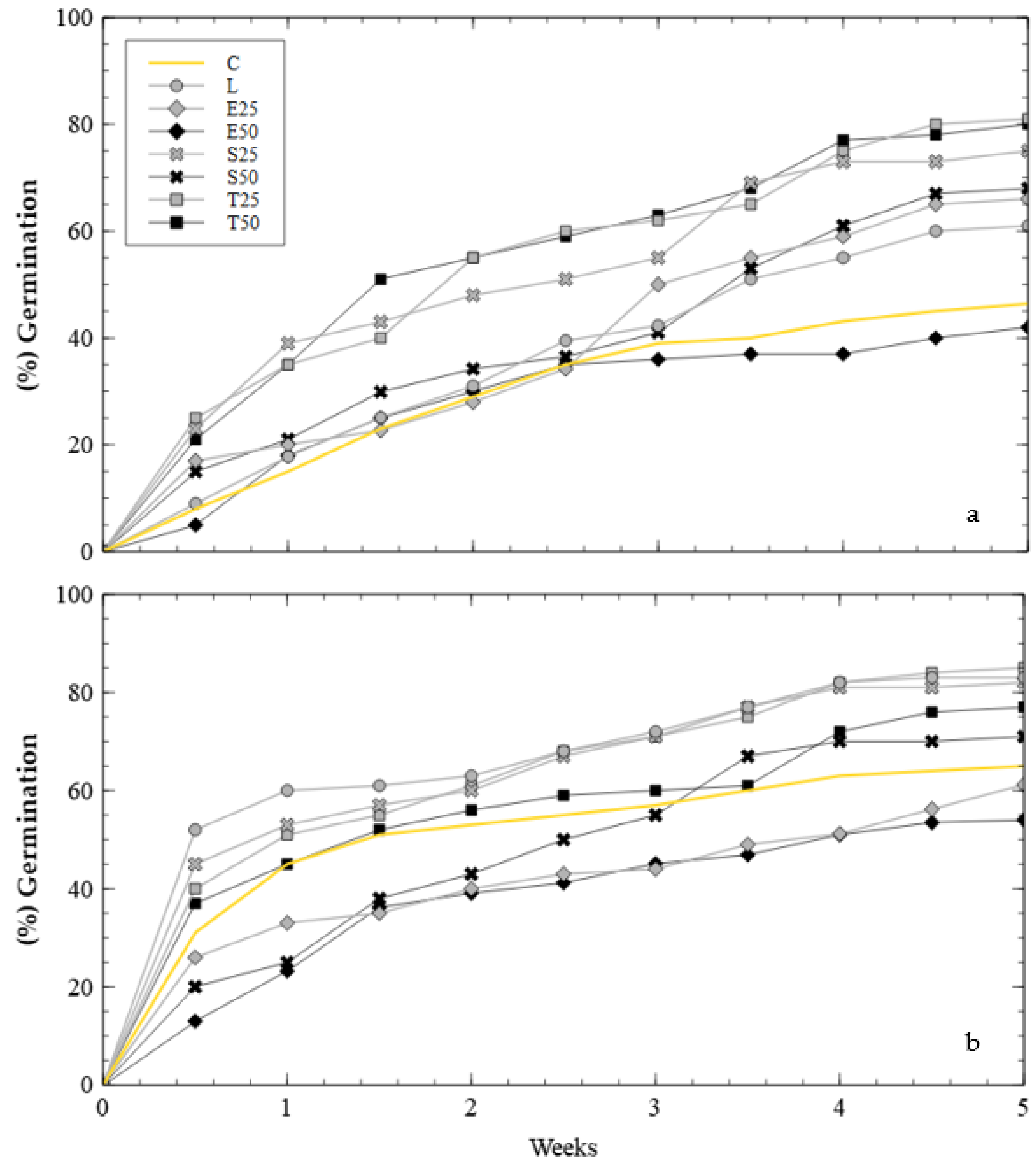
2.4. Test 2: Soil Amendment and Nutrient Source
2.5. Statiscal Analysis
3. Results and Discussion
3.1. Liming Agent
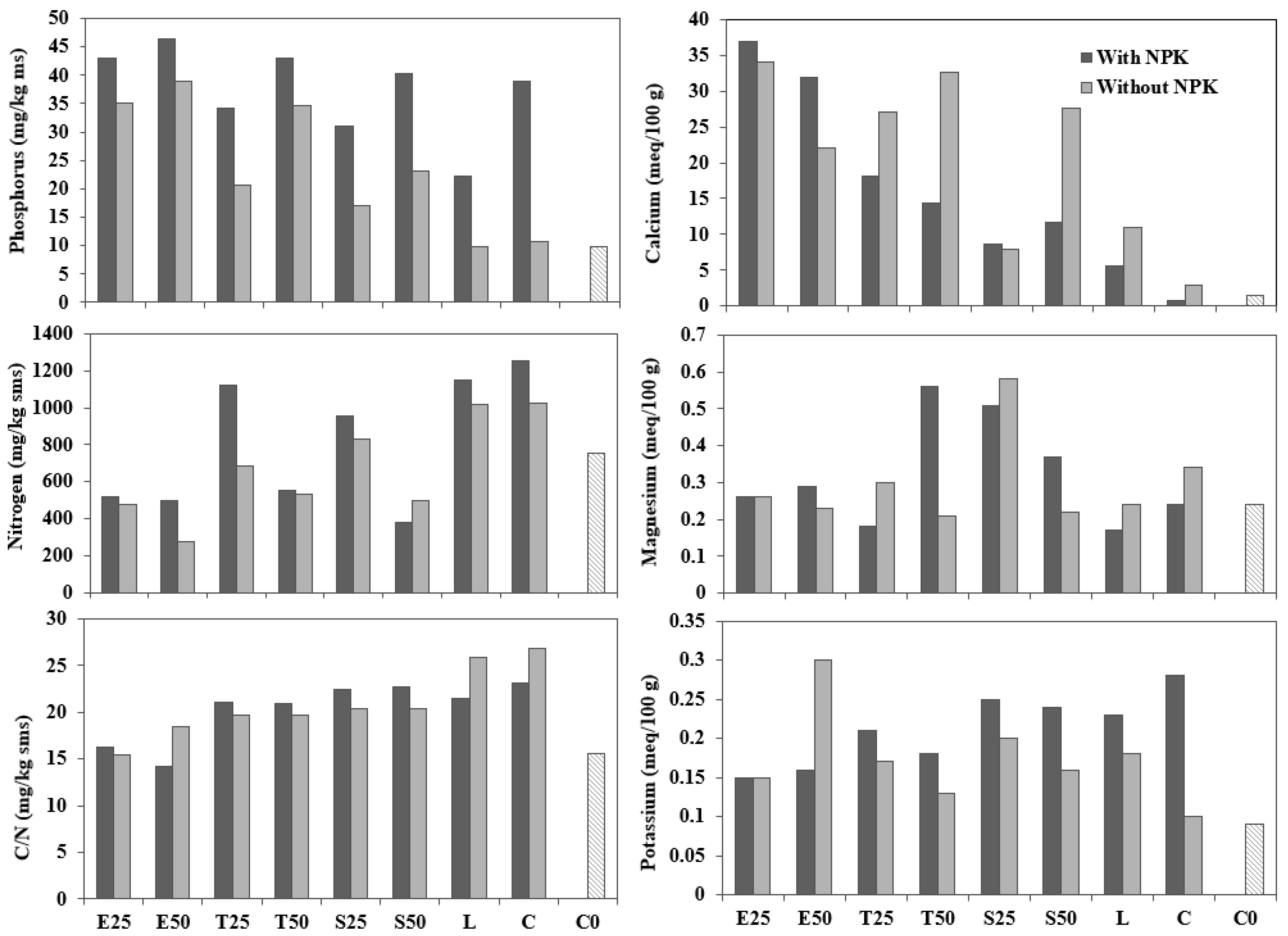
3.2. Soil Amendment and Nutrient Source
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Prăvălie, R.; Patriche, C.; Borrelli, P.; Panagos, P.; Roșca, B.; Dumitraşcu, M.; Nita, I.-A.; Săvulescu, I.; Birsan, M.-V.; Bandoc, G. Arable lands under the pressure of multiple land degradation processes. A global perspective. Environ. Res. 2021, 194, 110697. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, X.; Zhang, K. Understanding global land degradation processes interacted with complex biophysics and socioeconomics from the perspective of the normalized difference vegetation index (1982–2015). Glob. Planet. Chang. 2021, 198, 103431. [Google Scholar] [CrossRef]
- Nkonya, E.; Anderson, W.; Kato, E.; Koo, J.; Mirzabaev, A.; von Braun, J.; Meyer, S. Global Cost of Land Degradation. In Economics of Land Degradation and Improvement—A Global Assessment for Sustainable Development; Nkonya, E., Mirzabaev, A., von Braun, J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 117–165. ISBN 978-3-319-19168-3. [Google Scholar]
- Zhu, H.; Chen, C.; Xu, C.; Zhu, Q.; Huang, D. Effects of soil acidification and liming on the phytoavailability of cadmium in paddy soils of Central Subtropical China. Environ. Pollut. 2016, 219, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Li, F.; Liu, C.; Feng, X.; Gu, L.; Wang, B.; Wen, S.; Xu, M. Mitigation of soil acidification through changes in soil mineralogy due to long-term fertilization in Southern China. CATENA 2019, 174, 227–234. [Google Scholar] [CrossRef]
- Rengel, Z. Handbook of Soil Acidity; CRC Press: Boca Raton, FL, USA, 2003; ISBN 978-0-203-91231-7. [Google Scholar]
- Dai, Z.; Zhang, X.; Tang, C.; Muhammad, N.; Wu, J.; Brookes, P.C.; Xu, J. Potential role of biochars in decreasing soil acidification—A Critical Review. Sci. Total Environ. 2017, 581–582, 601–611. [Google Scholar] [CrossRef]
- Edmeades, D.C.; Blarney, F.P.C.; Farina, M.P.W. Techniques for Assessing Plant Responses on Acid Soils. In Plant-Soil Interactions at Low pH: Principles and Management, Proceedings of the Third International Symposium on Plant-Soil Interactions at Low pH, Brisbane, QLD, Australia, 12–16 September 1993; Date, R.A., Grundon, N.J., Rayment, G.E., Probert, M.E., Eds.; Developments in Plant and Soil Sciences; Springer: Dordrecht, The Netherlands, 1995; pp. 221–233. ISBN 978-94-011-0221-6. [Google Scholar]
