Combining Remote Sensing and Species Distribution Modelling to Assess Pinus hartwegii Response to Climate Change and Land Use from Izta-Popo National Park, Mexico
Abstract
:1. Introduction
2. Materials and Methods
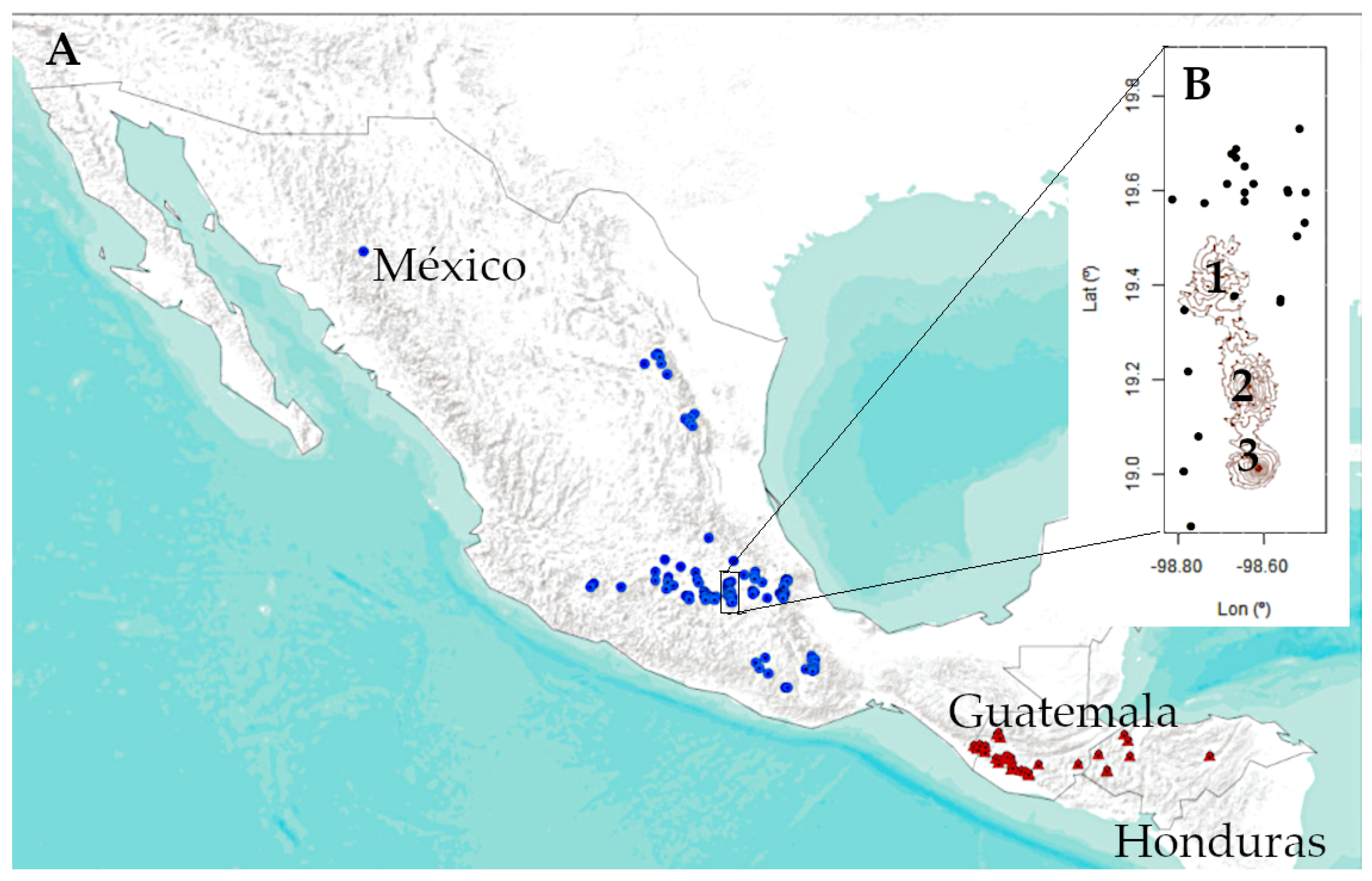
2.1. Species and Study Area
2.2. Remote Sensing-Based Maps
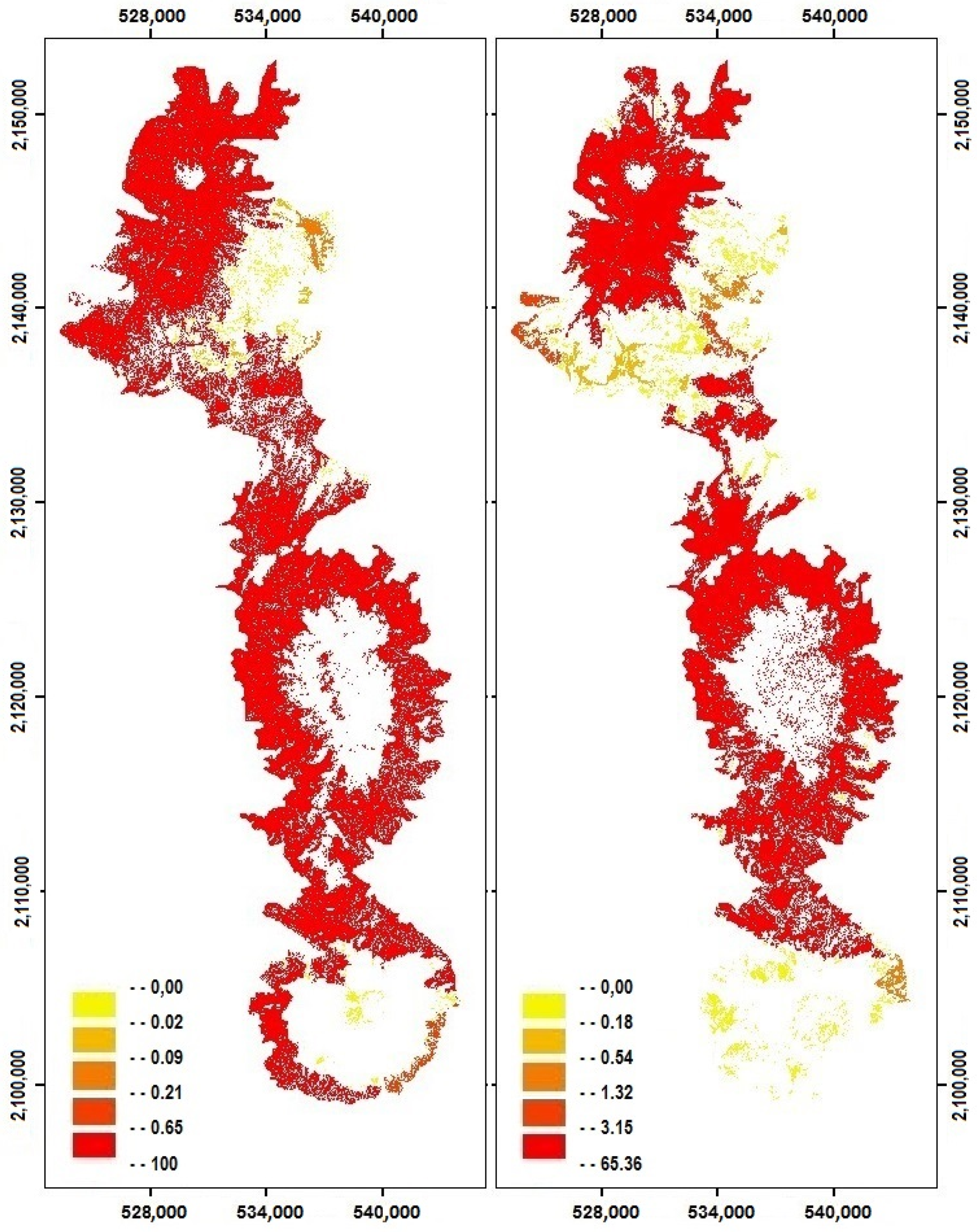
2.3. Changes in Habitat Availability
2.4. Species Distribution Models
2.5. Climate Change Impact on P. hartwegii Distribution
3. Results
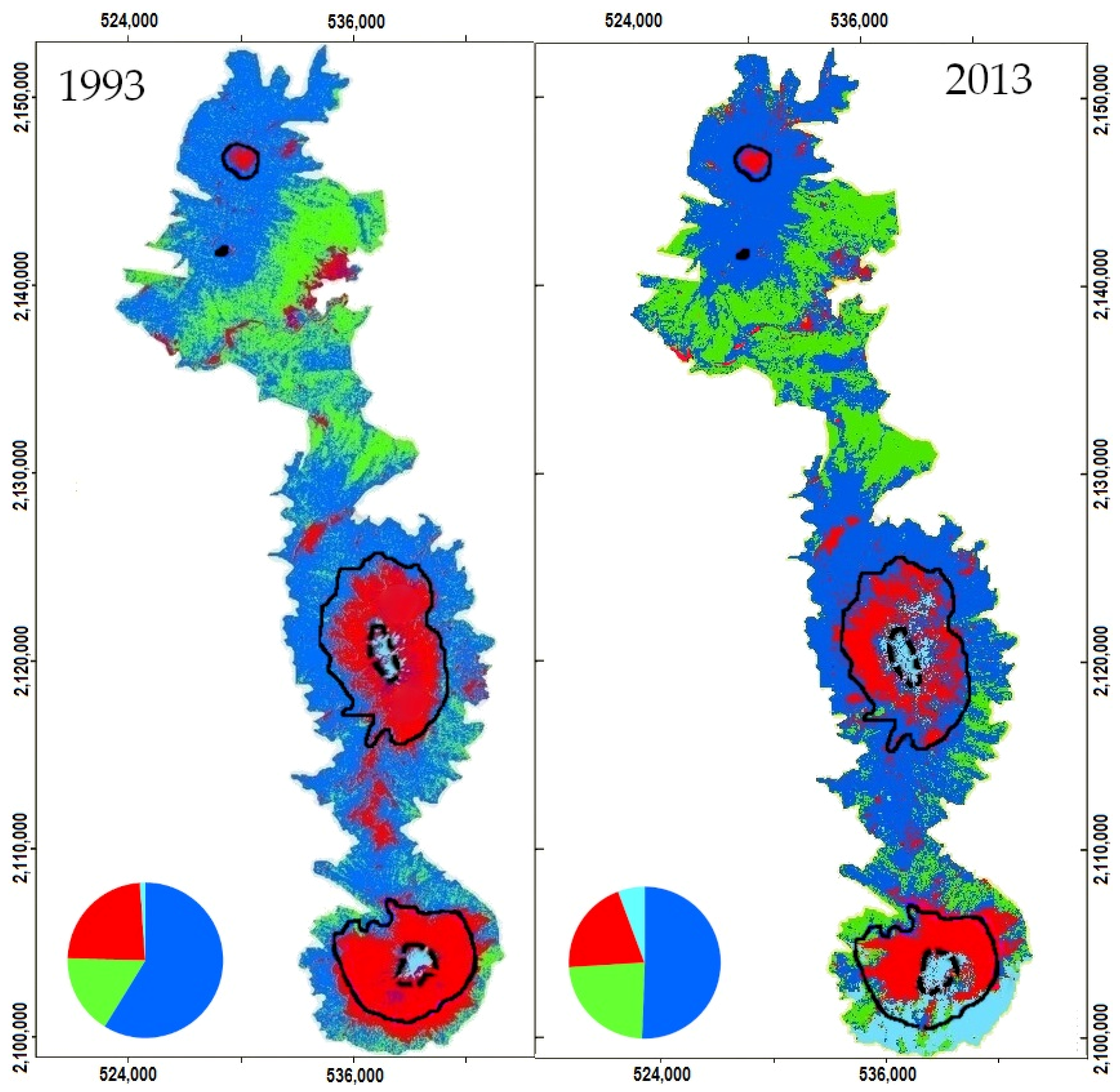
3.1. Remote Sensing Data
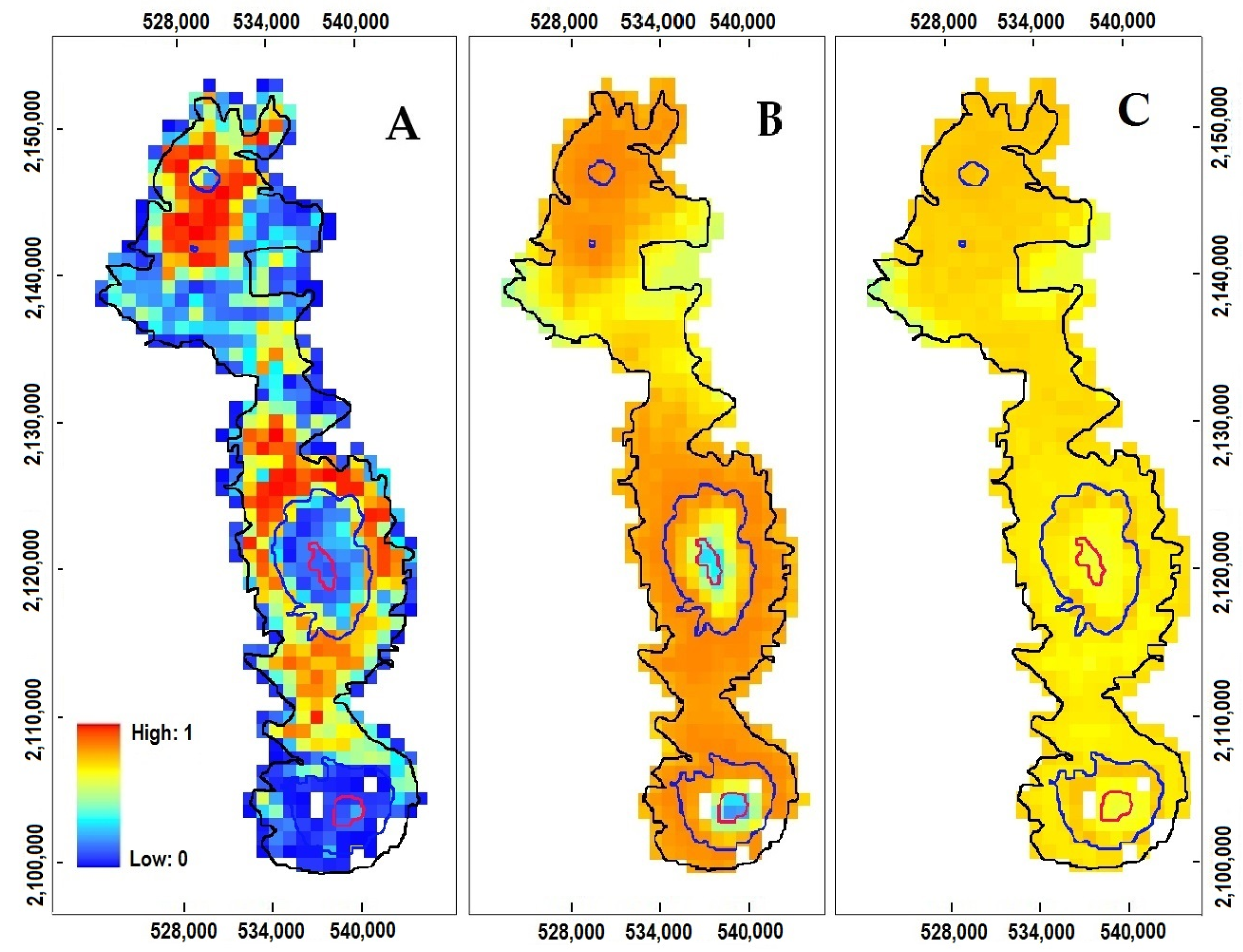
3.2. P. hartwegii Habitat Suitability Models
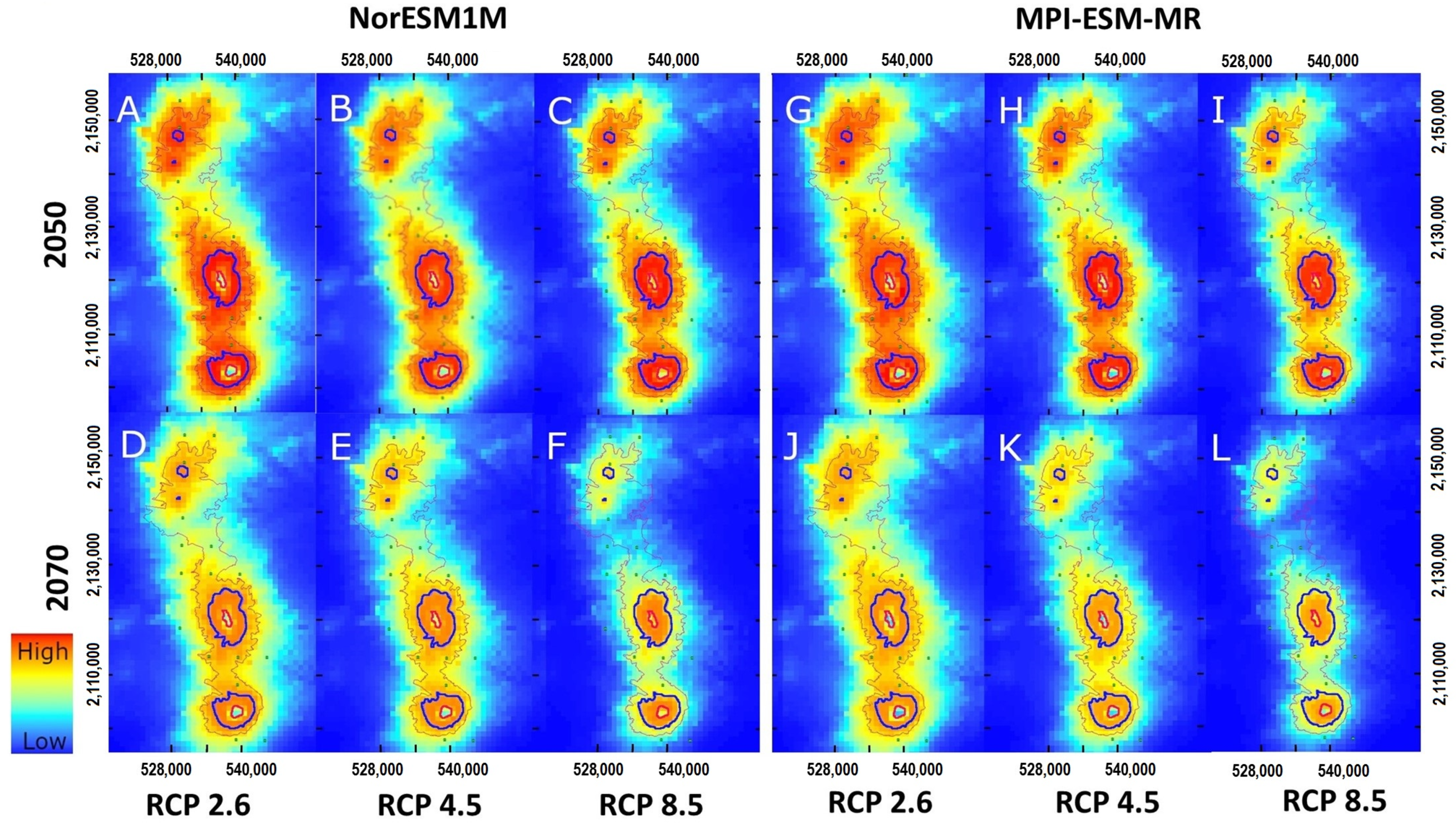
3.3. Projections to Present and Future Climate Conditions
4. Discussion
4.1. The Use of Remote Sensing Data to Validate Species Distribution Models
4.2. Species Distribution Modeling
4.3. Impact of Cover Change on Habitat Availability and Reachability
4.4. The Impact of Climate Change
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shukla, P.; Skea, J.; Calvo Buendia, E.; Masson-Delmotte, V.; Pörtner, H.; Roberts, D.; Zhai, P.; Slade, R.; Connors, S.; Van Diemen, R. IPCC, 2019: Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Report; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2019; pp. 1–864. [Google Scholar]
- Foden, W.B.; Young, B.E.; Akçakaya, H.R.; Garcia, R.A.; Hoffmann, A.A.; Stein, B.A.; Thomas, C.D.; Wheatley, C.J.; Bickford, D.; Carr, J.A.; et al. Climate change vulnerability assessment of species. Wiley Interdiscip. Rev. Clim. Chang. 2019, 10, 551. [Google Scholar] [CrossRef] [Green Version]
- Pederson, G.T.; Graumlich, L.J.; Fagre, D.B.; Kipfer, T.; Muhlfeld, C.C. A Century of Climate and Ecosystem Change in Western Montana: What Do Temperature Trends Portend? Clim. Change 2010, 98, 133–154. [Google Scholar] [CrossRef]
- Kohler, T.; Giger, M.; Hurni, H.; Ott, C.; Wiesman, U.; von Dach, S.W.; Maselli, D. Mountains and Climate Change: A Global Concern. Mt. Res. Dev. 2010, 30, 53–55. [Google Scholar] [CrossRef] [Green Version]
- Titeux, N.; Maes, D.; Van Daele, T.; Onkelinx, T.; Heikkinen, R.K.; Romo, H.; García-Barros, E.; Munguira, M.L.; Thuiller, W.; van Swaay, C.A. The Need for Large-scale Distribution Data to Estimate Regional Changes in Species Richness under Future Climate Change. Divers. Distrib. 2017, 23, 1393–1407. [Google Scholar] [CrossRef] [Green Version]
