Abstract
Throughout the Mediterranean basin, the long-term interaction between human activities and natural processes has led to the formation of unique ecosystems whose biodiversity may be higher than that of the “original” systems. This is particularly true in the case of transformations of continuous stretches of closed forest into a complex mosaic of open and closed habitat over the course of centuries. In this study, we assessed the variation in diversity of ant assemblages in a typical patchy landscape, sampling ants in the three most important constituting habitats: olive plantation, harvested forest, and mature forest. In the study we used two different sampling methods—pitfall traps and observation at baits—which provided information on species presence at different temporal scales. The three habitats displayed different species assemblages, and considerable variation in species composition was observed at different times of the day, particularly in the harvested forest. Functional group analysis showed that the olive plantation, although the most artificial habitat, displayed the highest number of functional groups, suggesting a wider spectrum of available ecological niches for ant species within this habitat type. Overall, it was concluded that each of the three habitats contributes to enhance diversity at the landscape scale, which is greater than that expected from a more homogeneous habitat composition.
1. Introduction
Agricultural and forestry landscapes are two of the most relevant land use types in terms of human-centered sustainable development in the world [1]. Throughout the Mediterranean basin, the long-term interaction between human activities and natural processes has led to the formation of unique ecosystems whose biodiversity may be higher than that of the “original” systems [2]. This is particularly true in the case of transformations of continuous stretches of closed forest into a complex mosaic of open and closed habitat over the course of centuries [3]. This type of landscape characterizes several parts of the Mediterranean basin and is particularly prevalent in many parts of Central Italy [4,5]. The overall biodiversity of such a landscape depends on a plethora of factors, which include the dimensions of the habitat patches and their ecological integrity, the extension of urbanized areas, the presence of ecological barriers, and the type of agriculture [6,7,8,9]. Additionally, variation in vegetation type and canopy cover are key environmental elements promoting the increase of taxonomic and functional diversity at different spatial scales [10,11].
Ants are a key component of all terrestrial systems, where they exert a strong direct influence on the composition and dynamics of arthropod communities and can influence plant assemblages and ecosystem dynamics [12,13,14,15]. A better understanding of how ant communities function within these systems is therefore of the upmost importance [16]. Not surprisingly, the effects of common environmental features of the Mediterranean agroforestry landscapes on the composition and dynamics of ant communities have been studied from different angles [17,18,19]. For example, the occurrence of a frequent alternation of shaded and sunny patches can promote community differentiation because of the interplay between competitive strength and the thermal tolerance of the species [20,21,22,23]. This effect is evident at the landscape scale, where habitats differ in their associated ant assemblages, but is also visible at a micro-scale [24]. Moreover, due to their ecological relevance, and several of their ecological features, ants are also used as indicators of ecosystem functioning and human impact, although their importance is often overlooked when assessing the sustainability of agroecosystems [25,26,27,28].
The issue of how different factors operate in structuring Mediterranean ant communities at different spatial scales has been debated. Recently, Boet et al. [29] showed that abiotic environmental filtering is most important at a large scale, biotic factors are strongest at a medium scale, and neither prevail at a local scale. Over the last few years, the importance of biotic factors such as competition in shaping ant communities has been questioned, in light of weak evidence for clear pressure exerted by these factors [30,31]. On the contrary, evidence of the effect of abiotic factors such as human management is increasing [32,33]. Moreover, other natural factors (such as the day-night cycle) seem to be central in differentiating ant assemblages, and the available studies agree that temperature is probably the main determinant affecting community structure [21,22,34,35]. At a small spatial scale, all the species are subject to the same set of abiotic factors operating at a larger scale (e.g., climatic), but local changes can significantly alter the strength, and hence the effect, of some of these. For example, local changes in tree cover alter the exposure to direct sunlight, thus favoring only some of the species [36]. Analyzing diversity changes at a very local scale may therefore help in elucidating the role of these different factors. To the best of our knowledge, however, comparative studies focusing on how ant assemblage and diversity varies among neighboring habitats at local scale according to the daily cycle are rare.
With this study, we aimed to fill this gap by assessing how, at a local scale, bordering habitat patches composing a typical Mediterranean agroforestry landscape in Tuscany (Central Italy) differed in ant assemblage composition. We also investigated whether the day-night temporal variability of climatic conditions contributed to assemblage variation, and if so, to what extent. We selected three common habitats in this region: a low-managed olive orchard, a recently harvested forest, and a mature forest. Such systems represent important components of the ecosystem of this area, which is a complex mosaic of tilled patches, vineyards, olive orchards, pastures, and a few dense urban centers, interspersed throughout an extended matrix of natural and semi-natural forest [4,5]. We applied two different sampling methods—pitfall traps and food baits—to get a clearer picture of the whole community structure and species turnover throughout the day [37]. We also characterized ant species, assigning each of them to its functional group [17,38,39]. This approach helped to explain the variations in species assemblage according to the functional role of each species. Our expectations were to find a detectable difference in ant species assemblage according to the type of habitat. Also, we expected to find remarkable changes in composition due to variations in abiotic factors throughout the day-night cycle. We also expected the ant assemblage variation to be greater in the olive plantations than in woodlands due to the greater daily temperature changes in the former.
2. Material and Methods
2.1. Study Area and Sampling Design
The study was carried out from June to July in an agricultural area located about 8 km from the center of Florence, Italy (43.885000 N, 11.160000 E), where plots hosting olive plantations and variously-managed woodlands coexist. The majority of ant species were highly active during this period [22]. The olive plantation (OL) was lightly managed, with a single mowing during late spring, tree-pruning every two to three years, and no use of chemicals for soil manuring or plant parasite control, with the exception of occasional use of copper sulfate [40]. The trees were arranged in regular lines with approximately 6 m between plants. On the whole, the olive plantation covered about 30 ha, and was bordered by a mixed oak-pine forest (Corine Land Cover code 3.1.1.2), in which the dominant species are Quercus pubescens, Quercus cerris and Quercus ilex, with irregularly scattered maritime pines (Pinus pinaster) and a few cypresses (Cupressus semprevirens L.). Part of the forest had been harvested two years before this study, leaving scattered arboreal vegetation (mainly Pinus, Cupressus, and a few oaks). In large portions of the harvested areas, an incipient undergrowth comprised of brooms (Spartium junceum L.) and privets (Ligustrum ovalifolium Hassk.) was present. In contrast, the unharvested forest was characterized by a greater density of trees, with a thin undergrowth and higher incidence of sciophilous plants, such as ivy (Hedera helix) or butcher’s broom (Ruscus aculeatus). For each type of habitat (olive plantation: OL, unharvested forest: MF, harvested forest: HF) we selected two plots of at least 2.5 ha, located at a distance of at least 100 m from each other. Canopy cover was measured in 10 randomly chosen points per habitat type (five per plot), using a Model-A spherical crown densitometer, and differences among habitats were assessed by a Poisson GLM—the habitat was the main factor and the plot was the nested random factor—followed by computation of the analysis of deviance, with the F test to assess the significance. Then, Tukey post-hoc tests were performed to compare habitats in pairs.
