Dissipation of Micropollutants in a Rewetted Fen Peatland: A Field Study Using Treated Wastewater
Abstract
:1. Introduction
2. Materials and Methods
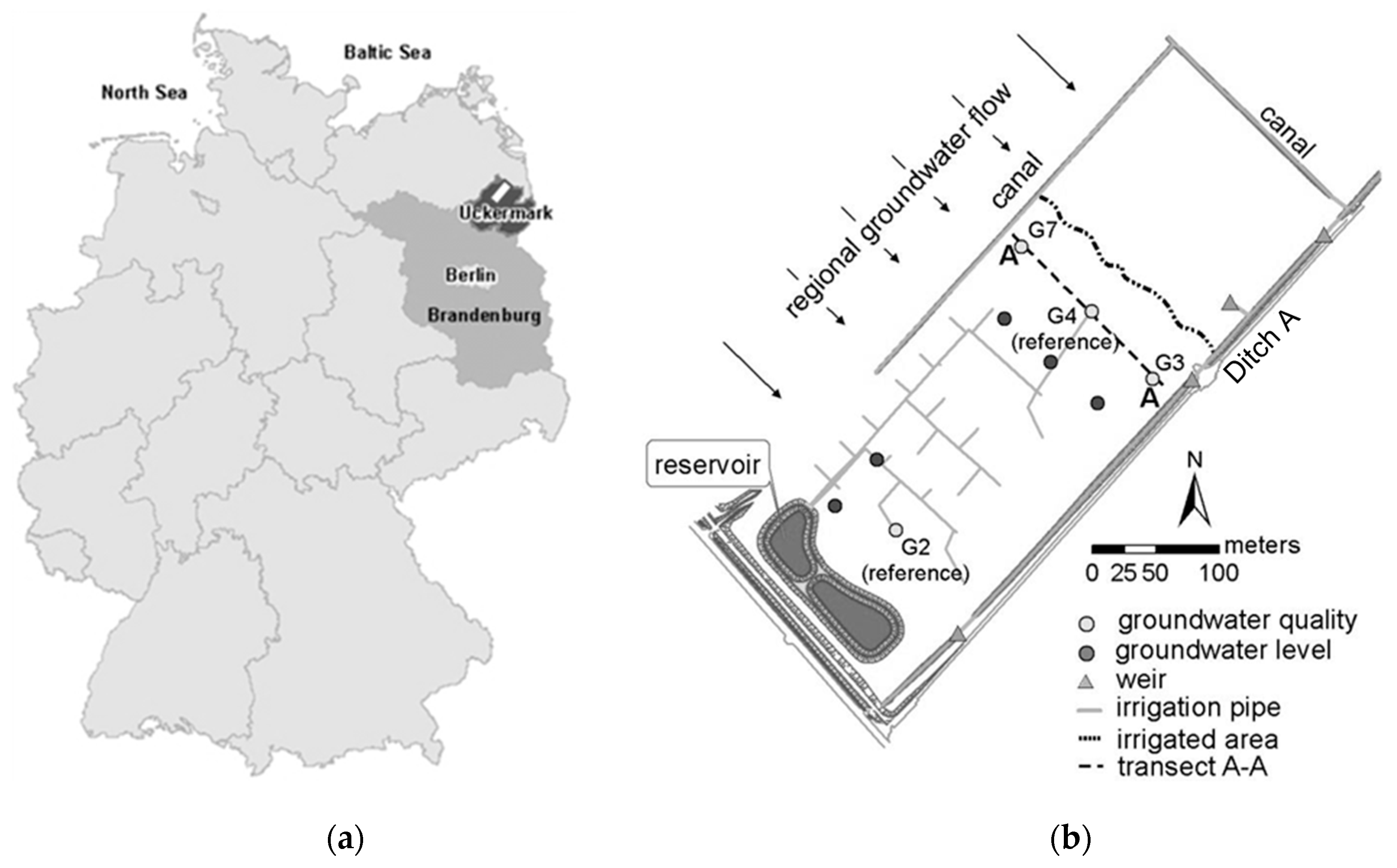
2.1. Study Site Description
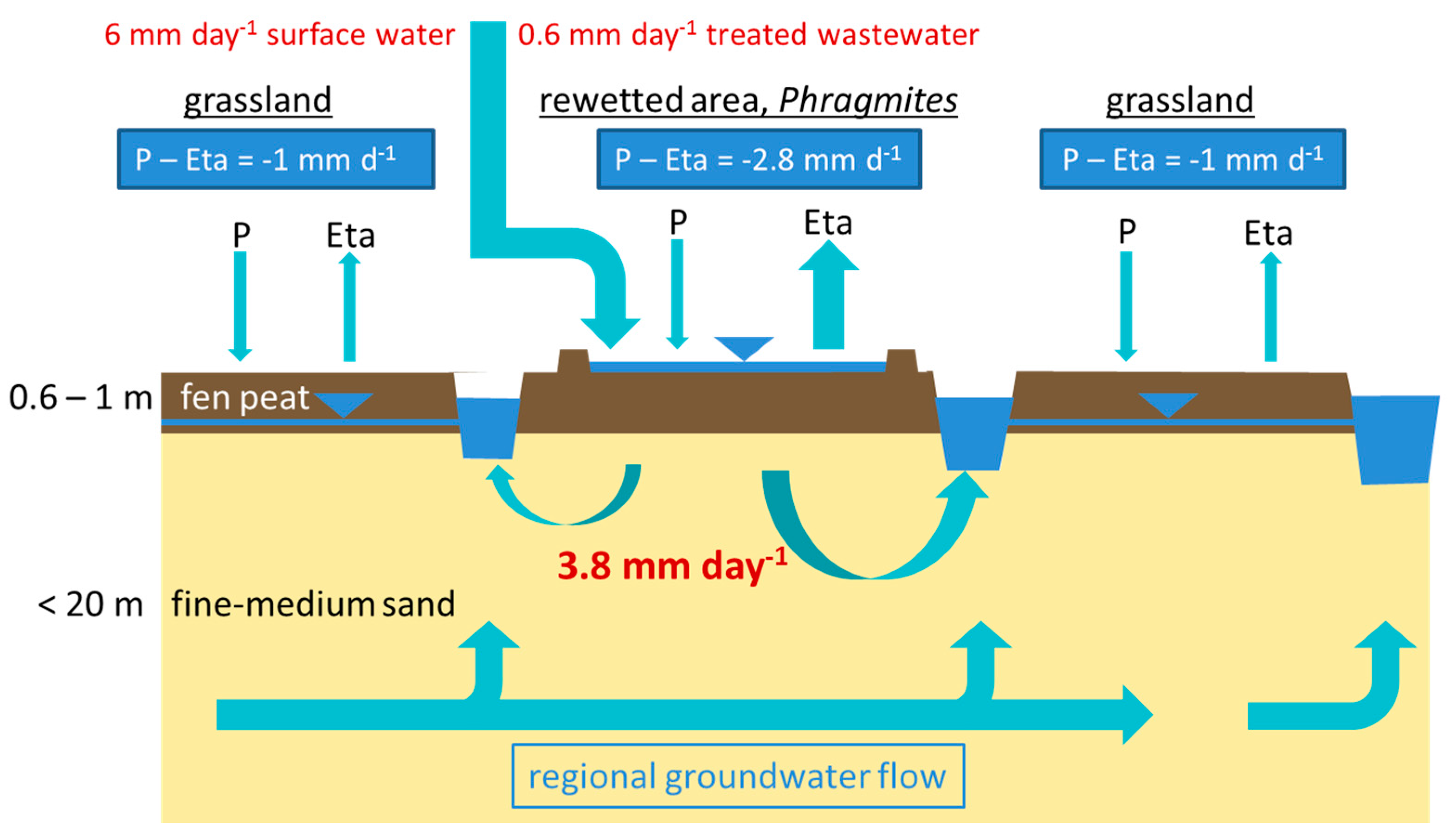
2.2. Flooding Regime
2.3. Monitoring Program
2.4. Micropollutants in Water and Soil
2.5. Biotests with Soil before and after Flooding
