Abstract
Maytenus senegalensis subsp. europaea is a shrub belonging to the Celastraceae family, whose only European populations are distributed discontinuously along the south-eastern coast of the Iberian Peninsula, forming plant communities with great ecological value, unique in Europe. As it is an endangered species that makes up plant communities with great palaeoecological significance, the development of species distribution models is of major interest under different climatic scenarios, past, present and future, based on the fact that the climate could play a relevant role in the distribution of this species, as well as in the conformation of the communities in which it is integrated. Palaeoecological models were generated for the Maximum Interglacial, Last Maximum Glacial and Middle Holocene periods. The results obtained showed that the widest distribution of this species, and the maximum suitability of its habitat, occurred during the Last Glacial Maximum, when the temperatures of the peninsular southeast were not as contrasting as those of the rest of the European continent and were favored by higher rainfall. Under these conditions, large territories could act as shelters during the glacial period, a hypothesis reflected in the model’s results for this period, which exhibit a further expansion of M. europaea’s ecological niche. The future projection of models in around 2070, for four Representative Concentration Pathways according to the fifth report of the Intergovernmental Panel on Climate Change, showed that the most favorable areas for this species would be Campo de Dalías (southern portion of Almería province) as it presents the bioclimatic characteristics of greater adjustment to M. europaea’s ecological niche model. Currently, some of the largest specimens of the species survive in the agricultural landscapes in the southern Spain. These areas are almost totally destroyed and heavily altered by intensive agriculture greenhouses, also causing a severe fragmentation of the habitat, which implies a prospective extinction scenario in the near future.
1. Introduction
Maytenus senegalensis subsp. europaea (Boiss.) Rivas Martínez ex Güemes and Crespo (≡Celastrus europaeus Boissier, Elench. Pl. Nov.: 29. 1838; Catha europaea (Boiss.) Boissier, Voy. Bot. Espagne 2: 725. 1845; Gymnosporia europaea (Boiss.) Masf., Anales Soc Esp. Hist. Nat. 10: 176. 1881; Gymnosporia senegalensis var. europaea (Boiss.) Jahand. and Maire, Cat. Pl. Maroc 2: 474. 1932), represents a taxon with a subspecific rank and disjunct distribution of Gymnosporia senegalensis (Lam.) Loes. (≡Celastrus senegalensis Lamarck, Encycl. 1: 661. 1785; Maytenus senegalensis (Lam.) Exell in Bol. Soc. Brot., ser. 2, 26: 223. 1952) native to the African tropical savannahs [,,].
This plant species belonging to the Celastraceae family is a very thorny deciduous shrub, which grows up to three meters. It differs significantly from the senegalensis subspecies both in phenomorphological adaptations to the arid Mediterranean climate, modulated by a high proportion of dewfall from marine origin and no-frost events [,,,,,,]; and in genetic diversity since this taxon presents a high variability, even within the Iberian range [], so that the segregation of the European lineage would be justified.
In Europe, the populations of this plant species are found specifically in Spain, in the most thermal areas of the southeast coastal region, from Malaga to Alicante provinces, growing between sea level and 300 (600) masl [,,,,,,,,]. The presence of M. europaea in the Iberian flora dates back to the Lower Cretaceous and is related to the palaeogeographic and paleoclimatic history of the Mediterranean Basin [,]. In the last period of the quaternary, the Holocene, studies about paleoclimatic and paleoecological trends even confirm the coexistence of this species in deciduous Quercus forests [,].
M. europaea communities are extraordinary vegetal formations in Europe, considered by Directive 92/43/EEC [] as Priority Habitat (ANNEX I. cod. 5220* Arborescent Scrubs with Ziziphus), which shows a unique and outstanding landscape in the European context, resulting from the arrangement of this vegetation in hemispherical clusters [,], in a way that is difficult to interpret, and which has resulted in different readings about its dynamics [,,,,,,,]. In addition to the landscape value, this habitat is able to maintain a singular biodiversity in terms of richness and rarity of species in arid ecosystems []; moreover, this habitat’s conservation may have positive effects that stretch beyond the characteristic community of plant species. For instance, authors like Rey et al. [] highlighted that a satisfactory management of this kind of bunches will probably lead to the conservation of many other species. Some studies show that up to 25 woody plants species present in this habitat can be gathered beneath their canopy [], and more than 80 insect species use floral resources []. Wild flora has proven to be a reservoir of beneficial invertebrates for the different agricultural systems, including not only pollinators, which play a fundamental role in the functioning of ecosystems being responsible for pollinator service of numerous plant species [,], but also predators that might contribute positively to the Biological Control as an effective way to reduce pest populations surrounding orchards [], vineyards [] or even greenhouses []. The loss of key (or minority) invertebrates consequently leads to the loss of those functions performed by them (functional diversity), which weakens and endangers the stability of these ecosystems if they cannot be replaced by other taxa []. Furthermore, this habitat represents a traditional agro-cultural system with ethnographic values that provides a number of ecosystem services [].
From the conservationist point of view, several acts in Spain, enacted at regional and national level, currently protect this plant species [,,,,], which is considered to be under vulnerable conservation status, essentially due to key threat factors such as land use changes and habitat loss, related to agricultural development. Thus, this species is an endangered taxon, being an essential part of an extremely threatened habitat [,,,]. In addition, this plant is included in several Spanish Red Lists of vascular flora [,,,,,].
The present case represents an interesting and complex study of the distribution dynamics of a severely threatened plant species, for which the implementation of a species distribution model could foster further understanding. Thus, for a better understanding of the distribution dynamics based on the optimal bioclimatic conditions over time and to predict the conservation status in future, the aims of this study were to (i) model the ecological niche of M. europaea in the Iberian Peninsula using three projections in the past—Mid-Holocene (6 ka), Last Glacial Maximum (LGM) (22 ka), and Interglacial Maximum (120 ka); (ii) evaluate the variations in the potential habitat of M. europaea modelled for the future (year 2070) in the four possible scenarios according to the representative concentration pathways (RCP2.6, RCP4.5, RCP6.0, RCP8.5); (iii) compare the retrospective and prospective results with the ecological niche model obtained from bioclimatic current data.
2. Materials and Methods
2.1. Study Area
The study area is located in the south and southeast of Spain. More specifically, it corresponds to the coastal area biogeographically encompassed by the Baetic and Murcian-Almeriensian provinces. This territory is characterized by a Mediterranean climate, with thermo-Mediterranean thermotype, modulated from dry to semiarid ombrotype, according to the bioclimatic classification proposed by Rivas-Martínez et al. []. It coincides with hotspots for plant biodiversity in southern Spain in terms of rarity and endemicity [,,], and includes semiarid, as well as arid areas, which are considered among the most vulnerable ecosystems to global change drivers [,,].
2.2. Species Distribution Models
Species distribution models (hereinafter SDMs) have been globally recognized as a useful tool in nature conservation and management, for instance, to refine the threat status of a species [,,,]. When applied to distribution data, they can predict distributions across geographic landscapes by multiple responses, improve image analysis or remote-sensing in order to lead the search for poorly known species [,,,], thus providing perceptions into the species’ habitat, range and abundance [,,,,]. Furthermore, several authors like Elith and Leathwick [], Benito et al. [], Fois et al. [], and De Luis et al. [,] used SDMs based on the extant localities, as well as the respective current and future climate scenarios to predict the possible variation in the environmental niche of certain plant species, inferring ecological and evolutionary insights.
