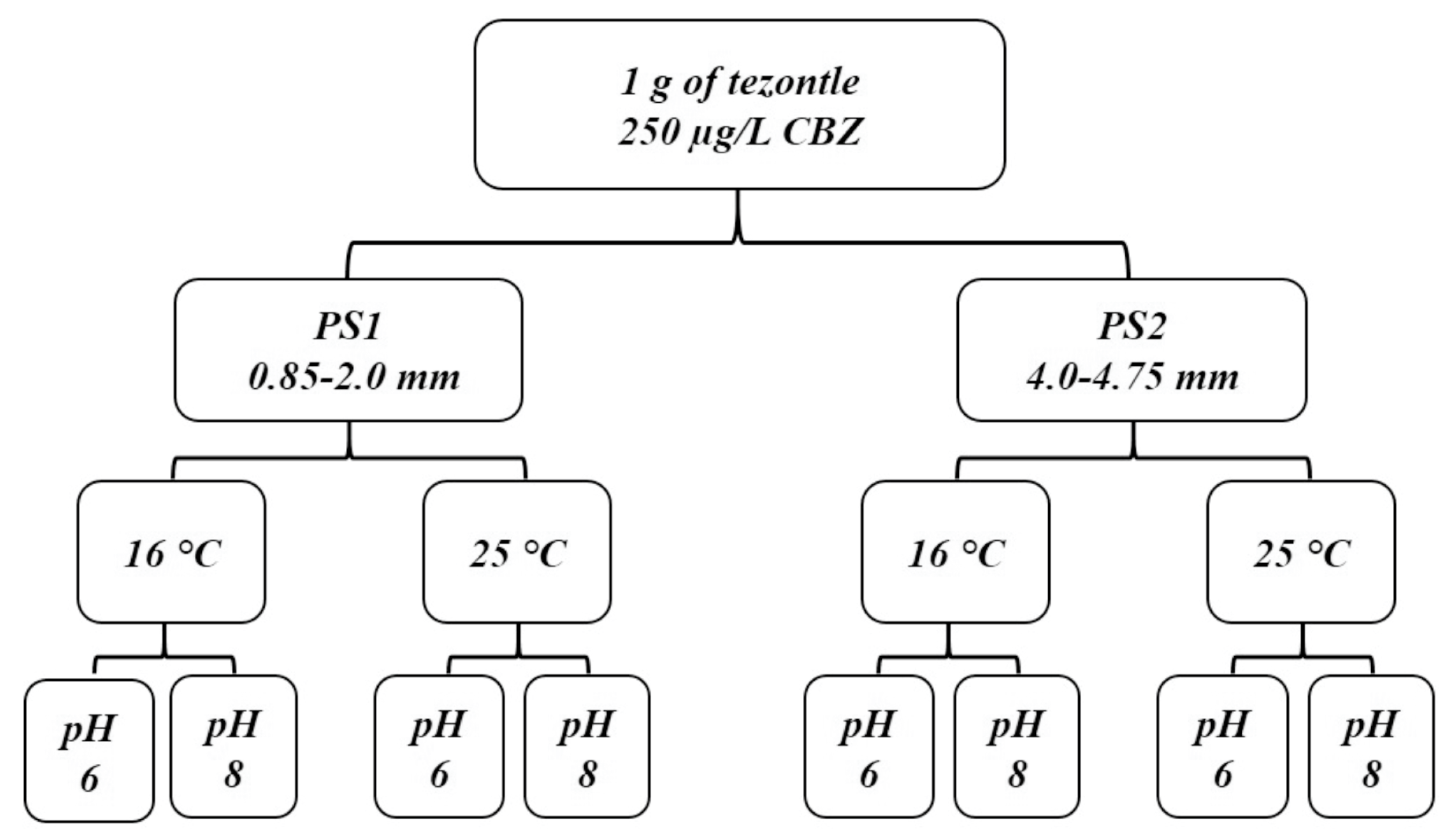
3.1. Structural and Textural Properties of the Ground Tezontle
The structural and textural properties of the material used in this study are shown in
Table 1. According to the particle size distribution, the ground
tezontle has a wide particle size distribution, with 92.73% of the material having diameters between 0.425 and 4 mm. With regard to d
10, d
60, and the uniformity coefficient, they fall into the range of recommendable values for filter media used in CWs [
25]. In addition, the apparent porosity was more than 50%, which is higher than those values reported for gravel; in this way, ground
tezontle exhibits advantages as a support matrix in CWs, because, the higher amount of void space, the better the hydraulic conductivity [
2].