- Pearson, R.G.; Dawson, T.P.; Berry, P.M.; Harrison, P. Species: A Spatial Evaluation of Climate Impact on the Envelope of Species. Ecol. Model. 2002, 154, 289–300. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Predicting the Impacts of Climate Change on the Distribution of Species: Are Bioclimate Envelope Models Useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Bambach, N.; Meza, F.J.; Gilabert, H.; Miranda, M. Impacts of Climate Change on the Distribution of Species and Communities in the Chilean Mediterranean Ecosystem. Reg. Environ. Change 2013, 13, 1245–1257. [Google Scholar] [CrossRef]
- Anderson, R.P. A Framework for Using Niche Models to Estimate Impacts of Climate Change on Species Distributions. Ann. N. Y. Acad. Sci. 2013, 1297, 8–28. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Acevedo, P.; Barbosa, A.M.; Lobo, J.M.; Real, R. Discrimination Capacity in Species Distribution Models Depends on the Representativeness of the Environmental Domain. Glob. Ecol. Biogeogr. 2013, 22, 508–516. [Google Scholar] [CrossRef] [Green Version]
- Peterson, A.; Soberón, J. Interpretation of Models of Fundamental Ecological Niches and Species Distribution Areas. Biodivers. Inform. 2005, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive Habitat Distribution Models in Ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Mateo, R.G.; Felicisimo, A.M.; Munoz, J. Species Distributions Models: A Synthetic Revision. Rev. Chil. Hist. Nat. 2011, 84, 217–240. [Google Scholar] [CrossRef] [Green Version]
- Yates, K.L.; Bouchet, P.J.; Caley, M.J.; Mengersen, K.; Randin, C.F.; Parnell, S.; Fielding, A.H.; Bamford, A.J.; Ban, S.; Barbosa, A.M. Outstanding Challenges in the Transferability of Ecological Models. Trends Ecol. Evol. 2018, 33, 790–802. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J.; Dudík, M. Modeling of Species Distributions with Maxent: New Extensions and a Comprehensive Evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological Niche Modeling in Maxent: The Importance of Model Complexity and the Performance of Model Selection Criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.; Qiao, H. Effect of the Maxent model’s complexity on the prediction of species potential distributions. Biodivers. Sci. 2016, 24, 1189–1196. [Google Scholar] [CrossRef] [Green Version]
