Abstract
Soil microbial residues play an important role in the formation and stabilization of soil organic matter and can be quantitatively characterized by amino sugars. However, the response of soil microbial residues to agricultural cultivation in karst areas remains unclear. In this study, we collected soil samples from natural reserved land as well as five plantation forests dominated by Citrus trees cultivated for 0, 1, 5, 15, 30 years to examine the effects of agricultural cultivation on the content of microbial residues (amino sugar analysis). Results showed that: (1) Soil Amino Sugars (ASs) contents were significantly reduced after agricultural cultivation along with the sharp decrease in soil organic carbon (SOC). After 30 years of cultivation, the contents of total ASs, glucosamine (GluN), galactosamine (GalN), and muramic acid (MurA) in cultivated soils decreased by 58.22%, 55.30%, 27.11%, respectively, compared with 0 yr.; (2) Microbial residual carbon contribution to SOC increased from 34.11% to 81.33% after 30 years of cultivation, including fungal residual carbon (FRC) (25.79% to 48.6%) and bacterial residual carbon (BRC) (8.32% to 32.72%); (3) Soil GluN/MurA values tended to decrease with increasing cultivation years. The results highlight the significant effect of cultivation years on amino sugar accumulation. It indicates that the years of reclamation in karst areas have different impacts on the organic fractions derived from various microbial communities in the soil organic matter pool, and the microbial residues indicated by amino sugar are of great significance for the interception of soil organic matter.
1. Introduction
Microorganisms are key contributors to the biogeochemical cycle of soil components and play an indispensable role in the conversion of soil organic matter (SOM) through catabolic and anabolic activities [1,2,3]. Microorganisms promote the degradation of SOM through catabolism, thus making the available C sources in soil into more resilient organic matter through anabolism [3]. Microbial residues are an important source of SOC compared to the biomass of in vivo microorganisms in the soil [4,5]. The formation and decomposition of microbial residues can considerably affect SOC accumulation [6]. Fungal and bacterial residues are important components of the stable SOC pool of soils [7,8]. Changes in environmental factors and surface vegetation conditions can have an impact on the activity, abundance, and community composition of soil microorganisms, resulting in corresponding changes in the quantity and composition of microbial residues [9].
Soil Amino Sugars are important microbial cell wall components that remain relatively stable in the soil and are therefore used to characterize microbial residual carbon (MRC) to indicate the contribution of microorganisms to SOC [3,5]. Among the 26 soil ASs that have been identified so far, only glucosamine, galactosamine, muramic acid, and mannosamine (ManN) can be measured quantitatively. MurA originates only from bacterial cell walls and is used to indicate bacterial residues; GluN, on the other hand, is present in large amounts in the chitin in fungal cell walls and is used to indicate fungal residues; however, the origin of ManN and GalN is currently unclear [10,11,12]. The heterogeneity of soil ASs is used to distinguish the relative contribution of fungi and bacteria to SOC transformations [2,5]. Bacteria and fungi differ in their adaptation to the soil environment. There is a differential efficiency in their utilization of organic matter [5,13]. The ratio of GluN to MurA is commonly used in previous studies to evaluate the relative contribution of fungi or bacteria to SOM transformation, which, when the ratio increases, indicates that the contribution of fungal residues to SOM accumulation is greater than that of bacteria; conversely, it indicates a greater contribution of bacterial residues [11,14,15]. The accumulation characteristics of soil ASs reflect the regulation that microorganisms impose on the SOM cycle [16].
Karst areas account for 12% of the world’s total terrestrial area [17], and there are about 3.44 million km2 of carbonate rock areas including buried, covered, and exposed in China, accounting for 15.6% of the world’s karst areas, of which 83% are distributed in southwest China [18,19]. The karst ecosystem is a typical fragile ecosystem with notable contradictions between humans and land, low soil productivity, and severe soil degradation [18,20]. The soil in karst areas is richer in nutrients than other types of soil at the same latitude, but soil carbon (C) and nitrogen (N) can be easily lost from the subsurface through leaching due to the special binary hydrogeological structure of karst ecosystems [18]. Residents have reclaimed large areas of natural reserved land and planted crops in karst mountain areas to sustain their living. Intensive agricultural activities are considered to be the main driving factor for soil organic carbon (SOC) loss [21,22]. It is the severe soil degradation in fragile karst ecosystems that establishes the status of microorganisms as playing an important role in nutrient regulation and enhancing ecological conservation in karst areas. Previous studies have compared differences in soil microbial communities in karst and non-karst areas and studied the changes stimulated by land use changes. However, studies during long-term continuous cultivation are still deficient [15,23,24]. What is perceived is that insufficient carbon sources are likely to cause a reduction in the metabolic rate of the microbial community [25]. Environmental factor conditions caused by high contents of calcium (Ca) and magnesium (Mg) ions content and high pH value may result in distinct soil microbial abudance [20,26]. We, therefore, hypothesized that: (1) the reclamation and continued cultivation of natural woodlands in karst ecosystems will lead to a decrease in microbial residues. (2) There are differences in the stability of fungal and bacterial-derived residues in response to environmental changes. (3) pH value, the content of cationic minerals, and soil texture may affect the accumulation of MRC.
