Land Use Pattern Affects Microplastic Concentrations in Stormwater Drains in Urban Catchments in Perth, Western Australia
Abstract
1. Introduction
- What is the concentration of microplastics in selected stormwater drains across the Perth and Peel region?
- What plastic shapes, sizes, colours and polymer types are identified in the drainage systems?
- Is there a significant difference in microplastic concentrations within or between any of the drainage systems?
- Does the land use pattern, as defined by catchment area, catchment population, pre-sampling rainfall, and the proportions of residential, industrial, commercial, agricultural, natural land, and public open spaces, affect stormwater plastic concentrations?
2. Materials and Methods
2.1. Sample Sites
2.2. Sampling Protocol
2.3. Sample Processing
2.4. Sample Analysis
2.5. Blanks
2.6. Microplastic Identification and Characterisation
2.7. Data Analysis
3. Results
3.1. Catchment Modelling
3.2. Background Contamination and Raman Spectroscopy
3.3. Statistical Analyses of Factors Affecting Microplastic Concentrations
- (a)
- Microplastic concentrations across and within drainage catchments.
- (b)
- Analysis of Covariates
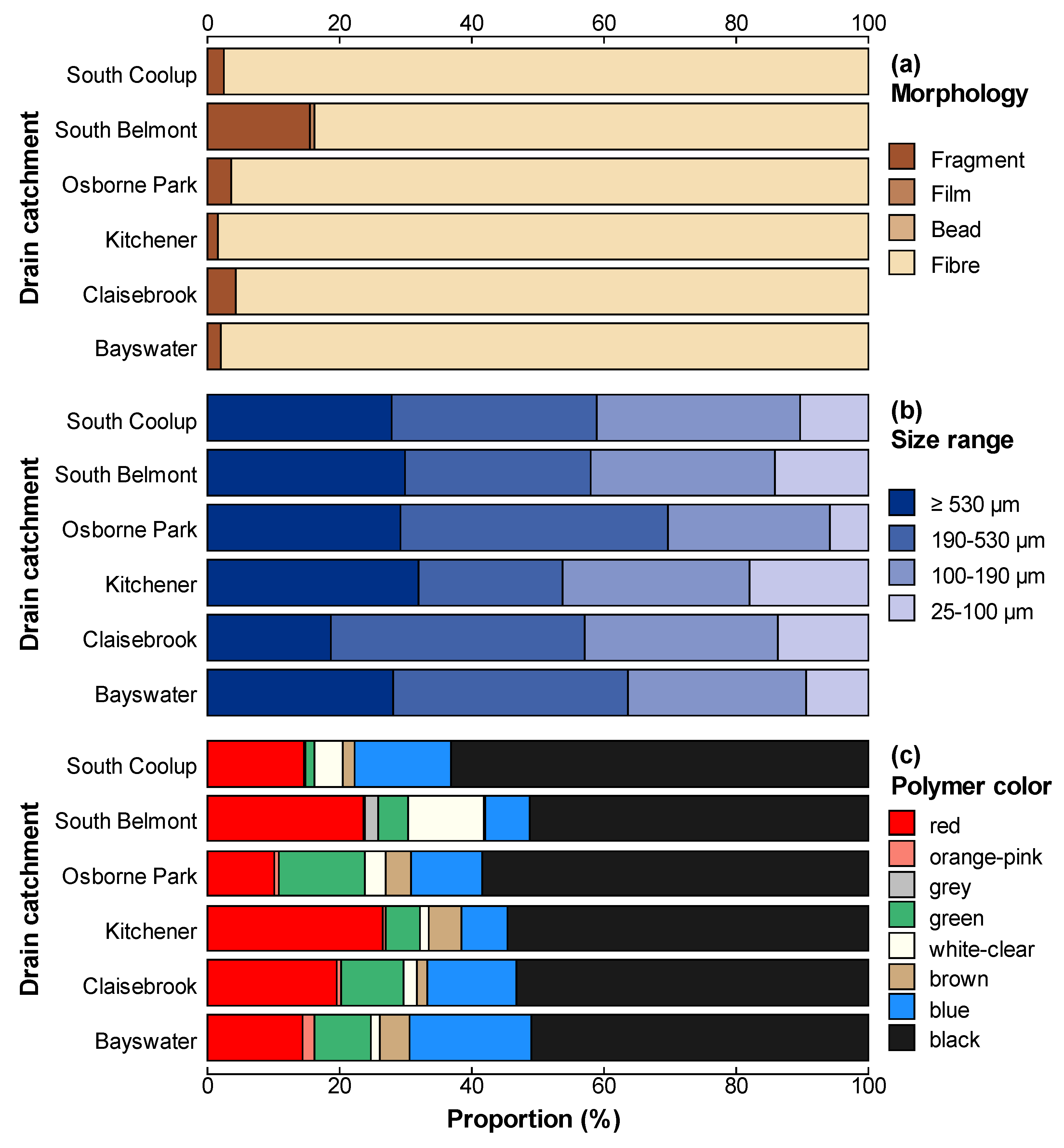
3.4. Microplastic Characteristics
4. Discussion
4.1. Catchment and Site Variation in Microplastic Concentrations
4.2. Factors Affecting Microplastic Concentrations
4.3. Microplastic Concentrations
4.4. Polymer Types
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, W.J.; Clout, M.; Côté, I.M.; Daszak, P.; Depledge, M.H.; Fellman, L.; Fleishman, E.; Garthwaite, R.; Gibbons, D.W.; De Lurio, J.; et al. A horizon scan of global conservation issues for 2010. Trends Ecol. Evol. 2010, 25, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Shi, H.; Peng, J.; Wang, Y.; Xiong, X.; Wu, C.; Lam, P.K.S. Microplastic pollution in China’s inland water systems: A review of findings, methods, characteristics, effects, and management. Sci. Total Environ. 2018, 630, 1641–1653. [Google Scholar] [CrossRef] [PubMed]
- Arthur, C.; Baker, J.; Bamford, H. (Eds.) Proceedings of the International Research Workshop on the Occurrence, Effects and Fate of Microplastic Marine Debris; National Oceanic and Atmospheric Administration (NOAA): Tacoma, WA, USA, 2008; Volume NOS-OR&R-30, p. 528. [Google Scholar]
- Qi, R.; Jones, D.L.; Li, Z.; Liu, Q.; Yan, C. Behavior of microplastics and plastic film residues in the soil environment: A critical review. Sci. Total Environ. 2020, 703, 134722. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Teuten, E.L.; Rowland, S.J.; Galloway, T.S.; Thompson, R.C. Potential for Plastics to Transport Hydrophobic Contaminants. Environ. Sci. Technol. 2007, 41, 7759–7764. [Google Scholar] [CrossRef]