- Araújo, M.B.; Pearson, R.G.; Thuiller, W.; Erhard, M. Validation of Species–Climate Impact Models under Climate Change. Glob. Chang. Biol. 2005, 11, 1504–1513. [Google Scholar] [CrossRef] [Green Version]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENM Eval: An R Package for Conducting Spatially Independent Evaluations and Estimating Optimal Model Complexity for Maxent Ecological Niche Models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Lobo, J.M.; Jiménez-Valverde, A.; Real, R. AUC: A Misleading Measure of the Performance of Predictive Distribution Models. Glob. Ecol. Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Ahrends, A.; Bulling, M.T.; Platts, P.J.; Swetnam, R.; Ryan, C.; Doggart, N.; Hollingsworth, P.M.; Marchant, R.; Balmford, A.; Harris, D.J. Detecting and Predicting Forest Degradation: A Comparison of Ground Surveys and Remote Sensing in Tanzanian Forests. Plants People Planet 2021, 3, 268–281. [Google Scholar] [CrossRef]
- Cord, A.F.; Brauman, K.A.; Chaplin-Kramer, R.; Huth, A.; Ziv, G.; Seppelt, R. Priorities to Advance Monitoring of Ecosystem Services Using Earth Observation. Trends Ecol. Evol. 2017, 32, 416–428. [Google Scholar] [CrossRef]
- Iverson, L.R.; Graham, R.L.; Cook, E.A. Applications of Satellite Remote Sensing to Forested Ecosystems. Landsc. Ecol. 1989, 3, 131–143. [Google Scholar] [CrossRef]
- McRoberts, R.E.; Wendt, D.G.; Nelson, M.D.; Hansen, M.H. Using a Land Cover Classification Based on Satellite Imagery to Improve the Precision of Forest Inventory Area Estimates. Remote Sens. Environ. 2002, 81, 36–44. [Google Scholar] [CrossRef]
- Chirici, G.; Mura, M.; McInerney, D.; Py, N.; Tomppo, E.O.; Waser, L.T.; Travaglini, D.; McRoberts, R.E. A Meta-Analysis and Review of the Literature on the k-Nearest Neighbors Technique for Forestry Applications That Use Remotely Sensed Data. Remote Sens. Environ. 2016, 176, 282–294. [Google Scholar] [CrossRef]
- Viña, A.; Gitelson, A.A.; Nguy-Robertson, A.L.; Peng, Y. Comparison of Different Vegetation Indices for the Remote Assessment of Green Leaf Area Index of Crops. Remote Sens. Environ. 2011, 115, 3468–3478. [Google Scholar] [CrossRef]
- Recuero, L.; Litago, J.; Pinzón, J.E.; Huesca, M.; Moyano, M.C.; Palacios-Orueta, A. Mapping Periodic Patterns of Global Vegetation Based on Spectral Analysis of NDVI Time Series. Remote Sens. 2019, 11, 2497. [Google Scholar] [CrossRef] [Green Version]