Ants were sampled by pitfall traps made of 50 mL Falcon centrifuge tubes (2.8 cm diameter, 11.5 cm height) buried in the soil with the edge matching the ground level. The tubes were filled with 30 mL of a preserving solution (70% ethylic alcohol, 28% water, 2% glycerol) and left closed for three days after placement to avoid the digging-in effect [41]. After this period, the trap lids were removed, and the traps left open for five consecutive days. Halfway through this period, traps were checked, and detritus, dead leaves or other debris carefully removed from the tubes. The solution was refilled when necessary. The sampling unit consisted of a grid made by five traps positioned at the corners and the center of a 5 m × 5 m square. Three different grids, separated by at least 20 m, were randomly positioned within each plot following a nested sampling design, for a total of six grids per habitat. All ants were identified at the species level, and each was assigned to a functional group (sensu [38]). The attribution to a functional group was based on the list of genera-functional groups provided [42], and following Gomez et al. [17] and Ottonetti et al. [39].
Finally, to assess the daily activity of ants in the three habitats, we observed ant presence at baits. Baiting was performed in the same plots used for pitfall trapping, two weeks after the conclusion of pitfall sampling to reduce the effect of disturbance. At each plot, we randomly placed two linear transects of nine baits each (distance between baits 3 m), with each bait consisting of a 5 cm × 5 cm quadrat of white paper bearing a small piece of tuna, following Ottonetti et al. [43]. Bait position was marked with a wooden stick, and maintained for all the duration of the sampling. In total, we observed 36 baits per habitat for each sampling occasion. Baits were observed for three consecutive hours at 30 min intervals, noting every ant moving on the paper card. For each species, at least three specimens were collected for identification. Baiting was repeated at three different times of the day: morning, between 6:00 a.m. and 9:00 a.m., afternoon, between 1:00 p.m. and 4:00 p.m., and evening, between 8:00 p.m. and 11:00 p.m. Morning, afternoon, and evening samplings in the same transect were performed during different days, separated by one week (with time of sampling randomized), to reduce disturbance due to repeated sampling in the same place. For bait within each habitat/time period combination, a species was considered present if observed at least once. Then, we calculated the incidence of each species in each transect by assigning to the species a total score from 0 to 9 according to the number of baits they visited.
2.2. Statistical Analysis
Captures in pitfall traps of the same grid were pooled into a single data matrix, and species abundances were transformed into presence/absence data. The dataset was therefore a species-x-plot matrix containing the presence/absence of each species in each plot. The number of ants that fed on each bait was standardized by incidence on baits (i.e., the number of baits where a species was observed in a transect). Both methods were applied to minimize the error due to the presence of a colony in close proximity to the baits [39]. In particular, we built separate datasets for each time of the day (morning, afternoon, and evening). For both types of datasets, ant species richness and diversity were estimated using the method described by Chao et al. [44], as implemented in the iNEXT R package. This analytical method, based on the estimation of Hill’s numbers, qD, yields estimates of total (rarefied and extrapolated) species richness (q = 0), the exponential of Shannon diversity index (q = 1), and Simpson diversity index (q = 2). The 95% confidence intervals were obtained by a bootstrap method based on 4999 replications of the reference sample set [45]. For this analysis, species incidences in all the four transects of each time/habitat combination were pooled together. The β-diversity was computed as a multivariate dispersion, following Anderson et al. [45]. This method is based on the average dissimilarity from individual observation units to their group centroid in multivariate space, using an appropriate dissimilarity measure (see below). Dispersions of groups are compared by permuting model residuals to generate a permutation distribution of F under the null hypothesis of no difference in dispersion between groups. The described statistical methods were used for assessing the diversity among habitats, both for the pitfall trap sampling dataset and the three-day baiting sampling dataset, separately. The datasets used for this analysis were the same described above (presence/absence and incidence), but records from the transects were not pooled together, and the matrix was complete (i.e., for each species in each transect there was an incidence value). Then, the Bray-Curtis index was used as a measure of dissimilarity for both matrixes. The β-diversity was compared among sites for the trap sampling dataset, and among sites within all the three times of the day—and vice versa—for the bait sampling dataset.
The value of each species as an indicator of habitat type was calculated using the IndVal index [46,47]. This method combines measurements of the degree of specificity of a species to a habitat type and its fidelity within that habitat. Species with high specificity and fidelity within a habitat have a high indicator value. Indicator species reflect the biotic or abiotic conditions of the environment, provide evidence for the impacts of environmental change, and predict the diversity of other species or communities within an area. For this analysis, we used the same datasets used for the β-diversity analysis.
Compositional differences among habitats for the pitfall dataset and the three-day baiting dataset were evaluated using multivariate techniques. For the pitfall traps dataset, the species abundance was transformed into presence/absence data, whereas for the bait sampling dataset the incidence value in each transect was used. Multivariate distances among samples were computed with the Bray-Curtis dissimilarity index. The resulting distance matrix was analyzed by non-Metric Multidimensional Scaling (nMDS) according to Clarke et al. [48]. Species composition differences were tested with a permutation-based nonparametric multivariate analysis of variance (npMANOVA), as described by Anderson [49]. For the pitfall trap sampling dataset, we added the plot as a nested random factor. For the baiting dataset, we added the time of day as a second fixed factor, and the significance of the interaction between this factor and the type of habitat was also assessed.
All the analyses were carried out with the R software package (ver. 3.5.3, [50]) using the libraries BiodiversityR (ver. 2.5), iNEXT (2.0), indicspecies (1.7), labdsv (1.6), RVAideMemoire (0.9), and vegan (2.2).
3. Results
Canopy cover scores ranged between 0 and 24, and the mean of observed values were 6.2 (±SD 1.24), 13.7 (±SD 0.955), and 21 (±SD 0.515) for OL, HF, and MF, respectively. Mean canopy cover scores differed significantly with respect to habitat (F2,27 = 42.53, p < 0.001, Tukey post-hoc tests, p < 0.001 for all comparisons), whereas no effect of the plot was found (F3,24 = 0.030, p = 0.993).