- Khalid, N.; Aqeel, M.; Noman, A.; Khan, S.M.; Akhter, N. Interactions and effects of microplastics with heavy metals in aquatic and terrestrial environments. Environ. Pollut. 2021, 290, 118104. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Tassin, B. Sources and Fate of Microplastics in Urban Areas: A Focus on Paris Megacity. In Freshwater Microplastics: Emerging Environmental Contaminants? Wagner, M., Lambert, S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 69–83. [Google Scholar]
- de Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Change Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Sutton, R.; Mason, S.A.; Stanek, S.K.; Willis-Norton, E.; Wren, I.F.; Box, C. Microplastic contamination in the San Francisco Bay, California, USA. Mar. Pollut. Bull. 2016, 109, 230–235. [Google Scholar] [CrossRef]
- Lasee, S.; Mauricio, J.; Thompson, W.; Karnjanapiboonwong, A.; Kasumba, J.; Subbiah, S.; Morse, A.; Anderson, T. Microplastics in a freshwater environment receiving treated wastewater effluent: Microplastics in Urban Surface Water. Integr. Environ. Assess. Manag. 2017, 13, 528–532. [Google Scholar] [CrossRef]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; International Union for Conservation of Nature and Natural Resources (IUCN): Gland, Switzerland, 2017; p. 43. [Google Scholar]
- Smyth, K.; Drake, J.; Li, Y.; Rochman, C.; Van Seters, T.; Passeport, E. Bioretention cells remove microplastics from urban stormwater. Water Res. 2021, 191, 116785. [Google Scholar] [CrossRef]
- Horton, A.A.; Dixon, S.J. Microplastics: An introduction to environmental transport processes. WIREs Water 2018, 5, e1268. [Google Scholar] [CrossRef]
- Monira, S.; Bhuiyan, M.A.; Haque, N.; Shah, K.; Roychand, R.; Hai, F.I.; Pramanik, B.K. Understanding the fate and control of road dust-associated microplastics in stormwater. Process Saf. Environ. Protect. 2021, 152, 47–57. [Google Scholar] [CrossRef]
- Kowalski, N.; Reichardt, A.M.; Waniek, J.J. Sinking rates of microplastics and potential implications of their alteration by physical, biological, and chemical factors. Mar. Pollut. Bull. 2016, 109, 310–319. [Google Scholar] [CrossRef]
- Lutz, N.; Fogarty, J.; Rate, A. Accumulation and potential for transport of microplastics in stormwater drains into marine environments, Perth region, Western Australia. Mar. Pollut. Bull. 2021, 168, 112362. [Google Scholar] [CrossRef]
- Piñon-Colin, T.d.J.; Rodriguez-Jimenez, R.; Rogel-Hernandez, E.; Alvarez-Andrade, A.; Wakida, F.T. Microplastics in stormwater runoff in a semiarid region, Tijuana, Mexico. Sci. Total Environ. 2020, 704, 135411. [Google Scholar] [CrossRef]
- Treilles, R.; Gasperi, J.; Gallard, A.; Saad, M.; Dris, R.; Partibane, C.; Breton, J.; Tassin, B. Microplastics and microfibers in urban runoff from a suburban catchment of Greater Paris. Environ. Pollut. 2021, 287, 117352. [Google Scholar] [CrossRef]
- Data WA. Water Corporation: Drainage Open Channel (WCORP-083). Government of Western Australia: Perth, Australia. Available online: https://catalogue.data.wa.gov.au/dataset/drain-open-channel (accessed on 7 October 2022).