MaxEnt 3.4.1 [] was used to model the potential habitat of M. europaea in the different proposed scenarios. The application of the Maximum Entropy principle to estimate ecological niche modelling and potential distribution areas follows the studies of Phillips et al. [] and Phillips and Dudík []. Said software has been used in many fields of science which proved its validity [,,,,], being widely recognized as the most used tool, even for small sample sizes and poorly known species’ distributions []. The modelling process is able to indicate suitable conditions for species in areas where their presence has not been registered or evidenced; several studies supported the reliability of SDMs [,,]. Since this process is iterative, the modelling outcomes can highlight other factors related to the physical environment, anthropogenic influences or conditions of growth and reproduction of the species [,,].
MaxEnt can operate with information about presence, which often represents the largest set of available data []. Only-presence-data algorithms usually represent the spatial distribution of the fundamental ecological niche of a species, while algorithms based on presence-absence data characterize more closely the distribution of the effective ecological niche []. The present study has been conducted with presence data alone, since a presence-absence modelling might generate controversies in light of the recent dynamics of the largest M. europaea population. Land changes due to the evolution of intensive crops in the province of Almería [,], which may result in defining an area with optimal characteristics for this species as an area of absence, could offer an incorrect outcome and decrease biological significance in the interpretation of the model [,].
In the MaxEnt configuration, 1000 was established as the maximum iteration parameter; the convergence limit was set at 0.00001 with a value of 0.001 for regularization []. The precision of the predictions was evaluated by using the area under the curve (AUC) []. AUC values below 0.7 were considered as poor, values between 0.7 and 0.9 were moderate and >0.9 were considered as high []. Hinge and threshold functions were disabled, leading to easily interpretable response curves and further adjustment to the ecological niche theory [] (Supplementary Materials Figure S1). The program produced a set of maps in which each pixel represents a value between 0 and 1, the closer values to 1 indicate the greater suitability for the species, being thus interpreted as an index of habitat potentiality. It also allowed for the generation of response curves for the species in relation to the variables used, noting their suitability, the evaluation of optimal values, the tolerance intervals and the various variables’ thresholds.
2.3. Presence Data
Presence data were collected through a huge bibliographical search combined with intensive fieldwork developed between 2011 and 2018 in Almería, Granada, Malaga, Murcia and Alicante provinces. Field data were geo-referenced using a GPS device (Garmin GPSMAP 60CX, 2 m error). Furthermore, digital sources were checked to gather distribution information on the species, such as GBIF [], ANTHOS [], and FAME project (Database of Threatened Flora of Andalusia) [], and adding details about herbaria records (HUAL, GDA, GDAC, JAEN, MGC, and MUB). The bibliographical data, particularly those taken from the Internet, were carefully checked by QGIS [] and using the latest aerial orthophoto, to only process reliable information. In order to avoid errors and remove duplicated occurrence records all the information was carefully filtered. A total of 1819 geo-referenced presence records of M. europaea were selected (Figure 1). The dataset of presence records was analyzed by using a spatial autocorrelation analysis.
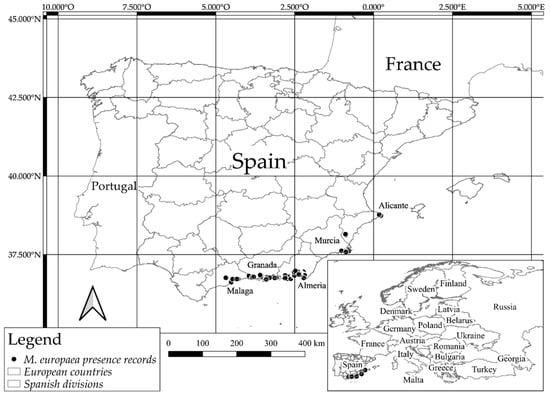
Figure 1.
Presence records of M. europaea in Europe. The localities are represented by dots and detailed in Table S1.
2.4. Environmental Variables
WorldClim [] provides data on 19 bioclimatic variables considered as broadly significant from the biological point of view when modelling distribution areas [,,]. Past bioclimatic data for the Mid-Holocene (ca. 6 ka AP), Last Glacial Maximum (LGM) (ca. 22 ka AP), and Interglacial Maximum (ca. 120 ka AP) were used in the present study. For the current distribution modelling, bioclimatic variables were generated from the climatic data period (1970–2000). The future estimation (2070) assumed the four Representative Concentration Pathways for emissions (RCPs), according to the fifth report from the IPCC []; RCP2.6 (a stringent pathway that requires that carbon dioxide (CO2) emissions start declining by 2020 and reach zero by 2100, RCP4.5 (an intermediate scenario that requires emissions to start declining by approximately 2045 to reach roughly half of the levels of 2050 by 2100), RCP6.0 (a scenario where emissions peak around 2080 and then decline), RCP8.5 (it represents the basis for worst-case climate change scenarios, where emissions continue to rise throughout the 21st century).
Bioclimatic data were downloaded from the Community Climate System Model (CCSM4) as the main reference for the distribution tests. This model simulates the global climate using four separate sub-models for the atmosphere, earth, oceans and sea ice []. These bioclimatic data had a 30-s resolution, except for the LGM, for which only data at 2.5 min were available. Each environmental variable map was adjusted to the Iberian Peninsula mask without Portugal.
To rule out the multicollinearity on the bioclimatic variables, a variance inflation factors analysis (VIF) was used. VIF analysis calculates variance-inflation and generalized variance-inflation factors for linear, generalized linear, and other statistical models able to discriminate and select the variables []. VIF analysis was performed with the R software [,,]. According to this methodology VIF value must be under 5; thus, six bioclimatic variables were selected (BIO2, BIO3, BIO8, BIO9, BIO15, BIO19) to execute the modelling process (Table 1).

Table 1.
Bioclimatic variables selected in the variance inflation factors (VIF) analysis (*) from the 19 variables available on WorldClim website.
2.5. Potential Distribution of M. europaea
MaxEnt models’ results for M. europaea distribution were adjusted by comparing its limits with the presence-absence of species that characterize different habitats together with [,,]. Such species were Periploca angustifolia Labil. that coexist in the association Mayteno europaei-Periplocetum angustifoliae Rivas Goday and Esteve; Ziziphus lotus (L.) Lam, both species subsist in the association Gymnosporio europaei-Ziziphetum loti F. Casas, and mesophilic species, such as Buxus balearica Lam. and Cneorum tricoccon L., these three species coexist in the association Cneoro tricocci-Buxetum balearicae Rivas Goday and Rivas Mart. Chorological data for these plant species were obtained from GBIF and ANTHOS databases [,] (Figure S2). In addition, the current final map that describes the potential habitat of M. europaea and its decrease in south and southeast of Spain were designed as contiguous areas by combining presence records, species modelling, and photo-interpretation of polygons performed in the national SIOSE project [] as was done by Mendoza-Fernández et al. [].
3. Results
3.1. M. europaea SDMs in the Past
Figure 2 shows the plots obteined for potential habitat of M. europaea in the four scenarios explored for the past.