To explore the differences in bacterial and fungal abundance and communities in the continued cultivation, a space-for-time substitution approach has often been used as an alternative to long-term studies [27]. We used amino sugar as an indicator of microbial residues and natural forest land as a comparison to investigate the characteristics of soil ASs quantity changes with different cultivation years (0 yr., 1 yr., 5 yrs., 15 yrs., 30 yrs.) in karst areas, to explore the impact of cultivation years on the organic matter accumulation and the corresponding contribution of microbial communities, aiming to supply a theoretical reference for the biogeochemical processes and microbial regulatory mechanisms on soil quality after the conversion from natural reserved land to cultivated land in degraded karst areas.
2. Materials and Methods
2.1. Site Description
This study was conducted in the field of scientific observation and research station of Southwest Karst Rocky Desertification of the Ministry of Science and Technology (110°32′27″ E, 25°12′33″ N), located in the Dengming village, 30 km to the southeast of Guilin, Guangxi Zhuang Autonomous Region, China (Figure 1a). It has a typical subtropical monsoon climate, with an annual mean temperature of 18.6 °C, and an average temperature in January is around 7.9 °C, while in July, it is about 28 °C. The annual mean precipitation ranges from 1800 to 2000 mm, with 60–70% occurring between April and September. The study area is characterized by a typical karst landscape with gentle alleys by steep hills. The sampling site was on a slight slope ranging from 15–20°. The soil type was classified as Leptosols (calcareous soil) according to the FAO World Reference Base for Soil Resources [28].
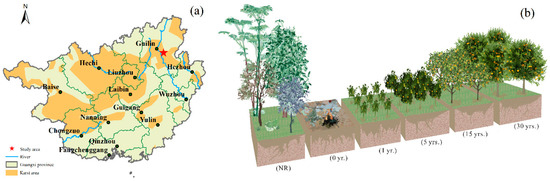
Figure 1.
Location of sampling sites in Guilin, Guangxi Zhuang Autonomous Region, China, where (a) is the map of Guangxi Province, the red star in figure (a) represents the study area, and (b) represents different land use types in sampling sites.
The continuous depression used to be a natural forest with shrubs and trees when no man-made disturbances were received. It remained woodland until residents burnt the forest for the cultivation of citrus orchards for economic purposes. We took soil samples from 0–10 cm below the soil, which had a temperature of roughly 22–26 °C.
2.2. Sampling
The sample sites were set on a space-for-time substitution method and soil sampling was carried out in July 2018. Sites with different cultivation years (0 yr.; 1 yr.; 5 yrs.; 15 yrs.; 30 yrs.) were established in this region with natural reserved forest (NR) as a comparison (Figure 1b). To ensure the similarity of soil samples, taking environmental conditions, lithology, soil type, and previous cultivation stages into account, 3 or 5 representative sample points were selected as spatial repetition. Three quadrats in the size of 1 × 1 m were randomly selected at intervals of 5 m per sample point. Each soil sample was gathered randomly by a composite of 3 sub-samples at 0–10 cm soil depth in each quadrat, removing the surface humus and visible gravel, fine roots, and animal residues. A total of 20 soil samples were collected from 6 sample plots. Each composite sample was air dried and then sieved through a 2 mm mesh before analysis.
2.3. Soil Analysis
Soil pH value was determined at a 1:2.5 (w/v) soil/water ratio by a digital seven Excellence pH/mV detector (METTLER TOLEDO, Shanghai, China). SOC and TN were analyzed by a Sercon SL C/N Elemental Analyzer (SerCon Integra 2, Crewe, England) after removing carbonate through the pretreatment with 1 mol/L HCl. Soil water content (SWC) and soil water holding capacity (SWHC) were measured by the drying method. The contents of Ca, Mg, P, Fe, Al, and Si in soil were determined using total X-ray Fluorescence Spectroscopy. Soil texture (Silt, Clay, Sand) was determined by Laser Particle Characterization Analyzer (Beckman Coulter LS-230, Brea, CA, USA). During the pretreatment process, the soil samples required to be removed from the organic matter and carbonates with H2O2 and dilute HCl.
Soil ASs were determined according to the method described by Zhang and Amelung [29]. Briefly, soil samples (about 0.2 g through 0.25 mm sieve) were hydrolyzed with 6 M hydrochloric acid (10 mL) at 105 °C for 8 h. After purification treatments of centrifugation, freeze-drying, and nitrogen blowing, the extracted soil ASs were configured with methanol (3 mL) as a derivatizing agent and finally solubilized with a mixture of aldononitrile acetates solvent (v/v = 1:1, 150 μL) and used for gas chromatographic determination.
As in many previous studies, the very low content of ManN in this research was not considered [2,14,30]. The concentrations of Total ASs were calculated by the sum of GluN, GalN, and MurA [31]. GluN/MurA values were used to infer the corresponding contribution of fungi and bacteria to SOM [5]. Moreover, the amounts of Fungal residue carbon (FRC) and Bacterial residue carbon (BRC) were derived from the content of ASs based on the values of GluN and MurA:
where MurA and GluN were assumed to occur in bacterial cells in an average molar ratio of 1:2 [12], 179.2 and 251 were the molecular weights of GluN and MurA, respectively, and 9 was the conversion factor of fungal GluN to FRC. Besides, the microbial residual carbon (MRC) was the sum of F-MBC and B-MBC [2,23].