3. Results
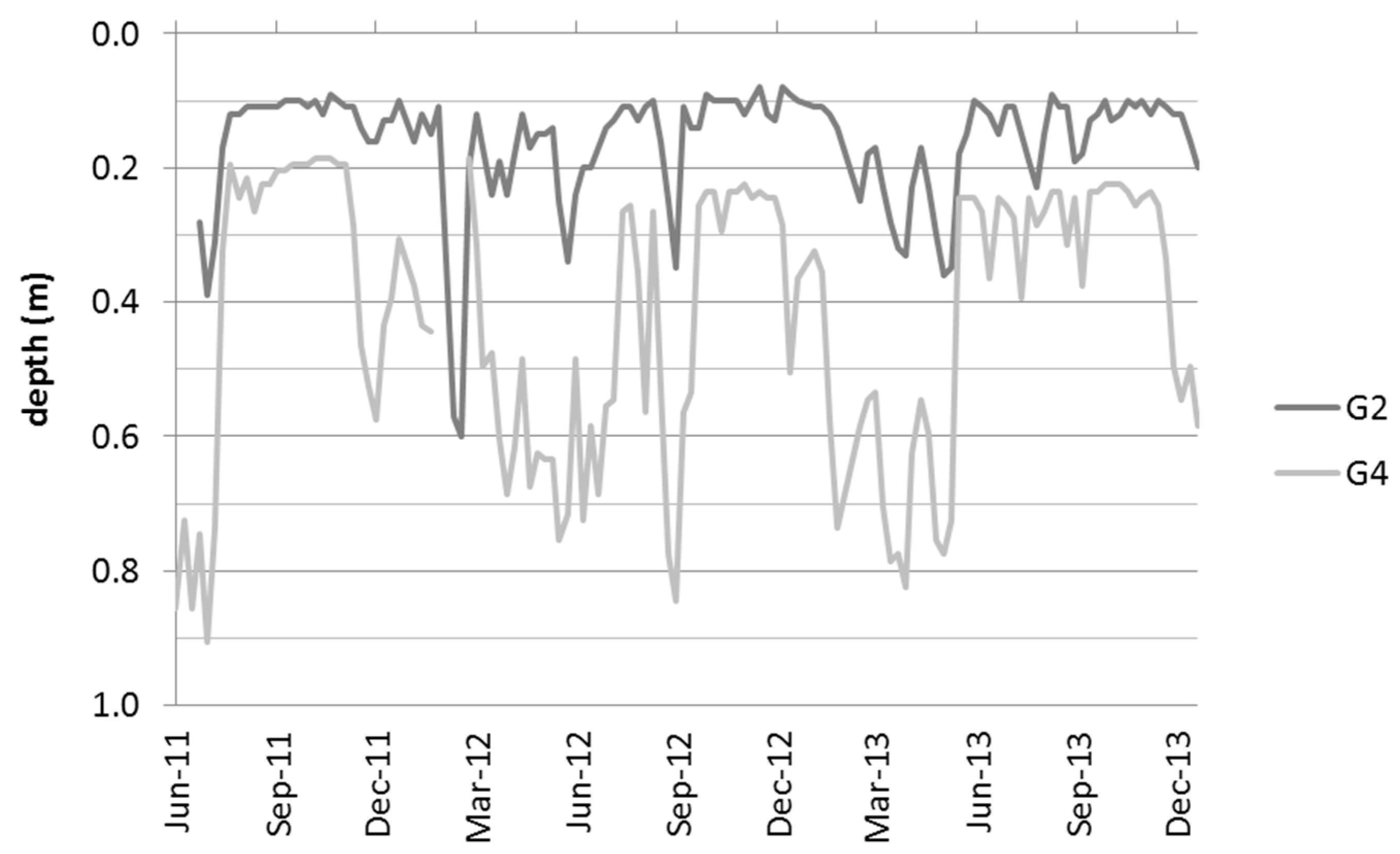
3.1. Hydrological and Geohydraulic Conditions, and Physio-Chemical Soil Properties
3.2. Micropollutants in the Treated Wastewater, Groundwater, Surface Water and Peat Soil
3.3. Microbial Activity
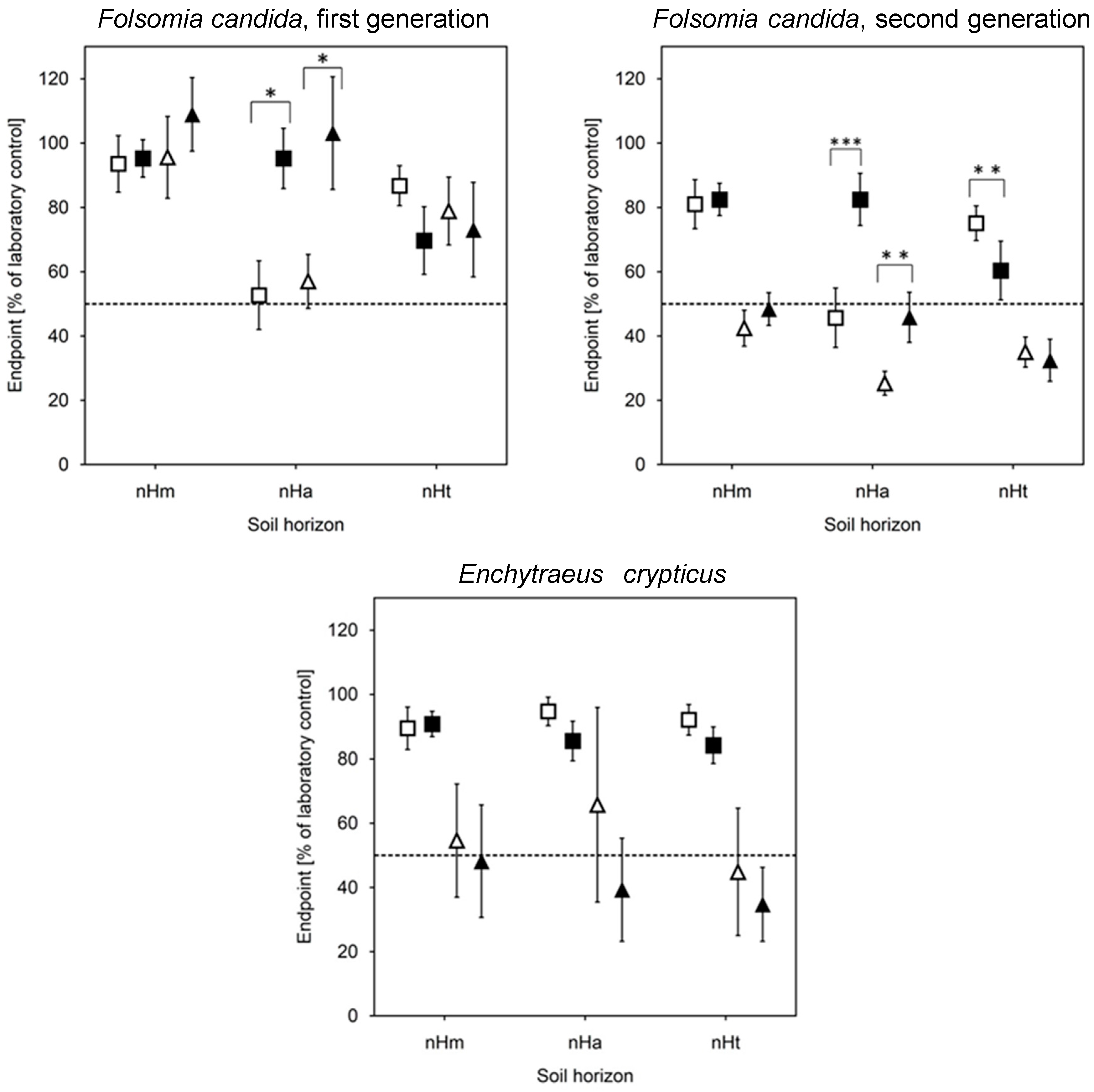
3.4. Biotests with Soil (Risk Assessment)
4. Discussion
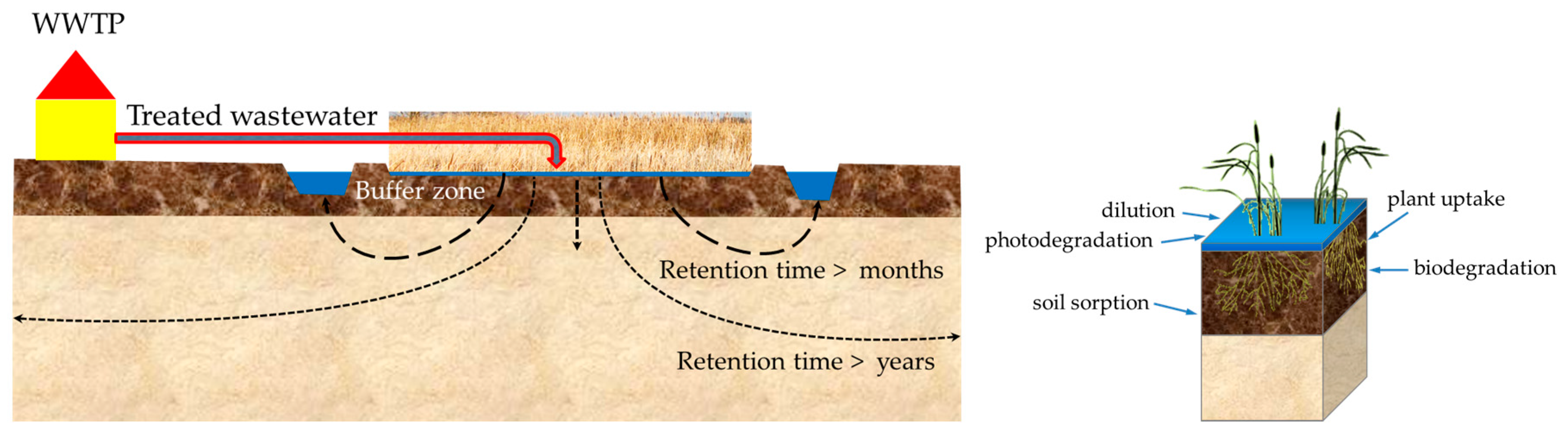
4.1. Dissipation
- First group, where the concentration in the groundwater was approximately identical with the TMC and higher than the LoQ: diclofenac, p-TSA + o-TSA, AA, metoprolol, and bezafibrate.
- Second group, where the measured concentration in the groundwater is lower than the TMC: acesulfame, diatrizoate, FAA, tolyltriazole, carbamazepine, benzotriazole, PBSA, and AAA.
4.2. Microbial Activity and Potential for the Degradation of Micropollutants in Peat Soils
4.3. Ecotoxicological Impacts of Wastewater Application
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions—A Blueprint to Safeguard Europe’s Water Resources, COM (2012) 673 Final. Available online: http://ec.europa.eu/environment/water/blueprint/ia_en.htm (accessed on 1 February 2017).