On the other hand, as expected, the BET specific surface area was very small in comparison to adsorbents prepared or synthetized for drug removal but alike to the value reported by Alemayehu & Lennartz [
26] for a similar volcanic scoria. In addition, the N
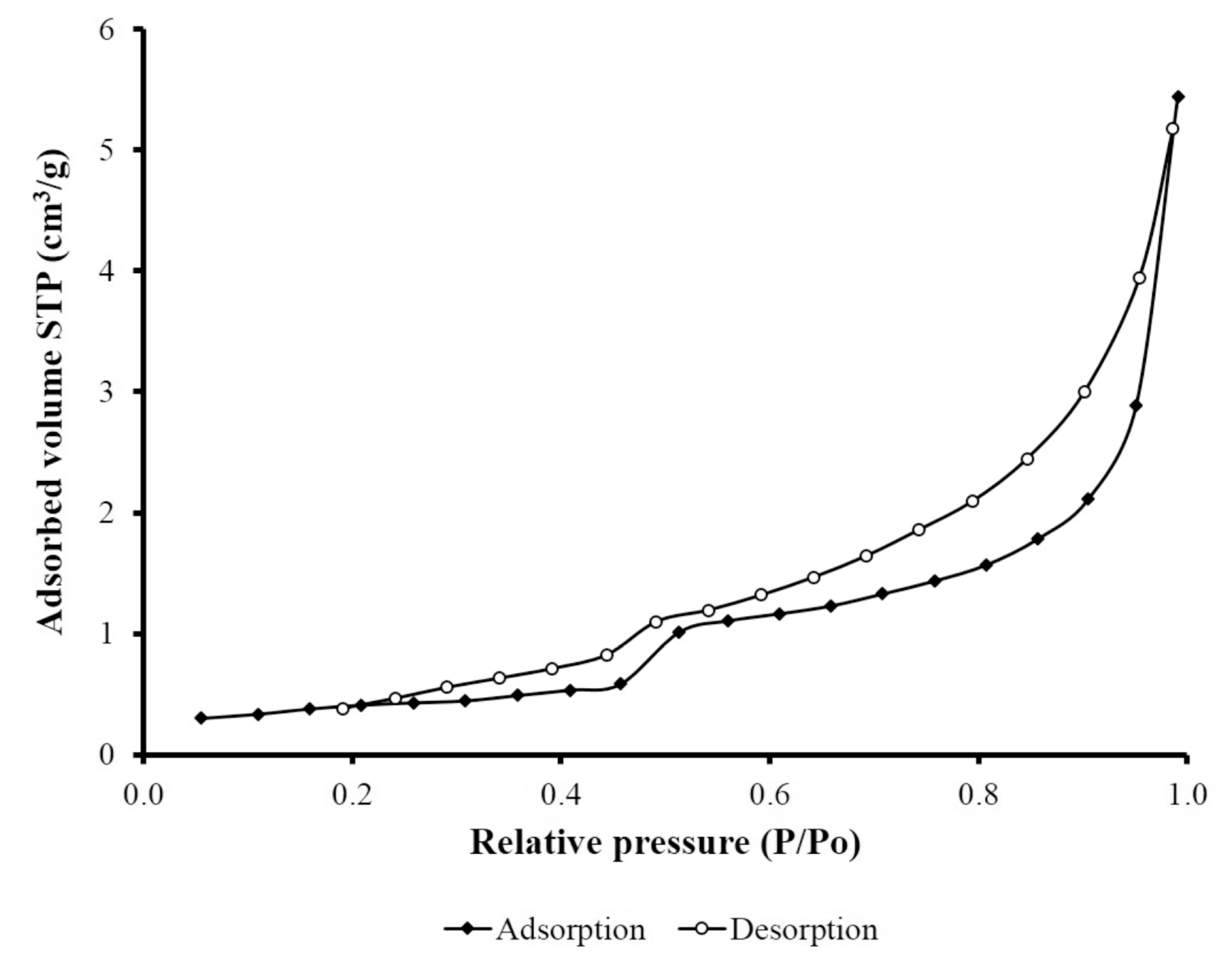
2 adsorption–desorption isotherm of
tezontle (
Figure 2) was of the type IV with a hysteresis loop of H3-type, according to the IUPAC classification [
27]. The hysteresis loop of H3-type indicates the presence of non-rigid aggregates of plate-like particles giving rise to slit-type shaped pores [
28]. The pore sizes calculated from the desorption branch of N
2 sorption isotherm were in the mesopore range with values between 2 and 50 nm, which is in line with the values reported by Vilchis-Granados et al. [