3.1. Pitfall Trap Sampling
A total of 1259 ants were collected (292 in OL, 628 in HF, and 339 in MF), belonging to 2 subfamilies, 14 genera, and 28 species. A total of 11 species were found in OL, 14 in HF, and 18 in MF. A list of the species collected is reported in Table 1. The only species collected in all three habitats was Aphaenogaster subterranea, which was found in 15 out of 18 sampling grids. Eight species were shared between HF and MF, five between OL and HF, and three between OL and MF. A total of 12 species were collected in only one of the three habitats (Table 1). Figure 1A shows sample-based estimation of ant species richness and diversity based on Hill’s numbers for the three habitats. OL had the lowest richness and diversity, while MF had the highest values, although there was considerable variability in the estimated values. As for species richness in particular, the results suggest that the mature forest had significantly more species than the olive orchard. MF had intermediate values and its confidence bands overlapped with those of both the other two habitats. On the contrary, results were less sharp when looking at Shannon and Simpson diversity, where the order of diversity was retained (MF > HF > OL), but with some overlap among the confidence intervals of the three habitats. The β-diversity did not differ among habitats (F2,15 = 0.766, p = 0.47).

Table 1.
Functional group (FG), IndVal index (IndVal), and its significance (p) of the species collected in the three habitats by pitfall trap sampling. Form. = Formicinae; Myrm. = Myrmicinae; Significance levels: ns, not significant; * < 0.05; ** < 0.01. Functional groups: cc, cold-climate specialist; cr, cryptic species; hc, hot-climate specialist; gm, generalized Myrmicinae, op, opportunist; sc, subordinate Camponotini.
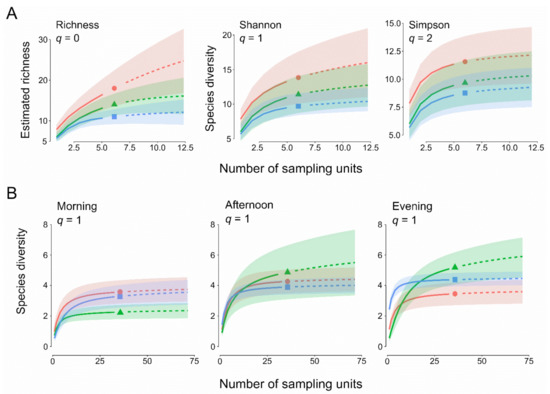
Figure 1.
Sample-based plots for ant species diversity based on Hill numbers (q). (A) Pitfall trap sampling, (B) bait sampling, subdivided by daily period (morning, afternoon, and evening). For bait sampling, only q = 1 (Simpson index) is reported. Red circles, lines, and buffers: mature forest (MF); Green triangles, lines, and buffers: harvested forest (HF); blue squares, lines, and buffers: olive plantations (OL).
Five species were recognized as indicators of OL (Table 1), and two of these, Plagiolepis pygmaea and Pheidole pallidula, had high index values (>0.90). Three species were considered indicators for MF (Stenamma striatula, Temnothorax lichtensteini, and Temnothorax parvulus), while only one, Formica gagates, was considered an indicator for HF.
Figure 2A shows the nMDS ordination plot (nMDS stress: 0.09). OL samples are clearly separated from those of HF and MF, which are gathered together on the right side of the plot. Results of the npMANOVA indicated that the effect of habitat type was significant (F2,12 = 17.39, p < 0.001), and the plot also had a significant effect (F3,12 = 2.59, p = 0.044).
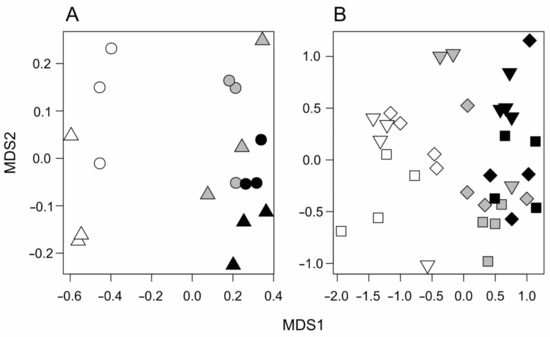
Figure 2.
Non-Metric Multidimensional Scaling (nMDS) ordination plots for (A) pitfall traps (nMDS stress: 0.09), and (B) baits (nMDS stress 0.10). White symbols, olive plantation (OL); grey symbols, harvested forest (HF); black symbols, mature forest (MF). In (A), each symbol is a grid of pitfall traps, and circles and triangles represent the two different plots within each habitat. In (B), each symbol is a transect, and different symbols represent the three daily periods (squares, morning; diamonds, afternoon; triangles, evening).
Six functional groups were identified: cc, cold-climate specialist; cr, cryptic species; hc, hot-climate specialist; gm, generalized Myrmicinae, op, opportunist; sc, subordinate Camponotini. Only one functional group (hc) was exclusive to a single habitat (OL). Three groups were shared between two habitats (sc: OL and MF; gm: OL and HF; cc: HF and MF), while the last two groups (cr and op) were detected in all three habitats, in different proportions.
3.2. Bait Sampling
Baiting yielded a lower number of species than pitfall traps (17, belonging to 2 subfamilies and 12 genera, Table 2). In the olive orchard, Pheidole pallidula, Crematogaster scutellaris, and P. pygmaea were present in all three daily periods, with the first species being the most represented species. In both of the other two habitats, only C. scutellaris was always present. Sample-based estimation of ant species diversity based on Hill’s numbers for the three habitats in the three daily periods showed a large overlap between OL and MF, while diversity in HF appeared to increase from morning to evening (Figure 1B). The β-diversity did not differ among habitats in all three daily periods (morning: F2,9 = 2.381, p = 0.133; afternoon: F2,9 = 1.720, p = 0.212; evening: F2,9 = 0.53, p = 0.614), but in HF a significant difference among daily periods was found (OL: F2,9 = 0.750, p = 0.545; HF: F2,9 = 5.153, p = 0.015; MF: F2,9 = 1.726, p = 0.221). Multiple comparisons showed that this difference was significant only between morning and evening (Tukey HSD test, p = 0.002).

Table 2.
Functional group (FG), IndVal index (InV), and its significance (p) of the species collected by baiting in three habitats. Mo = Morning; Af = afternoon; Ev = evening; Form. = Formicinae; Myrm. = Myrmicinae; Significance levels: ns, not significant; * < 0.05; ** < 0.01. Functional groups: cc, cold-climate specialist; cr, cryptic species; hc, hot-climate specialist; gm, generalized Myrmicinae, op, opportunist; sc, subordinate Camponotini.
