Abstract
Tree-of-heaven (Ailanthus altissima) is one of the most dangerous and widespread invasive woody plant species in Europe. Despite the fact that A. altissima is in the focus of an increasing number of research projects, the impact of its mass spread on native vegetation, its diversity, and changes in soil quality are still incomplete. The current study addresses the effects of this invasive species on plant diversity and soil parameters simultaneously. The main objective of our research is to determine the impact of cover and mass of A. altissima on the diversity of each forest layer; the examined soil parameters and on other selected environmental variables. For botanical and pedological investigations we selected nine A. altissima-dominated sites in Central Europe, in the Pannonian Biogeographical Region. Based on our results, it can be stated that fully grown A. altissima-dominated stands can displace other taxa by their shading and allelopathy, thereby reducing canopy layer diversity. The increase in the species richness of the shrub layer had a positive correlation with the diversity of the floor layer and also with the humus and ammonia content of the soil. As the diversity of shrub layer and floor layer positively correlated with many soil parameters, the diverse vegetation of these layers can represent a potential opportunity for the regeneration of areas infected with A. altissima.
1. Introduction
Ailanthus altissima (Mill.) Swingle is currently one of the most dangerous invasive woody plant species in Europe [1,2,3] and is also included in the list of invasive alien species of European Union concern [4]. This species is native in Northeast and Central China, as well as Korea, but its synanthropic area currently includes temperate and Mediterranean climates on five continents [5]. The species often spreads from populated areas along roads [1].
With its appearance, aggressive spread, and allelopathic properties, A. altissima displaces the potential species of the given habitat, thus affecting the composition of the vegetation, but also its diversity. Therefore, it causes increasing ecological damage in both protected and non-protected areas, as has been confirmed by several studies [6,7,8,9,10]. A Central European study showed that in A. altissima stands disturbance tolerant species, generalists, and weed species are common, but even alien competitors (according to Borhidi’s social behaviour types of plant species) also appear [11,12].
Despite the fact that an increasing number of research projects focus on the allelopathic effect of A. altissima, only a few studies have examined the effects of Ailanthus-dominated stands on the floral diversity and soil properties.
Motard et al. [13] studied the effects of A. altissima invasion in the Fontainebleau Forest near Paris, France. Vegetation under this species was found to be significantly poorer and composed of more common species than under other tree species, and the composition was significantly different. As the number of A. altissima root shoots increased, the plant species richness decreased significantly. This effect is due to the strong competitive abilities and the allelopathic compounds it allocates to the soil. In their study of the Mediterranean region, Vilà et al. [14] found that A. altissima reduced the level of diversity of native plant species, while Traveset et al. [15] found that total plant species richness did not change. Constán-Nava et al. [16] examined the direct and indirect effects of the studied species invasion on ecosystem functioning in coastal, Mediterranean ecosystems. Flora characteristics (species richness, philodiversity), as well as several indicators of ecosystem functioning (undergrowth plant biomass, soil enzyme activity, available phosphorus, and organic matter) in A. altissima-infected and control areas, were examined. Based on their results, the presence of A. altissima was associated with lower plant species richness, phylodiversity, and multifunctionality. After analysing each function separately, it was found that biodiversity has the opposite effect of invasion on all measured functions, thus reducing the strength (either positive or negative) of the effect of A. altissima on them.
Mount [17] also examined the consequences of the invasion of A. altissima in an old-growth forest in Southeastern Kentucky, USA. Despite the fact that no significant correlations were found due to the presumably small number of samples, her results suggest that the species composition of floor layer changes after an invasion in open areas, while woody vegetation is less sensitive to the invasion of A. altissima.
The effects of A. altissima invasion were also studied by Brooks et al. [18] in Virginia, USA, in stands of different ages at infected and non-infected test points. On-site herbaceous- and woody-stemmed plant species were recorded, and soil samples were collected, the latter of which were then germinated in a greenhouse, and those grown species were also identified. They found an association with the invasion of A. altissima and the decrease in the number of individuals and species of native plants with respect to forest vegetation but not with respect to the seed bank. In the presence of A. altissima, diversity of non-native woody species increased; additionally, the impact on the nativity of the woody understory became more extreme over time.
Examining the seed bank of the invaded areas, Gioria et al. [19] found these to be poorer in native species and their densities and richer in non-native species and their abundance. The negative effects of A. altissima on undergrowth and seed banks may become more severe over time [20].