FRC = [GluN/179.2 − (2 × MurA)/251.2)] × 179.2 × 9;
BRC = MurA × 45;
2.4. Statistical Analysis
One-way analysis of variance (ANOVA) was used to test all the parameters. When significant differences exist, the separation of means was subjected to Duncan’s honestly significant differences test (p < 0.05). Data were all subjected to statistical analysis using SPSS 19.0 software (SPSS Inc. Chicago, IL, USA). Origin 2022 software (Origin Lab., Northampton, MA, USA) was used for data visualization. Three replicates were separated for independent testing in the laboratory and statistical analysis.
3. Results
3.1. Differences in ASs, MRC and Their Contribution to SOC
In terms of soil physical and chemical properties (Table 1), soil pH value increased from NR to 1 yr. and then decreased from 1 yr. to 30 yrs. Soil C/N also raised after the reclamation from NR, but constantly dropped with increasing cultivation years. SWC and FWHC did not change significantly among the six types of soil samples. The results for soil texture showed a significant reduction in soil Sand content, which resulted in an increase in SBD.

Table 1.
Soil physical and chemical properties.
The contents of soil GluN, GalN, and MurA ranged from 1075.07 to 2842.05 mg·kg−1, 688.29 to 1671.29 mg·kg−1, and 121.47 to 205.65 mg·kg−1, with GluN contributing the highest to the total ASs of 55.76%~62.40% (Figure 2). The contents of GluN, GalN, and MurA in the soil increased after 1 year of cultivation, which increased by 12.75%, 15.20%, and 37.60%, respectively, compared to NR. However, they all showed a gradual decrease as the cultivation years continued to increase. The contents of GluN, GalN, and MurA decreased by 62.17%, 58.82%, and 40.94%, respectively, when compared with 1 yr. GluN, GalN, and MurA lost 58.22%, 55.30%, and 27.11%, respectively, during the 30 years of cultivation after this period.
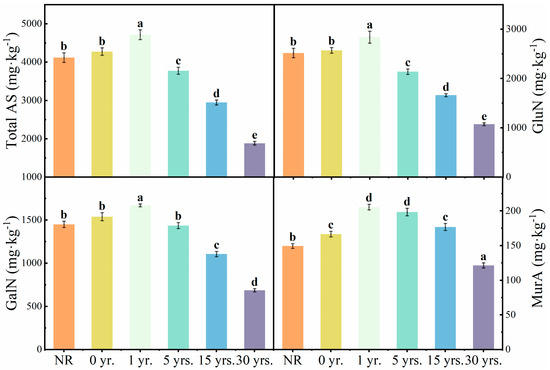
Figure 2.
Changes of soil total ASs and individual AS contents of NR and cultivated land. Note: Different letters indicate significant difference among the land use types at p < 0.05.
3.2. Differences in the Contribution of MRC, FRC, BRC to SOC
During the cultivation of natural reserved land for cultivating citrus trees, the FRC (8.12–22.94 g·kg−1), BRC (5.47–9.25 g·kg−1), and MRC (13.58–32.19 g·kg−1) contents showed a tendency to increase and then decrease significantly, with the highest point at 1 yr. and the lowest at 30 yrs. (Figure 3). GluN/MurA values (7.43 to 15.43) declined significantly during cultivation, with a 51.87% reduction in this ratio at 30 yrs. compared to NR (Figure 4). The GluN/MurA values showed a good linear fit (Figure 5) with Ca, Mg, Fe/Al oxides, pH, and Sand in the soil (R2 > 0.65, except for Fe oxides), indicating that the bacterial and fungal community structure may be impacted by the mentioned factors.
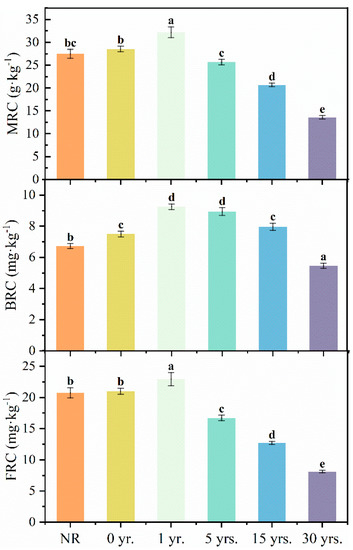
Figure 3.
Concentrations of MRC, BRC, and FRC in soils of natural reserved land and cultivated land. Note: Different letters indicate significant difference among the land use types at p < 0.05.
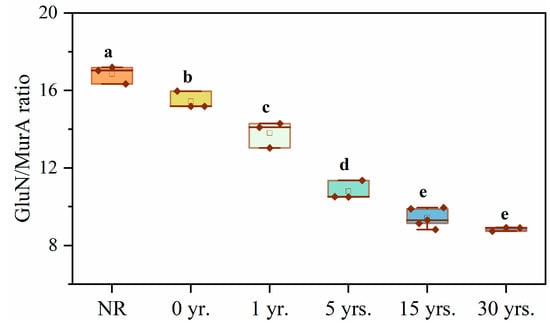
Figure 4.