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D.L. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- IRUG. Spectral Database Index. Available online: http://www.irug.org/search-spectral-database (accessed on 7 October 2022).
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.P.; Ribeiro-Claro, P.J.A. Identification of microplastics using Raman spectroscopy: Latest developments and future prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef]
- Geoscience Australia. Digital Elevation model (DEM) of Australia derived from LiDAR 5 Metre Grid; Geoscience Australia: Symonston, Australia, 2021. [Google Scholar]
- ESRI. ArcGIS Pro: Release 2.6.0; Environmental Systems Research Institute: Redlands, CA, USA, 2020. [Google Scholar]
- ABARES. The Australian Land Use and Management Classification Version 8; Department of Agriculture, Fisheries and Forestry: Canberra, Australia, 2016. [Google Scholar]
- DPIRD. Catchment Scale Land Use Mapping for Western Australia (DPIRD-067); Department of Primary Industries and Regional Development: Perth, Autralia, 2018. [Google Scholar]
- BOM. Climate Data Online. Available online: http://www.bom.gov.au/climate/data/ (accessed on 15 September 2021).
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.2.0; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Pinheiro, J.; Bates, D. R Core Team nlme: Linear and Nonlinear Mixed Effects Models; R Package Version 3.1-157; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Mora-Teddy, A.K.; Matthaei, C.D. Microplastic pollution in urban streams across New Zealand: Concentrations, composition and implications. N. Z. J. Mar. FreshWater Res. 2020, 54, 233–250. [Google Scholar] [CrossRef]
- Townsend, K.R.; Lu, H.; Sharley, D.J.; Pettigrove, V. Associations between microplastic pollution and land use in urban wetland sediments. Environ. Sci. Pollut. Res. 2019, 26, 22551–22561. [Google Scholar] [CrossRef]
- Liu, F.; Olesen, K.B.; Borregaard, A.R.; Vollertsen, J. Microplastics in urban and highway stormwater retention ponds. Sci. Total Environ. 2019, 671, 992–1000. [Google Scholar] [CrossRef]
- Free, C.M.; Jensen, O.P.; Mason, S.A.; Eriksen, M.; Williamson, N.J.; Boldgiv, B. High-levels of microplastic pollution in a large, remote, mountain lake. Mar. Pollut. Bull. 2014, 85, 156–163. [Google Scholar] [CrossRef]
- Yonkos, L.T.; Friedel, E.A.; Perez-Reyes, A.C.; Ghosal, S.; Arthur, C.D. Microplastics in Four Estuarine Rivers in the Chesapeake Bay, U.S.A. Environ. Sci. Technol. 2014, 48, 14195–14202. [Google Scholar] [CrossRef]
- Grbić, J.; Helm, P.; Athey, S.; Rochman, C.M. Microplastics entering northwestern Lake Ontario are diverse and linked to urban sources. Water Res. 2020, 174, 115623. [Google Scholar] [CrossRef]