Castro-Díez et al. [21] examined the nitrogen enrichment effect of falling foliage under two invasive species (A. altissima and Robinia pseudoacacia) and two native species (Ulmus minor and Fraxinus angustifolia) in Central Spain. The foliage of Ailanthus was found to degrade faster than that of U. minor, and the release of N per unit litter mass was higher under Ailanthus. However, soils collected under native and invasive trees did not differ in either the potential N mineralisation rate or the N mineral, as the released nitrogen was rapidly taken up by the vegetation and/or large amounts of organic matter accumulated in the soil prior to the invasion.
Montecchiari et al. [22] studied sub-Mediterranean and Mediterranean forest communities dominated by A. altissima in central Italy with different habitat conditions to shed light on the possible floristic vegetation autonomy of these communities. Based on their results, two new communities were distinguished: (1) a forest community with a stratified structure and high canopy density on the warmer slopes of hills, dry soil conditions, and low anthropogenic disturbance, and (2) forest communities with a monolayer structure of few species, typically close to agricultural and inhabited areas, on clayey, silty–sandy loam soils, soil moisture, and high anthropogenic disturbance. Montecchiari et al. [23] also highlighted the principal floristic and environmental differences between A. altissima forests vegetation and native forests typical of the hilly landscape of sub-Mediterranean bioclimate in Southern Europe (Italy). They found that floristic diversity (richness) was higher and annual ruderal plants occurred in higher proportions in A. altissima forests, while typical forest herbaceous plants were present in smaller numbers in comparison to the native forests. For topsoil parameters, they found lower total nitrogen and carbon and C/N ratio but no significant difference in soil pH in A. altissima plots. Their results suggest that this invasive species is capable of altering some soil properties.
Motard et al. [24] studied how the invasion of A. altissima transforms soil and litter communities in temperate forest ecosystems. Their study shows that increasing density of A. altissima is associated with lower soil microbial activity, a decrease in the number of degraders (Acari and Collembola) and above-ground predatory Coleoptera, and a decrease in the species richness of the terrestrial Gastropoda class, but resulted in higher abundance of Lumbricidae underground and above-ground coprophage Coleoptera. It has been found that invasion of A. altissima has an effect on the structure of the soil food chain, may accelerate the mineralisation of organic matter, and potentially favour nitrophilous plant species.
In our research, we sought answers to the following questions: (1) how similar are the study sites based on floristic composition? (2) to what extent did A. altissima become widespread in different layers of the studied stands? (3) to what extent did the cover of A. altissima at different layers determine the diversity of each layer, the studied soil parameters, and other environmental variables?
2. Materials and Methods
2.1. Study Sites
Based on the recommendation of foresters and conservationists, 9 heavily infected study sites by A. altissima with different characteristics and a minimum of 70% A. altissima total cover were selected in Hungary (Pannonian Region): Bócsa (B), Fóti-Somlyó (F), Galgahévíz (G), Gyermely (GY), Isaszeg (I), Makád (M), Tök (T), Tököl (TL), and Várvölgy (V) (Figure 1, Table 1). Sites close to the roads were previously excluded in order to avoid the effect of this kind of disturbance on our results.
Figure 1.
Location of the study sites.

Table 1.
Basic data of the study sites (sources [25,26,27]).
2.2. Botanical Sampling
The botanical survey was conducted in the summer of 2016 and 2017, in June and July. A total of 50 quadrats were marked out for investigation, and a minimum of 3 quadrats was recorded in each study site. After selecting the quadrats, the corners of the typically square shaped, 10 × 10 metre-sized quadrats were visibly marked with a colourful marker, and then the GPS coordinates of their centre were recorded. For the latter, Garmin eTrex 20x (Xizhi Dist., New Taipei City, Taiwan) was used. Thereafter, in each quadrat in each layer (canopy, shrub layer, and floor), the percentage cover was visually estimated for each vascular plant species.
2.3. Measurements of Biotic and Abiotic (Soil and Other Environmental Parameters) Data
Exposure, slope angle, altitude, the height of A. altissima stands, mean and maximum diameter (at 130 cm height) of A. altissima trees, total coverage (%) of vegetation, and coverage (%) of each present species on different layers were recorded for each quadrat. The tree height was determined using a triangulation method and a laser altimeter (Nikon Forestry Pro, Shinagawa, Tokyo, Japan). The diameter of the trees was measured with a 5 m long tape measure (Ningbo, Yuyao, China) along the diagonals of the quadrats, measuring the diameter of a minimum of 10 individuals.