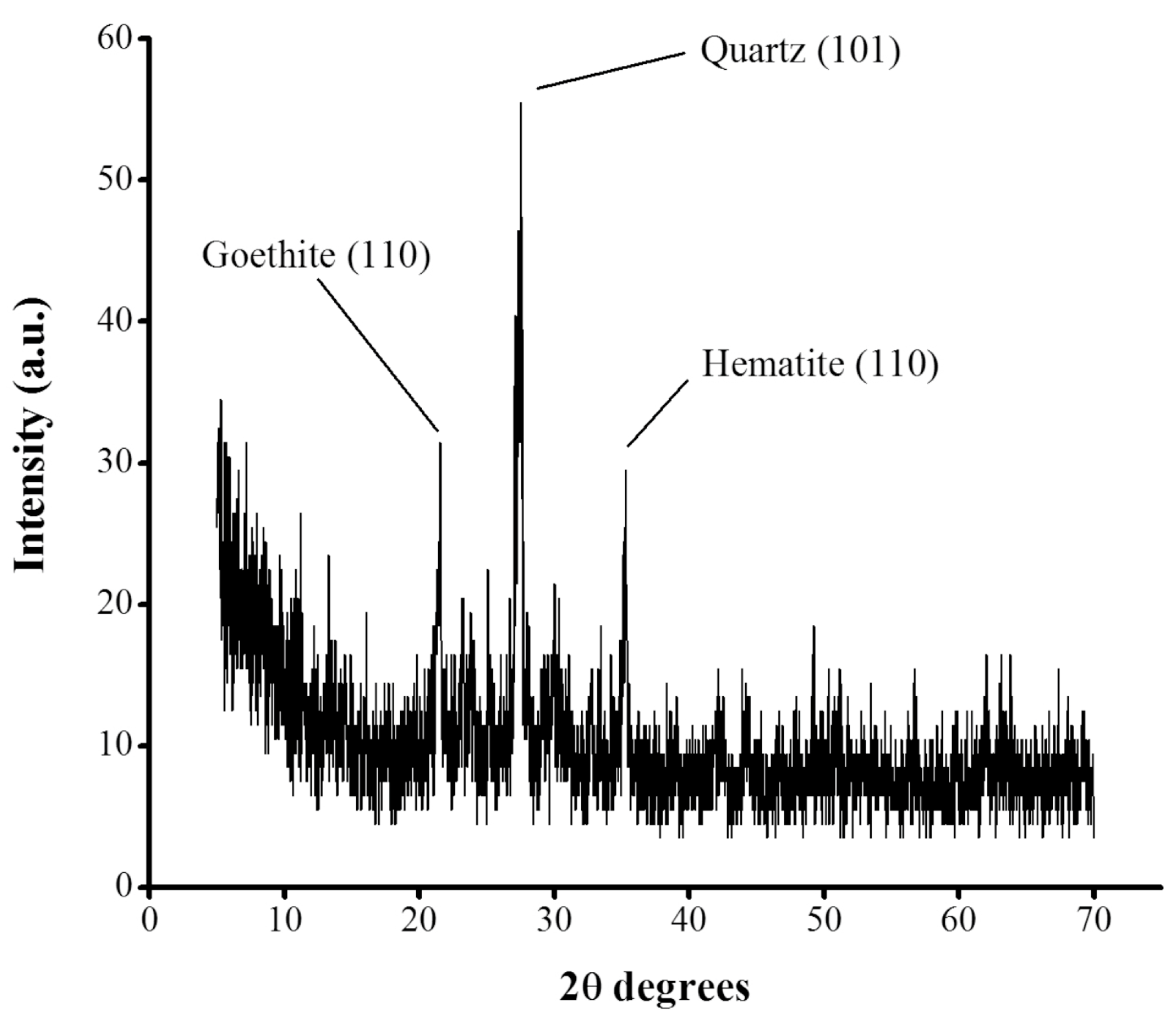
29]. Finally, the X-ray diffraction pattern of
tezontle evaluated in this study (
Figure 3) revealed the crystalline structure of the material with crystalline peaks located at 2θ angles of 21.6°, 27.5°, and 35.3°. The diffraction peak at 2θ = 21.6° corresponds to the plane (110) of goethite (FeO(OH)), while the peak at 27.5° belongs to the plane (101) of quartz (SiO
2) according to Brooks et al. [
30]. On the other hand, the peak at 35.3° corresponds to the crystalline plane (110) of hematite (Fe
2O
3) according to Farahmandjou & Soflaee [
31]. These results, are in line to those crystalline planes reported for
tezontle by Ponce et al. [
32] who proposed a preliminary composition consisted mainly of quartz (SiO
2) and ferric oxides like hematite (Fe
2O
3).