As for the IndVal analysis, Camponotus aethiops was the only indicator species for OL, although only during the afternoon. In HF, F. gagates and C. vagus were indicators, in the morning and in the afternoon, respectively. Finally, in MF, only A. subterranea was found to be an indicator, and only in the evening.
The nMDS from bait samples broadly paralleled the one obtained from pitfall traps (Figure 2B, nMDS stress: 0.10). Olive orchard samples gathered on the left side of the plot, whereas both types of forests were close to one another on the right side, although samples were separated according to habitat type. In the plot, the categorization of each sample according to daily period is reported (Figure 2B). Overall, samples appeared to gather together according to daily period, though a clear separation did not emerge. The result of the npMANOVA test detected significant differences in both the main factors and in their interaction (habitat type: F2,26 = 12.467, p = 0.001; daily period: F2,26 = 3.226, p = 0.006; interaction: F4,26 = 1.945, p = 0.014).
The same six functional groups as those found with pitfall sampling were collected from baiting sampling. In Figure 3, all species found using baiting sampling, their proportion with respect to the total, and their functional group are summarized by pie charts.
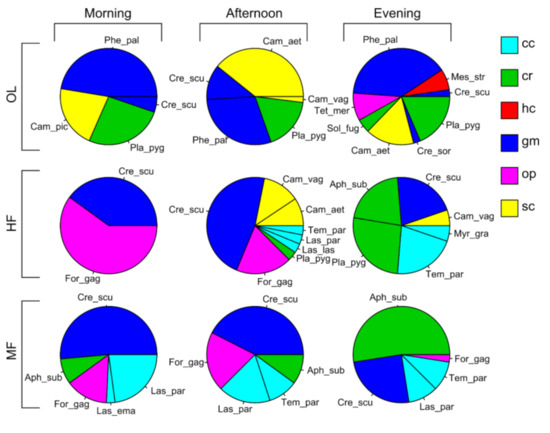
Figure 3.
Pie charts of the proportion of incidence of species on the total (i.e., the sum of the incidence of each species in the two transects in that habitat and in that period) in the three habitats and for the three daily periods. Different colors represent functional groups: cc, cold-climate specialist; cr, cryptic species; hc, hot-climate specialist; gm, generalized Myrmicinae, op, opportunist; sc, subordinate Camponotini. Species abbreviations: Aph_sub, Aphenogaster subterranea; Cam_aet, Camponotus aethiops; Cam_pic, Camponotus piceus; Cam_vag, Camponotus vagus; Cre_scu, Crematogaster scutellaris; Cre_sor, Crematogaster sordidula; For_gag, Formica gagates; Phe_pal, Pheidole pallidula; Las_las, Lasius lasioides; Las_par, Lasius paralienus; Myr_gra, Myrmecina graminicola; Pla_pyg, Plagiolepis pygmaea; Sol_fug, Solenopsis fugax; Tem_par, Temnothorax parvulus: Tet_mer, Tetramorium meridionalis.
4. Discussion
The ant assemblages characterizing the three habitats differed markedly, and both types of sampling methods confirmed this result. In particular, the species assemblage of the olive plantation was considerably different from those characterizing the two types of woods, which differed slightly but significantly from each other. Habitat-related differences due to forest management are known, and the result was expected given the sensitivity of ants as bioindicators [51]. Tree cover management is a factor that can affect assemblage composition, as it alters microclimate, food availability and quality, and the availability of nesting sites [52,53,54]. Forests provide greater shading due to canopy cover and litter layer [55,56,57,58], the different thickness of which can alter ant communities [59,60]. Olive plantations, which are characterized by sparse trees and wide portions of bare ground exposed to direct sunlight, are more similar to open fields than to woody habitats. Hence, it is not surprising that the associated ant community differed from those of both types of woods. Interestingly, the rarefaction of trees in the harvested forest, despite being insufficient to completely change assemblage composition, may have created suitable habitats for species typical of open habitats, such as Formica cunicularia [61], which was found both in the olive plantation and in the harvested forest, but not in the mature forest.
Univariate analyses provided a complex picture of diversity patterns in the studies habitat types. When the time of the day was not taken into account, estimated species richness in the mature forest was approximately double than that of the olive plantation, probably reflecting the greater complexity of this former. Differences in both Simpson and Shannon diversity were, however, less sharp, although the order of diversity was still consistent: mature forest values were greater than those of the harvested forest, which in turn were higher than those measured in the olive orchard. This point confirms that such a multifaceted Mediterranean ecosystem can support high levels of biodiversity at the landscape level [62,63], and also that some habitats like olive orchards, where anthropogenic disturbance is higher, can partly contribute to high biodiversity. Moreover, habitats like olive orchards simultaneously provides suitable habitat for both open-habitat species such as Messor spp., which require large, unshaded portions of grasslands [64], and tree-nesting species such as Crematogaster scutellaris [65,66]. These results agree with the findings of Reyes-López et al. [24], who found that the presence of a few isolated trees in an open habitat can produce a significant increase in local ant diversity. Interestingly, the olive plantation was also the habitat with the highest number of indicator species (five), suggesting that this habitat might provide opportunities for several species having unique ecological constraints. Furthermore, as is the case of most olive groves in Central Italy, the olive orchards used in this study were managed in a style approximating some recognized organic management method, for example by limiting the use of chemical treatments and machinery. Several studies have described a decline in biological diversity—measured as either species richness, abundance of indicator taxa, or other indicator of community structure—in conventional compared to organically-managed plantations, with adverse effects evident both on plants [67,68,69] and several invertebrate taxa including ants [26,70,71,72,73]. One of the crucial practices responsible for detrimental effects on arthropod fauna is the use of chemical treatments, particularly insecticides [27,71]. The effect of management type on olive groves (i.e., organic, conventional, integrated, etc.) in particular has been demonstrated to affect arthropod communities [74,75], though the large spectrum of farming practices within each of these management types and environmental contexts probably have greater influence [76]. Nonetheless, less intense management of olive trees seems to favor arthropod diversity [77]. As a rule, in permanent crops exposed to intense farming, the homogenization of the habitat can have detrimental effects on arthropod communities, particularly on ants [7]. In the case of the olive groves in this study, chemical compounds were not used, with the exception of copper sulfate as a fungicide, concentrations of which seem to be effectively regulated in ant tissues [78,79].