Soil samples for laboratory analysis were collected in summer 2017. Three samples per site, each of about 100 cm3, were taken randomly within the quadrats from the 0–10 cm layer. The laboratory analysis was carried out at the Department of Agrochemistry of the Hungarian University of Agriculture and Life Sciences. After the mechanical preparation (sieving, chopping) and the drying of the samples, soil pH was measured in a 1:2.5 ratio (w/v) soil: water and 1 M KCl suspension with a digital pH meter, Radelkis OP-211/2 [28]. Soil carbonate content was determined with a Scheibler calcimeter method [28]. To measure the available nitrogen content, we prepared soil extracts by dissolving them with 1 M KCl solution; then, samples were mechanically shaken for 1 h and filtered through 0.45 μm membrane filters. We measured the ammoniacal nitrogen (NH4+-N) and nitrate-nitrogen (NO3−-N) content with a Parnas–Wagner device, using FeSO4 and CuSO4 for NO3 reduction [29]. Available potassium (K2O) and phosphorus (P2O5) were estimated based on the ammonium–lactate solution method (AL method) using flame photometer (Jenway PFP 7, Jenway, UK), respectively [29]. The Arany-type soil texture coefficient (KA) describes the distribution of soil particles. The coefficient was determined by the amount of distilled water added to the air-dried soil sample until it reached plasticity. The organic matter (OM) content was determined by the Tyurin method which is based on the decomposition caused by potassium dichromate, followed by titration with Mohr salt, based on Hungarian standard [30].
2.4. Statistical Analysis
Statistical and visualisation analyses were performed using the Paleontological Statistics (PAST) Version 3.21 and 4.05 [31,32,33] statistical software packages.
To better understand the similarities between the vegetation of different study sites, the data sets were analysed with distance-based classical cluster analysis (unweighted pair-group average (UPGMA)) [34] using Euclidean mean distance.
To provide an overview of the distribution of the A. altissima between each area and layer, a radar chart was created with polar grid type and fine grid density, and the areas were separated by a red line for better transparency.
The diversity was also examined with the diversity module of the PAST, specifically for the most commonly used Shannon and Simpson diversity indices, as well as Shannon evenness.
To analyse the correlation between vegetation, environmental, and soil parameters, the univariate menu’s correlation module and linear r (Pearson) correlation method of PAST was used, the results given with r statistic (degrees of freedom) and p value (significance).
3. Results
3.1. Composition of Vegetation and Similarity of Study Sites
Based on the distance-based classification analysis, the recorded stands fall into two large groups (Figure 2). The smaller group on the right side of the figure (box A–G, GY, T) was separated from the vegetation of the other study sites due to the characteristic and/or massive occurrence of plant species (Acer negundo in the canopy layer; Acer negundo, Cornus sanguinea, and Ligustrum vulgare in the shrub layer; nitrophilous Chaerophyllum temulum, and Urtica dioica in the floor layer). The group which contained stands from several study sites (box B) owed its isolation to A. altissima. Among the study sites of the larger group, the Tököl (TL) area was separated from the other study sites by Populus canadensis, which predominated at the uppermost canopy layer, while the Isaszeg (I) site was separated from the other sites by the prevalence of nitrophilous Bromus sterilis.
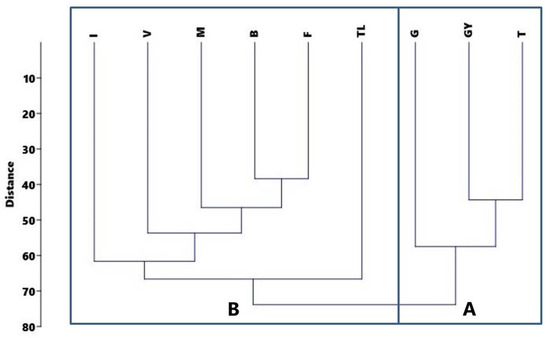
Figure 2.
UPGMA analysis of the study sites’ vegetation: boxes—main distinct groups.