- Naiman, C.P.J.; Decamps, H.; Mc Clain, M.E. Ecology, Conservation, and Management of Streamside Communities, Riparia 1st ed.; Elsevier Academic Press: London, UK, 2005. [Google Scholar]
- Roebeling, P.C.; Cunha, M.C.; Arroja, L.; van Grieken, M.E. Agricultural water pollution treatment for efficient water quality improvement in linked terrestrial and marine ecosystems. J. Coast. Res. 2011, 64, 1936–1940. [Google Scholar]
- Couwenberg, J.; Fritz, C. Towards developing IPCC methane ‘emission factors’ for peatlands (organic soils). Mires Peat 2012, 10, 1–17. [Google Scholar]
- Maassen, S.; Balla, D.; Dannowski, R. Long-term behavior of groundwater chemistry in a periodically rewetted fen area covered with macrophytes. J. Environ. Manag. 2015, 150, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Pharmaceuticals in the environment. Annu. Rev. Environ. Resour. 2010, 35, 57–75. [Google Scholar] [CrossRef]
- Ternes, T.; Joss, A. Human Pharmaceuticals, Hormones and Fragrances—The Challenge of Micropollutants in Urban Waste Water Manag; IWA Publishing: London, UK, 2007. [Google Scholar]
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; von Gunten, U.; Wehrli, B. The challenge of micropollutants in aquatic systems. Science 2006, 313, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Noguera-Oviedo, K.; Aga, D.S. Lessons learned from more than two decades of research on emerging contaminants in the environment. J. Hazard. Mater. 2016, 316, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Lahti, M.; Oikari, A. Microbial transformation of pharmaceuticals naproxen, bisoprolol, and diclofenac in aerobic and anaerobic environments. Arch. Environ. Contam. Toxicol. 2011, 61, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Wick, A.; Fink, G.; Joss, A.; Siegrist, H.; Ternes, A.T. Fate of beta blockers and psycho-active drugs in conventional wastewater treatment. Water Res. 2009, 43, 1060–1074. [Google Scholar] [CrossRef] [PubMed]
- Imfeld, G.; Braeckevelt, M.; Kuschk, P.; Richnow, H.H. Monitoring and Assessing Processes of Organic Chemicals Removal in Constructed Wetlands. Chemosphere 2009, 74, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Schröder, P.; Navarro-Aviñó, J.; Azeizeh, H.; Goldhirsch, A.G.; DiGrigorio, S.; Komives, T.; Langergraber, G.; Lenz, A.; Maestri, E.; Memon, A.R.; et al. Using phytoremediation technologies to upgrade water treatment in Europe. Environ. Sci. Pollut. Res. 2007, 14, 490–497. [Google Scholar] [CrossRef]
- Carvalho, P.N.; Basto, M.C.P.; Almeida, C.M.R.; Brix, H. A Review of plant-pharmaceutical interactions: From uptake and effects in crop plants to phytoremediation in constructed wetlands. Environ. Sci. Pollut. Res. 2014, 21, 11729–11763. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, M.I.; Lambrianides, A.; Schneider, M.; Kümmerer, K.; Fatta-Kassinos, D. Environmental side effect of o pharmaceutical coctails: What we know and what we should know. J. Hazard. Mater. 2014, 279, 169–189. [Google Scholar] [CrossRef] [PubMed]
- Verlicchi, P.; Zambello, E. How efficient are constructed wetlands in removing pharmaceuticals from untreated and treated urban wastewaters? A review. Sci. Total Environ. 2014, 470, 1281–1306. [Google Scholar] [CrossRef] [PubMed]
- Maassen, S.; Balla, D. Impact of hydrodynamics (ex- and infiltration) on the microbially controlled phosphorus mobility in running water sediments of a cultivated northeast german wetland. Ecol. Eng. 2010, 36, 1146–1155. [Google Scholar] [CrossRef]
- Zeitz, J.; Velty, S. Soil properties of drained and rewetted fen soils. J. Plant Nutr. Soil Sci. 2002, 165, 618–626. [Google Scholar] [CrossRef]
- Shareef, A.; Page, D.; Vanderzalm, J.; Williams, M.; Gupta, V.V.S.R.; Dillon, P.; Kookana, R. Biodegradation of Simazine and Diuron Herbicides Under Aerobic and Anoxic Conditions Relevant to Managed Aquifer Recharge of Storm Water. Clean Soil, Air Water 2014, 42, 745–752. [Google Scholar] [CrossRef]
- Siemens, J.; Huschek, G.; Walshe, G.; Siebe, C.; Kasteel, R.; Wulf, S.; Clemens, J.; Kaupenjohann, M. Transport of Pharmaceuticals in Columns of a Wastewater-Irrigated Mexican Clay Soil. J. Environ. Qual. 2010, 39, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, V.; Caselles-Osorio, A.; García, J.; Bayona, J.M. Behaviour of Pharmaceutical products and biodegradation intermediates in horizontal subsurface flow constructed wetland. A microcosmexperiment. Sci. Tot. Environ. 2008, 394, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Greskowiak, J.; Prommer, H.; Massmann, G.; Nützmann, G. Modeling seasonal redox dynamics and the corresponding fate of the pharmaceutical residue during artificial recharge of groundwater. Environ. Sci. Technol. 2006, 40, 6615–6621. [Google Scholar] [CrossRef] [PubMed]
- Balla, D.; Maassen, S.; Dannowski, R. Value-adding of rewetted fen peatlands using their purification potential with respect to surface water. In Peatlands in Balance, Proceedings of the 14th International Peat Congress, Stockholm, Sweden, 3–8 June 2012; International Peat Society: Jyväskylä, Finland, 2012; Abstract 423. [Google Scholar]
- World Reference Base for Soil Resources. Available online: http://www.fao.org/docrep/w8594e/w8594e00.HTM (accessed on 1 February 2017).
- Arbeitsgruppe Boden. Bodenkundliche Kartieranleitung, 4th ed.; Publisher: Bundesanstalt für Geowissenschaften und Rohstoffe und Geologische Landesämter: Hannover, Germany, 1994. (In German) [Google Scholar]
- Dannowski, R.; Balla, D. Wasserhaushalt und geohydraulische Situation einer vernässten Niedermoorfläche mit Schilfanbau in Nordost-Brandenburg. Arch. Nat. Conserv. Landsc. Res. 2004, 43, 27–40. (In German) [Google Scholar]
- Grundwasserverordnung vom 9. November 2010 (BGBl. I S. 1513). Available online: https://www.gesetze-im-internet.de/bundesrecht/grwv_2010/gesamt.pdf (accessed on 1 February 2017).