The analysis of bait data suggested a temporal change of the species assemblage at the different times of the day. As described in previous studies, the most apparent explanation for this observation is that these changes are driven by the different thermal tolerances, probably more specific for the species within the olive plantation than those within the wooded area assemblage [21,22], and by the interaction of this factor with competitive ability [80,81]. One of the most interesting results is the significant increase in β-diversity from the morning to the evening in the harvested forest, in contrast to the stability in species diversity over the three daily periods in both the olive plantation and mature forest. Summarizing, results indicate that in the olive plantation some environmental filters acted on ant community modifying their assemblage but not their diversity, whereas the opposite occurred in the harvested forest. In the mature forest, instead, neither of the two parameters varied. This may be due to more stable conditions in the mature forest than in the two other habitats, such as lower thermal excursions during the day-night cycle [37]. In the olive plantation, instead, the highest number of functional groups suggests that high availability of ecological niches might allow for changes in species makeup thorough the day. In the harvested forest, the recent management might have affected the available niches of some species more active in particular times of the day, but this point needs to be investigated in details. However, the analysis of species turnover revealed by the activity at the baits provides further insights to better understand and interpret the differences observed at the community level. For example, the olive plantation was the only habitat with an exclusive functional group, the “hot-climate specialists”, represented by Messor sp. However, baiting showed that M. structor concentrated its activity during the evening, suggesting that though this species is adapted to hot climates, it prefers cooler hours for its activity, probably because of the too high temperatures during the daytime in the olive plantation [82]. Similarly, the wood specialist Aphaenogaster subterranea was active only during the evening/night as known [83], whereas the forest species Formica gagates concentrated its activity in the morning hours. This last point agrees with the niche partitioning of the species by their thermal tolerance [37].
Despite the fact that the two methods yielded similar and largely interchangeable results, it is worth emphasizing their differences. Pitfall trapping allowed detection of many more species than baits, whereas baiting gave the most interesting information on the temporal pattern of species occurrence, and therefore on the constraints that limit their activity. The “take-home” message is therefore that a thorough comprehension of the dynamics of ant assemblages in the Mediterranean region requires the simultaneous adoption of different collection methods [84].
A final point is related to information provided by the analysis of functional groups. Functional groups were hypothesized to change among different habitats. These differences were in part observed; for example, we found hot-climate specialists in the olive plantation and cold-climate specialists in the forests. However, despite the functional group approach proven useful to compare diversity changes across habitats, the attribution of the different species to specific groups is still problematic, as it is largely based on functional details at the genus level. A fine-tuning of the method, based on the careful analysis of the functional traits of each species would therefore be needed (see e.g., [85] for an interesting analysis). However, we think that a more integrated approach involving the direct measure of both behavioral and functional traits would probably be the best solution [86,87,88,89] for future studies.
5. Conclusions
The results of this study show that the three analyzed habitats hosted different ant assemblages, despite similarities in overall richness and diversity. Analysis of daily activity showed interesting variations in the harvested forest not present in the more temporally-stable structure of both the olive plantation and mature forest. These results answer our main question and suggest that even at the local scale, habitat arrangement plays a central role in shaping ant communities. Furthermore, by comparing the two sampling methods, we can strongly recommend the integrated use of more than one sampling method, since pitfall traps appear to be more efficient for capturing the species diversity of habitats, but baits give more information about the daily turnover of these species. Finally, the role of eco-friendly approaches, both in forest management and in farming, is emphasized, as it is one of the most important factors allowing maintenance of such high diversity and complexity. Hence, we support efforts toward promoting a traditional low-impact approach to agroforestry management and regard the protection of local “biodiversity islands” as a central challenge for future policies.
Author Contributions
Conceptualization, F.F., L.O., L.T. and G.S.; methodology, A.M., F.F., L.O., L.T., and G.S.; formal analysis, F.F. and G.S.; resources, A.M. and F.F.; writing—original draft preparation, F.F.; writing—review and editing, A.M., F.F., and G.S.; visualization, A.M., F.F. and G.S.; supervision, G.S.; project administration, G.S. All authors have read and agreed to the published version of the manuscript.
Funding
The study was partly supported by grants from the University of Florence.
Institutional Review Board Statement
The study did not require ethical approval.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available from the Authors upon request from.
Acknowledgments
We are grateful to ‘Fattoria di Travalle’ for permission to work on their property.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Miranda, D.; Moragues-Faus, A.; Arnalte-Alegre, E. Chapter 12 Agriculture in Mediterranean Europe: Challenging Theory and Policy. In Agriculture in Mediterranean Europe: Between Old and New Paradigms (Research in Rural Sociology and Development, Vol. 19); Emerald Group Publishing Limited: Bingley, UK, 2013; Volume 19, pp. 295–310. [Google Scholar]
- Dufour-Dror, J.M. A quantitative classification of Mediterranean mosaic-like landscapes. J. Med. Ecol. 2002, 3, 3–12. [Google Scholar]
- Galli, M.; Bonari, E.; Marraccini, E.; Debolini, M. Characterisation of Agri-Landscape Systems at a Regional Level: A Case Study in Northern Tuscany. Ital. J. Agron. 2010, 5, 285–294. [Google Scholar] [CrossRef]