- Ulrich, B.; Sumner, M.E. Soil Acidity; Springer Science & Business Media: Berlin/Heilderberg, Germany, 2012; ISBN 978-3-642-74442-6. [Google Scholar]
- Sandoval López, D.M.; Arturi, M.F.; Goya, J.F.; Pérez, C.A.; Frangi, J.L. Eucalyptus Grandis Plantations: Effects of Management on Soil Carbon, Nutrient Contents and Yields. J. For. Res. 2020, 31, 601–611. [Google Scholar] [CrossRef]
- Wei, X.; Liu, S.; Müller, K.; Song, Z.; Guan, G.; Luo, J.; Wang, H. Urbanization-Induced Acid Rain Causes Leaching Loss of Calcium from Limestone-Derived Soil in South China. J. Soils Sediments 2019, 19, 3797–3804. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Li, K.; Ruan, Y.; Kong, Y.; Liu, M.; Ling, N.; Shen, Q. Distinct Response Patterns of Soil Bacteria to Oxalate Imply Their Role in Buffering Soil Acidification: Evidence from Red Soils with Long-Term Fertilisation Regimes. Land Degrad. Dev. 2019, 30, 1632–1641. [Google Scholar] [CrossRef]
- Zeng, M.; de Vries, W.; Bonten, L.T.C.; Zhu, Q.; Hao, T.; Liu, X.; Xu, M.; Shi, X.; Zhang, F.; Shen, J. Model-Based Analysis of the Long-Term Effects of Fertilization Management on Cropland Soil Acidification. Environ. Sci. Technol. 2017, 51, 3843–3851. [Google Scholar] [CrossRef]
- Hao, T.; Zhu, Q.; Zeng, M.; Shen, J.; Shi, X.; Liu, X.; Zhang, F.; de Vries, W. Quantification of the Contribution of Nitrogen Fertilization and Crop Harvesting to Soil Acidification in a Wheat-Maize Double Cropping System. Plant Soil 2019, 434, 167–184. [Google Scholar] [CrossRef]
- Meza-Palacios, R.; Aguilar-Lasserre, A.A.; Morales-Mendoza, L.F.; Rico-Contreras, J.O.; Sánchez-Medel, L.H.; Fernández-Lambert, G. Decision Support System for NPK Fertilization: A Solution Method for Minimizing the Impact on Human Health, Climate Change, Ecosystem Quality and Resources. J. Environ. Sci. Health Part A 2020, 55, 1267–1282. [Google Scholar] [CrossRef] [PubMed]
- Vlek, P.L.G.; Rodríguez-Kuhl, G.; Sommer, R. Energy Use and CO2 Production in Tropical Agriculture and Means and Strategies for Reduction or Mitigation. Environ. Dev. Sustain. 2004, 6, 213–233. [Google Scholar] [CrossRef]
- Rakshit, A.; Sarkar, B.; Abhilash, P. Soil Amendments for Sustainability: Challenges and Perspectives; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1-351-02701-4. [Google Scholar]