- Sang, W.; Chen, Z.; Mei, L.; Hao, S.; Zhan, C.; Zhang, W.b.; Li, M.; Liu, J. The abundance and characteristics of microplastics in rainwater pipelines in Wuhan, China. Sci. Total Environ. 2021, 755, 142606. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, S.; Zhang, Y.; Lo, Y.; Liu, M.; Ma, Y.; Chau, H.S.; Cao, Y.; Xu, X.; Wu, R.; et al. A systematic study of microplastic occurrence in urban water networks of a metropolis. Water Res. 2022, 223, 118992. [Google Scholar] [CrossRef] [PubMed]
- Shruti, V.C.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Kutralam-Muniasamy, G. Current trends and analytical methods for evaluation of microplastics in stormwater. Trends Environ. Anal. Chem. 2021, 30, e00123. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Drapper, D.; Hornbuckle, A.; Rintoul, L.; Leusch, F.D.L. Microplastic pollution in a stormwater floating treatment wetland: Detection of tyre particles in sediment. Sci. Total Environ. 2020, 713, 136356. [Google Scholar] [CrossRef] [PubMed]
- Erni-Cassola, G.; Zadjelovic, V.; Gibson, M.I.; Christie-Oleza, J.A. Distribution of plastic polymer types in the marine environment; A meta-analysis. J. Hazard. Mater. 2019, 369, 691–698. [Google Scholar] [CrossRef]
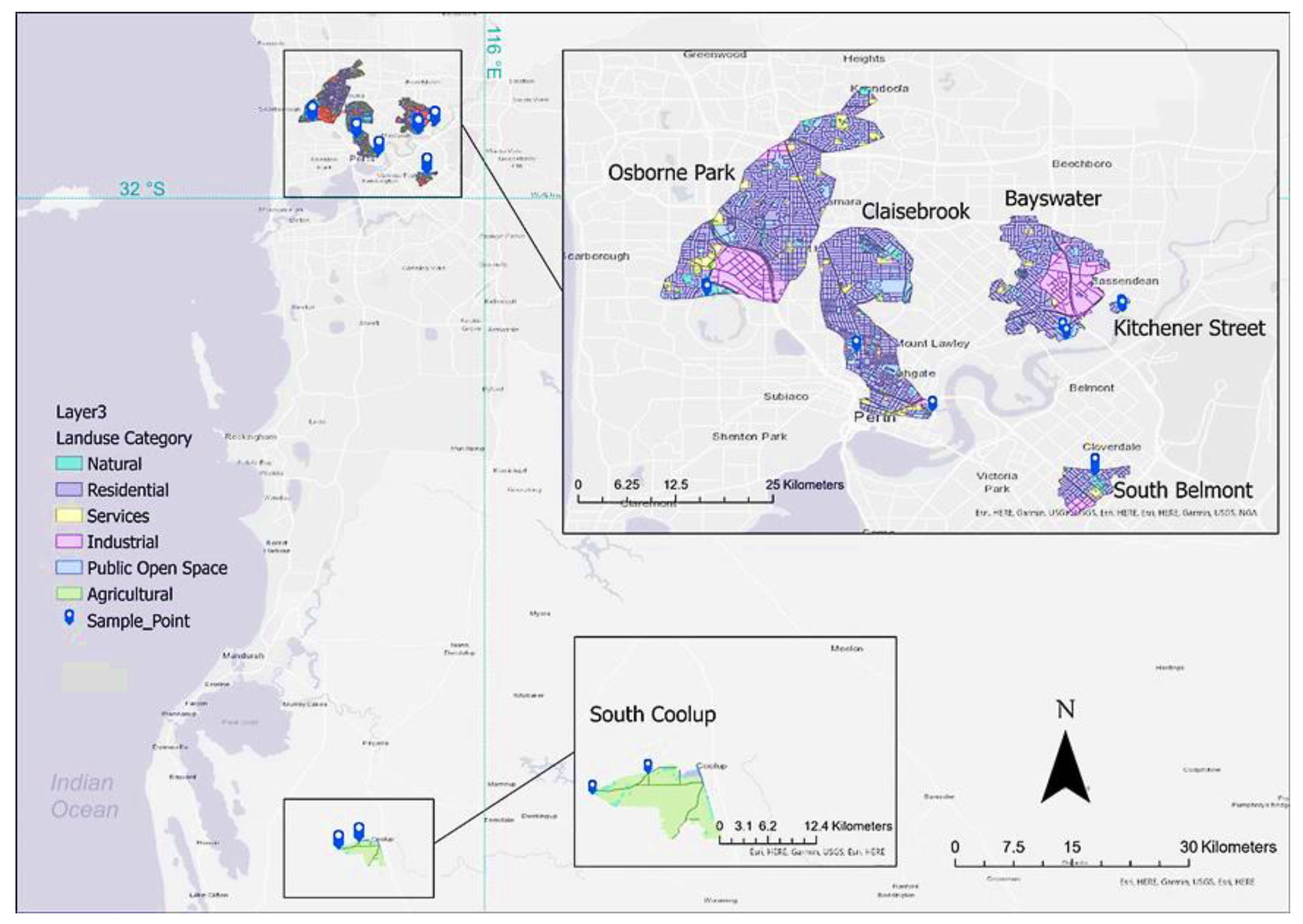
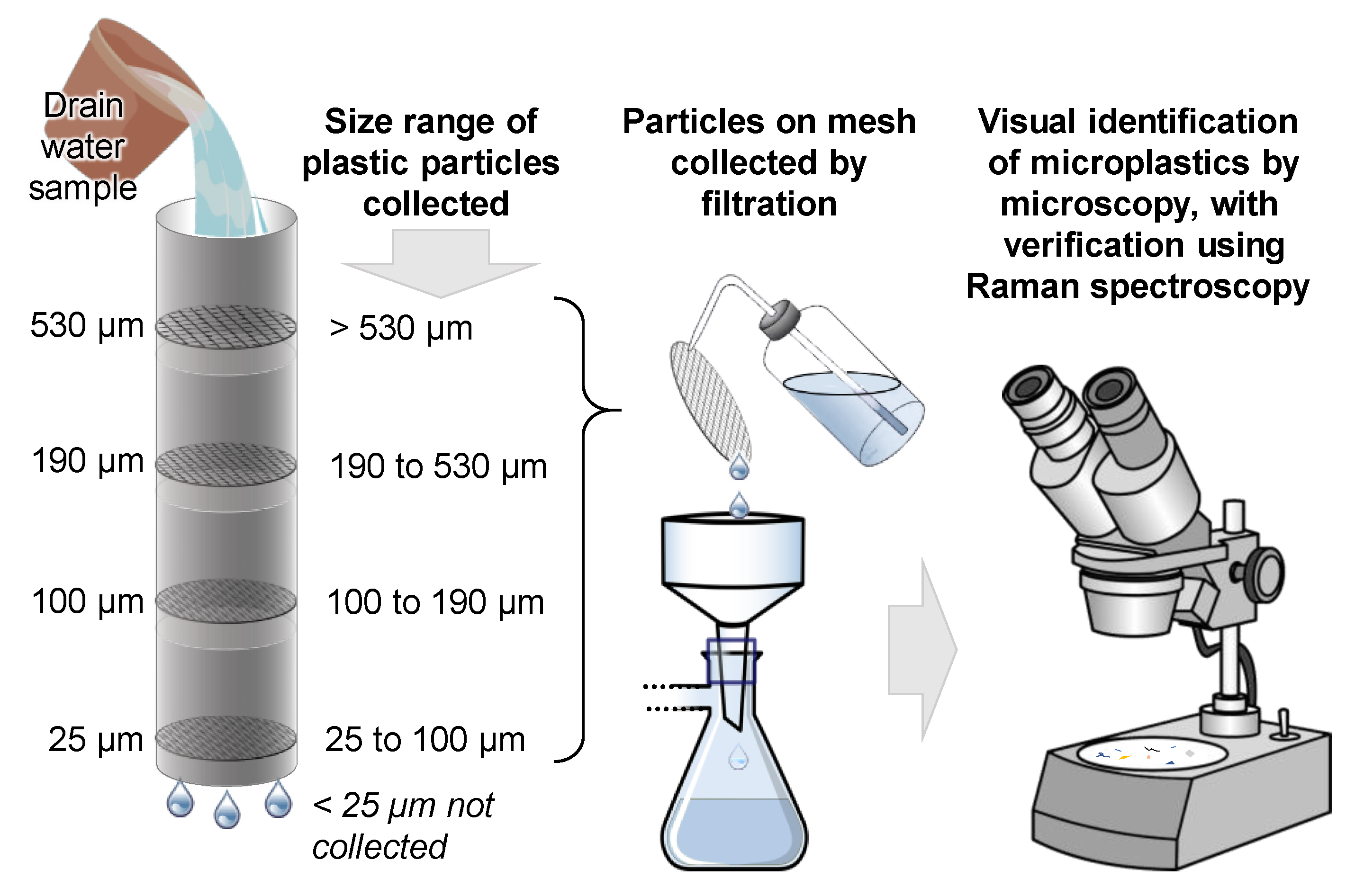
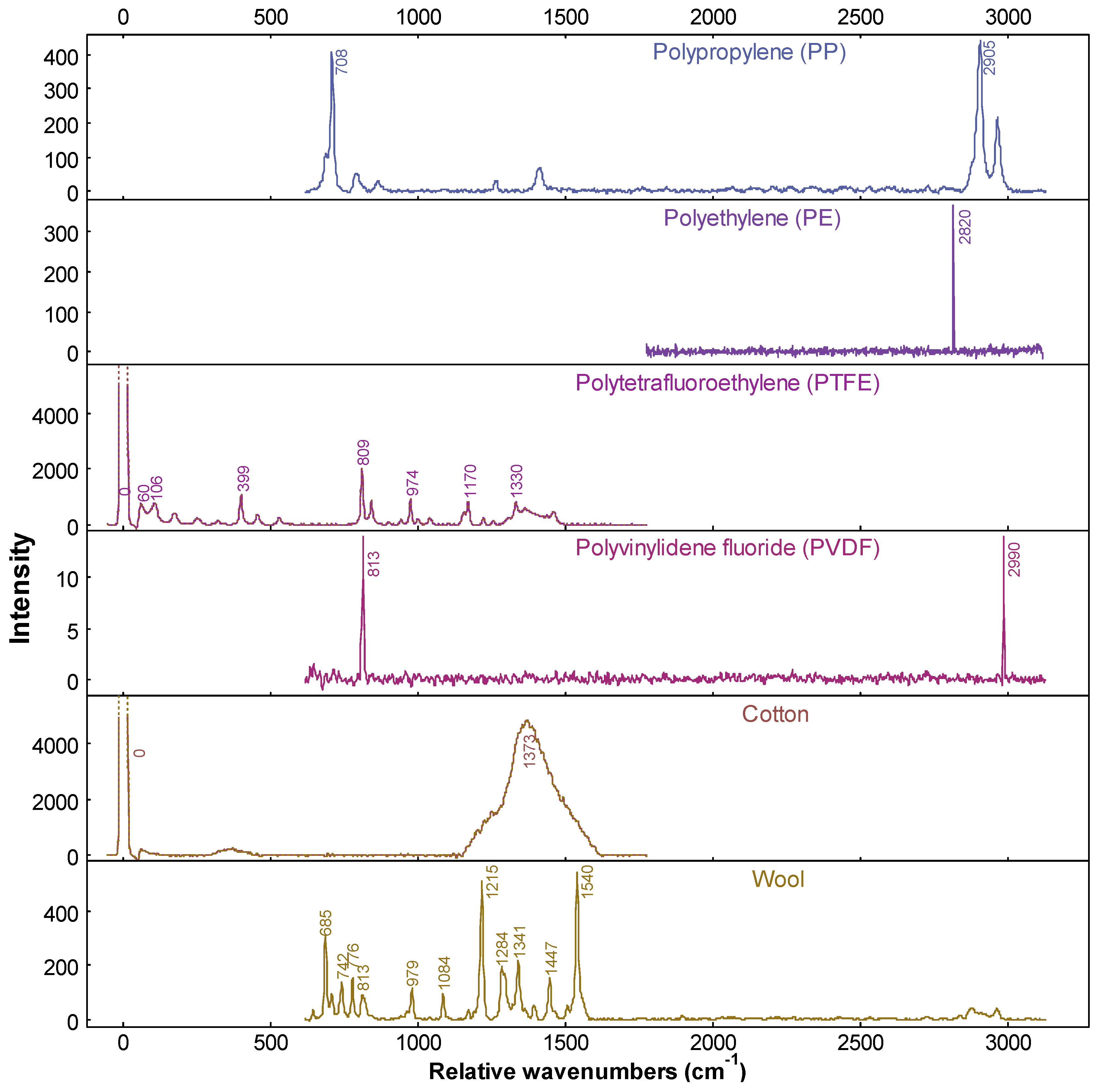
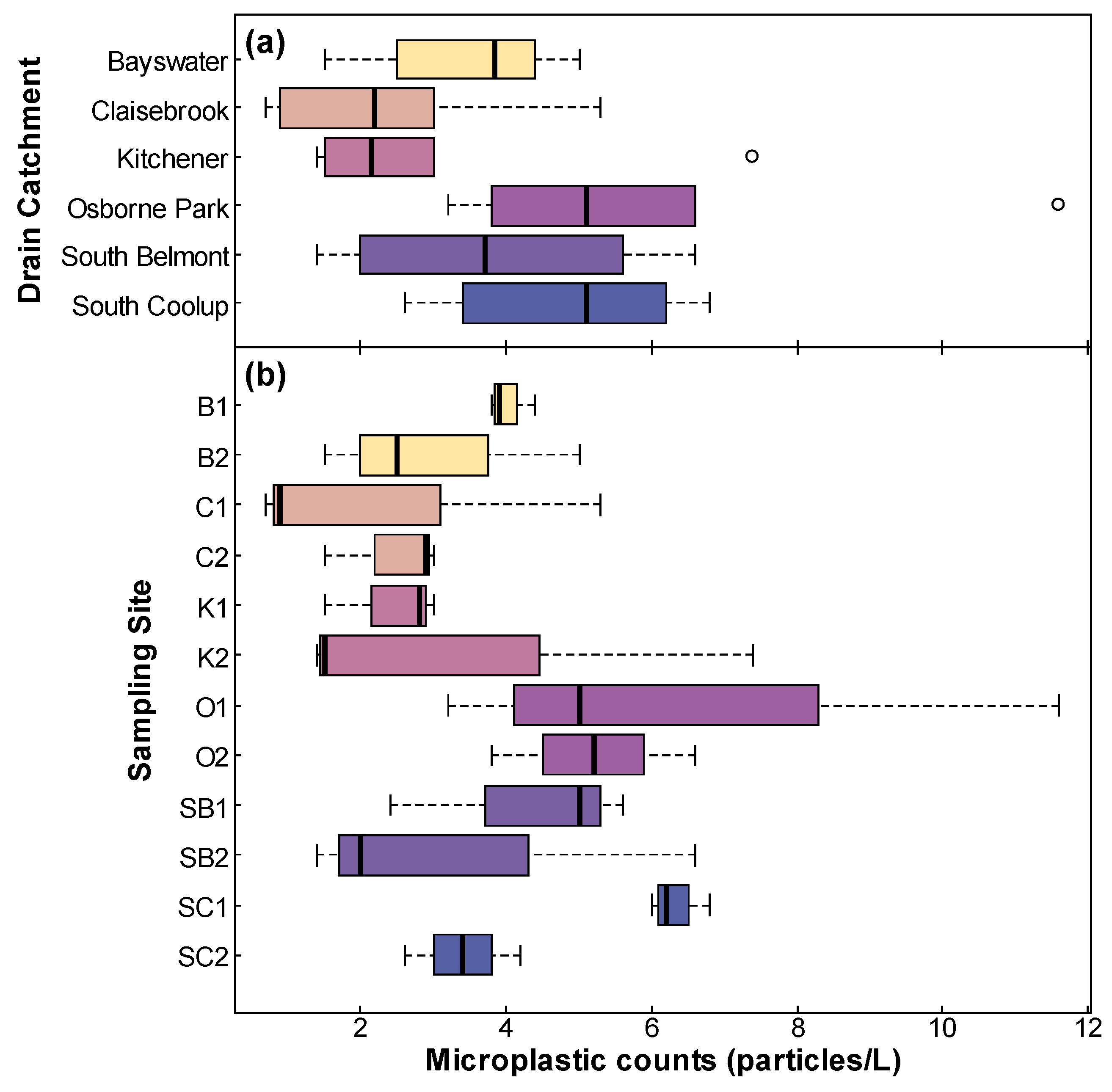
| Sample Catchment | Site Number | Site Code | Coordinates (Decimal Degrees, WGS84) | Sample Size | |