- Agnoletti, M. Rural landscape, nature conservation and culture: Some notes on research trends and management approaches from a (southern) European perspective. Landsc. Urban Plan. 2014, 126, 66–73. [Google Scholar] [CrossRef]
- Zamora, J.; Verdú, J.R.; Galante, E. Species richness in Mediterranean agroecosystems: Spatial and temporal analysis for biodiversity conservation. Biol. Conserv. 2007, 134, 113–121. [Google Scholar] [CrossRef]
- Cerdá, X.; Palacios, R.; Retana, J. Ant Community Structure in Citrus Orchards in the Mediterranean Basin: Impoverishment as a Consequence of Habitat Homogeneity. Environ. Èntomol. 2009, 38, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Datry, T.; Bonada, N.; Heino, J. Towards understanding the organisation of metacommunities in highly dynamic ecological systems. Oikos 2016, 125, 149–159. [Google Scholar] [CrossRef]
- Rey, P.J.; Manzaneda, A.J.; Valera, F.; Alcántara, J.M.; Tarifa, R.; Isla, J.; Molina-Pardo, J.L.; Calvo, G.; Salido, T.; Gutiérrez, J.E.; et al. Landscape-moderated biodiversity effects of ground herb cover in olive groves: Implications for regional biodiversity conservation. Agric. Ecosyst. Environ. 2019, 277, 61–73. [Google Scholar] [CrossRef]
- Arnan, X.; Cerdá, X.; Retana, J. Ant functional responses along environmental gradients. J. Anim. Ecol. 2014, 83, 1398–1408. [Google Scholar] [CrossRef]
- Blatrix, R.; Lebas, C.; Galkowski, C.; Wegnez, P.; Pimenta, R.; Morichon, D. Vegetation cover and elevation drive diversity and composition of ant communities (Hymenoptera: Formicidae) in a Mediterranean ecosystem. Myrmecol. News 2016, 22, 119–127. [Google Scholar]
- Cammeraat, E.L.H.; Risch, A.C. The impact of ants on mineral soil properties and processes at different spatial scales. J. Appl. Èntomol. 2008, 132, 285–294. [Google Scholar] [CrossRef]
- Frouz, J.; Jilková, V. The effect of ants on soil properties and processes (Hymenoptera: Formicidae). Myrmecol. News 2008, 11, 191–199. [Google Scholar]
- Chomicki, G.; Renner, S.S. The interactions of ants with their biotic environment. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170013. [Google Scholar] [CrossRef]
- Griffiths, H.M.; Ashton, L.A.; Walker, A.E.; Hasan, F.; Evans, T.A.; Eggleton, P.; Parr, C.L. Ants are the major agents of resource removal from tropical rainforests. J. Anim. Ecol. 2018, 87, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Wills, B.D.; Landis, D.A. The role of ants in north temperate grasslands: A review. Oecologia 2017, 186, 323–338. [Google Scholar] [CrossRef]
- Gómez, C.; Casellas, D.; Oliveras, J.; Bas, J.M. Structure of ground-foraging ant assemblages in relation to land-use change in the northwestern Mediterranean region. Biodivers. Conserv. 2003, 12, 2135–2146. [Google Scholar] [CrossRef]
- Verdú, J.R.; Numa, C.; Hernández-Cuba, O. The influence of landscape structure on ants and dung beetles diversity in a Mediterranean savanna—Forest ecosystem. Ecol. Indic. 2011, 11, 831–839. [Google Scholar] [CrossRef]
- Hevia, V.; Carmona, C.P.; Azcárate, F.M.; Torralba, M.; Alcorlo, P.; Ariño, R.; Lozano, J.; Castro-Cobo, S.; González, J.A. Effects of land use on taxonomic and functional diversity: A cross-taxon analysis in a Mediterranean landscape. Oecologia 2016, 181, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Cerdá, X.; Retana, J.; Cros, S. Thermal Disruption of Transitive Hierarchies in Mediterranean Ant Communities. J. Anim. Ecol. 1997, 66, 363–374. [Google Scholar] [CrossRef]
- Cerdá, X.; Retana, J.; Cros, S. Critical thermal limits in Mediterranean ant species: Trade-off between mortality risk and foraging performance. Funct. Ecol. 1998, 12, 45–55. [Google Scholar] [CrossRef]
- Santini, G.; Tucci, L.; Ottonetti, L.; Frizzi, F. Competition trade-offs in the organisation of a Mediterranean ant assemblage. Ecol. Èntomol. 2007, 32, 319–326. [Google Scholar] [CrossRef]
- Parr, C.L.; Gibb, H. The discovery-dominance trade-off is the exception, rather than the rule. J. Anim. Ecol. 2012, 81, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Reyes-López, J.; Ruiz, N.; Fernández-Haeger, J. Community structure of ground-ants: The role of single trees in a Mediterranean pastureland. Acta Oecol. 2003, 24, 195–202. [Google Scholar] [CrossRef]
- De Bruyn, L.L. Ants as bioindicators of soil function in rural environments. In Invertebrate Biodiversity as Bioindicators of Sustainable Landscapes; Elsevier: Amsterdam, The Netherlands, 1999; pp. 425–441. [Google Scholar]
- Chong, C.-S.; D’Alberto, C.F.; Thomson, L.J.; Hoffmann, A.A. Influence of native ants on arthropod communities in a vineyard. Agric. For. Èntomol. 2010, 12, 223–232. [Google Scholar] [CrossRef]
- Masoni, A.; Frizzi, F.; Brühl, C.; Zocchi, N.; Palchetti, E.; Chelazzi, G.; Santini, G. Management matters: A comparison of ant assemblages in organic and conventional vineyards. Agric. Ecosyst. Environ. 2017, 246, 175–183. [Google Scholar] [CrossRef]
- Martínez-Núñez, C.; Rey, P.J.; Salido, T.; Manzaneda, A.J.; Camacho, F.M.; Isla, J. Ant community potential for pest control in olive groves: Management and landscape effects. Agric. Ecosyst. Environ. 2021, 305, 107185. [Google Scholar] [CrossRef]
- Boet, O.; Arnan, X.; Retana, J. The role of environmental vs. biotic filtering in the structure of European ant communities: A matter of trait type and spatial scale. PLoS ONE 2020, 15, e0228625. [Google Scholar] [CrossRef]
- Cerdá, X.; Arnan, X.; Retana, J. Is competition a significant hallmark of ant (Hymenoptera: Formicidae) ecology. Myrmecol. News 2013, 18, 131–147. [Google Scholar]
- Stuble, K.L.; Juric, I.; Cerdá, X.; Sanders, N.J. Dominance hierarchies are a dominant paradigm in ant ecology (Hymenoptera: Formicidae), but should they be? and what is a dominance hierarchy anyways. Myrmecol. News 2017, 24, 71–81. [Google Scholar]
- Verdinelli, M.; Yakhlef, S.E.B.; Cossu, C.S.; Pilia, O.; Mannu, R. Variability of ant community composition in cork oak woodlands across the Mediterranean region: Implications for forest management. iForest 2017, 10, 707–714. [Google Scholar] [CrossRef]
- Hevia, V.; Ortega, J.; Azcárate, F.M.; López, C.A.; González, J.A. Exploring the effect of soil management intensity on taxonomic and functional diversity of ants in Mediterranean olive groves. Agric. For. Èntomol. 2019, 21, 109–118. [Google Scholar] [CrossRef]
- Spotti, F.A.; Castracani, C.; Grasso, D.A.; Mori, A. Daily activity patterns and food preferences in an alpine ant community. Ethol. Ecol. Evol. 2014, 27, 306–324. [Google Scholar] [CrossRef]
- Calazans, E.G.; Da Costa, F.V.; Cristiano, M.P.; Cardoso, D.C. Daily Dynamics of an Ant Community in a Mountaintop Ecosystem. Environ. Èntomol. 2020, 49, 383–390. [Google Scholar] [CrossRef]
- Wendt, C.F.; Frizzi, F.; Aiello, G.; Balzani, P.; Santini, G. Ant species but not trait diversity increases at the edges: Insights from a micro-scale gradient in a semi-natural Mediterranean ecosystem. Ecol. Entomol. in press.