3.2. M. europaea SDMs in the Present
In the context of the present situation, the results for the M. europaea potential extend of occurrence (EOO) are expressed in two ways; values format by territory (see Table 2), and detailed distribution map (Figure 3).
3.3. M. europaea SDMs Towards the Future (2070)
Plots of the potential habitat projected into the future according to the representative concentration pathways are illustrated in Figure 4.
4. Discussion
4.1. Distribution Dynamics
The model obtained for the LGM showed a significant dominance of the M. europaea habitat towards the southeast and the east of the Iberian Peninsula (Figure 2), exhibiting high suitability in areas nowadays submerged, and along the coastal plains of the Almería province. Probably, the populations were more stable in said area, acting as a genetic diversity reservoir in the glacial period. Médail and Diadema [] identified the existence of such shelters as a crucial event in a context of global change. Furthermore, since the LGM possibly represented the most favorable scenario for this plant, the idea that M. europaea could not be as xerophyte an element as considered may be reinforced. During this period, weather conditions became more humid than in the Mid-Holocene and the Interglacial Maximum. In addition, in the case of the southern Iberian Peninsula, the temperature decrease was not as extreme as in other parts of Europe, favoring a climate characterized by relatively mild temperatures and intense rainfall []. On the contrary, the resulting model for both the Mid-Holocene and the Last Interglacial periods did not show such habitat suitability for the plant. While the results for both periods were quite similar, it should be noted that the Mid-Holocene scenario presented a medium suitability for this plant since the modelling process was very strict with the bioclimatic variables developed for this time interval. The outcome of this period subsequent to the LGM, where the niche model showed the greatest habitat amplitude for M. europaea in the southeast of Spain, may be understood as the consequence of a delay in the dynamics of the M. europaea populations from the LGM through the Mid-Holocene period, as previous studies in Sierra de Gádor suggest [,], which shows the gradual decline of M. europaea and other deciduous Quercus genus species that might have been more widely distributed during the LGM.
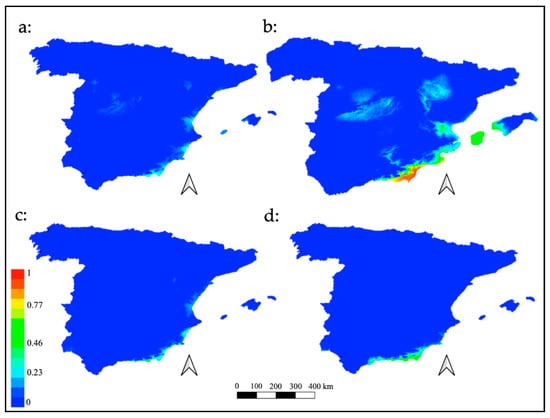
Figure 2.
Plots of potential habitat of M. europaea in the past. (a) Interglacial Maximum (ca. 120 ka BP); (b) LMG (ca. 22 ka BP); (c) Mid-Holocene (ca. 6 ka BP); (d) present.
Otherwise, the results achieved by combining presence records, species modelling, and photo-interpretation of polygons demonstrated that M. europaea’s current extent of occurrence (EOO) in Spain is approximately 166,721.2 ha, less than 47% of the suitable potential area (Table 2 and Figure 3). As shown in Figure 1, there is no presence of M. europaea in the east coast of the province of Almería or in a significant part of the Murcian coast. This is an important point to take into consideration when interpreting the modelling results, since this area is the driest in southern Spain, but where Z. lotus and P. angustifolia plant communities are able to develop and grow. Unifying the distribution of the aforementioned species in a single map helped to realize that M. europaea exhibits a less xerophytic behavior than Z. lotus and P. angustifolia, since the former does not coexist with the latter in the Murcian-Almeriensian bordering zone, thus proving M. europaea’s more mesophilic character. The slight probability of presence recorded to the north and west of Spain may be caused by common temperature variables or the coincidence of the summer drought period, related to the savannoid character of this plant, since there is no evidence of any past or current presence in these areas.

Table 2.
EOO of M. europaea in Spanish provinces and Europe. Values calculated from SIOSE project.
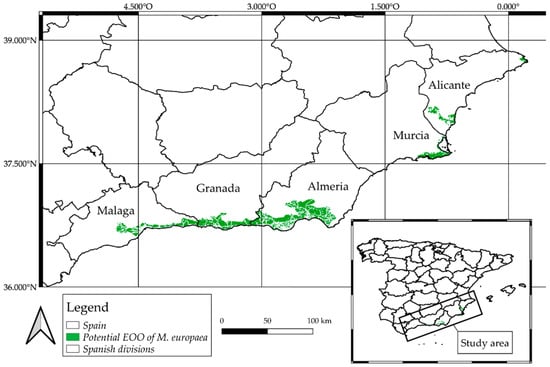
Figure 3.
M. europaea potential extend of occurrence (EOO). Green polygons indicate available habitat.
In order to achieve higher accuracy in establishing the influence of bioclimatic scenarios, models of singular thermophilic and xerophilous species that coexist with M. europaea could be compared in equal space-time conditions []. Hence, several models from different communities may be obtained, and thus their spatial-temporal dynamics analyzed [,]. In addition, indirect gradient variables corresponding to the physical characteristics of the territory (elevation, orientation, slope, geology, etc.) could be included in the model (at least at present and future predictions), since they might show a good correlation with the patterns of species distribution by combining resource gradients and direct gradients [].
Finally, the results of the future models with a projection for the year 2070 showed a slight variation in suitability among them, in which neither considerable habitats increase nor a significant decrease of it was observed. In the different scenarios, the zones exhibited small geographical variations, which slightly expand or reduce M. europaea’s ecological niche (Figure 4). However, habitat suitability was not altered, remaining permanent, with intermediate values in the four models. The results reinforce the thesis of M. europaea’s mesophilic behavior, and contrary to expectations, the global temperature increases projected in the different RCPs scenarios could predict the probable deterioration of the habitat. Nevertheless, a specific area was highlighted, the coastal plain in the south of the province of Almería (from sea level to 350 masl. approx.), where the four models corresponding to each RCPs scenario remained constant in terms of suitability, and offered the maximum values for all models. This fact makes this area a very probable one where potential M. europaea habitat is predicted in any of the 2070 scenarios. In addition, it supports the idea that this would be the most suitable area for the plant, in light of the four possible futures with an irradiative effect increase. Despite the fact that this area might be treated in the short term as one of the few habitats in the European continent available for this plant, it is currently a very altered territory, especially due to land-use changes resulting from the proliferation of tropical crops and intensive agriculture even in protected natural areas (an example of the deficient state of conservation in the Site of Community Importance (SCI) Artos de El Ejido (in Campo de Dalías, Almería, Spain) is shown in Figure 5), which has reached up to 90% of the potential area of this species [,,,].
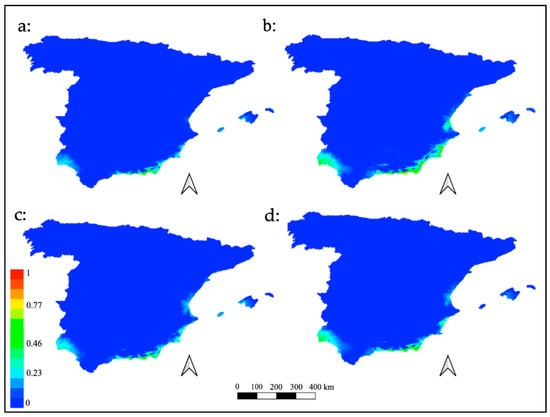
Figure 4.