3.2. Adsorption Kinetics of CBZ onto Tezontle without Biofilm
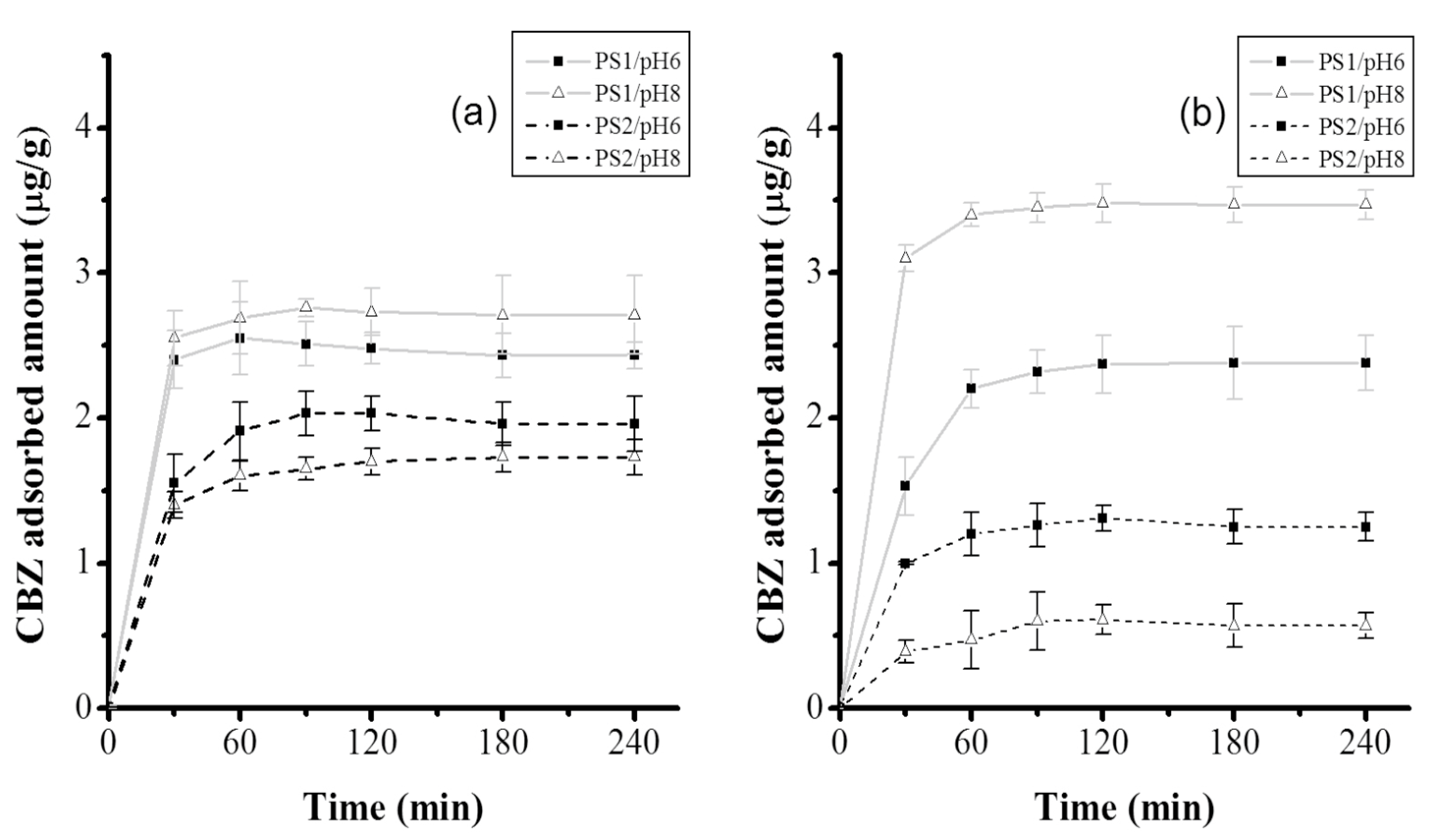
The kinetics of CBZ adsorption by
tezontle under the different assessed conditions showed a similar behavior with respect to the time that the equilibrium was accomplished (
Figure 4). With regard to the reached equilibrium concentrations, they showed in general, a low adsorption capacity of
tezontle, with small variations at 16 °C irrespective of pH or particle size (
Table 2); higher variations were observed at 25 °C. However, for both temperatures, the adsorption capacity of
tezontle was higher with PS1; such results were expected, since it is well known that smaller particle sizes have larger surface areas available for adsorbate-adsorbent interactions.
The mathematical model of the factorial design [
33] used to find the optimal conditions for CBZ removal by
tezontle is shown in Equation (1). In addition, the experimental design along with the data obtained in the CBZ adsorption experiments are shown in
Table 3.
i = 1 and 2.
j = 1 and 2.
k = 1 and 2 for this particular case.
µ, is the overall mean effect.
, are the effects of the
i th level of factor A (particle size),
; of the
j th level of factor B (temperature),
; and, of the
k th level of factor C (pH),
.
, are the effects of the interactions between A × B, A × C, B × C and A × B × C, respectively.
, is the random error in the combination
ijkl and
l are the replicates.