- Retana, J.; Cerdá, X. Patterns of diversity and composition of Mediterranean ground ant communities tracking spatial and temporal variability in the thermal environment. Oecologia 2000, 123, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.N. A Classification of Australian Ant Communities, Based on Functional Groups Which Parallel Plant Life-Forms in Relation to Stress and Disturbance. J. Biogeogr. 1995, 22, 15. [Google Scholar] [CrossRef]
- Ottonetti, L.; Tucci, L.; Santini, G. Recolonization Patterns of Ants in a Rehabilitated Lignite Mine in Central Italy: Potential for the Use of Mediterranean Ants as Indicators of Restoration Processes. Restor. Ecol. 2006, 14, 60–66. [Google Scholar] [CrossRef]
- Santini, G.; Ramsay, P.M.; Tucci, L.; Ottonetti, L.; Frizzi, F. Spatial patterns of the ant Crematogaster scutellaris in a model ecosystem. Ecol. Èntomol. 2011, 36, 625–634. [Google Scholar] [CrossRef]
- Greenslade, P.J.M. Sampling ants with pitfall traps: Digging-in effects. Insectes Sociaux 1973, 20, 343–353. [Google Scholar] [CrossRef]
- Brown, W.L. Diversity of ants. In Ants. Standard Methods for Measuring and Monitoring Biodiversity; Agosti, D., Majer, J.D., Alonso, L., Schultz, R., Eds.; Smithsonian Institution Press: Washington, DC, USA, 2000; pp. 45–79. [Google Scholar]
- Ottonetti, L.; Tucci, L.; Frizzi, F.; Chelazzi, G.; Santini, G. Changes in ground-foraging ant assemblages along a disturbance gradient in a tropical agricultural landscape. Ethol. Ecol. Evol. 2010, 22, 73–86. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Anderson, M.J.; Ellingsen, K.E.; McArdle, B.H. Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 2006, 9, 683–693. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- De Cáceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N.; Somerfield, P.J.; Warwick, R.M. Change in Marine Communities. An Approach to Statistical Analysis and Interpretation, 3rd ed.; PRIMER-E Ltd: Plymouth, UK, 2014; p. 262. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org (accessed on 9 February 2021).
- Gerlach, J.; Samways, M.J.; Pryke, J. Terrestrial invertebrates as bioindicators: An overview of available taxonomic groups. J. Insect Conserv. 2013, 17, 831–850. [Google Scholar] [CrossRef]
- Andersen, A.N.; Majer, J.D. Ants show the way Down Under: Invertebrates as bioindicators in land management. Front. Ecol. Environ. 2004, 2, 291–298. [Google Scholar] [CrossRef]
- Arnan, X.; Gracia, M.; Comas, L.; Retana, J. Forest management conditioning ground ant community structure and composition in temperate conifer forests in the Pyrenees Mountains. For. Ecol. Manag. 2009, 258, 51–59. [Google Scholar] [CrossRef]
- Schmidt, F.A.; Ribas, C.R.; Schoereder, J.H. How predictable is the response of ant assemblages to natural forest recovery? Implications for their use as bioindicators. Ecol. Indic. 2013, 24, 158–166. [Google Scholar] [CrossRef]
- Théry, M. Forest light and its influence on habitat selection. In Tropical Forest Canopies: Ecology and Management; Springer: Dordrecht, The Netherlands, 2001; pp. 251–261. [Google Scholar] [CrossRef]
- Härdtle, W.; Von Oheimb, G.; Westphal, C. The effects of light and soil conditions on the species richness of the ground vegetation of deciduous forests in northern Germany (Schleswig-Holstein). For. Ecol. Manag. 2003, 182, 327–338. [Google Scholar] [CrossRef]
- Wulf, M.; Naaf, T. Herb layer response to broadleaf tree species with different leaf litter quality and canopy structure in temperate forests. J. Veg. Sci. 2009, 20, 517–526. [Google Scholar] [CrossRef]
- Durak, T. Changes in diversity of the mountain beech forest herb layer as a function of the forest management method. For. Ecol. Manag. 2012, 276, 154–164. [Google Scholar] [CrossRef]
- Arnan, X.; Rodrigo, A.; Retana, J. Uncoupling the effects of shade and food resources of vegetation on Mediterranean ants: An experimental approach at the community level. Ecography 2007, 30, 161–172. [Google Scholar] [CrossRef]
- Campos, R.B.; Schoereder, J.H.; Sperber, C.F. Small-scale patch dynamics after disturbance in litter ant communities. Basic Appl. Ecol. 2007, 8, 36–43. [Google Scholar] [CrossRef]
- Kvamme, T.; Collingwood, C.A. The first records in Norway of Myrmica specioides Bondroit, 1918 and Formica cunicularia Latreille, 1798 (Hymenoptera, Formicidae). Nor. J. Entomol. 2009, 56, 65–68. [Google Scholar]
- Tomei, P.E.; Bertacchi, A. The Protection of Biodiversity in Tuscany. In Managing European Coasts; Springer: Berlin, Germany, 2007; pp. 87–89. [Google Scholar]
- Viciani, D.; Lastrucci, L.; Dell’Olmo, L.; Ferretti, G.; Foggi, B. Natura 2000 habitats in Tuscany (central Italy): Synthesis of main conservation features based on a comprehensive database. Biodivers. Conserv. 2014, 23, 1551–1576. [Google Scholar] [CrossRef]
- Detrain, C.; Pasteels, J.M. Seed preferences of the harvester ant Messor barbarus in a Mediterranean mosaic grassland (Hymenoptera: Formicidae). Sociobiology 2000, 35, 35–48. [Google Scholar]
- Masoni, A.; Frizzi, F.; Turillazzi, S.; Santini, G. Making the right choice: How Crematogaster scutellaris queens choose to co-found in relation to nest availability. Insectes Sociaux 2019, 66, 257–263. [Google Scholar] [CrossRef]
- Frizzi, F.; Ciofi, C.; Dapporto, L.; Natali, C.; Chelazzi, G.; Turillazzi, S.; Santini, G. The Rules of Aggression: How Genetic, Chemical and Spatial Factors Affect Intercolony Fights in a Dominant Species, the Mediterranean Acrobat Ant Crematogaster scutellaris. PLoS ONE 2015, 10, e0137919. [Google Scholar] [CrossRef]