- Reyer, C.P.O.; Silveyra Gonzalez, R.; Dolos, K.; Hartig, F.; Hauf, Y.; Noack, M.; Lasch-Born, P.; Rötzer, T.; Pretzsch, H.; Meesenburg, H.; et al. The Profound Database for Evaluating Vegetation Models and Simulating Climate Impacts on European Forests. Earth Syst. Sci. Data 2020, 12, 1295–1320. [Google Scholar] [CrossRef]
- Matasci, G.; Hermosilla, T.; Wulder, M.A.; White, J.C.; Hobart, G.W.; Zald, H.S.J.; Coops, N.C. A Space-Time Data Cube: Multi-Temporal Forest Structure Maps from Landsat and Lidar. In Proceedings of the 2017 IEEE International Geoscience and Remote Sensing Symposium (IGARSS); IEEE: Piscataway, NJ, USA, 2017; pp. 2581–2584. [Google Scholar] [CrossRef]
- Cord, A.F.; Klein, D.; Gernandt, D.S.; de la Rosa, J.A.P.; Dech, S. Remote Sensing Data Can Improve Predictions of Species Richness by Stacked Species Distribution Models: A Case Study for Mexican Pines. J. Biogeogr. 2014, 41, 736–748. [Google Scholar] [CrossRef]
- West, A.M.; Evangelista, P.H.; Jarnevich, C.S.; Young, N.E.; Stohlgren, T.J.; Talbert, C.; Talbert, M.; Morisette, J.; Anderson, R. Integrating Remote Sensing with Species Distribution Models; Mapping Tamarisk Invasions Using the Software for Assisted Habitat Modeling (SAHM). J. Vis. Exp. 2016, 116, e54578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto-Ledezma, J.N.; Cavender-Bares, J. Using Remote Sensing for Modeling and Monitoring Species Distributions. In Remote Sensing of Plant Biodiversity; Cavender-Bares, J., Gamon, J., Townsend, P., Eds.; Springer: New York, USA, 2020; pp. 199–233. [Google Scholar]
- de Araujo Barbosa, C.C.; Atkinson, P.M.; Dearing, J.A. Remote Sensing of Ecosystem Services: A Systematic Review. Ecol. Indic. 2015, 52, 430–443. [Google Scholar] [CrossRef]
- Defries, R.S.; Hansen, M.C.; Townshend, J.R.G.; Janetos, A.C.; Loveland, T.R. A New Global 1-Km Dataset of Percentage Tree Cover Derived from Remote Sensing. Glob. Change Biol. 2000, 6, 247–254. [Google Scholar] [CrossRef]
- Durán-Medina, E.; Mas, J.F.; Velázquez, A. Cambios En Las Coberturas de Vegetación y Usos Del Suelo En Regiones Con Manejo Forestal Comunitario y Áreas Naturales Protegidas de México. In Los Bosques Comunitarios de México: Manejo Sustentable de Paisajes Forestales; Barton Bray, D., Merino Pérez, L., Barry, D., Eds.; Instituto Nacional de Ecología: Ciudad de México, México, 2007; pp. 267–299. [Google Scholar]
- Dyke, A.S. Late Quaternary Vegetation History of Northern North America Based on Pollen, Macrofossil, and Faunal Remains. Géogr. Phys. Quat. 2005, 59, 211–262. [Google Scholar] [CrossRef] [Green Version]