- Drapanauskaitė, D.; Bunevičienė, K.; Mažeika, R. Influence of Different Liming Material on Soil PH and Spring Barley Yield under Different Soil Moisture Conditions. Žemės Ūkio Moksl. 2020, 27. [Google Scholar] [CrossRef]
- Moore, A.; Pirelli, G.J.; Filley, S.; Fransen, S.; Sullivan, D.M.; Fery, M.; Thomson, T. Nutrient Management for Pastures: Western Oregon and Western Washington; Oregon State University: Corvallis, OR, USA, 2019. [Google Scholar]
- Li, J.-Y.; Wang, N.; Xu, R.-K.; Tiwari, D. Potential of Industrial Byproducts in Ameliorating Acidity and Aluminum Toxicity of Soils Under Tea Plantation. Pedosphere 2010, 20, 645–654. [Google Scholar] [CrossRef]
- Pędziwiatr, A.; Potysz, A.; Uzarowicz, Ł. Combustion Wastes from Thermal Power Stations and Household Stoves: A Comparison of Properties, Mineralogical and Chemical Composition, and Element Mobilization by Water and Fertilizers. Waste Manag. 2021, 131, 136–146. [Google Scholar] [CrossRef]
- Olofinnade, O.; Morawo, A.; Okedairo, O.; Kim, B. Solid Waste Management in Developing Countries: Reusing of Steel Slag Aggregate in Eco-Friendly Interlocking Concrete Paving Blocks Production. Case Stud. Constr. Mater. 2021, 14, e00532. [Google Scholar] [CrossRef]
- Liu, J.; Wang, D. Influence of Steel Slag-Silica Fume Composite Mineral Admixture on the Properties of Concrete. Powder Technol. 2017, 320, 230–238. [Google Scholar] [CrossRef]
- Branca, T.A.; Colla, V.; Algermissen, D.; Granbom, H.; Martini, U.; Morillon, A.; Pietruck, R.; Rosendahl, S. Reuse and Recycling of By-Products in the Steel Sector: Recent Achievements Paving the Way to Circular Economy and Industrial Symbiosis in Europe. Metals 2020, 10, 345. [Google Scholar] [CrossRef] [Green Version]
- Naidu, T.S.; Sheridan, C.M.; van Dyk, L.D. Basic Oxygen Furnace Slag: Review of Current and Potential Uses. Miner. Eng. 2020, 149, 106234. [Google Scholar] [CrossRef]
- Satish Kumar, D.; Sah, R.; Sanyal, S.; Prasad, G. Measurement of Metallic Iron in Steel Making Slags. Measurement 2019, 131, 156–161. [Google Scholar] [CrossRef]
- Shen, W.; Liu, Y.; Wu, M.; Zhang, D.; Du, X.; Zhao, D.; Xu, G.; Zhang, B.; Xiong, X. Ecological Carbonated Steel Slag Pervious Concrete Prepared as a Key Material of Sponge City. J. Clean. Prod. 2020, 256, 120244. [Google Scholar] [CrossRef]
- Lai, M.H.; Zou, J.; Yao, B.; Ho, J.C.M.; Zhuang, X.; Wang, Q. Improving Mechanical Behavior and Microstructure of Concrete by Using BOF Steel Slag Aggregate. Constr. Build. Mater. 2021, 277, 122269. [Google Scholar] [CrossRef]