According to the ANOVA (
Table 4), out of the three factors, only the particle size was significant (
p < 0.05) for CBZ adsorption, confirming that PS1 functions better that PS2 as was aforementioned with regard to
Figure 4. However, even more important, the interaction between the evaluated three factors (particle size, temperature, and pH) was significant (
p < 0.05), and the best experimental conditions for CBZ adsorption onto
tezontle was the combination of PS1, pH 8, and 25 °C. Under such conditions, the lowest equilibrium concentration and the corresponding highest removal of CBZ was reached (
Table 2). It is important to point out that although the adsorptive capacity of
tezontle was found to be low, the contribution of this porous medium to CBZ removal remains important because of the quantity of filter medium required in CWs, as well as the concentration of this drug in wastewater.
The increase in
tezontle adsorption capacity with temperature is probably due to the fact that temperature enhances the mobility of organic compounds, which may lead to a higher adsorption [
15]. Nevertheless, this behavior was only observed in this particular case (PS1, pH 8), while in the other cases the adsorption capacity decreased with the increase in temperature. In this way, apparently temperature does not have a unique effect on the adsorption of CBZ onto
tezontle. On the other hand, a better result at pH 8 in comparison to pH 6 coincides with the findings of other authors who affirm that under acidic conditions, the positively-charged adsorbent surface do not favor pharmaceutical sorption [
13], in particular for neutral-organic compounds, such as CBZ [
2].
With respect to the kinetic of the adsorption of CBZ on
tezontle, the two most common models were evaluated, i.e., Lagergren pseudo-first-order model (Equation (2)) and pseudo second-order model (Equation (3)).
where
K1 is Lagergren rate constant (min
−1);
K2 is pseudo second-order rate constant (g/µg·min);
qe and
qt are the amount of pollutant adsorbed at equilibrium (µg/g) and at time
t (min), respectively.
The pseudo-first-order model assumes a proportional relation for the rate of adsorption, while the pseudo-second-order equation suggests that the rate of adsorption is proportional to the square of the number of unoccupied sites [
34]. According to the correlation coefficient R
2, the pseudo-first order model does not fit well to the data obtained from the experiments (
Table 5). In contrast, a better fit to pseudo-second order model was found for all assays (
Table 5), which suggests a CBZ chemisorption [
35,
36] onto
tezontle.
The previous results were confirmed through the quantification of actives sites (in PS1), which determines the extent of a chemisorption process [
37]. Similar to activated carbons [
38], both acid and basic sites were found, in this case in 0.087 and 0.147 meq/g, respectively. Due to the capability of the carboxamide group present in the molecule of CBZ, in particular the -NH
2 group of forming hydrogen bond [
39], one probable mechanism for CBZ adsorption was the formation of hydrogen bonds with π electrons from BAS on the