|---|---|---|---|---|---|
| Longitude | Latitude | ||||
| Bayswater Main Drain | 1 | B1 | 115.92135 | −31.92585 | 10 L |
| Bayswater Main Drain | 2 | B2 | 115.92186 | −31.92730 | 10 L |
| Claisebrook Main Drain | 1 | C1 | 115.85102 | −31.93210 | 10 L |
| Claisebrook Main Drain | 2 | C2 | 115.87702 | −31.95256 | 10 L |
| Osborne Park Branch Drain | 1 | O1 | 115.79987 | −31.91233 | 5 L |
| Osborne Park Branch Drain | 2 | O2 | 115.80029 | −31.91306 | 5 L |
| Kitchener St Drain | 1 | K1 | 115.94133 | −31.91799 | 10 L |
| Kitchener St Drain | 2 | K2 | 115.94171 | −31.91855 | 10 L |
| South Belmont Main Drain | 1 | SB1 | 115.93236 | −31.97443 | 10 L |
| South Belmont Main Drain | 2 | SB2 | 115.93233 | −31.97202 | 5L |
| South Coolup Main Drain | 1 | SC1 | 115.85390 | −32.74431 | 5 L |
| South Coolup Main Drain | 2 | SC2 | 115.83038 | −32.75319 | 5 L |
| B1 | B2 | C1 | C2 | K1 | K2 | O1 | O2 | SB1 | SB2 | SC1 | SC2 | |
| Catchment Characteristics | ||||||||||||
| Area (ha) | 1089 | 1105 | 1459 | 990 | 20 | 21 | 2227 | 2324 | 223 | 238 | 899 | 631 |
| Population | 21039 | 21372 | 34370 | 24548 | 298 | 319 | 45963 | 49291 | 3221 | 3387 | 91 | 64 |
| Rainfall (mm) ¹ | 31.2 | 31.2 | 56.8 | 11.4 | 31.8 | 31.8 | 44.6 | 6.8 | 31.8 | 5.4 | 14.8 | 14.8 |
| Land Use Proportion (%) | ||||||||||||
| Public open | 8.5 | 9.2 | 16.6 | 17.9 | 0.00 | 0.00 | 9.6 | 9.3 | 8.7 | 8.1 | 0.00 | 0.00 |