- FEFLOW. Available online: https://www.mikepoweredbydhi.com (accessed on 1 February 2017).
- Wode, F.; Reilich, C.; van Baar, P.; Dünnbier, U.; Jekel, M.; Reemtsma, T. Multiresidue analytical method for the simultaneous determination of 72 micropollutants in aqueous samples with ultra high performance liquid chromatography–high resolution mass spectrometry. J. Chromatogr. A 2012, 1270, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Rühmland, S.; Wick, A.; Ternes, T.A.; Barjenbruch, M. Fate of pharmaceuticals in a subsurface flow constructed wetland and two ponds. Ecol. Eng. 2015, 80, 125–139. [Google Scholar] [CrossRef]
- Braguglia, C.M.; Coors, A.; Gallipoli, A.; Gianico, A.; Guillon, E.; Kunkel, U.; Mascolo, G.; Richter, E.; Ternes, T.A.; Tomei, M.C.; Mininni, G. Quality Assessment of digested sludges produced by advanced stabilization processes. Environ. Sci. Pollut. Res. Int. 2015, 22, 7216–7235. [Google Scholar] [CrossRef] [PubMed]
- Analyses Data Sheet for Standard Soils according to GLP. Available online: http://www.lufa-speyer.de/images/stories/2016-STB-Bo-data_sheet.pdf (accessed on 1 February 2017).
- ISO 17616. Soil Quality - Guidance on the Choice and Evaluation of Bioassays for Ecotoxicological Characterization of Soils and Soil Materials (2008). Available online: http://www.iso.org/iso/catalogue_detail.htm?csnumber=38417 (accessed on 1 February 2017).
- Succow, M.; Joosten, H. Landschaftsökologische Moorkunde; Schweizerbart‘sche Verlagsbuchhandlung: Stuttgart, Germany, 2010. (In German) [Google Scholar]
- Schindler, U.; Behrendt, A.; Müller, L. Change of soil hydrological properties of fens as a result of soil development. J. Plant Nutr. Soil Sci. 2003, 166, 357–363. [Google Scholar] [CrossRef]
- Umweltbundesamt. Bewertung der Abwesenheit teil- oder nicht bewertbarer Stoffe im Trinkwasser aus gesundheitlicher Sicht—Empfehlungen der Trinkwasserkommission des Umweltbundesamtes. Bundesgesundheitsbl-Gesundheitsforsch-Gesundheitsschutz 2003, 46, 249–251. (In German) [Google Scholar]
- Working Group of the Federal States on Water Issues (LAWA). Determination of Insignificance Thresholds for Groundwater. Available online: http://www.lawa.de/documents/GFS-Report_8df.pdf (accessed on 17 October 2016).
- Radke, M.; Maier, M. Lessons learned from sediment/water-testing of pharmaceuticals. Wat. Res. 2014, 55, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Scheytt, T.; Mersmann, P.; Lindstädt, R.; Heberer, T. Determination of Sorption Coefficients of Pharmaceutically Active Substances Carbamazepine, Diclofenac, and Ibuprofen, in Sandy Sediments. Chemosphere 2005, 60, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Falås, P.; Wick, A.; Castronovo, S.; Habermacher, J.; Ternes, T.A.; Joss, A. Tracing the Limits of Organic Micropollutants Removal in Biological Wastewater Treatment. Water Res. 2016, 95, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Jewell, K.S.; Falås, P.; Wick, A.; Joss, A.; Ternes, T.A. Transformation of Diclofenac in Hybrid Biofilm-Activated Sludge Processes. Water Res. 2016, 105, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Boreen, A.L.; Arnold, W.A.; McNeill, K. Photodegradation of pharmaceuticals in the aquatic environment: A review. Aquat. Sci. 2003, 65, 320–341. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Hua, T.; Gersberg, R.M.; Zhu, J.; Ng, W.J.; Tan, S.K.D. Fate of diclofenac in wetland mesocosm planted with Scirpus validus. Ecol. Eng. 2012, 49, 59–64. [Google Scholar] [CrossRef]
- Meffe, R.; Massmann, G.; Kohfahl, C.; Taute, T.; Richter, D.; Dünnbier, U.; Pekdeger, A. Investigation the redox sensitivity of para-toluenesulfonamide(p-TSA) in groundwater. Environ. Earth Sci. 2012, 65, 861–870. [Google Scholar] [CrossRef]
- Tolls, J. Sorption of Veterinary Pharmaceuticals in Soils: A Review. Environ. Sci. Technol. 2001, 35, 3397–3406. [Google Scholar] [CrossRef] [PubMed]
- Kah, M.; Brown, C.D. Prediction of the adsorption of ionizable pesticides in soils. J. Agric. Food Chem. 2007, 55, 2312–2322. [Google Scholar] [CrossRef] [PubMed]