tezontle surface. From studies on carbon surfaces, it has been found that basic properties are associated with Lewis sites located at the π electron-rich regions [
38], characteristic of oxygen-containing functionality capable of acting as a basic center [
40]. In this case, the presence of oxygen in a
tezontle surface could be due to its main components, i.e., quartz and ferric oxides [
32].
3.3. Adsorption Kinetic Assays of CBZ onto Tezontle with Biofilm
The characteristics of the sedimented wastewater used to promote biofilm formation on the
tezontle with PS1 inside the columns are shown in
Table 6. The characteristics were similar to those of the wastewater used in pilot-scale hybrid wetlands for CBZ removal [
9].
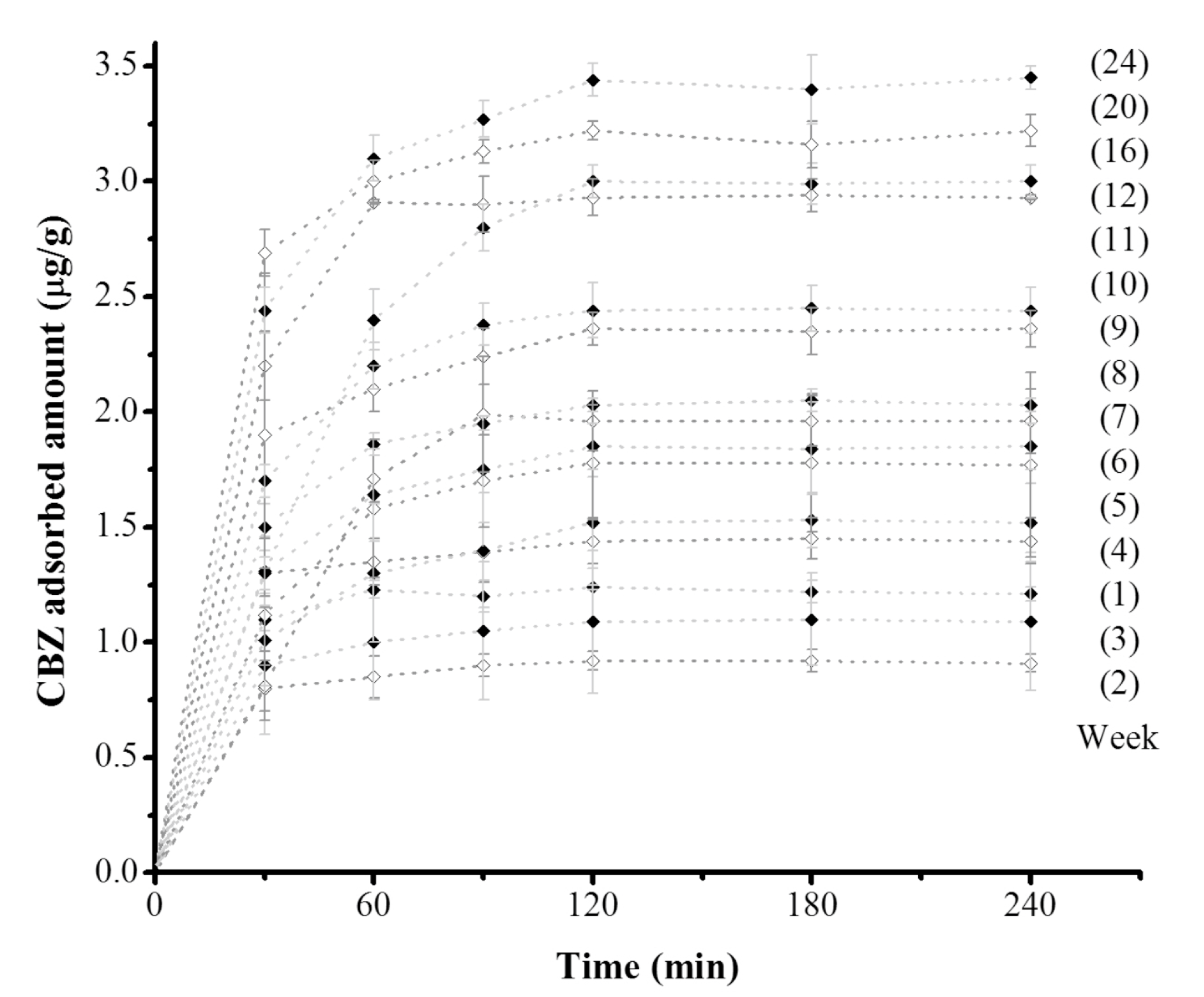
Similar to the essays with
tezontle without biofilm growth, the time for the equilibrium to be reached was 120 min for all of the assays. However, the CBZ equilibrium concentrations varied according to the exposition period of
tezontle to wastewater. In general, the higher the time of exposition, the larger the adsorption capacity (
Figure 5). These results indicate a modification on the basal state of contact surface of
tezontle when being exposed to wastewater and suggest the presence of biofilm. It is recognized that the presence of biofilm implies the attachment and deposition of extracellular polymeric substances (EPS) along with bacterial cells and this complex matrix modify the physicochemical characteristics of carrier surfaces [
41]. Moreover, the evolution in the sorption capacity of
tezontle is probably related to the time required for the biofilm growth. Although the biofilm formation begins within a few minutes, the complete process to reach a mature biofilm capable of produce EPS, responsible of the sorption process, might require days [
42].
On the other hand, despite the increase in the adsorption capacity of
tezontle along the time, with a noticeable increase in the removal percent of CBZ, the maximum value reached after 24 weeks was smaller than the value obtained with
tezontle without biofilm.
Figure 6 shows increments in the percent of CBZ removal, almost with a linear tendency (R
2 = 0.95) over time, starting in the second week until the end of the experiment. These results suggest that the removal percentage could probably reach and possibly surpass that obtained with free-biofilm
tezontle with larger periods for biofilm growth.
Furthermore, as expected, the experimental data obtained from each adsorption kinetic showed a better fit to pseudo-second order kinetic model (
Table 7) alike to the adsorption kinetic with free-biofilm
tezontle, indicating a chemisorption process. Once more, these results were confirmed through the quantification of active sites in each sample of
tezontle with biofilm growth, throughout the study. After the first week, the concentration of BAS as well as the concentration of AAS showed a visible decrease in comparison to the concentration in free-biofilm
tezontle and then, even more after two weeks (
Figure 7); possibly as a consequence of the coating of basal active sites in the
tezontle surface by bacterial attachment which changed its physicochemical characteristics as was aforementioned.
After this general reduction in the active sites concentration, the AAS showed a slight increase during the next two weeks and then, a clear tendency of decreasing until almost its disappearance after ~10 weeks, suggesting their minimal contribution to the adsorption process. In contrast, a noticeable increase was found in the BAS concentration along the time, suggesting that the BAS presence after the second week was due to the biofilm growth onto the
tezontle exposed to wastewater and specifically, due to the release of EPS by forming-biofilm microbes. EPS are high-molecular-weight molecules consisting mainly of polysaccharides (40%), DNA, proteins, lipids, and humic acids [
41,
43]. Charged or hydrophobic polysaccharides and proteins are particularly responsible of organic compound sorption [
44]. Some specific polysaccharide monomers detected by Andersson et al. [
45] in EPS released by microbial consortia developed in wastewater are rhamnose, arabinose, galactose, glucose, mannose, ribose among others. In this way, this chemical structures with large π electron-rich region could have participated in the CBZ chemisorption process through hydrogen bonds [
23]. In addition, a clear relationship was observed between BAS concentration and CBZ removal percent, highlighting this pathway as the main mechanism of adsorption.
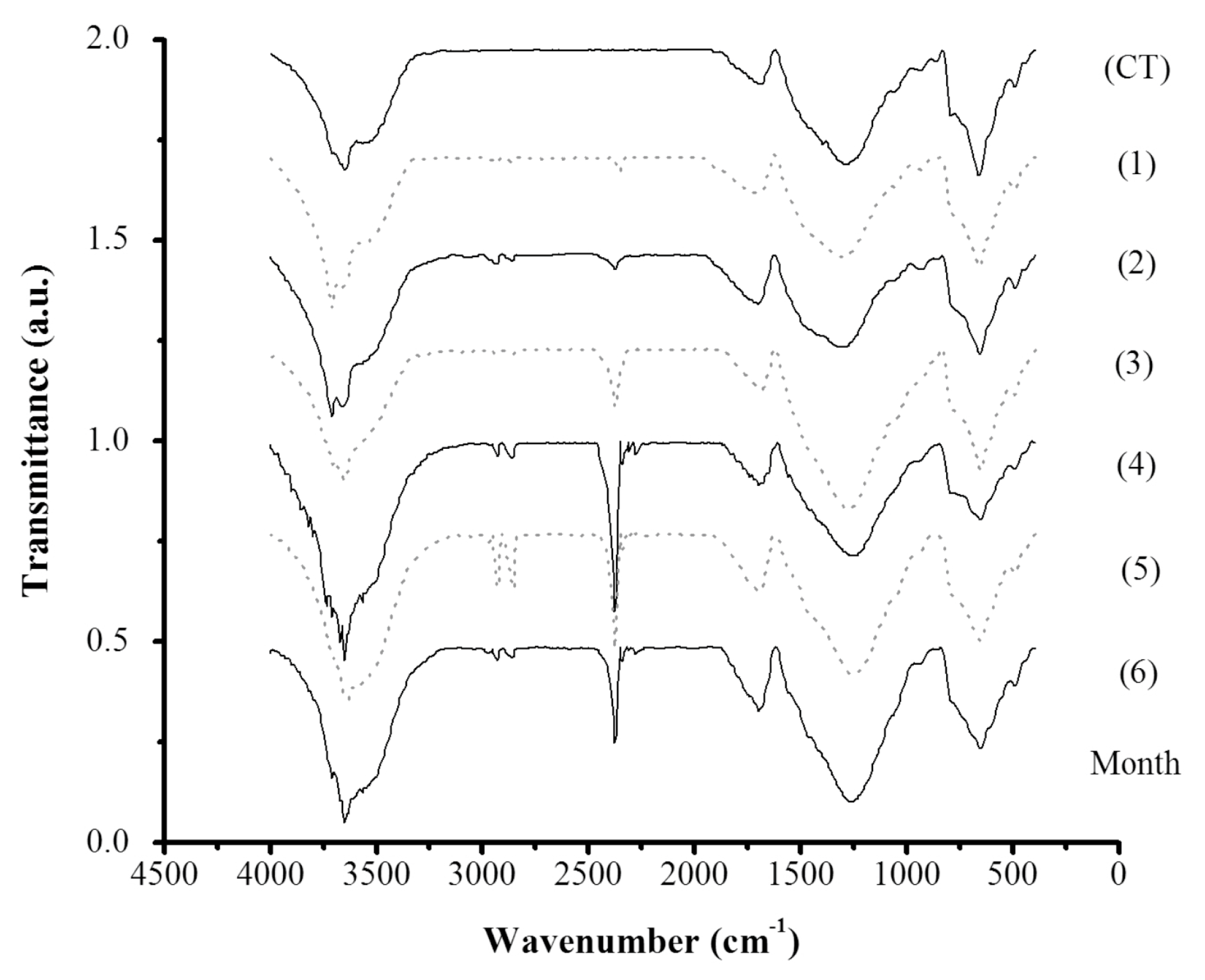
The presence of biofilm on
tezontle was confirmed through FTIR analysis by which the presence of characteristic biofilms peaks was observed (
Figure 8). The small bands between 2900 and 3000 cm
−1 are related to C-H stretching vibration [
41] associated with bacterial biomass [
46], whereas the peak at around 2400 cm
−1 is due to the vibration of C-O functional groups likely from carboxylic acids which has been reported as a sorption active site present in the cell wall of Gram-positive bacteria [
46]. Other characteristic biofilm bands corresponding to proteins (1637–1660 and 1272–1288 cm
−1) and polysaccharides (1000–1132 cm
−1) have been reported in the literature [
41]; however, in this study they were not detected, apparently because of the wide and intense bands in the
tezontle FTIR fingerprint between 400 and 1750 cm
−1, which interfered with the detection of these bands.