3.2. Mass of A. altissima at Different Layers of the Studied Forest Sites
Typically, the cover of A. altissima was most massive in the canopy layer of the studied stands (Figure 3). In the canopy layer, its average cover was between 38.33% (TL) and 104.60% (M). In the shrub layer, the cover of the species was higher than in the floor layer in most sites. In one of the sites (V), invasive species had a massive appearance in the canopy layer as well as in the shrub and floor layer. The lowest cover of A. altissima was 3.17% (G), and the highest was 66.13% (TL) in the shrub layer, while in the floor layer, these variables were 2.00% (M) and 23.00% (V).
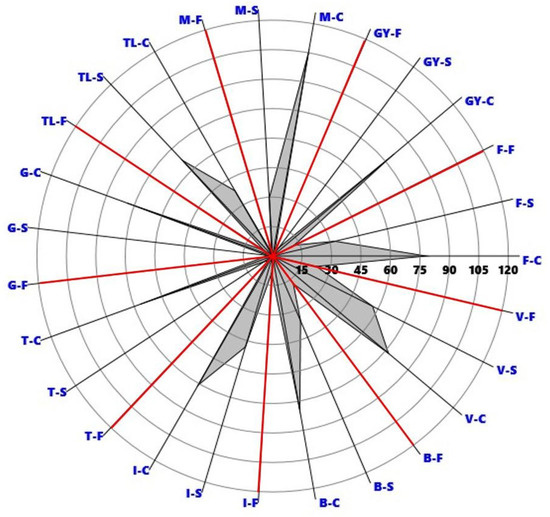
Figure 3.
Polar diagram of Ailanthus altissima cover % per study site and forest layer: C—canopy layer; S—shrub layer; F—forest floor layer; for the abbreviations of the study sites see Figure 1.
3.3. The Diversity of Each Layer Depending on the Mass of A. altissima
Shannon’s and Simpson’s diversity values were lowest in the canopy layer and highest in the floor layer (Figure 4). In two stands, the A. altissima was monodominant (I and V) at the canopy layer, so their diversity cannot be interpreted. In the stands with also very low canopy diversity, a tree species (Celtis occidentalis (F), Robinia pseudoacacia (F), and B) is present with low cover. Shannon’s diversity values were lower than 1.02 in the canopy layer, 0.49 to 2.00 in the shrub layer, and 1.65 to 2.84 in the floor layer. Simpson’s diversity values were below 0.63 in the canopy layer, 0.22 to 0.84 in the shrub layer, and 0.65 to 0.90 in the floor layer.
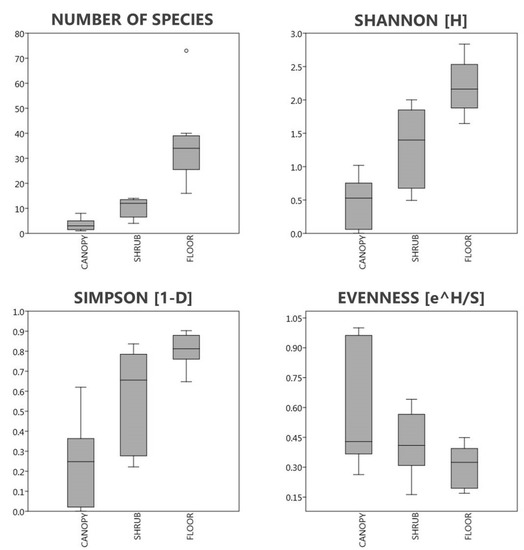
Figure 4.
Number of species, Shannon’s and Simpson’s diversity values, and Shannon’s evenness of the vegetation layers.
3.4. Correlation between the Examined Biotic and Abiotic Variables
Regarding the dendrology data of the stands and the environmental factors (Figure 5) we found positive correlation slope angle with mean diameter (r = 0.6691, p = 0.0487), slope angle with ground species richness (r = 0.6840, p = 0.0422), MASL with humus (r = 0.7337, p = 0.0244), stand height with mean diameter (r = 0.7721, p = 0.0148), and stand height with max diameter (r = 0.7368, p = 0.0235). In addition, a very strong positive correlation between the mean and max diameters (r = 0.9265, p = 0.0003) was found. Positive correlations between the studied soil parameters were also found as follows: total soil N with humus (r = 0.6725, p = 0.0472); soil CaCO3 with humus (r = 0.7376, p = 0.0233); soil KA with humus (r = 0.7646, p = 0.0164); total soil N with soil KA (r = 0.9458, p = 0.0001); soil pH KCl with soil pH H2O (r = 0.9881, p = 0.0000006).