Future distribution models for M. europaea. Plots of the potential habitat projected into the future according to the representative concentration pathways: (a) RCP2.6; (b) RCP4.5; (c) RCP6.0; (d) RCP8.5.
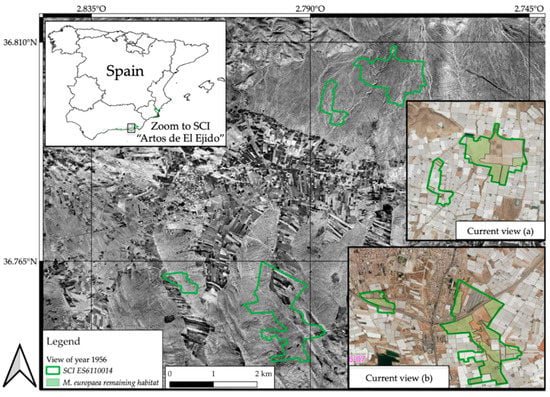
Figure 5.
Land changes in the Site of Community Importance (SCI) ES611001 (Artos de El Ejido), sited in Campo de Dalías (Almería). Evolution of M. europaea habitat loss inside this SCI from 1956 to the present.
4.2. Evolution of Distribution Patterns over Time
From a paleoecological point of view, the glaciation conditions could favor the extension of the potential niche for M. europaea in the Iberian southeast, corroborating that this plant does not present as xerophytic a behavior as Z. lotus or P. angustifolia, since the LGM model is the one that presents the highest suitability. This fact may be related to an increase in humidity in that period []. This supposition is consistent with two other types of evidence:
- The presence of M. europaea growing and showing its maximum abundance dovetailing with that of the deciduous forests located in Sierra de Gádor during the Mid-Holocene according to palynological studies,
- The link between M. europaea with C. tricoccon and B. balearica, which have mesothermophilic characteristics, capable of generating debate on the Maytenus-Periploca-Ziziphus trilogy, which was considered until now as a set of Ibero-African species with a hyperxerophytic conduct. This could explain the absence of M. europaea from Cabo de Gata to the Murcia region, being this area the most arid and with the smallest rainfall in the entire Spanish southeast, where conversely both Z. lotus and P. angustifolia are frequent.
In the near future, still affected by global change, the southern part of the province of Almería may become the most suitable area for M. europaea in the Iberian Peninsula. However, the natural area available for this plant´s development might be limited by the proliferation of intensive crops, which already occupy more than 90% of the habitat in this zone. This might cause a serious conservation problem for this species and increase its extinction risk due to massive occupation, fragmentation and land-use changes.
4.3. Considerations for Conservation of This Plant Species in an Agricultural Matrix
Preservation of some semi-natural strongholds for the Spanish M. europaeea communities in the area known as Campo de Dalías (southern portion of Almería province) is fundamental for the conservation of the species. In addition, there are two additional strategies that may favor its preservation. The first one is related to the use of this species with the aim to establish hedges, vegetal fences [] or small clusters that serve as a refuge for pollinators and predatory species of insects or other harmful pests for crops. In general, insects are considered one of the most effective animal groups in the pollination processes, thus their presence in natural systems is essential. Nevertheless, intensive agriculture and continuous landscape modification through land-use changes are associated with the decrease in the population of pollinators [], and/or severe alterations in their population concentrations []. Moreover, the agricultural use of pesticides and broad-spectrum chemicals produces a decrease not only in pest insects but also in all the entomofauna associated with these systems, thus affecting the insects of less altered neighboring habitats []. The combination of Maytenus and Ziziphus bunches could considerably improve this strategy, and hence favor a more sustainable development model in an area where highly intensive agriculture production can be understood as an industrial system if inputs and residues are taken account of []. The second strategy of great interest would be the creation of green spaces, by means of peri-urban parks, where some of the remaining fragments of this community that cannot be conserved within a legally protected area, may be integrated.
5. Conclusions
We can conclude that although the Campo de Dalías area has suffered the greatest habitat loss in terms of the extent of occurrence and occupancy area, this is a key territory for the future of the species, as demonstrated by the distribution models generated throughout this research. Therefore, safeguarding the last remaining redoubts of the Spanish M. europaea communities in this zone is considered absolutely essential.
Moreover, it is revealed that the already deficient conservation status of M. europaea, a unique plant species in Europe, could worsen if the current global change drivers were to intensify, although the greatest threat factor for this species continues to be the loss of available habitat as a result of changes in land use. The limits of this research may be due to the enormous asymmetry in terms of spatial information of the taxon presence. This fact is most likely due to the deforestation of the coastal areas and the intense land changes that the south and southeast of Spain have suffered. Future lines of research could be aimed at observing how changes in climate are affecting the phenology of this species, designing ex situ conservation techniques and translocation of individuals, and at improving knowledge to determine if M. europea is in fact a relict “savannoid” paleotropical element rather than a “xerothermic scrub”, and further detailing its distribution in Spain in order to guarantee the preservation of the highest number of populations and the maximum genetic diversity.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-445X/10/1/1/s1, Figure S1: Results of gains and Roc curve. MaxEnt analysis of the variables. Layers of the 6 bioclimatic variables selected in the VIF analysis; Figure S2: Distribution of Z. lotus, C. tricoccon, B. balearica and P. angustifolia. Records at 10 km2 in south and east of Spain; Table S1: Presence records of M. europaea. Geographic coordinates of presence records gathered from several sources.
Author Contributions
Conceptualization, J.F.M., F.M-H. and A.J.M.-F.; methodology, J.F.M., F.M.-H. and A.J.M.-F.; software, F.M.-H. and B.T.; validation, J.F.M., M.E.M., F.J.P.-G. and E.S.-S.; formal analysis, J.F.M., M.E.M. and A.J.M.-F.; investigation, A.J.M.-F. and F.M.-H.; resources, F.M.-H. and B.T.; data curation, F.J.P.-G. and E.S.-S.; writing—original draft preparation, A.J.M.-F., F.M.-H., E.S.-S. and J.F.M.; writing—review and editing, A.J.M.-F., F.M.-H., E.S.-S. and J.F.M.; visualization, M.E.M. and J.F.M.; supervision, J.F.M.; project administration, J.F.M.; funding acquisition, J.F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CEI·MAR, grant numbers CEIJ-012 and CEIJ-009, Integrated study of coastal sands vegetation (AREVEG I and II).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in https://susy.mdpi.com/user/manuscripts/displayFile/fa7da24ac6710de1cf1e3d8e7c05827c/supplementary.
Acknowledgments
This study has been made possible through the projects ‘Assessment, Monitoring and Applied Scientific Research for Ecological Restoration of Gypsum Mining Concessions (Majadas Viejas and Marylen) and Spreading of Results (ECORESGYP)’ sponsored by the company EXPLOTACIONES RÍO DE AGUAS S.L. (TORRALBA GROUP) and ‘Provision of services, monitoring and evaluation of the environmental restoration of the mining concessions Los Yesares, María Morales and El Cigarrón’ sponsored by the company Saint Gobain Placo Iberica S.A. We thank Beatrice Antolin for reviewing the English translation of the text.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Archibold, O.W. Ecology of World Vegetation; Chapman and Hall: London, UK, 1995. [Google Scholar] [CrossRef]
- Bobe, R. The evolution of arid ecosystems in eastern Africa. J. Arid. Environ. 2006, 66, 564–584. [Google Scholar] [CrossRef]
- Verdcourt, B.; Trump, E.C. Common Poisonous Plants of East Africa; Collins: London, UK, 1969. [Google Scholar]
- Blanca, G.; Cabezudo, B.; Cueto, M.; Fernández López, C.; Morales Torres, C. Flora Vascular de Andalucía Oriental; Consejería de Medio Ambiente, Junta de Andalucía: Sevilla, Spain, 2009. [Google Scholar]
- Castroviejo, S. Flora Iberica. Plantas Vasculares de la Península Ibérica e Islas Baleares; Real Jardín Botánico de Madrid: Madrid, Spain, 2012. [Google Scholar]
- Díez-Garretas, B.; Asensi, A.; Rivas-Martínez, S. Las comunidades de Maytenus senegalensis subsp. europaeus (Celastraceae) en la Península Ibérica. Lazaroa 2005, 26, 83. [Google Scholar]
- Ferrer-Gallego, P.; Laguna, E. Remarks on the nomenclatural types of Celastrus senegalensis and C. europaeus (Celastroideae-Celastraceae). Acta Bot. Malac. 2020, 45, 194–202. [Google Scholar] [CrossRef]
- Pérez-Latorre, A.V.; Gavira, O.; Cabezudo, B. Phenomorphology and ecomorphological characters of Maytenus senegalensis L. shrublands in the Iberian Peninsula: A comparison with other Mediterranean plant communities. Flora 2010, 205, 200–210. [Google Scholar] [CrossRef]
- Ruiz de la Torre, J. Flora Mayor; Organismo Autónomo de Parques Nacionales, Dirección General para la Biodiversidad: Madrid, Spain, 2006. [Google Scholar]
- Uclés, O.; Villagarcía, L.; Moro, M.J.; Canton, Y.; Domingo, F. Role of dewfall in the water balance of a semiarid coastal steppe ecosystem. Hydrol. Process. 2014, 4, 2271–2280. [Google Scholar] [CrossRef]
- Pérez Salmerón, E. Efectos de la Fragmentación del Habitat Sobre la Diversidad Genética de Maytenus sengalensis (Lam.) Exell (CELASTRACEAE) en el sur de la Peninsula Ibérica. Implicaciones en su Conservación. UAL. 2017. Available online: https://www.ual.es/estudios/grados/presentacion/plandeestudios/trabajofinestudios/curso/4509/2016-17 (accessed on 21 January 2020).
- Esteve Chueca, F. Descripción de comunidades con Gymnosporia europaea Webb y Periploca laevigata Ait. en el semiárido de la costa de Murcia. An. Jard. Bot. Madr. 1955, 12, 265–291. [Google Scholar]
- GBIF Home Page. Available online: www.gbif.org (accessed on 10 November 2019).
- Güemes, J.; Crespo, B. Maytenus senegalensis (lam.) Exell subsp. europaeus (Boiss.) Rivas Martínez, comb. nov. (Celastraceae), y noticias diversas acerca del mismo. An. Jard. Bot. Madr. 1990, 48, 86–88. [Google Scholar]
- López González, G.A. Los árboles y Arbustos de la Península Ibérica e Islas Baleares (Especies Silvestres y las Principales Cultivadas) Tomo II; Mundi-Prensa: Madrid, Spain, 2001. [Google Scholar]
- Pérez Latorre, A.V.; Navas, D.; Gavira, O.; Caballero, G.; Cabezudo, B. Vegetación del Parque Natural de las sierras Tejeda, Almijara y Alhama (Málaga-Granada) España. Acta Bot. Malac. 2004, 29, 117–191. [Google Scholar] [CrossRef]
- Pérez García, F.J.; Cueto, M.; Jiménez Sánchez, M.L.; Garrido, J.; Martínez-Hernández, F.; Medina-Cazorla, J.M.; Rodríguez-Tamayo, M.L.; Sola, A.J.; Mota Poveda, J.F. Contribución al conocimiento de la flora de Andalucía: Citas novedosas e interesantes de la provincia de Almería. Acta Bot. Malac. 2003, 28, 233–237. [Google Scholar] [CrossRef]
- Von Raab-Straube, E. Celastraceae. In Euro+Med Plantbase-the Information Resource for Euro-Mediterranean Plant Diversity; 2018; Available online: https://www.emplantbase.org/home.html (accessed on 15 January 2020).
- Manzano Cano, J. Maytenus senegalensis subsp. europaea en la peninsula ibérica: Distribución, ecología, fitosociología y conservación. Acta Bot. Malac. 2020, 45. [Google Scholar] [CrossRef][Green Version]
- Quézel, P. Definition of the Mediterranean region and the origin of its flora. In Plant Conservation in the Mediterranean Area; Gomez-Campo, C., Junk, W., Eds.; Geobotany 7; Springer: Dordrecht, The Netherlands, 1985; pp. 9–24. [Google Scholar]
- Blanca, G. Origen de la Flora de Andalucía; Valdés Bermejo, E., Ed.; Junta de Andalucía: Sevilla, Spain, 1993; pp. 19–35.