| Industrial | 31.4 | 30.9 | 1.4 | 0.00 | 0.00 | 0.00 | 13.0 | 16.0 | 17.4 | 16.3 | 0.47 | 0.50 |
| Residential | 58.0 | 58.0 | 76.8 | 77.7 | 84.2 | 84.2 | 68.5 | 65.9 | 64.6 | 67.0 | 1.9 | 2.7 |
| Services | 2.2 | 2.1 | 3.9 | 3.0 | 15.8 | 15.8 | 6.7 | 6.4 | 5.1 | 4.8 | 0.00 | 0.00 |
| Natural | 0.00 | 0.10 | 1.4 | 1.5 | 0.00 | 0.00 | 2.2 | 2.4 | 4.1 | 3.8 | 2.7 | 2.5 |
| Agricultural | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 94.9 | 94.3 |
| Bayswater | Claisebrook | Kitchener | Osborne | S. Belmont | S. Coolup | |
|---|---|---|---|---|---|---|
| Mean (MP/L) | 12.6 | 12.8 | 9.2 | 20 | 12.8 | 18 |
| Standard deviation | 3.6 | 4.7 | 2.1 | 12 | 4.2 | 10 |
| B1 | B2 | C1 | C2 | K1 | K2 | O1 | O2 | SB1 | SB2 | SC1 | SC2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (MP/L) | 13.9 | 11.2 | 14.7 | 11.0 | 9.7 | 8.8 | 25 | 15.2 | 12.9 | 12.7 | 25 | 10.3 |
| Standard deviation | 3.6 | 3.8 | 5.4 | 4.0 | 2.2 | 2.4 | 16 | 2.6 | 1.5 | 6.5 | 10 | 2.2 |
| Covariate | Model Specifications | Covariate Coefficient | p-Value |
|---|---|---|---|
| Catchment population ÷ 1000 | Heteroskedastic, variation at site level | 0.1041 | 0.005 |
| Catchment area | Heteroskedastic, variation at site level | 0.0025 | 0.0037 |
| Rainfall | Heteroskedastic, variation at site level | −0.0109 | 0.86 |
| Residential proportion | Heteroskedastic, variation at catchment level | −0.1168 | 0.0169 |