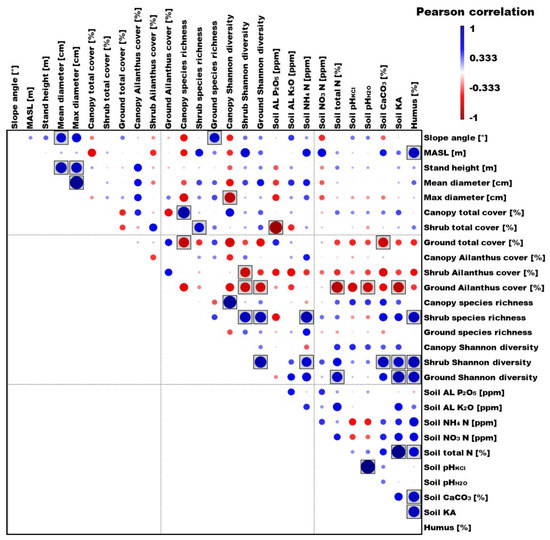
Figure 5.
Linear (Pearson) correlation of the examined biotic and abiotic factors: blue—circle size proportionally scaled; red—positive correlation; boxed—negative correlation; p < 0.005 significance.
Regarding the correlation between different vegetation indicators and environmental parameters, two major groups of results were separated: plant cover (especially A. altissima cover) and species richness diversity. We found positive correlation for shrub total cover with shrub species richness (r = 0.6667, p = 0.0499) and an even stronger correlation between the canopy total cover and the canopy species richness (r = 0.8285, p = 0.0058). In the case of ground total cover, we found a significant negative correlation with canopy species richness (r = −0.7183, p = 0.0293) and soil CaCO3 (r = −0.6888, p = 0.0402), while in the case of shrub total cover, a stronger negative correlation was found with soil AL P2O5 (r = −0.8814, p = 0.0017). The shrub cover characteristic of A. altissima negatively correlated with the Shannon diversity of the shrub layer (r = −0.6895, p = 0.0399). The ground cover of A. altissima significantly and negatively correlated with the following parameters: shrub Shannon diversity (r = −0.6801, p = 0.0438); ground Shannon diversity (r = −0.6723, p = 0.0473); total soil N (r = −0.7861, p = 0.0120); soil pH H2O (r = −0.6844, p = 0.0420); soil KA (r = −0.7353, p = 0.0240).
Examining species richness, the results show that there was a positive significant correlation for canopy species richness with canopy Shannon diversity (r = 0.8960, p = 0.0011). Interestingly, the canopy species richness had a significant, negative correlation with the maximum diameter (r = −0.7443, p = 0.0215). Shrub species richness was positively correlated with shrub Shannon diversity (r = 0.7491, p = 0.0202); ground Shannon diversity (r = 0.7639, p = 0.0166); humus (r = 0.7534, p = 0.0191), while soil NH4N showed an even stronger significant correlation (r = 0.8028, p = 0.0092) with it. The Shannon diversity of the shrub layer also showed positive correlation with soil NH4N (r = 0.6859, p = 0.0414); CaCO3 (r = 0.7493, p = 0.0201); KA (r = 0.7036, p = 0.0344); humus (r = 0.7964, p = 0.0102) parameters; ground Shannon diversity had a significant correlation with total soil N (r = 0.7179, p = 0.0294) and humus (r = 0.7618, p = 0.0171) parameters, while soil KA had even more strongly significant correlation (r = 0.8075, p = 0.0085), as did shrub Shannon diversity with ground Shannon diversity (r = 0.8150, p = 0.0074).
4. Discussion
Based on the evaluation of the study sites, the role of A. altissima was very significant in all stands; therefore, its appearance can have invasive and serious negative effects on diversity. Based on the mass conditions of A. altissima observed in the canopy layer (Figure 2), it can be assumed that the A. altissima trees in the floor layer and shrub layer already came from the internal propagation pool of the stand. Stands in which the trunk diameter of the species had the largest diameter at the canopy layer were presumably colonised with this invasive species at the earliest. Nitrophilous species, which were abundant in places in the floor layer, confirmed the results of Motard et al. [24] and Montecchiari et al. [22,23], i.e., a massive proliferation of A. altissima in an area could potentially benefit nitrophilous plant species in the understory; however, this may be related to soil parameters in our case (Table 1).