- Carrión, J.S.; Munuera, M.; Dupré, M.; Andrade, A. Abrupt vegetation changes in the Segura Mountains of southern Spain throughout the Holocene. J. Ecol. 2001, 89, 783–797. [Google Scholar] [CrossRef]
- Carrión, J.S.; Sánchez-Gómez, P.; Mota, J.F.; Yll, R.; Chaín, C. Holocene vegetation dynamics, fire and grazing in the Sierra de Gádor, southern Spain. Holocene 2003, 13, 839–849. [Google Scholar] [CrossRef]
- Anon. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. DOCE 1992, 206, 7–50. [Google Scholar]
- Mota, J.F.; Peñas, J.; Castro, H.; Cabello, J.; Guirado, J.S. Agricultural development vs biodiversity conservation: The Mediterranean semiarid vegetation in El Ejido (Almería, southeastern Spain). Biodivers. Conserv. 1996, 5, 1597–1617. [Google Scholar] [CrossRef]
- Mendoza-Fernández, A.J.; Salmerón-Sánchez, E.; Martínez-Hernández, F.; Pérez-García, F.J.; Lahora, A.; Merlo, E.; Mota, J.F. Intensive Habitat Loss in South Spain: Arborescent Scrubs with Ziziphus (5220*). In Habitats of the World-Biodiversity and Threats; Musarella, C.M., Cano Ortiz, A., Quinto Canas, R., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Parras, J.M.; Peinado, M.; Alcaraz, F. Sobre la vegetación termófila de la cuenca mediterránea de Granada y sus áreas limítrofes. Lazaroa 1985, 8, 251–268. [Google Scholar]
- Peinado, M.; Alcaraz, F.; Martínez-Parras, J.M. Vegetation of Southeastern Spain. In Flora et Vegetatio Mundi Volume X; J. Cramer: Berlin, Germany; Stuttgart, Germany, 1992. [Google Scholar]
- Rivas Goday, S.; Rivas-Martínez, S. Matorrales y tomillares de la Península Ibérica comprendidos en la clase Ononido-Rosmarinetea Br.-Bl. An. Inst. Bot. Cavanilles 1968, 1025, 1–297. [Google Scholar]
- Rivas-Martínez, S.; Fernández-González, F.; Loidi, J.; Lousã, M.; Penas, A. Syntaxonomical checklist of vascular plant communities of Spain and Portugal to association level. Itinera Geobot. 2001, 14, 5–341. [Google Scholar]
- Rivas-Martínez, S.; Díaz González, T.E.; Fernández-González, F.; Izco, J.; Loidi, J.; Lousã, M.; Penas, A. Vascular plant communities of Spain and Portugal. Itinera Geobot. 2002, 15, 5–432. [Google Scholar]
- Mendoza-Fernández, A.J.; Pérez-García, F.J.; Martínez-Hernández, F.; Medina-Cazorla, J.M.; Garrido-Becerra, J.A.; Merlo Calvente, M.E.; Romero, J.S.; Mota, J.F. Threatened plants of arid ecosystems in the Mediterranean Basin: A case study of the south-eastern Iberian Peninsula. Oryx 2014, 48, 548–554. [Google Scholar] [CrossRef]
- Rey, P.J.; Cancio, I.; Manzaneda, A.J.; González-Robles, A.; Valera, F.; Salido, T.; Alcántara, J.M. Regeneration of a keystone semiarid shrub over its range in Spain: Habitat degradation overrides the positive effects of plant-animal mutualisms. Plant Biol. 2018, 20, 1083–1092. [Google Scholar] [CrossRef]
- Rey, P.J.; Alcántara, J.M.; Manzaneda, A.J.; Sánchez-Lafuente, A.M. Facilitation contributes to Mediterranean woody plant diversity but does not shape the diversity-productivity relationship along aridity gradients. New Phytol. 2016, 211, 464–476. [Google Scholar] [CrossRef] [PubMed]
- González-Robles, A. Disrupción de los Mutualismos Planta-Polinizador de Ziziphus lotus (L.) Lam Por Pérdida de Hábitat y Degradación del Paisaje: Consecuencias Para el Flujo Génico y la Conservación de sus Poblaciones en el Sureste Semiárido de España. Ph.D. Thesis, Universidad de Jaén, Jaén, Spain, 2019. Available online: https://dialnet.unirioja.es/servlet/tesis?codigo=255239 (accessed on 12 February 2020).
- Rathcke, B.J.; Jules, E.S. Habitat fragmentation and plant-pollinator interactions. Curr. Sci. 1993, 65, 273–277. [Google Scholar]
- Gill, R.J.; Baldock, K.C.R.; Brown, M.J.F.; Cresswell, J.E.; Dicks, L.V.; Fountain, M.T.; Grarratt, M.P.D.; Gough, L.A.; Heard, M.S.; Ollerton, J.; et al. Protecting an Ecosystem Service: Approaches to Understanding and Mitigating Threats to Wild Insect Pollinators. Adv. Ecol. Res. 2016, 54, 135–206. [Google Scholar] [CrossRef]
- Rojas Rodriguez, J.; Rossetti, M.R.; Videla, M. Importance of flowers in field margins for insect communities in agroecological farms from Cordoba, Argentina. Rev. Fac. Cienc. Agrar. Univ. Nac. Cuyo 2019, 51, 249–259. [Google Scholar]
- López García, G.P.; Mazzitelli, M.E.; Fruitos, A.; González, M.; Marcucci, B.; Giusti, R.; Alemanno, V.; Barrio, L.D.; Portela, J.; Debandi, G. Pollinator and predator insects biodiversity in vineyards agroecosystems of Mendoza, Argentina. Considerations for habitat management. Rev. Fac. Cienc. Agrar. Univ. Nac. Cuyo 2019, 51, 309–322. [Google Scholar]
- Cotes, B.; González, M.; Benítez, E.; De Mas, E.; Clemente-Orta, G.; Campos, M.; Rodríguez, E. Spider Communities and Biological Control in Native Habitats Surrounding Greenhouses. Insects 2018, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Loreau, M.; Mouquet, N.; Gonzales, A. Biodiversity as spatial insurance in heterogeneous landscapes. Proc. Natl. Acad. Sci. USA 2003, 100, 12765–12770. [Google Scholar] [CrossRef]
- López Martos, J.M. Artineras, mirar desde el cielo para reconstruir el paisaje primitivo del Campo de Dalías. Farua 2014, 17, 153–172. [Google Scholar]
- De Andalucía, J. Decreto 104/1994, de 10 de mayo, por el que se establece el Catálogo Andaluz de Especies de Flora Silvestre Amenazada. BOJA 1994, 107, 7948–7953. [Google Scholar]
- De Andalucía, Comunidad Autónoma. Ley 8/2003, de 28 de octubre, de la Flora y la Fauna Silvestres. BOE 2003, 288, 42808–42830. [Google Scholar]
- Del Estado, J. Ley 42/2007, de 13 de diciembre, del Patrimonio Natural y de la Biodiversidad. BOE 2007, 299, 51275–51327. [Google Scholar]
- Anon. Decreto 23/2012, de 14 de febrero, por el que se regula la conservación y el uso sostenible de la flora y la fauna silvestres y sus hábitats. BOJA 2012, 60, 114–163. [Google Scholar]
- Mendoza-Fernández, A.J.; Martínez-Hernández, F.; Pérez-García, F.J.; Garrido-Becerra, J.A.; Benito, B.M.; Salmerón-Sánchez, E.; Guirado, J.; Merlo, M.E.; Mota, J.F. Extreme habitat loss in a Mediterranean habitat: Maytenus senegalensis subsp. europaea. Plant Biosyst. 2015, 149, 503–511. [Google Scholar] [CrossRef]
- Mota, J.; Cabello, J.; Cueto, M.; Gómez, F.; Giménez, E.; Peñas, J. Datos Sobre la Vegetación del Sureste de Almería (Desiertos de Tabernas, Karst en Yesos de Sorbas y Cabo de Gata); Servicio Publicaciones Universidad de Almería: Almería, Spain, 1997. [Google Scholar]
- Blanca, G.; Cabezudo, B.; Hernández-Bermejo, J.E.; Herrera, C.M.; Muñoz, J.; Valdés, B. Libro Rojo de la Flora Silvestre Amenazada de Andalucía; Tomo II: Especies Vulnerables; Consejería de Medio Ambiente, Junta de Andalucía: Sevilla, Spain, 2000. [Google Scholar]
- Cabezudo, B.; Talavera, S.; Blanca, G.; Salazar, C.; Cueto, M.; Valdés, B.; Hernández Bermejo, J.E.; Rodríguez Hiraldo, C.; Navas Fernández, D.; Vega Durán, C. Lista Roja de la Flora Vascular de Andalucía; Consejería de Medio Ambiente, Junta de Andalucía: Sevilla, Spain, 2005. [Google Scholar]
- Hernández Bermejo, J.E.; Clemente, M. Protección de la Flora de Andalucía. Catálogo General de Especies de Recomendada Protección en Andalucía (Endémicas, Raras y Amenazadas de Extinción); Junta de Andalucía: Sevilla, Spain, 1994.