- Massmann, G.; Dünnbier, U.; Heberer, T.; Taute, T. Behavior and redox sensitivity of pharmaceutical residues during bank filtration—Investigation of Residues of Phenazone-Type Analgesics. Chemosphere 2008, 71, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Kodešová, R.; Grabic, R.; Kočárek, M.; Klement, A.; Golovko, O.; Fér, M.; Nikodem, A.; Jakšík, J. Pharmaceuticals’ sorption relative to soil properties of thirteen different Soils. Sci. Tot. Environ. 2015, 51, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Kodešová, R.; Kočárek, M.; Klement, A.; Golovko, O.; Koba, O.; Fér, M.; Nikodem, A.; Vondráčková, L.; Jakšík, J.; Grabic, R. An analysis of the dissipation of pharmaceuticals under thirteen different soil conditions. Sci. Total Environ. 2016, 544, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, M.; Boxberger, N.; Börnick, H.; Licha, T.; Worch, E. Sorption Influenced transport of ionizable pharmaceuticals onto natural sandy aquifer sediment at different pH. Chemosphere 2012, 8, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, M.; Börnick, H.; Nödler, K.; Licha, T.; Worch, E. Role of cation exchange processes on the sorption influenced transport of cationic ß-blockers in Aquifer Sediments. Water Res. 2012, 46, 5472–5482. [Google Scholar] [CrossRef] [PubMed]
- Wode, F. Screening und Quantifizierung Organischer Spurenstoffe Mittels UPLC-HRMS: Entwicklung von Methoden und Anwendung zur Unterscheidung Historischer und Aktueller Spurenstoffe in Einem Ehemaligen Rieselfeld. Ph.D. Thesis, TU Berlin, Berlin, Germany, 28 August 2014. (In German). [Google Scholar]
- Maeng, S.K.; Sharma, S.K.; Abel, C.D.T.; Magic-Knezev, A.; Amy, G.L. Role of biodegradation in the removal of pharmaceutically Active Compounds with Different Bulk Organic Matter Characteristics Trough Managed Aquifer Recharge. Batch and Column Studies. Water Res. 2011, 45, 4722–4736. [Google Scholar] [CrossRef] [PubMed]
- Heberer, T.; Adam, M. Transport and Attunuation of pharmaceuticals during artificial groundwater replenishment. Environ. Chem. 2004, 1, 22–25. [Google Scholar] [CrossRef]
- Müller, B.; Scheytt, T.; Asbrand, M. Pharmaceuticals as indicator of sewage-influenced groundwater. Hydrogeol. J. 2012, 20, 1117–1129. [Google Scholar] [CrossRef]
- Buerge, I.J.; Keller, M.; Buser, H.-R.; Müller, M.D.; Poiger, T. Saccharin and other Artificial Sweeteners in Soils: Estimated Inputs from Agriculture and Households, Degradation, and Leaching to Groundwater. Environ. Sci. Technol. 2011, 45, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Van Stempvoort, D.R.; Robertson, W.D.; Brown, S.J. Artificial sweeteners in a large septic plume. Ground Water Monit. Rem. 2011, 31, 95–102. [Google Scholar] [CrossRef]
- Jekel, M.; Dott, W.; Bergmann, A.; Dünnbier, U.; Gnirß, R.; Haist-Gulde, B.; Hamscher, G.; Letzel, M.; Licha, T.; Lyko, S.; et al. Selection of organic process and source indicator substances for the anthropogenically influenced water cycle. Chemosphere 2015, 125, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Gan, J.; Nguyen, V.T.; Chen, H.; You, L.; Duarah, A.; Zhang, L.; Gin, K.Y. Sorption and biodegradation of Artificial Sweeteners in Activated Sludge Processes. Bioresour. Technol. 2015, 197, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Scheurer, M.; Brauch, H.-J.; Lange, F.T. Analysis and occurrence of seven Artificial Sweeteners in German waste water and surface water and in soil aquifer treatment (SAT). Anal. Bioanal. Chem. 2009, 394, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Nguyen, V.T.; Urase, T.; Ngo, H.H. Role of Nitrification in the biodegradation of selected artificial sweetening agents in biological wastewater treatment process. Bioresour. Technol. 2014, 161, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Castronovo, S.; Wick, A.; Scheurer, M.; Nödler, K.; Schulz, M.; Ternes, T.A. Biodegradation of the artificial sweetener acesulfame in biological treatment and sandfilters. Water Res. 2017, 110, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Scheurer, M.; Storck, F.R.; Graf, C.; Brauch, H.-J.; Ruck, W.; Lev, O.; Lange, F.T. Correlation of six anthropogenic markers in wastewater, surface water, bank filtrate, and soil aquifer treatment. J. Environ. Monit. 2011, 13, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Redeker, M.; Wick, A.; Meermann, B.; Ternes, T.A. Removal if the iodinated X-ray contrast medium diatrizoate by anaerobic transformation. Environ. Sci. Technol. 2014, 48, 10145–10154. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Aagaard, P.; Breedveld, G.D. Sorption of triazoles to soil and iron minerals. Chemosphere 2007, 67, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Breedveld, G.D.; Roseth, R.; Sparrevik, M.; Hartnik, T.; Hem, L.J. Persistence of the De-icing additive benzotriazole at an abandoned airport. Water Air Soil Pollut. 2003, 3, 91–101. [Google Scholar] [CrossRef]
- Jafvert, C.T. Sorption of organic acid compounds to sediments: Initial model development. Environ. Toxicol. Chem. 1990, 9, 1259–1268. [Google Scholar] [CrossRef]