Ratios of glucosamine to muramic acid in soils of natural reserved land and cultivated land. Note: Different letters indicate significant difference among the land use types at p < 0.05.
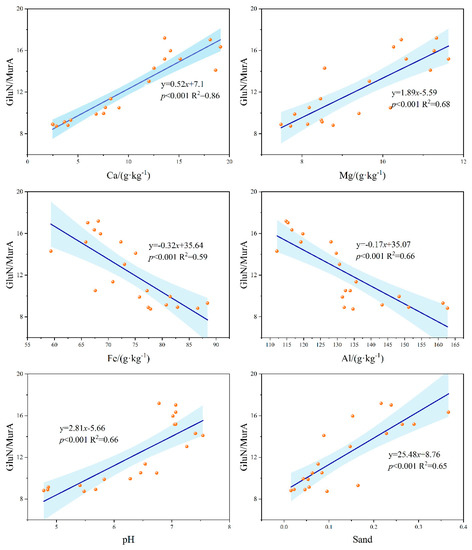
Figure 5.
The relationship between soil GluN/MurA value and metallic oxides, pH value, and soil sand content.
The contribution of MRC, FRC, and BRC to SOC in soils changed significantly, accompanied by a sudden loss of SOC in all six types of soil samples, respectively (Figure 6). The soils in NR had the highest content of SOC, but cultivation led to a dramatic loss of SOC, 80.24% compared to NR. The most obvious decline was observed after the soil was reclaimed for cultivation (0 yr.), with a loss of 22.80%.
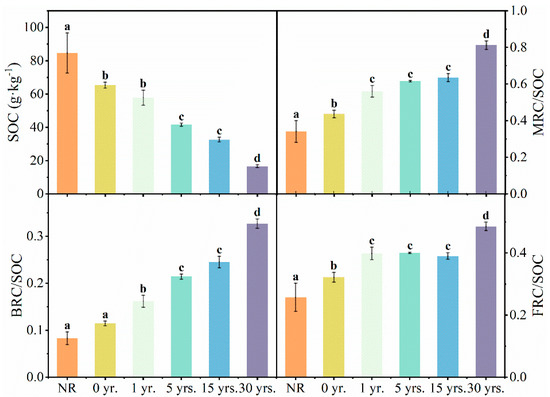
Figure 6.
Contribution of fungal, bacterial, and microbial residue C to SOC in soils of natural reserved land and cultivated land. Note: Different letters indicate significant difference among the land use types at p < 0.05.
The ratio of MRC to SOC (0.34 to 0.81) continued to increase in response to the dramatic loss of SOC, with a total increase of 138.43% over the 30-year cultivation. The BRC (from 0.08 to 0.33) and FRC (from 0.26 to 0.49), respectively, had an overall significant rise in the share of SOC. It indicated that the loss of other organic fractions in the soil has continued to grow since the natural reserved land was cultivated.
4. Discussion
4.1. Cultivation Effect on the Acumination of Total ASs
In this study, total and individual amino sugar content increased after the cultivation of natural reserved land. It was because, after fire reclamation, plant ash as an external organic substance (plant-derived C) can provide a supply for soil microorganisms, which can be transformed into soluble sugar, phenolics, organic acids, and free amino acids after decomposition by extracellular enzymes, being rapidly absorbed and used by microorganisms to synthesize microbial biomass through their growth and metabolism [32,33,34]. The accumulation of ASs, as stable microbial residual substances, was controlled by the increased synthesis of microorganisms for ASs or diminished catabolic mineralization [5,35]. Both total and individual ASs in the soil were increased in a short time after NR reclamation when microbial multiplication became significantly more frequent due to the supplementary of sufficient C sources [3,13,25].
On the other hand, the amount of total ASs and individual AS tended to decrease with the increase of cultivation years. In the absence of nutrients in the soil, ASs could be utilized preferentially as an energy source to supply microorganisms with C and N [2,36]. Especially when soil N is deficient, microbial residues (i.e., cell wall material) will be preferred for decomposition and metabolism by soil microorganisms [37]. Soil microbial residues contain more than 60% of the total N and were mainly distributed in proteins, nucleic acids, glycoproteins, fungal chitin, and bacterial peptidoglycans, making microbial reuse of residues more efficient as compared to decomposing other stable organic materials (persistent SOM) [37,38,39]. Karst ecosystems are vulnerable to rapid degradation, resulting in irreversible loss of SOM and nutrients [18,40]. When there is a deficiency of available nutrients in the soil, it will constrain microbial growth and lead to a reduction of microbial mass metabolism, and then the accumulated microbial cell residues will be decomposed for energy supply to survive for the living microorganisms.
4.2. Factors Regulating the Contribution of MRC to SOC
The contribution of MRC to SOC increased during cultivation (34.11% to 81.33%), especially after the 15 years of cultivation, with the share of MRC in SOC increasing by 17.69% over the 15 years after cultivation, slightly higher than that in some previous studies [31,41]. This suggested that the formation and decomposition of soil microbial residues bring about a dynamic response in microbial abundance and subsequent turnover, which then was of great contribution to a stable SOC pool [39,42,43,44]. As with other studies, the accumulation of MRC was closely related to the stability of SOC [4,31]. The limitation of soil substrates in the presence of unstable SOM pools would strongly lead to the reuse of MRC when the SOM pool has not yet stabilized, causing a sharp decline in MRC [11,42,45]. Karst areas often suffer from soil erosion and reduction of water storage capacity due to the special hydrogeological background and the well-developed underground fissures. Along with the disturbance of anthropogenic activities, it further causes irreversible loss of SOM and nutrients [18]. In general, cultivation is detrimental to the maintenance of SOM in karst areas, which also reinforces the contributing role of microbial-derived SOC in the SOM pool.
It is generally believed that the stability of MRC in soils is mainly maintained by the interaction of minerals or mineral oxides [45,46]. Neutral-alkaline soils in karst areas have a high content of Ca and Mg ions formed from the weathering of carbonate rocks, which can be rapidly lost along with the increase of cultivation years [47]. Ca and Mg regulated the accumulation of MRC mainly indirectly by controlling the availability of SOC [24,47,48]. Growing studies have shown that geochemical factors such as Al/Fe-oxides can not only adsorb SOC functional groups of SOC to stabilize them more, but also protect them from microbial decomposition [49,50]. Minerals block the access between microorganisms and nutrients, ultimately inhibiting the formation of microbial biomass and the accumulation of microbial residues [50,51].
4.3. Contribution of Fungal, and Bacterial Residue C to SOC
The contribution of FRC (25.79% to 48.6%) to SOC was higher than that of BRC (8.32% to 32.72%) at different years of cultivation. It had been established that fungi can utilize recalcitrant components as energy supply better than bacteria. Citrus trees were planted mainly after agricultural cultivation in this study area, and the litter mostly originated from leaves and decaying fruits, making the soil contain more lignin and cellulose, which were difficult to be decomposed for substances [5,52]. Therefore, fungal communities in cultivated soils are at a much higher competitive advantage, leading to a higher contribution of FRC to SOC. In addition, the bacterial cell wall is composed mainly of peptidoglycan, and BRC is more easily broken down, whereas the cell wall of fungi contains chitin, which is difficult to be decomposed. Therefore, BRC will be more easily decomposed, promoting the accumulation of FRC residues in the soil [52,53]. Moreover, FRC can be physically protected by soil aggregates than BRC because extracellular mycelium and polysaccharides from fungi are engaged in the formation of soil aggregates [31]. Overall, FRC contributes more to SOC than BRC.
Significant decreases in GluN/MurA values were observed with increasing years of cultivation in this study. GluN decreased more (62.17%) than MurA (40.94%), indicating that bacterial residues gradually exceeded the relative contribution of fungal residues to SOM during long-term cultivation [11]. Soil active organic matter fraction is more likely to accumulate in MurA than in GluN or GalN under nutrient-rich soil conditions. When the soil environment is depleted of nutrients, MurA will theoretically be decomposed together with the active organic matter [11]. At the same time, they are more easily protected from microbial decomposition by being adsorbed on the surface of the soil mineral particles or in micropores and nanopores [5,54].
Correlation analysis showed that GluN/MurA values had a negative correlation with Clay and Silt, but a significant positive correlation with the content of Sand (p < 0.01), indicating that the soil pores are conducive to the extension of fungal mycelium, while soils with high clay particle content are more favorable for the adsorption and preservation of bacteria [13,31]. It was reported in previous studies that different microbial species have different pH adaptation intervals and energy requirements [55]. Soil microbial communities in alkaline and neutral soils tend to be dominated by bacteria and are associated with lower fungal to bacterial residual carbon ratios [41]. Roth et al. have also suggested a rapid turnover of fungi in high pH floodplain sediments compared to bacterial residues [56]. The GluN/MurA values in the study consistently decreased since cultivation, indicating that the fungal residues, whose cell wall that are once difficult to be decomposed, turns into the supply of soil nutrients, and plays a critical regulatory role in nutrient cycling and organic matter turnover. It is thus clear that long-term cultivation is not conducive to the storage and stability of SOC in karst ecosystems.
5. Conclusions
In this work, we analyzed the total ASs including GluN, MurA, and GalN with different cultivation years. Our results showed that the total ASs content of the soil increased slightly at the beginning of reclamation (0–1 yr) and showed a significant decrease with the increase of reclamation years, especially between 5 and 30 yrs., indicating that different species of microorganisms (fungi and bacteria) responded differently to woodland reclamation in karst areas. The GluN/MurN values decreased significantly with the increase of cultivation years, indicating that the accumulation of bacterial residues would be significantly dominant with long-term continuous cultivation, which suggested that bacteria gradually dominated the microbial community and the contribution of bacterial residues to the accumulation of soil organic matter increased significantly. Such results also confirmed that long-term cultivation in karst areas has different impacts on the organic matters derived from various microbial communities. Microbial residues with amino sugars as indicators have an important role in the interception of soil organic matter. In addition, pH, Ca, Mg, and Fe/Al oxides were significantly correlated with total ASs and individual ASs in this study, thus implying that the geochemical background of calcium-rich alkaline soils in karst areas cannot be neglected in the accumulation of microbial residues. Therefore, the change of amino sugar could be an important evaluation index for the evaluation of organic matter accumulation and transformation during soil degradation in karst areas.
Author Contributions
Conceptualization, M.Z. and H.Y.; Data curation, H.Y. and T.Z.; Methodology, M.Z. and H.Y.; Validation, H.Y., T.Z. and C.Z.; Visualization, M.Z. and D.Z.; Writing—original draft, M.Z.; Writing—review and editing, H.Y., T.Z., C.Z. and D.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Natural Scientific Foundation of China under Grant No. U2102209, Guangxi Key Research and Development Program under Grant No. GuikeAB22035004, Guangxi Science and Technology Base and Talent Special Project, under Grant No. Guike AD20297090.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Acknowledgments
This work was supported by Natural Scientific Foundation of China under Grant No. U2102209, Guangxi Key Research and Development Program under Grant No. GuikeAB22035004, Guangxi Science and Technology Base and Talent Special Project, under Grant No. Guike AD20297090. We would like to thank the reviewers who read the first draft of this paper for their constructive comments that helped improve the manuscript. Thanks are also to Fen Huang and Chunlai Zhang for their help in field work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bin, Z.; Qi, C.; Xueli, D.; Hongbo, H.; Xudong, Z. Research progress on accumulation, turnover and stabilization of mi-crobial residues in soil. Acta Pedologica Sinica. 2022. Available online: https://kns.cnki.net/kcms/detail/32.1119.P.20210707.1121.004.html (accessed on 22 September 2022). (In Chinese).
- Joergensen, R.G. Amino sugars as specific indices for fungal and bacterial residues in soil. Biol. Fert. Soils 2018, 54, 559–568. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; An, S.; Liang, C.; Liu, Y.; Kuzyakov, Y. Microbial necromass as the source of soil organic carbon in global ecosystems. Soil Biol. Biochem. 2021, 162, 108422. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Balser, T.C. Net Microbial Amino Sugar Accumulation Process in Soil as Influenced by Different Plant Material Inputs. Biol. Fert. Soils 2007, 44, 1–7. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, Z.; Liu, Y.; Luo, Y.; Deng, Y.; Xu, X.; Liu, S.; Richter, A.; Shibistova, O.; Guggenberger, G.; et al. C:N:P stoichiometry regulates soil organic carbon mineralization and concomitant shifts in microbial community composition in paddy soil. Biol. Fert. Soils. 2020, 56, 1093–1107. [Google Scholar] [CrossRef]
- Kindler, R.; Miltner, A.; Richnow, H.H.; Kästner, M. Fate of gram-negative bacterial biomass in soil-mineralization and contribution to SOM. Soil Biol. Biochem. 2006, 38, 2860–2870. [Google Scholar] [CrossRef]
- Schweigert, M.; Herrmann, S.; Miltner, A.; Fester, T.; Kästner, M. Fate of ectomycorrhizal fungal biomass in a soil bioreactor system and its contribution to soil organic matter formation. Soil. Biol. Biochem. 2015, 88, 120–127. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, B.; Wei, Z.; He, H.; Filley, T.R. Conversion of grassland into cropland affects microbial residue carbon retention in both surface and subsurface soils of a temperate agroecosystem. Biol. Fert. Soils 2020, 56, 137–143. [Google Scholar] [CrossRef]
- Li, L.; Wilson, C.B.; He, H.; Zhang, X.; Zhou, F.; Schaeffer, S.M. Physical, biochemical, and microbial controls on amino sugar accumulation in soils under long-term cover cropping and no-tillage farming. Soil Biol. Biochem. 2019, 135, 369–378. [Google Scholar] [CrossRef]
- He, H.; Zhang, W.; Zhang, X.; Xie, H.; Zhuang, J. Temporal responses of soil microorganisms to substrate addition as indicated by amino sugar differentiation. Soil Biol. Biochem. 2011, 43, 1155–1161. [Google Scholar] [CrossRef]
- Engelking, B.; Flessa, H.; Joergensen, R.G. Shifts in amino sugar and ergosterol contents after addition of sucrose and cellulose to soil. Soil Biol. Biochem. 2007, 39, 2111–2118. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Rubert, K.F.; Balser, T.C. Effect of plant materials on microbial transformation of amino sugars in three soil microcosms. Biol. Fert. Soils 2007, 43, 631–639. [Google Scholar] [CrossRef]
- Zhang, X.; Amelung, W.; Yuan, Y.; Samson-Liebig, S.; Brown, L.; Zech, W. Land-use effects on amino sugars in particle size fractions of an Argiudoll. Appl. Soil Ecol. 1999, 11, 271–275. [Google Scholar] [CrossRef]
- Miltner, A.; Bombach, P.; Schmidt-Brücken, B.; Kästner, M. SOM genesis: Microbial biomass as a significant source. Biogeochemistry 2012, 111, 41–55. [Google Scholar] [CrossRef]