- Moreno, J.C. (Ed.) Lista Roja 2008 de la Flora Vascular Española; Dgmnpf, Mmamrm, Sebicop: Madrid, Spain, 2008. [Google Scholar]
- Sánchez-Gómez, P.; Carrión Vilches, M.A.; Hernández González, A.; Guerra Montes, J. Libro Rojo de la Flora Silvestre Protegida de la Región de Murcia; Dirección General del Medio Natural: Murcia, Spain, 2002. [Google Scholar]
- Rivas-Martínez, S.; Asensi, A.; Díaz-Garretas, B.; Molero, J.; Valle, F.; Cano, E.; Costa Talens, M.; López, M.L. Mapa de series, geoseries y geopermaseries de vegetación de España, Memoria del mapa de vegetación potencial de España. Itinera Geobot. 2007, 17, 5–436. [Google Scholar]
- Cañadas, E.M.; Giuseppe, F.; Peñas, J.; Lorite, J.; Mattana, E.; Bacchetta, G. Hotspots within hotspots: Endemic plant richness, environmental drivers, and implications for conservation. Biol. Conserv. 2014, 170, 282–291. [Google Scholar] [CrossRef]
- Médail, F.; Quézel, P. Biodiversity hotspots in the Mediterranean basin: Setting global conservation priorities. Conserv. Biol. 1999, 13, 1510–1513. [Google Scholar] [CrossRef]
- Mendoza-Fernández, A.J.; García, F.J.P.; Martínez-Hernández, F.; Salmerón-Sánchez, E.; Medina-Cazorla, J.M.; Garrido-Becerra, J.A.; Martínez-Nieto, M.I.; Calvente, M.E.M.; Mota, J.F. Areas of endemism and threatened flora in a Mediterranean hotspot: Southern Spain. J. Nat. Conserv. 2015, 23, 35–44. [Google Scholar] [CrossRef]
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global consequences of land use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef]
- Kefi, S.; Rietkerk, M.; Alados, C.L.; Pueyo, Y.; Papanastasis, V.P.; El Aich, A.; De Ruiter, P.C. Spatial vegetation patterns and imminent desertification in Mediterranean arid ecosystems. Nature 2007, 449, 213–217. [Google Scholar] [CrossRef]
- Klausmeyer, K.R.; Shaw, M.R. Climate Change, Habitat Loss, Protected Areas and the Climate Adaptation Potential of Species in Mediterranean Ecosystems Worldwide. PLoS ONE 2009, 4, e0006392. [Google Scholar] [CrossRef]
- Peñas, J.; Benito, B.; Lorite, J.; Ballesteros, M.; Cañadas, E.M.; Martinez-Ortega, M. Habitat fragmentation in arid zones: A case study of Linaria nigricans under land use changes (SE Spain). Environ. Manag. 2011, 48, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Peterman, W.E.; Crawford, J.A.; Kuhns, A.R. Using species distribution and occupancy modeling to guide survey efforts and assess species status. J. Nat. Conserv. 2013, 21, 114–121. [Google Scholar] [CrossRef]
- Smeraldo, S.; Bosso, L.; Fraissinet, M.; Bordignon, L.; Brunelli, M.; Ancillotto, L.; Russo, D. Modelling risks posed by wind turbines and electric power lines to soaring birds: The black stork (Ciconia nigra) in Italy as a case study. Biodiv. Conserv. 2020, 29, 1959–1976. [Google Scholar] [CrossRef]
- Zhang, Z.; Mammola, S.; Liang, Z.; Capinha, C.; Wei, Q.; Wu, Y.; Zhou, J.; Wang, C. Future climate change will severely reduce habitat suitability of the Critically Endangered Chinese giant salamander. Freshw. Biol. 2020, 65, 971–980. [Google Scholar] [CrossRef]
- De Siqueira, M.F.; Durigan, G.; de Marco Júnior, P.; Peterson, A.T. Something from nothing: Using landscape similarity and ecological niche modeling to find rare plant species. J. Nat. Conserv. 2009, 17, 25–32. [Google Scholar] [CrossRef]
- Williams, J.N.; Seo, C.; Thorne, J.; Nelson, J.K.; Erwin, S.; O’Brien, J.M.; Schwartz, M.W. Using species distribution models to predict new occurrences for rare plants. Divers. Distrib. 2009, 15, 565–576. [Google Scholar] [CrossRef]
- Fois, M.; Cuena-Lombraña, A.; Fenu, G.; Bacchetta, G. Using species distribution models at local scale to guide the search of poorly known species: Review, methodological issues and future directions. Ecol. Model. 2018, 385, 124–132. [Google Scholar] [CrossRef]
- Raffini, F.; Bertorelle, G.; Biello, R.; D’Urso, G.; Russo, D.; Bosso, L. From Nucleotides to Satellite Imagery: Approaches to identify and manage the invasive pathogen Xylella fastidiosa and its insect vectors in Europe. Sustainability 2020, 12, 4508. [Google Scholar] [CrossRef]
- Guirado, E.; Alcaraz-Segura, D.; Rigol-Sanchez, J.P.; Gisbert, J.; Martínez-Moreno, F.; Galindo-Zaldívar, J.; González-Castillo, L.; Cabello, J. Remote-sensing-derived fractures and shrub patterns to identify groundwater dependence. Ecohydrology 2018, 11, e1933. [Google Scholar] [CrossRef]
- Guirado, E.; Blanco-Sacristán, J.; Rigol-Sánchez, J.P.; Alcaraz-Segura, D.; Cabello, J. A multi-temporal object-based image analysis to detect long-lived shrub cover changes in drylands. Remote Sens. 2019, 11, 2649. [Google Scholar] [CrossRef]
- Elith, J.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijimans, R.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; Li, J. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Guisan, A.; Tingley, R.; Baumgartner, J.B.; Naujokaitis-Lewis, I.; Sutcliffe, P.R.; Tulloch, A.I.T.; Regan, T.J.; Brotons, L.; McDonald-Madden, E.; Mantyka-Pringle, C.; et al. Predicting species distributions for conservation decisions. Ecol. Lett. 2013, 16, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Benito, B.M.; Lorite, J.; Pérez-Pérez, R.; Gómez-Aparicio, L.; Peñas, J. Forecasting plant range collapse in a Mediterranean hotspot: When dispersal uncertainties matter. Divers. Distrib. 2014, 20, 72–83. [Google Scholar] [CrossRef]
- Fois, M.; Cuena-Lombraña, A.; Fenu, G.; Cogoni, D.; Bacchetta, G. The reliability of conservation status assessments at regional level: Past, present and future perspectives on Gentiana lutea L. ssp. lutea in Sardinia. J. Nat. Conserv. 2016, 33, 1–9. [Google Scholar] [CrossRef]
- De Luis, M.; Bartolomé, C.; Óscar, C.; Álvarez Jiménez, J. Gypsophila bermejoi G. López: A possible case of speciation repressed by bioclimatic factors. PLoS ONE 2018, 13, e0190536. [Google Scholar] [CrossRef]
- De Luis, M.; Álvarez Jiménez, J.; Labarga, J.M.; Carmen, B. Four climate change scenarios for Gypsophila bermejoi G. López (Caryophyllaceae) to address whether bioclimatic and soil suitability will overlap in the future. PLoS ONE 2019, 14, e0218160. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. Maxent Software for Modeling Species Niches and Distributions (VERSION 3.4.1). c2020. Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 15 March 2019).