- Liu, Y.; Ying, G.; Shareef, A.; Kookana, R.S. Biodegradation of three selected benzotriazoles in aquifer material under aerobic and anaerobic conditions. J. Contam. Hydrol. 2013, 151, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Fink, G.; Wintgens, T.; Melin, T.; Ternes, T.A. Sorption behavior of potential organic waste water indicators with soils. Water Res. 2009, 43, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.; Lema, J.M.; Omil, F. Removal of pharmaceutical and personal care products (PPCPs) under nitrifying and denitrifying conditions. Water Res. 2010, 44, 3214–3224. [Google Scholar] [CrossRef] [PubMed]
- Hamann, E.; Stuyfzand, P.J.; Greskowiak, J.; Timmer, H.; Massmann, G. The Fate of organic micropollutants during long-term/long-distance river bank filtration. Sci. Total Environ. 2016, 545–546, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Grossberger, A.; Hadar, Y.; Borch, T.; Chefetz, B. Biodegradability of pharmaceutical compounds in agricultural soils irrigated with treated waste water. Environ. Pollut. 2014, 185, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Revitt, D.M.; Balogh, T.; Jones, H. Sorption Behavior and transport potential for selected pharmaceuticals and triclosan in two sterilized soils. J. Soils Sediments 2015, 15, 594–606. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Gschwend, P.M.; Imboden, D.M. Environmental Organic Chemistry, 2nd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Borisover, M.; Sela, M.; Chefetz, B. Enhancement Effect of water associated with natural organic matter (NOM) on organic compound-NOM Interactions: A case study with carbamazepine. Chemosphere 2011, 82, 1454–1460. [Google Scholar] [CrossRef] [PubMed]
- Aristilde, L.; Sposito, G. Complexes of the antimicrobial ciprofloxacin with soil, peat, and aquatic humic substances. Environ. Toxicol. Chem. 2013, 32, 1467–1478. [Google Scholar]
- Żabczyński, S.; Buntner, D.; Miksch, K.; Roig, B. Performance of the conventional treatment processes of the most resistant PPs. In Pharmaceuticals in the Envir. Current Knowledge and Need Assessment to Reduce Presence and Impact; Roig, B., Ed.; IWA publishing: London, UK, 2010; pp. 87–114. [Google Scholar]
- Sauvêtre, A.; Schröder, P. Uptake of Carbamazepine by rhizomes and endophytic bacteria of phragmites australis. Front. Plant. Sci. 2015, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.; Hecht, F.; Schnellbacher, N.; Ternes, T.A.; Wick, A.; Wode, F.; Coors, A. Assessing the ecological long-term impact of wastewater irrigation on soil and water based on bioassays and chemical analyses. Water Res. 2015, 84, 33–42. [Google Scholar] [CrossRef] [PubMed]
- König, A.; Weidbauer, C.; Seiwert, B.; Reemtsma, T.; Unger, T.; Jekel, M. Reductive Transformation of carbamazepine by biotic and abiotic processes. Water Res. 2106, 101, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Wick, A.; Marincas, O.; Moldovan, Z.; Ternes, T.A. Sorption of biocides, triazine, and phenylurea herbicides, and UV-filters onto secondary sludge. Water Res. 2011, 45, 3638–3652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, J.; Wang, Y. Humic Acids decrease the photodegradation of the sunscreen UV filter 2-phenylbenzimidazole-5-sulfonic acid in natural waters. Environ. Chem. Lett. 2012, 10, 389–394. [Google Scholar] [CrossRef]
- Barret, M.; Delgadillo-Mirquez, L.; Trably, E.; Delgenes, N.; Braun, F.; Cea-Barcia, G.; Steyer, J.P.; Patureau, D. Anaerobic Removal of trace organic contaminants in sewage sludge: 15 Years of Experience. Pedosphere 2012, 22, 508–517. [Google Scholar] [CrossRef]
- Delgadillo-Mirquez, L.; Lardon, L.; Steyer, J.P.; Patureau, D. A New dynamic model for bioavailability and cometabolism of micropollutants during anaerobic digestion. Water Res. 2011, 45, 4511–4521. [Google Scholar] [CrossRef] [PubMed]
- Stasinakis, A.S. Review on the Fate of Emerging Contaminants during Sludge Anaerobic Digestion. Bioresour. Technol. 2012, 121, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Helbling, D.E.; Johnson, D.R.; Honti, M.; Fenner, K. Micropollutant biotransformation kinetics associate with WWTP process parameters and microbial community characteristics. Environ. Sci. Technol. 2012, 46, 10579–10588. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Harper, W.; Holbrook, R.; Love, N. Role of Particle size and ammonium oxidation in removal of a-ethinyl estradiol in bioreactors. J. Environ. Eng. 2006, 132, 1527–1529. [Google Scholar] [CrossRef]
- Reiche, M.; Hädrich, A.; Lischeid, G.; Küsel, K. Impact of manipulated drought and heavy rainfall events on peat mineralization processes and source-sink functions of an acidic fen. J. Geophys. Res. 2014, G02021. [Google Scholar] [CrossRef]
- Hill, B.H.; Elonen, C.M.; Jicha, T.M.; