Remote Sensing-Based Estimation of Advanced Perennial Grass Biomass Yields for Bioenergy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data
2.3. Image Processing and Model Development
3. Results
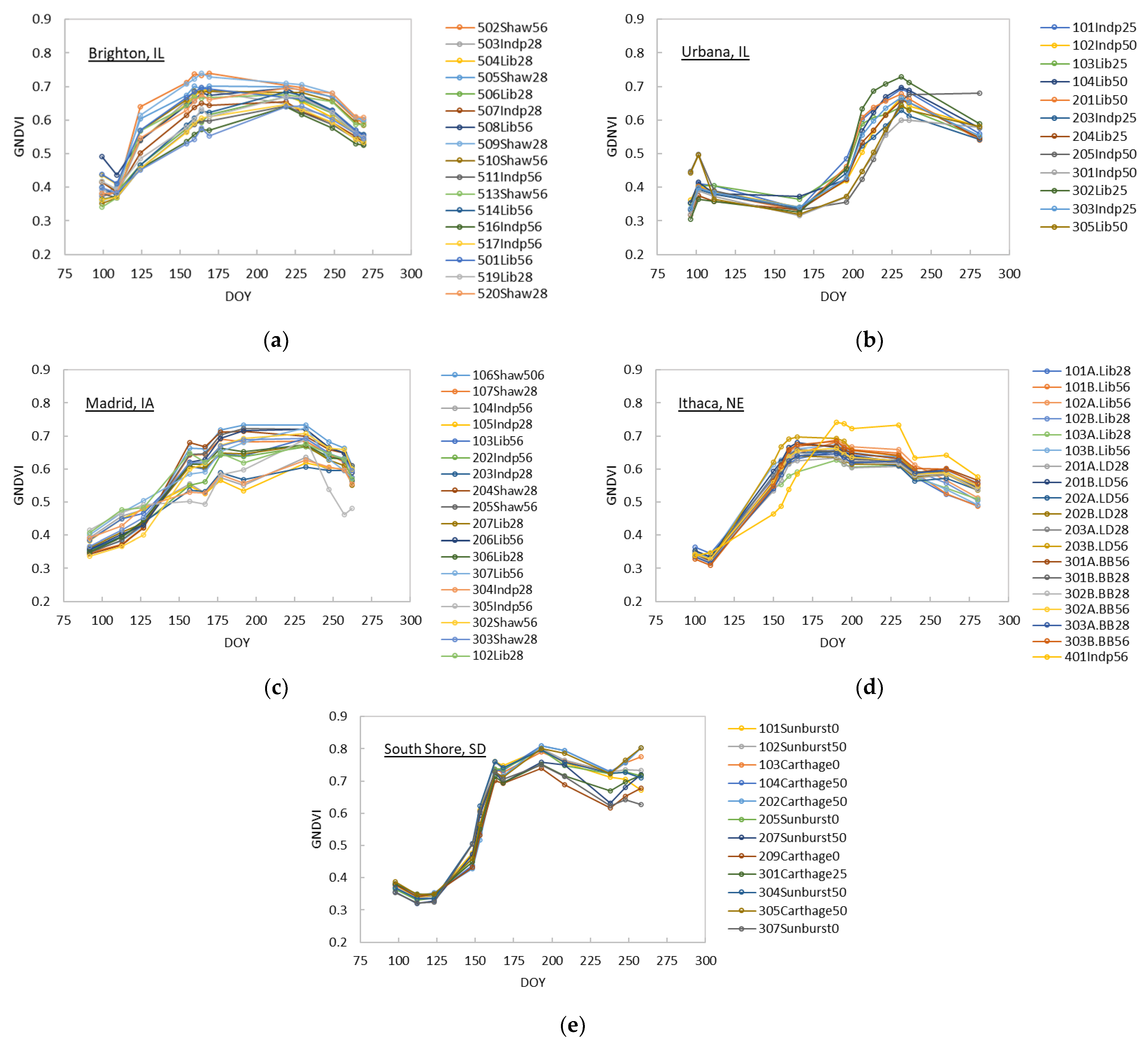
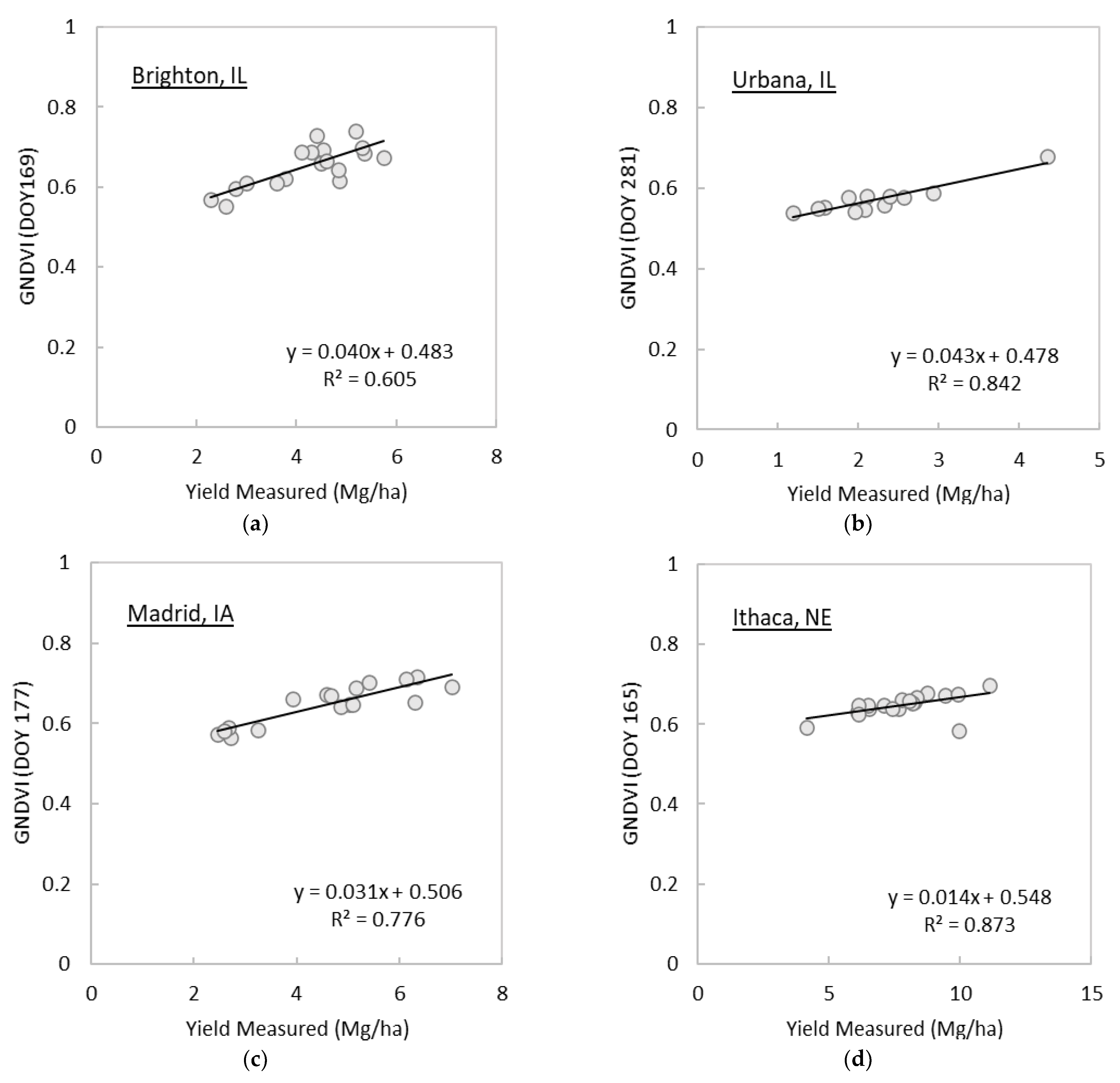
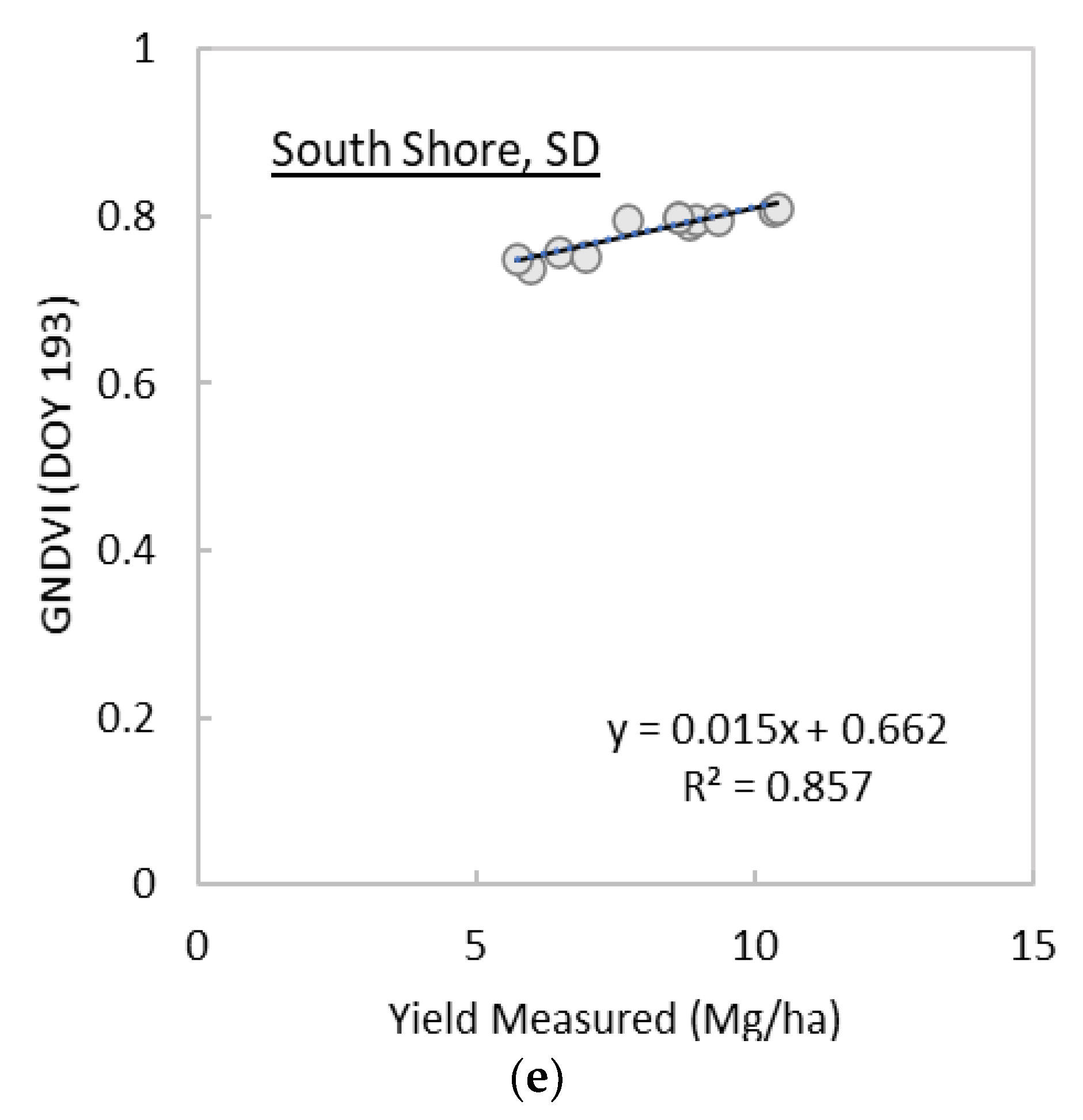
3.1. Analysis of Spectral Vegetation Indices in Relation to Switchgrass Yields
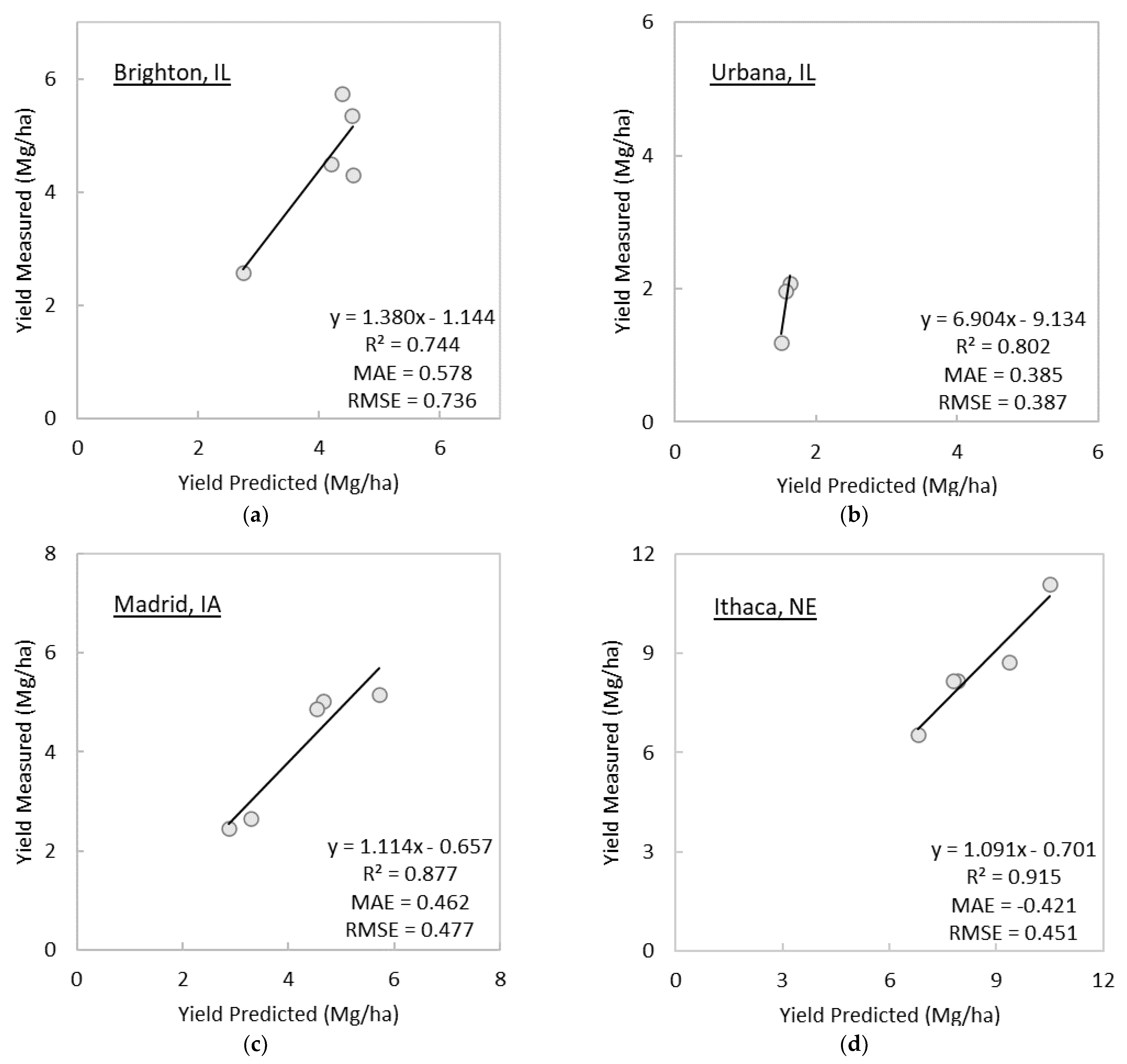
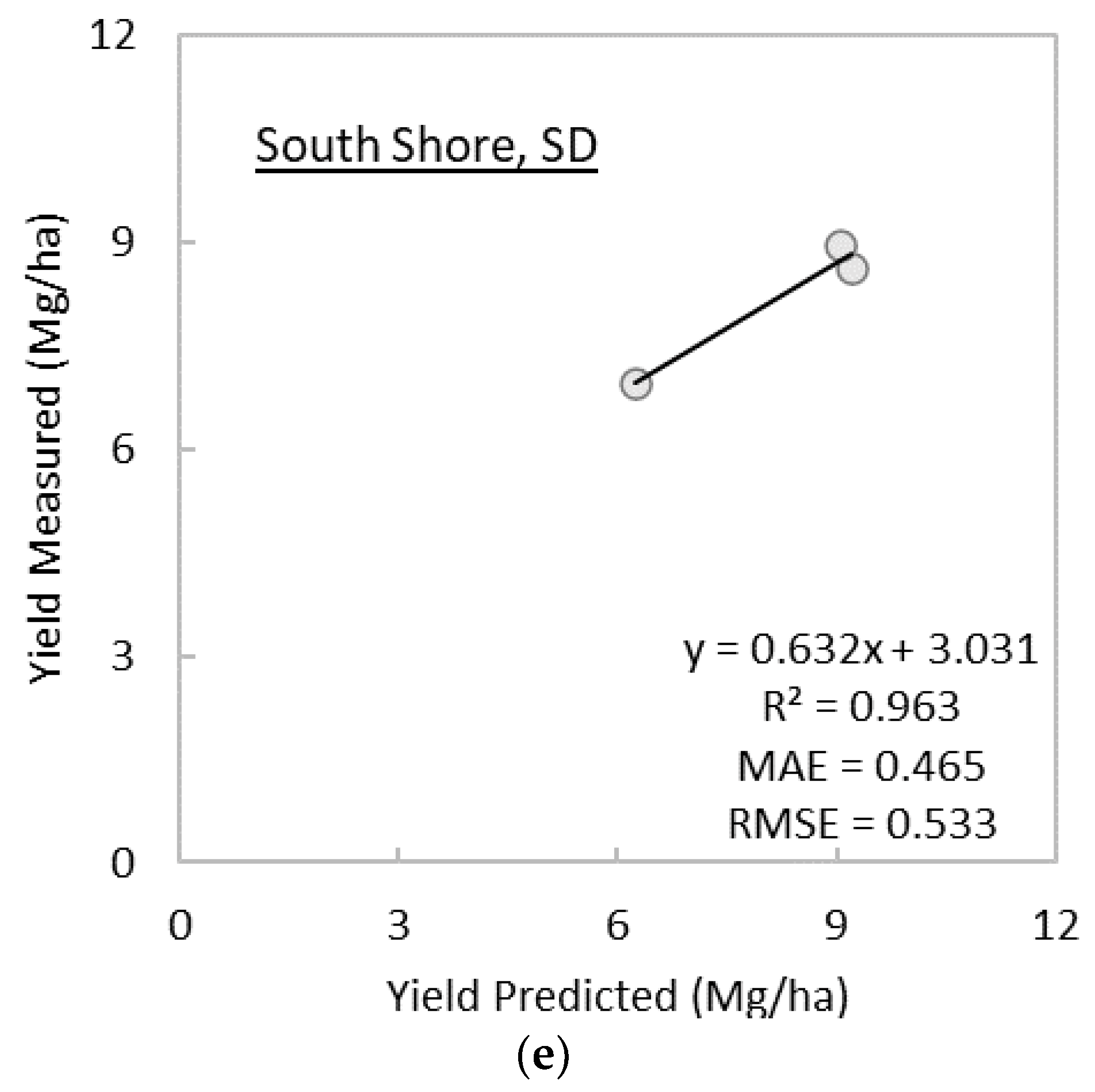
3.2. Prediction of Switchgrass Dry Biomass Yields at Harvest
4. Discussion
5. Conclusions
- The linear regression model using midsummer GNDVI predicted at-harvest perennial grass yield with R2 as high as 0.879 and MAE and RMSE as low as 0.539 Mg/ha and 0.616 Mg/ha, respectively, except for the establishment year. The selection of image date in this study was based on image availability; thus, there is a possibility that the GNDVI linear regression models has a greater predictive power for at-harvest yields than presented in this study.
- The GNDVI linear regression model predicted at-harvest switchgrass yields as early as 152 days before the date of harvest on average, except for the year of establishment. More frequent cloud-free imagery than used in the present study or simulated seasonal trajectories of spectral vegetation indices could allow us to answer more precisely how early and accurately at-harvest yields can be predicted using remote sensing.
- While the NIR spectral band was found to be one of the key spectral bands, the green band appeared to have a greater contribution for predicting at-harvest switchgrass and other perennial grass dry biomass yields than the red band. This is consistent with existing studies [58,59] demonstrating that the sensitivity of the green spectral reflectance to plant chlorophyll content, which is indicative of green biomass volume, is greater than that of the red spectral reflectance.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dietz, T.; Börner, J.; Förster, J.J.; Von Braun, J. Governance of the bioeconomy: A global comparative study of national bioeconomy strategies. Sustainability 2018, 10, 3190. [Google Scholar] [CrossRef]
- Duncan, M. US Federal initiatives to support biomass research and development. J. Ind. Ecol. 2003, 7, 193–201. [Google Scholar] [CrossRef]
- Board, B. The Bioeconomy Initiative: Implementation Framework. 2018. Available online: https://biomassboard.gov/sites/default/files/pdfs/Bioeconomy_Initiative_Implementation_Framework_FINAL.pdf (accessed on 4 December 2020).
- Golden, J.S.; Handfield, R.B.; Daystar, J.; McConnell, T.E. An economic impact analysis of the US biobased products industry: A report to the Congress of the United States of America. Ind. Biotechnol. 2015, 11, 201–209. [Google Scholar] [CrossRef]
- Langholtz, M.H.; Stokes, B.J.; Eaton, L.M. 2016 Billion-ton report: Advancing domestic resources for a thriving bioeconomy, Volume 1: Economic availability of feedstock. In Oak Ridge National Laboratory, Oak Ridge, Tennessee, Managed by UT-Battelle, LLC for the US Department of Energy (DOE); U.S. DOE Energy Efficiency and Renewable Energy Bioenergies Technologies Office: Washington, DC, USA, 2016; Volume 2016, pp. 1–411. [Google Scholar]
- Board, B. Increasing Feedstock Production for Biofuels: Economic Drivers, Environmental Implications, and the Role of Research. 2010. Available online: https://afdc.energy.gov/files/pdfs/increasing_feedstock_revised.pdf (accessed on 1 June 2021).
- Mitchell, R.; Schmer, M.; Anderson, W.; Jin, V.; Balkcom, K.; Kiniry, J.; Coffin, A.; White, P. Dedicated energy crops and crop residues for bioenergy feedstocks in the central and eastern USA. Bioenergy Res. 2016, 9, 384–398. [Google Scholar] [CrossRef]
- Berndes, G.; Bird, N.; Cowie, A. Bioenergy, Land Use Change and Climate Change Mitigation; IEA Bioenergy Report; ExCo:2010:03. Available online: https://www.osti.gov/etdeweb/servlets/purl/22110326 (accessed on 1 June 2021).
- Ssegane, H.; Negri, M.C.; Quinn, J.; Urgun-Demirtas, M. Multifunctional landscapes: Site characterization and field-scale design to incorporate biomass production into an agricultural system. Biomass Bioenergy 2015, 80, 179–190. [Google Scholar] [CrossRef]
- Cacho, J.F.; Youssef, M.A.; Chescheir, G.M.; Skaggs, R.W.; Appelboom, T.W.; Leggett, Z.H.; Sucre, E.B.; Nettles, J.E.; Arellano, C. Effects of forest-based bioenergy feedstock production on shallow groundwater quality of a drained forest soil. Sci. Total Environ. 2018, 631, 13–22. [Google Scholar] [CrossRef]
- Cacho, J.; Negri, M.; Zumpf, C.; Campbell, P. Introducing perennial biomass crops into agricultural landscapes to address water quality challenges and provide other environmental services. Wiley Interdiscip. Rev. Energy Environ. 2018, 7, e275. [Google Scholar] [CrossRef]
- Englund, O.; Dimitriou, I.; Dale, V.H.; Kline, K.L.; Mola-Yudego, B.; Murphy, F.; English, B.; McGrath, J.; Busch, G.; Negri, M.C. Multifunctional perennial production systems for bioenergy: Performance and progress. Wiley Interdiscip. Rev. Energy Environ. 2020, 9, e375. [Google Scholar] [CrossRef]
- Ssegane, H.; Negri, M.C. An integrated landscape designed for commodity and bioenergy crops for a tile-drained agricultural watershed. J. Environ. Qual. 2016, 45, 1588–1596. [Google Scholar] [CrossRef]
- Schmer, M.R.; Mitchell, R.; Vogel, K.; Schacht, W.H.; Marx, D.B. Spatial and temporal effects on switchgrass stands and yield in the Great Plains. BioEnergy Res. 2010, 3, 159–171. [Google Scholar] [CrossRef][Green Version]
- Ahamed, T.; Tian, L.; Zhang, Y.; Ting, K. A review of remote sensing methods for biomass feedstock production. Biomass and Bioenergy 2011, 35, 2455–2469. [Google Scholar] [CrossRef]
- Basso, B.; Cammarano, D.; Carfagna, E. Review of crop yield forecasting methods and early warning systems. In Proceedings of the First Meeting of the Scientific Advisory Committee of the Global Strategy to Improve Agricultural and Rural Statistics, FAO Headquarters, Rome, Italy, 18–19 June 2013. [Google Scholar]
- Campbell, J.B. Introduction to Remote Sensing; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Slaton, M.R.; Raymond Hunt, E., Jr.; Smith, W.K. Estimating near-infrared leaf reflectance from leaf structural characteristics. Am. J. Bot. 2001, 88, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, J.F.; Schepers, J.S.; Francis, D.D.; Varvel, G.E.; Wilhelm, W.W.; Tringe, J.M.; Schlemmer, M.R.; Major, D.J. Use of remote-sensing imagery to estimate corn grain yield. Agron. J. 2001, 93, 583–589. [Google Scholar] [CrossRef]
- Hamada, Y.; Ssegane, H.; Negri, M.C. Mapping intra-field yield variation using high resolution satellite imagery to integrate bioenergy and environmental stewardship in an agricultural watershed. Remote Sens. 2015, 7, 9753–9768. [Google Scholar] [CrossRef]
- Johnson, D.M. An assessment of pre-and within-season remotely sensed variables for forecasting corn and soybean yields in the United States. Remote Sens. Environ. 2014, 141, 116–128. [Google Scholar] [CrossRef]
- Gao, F.; Anderson, M.; Daughtry, C.; Johnson, D. Assessing the variability of corn and soybean yields in central Iowa using high spatiotemporal resolution multi-satellite imagery. Remote Sens. 2018, 10, 1489. [Google Scholar] [CrossRef]
- Cicek, H.; Sunohara, M.; Wilkes, G.; McNairn, H.; Pick, F.; Topp, E.; Lapen, D. Using vegetation indices from satellite remote sensing to assess corn and soybean response to controlled tile drainage. Agric. Water Manag. 2010, 98, 261–270. [Google Scholar] [CrossRef]
- Smith, R.; Adams, J.; Stephens, D.; Hick, P. Forecasting wheat yield in a Mediterranean-type environment from the NOAA satellite. Aust. J. Agric. Res. 1995, 46, 113–125. [Google Scholar] [CrossRef]
- Dempewolf, J.; Adusei, B.; Becker-Reshef, I.; Hansen, M.; Potapov, P.; Khan, A.; Barker, B. Wheat yield forecasting for Punjab Province from vegetation index time series and historic crop statistics. Remote Sens. 2014, 6, 9653–9675. [Google Scholar] [CrossRef]
- Salazar, L.; Kogan, F.; Roytman, L. Use of remote sensing data for estimation of winter wheat yield in the United States. Int. J. Remote Sens. 2007, 28, 3795–3811. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, X.; Dong, Z.; Guo, W. Estimation of biomass in wheat using random forest regression algorithm and remote sensing data. Crop J. 2016, 4, 212–219. [Google Scholar]
- Mosleh, M.K.; Hassan, Q.K.; Chowdhury, E.H. Development of a remote sensing-based rice yield forecasting model. Span. J. Agric. Res. 2016, 14, e0907. [Google Scholar] [CrossRef]
- Noureldin, N.; Aboelghar, M.; Saudy, H.; Ali, A. Rice yield forecasting models using satellite imagery in Egypt. Egypt. J. Remote Sens. Space Sci. 2013, 16, 125–131. [Google Scholar] [CrossRef]
- Tennakoon, S.; Murty, V.; Eiumnoh, A. Estimation of cropped area and grain yield of rice using remote sensing data. Int. J. Remote Sens. 1992, 13, 427–439. [Google Scholar] [CrossRef]
- Cicore, P.; Serrano, J.; Shahidian, S.; Sousa, A.; Costa, J.L.; da Silva, J.R.M. Assessment of the spatial variability in tall wheatgrass forage using LANDSAT 8 satellite imagery to delineate potential management zones. Environ. Monit. Assess. 2016, 188, 513. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Shahidian, S.; Marques da Silva, J. Monitoring seasonal pasture quality degradation in the Mediterranean montado ecosystem: Proximal versus remote sensing. Water 2018, 10, 1422. [Google Scholar] [CrossRef]
- Serrano, J.; Shahidian, S.; Marques da Silva, J. Evaluation of normalized difference water index as a tool for monitoring pasture seasonal and inter-annual variability in a Mediterranean agro-silvo-pastoral system. Water 2019, 11, 62. [Google Scholar] [CrossRef]
- Massey, J.; Antonangelo, J.; Zhang, H. Nitrogen Fertilization and Harvest Timing Affect Switchgrass Quality. Resources 2020, 9, 61. [Google Scholar] [CrossRef]
- Guretzky, J.A.; Biermacher, J.T.; Cook, B.J.; Kering, M.K.; Mosali, J. Switchgrass for forage and bioenergy: Harvest and nitrogen rate effects on biomass yields and nutrient composition. Plant Soil 2011, 339, 69–81. [Google Scholar] [CrossRef]
- Hedtcke, J.L.; Sanford, G.R.; Hadley, K.E.; Thelen, K.D. Maximizing land use during switchgrass establishment in the north central United States. Agron. J. 2014, 106, 596–604. [Google Scholar] [CrossRef]
- Sarath, G.; Baird, L.M.; Mitchell, R.B. Senescence, dormancy and tillering in perennial C4 grasses. Plant Sci. 2014, 217, 140–151. [Google Scholar] [CrossRef]
- Serapiglia, M.J.; Boateng, A.A.; Lee, D.K.; Casler, M.D. Switchgrass harvest time management can impact biomass yield and nutrient content. Crop Sci. 2016, 56, 1970–1980. [Google Scholar] [CrossRef]
- Wilson, D.M.; Heaton, E.A.; Liebman, M.; Moore, K.J. Intraseasonal changes in switchgrass nitrogen distribution compared with corn. Agron. J. 2013, 105, 285–294. [Google Scholar] [CrossRef]
- Wang, Z.; Smyth, T.J.; Crozier, C.R.; Gehl, R.J.; Heitman, A.J. Yield and nitrogen removal of bioenergy grasses as influenced by nitrogen rate and harvest management in the coastal plain region of North Carolina. BioEnergy Res. 2018, 11, 44–53. [Google Scholar] [CrossRef]
- Zumpf, C.; Lee, M.S.; Thapa, S.; Guo, J.; Mitchell, R.; Volenec, J.J.; Lee, D. Impact of warm-season grass management on feedstock production on marginal farmland in Central Illinois. GCB Bioenergy 2019, 11, 1202–1214. [Google Scholar] [CrossRef]
- Sibanda, M.; Mutanga, O.; Dube, T.; Mafongoya, P.L. Spectrometric proximally sensed data for estimating chlorophyll content of grasslands treated with complex fertilizer combinations. J. Appl. Remote Sens. 2020, 14, 024517. [Google Scholar] [CrossRef]
- Orsini, R.; Fiorentini, M.; Zenobi, S. Testing vegetation index categories as influenced by soil management and nitrogen fertilization in cereal based cropping systems. In Proceedings of the 2019 IEEE International Workshop on Metrology for Agriculture and Forestry (MetroAgriFor), Portici, Italy, 24–26 October 2019; pp. 13–18. [Google Scholar]
- Orsini, R.; Fiorentini, M.; Zenobi, S. Evaluation of Soil Management Effect on Crop Productivity and Vegetation Indices Accuracy in Mediterranean Cereal-Based Cropping Systems. Sensors 2020, 20, 3383. [Google Scholar] [CrossRef]
- Schut, A.G.; Traore, P.C.S.; Blaes, X.; Rolf, A. Assessing yield and fertilizer response in heterogeneous smallholder fields with UAVs and satellites. Field Crops Res. 2018, 221, 98–107. [Google Scholar] [CrossRef]
- Vogel, K.P.; Masters, R.A. Frequency grid--a simple tool for measuring grassland establishment. Rangel. Ecol. Manag. J. Range Manag. Arch. 2001, 54, 653–655. [Google Scholar] [CrossRef]
- Illinois Climate Network: Water and Atmospheric Resources Monitoring Program. Illinois State Water Survey, 2204 Griffith Drive, Champaign, IL 61820-7495. Available online: https://doi.org/10.13012/J8MW2F2Q (accessed on 30 June 2021).
- Automated Weather Data Network: High Plains Regional Climate Center. Available online: https://hprcc.unl.edu/awdn/ (accessed on 1 July 2021).
- Iowa Environmental Mesonet: Iowa State University. Available online: https://mesonet.agron.iastate.edu/agclimate/hist/daily.php (accessed on 15 June 2021).
- South Dakota Mesonet. Available online: https://climate.sdstate.edu/ (accessed on 15 June 2021).
- Kaufman, Y.J.; Tanre, D. Atmospherically resistant vegetation index (ARVI) for EOS-MODIS. IEEE Trans. Geosci. Remote Sens. 1992, 30, 261–270. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Jiang, Z.; Huete, A.R.; Didan, K.; Miura, T. Development of a two-band enhanced vegetation index without a blue band. Remote Sens. Environ. 2008, 112, 3833–3845. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Sripada, R.P. Determining In-Season Nitrogen Requirements for Corn Using Aerial Color-Infrared Photography; North Carolina State University: Raleigh, NC, USA, 2005. [Google Scholar]
- Gitelson, A.; Merzlyak, M.N. Spectral reflectance changes associated with autumn senescence of Aesculus hippocastanum L. and Acer platanoides L. leaves. Spectral features and relation to chlorophyll estimation. J. Plant Physiol. 1994, 143, 286–292. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote sensing of chlorophyll concentration in higher plant leaves. Adv. Space Res. 1998, 22, 689–692. [Google Scholar] [CrossRef]
- Sripada, R.P.; Heiniger, R.W.; White, J.G.; Meijer, A.D. Aerial color infrared photography for determining early in-season nitrogen requirements in corn. Agron. J. 2006, 98, 968–977. [Google Scholar] [CrossRef]
- Crippen, R.E. Calculating the vegetation index faster. Remote Sens. Environ. 1990, 34, 71–73. [Google Scholar] [CrossRef]
- Yang, Z.; Willis, P.; Mueller, R. Impact of band-ratio enhanced AWIFS image to crop classification accuracy. In Proceedings of the Pecora 17 The Future of Land Imaging Going Operational, Denver, CO, USA, 18–20 November 2008; pp. 1–11. [Google Scholar]
- Qi, J.; Chehbouni, A.; Huerte, A.; Kerr, Y.; Sorooshian, S. A modified soil adjusted vegetation index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Chen, J.M. Evaluation of vegetation indices and a modified simple ratio for boreal applications. Can. J. Remote Sens. 1996, 22, 229–242. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. NASA Spec. Publ. 1974, 351, 309. [Google Scholar]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Roujean, J.-L.; Breon, F.-M. Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Huete, A. A Soil-Adjusted Vegetation Index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Birth, G.S.; McVey, G.R. Measuring the color of growing turf with a reflectance spectrophotometer 1. Agron. J. 1968, 60, 640–643. [Google Scholar] [CrossRef]
- Gitelson, A.; Stark, R.; Grits, U.; Rundquist, D.; Kaufman, Y.; Derry, D. Vegetation and soil lines in visible spectral space: A concept and technique for remote estimation of vegetation fraction. Int. J. Remote Sens. 2002, 23, 2537–2562. [Google Scholar] [CrossRef]
- Gitelson, A.A. Wide dynamic range vegetation index for remote quantification of biophysical characteristics of vegetation. J. Plant Physiol. 2004, 161, 165–173. [Google Scholar] [CrossRef]
- Gamon, J.; Surfus, J. Assessing leaf pigment content and activity with a reflectometer. New Phytol. 1999, 143, 105–117. [Google Scholar] [CrossRef]
- Xue, J.; Su, B. Significant remote sensing vegetation indices: A review of developments and applications. J. Sens. 2017, 2017, 1353691. [Google Scholar] [CrossRef]
- Casler, M.D.; Vogel, K.P.; Harrison, M. Switchgrass germplasm resources. Crop Scie. 2015, 55, 2463–2478. [Google Scholar] [CrossRef]
- Liatukas, Ž.; Lemežienė, N.; Butkutė, B.; Cesevičienė, J.; Dabkevičienė, G. Chlorophyll values as a measure of genetic variation of switchgrass (Panicum virgatum L.) populations under cool temperate climate conditions. Zemdirb. Agric. 2015, 102, 159–166. [Google Scholar] [CrossRef]
- Mitchell, R.B.; Lee, D.; Casler, M. Switchgrass. In Cellulosic Energy Cropping Systems; Karlen, D.L., Ed.; John Wiley & Sons, Ltd.: West Sussex, UK, 2014; pp. 75–85. [Google Scholar]
- Vogel, K.P.; Sarath, G.; Saathoff, A.J.; Mitchell, R.B. Chapter 17 Switchgrass. In RSC Energy and Environmental Series No.3: Energy Crops; Halford, N.G., Karp, A., Eds.; RSC Publishing: Cambridge, UK, 2011; pp. 341–369. [Google Scholar]
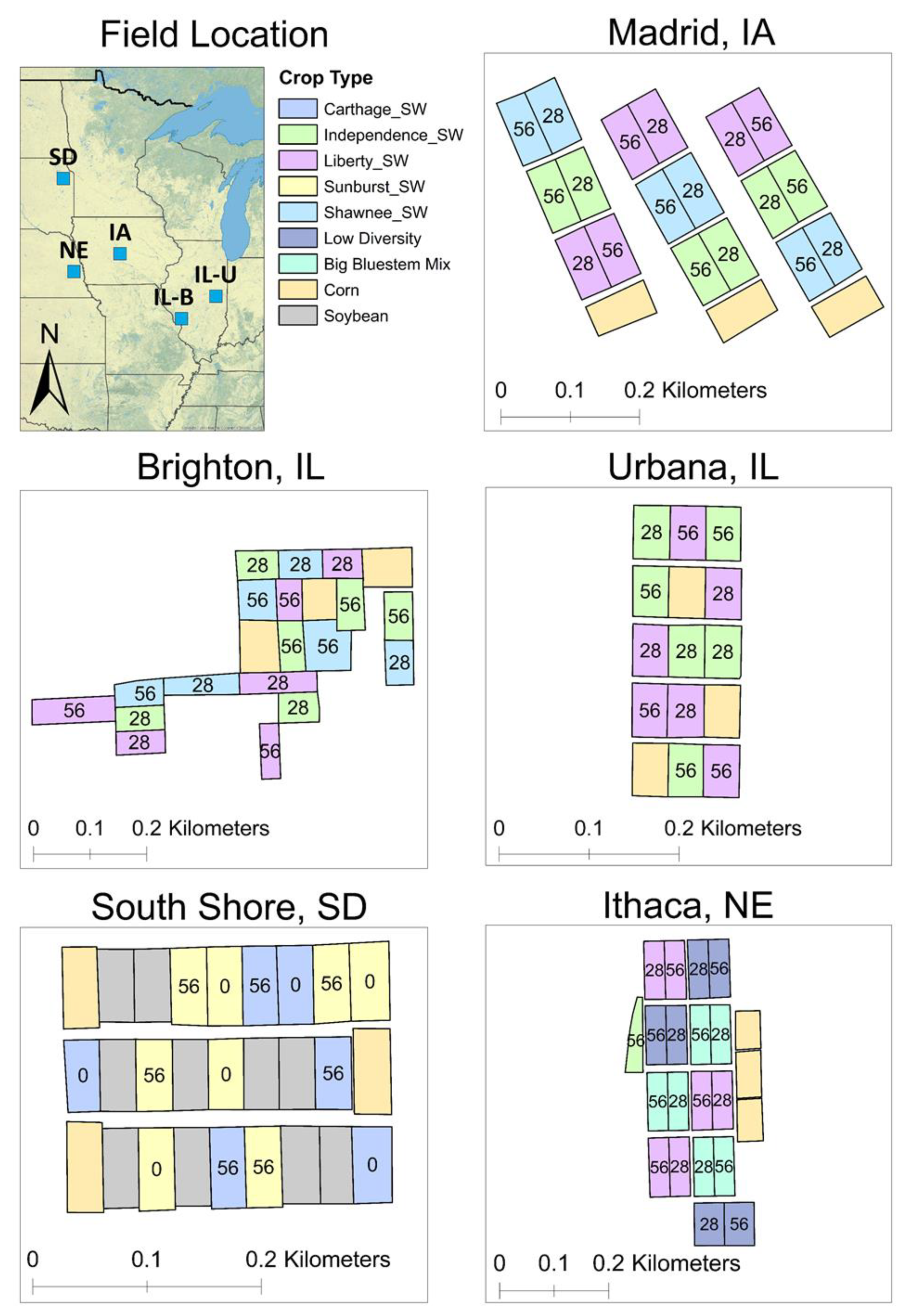
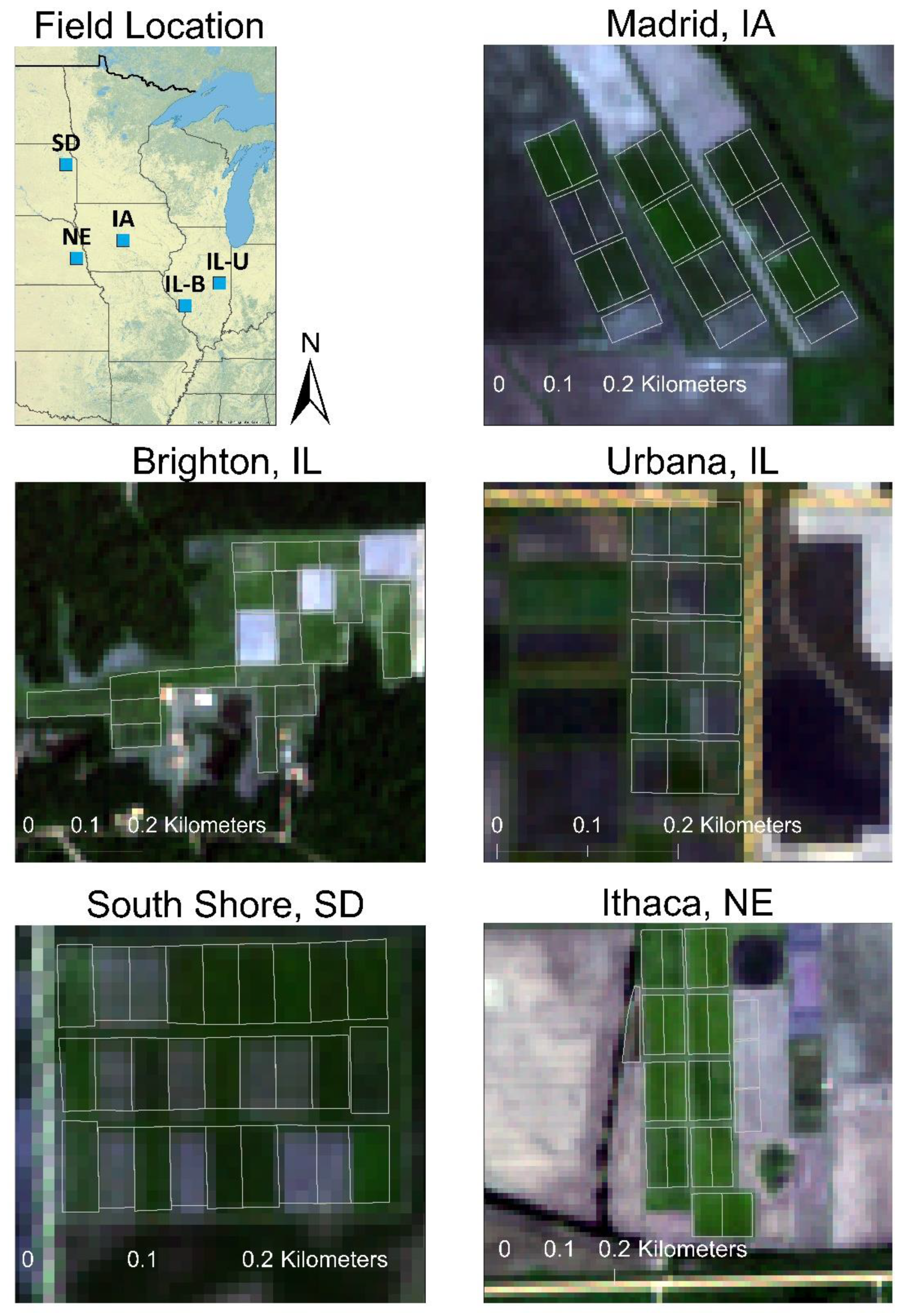
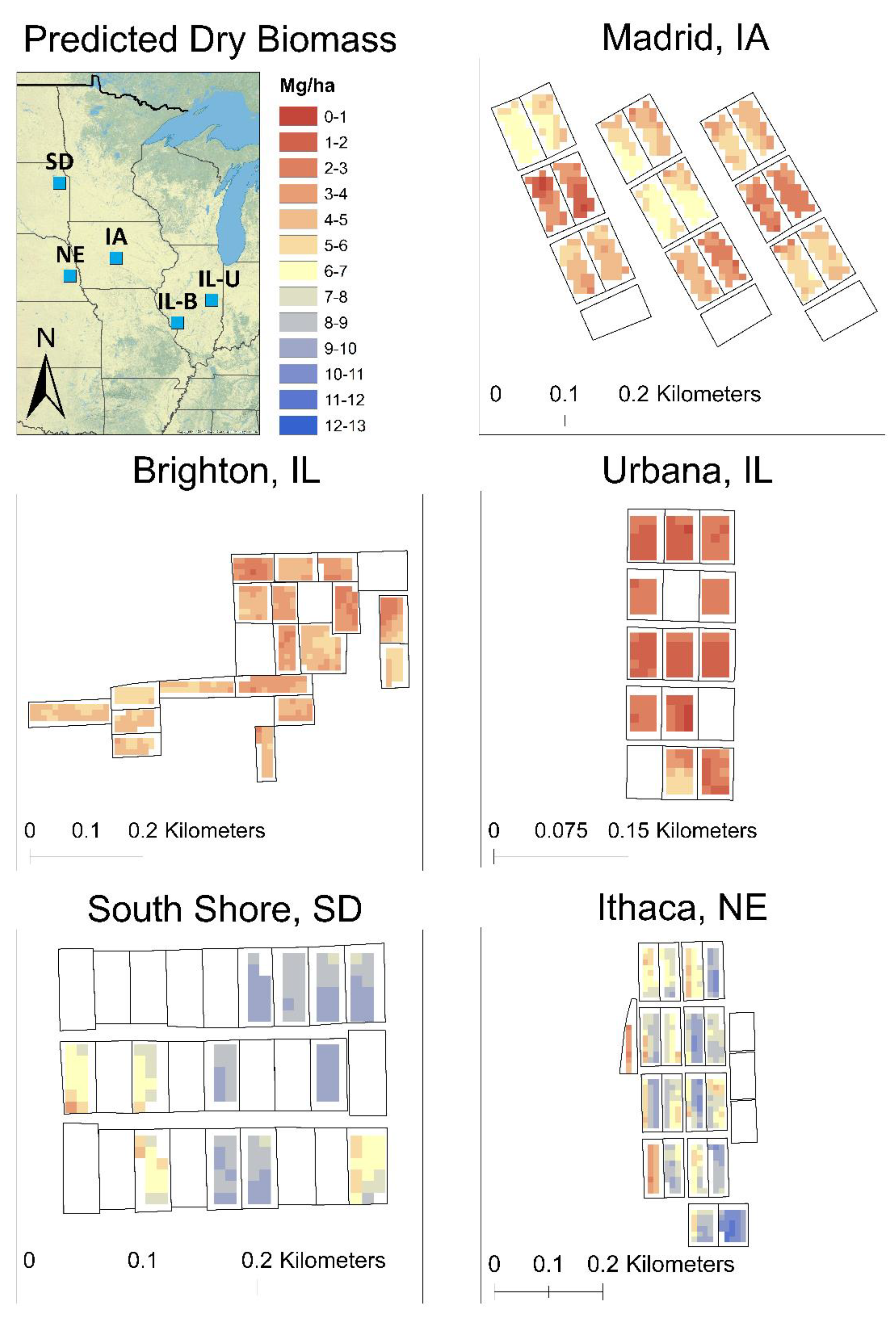
| Site | Coordinates | Field Size (Plot Size) (ha) | Rainfall + Snow Melt * (mm) | Cropping History | Planting Date | Harvest Date |
|---|---|---|---|---|---|---|
| Brighton, IL | 39°3′23.23″ N, 90°11′7.62″ W | 8.5 (0.4) | 1158 € | Corn/soybean rotation | 28 May 2019 | 9 December 2020 |
| Urbana, IL | 40°4′7.68″ N, 88°11′26.78″ W | 6.1 (0.2) | 731 | Perennial grass plots Soybean (2018) Corn (2019) | 30 May 2020 | 7 December 2020 |
| Madrid, IA | 41°55′52.17″ N, 93°45′49.28″ W | 8.5 (0.4) | 730 Ꞩ | Corn/soybean rotation | 13 June 2019 | 20 November 2020 |
| Ithaca, NE | 41°8′57.54″ N, 96°27′14.07″ W | 8.9 (0.4) | 467 £ | Corn/soybean rotation | 14 June 2019 (‘Independence’) 18 April 2012 | 16 November 2020 |
| South Shore, SD | 45°6′20.30″ N, 97°3′42.41″ W | 3.6 (0.2) | 533 ꞎ | Wheat/soybean rotation | (all other grasses) 3 June 2019 | 19 November 2020 |
| Site | Plot Count | Yield (Mg/ha) | ||
|---|---|---|---|---|
| Min., Max. (Range) | Mean | Standard Deviation | ||
| Brighton, IL | 18 | 2.27, 5.75 (3.48) | 4.20 | 1.45 |
| Urbana, IL | 12 | 1.19, 4.35 (3.16) | 2.24 | 0.82 |
| Madrid, IA | 18 | 2.45, 7.01 (4.56) | 4.62 | 1.86 |
| Ithaca, NE | 19 1 | 4.14, 11.1 (6.96) | 7.75 | 1.67 |
| South Shore, SD | 12 | 5.74, 10.4 (4.66) | 8.16 | 1.59 |
| Site | April | May | June | July | August | September | October | Total |
|---|---|---|---|---|---|---|---|---|
| Brighton, IL | 8, 18 | 23 | 2, 7, 12, 17 | no image | 6, 16 | 5, 20, 25 | no image | 12 |
| Urbana, IL | 5, 10, 20 | no image | 14 | 14, 24, 29 | 8, 18, 23 | no image | 7 | 11 |
| Madrid, IA | 1, 12 | 6 | 6, 15, 25 | 10 | 19 | 3, 13, 18 | no image | 11 |
| Ithaca, NE | 9, 19 | 19 | 3, 8, 13 | 8, 13, 18 | 17, 27 | 9, 16 | 6 | 14 |
| South Shore, SD | 7, 22 | 2, 27 | 1, 11, 16 | 11, 16 | 10, 25 | 4, 14 | no image | 13 |
| Index | Formula | Source |
|---|---|---|
| Atmospherically resistant vegetation index (ARVI) | [NIR − (R − B)]/[NIR + (R − B)] | [51] |
| Difference vegetation index (DVI) | NIR − R | [52] |
| Enhanced vegetation index (EVI) | 2.5 × [(NIR − R)/(NIR + 6 × R − 7.5 × B + 1)] | [53] |
| Enhanced vegetation index 2 (EVI2) | 2.5 × [(NIR − R)/(NIR + 2.4 × R + 1)] | [54] |
| Green atmospherically resistant index (GARI) | {NIR − [G − 1.7 × (B − R)]}/{NIR + [G − 1.7 × (B − R)]} | [55] |
| Green chlorophyll index (GCI) | NIR/G − 1 | [56] |
| Green difference vegetation index (GDVI) | NIR − G | [57] |
| Green normalized difference vegetation index (GNDVI) | (NIR − G)/(NIR + G) | [58,59] |
| Green red ratio vegetation index (GRVI) | G/R | [60] |
| Infrared percentage vegetation index (IPVI) | NIR/(NIR + R) | [61] |
| Modified non-linear index (MNLI) | [(NIR2 − R) × (1 + 0.5)]/(NIR2 + R + 0.5) | [62] |
| Modified soil-adjusted vegetation index (MSAVI) | {2 × NIR + 1 − sqrt [(2 × NIR + 1)2 − 8 × (NIR − R)]}/2 | [63] |
| Modified simple ratio (MSR) | [(NIR/R) − 1]/{[sqrt(NIR/R)] + 1} | [64] |
| Normalized difference vegetation index (NDVI) | (NIR − R)/(NIR + R) | [65] |
| Optimized soil-adjusted vegetation index (OSAVI) | (NIR − R)/(NIR + R + 0.16) | [66] |
| Renormalized difference vegetation index (RDVI) | (NIR − R)/[sqrt(NIR + R)] | [67] |
| Soil-adjusted vegetation index (SAVI) | (1 + 0.5) × [(NIR − R)/(NIR + R + 0.5)] | [68] |
| Simple ratio (SR) | NIR/R | [69] |
| Visible atmospherically resistant index (VARI) | (G − R)/(G + R − B) | [70] |
| Wide dynamic range vegetation index (WDRVI) | (0.2 × NIR − R)/(0.2 × NIR + R) | [71] |
| Spectral Index | Spectral Band Used in Index | Brighton, IL | Urbana, IL | Madrid, IA | Ithaca, NE | South Shore, SD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | G | R | NIR | DOY | R2 | DOY | R2 | DOY | R2 | DOY | R2 | DOY | R2 | |
| ARVI | x | x | x | 154 | 0.660 | 281 | 0.559 | 177 | 0.713 | 230 | 0.718 | 248 | 0.813 | |
| EVI | x | x | x | 154 | 0.640 | 281 | 0.633 | 177 | 0.715 | 230 | 0.728 | 248 | 0.839 | |
| EVI2 | x | x | 154 | 0.624 | 281 | 0.706 | 177 | 0.728 | 165 | 0.810 | 223 | 0.802 | ||
| GARI | x | x | x | x | 154 | 0.614 | 281 | 0.696 | 177 | 0.649 | 165 | 0.840 | 193 | 0.909 |
| GCI | x | x | 169 | 0.515 | 281 | 0.828 | 192 | 0.739 | 165 | 0.884 | 193 | 0.878 | ||
| GDVI | x | x | 229 | 0.451 | 281 | 0.626 | 192 | 0.636 | 165 | 0.869 | 208 | 0.932 | ||
| GLI | x | x | x | 154 | 0.622 | 96 | 0.614 | 177 | 0.648 | 165 | 0.576 | 238 | 0.428 | |
| GNDVI | x | x | 169 | 0.605 | 281 | 0.842 | 177 | 0.776 | 165 | 0.873 | 193 | 0.857 | ||
| GRRVI | x | x | 154 | 0.553 | 96 | 0.614 | 177 | 0.620 | 165 | 0.638 | 238 | 0.628 | ||
| IPVI | x | x | 154 | 0.643 | 281 | 0.681 | 177 | 0.737 | 165 | 0.803 | 223 | 0.800 | ||
| MNLI | x | x | 154 | 0.637 | 281 | 0.659 | 177 | 0.725 | 195 | 0.772 | 208 | 0.887 | ||
| MSAVI | x | x | 154 | 0.661 | 281 | 0.647 | 177 | 0.738 | 165 | 0.795 | 223 | 0.797 | ||
| MSR | x | x | 154 | 0.532 | 281 | 0.743 | 262 | 0.070 | 165 | 0.827 | 248 | 0.820 | ||
| NDVI | x | x | 154 | 0.643 | 281 | 0.681 | 177 | 0.731 | 165 | 0.803 | 223 | 0.800 | ||
| OSAVI | x | x | 154 | 0.643 | 281 | 0.681 | 177 | 0.737 | 165 | 0.803 | 223 | 0.800 | ||
| RDVI | x | x | 154 | 0.527 | 281 | 0.716 | 177 | 0.680 | 165 | 0.841 | 208 | 0.910 | ||
| SAVI | x | x | 154 | 0.643 | 281 | 0.681 | 177 | 0.737 | 165 | 0.803 | 223 | 0.800 | ||
| SR | x | x | 154 | 0.484 | 281 | 0.752 | 177 | 0.648 | 165 | 0.826 | 248 | 0.829 | ||
| VARI | x | x | x | 154 | 0.593 | 96 | 0.617 | 177 | 0.685 | 165 | 0.641 | 238 | 0.639 | |
| WDRVI | x | x | 154 | 0.597 | 281 | 0.727 | 177 | 0.723 | 165 | 0.818 | 223 | 0.805 | ||
| Site | Image Date (DOY) | R2 | MAE | RMSE |
|---|---|---|---|---|
| Brighton, IL | 169 | 0.592 | 0.589 | 0.685 |
| Urbana, IL | 281 | 0.694 | 0.377 | 0.399 |
| Madrid, IA | 177 | 0.835 | 0.555 | 0.645 |
| Ithaca, NE | 165 | 0.870 | 0.539 | 0.616 |
| South Shore, SD | 193 | 0.879 | 0.579 | 0.653 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamada, Y.; Zumpf, C.R.; Cacho, J.F.; Lee, D.; Lin, C.-H.; Boe, A.; Heaton, E.; Mitchell, R.; Negri, M.C. Remote Sensing-Based Estimation of Advanced Perennial Grass Biomass Yields for Bioenergy. Land 2021, 10, 1221. https://doi.org/10.3390/land10111221
Hamada Y, Zumpf CR, Cacho JF, Lee D, Lin C-H, Boe A, Heaton E, Mitchell R, Negri MC. Remote Sensing-Based Estimation of Advanced Perennial Grass Biomass Yields for Bioenergy. Land. 2021; 10(11):1221. https://doi.org/10.3390/land10111221
Chicago/Turabian StyleHamada, Yuki, Colleen R. Zumpf, Jules F. Cacho, DoKyoung Lee, Cheng-Hsien Lin, Arvid Boe, Emily Heaton, Robert Mitchell, and Maria Cristina Negri. 2021. "Remote Sensing-Based Estimation of Advanced Perennial Grass Biomass Yields for Bioenergy" Land 10, no. 11: 1221. https://doi.org/10.3390/land10111221
APA StyleHamada, Y., Zumpf, C. R., Cacho, J. F., Lee, D., Lin, C.-H., Boe, A., Heaton, E., Mitchell, R., & Negri, M. C. (2021). Remote Sensing-Based Estimation of Advanced Perennial Grass Biomass Yields for Bioenergy. Land, 10(11), 1221. https://doi.org/10.3390/land10111221