- Zechmeister, H.G.; Schmitzberger, I.; Steurer, B.; Peterseil, J.; Wrbka, T. The influence of land-use practices and economics on plant species richness in meadows. Biol. Conserv. 2003, 114, 165–177. [Google Scholar] [CrossRef]
- Roschewitz, I.; Gabriel, D.; Tscharntke, T.; Thies, C. The effects of landscape complexity on arable weed species diversity in organic and conventional farming. J. Appl. Ecol. 2005, 42, 873–882. [Google Scholar] [CrossRef]
- Nascimbene, J.; Marini, L.; Paoletti, M.G. Organic Farming Benefits Local Plant Diversity in Vineyard Farms Located in Intensive Agricultural Landscapes. Environ. Manag. 2012, 49, 1054–1060. [Google Scholar] [CrossRef]
- Ruano, F.; Lozano, C.; Tinaut, A.; Peña, A.; Pascual, F.; García, P.; Campos, M. Impact of pesticides on beneficial arthropod fauna of olive groves. IOBC/WPRS Bull. 2001, 24, 113–120. [Google Scholar]
- Santos, S.A.; Cabanas, J.E.; Pereira, J.A. Abundance and diversity of soil arthropods in olive grove ecosystem (Portugal): Effect of pitfall trap type. Eur. J. Soil Biol. 2007, 43, 77–83. [Google Scholar] [CrossRef]
- Trivellone, V.; Paltrinieri, L.P.; Jermini, M.; Moretti, M. Management pressure drives leafhopper communities in vineyards in Southern Switzerland. Insect Conserv. Divers. 2012, 5, 75–85. [Google Scholar] [CrossRef]
- Caprio, E.; Nervo, B.; Isaia, M.; Allegro, G.; Rolando, A. Organic versus conventional systems in viticulture: Comparative effects on spiders and carabids in vineyards and adjacent forests. Agric. Syst. 2015, 136, 61–69. [Google Scholar] [CrossRef]
- Redolfi, I.; Tinaut, A.; Pascual, F.; Campos, M. Qualitative aspects of myrmecocenosis (Hym., Formicidae) in olive orchards with different agricultural management in Spain. J. Appl. Èntomol. 1999, 123, 621–627. [Google Scholar] [CrossRef]
- Jerez-Valle, C.; García, P.A.; Campos, M.; Pascual, F. A simple bioindication method to discriminate olive orchard management types using the soil arthropod fauna. Appl. Soil Ecol. 2014, 76, 42–51. [Google Scholar] [CrossRef]
- Gkisakis, V.; Volakakis, N.; Kollaros, D.; Bàrberi, P.; Kabourakis, E.M. Soil arthropod community in the olive agroecosystem: Determined by environment and farming practices in different management systems and agroecological zones. Agric. Ecosyst. Environ. 2016, 218, 178–189. [Google Scholar] [CrossRef]
- Gkisakis, V.D.; Kollaros, D.; Bàrberi, P.; Livieratos, I.C.; Kabourakis, E.M. Soil Arthropod Diversity in Organic, Integrated, and Conventional Olive Orchards and Different Agroecological Zones in Crete, Greece. Agroecol. Sustain. Food Syst. 2015, 39, 276–294. [Google Scholar] [CrossRef]
- Gramigni, E.; Calusi, S.; Gelli, N.; Giuntini, L.; Massi, M.; Delfino, G.; Chelazzi, G.; Baracchi, D.; Frizzi, F.; Santini, G. Ants as bioaccumulators of metals from soils: Body content and tissue-specific distribution of metals in the ant Crematogaster scutellaris. Eur. J. Soil Biol. 2013, 58, 24–31. [Google Scholar] [CrossRef]
- Frizzi, F.; Masoni, A.; Çelikkol, M.; Palchetti, E.; Ciofi, C.; Chelazzi, G.; Santini, G. Serpentine soils affect heavy metal tolerance but not genetic diversity in a common Mediterranean ant. Chemosphere 2017, 180, 326–334. [Google Scholar] [CrossRef]
- Kaspari, M.; Clay, N.A.; Lucas, J.; Yanoviak, S.P.; Kay, A. Thermal adaptation generates a diversity of thermal limits in a rainforest ant community. Glob. Chang. Biol. 2015, 21, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Frizzi, F.; Bartalesi, V.; Santini, G. Combined effects of temperature and interspecific competition on the mortality of the invasive garden ant, Lasius neglectus: A laboratory study. J. Therm. Biol. 2017, 65, 76–81. [Google Scholar] [CrossRef]
- Frizzi, F.; Rispoli, A.; Chelazzi, G.; Santini, G. Effect of water and resource availability on ant feeding preferences: A field experiment on the Mediterranean ant Crematogaster scutellaris. Insectes Sociaux 2016, 63, 565–574. [Google Scholar] [CrossRef]
- Czechowski, W.; Radchenko, A.; Czechowska, W. The Ants (Hymenoptera, Formicidae) of Poland; Museum and Institute of Zoology: Warsaw, Poland, 2002; p. 496. [Google Scholar]
- Salata, S.; Kalarus, K.; Borowiec, L.; Trichas, A.; Kujawa, K. How estimated ant diversity is biased by the sampling method? A case study of Crete: A Mediterranean biodiversity hotspot. Biodivers. Conserv. 2020, 29, 3031–3050. [Google Scholar] [CrossRef]
- Roig, X.; Espadaler, X. Proposal of functional groups of ants for the Iberian Peninsula and Balearic Islands, and their use as bioindicators. Iberomyrmex 2010, 2, 28–29. [Google Scholar]
- Wiescher, P.T.; Pearce-Duvet, J.M.C.; Feener, D.H. Assembling an ant community: Species functional traits reflect environmental filtering. Oecologia 2012, 169, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Parr, C.L.; Dunn, R.R.; Sanders, N.J.; Weiser, M.D.; Photakis, M.; Bishop, T.R.; Fitzpatrick, M.C.; Arnan, X.; Baccaro, F.; Brandão, C.R.F.; et al. GlobalAnts: A new database on the geography of ant traits (Hymenoptera: Formicidae). Insect Conserv. Divers. 2017, 10, 5–20. [Google Scholar] [CrossRef]
- Wendt, C.F.; Nunes, A.; Verble, R.; Santini, G.; Boieiro, M.; Branquinho, C. Using a space-for-time approach to select the best biodiversity-based indicators to assess the effects of aridity on Mediterranean drylands. Ecol. Indic. 2020, 113, 106250. [Google Scholar] [CrossRef]
- Wendt, C.F.; Ceia-Hasse, A.; Nunes, A.; Verble, R.; Santini, G.; Boieiro, M.; Branquinho, C. Local environmental variables are key drivers of ant taxonomic and functional beta-diversity in a Mediterranean dryland. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).