- Reese, H.; Nilsson, M.; Sandström, P.; Olsson, H. Applications Using Estimates of Forest Parameters Derived from Satellite and Forest Inventory Data. Comput. Electron. Agric. 2002, 37, 37–55. [Google Scholar] [CrossRef] [Green Version]
- Tian, F.; Brandt, M.; Liu, Y.Y.; Rasmussen, K.; Fensholt, R. Mapping Gains and Losses in Woody Vegetation across Global Tropical Drylands. Glob. Chang. Biol. 2017, 23, 1748–1760. [Google Scholar] [CrossRef]
- Cisneros-Araujo, P.; Ramirez-Lopez, M.; Juffe-Bignoli, D.; Fensholt, R.; Muro, J.; Mateo-Sánchez, M.C.; Burgess, N.D. Remote Sensing of Wildlife Connectivity Networks and Priority Locations for Conservation in the Southern Agricultural Growth Corridor (SAGCOT) in Tanzania. Remote Sens. Ecol. Conserv. 2021, 7, 430–444. [Google Scholar] [CrossRef]
- Crooks, K.R.; Sanjayan, M.A. Connectivity Conservation: Maintaining Connections for Nature; Cambridge University Press: Cambridge, UK, 2006; pp. 1–20. [Google Scholar]
- Osipova, L.; Okello, M.M.; Njumbi, S.J.; Ngene, S.; Western, D.; Hayward, M.W.; Balkenhol, N. Using Step-Selection Functions to Model Landscape Connectivity for African Elephants: Accounting for Variability across Individuals and Seasons. Anim. Conserv. 2019, 22, 35–48. [Google Scholar] [CrossRef]
- de la Fuente, B.; Mateo-Sánchez, M.C.; Rodríguez, G.; Gastón, A.; de Ayala, R.P.; Colomina-Pérez, D.; Melero, M.; Saura, S. Natura 2000 Sites, Public Forests and Riparian Corridors: The Connectivity Backbone of Forest Green Infrastructure. Land Use Policy 2018, 75, 429–441. [Google Scholar] [CrossRef]
- Zeller, K.A.; McGarigal, K.; Whiteley, A.R. Estimating Landscape Resistance to Movement: A Review. Landsc. Ecol. 2012, 27, 777–797. [Google Scholar] [CrossRef]
- Saura, S.; Estreguil, C.; Mouton, C.; Rodríguez-Freire, M. Network Analysis to Assess Landscape Connectivity Trends: Application to European Forests (1990–2000). Ecol. Indic. 2011, 11, 407–416. [Google Scholar] [CrossRef]
- Critchfield, W.; Little, E., Jr. Geographic Distribution of the Pines of the World; US Department of Agriculture, Forest Service: Washington, DC, USA, 1966.
- Farjon, A.; Filer, D. An Atlas of the World’s Conifers: An Analysis of Their Distribution, Biogeography, Diversity and Conservation Status; Brill: Leiden, The Netherlands, 2013. [Google Scholar]
- Comisión Nacional de Áreas Naturales Protegidas. Programa de Manejo Parque Nacional Izta-Popo; CONANP: Jalisco, Mexico, 2013.
- Palacio-Prieto, J.L.; Bocco, G.; Velázquez, A.; Mas, J.-F.; Takaki-Takaki, F.; Victoria, A.; Luna-González, L.; Gómez-Rodríguez, G.; López-García, J.; Muñoz, M.P.; et al. La Condición Actual de Los Recursos Forestales En México: Resultados Del Inventario Forestal Nacional 2000. Investig. Geogr. 2000, 43, 183–203. [Google Scholar] [CrossRef]
- Comisión Nacional Forestal (CONAFOR). El Inventario Nacional Forestal y de Suelos de México 2004–2009. Una Herramienta Que Da Certeza a La Planeación, Evaluación y El Desarrollo Forestal de México; CONAFOR: Jalisco, Mexico, 2009.
- WorldClim 1.4 Climate Data. Available online: https://www.worldclim.org/data/v1.4/worldclim14.html (accessed on 8 January 2012).
- Rzedowski, J.; Huerta, L. Vegetación de México; Limusa: Distrito Federal, México, 1978. [Google Scholar]
- USGS. Global Visualization Viewer (GloVis). Available online: http://Glovis.Usgs.Gov/ (accessed on 9 September 2013).
- Chuvieco, E. Teledeteccion Ambiental. La Observacion de La Tierra Desde El Espacio; Ariel: Barcelona, Spain, 2002. [Google Scholar]
- Congalton, R.; Green, K. Assessing the Accuracy of Remotely Sensed Data: Principles and Practices; Lewis Publishing Company: Boca Ratón, FL, USA, 1999. [Google Scholar]
- ESRI ArcGIS Desktop: 10. ArcMap 10.1.; Environmental Systems Research Institute: New York, NY, USA, 2011. [Google Scholar]
- Urban, D.; Keitt, T. Landscape connectivity: A graph-theoretic perspective. Ecology 2001, 82, 1205–1218. [Google Scholar] [CrossRef]
- Bullock, J.M.; Mallada González, L.; Tamme, R.; Götzenberger, L.; White, S.M.; Pärtel, M.; Hooftman, D.A.P. A Synthesis of Empirical Plant Dispersal Kernels. J. Ecol. 2017, 105, 6–19. [Google Scholar] [CrossRef]
- Clark, J.S.; Macklin, E.; Wood, L. Stages and Spatial Scales of Recruitment Limitation in Southern Appalachian Forests. Ecol. Monogr. 1998, 68, 213–235. [Google Scholar] [CrossRef]
- Fajardo, J.; Mateo, R.G.; Vargas, P.; Fernández-Alonso, J.L.; Gómez-Rubio, V.; Felicísimo, Á.M.; Muñoz, J. The Role of Abiotic Mechanisms of Long-Distance Dispersal in the American Origin of the Galápagos Flora. Glob. Ecol. Biogeogr. 2019, 28, 1610–1620. [Google Scholar] [CrossRef]
- Wang, B.C.; Smith, T.B. Closing the Seed Dispersal Loop. Trends Ecol. Evol. 2002, 17, 379–386. [Google Scholar] [CrossRef]
- Thomson, F.J.; Moles, A.T.; Auld, T.D.; Kingsford, R.T. Seed dispersal distance is more strongly correlated with plant height than with seed mass. J. Ecol. 2011, 99, 1299–1307. [Google Scholar] [CrossRef]
- Saura, S.; Pascual-Hortal, L. A New Habitat Availability Index to Integrate Connectivity in Landscape Conservation Planning: Comparison with Existing Indices and Application to a Case Study. Landsc. Urban Plan. 2007, 83, 91–103. [Google Scholar] [CrossRef]
- Saura, S.; Rubio, L. A Common Currency for the Different Ways in Which Patches and Links Can Contribute to Habitat Availability and Connectivity in the Landscape. Ecography 2010, 33, 523–537. [Google Scholar] [CrossRef]
- Saura, S.; Torné, J. Conefor Sensinode 2.2: A Software Package for Quantifying the Importance of Habitat Patches for Landscape Connectivity. Environ. Model. Softw. 2009, 24, 135–139. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Amat, E.; Mateo, R.G.; Nieto-Lugilde, D.; Morueta-Holme, N.; Svenning, J.C.; García-Amorena, I. Impact of Model Complexity on Cross-Temporal Transferability in Maxent Species Distribution Models: An Assessment Using Paleobotanical Data. Ecol. Model. 2015, 312, 308–317. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Elith, J. Species Distribution Modeling with R; R CRAN Project; Spanish National Research Network (Mirror): Madrid, Spain, 2013. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Henry, M.; Stevens, H.; et al. Vegan: Community Ecology Package. Ordination Methods, Diversity Analysis and Other Functions for Community and Vegetation Ecologists; Comprehensive R Archive Network: Berkeley, CA, USA, 2016. [Google Scholar]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very High Resolution Interpolated Climate Surfaces for Global Land Areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Morueta-Holme, N.; Fløjgaard, C.; Svenning, J.-C. Climate Change Risks and Conservation Implications for a Threatened Small-Range Mammal Species. PLoS ONE 2010, 5, e10360. [Google Scholar] [CrossRef] [PubMed]
- Heiberger, R.M.; Holland, B. Statistical Analysis and Data Display An Intermediate Course with Examples in R; Springer: New York, NY, USA, 2015. [Google Scholar]
- Fielding, A.H.; Bell, J.F. A Review of Methods for the Assessment of Prediction Errors in Conservation Presence/Absence Models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Johnson, J.B.; Omland, K.S. Model Selection in Ecology and Evolution. Trends Ecol. Evol. 2004, 19, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Zurell, D.; Franklin, J.; König, C.; Bouchet, P.J.; Dormann, C.F.; Elith, J.; Fandos, G.; Feng, X.; Guillera-Arroita, G.; Guisan, A.; et al. A Standard Protocol for Reporting Species Distribution Models. Ecography 2020, 43, 1261–1277. [Google Scholar] [CrossRef]
- Jungclaus, J.H.; Fischer, N.; Haak, H.; Lohmann, K.; Marotzke, J.; Matei, D.; Mikolajewicz, U.; Notz, D.; von Storch, J.S. Characteristics of the Ocean Simulations in the Max Planck Institute Ocean Model (MPIOM) the Ocean Component of the MPI-Earth System Model. J. Adv. Model. Earth Syst. 2013, 5, 422–446. [Google Scholar] [CrossRef]
- Iversen, T.; Bentsen, M.; Bethke, I.; Debernard, J.B.; Kirkevåag, A.; Seland, Ø.; Drange, H.; Kristjansson, J.E.; Medhaug, I.; Sand, M.; et al. The Norwegian Earth System Model, NorESM1-M–Part 2: Climate Response and Scenario Projections. Geosci. Model Dev. 2013, 6, 389–415. [Google Scholar] [CrossRef] [Green Version]
- WorldClim. Available online: http://www.worldclim.org/Cmip5_30s (accessed on 1 October 2014).
- Ribalaygua, J.; Gaitán, E.; Pórtoles, J.; Monjo, R. Climatic Change on the Gulf of Fonseca (Central America) Using Two-Step Statistical Downscaling of CMIP5 Model Outputs. Theor. Appl. Climatol. 2018, 132, 867–883. [Google Scholar] [CrossRef]
- Flato, G.; Marotzke, J.; Abiodun, B.; Braconnot, P.; Chou, S.C.; Collins, W.; Cox, P.; Driouech, F.; Emori, S.; Eyring, V.; et al. Evaluation of Climate Models. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 741–866. [Google Scholar]
- Mosier, T.M.; Hill, D.F.; Sharp, K.V. 30-Arcsecond Monthly Climate Surfaces with Global Land Coverage. Int. J. Climatol. 2014, 34, 2175–2188. [Google Scholar] [CrossRef]
- Felicísimo, A.M.; Muñoz, J.; Villaba, C.J.; Mateo, R.G. Impactos del Cambio Climático Sobre la Flora Española; Oficina Española de Cambio Climático, Ministerio de Medio Ambiente y Medio Rural y Marino: Madrid, Spain, 2011.
- Settele, J.; Kudrna, O.; Harpke, A.; Kühn, I.; Van Swaay, C.; Verovnik, R.; Warren, M.; Wiemers, M.; Hanspach, J.; Hickler, T.; et al. Climatic Risk Atlas of European Butterflies; Pensoft Publishers: Sofia, Bulgaria, 2008; p. 710. [Google Scholar]
- Nenzén, H.K.; Araújo, M.B. Choice of Threshold Alters Projections of Species Range Shifts under Climate Change. Ecol. Model. 2011, 222, 3346–3354. [Google Scholar] [CrossRef]
- Vale, C.G.; Tarroso, P.; Brito, J.C. Predicting Species Distribution at Range Margins: Testing the Effects of Study Area Extent, Resolution and Threshold Selection in the Sahara–Sahel Transition Zone. Divers. Distrib. 2014, 20, 20–33. [Google Scholar] [CrossRef]
- Sitch, S.; Smith, B.; Prentice, I.C.; Arneth, A.; Bondeau, A.; Cramer, W.; Kaplan, J.O.; Levis, S.; Lucht, W.; Sykes, M.T.; et al. Evaluation of Ecosystem Dynamics, Plant Geography and Terrestrial Carbon Cycling in the LPJ Dynamic Global Vegetation Model. Glob. Chang. Biol. 2003, 9, 161–185. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.K.; Roy, D.P.; Yan, L.; Huang, H.; Li, J. Landsat 15-m Panchromatic-Assisted Downscaling (LPAD) of the 30-m Reflective Wavelength Bands to Sentinel-2 20-m Resolution. Remote Sens. 2017, 9, 755. [Google Scholar] [CrossRef] [Green Version]
- Immitzer, M.; Vuolo, F.; Atzberger, C. First Experience with Sentinel-2 Data for Crop and Tree Species Classifications in Central Europe. Remote Sens. 2016, 8, 166. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at High Resolution for the Earth’s Land Surface Areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Valverde, A.; Lobo, J.M.; Hortal, J. Not as Good as They Seem: The Importance of Concepts in Species Distribution Modelling. Divers. Distrib. 2008, 14, 885–890. [Google Scholar] [CrossRef]
- Hernández, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The Effect of Sample Size and Species Characteristics on Performance of Different Species Distribution Modeling Methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Magory Cohen, T.; McKinney, M.; Kark, S.; Dor, R. Global Invasion in Progress: Modeling the Past, Current and Potential Global Distribution of the Common Myna. Biol. Invasions 2019, 21, 1295–1309. [Google Scholar] [CrossRef]
- Begg, G.S.; Cook, S.M.; Dye, R.; Ferrante, M.; Franck, P.; Lavigne, C.; Lövei, G.L.; Mansion-Vaquie, A.; Pell, J.K.; Petit, S.; et al. A Functional Overview of Conservation Biological Control. Crop Prot. 2017, 97, 145–158. [Google Scholar] [CrossRef]
- Velasco, J.A.; González-Salazar, C. Akaike Information Criterion Should Not Be a “Test” of Geographical Prediction Accuracy in Ecological Niche Modelling. Ecol. Inform. 2019, 51, 25–32. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Casassa, J.; Karoly, D.J.; Imeson, A.; Liu, A.; Menzel, A.; Rawlins, S.; Root, T.L.; Seguin, B.; Tryjanowski, P. Assessment of Observed Changes and Responses in Natural and Managed Systems. In Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry, M.L., Canziani, O.F., Palutikof, J.P., van der Linden, P.J., Hanson, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 79–131. [Google Scholar]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Biodiversity Synthesis; World Resources Institute: Washington, DC, USA, 2005; p. 100. [Google Scholar]
- Kindlmann, P.; Burel, F. Connectivity Measures: A Review. Landsc. Ecol. 2008, 23, 879–890. [Google Scholar] [CrossRef] [Green Version]
- Wallace, J.; Aquilué, N.; Archambault, C.; Carpentier, S.; Francoeur, X.; Greffard, M.-H.; Laforest, I.; Galicia, L.; Messier, C. Present Forest Management Structures and Policies in Temperate Forests of Mexico: Challenges and Prospects for Unique Tree Species Assemblages. For. Chron. 2015, 91, 306–317. [Google Scholar] [CrossRef] [Green Version]
- CSF (Conservación Estratégica). Valoración de Servicios Ambientales del Parque Nacional Iztaccíhuatl–Popocatépetl; Conservation Strategy Fund: Arcata, CA, USA, 2017. [Google Scholar]
- Huntley, B.; Birks, H.J.B. Atlas of Past and Present Pollen Maps for Europe, 0–13,000 Years Ago; Cambridge University Press: Cambridge, UK, 1983. [Google Scholar]
- Davis, M.B.; Shaw, R.G. Range Shifts and Adaptive Responses to Quaternary Climate Change. Science 2001, 292, 673–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenoir, J.; Gégout, J.; Guisan, A.; Vittoz, P.; Wohlgemuth, T.; Zimmermann, N.E.; Dullinger, S.; Pauli, H.; Willner, W.; Svenning, J. Going against the Flow: Potential Mechanisms for Unexpected Downslope Range Shifts in a Warming Climate. Ecography 2010, 33, 295–303. [Google Scholar] [CrossRef]
- Chen, I.-C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid Range Shifts of Species Associated with High Levels of Climate Warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- OPPLA. Izta-Popo-Replenishing Groundwater through Reforestation in Mexico. EU Repository of Nature-Based Solutions. 2021. Available online: https://oppla.eu/casestudy/18030 (accessed on 22 June 2021).
| Class | Description |
|---|---|
| Pine forest | Areas dominated by Pinus hartwegii |
| Pino-Encino-Oyamel forest | Mixed formations dominated by P. hartwegii, Pinus ayacahuite Ehrenb. ex Schltdl., P. montezumae Lamb., Quercus crassifolia Benth., Quercus laurina M. Martens & Galeotti, and Abies religiosa Kunth Schltdl. et Chamy |
| Urban zones | |
| Crops | |
| Pastures | Areas dominated by Festuca spp., Camalagrostis tolucensis Trin. ex Steud., Agrostis tolucensis Kunth., and Juniperus montícola Martínez |
| Clouds | Clouds and glaciers |
| Shadows |
| Wc. V | Description | S1 | S2 |
|---|---|---|---|
| BIO 01 | Annual Mean Temperature | X | |
| BIO 02 | Mean Diurnal Range (Mean of monthly: max temp—min temp) | ||
| BIO 03 | Isothermality (BIO2/BIO7) (×100) | ||
| BIO 04 | Temperature Seasonality (standard deviation ×100) | ||
| BIO 05 | Max Temperature of Warmest Month | X | X |
| BIO 06 | Min Temperature of Coldest Month | X | |
| BIO 07 | Temperature Annual Range (BIO5-BIO6) | ||
| BIO 08 | Mean Temperature of Wettest Quarter | X | |
| BIO 09 | Mean Temperature of Driest Quarter | X | X |
| BIO 10 | Mean Temperature of Warmest Quarter | ||
| BIO 11 | Mean Temperature of Coldest Quarter | ||
| BIO 12 | Annual Precipitation | X | X |
| BIO 13 | Precipitation of Wettest Month | ||
| BIO 14 | Precipitation of Driest Month | ||
| BIO 15 | Precipitation Seasonality (Coefficient of Variation) | X | X |
| BIO 16 | Precipitation of Wettest Quarter | ||
| BIO 17 | Precipitation Driest Quarter | ||
| BIO 18 | Precipitation of Warmest Quarter | X | |
| BIO 19 | Precipitation of Coldest Quarter | X |
| Yr | D (m) | dPCintra | dPCflux | dPCconn |
|---|---|---|---|---|
| 1993 | 19.1 | 98.79 | 1.03 | 0.18 |
| 1993 | 52.72 | 95.76 | 3.47 | 0.76 |
| 2013 | 19.1 | 94.81 | 4.67 | 0.52 |
| 2013 | 52.72 | 77.29 | 17.80 | 4.91 |
| Model Id. | Occ. | n | Features | β | AUC | AIC | p | rHS-PO |
|---|---|---|---|---|---|---|---|---|
| 1 | 121 | 7 | Auto-features | 0 | 0.981 | - | 159 | 0.180 |
| 2 | 121 | 7 | Auto-features | 1 | 0.986 | 3274.54 | 33 | 0.395 |
| 3 | 121 | 7 | Auto-features | 5 | 0.986 | 2908.33 | 14 | 0.403 |
| 4 | 121 | 7 | L.Q.P | 0 | 0.988 | 2846.66 | 12 | 0.443 |
| 5 | 121 | 7 | L.Q.P | 1 | 0.988 | 2839.50 | 10 | 0.446 |
| 6 | 121 | 7 | L.Q.P | 5 | 0.988 | 2858.54 | 8 | 0.421 |
| 13 | 121 | 6 | Auto-features | 0 | 0.981 | - | 140 | 0.233 |
| 14 | 121 | 6 | Auto-features | 1 | 0.988 | 2960.42 | 28 | 0.363 |
| 15 | 121 | 6 | Auto-features | 5 | 0.988 | 2868.96 | 9 | 0.330 |
| 16 | 121 | 6 | L.Q.P | 0 | 0.988 | 2867.29 | 12 | 0.357 |
| 17 | 121 | 6 | L.Q.P | 1 | 0.989 | 2861.54 | 7 | 0.343 |
| 18 | 121 | 6 | L.Q.P | 5 | 0.989 | 2868.03 | 6 | 0.321 |
| 7 | 97 | 7 | Auto-features | 0 | 0.994 | 3071.03 | 79 | 0.340 |
| 8 | 97 | 7 | Auto-features | 1 | 0.994 | 2369.42 | 18 | 0.213 |
| 9 | 97 | 7 | Auto-features | 5 | 0.994 | 2257.98 | 11 | 0.325 |
| 10 | 97 | 7 | L.Q.P | 0 | 0.994 | 2276.60 | 14 | 0.351 |
| 11 | 97 | 7 | L.Q.P | 1 | 0.993 | 2322.88 | 9 | 0.397 |
| 12 | 97 | 7 | L.Q.P | 5 | 0.993 | 2279.90 | 8 | 0.381 |
| 19 | 97 | 6 | Auto-features | 0 | 0.992 | 2688.57 | 69 | 0.240 |
| 20 | 97 | 6 | Auto-features | 1 | 0.992 | 2282.71 | 18 | 0.153 |
| 21 | 97 | 6 | Auto-features | 5 | 0.992 | 2279.01 | 8 | 0.225 |
| 22 | 97 | 6 | L.Q.P | 0 | 0.993 | 2284.95 | 11 | 0.342 |
| 23 | 97 | 6 | L.Q.P | 1 | 0.992 | 2313.13 | 9 | 0.285 |
| 24 | 97 | 6 | L.Q.P | 5 | 0.992 | 2333.28 | 5 | 0.265 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Amorena, I.; Moreno-Amat, E.; Aulló-Maestro, M.E.; Mateo-Sánchez, M.C.; Merino-De-Miguel, S.; Ribalaygua, J.; Marchant, R. Combining Remote Sensing and Species Distribution Modelling to Assess Pinus hartwegii Response to Climate Change and Land Use from Izta-Popo National Park, Mexico. Land 2021, 10, 1037. https://doi.org/10.3390/land10101037
García-Amorena I, Moreno-Amat E, Aulló-Maestro ME, Mateo-Sánchez MC, Merino-De-Miguel S, Ribalaygua J, Marchant R. Combining Remote Sensing and Species Distribution Modelling to Assess Pinus hartwegii Response to Climate Change and Land Use from Izta-Popo National Park, Mexico. Land. 2021; 10(10):1037. https://doi.org/10.3390/land10101037
Chicago/Turabian StyleGarcía-Amorena, Ignacio, Elena Moreno-Amat, María Encina Aulló-Maestro, María Cruz Mateo-Sánchez, Silvia Merino-De-Miguel, Jaime Ribalaygua, and Robert Marchant. 2021. "Combining Remote Sensing and Species Distribution Modelling to Assess Pinus hartwegii Response to Climate Change and Land Use from Izta-Popo National Park, Mexico" Land 10, no. 10: 1037. https://doi.org/10.3390/land10101037