- Wei, X.; Deng, X.; Xiang, W.; Lei, P.; Ouyang, S.; Wen, H.; Chen, L. Calcium content and high calcium adaptation of plants in karst areas of southwestern Hunan, China. Biogeosciences 2018, 15, 2991–3002. [Google Scholar] [CrossRef]
- Jiang, Z.; Lian, Y.; Qin, X. Rocky Desertification in southwest China: Impacts, causes, and restoration. Earth-Sci. Rev. 2014, 132, 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Li, Z.; Liu, M.; Xu, C.; Zhang, R.; Luo, W. Effects of vegetation restoration on soil quality in degraded karst landscapes of southwest China. Sci. Total Environ. 2019, 10, 2657–2665. [Google Scholar] [CrossRef]
- Li, D.; Wen, L.; Yang, L.; Luo, P.; Xiao, K.; Chen, H.; Zhang, W.; He, X.; Chen, H.; Wang, K. Dynamics of soil organic carbon and nitrogen following agricultural abandonment in a karst region. J. Geophys. Res.-Biogeosci. 2017, 122, 230–242. [Google Scholar] [CrossRef]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta-analysis. Global Change Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Don, A.; Schumacher, J.; Freibauer, A. Impact of tropical land—Use change on soil organic carbon stocks—A meta analysis. Glob. Chang. Biol. 2011, 17, 1658–1670. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, C.; Duan, X.; Chen, H.; Li, D. Variation of microbial residue contribution to soil organic carbon seques-tration following land use change in a subtropical karst region. Geoderma 2019, 353, 340–346. [Google Scholar] [CrossRef]
- Xiao, D.; He, X.; Zhang, W.; Hu, P.; Sun, M.; Wang, K. Comparison of bacterial and fungal diversity and network connec-tivity in karst and non-karst forests in southwest China. Sci. Total Environ. 2022, 822, 153–179. [Google Scholar] [CrossRef] [PubMed]
- Peltre, C.; Gregorich, E.G.; Bruun, S.; Jensen, L.S.; Magid, J. Repeated application of organic waste affects soil organic matter composition: Evidence from thermal analysis, ftir-pas, amino sugars and lignin biomarkers. Soil Biol. Biochem. 2017, 104, 117–127. [Google Scholar] [CrossRef]
- Tang, J.; Tang, X.; Qin, Y.; He, Q.; Yi, Y.; Ji, Z. Karst rocky desertification progress: Soil calcium as a possible driving force. Sci. Total Environ. 2019, 649, 1250–1259. [Google Scholar] [CrossRef]
- Pickett, S.T.A. Space-for-time substitution as an alternative to long-term studies. In Long-Term Studies in Ecology; Likens, G.E., Ed.; Springer: New York, NY, USA, 1989. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. World Reference Base for Soil Resources 2014: International soil classification system for naming soils and creating legends for soil maps. In World Soil Resources Report; FAO: Rome, Italy, 2014; Volume 106, pp. 12–21. [Google Scholar]
- Zhang, X.; Amelung, W. Gas chromatographic determination of muramic acid, glucosamine, mannosamine, and galac-tosamine in soils. Soil Biol. Biochem. 1996, 28, 1201–1206. [Google Scholar] [CrossRef]
- Guggenberger, G.; Frey, S.D.; Six, J.; Paustian, K.; Elliott, E.T. Bacterial and fungal cell-wall residues in conventional and no-tillage agroecosystems. Soil Sci. Soc. Am. J. 1999, 63, 1188–1198. [Google Scholar] [CrossRef]
- Liang, C.; Amelung, W.; Lehmann, J.; Kästner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Chang. Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef]
- Averill, C.; Turner, B.L.; Finzi, A.C. Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 2014, 505, 543–545. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The microbial efficiency-matrix stabilization (mems) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Luo, J.; Lindsey, S.; Zhou, F.; Xie, H.; Li, Y.; Zhu, P.; Wang, L.; Shi, Y.; et al. Differential accumulation of microbial necromass and plant lignin in synthetic versus organic fertilizer-amended soil. Soil Biol. Biochem. 2020, 149, 107967. [Google Scholar] [CrossRef]
- Hobara, S.; Osono, T.; Hirose, D.; Noro, K.; Hirota, M.; Benner, R. The roles of microorganisms in litter decomposition and soil formation. Biogeochemistry 2014, 118, 471–486. [Google Scholar] [CrossRef]
- Fernandez, C.W.; Koide, R.T. Initial melanin and nitrogen concentrations control the decomposition of ectomycorrhizal fungal litter. Soil Biol. Biochem. 2014, 77, 150–157. [Google Scholar] [CrossRef]
- Zeglin, L.H.; Myrold, D.D. Fate of decomposed fungal cell wall material in organic horizons of old-Growth douglas-fir forest soils. Soil Sci. Soc. Am. J. 2013, 77, 489–500. [Google Scholar] [CrossRef]
- Brabcová, V.; Štursová, M.; Baldrian, P. Nutrient content affects the turnover of fungal biomass in forest topsoil and the composition of associated microbial communities. Soil Biol. Biochem. 2018, 118, 187–198. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, Z.; Xu, X.; Liu, S.; Jones, D.L.; Kuzyakov, Y.; Shibistova, O.; Wu, J.; Ge, T. Carbon and nitrogen recycling from microbial necromass to cope with C: N stoichiometric imbalance by priming. Soil Biol. Biochem. 2020, 142, 107720. [Google Scholar] [CrossRef]
- Peng, J.; Xu, Y.Q.; Zhang, R.; Xiong, K.N.; Lan, A.J. Soil erosion monitoring and its implication in a limestone land suffering from rocky desertification in the Huajiang Canyon, Guizhou, southwest China. Environ. Earth Sci. 2013, 69, 831–841. [Google Scholar] [CrossRef]
- Khan, K.S.; Mack, R.; Castillo, X.; Kaiser, M.; Joergensen, R.G. Microbial biomass, fungal and bacterial residues, and their relationships to the soil organic matter C/N/P/S ratios. Geoderma 2016, 271, 115–123. [Google Scholar] [CrossRef]
- Angst, G.; Messinger, J.; Greiner, M.; Häusler, W.; Hertel, D.; Kirfel, K.; Kögel-Knabner, I.; Leuschner, C.; Rethemeyer, J.; Mueller, C.W. Soil organic carbon stocks in topsoil and subsoil controlled by parent material, carbon input in the rhizosphere, and microbial-derived compounds. Soil Biol. Biochem. 2018, 122, 19–30. [Google Scholar] [CrossRef]
- Buckeridge, K.M.; Mason, K.E.; McNamara, N.P.; Ostle, N.; Puissant, J.; Goodall, T.; Griffiths, R.I.; Stott, A.W.; Whitaker, J. Environmental and microbial controls on microbial necromass recycling, an important precursor for soil carbon stabilization. Commun. Earth Environ. 2020, 1, 36. [Google Scholar] [CrossRef]
- Craig, M.E.; Geyer, K.M.; Beidler, K.V.; Brzostek, E.R.; Frey, S.D.; Stuart Grandy, A.; Liang, C.; Phillips, R.P. Fast-decaying plant litter enhances soil carbon in temperate forests but not through microbial physiological traits. Nat. Commun. 2022, 13, 1229. [Google Scholar] [CrossRef] [PubMed]
- Kallenbach, C.M.; Frey, S.D.; Grandy, A.S. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat. Commun. 2016, 7, 13630. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Walter, K.; Ellerbrock, R.H.; Sommer, M. Effects of land use and mineral characteristics on the organic carbon content, and the amount and composition of Na-pyrophosphate-soluble organic matter, in subsurface soils. Eur. J. Soil Sci. 2011, 62, 226–236. [Google Scholar] [CrossRef]
- Lützow, M.V.; Kögel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions—A review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Wen, L.; Li, D.; Yang, L.; Luo, P.; Chen, H.; Xiao, K.; Song, T.; Zhang, W.; He, X.; Chen, H.; et al. Rapid recuperation of soil nitrogen following agricultural abandonment in a karst area, southwest China. Biogeochemistry 2016, 129, 341–354. [Google Scholar] [CrossRef]
- Totsche, K.U.; Amelung, W.; Gerzabek, M.H.; Guggenberger, G.; Klumpp, E.; Knief, C.; Lehndorff, E.; Mikutta, R.; Peth, S.; Prechtel, A.; et al. Microaggregates in soils. J. Plant Nutr. Soil Sci. 2018, 181, 104–136. [Google Scholar] [CrossRef]
- Fang, K.; Qin, S.; Chen, L.; Zhang, Q.; Yang, Y. Al/Fe mineral controls on soil organic carbon stock across Tibetan alpine grasslands. J. Geophys. Res.-Biogeosci. 2019, 124, 247–259. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Mineral–organic associations: Formation, properties, and relevance in soil environments. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2015; Volume 130, pp. 1–140. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef]
- Li, N.; Xu, Y.Z.; Han, X.Z.; He, H.B.; Zhang, X.D.; Zhang, B. Fungi contribute more than bacteria to soil organic matter through necromass accumulation under different agricultural practices during the early pedogenesis of a mollisol. Eur. J. Soil Biol. 2015, 67, 51–58. [Google Scholar] [CrossRef]
- Kravchenko, A.N.; Guber, A.K.; Razavi, B.S.; Koestel, J.; Quigley, M.Y.; Robertson, G.P.; Kuzyakov, Y. Microbial spatial footprint as a driver of soil carbon stabilization. Nat. Commun. 2019, 10, 3121. [Google Scholar] [CrossRef]
- Hu, Y.; Zheng, Q.; Zhang, S.; Noll, L.; Wanek, W. Significant release and microbial utilization of amino sugars and D-amino acid enantiomers from microbial cell wall decomposition in soils. Soil Biol. Biochem. 2018, 123, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Roth, P.J.; Lehndorff, E.; Cao, Z.H.; Zhuang, S.; Bannert, A.; Wissing, L.; Schloter, M.; Kögel-Knabner, I.; Amelung, W. Accumulation of nitrogen and microbial residues during 2000 years of rice paddy and non-paddy soil development in the Yangtze River Delta, China. Glob. Chang. Biol. 2011, 17, 3405–3417. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