On the other hand, there is a clear difference between the spectra of free-biofilm tezontle and those of tezontle with biofilm growth, in the sense that the first one does not present the aforementioned bands that showed a noticeable evolution in the FTIR spectra along the time of experimentation.
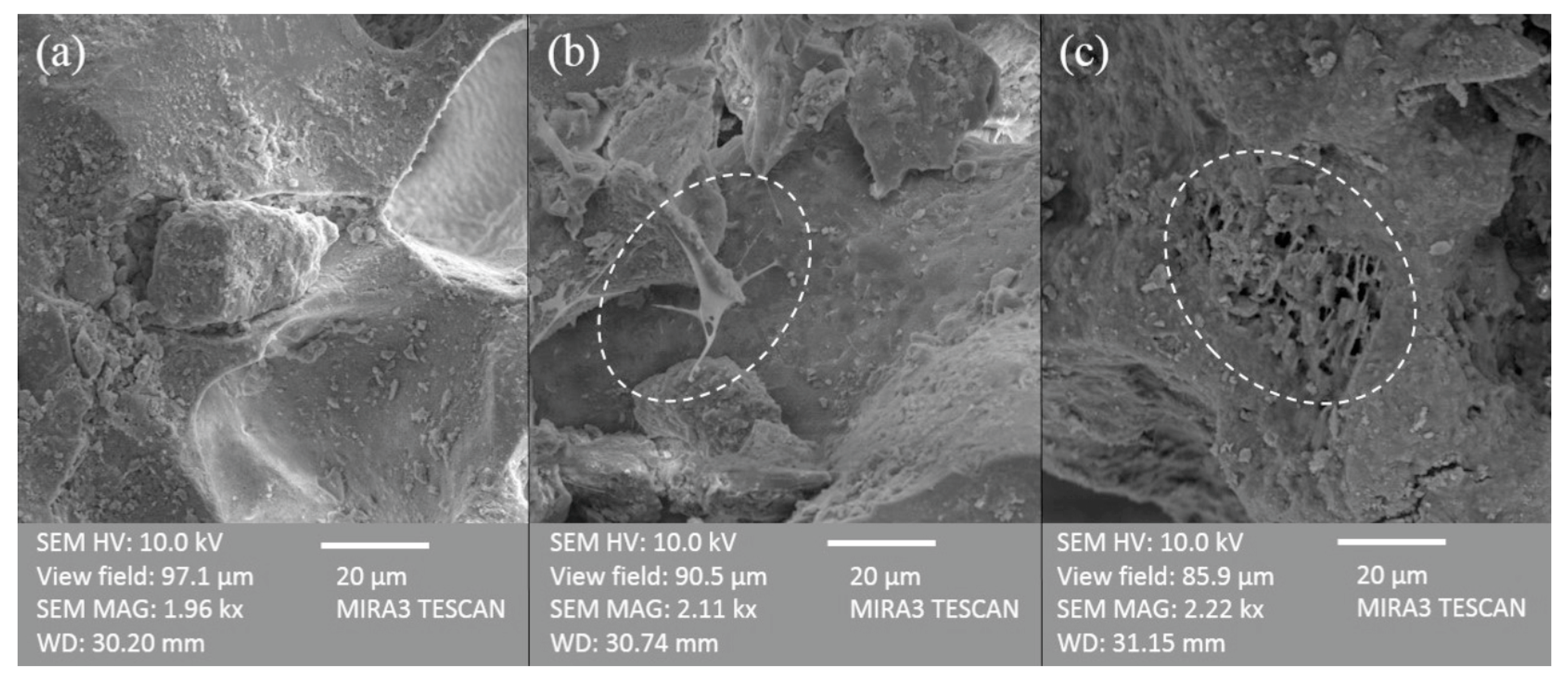
Finally, the FE-SEM micrographs revealed the presence of bridge-shape structures on the
tezontle surface, which have been reported as a common physical structure of biofilms whose number usually increases through the time [
47]. A comparison between free-biofilm
tezontle and
tezontle with two different periods of biofilm development (4 and 24 weeks) shows the highest density of biomass after 24 weeks (
Figure 9).