- Jiang, Y.; Ling, T.-C.; Shi, C.; Pan, S.-Y. Characteristics of Steel Slags and Their Use in Cement and Concrete—A Review. Resour. Conserv. Recycl. 2018, 136, 187–197. [Google Scholar] [CrossRef]
- Das, S.; Kim, G.W.; Hwang, H.Y.; Verma, P.P.; Kim, P.J. Cropping with Slag to Address Soil, Environment, and Food Security. Front. Microbiol. 2019, 10, 1320. [Google Scholar] [CrossRef]
- Ito, K. Steelmaking Slag for Fertilizer Usage. Nippon. Steel Sumitomo Met. Tech. Rep. 2015, 7, 130–136. [Google Scholar]
- Das, S.; Gwon, H.S.; Khan, M.I.; Jeong, S.T.; Kim, P.J. Steel Slag Amendment Impacts on Soil Microbial Communities and Activities of Rice (Oryza sativa L.). Sci. Rep. 2020, 10, 6746. [Google Scholar] [CrossRef] [Green Version]
- Ning, D.; Liang, Y.; Liu, Z.; Xiao, J.; Duan, A. Impacts of Steel-Slag-Based Silicate Fertilizer on Soil Acidity and Silicon Availability and Metals-Immobilization in a Paddy Soil. PLoS ONE 2016, 11, e0168163. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, J.; Nguyen, T.B.T.; Honeyands, T.; Monaghan, B.; O’Dea, D.; Rinklebe, J.; Vinu, A.; Hoang, S.A.; Singh, G.; Kirkham, M.B.; et al. Production, Characterisation, Utilisation, and Beneficial Soil Application of Steel Slag: A Review. J. Hazard. Mater. 2021, 419, 126478. [Google Scholar] [CrossRef]
- Wang, D.; Chang, J.; Ansari, W.S. The Effects of Carbonation and Hydration on the Mineralogy and Microstructure of Basic Oxygen Furnace Slag Products. J. CO2 Util. 2019, 34, 87–98. [Google Scholar] [CrossRef]
- Jia, R.; Liu, J.; Jia, R. A Study of Factors That Influence the Hydration Activity of Mono-Component CaO and Bi-Component CaO/Ca2Fe2O5 Systems. Cem. Concr. Res. 2017, 91, 123–132. [Google Scholar] [CrossRef]
- Andrés-Vizán, S.M.; Villanueva-Balsera, J.M.; Álvarez-Cabal, J.V.; Martínez-Huerta, G.M. Classification of BOF Slag by Data Mining Techniques According to Chemical Composition. Sustainability 2020, 12, 3301. [Google Scholar] [CrossRef] [Green Version]
- Ndlovu, S.; Simate, G.S.; Matinde, E. Waste Production and Utilization in the Metal. Extraction Industry; Routledge: London, UK, 2017; ISBN 978-1-4987-6729-3. [Google Scholar]
- Ding, Y.-C.; Cheng, T.-W.; Liu, P.-C.; Lee, W.-H. Study on the Treatment of BOF Slag to Replace Fine Aggregate in Concrete. Constr. Build. Mater. 2017, 146, 644–651. [Google Scholar] [CrossRef]