The canopy layer had the highest A. altissima cover, while the shrub layer had smaller, and the floor layer had the smallest A. altissima cover; the Shannon and Simpson diversity values varied inversely with the species cover values. Thus, the values of the two diversity indexes studied were higher where the mass of the A. altissima was low, while they were lower where the presence of A. altissima proved to be more severe. This confirms the results of Vilà et al. [14], Motard et al. [13], and Constán-Nava et al. [16] about the negative impact of the studied species on diversity.
The larger diameter of the A. altissima trunks and the smaller canopy Shannon diversity may indicate, on the one hand, that the presence of A. altissima may displace tree species from the stand in the long run; on the other hand, it also presumably indicates older stands. The former is consistent with the result of Brooks et al. [18] but contrasts with the North American observation of Mount [17].
Our results related to vegetation and environmental parameters show that the full cover of both shrub and canopy layer had a positive correlation with the species richness of the given layer. The increase in the species richness of the shrub layer had a positive correlation with the diversity of the floor layer and also increased the humus and ammonia content of the soil through more intensive root formation and significant foliage formation. The significant negative correlation of ground total cover with canopy species richness and soil CaCO3 may indicate that the number of species in the canopy layer can seriously affect the total floor layer cover, and floor layer condition may have a strong effect on soil cover. The mass presence of A. altissima in the floor layer negatively correlated with the diversity of the floor layer and shrub layer but also correlated with a number of soil parameters in an unfavourable direction, including soil pH and total nitrogen content. Our results confirm the observation of Motard et al. [13]. The significant changes obtained for the soil parameters are in contrast with the perception of Castro-Díez et al. [21], who draw attention to the inhibitory effect of secondary metabolites on soil N enrichment in the Pannonian habitats under study. Based on the examined correlations, it can be stated that the condition of both the shrub and floor layers, as well as their Shannon diversity values, had a positive correlation with many soil parameters, including humus and nitrogen contents of the soil, while they were also relatively strongly correlated with each other.
5. Conclusions
Based on our results, it appears that older A. altissima-dominated stands may displace other taxa by their shading and allelopathy, thereby reducing canopy layer diversity. The species richness of the shrub layer might have influenced certain soil parameters and is related to the Shannon diversity of the floor layer. As A. altissima becomes more dominant in the floor layer, it probably affects the plant diversity of all three layers and changes many parameters of the soil cover in a negative direction, making it difficult to manage the area later and to regenerate it. As the diversity of shrub and floor layers positively correlated with many soil parameters, the diverse vegetation of these layers can represent a potential opportunity for the regeneration of areas which are infected with A. altissima.
Due to the high level of A. altissima propagule pressure observed, the management of the stands requires special care, as the studied species is extremely invasive and can also form monodominant stands. In the case of this type of invasive tree species, the opening of larger gaps and clear cutting should be avoided. Based on our research, it can be assumed that more diverse shrub and floor layers result in soil enrichment. In contrast, with the increasing dominance of the A. altissima, the soil of the forest stand is expected to be improved.
Author Contributions
Conceptualization, E.T.K. and S.C.; methodology, S.C.; software, D.S.; formal analysis, D.S.; investigation, A.D.; data curation, A.D.; writing—original draft preparation, A.D., D.S., E.T.K., O.S., S.M., K.R., K.V.N., B.S., Z.S. and S.C.; writing—review and editing, A.D., D.S., E.T.K., P.T. and S.C.; visualization, D.S., O.S., S.M., K.R., K.V.N., B.S. and Z.S.; supervision, S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Acknowledgments
The research was supported by the Doctoral School of Environmental Sciences of the Hungarian University of Agriculture and Life Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kowarik, I.; Säumel, I. Biological flora of Central Europe: Ailanthus altissima (Mill.) Swingle. Perspect. Plant Ecol. 2007, 8, 207–237. [Google Scholar] [CrossRef]
- Lambdon, P.W.; Pyšek, P.; Basnou, C.; Hejda, M.; Arianoutsou, M.; Essl, F.; Jarošík, V.; Pergl, J.; Winter, M.; Anastasiu, P.; et al. Alien flora of Europe: Species diversity, temporal trends, geographical patterns and research needs. Preslia 2008, 80, 101–149. Available online: http://www.preslia.cz/P082Lam.pdf (accessed on 24 August 2021).
- Sladonja, B.; Sušek, M.; Guillermic, J. Review on Invasive Tree of Heaven (Ailanthus altissima (Mill.) Swingle) Conflicting Values: Assessment of Its Ecosystem Services and Potential Biological Threat. Environ. Manag. 2015, 56, 1009–1034. [Google Scholar] [CrossRef] [PubMed]
- List of Invasive Alien Species of Union Concern. Available online: https://ec.europa.eu/environment/nature/invasivealien/list/index_en.htm (accessed on 9 October 2021).
- Kowarik, I.; Böcker, R. Zur Verbreitung, Vergesellschaftung und Einbürgerung des Götterbaumes (Ailanthus altissima (Mill.) SWINGLE) in Mitteleuropa. Tuexenia 1984, 4, 9–29. [Google Scholar]
- Heisey, R.M. Allelopathic and Herbicidal Effects of Extracts from Tree of Heaven (Ailanthus altissima). Am. J. Bot. 1990, 77, 662–670. [Google Scholar] [CrossRef]
- Heisey, R.M. Evidence for allelopathy by tree-of-heaven (Ailanthus altissima). J. Chem. Ecol. 1990, 16, 2039–2055. [Google Scholar] [CrossRef]
- Gomez-Aparicio, L.; Canham, C.D. Neighbourhood analyses of the allelopathic effects of the invasive tree Ailanthus altissima in temperate forests. J. Ecol. 2008, 96, 447–458. [Google Scholar] [CrossRef] [Green Version]
- Csiszár, Á. Allelopathic effects of invasive woody plant species is Hungary. Acta Silv. Lign. Hung. 2009, 5, 9–17. [Google Scholar]
- Csiszár, Á.; Korda, M.; Schmidt, D.; Šporčić, D.; Süle, P.; Teleki, B.; Tiborcz, V.; Zagyvai, G.; Bartha, D. Allelopathic potential of some invasive neophytes occurring in Hungary. Allelopath. J. 2013, 31, 309–318. [Google Scholar]
- Demeter, A.; Falvai, D.; Trenyik, P.; Czóbel, S. Ecological indicator based comparative study of tree of heaven (Ailanthus altissima) stands’ herb layer. Columella J. Agric. Environ. Sci. 2017, 4, 15–20. [Google Scholar] [CrossRef]
- Borhidi, A. Social behaviour types, the naturalness and relative indicator values of the higher plants in the Hungarian Flora. Acta Bot. Hung. 1995, 39, 97–182. [Google Scholar]
- Motard, E.; Muratet, A.; Clair-Maczulajtys, D.; Machon, N. Does Invasive Species Ailanthus altissima Threaten Floristic Diversity of Temperate Peri-Urban Forests? Comptes Rendus Biol. 2011, 334, 872–879. [Google Scholar] [CrossRef]
- Vilà, M.; Tessier, M.; Suehs, C.M.; Brundu, G.; Carta, L.; Galanidis, A.; Lambdon, P.; Manca, M.; Médail, F.; Moragues, E.; et al. Local and regional assessments of the impacts of plant invaders on vegetation structure and soil properties of Mediterranean islands. J. Biogeogr. 2006, 33, 853–861. [Google Scholar] [CrossRef]
- Traveset, A.; Brundu, G.; Carta, L.; Mprezetou, I.; Lambdon, P.; Manca, M.; Médail, F.; Moragues, E.; Rodríguez-P’erez, J.; Siamantziouras, A.-S.D. Consistent performance of invasive plant species within and among islands of the Mediterranean basin. Biol. Invasions 2008, 10, 847–858. [Google Scholar] [CrossRef]
- Constán-Nava, S.; Soliveres, S.; Torices, R.; Serra, L.; Bonet, A. Direct and indirect effects of invasion by the alien tree Ailanthus altissima on riparian plant communities and ecosystem multifunctionality. Biol. Invasions 2015, 17, 1095–1108. [Google Scholar] [CrossRef]
- Mount, H. Impacts of Invasive Ailanthus altissima on Woody Plant Communities in an Old Growth Forest of Southeastern Kentucky. NSF-Research Experiences for Undergraduates, Disturbance Ecology in Central Appalachia 2019. p. 2. Available online: https://www.eku.edu/ (accessed on 24 August 2021).
- Brooks, R.K.; Barney, J.N.; Salom, S.M. The invasive tree, Ailanthus altissima, impacts understory nativity, not seedbank nativity. For. Ecol. Manag. 2021, 489, 119025. [Google Scholar] [CrossRef]
- Gioria, M.; Jarošík, V.; Pyšek, P. Impact of invasions by alien plants on soil seed bank communities: Emerging patterns. Perspect. Plant Ecol. Evol. Syst. 2014, 16, 132–142. [Google Scholar] [CrossRef]
- Strayer, D.L.; Eviner, V.T.; Jeschke, J.M.; Pace, M.L. Understanding the long-term effects of species invasions. Trends Ecol. Evol. 2006, 21, 645–651. [Google Scholar] [CrossRef]
- Castro-Díez, P.; Fierro-Brunnenmeister, N.; González-Muñoz, N.; Gallardo, A. Effects of exotic and native tree leaf litter on soil properties of two contrasting sites in the Iberian Peninsula. Plant Soil 2012, 350, 179–191. [Google Scholar] [CrossRef]
- Montecchiari, S.; Allegrezza, M.; Peliccia, V.; Tesei, G. First syntaxonomical contribution to the invasive Ailanthus altissima (Mill.) Swingle forest communities at its southern limit in Europe. Plant Sociol. 2020, 57, 145–160. [Google Scholar] [CrossRef]
- Montecchiari, S.; Tesei, G.; Allegrezza, M. Ailanthus altissima Forests Determine a Shift in Herbaceous Layer Richness: A Paired Comparison with Hardwood Native Forests in Sub-Mediterranean Europe. Plants 2020, 9, 1404. [Google Scholar] [CrossRef] [PubMed]
- Motard, E.; Dusz, S.; Geslin, B.; Akpa-Vinceslas, M.; Hignard, C.; Babiar, O.; Clair-Maczulajtys, D.; Michel-Salzat, A. How invasion by Ailanthus altissima transforms soil and litter communities in a temperate forest ecosystem. Biol. Invasions 2015, 17, 1817–1832. [Google Scholar] [CrossRef]
- Gyalog, L.; Síkhegyi, F. (Eds.) Surface Geological Maps of Hungary; Scale = 1:100.000; Hungarian Geological Institute: Budapest, Hungary, 2015. [Google Scholar]
- Bihari, Z.; Babolcsai, G.; Bartholy, J.; Ferenczi, Z.; Gerhátné Kerényi, J.; Haszpra, L.; Homokiné Ujváry, K.; Kovács, T.; Lakatos, M.; Németh, Á.; et al. Climate—Precipitation. In National Atlas of Hungary; Kocsis, K., Ed.; MTA CSFK Geographical Institute: Budapest, Hungary, 2018; Volume 2—Natural Environment, pp. 62–63. [Google Scholar]
- Bihari, Z.; Babolcsai, G.; Bartholy, J.; Ferenczi, Z.; Gerhátné Kerényi, J.; Haszpra, L.; Homokiné Ujváry, K.; Kovács, T.; Lakatos, M.; Németh, Á.; et al. Climate—Temperature. In National Atlas of Hungary; Kocsis, K., Ed.; MTA CSFK Geographical Institute: Budapest, Hungary, 2018; Volume 2—Natural Environment, pp. 60–61. [Google Scholar]
- Buzás, I. (Ed.) Soil and agrochemical test method book 2. In Physico-Chemical and Chemical Test Methods for Soils; Mezőgazdasági Kvk: Budapest, Hungary, 1988; p. 243. (In Hungarian) [Google Scholar]
- Egner, J.; Riehm, H.; Domingo, W. Untersuchungen über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nährst-offzustandes der Böden II. Chemische Extraktionsmethoden zur Phosphor-und Kaliumbestimmung. K. Lantbrukshögskolans Ann. 1960, 26, 199–215. (In German) [Google Scholar]
- MSZ-08-0210-1977; Testing Organic Carbon Content in Soils; Hungarian Standard; Hungarian Standards Institution: Budapest, Hungary, 1977; p. 6. (In Hungarian)
- Hammer, Ø. PAST—PAleontological STatictics Version 3.21 Reference Manual; Natural History Museum, University of Oslo: Oslo, Norway, 1999–2018; p. 225. [Google Scholar]
- Hammer, Ø. PAST—PAleontological STatictics Version 4.05 Reference Manual; Natural History Museum, University of Oslo: Oslo, Norway, 1999–2021; p. 284. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST—Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Harper, D.A.T. (Ed.) Numerical Palaeobiology; John Wiley & Sons: New York, NY, USA, 1999; p. 468. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).