| Agricultural proportion | Heteroskedastic, variation at site level | −0.0120 | 0.60 |
| Industrial proportion | Heteroskedastic, variation at site level | 0.1352 | 0.0107 |
| Natural proportion | Heteroskedastic, variation at site level | 0.7090 | 0.0248 |
| Services proportion | Heteroskedastic, variation at site level | −0.2761 | 0.0093 |
| Public Open Space proportion | Heteroskedastic, variation at site level | 0.3193 | 0.0051 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bond, C.; Li, H.; Rate, A.W. Land Use Pattern Affects Microplastic Concentrations in Stormwater Drains in Urban Catchments in Perth, Western Australia. Land 2022, 11, 1815. https://doi.org/10.3390/land11101815
Bond C, Li H, Rate AW. Land Use Pattern Affects Microplastic Concentrations in Stormwater Drains in Urban Catchments in Perth, Western Australia. Land. 2022; 11(10):1815. https://doi.org/10.3390/land11101815
Chicago/Turabian StyleBond, Cassandra, Hua Li, and Andrew W. Rate. 2022. "Land Use Pattern Affects Microplastic Concentrations in Stormwater Drains in Urban Catchments in Perth, Western Australia" Land 11, no. 10: 1815. https://doi.org/10.3390/land11101815
APA StyleBond, C., Li, H., & Rate, A. W. (2022). Land Use Pattern Affects Microplastic Concentrations in Stormwater Drains in Urban Catchments in Perth, Western Australia. Land, 11(10), 1815. https://doi.org/10.3390/land11101815