- Landon, J.R. Soil Chemistry. In Booker Tropical Soil Manual; Routledge: London, UK, 1991; ISBN 978-1-315-84684-2. [Google Scholar]
- Metson, A.J. New Zealand Department of Scientific and Industrial Research, Soil Bureau. In Methods of Chemical Analysis for Soil Survey Samples; D.S.I.R.: Wellington, New Zealand, 1956. [Google Scholar]
- Dean, W.E. Determination of Carbonate and Organic Matter in Calcareous Sediments and Sedimentary Rocks by Loss on Ignition; Comparison with Other Methods. J. Sediment. Res. 1974, 44, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A.; U.S. Department of Agriculture. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; U.S. Department of Agriculture: Washington, DC, USA, 1954.
- Kunhikrishnan, A.; Thangarajan, R.; Bolan, N.S.; Xu, Y.; Mandal, S.; Gleeson, D.B.; Seshadri, B.; Zaman, M.; Barton, L.; Tang, C.; et al. Chapter One—Functional Relationships of Soil Acidification, Liming, and Greenhouse Gas Flux. In Advances in Agronomy; Sparks, D.L., Ed.; Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2016; Volume 139, pp. 1–71. [Google Scholar]
- Bennett, J.; Greene, R.; Murphy, B.W.; Hocking, P.; Tongway, D. Influence of Lime and Gypsum on Long-Term Rehabilitation of a Red Sodosol, in a Semi-Arid Environment of New South Wales. Soil Res. 2014, 52, 120. [Google Scholar] [CrossRef]
- Mamatha, D.M.M.; Gowda, R.C.; Shivakumara, M.N. Effect of Basic Slag on Yield, Nutrient Status and Uptake by Paddy in Acid Soils of Karnataka, India. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2286–2292. [Google Scholar] [CrossRef] [Green Version]
- European Parliament and the Council. Official Journal of the European Union Regulation (EU) 2019/1009 of the European Parliament and of the Council; European Parliament and the Council: Brussels, Belgium, 2019. [Google Scholar]
- Holland, J.E.; Bennett, A.E.; Newton, A.C.; White, P.J.; McKenzie, B.M.; George, T.S.; Pakeman, R.J.; Bailey, J.S.; Fornara, D.A.; Hayes, R.C. Liming Impacts on Soils, Crops and Biodiversity in the UK: A Review. Sci. Total Environ. 2018, 610–611, 316–332. [Google Scholar] [CrossRef]
- Álvarez, E.; Viadé, A.; Fernández-Marcos, M.L. Effect of Liming with Different Sized Limestone on the Forms of Aluminium in a Galician Soil (NW Spain). Geoderma 2009, 152, 1–8. [Google Scholar] [CrossRef]
- Fertiliser Manual (RB209). Available online: https://www.gov.uk/government/publications/fertiliser-manual-rb209--2 (accessed on 31 October 2021).