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Baldwin, R.A. Use of maximum entropy modeling in wildlife research. Entropy 2009, 11, 854–866. [Google Scholar] [CrossRef]
- Deblauwe, V.; Barbier, N.; Couteron, P.; Lejeune, O.; Bogaert, J. The global biogeography of semi-arid periodic vegetation patterns. Global. Ecol. Biogeogr. 2008, 17, 715–723. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Riordan, E.C.; Rundel, P.W. Modelling the distribution of a threatened habitat: The California sage scrub. J. Biogeogr. 2009, 36, 2176–2188. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Saracino, A.; Bosso, L.; Russo, D.; Moroni, A.; Bonanomi, G.; Allevato, E. Coastal pine-oak glacial refugia in the Mediterranean basin: A biogeographic approach based on charcoal analysis and spatial modelling. Forests 2020, 11, 673. [Google Scholar] [CrossRef]
- Kaky, E.; Nolan, V.; Alatawi, A.; Gilbert, F. A comparison between Ensemble and MaxEnt species distribution modelling approaches for conservation: A case study with Egyptian medicinal plants. Ecol. Inform. 2020, 60, 101150. [Google Scholar] [CrossRef]
- Peterson, A.T.; Nakazawa, Y. Environmental data sets matter in ecological niche modelling: An example with Solenopsis invicta and Solenopsis richteri. Glob. Ecol. Biogeogr. 2008, 17, 135–144. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution 2008, 62, 2868–2883. [Google Scholar] [CrossRef]
- Bucklin, D.N.; Basille, M.; Benscoter, A.M.; Brandt, L.A.; Mazzotti, F.J.; Romañach, S.S.; Speroterra, C.; Watling, J.I. Comparing species distribution models constructed with different subsets of environmental predictors. Divers. Distrib. 2015, 21, 23–35. [Google Scholar] [CrossRef]
- Virgili, A.; Racine, M.; Authier, M.; Monestieza, P.; Ridoux, V. Comparison of habitat models for scarcely detected species. Ecol. Model. 2017, 346, 88–98. [Google Scholar] [CrossRef]
- Zaniewski, A.E.; Lehmann, A.; Overton, J.M. Predicting species spatial distributions using presence-only data: A case study of native New Zealand ferns. Ecol. Model. 2002, 157, 261–280. [Google Scholar] [CrossRef]
- Hirzel, A.H.; Hausser, J.; Chessel, D.; Perrin, N. Ecological-niche factor analysis: How to compute habitat- suitability maps without absence data? Ecology 2002, 83, 2027–2036. [Google Scholar] [CrossRef]
- Hirzel, A.; Guisan, A. Which is the optimal sampling strategy for habitat suitability modelling. Ecol. Model. 2002, 157, 331–341. [Google Scholar] [CrossRef]
- Lobo, J.M.; Jiménez-Valverde, A.; Real, R. AUC: A misleading measure of the performance of predictive distribution models. Glob. Ecol. Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Stohlgren, T.J.; Ma, P.; Kumar, S.; Rocca, M.; Morisette, J.T.; Jarnevich, C.S.; Benson, N. Ensemble habitat mapping of invasive plant species. Risk Anal. 2010, 30, 224–235. [Google Scholar] [CrossRef]
- ANTHOS. Information System of the Plants of Spain. Real Jardín Botánico, CSIC-Fundación Biodiversidad. Available online: www.anthos.es (accessed on 5 February 2020).
- FAME. Sistema de Información Sobre Flora Amenazada y de Interés en Andalucía. Available online: http://www.juntadeandalucia.es/medioambiente/site/rediam (accessed on 12 June 2019).
- QGIS Development Team. QGIS Geographic Information System. 2020. Open Source Geospatial Foundation Project. Available online: https://www.qgis.org/es/site/ (accessed on 20 March 2020).
- Worldclim. Global Climatic Data. Available online: http://www.worldclim.org (accessed on 10 December 2019).
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Booth, T.H. Why understanding the pioneering and continuing contributions of BIOCLIM to species distribution modelling is important. Austral Ecol. 2018, 43, 852–860. [Google Scholar] [CrossRef]
- Booth, T.H.; Nix, H.A.; Busby, J.R.; Hutchinson, M.F. BIOCLIM: The first species distribution modelling package, its early applications and relevance to most current MAXENT studies. Divers. Distrib. 2014, 20, 1–9. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. IPCC, 2013. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Gent, P.R.; Danabasoglu, G.; Donner, L.J.; Holland, M.M.; Hunke, E.C.; Jayne, S.R.; Lawrence, D.M.; Neale, R.B.; Rasch, P.J.; Vertenstein, M.; et al. The community climate system model version 4. J. Clim. 2011, 24, 4973–4991. [Google Scholar] [CrossRef]
- Li, J.; Fan, G.; He, Y. Predicting the current and future distribution of three Coptis herbs in China under climate change conditions, using the MaxEnt model and chemical analysis. Sci. Total Environ. 2020, 698, 134141. [Google Scholar] [CrossRef]
- Fox, J. Applied Regression Analysis and Generalized Linear Models; Sage Publications: Thousand Oaks, CA, USA, 2016. [Google Scholar]
- Fox, J.; Monette, G. Generalized collinearity diagnostics. J. Am. Stat. Assoc. 1992, 87, 178–183. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage Publications: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- SIOSE. Sistema de Información Sobre Ocupación del Suelo de España. Available online: www.siose.es (accessed on 20 January 2020).
- Médail, F.; Diadema, K. Glacial refugia influence plant diversity patterns in the Mediterranean Basin. J. Biogeogr. 2009, 36, 1333–1345. [Google Scholar] [CrossRef]
- Carrión, Y.; Ntinou, M.; Badal, E. Olea europaea L. in the north Mediterranean Basin during the Pleniglacial and the Early-Middle Holocene. Quat. Sci. Rev. 2010, 29, 952–968. [Google Scholar] [CrossRef]
- Quinto-Canas, R.; Mendes, P.; Cano-Ortiz, A.; Musarella, C.M.; Pinto-Gomes, C. Forest fringe communities of the southwestern Iberian Peninsula. Rev. Chapingo Ser. Cienc. For. Ambient. 2018, 24, 415–434. [Google Scholar] [CrossRef]
- Guisan, A.; Weiss, S.B.; Weiss, A.D. GLM versus CCA spatial modeling of plants species distribution. Plant Ecol. 1999, 143, 107–122. [Google Scholar] [CrossRef]
- Weniger, G.-C.; De Andrés-Herrero, M.; Bolín, V.; Kehl, M.; Otto, T.; Potì, A.; Tafelmaier, Y. Late Glacial rapid climate change and human response in the Westernmost Mediterranean (Iberia and Morocco). PLoS ONE 2019, 14, e0225049. [Google Scholar] [CrossRef]
- Aizen, M.A.; Garibaldi, L.A.; Cunningham, S.A.; Klein, A.M. How much does agriculture depend on pollinators? Lessons from long- term trends in crop production. Ann. Bot. 2009, 103, 1579–1588. [Google Scholar] [CrossRef]
- Winfree, R.; Bartomeus, I.; Cariveau, D. Native Pollinators in Anthropogenic Habitats. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 1–22. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).