- Chung, Y.S.; Choi, S.C.; Silva, R.R.; Kang, J.W.; Eom, J.H.; Kim, C. Case Study: Estimation of Sorghum Biomass Using Digital Image Analysis with Canopeo. Biomass Bioenergy 2017, 105, 207–210. [Google Scholar] [CrossRef]
- Patrignani, A.; Ochsner, T.E. Canopeo: A powerful new tool for measuring fractional green canopy cover. Agron. J. 2015, 107, 2312–2320. [Google Scholar] [CrossRef] [Green Version]
- Penn, C.J.; Camberato, J.J. A critical review on soil chemical processes that control how soil PH affects phosphorus availability to plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef] [Green Version]
- Moreira, A.; Moraes, L.A.C.; Aquino, G.S. Iron and Manganese Effect on Soil Chemical Properties, Yield Components, and Nutritional Status of Soybean. Commun. Soil Sci. Plant. Anal. 2018, 49, 1844–1854. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; Zia ur Rehman, M.; Waris, A.A. Zinc and Iron Oxide Nanoparticles Improved the Plant Growth and Reduced the Oxidative Stress and Cadmium Concentration in Wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA Soil Test for Zinc, Iron, Manganese, and Copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Dibb, D.W.; Thompson, W.R., Jr. Interaction of Potassium with Other Nutrients. In Potassium in Agriculture; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1985; pp. 515–533. ISBN 978-0-89118-247-4. [Google Scholar]
- Yildirim, I.Z.; Prezzi, M. Chemical, Mineralogical, and Morphological Properties of Steel Slag. Adv. Civil Eng. 2011, 2011, e463638. [Google Scholar] [CrossRef] [Green Version]
- Wacal, C.; Ogata, N.; Basalirwa, D.; Sasagawa, D.; Masunaga, T.; Yamamoto, S.; Nishihara, E. Growth and K Nutrition of Sesame (Sesamum indicum L.) Seedlings as Affected by Balancing Soil Exchangeable Cations Ca, Mg, and K of Continuously Monocropped Soil from Upland Fields Converted Paddy. Agronomy 2019, 9, 819. [Google Scholar] [CrossRef] [Green Version]
- Fageria, N.K.; Baligar, V.C.; Clark, R.B. Micronutrients in Crop Production. In Advances in Agronomy; Sparks, D.L., Ed.; Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2002; Volume 77, pp. 185–268. [Google Scholar]
- Dulac, J.; Hancock, D.; Harmon, D. White Clover Establishment and Management Guide. Available online: https://extension.uga.edu/publications.html (accessed on 25 November 2021).
- Pol, M.; Schmidtke, K.; Lewandowska, S. Plantago Lanceolata—An overview of its agronomically and healing valuable features. Open Agric. 2021, 6, 479–488. [Google Scholar] [CrossRef]
- Ayers, R.S.; Westcot, D.W. Miscellaneous Problems. In Water Quality for Agriculture; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 1985; ISBN 92-5-102263-1. [Google Scholar]
- European Commission. 2003/33/EC: Council Decision of 19 December 2002 Establishing Criteria and Procedures for the Acceptance of Waste at Landfills Pursuant to Article 16 of and Annex II to Directive 1999/31/EC; European Commission: Brussels, Belgium, 2002; Volume 011. [Google Scholar]

| % | BOF 0–20 mm | BOF 20–50 mm |
|---|---|---|
| SiO2 | 13.71 | 12.77 |
| Al2O3 | 8.38 | 7.07 |
| FeO | 9.50 | 11.57 |
| Fe2O3 | 1.83 | 1.69 |
| Fetotal | 14.38 | 19.53 |
| P2O5 | 1.22 | 1.27 |
| CaO | 45.98 | 44.37 |
| MgO | 2.97 | 2.62 |
| K2O | 0.041 | 0.051 |
| Na2O | 0.014 | 0.012 |
| MnO | 3.64 | 3.31 |
| TiO2 | 0.64 | 0.59 |
| BOF Slag | 0–20 mm | 20–50 mm |
|---|---|---|
| Untreated | 8.03 ± 0.07 | 7.54 ± 0.07 |
| Treated | 4.39 ± 0.07 | 4.52 ± 0.04 |
| Parameter | Unit | Value | Classification |
|---|---|---|---|
| Clay | % | 9.90 | - |
| Silt | % | 19.80 | - |
| Sand | % | 70.30 | - |
| pH | - | 4.20 | Very low |
| Conductivity | μS cm−1 | <70 | Very low |
| Organic matter | % | 2.03 | Normal |
| Nitrogen (N) | mg kg−1 | 752 | Low |
| Phosphorus (P) | mg kg−1 | 9.80 | Low |
| Potassium (K) | meq 100g−1 | 0.08 | Very low |
| Calcium (Ca) | meq 100g−1 | 1.48 | Very low |
| Magnesium (Mg) | meq 100g−1 | 0.24 | Very low |
| C/N | - | 15.60 | High |
| Soil Type | pH = 5.0 | pH = 5.5 | pH = 6.0 | pH = 6.2 | ||||
|---|---|---|---|---|---|---|---|---|
| Grass | Arable | Grass | Arable | Grass | Arable | Grass | Arable | |
| Organic soils | 600 | 1600 | 0 | 800 | 0 | 0 | 0 | 0 |
| Sands and loamy sands | 500 | 1000 | 300 | 700 | 0 | 400 | 0 | 300 |
| Sandy loams and sit loams | 600 | 1200 | 400 | 800 | 0 | 500 | 0 | 400 |
| Clay loams and clays | 700 | 1400 | 400 | 1000 | 0 | 600 | 0 | 400 |
| Material | % CaO | g m−2 | g pot−1 |
|---|---|---|---|
| BOF slag 0–20 mm | 50% | 900 | 77.6 |
| BOF slag 20–50 mm | 42% | 1071.5 | 92.4 |
| BOF slag all-one | 46% | 978.3 | 84.4 |
| Lime | 77% | 584.4 | 50.4 |
| Family | Species | Common Name | % |
|---|---|---|---|
| Fabacae | Trifolium repens | White clover | 5 |
| Medicago sativa | Alfalfa | 5 | |
| Trifolium pratense | Red clover | 5 | |
| Poaceae | Lolium perenne | English ryegrass | 20 |
| Festuca rubra | Red fescue | 15 | |
| Festuca arundinacea | Tall fescue | 15 | |
| Lolium multiflorum | Italian ryegrass | 13 | |
| Dactylis glomerata | Cat grass | 13 | |
| Agrostis tenuis | Colonial bent | 5 | |
| Holcus lanatus | Tufted grass | 2 | |
| Plantaginaceae | Plantago lanceolata | Ribwort plantain | 2 |
| Amendment | Fertiliser | Fe * | Mn * | Cu * | Zn * | pH | |
|---|---|---|---|---|---|---|---|
| E25 | 25% 0–20 mm BOF slag | With NPK | 97.50 | 12.40 | 0.20 | 1.11 | 10.20 |
| Without NPK | 118.00 | 6.80 | 0.20 | 0.20 | 10.10 | ||
| E50 | 50% 0–20 mm BOF slag | With NPK | 149.00 | 1.12 | 0.23 | 0.20 | 11.40 |
| Without NPK | 175.00 | 1.00 | 0.33 | 0.20 | 11.20 | ||
| T25 | 25% all-one BOF slag | With NPK | 60.60 | 13.31 | 0.20 | 6.35 | 8.31 |
| Without NPK | 79.40 | 24.80 | 0.20 | 0.85 | 7.92 | ||
| T50 | 50% all-one BOF slag | With NPK | 63.60 | 33.40 | 0.27 | 2.01 | 8.80 |
| Without NPK | 79.30 | 39.30 | 0.29 | 2.08 | 9.30 | ||
| S25 | 25% treated BOF slag | With NPK | 50.00 | 16.48 | 0.20 | 2.06 | 7.590 |
| Without NPK | 63.00 | 17.39 | 0.20 | 2.10 | 7.71 | ||
| S50 | 50% treated BOF slag | With NPK | 49.20 | 26.40 | 0.23 | 1.23 | 8.87 |
| Without NPK | 60.40 | 14.90 | 0.20 | 1.96 | 8.32 | ||
| L | Lime | With NPK | 74.00 | 1.00 | 0.20 | 2.62 | 8.12 |
| Without NPK | 87.00 | 1.09 | 0.20 | 1.76 | 8.15 | ||
| C | Soil control | With NPK | 48.20 | 2.81 | 0.26 | 6.78 | 4.46 |
| Without NPK | 44.70 | 5.28 | 0.20 | 6.54 | 4.30 | ||
| C0 | Initial soil | - | 51.00 | 1.00 | 0.29 | 1.72 | 4.20 |
| Amendment | Fertiliser | Cr | V | Cu | Sr | Al | Ni | As | Zn |
|---|---|---|---|---|---|---|---|---|---|
| E25 | With NPK | 0.0023 | 0.0105 | 0.0988 | 0.4239 | 0.2785 | 0.0092 | 0.0507 | 0.0332 |
| Without NPK | 0.0022 | 0.0398 | 0.0454 | 0.2180 | 0.2700 | 0.0075 | 0.0452 | 0.0499 | |
| E50 | With NPK | <0.002 | 0.0022 | 0.0818 | 0.7164 | 0.0799 | 0.0038 | 0.0377 | 0.0186 |
| Without NPK | <0.002 | <0.002 | 0.0976 | 2.3522 | 0.0579 | 0.0044 | 0.0389 | 0.0076 | |
| T25 | With NPK | 0.004 | 0.0286 | 0.0321 | 0.1485 | 0.2532 | 0.0073 | 0.0766 | 0.0514 |
| Without NPK | <0.002 | 0.022 | 0.007 | 0.1470 | 0.0311 | <0.002 | 0.0124 | 0.1292 | |
| T50 | With NPK | 0.0032 | 0.0037 | 0.0769 | 0.3636 | 0.2157 | 0.0081 | 0.0714 | 0.0569 |
| Without NPK | 0.0039 | 0.0112 | 0.0798 | 0.5984 | 0.3726 | 0.0086 | 0.0935 | 0.114 | |
| S25 | With NPK | 0.008 | 0.1428 | 0.0138 | 0.2005 | 0.1614 | 0.0042 | 0.0517 | 0.1561 |
| Without NPK | <0.002 | 0.0204 | 0.0063 | 0.1656 | 0.0335 | <0.002 | 0.0149 | 0.1833 | |
| S50 | With NPK | 0.0031 | 0.0935 | 0.0176 | 0.1425 | 0.0487 | 0.0033 | 0.0227 | 0.2728 |
| Without NPK | 0.0025 | 0.0666 | 0.0135 | 0.1282 | 0.0461 | <0.002 | 0.0137 | 0.2172 | |
| L | With NPK | 0.0062 | 0.0145 | 0.0043 | 0.6952 | 3.4287 | 0.0073 | <0.002 | 0.5077 |
| Without NPK | 0.0044 | 0.0053 | 0.0063 | 0.1399 | 1.0978 | <0.002 | <0.002 | 6.8505 | |
| C | With NPK | <0.002 | <0.002 | 0.0051 | 0.0056 | 0.5178 | <0.002 | 0.0095 | 0.1967 |
| Without NPK | <0.002 | 0.0028 | 0.0118 | 0.2415 | 0.4344 | 0.0037 | <0.002 | 0.1622 | |
| FAO | - | 0.100 | 0.100 | 0.200 | - | 5.000 | 0.200 | 0.100 | 2.000 |
| EU (2003/33/EC) | - | 0.100 | - | 0.600 | - | - | 0.120 | 0.060 | 1.200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Piloneta, M.; Ortega-Fernández, F.; Terrados-Cristos, M.; Álvarez-Cabal, J.V. Application of Steel Slag for Degraded Land Remediation. Land 2022, 11, 224. https://doi.org/10.3390/land11020224
Díaz-Piloneta M, Ortega-Fernández F, Terrados-Cristos M, Álvarez-Cabal JV. Application of Steel Slag for Degraded Land Remediation. Land. 2022; 11(2):224. https://doi.org/10.3390/land11020224
Chicago/Turabian StyleDíaz-Piloneta, Marina, Francisco Ortega-Fernández, Marta Terrados-Cristos, and Jose Valeriano Álvarez-Cabal. 2022. "Application of Steel Slag for Degraded Land Remediation" Land 11, no. 2: 224. https://doi.org/10.3390/land11020224
APA StyleDíaz-Piloneta, M., Ortega-Fernández, F., Terrados-Cristos, M., & Álvarez-Cabal, J. V. (2022). Application of Steel Slag for Degraded Land Remediation. Land, 11(2), 224. https://doi.org/10.3390/land11020224








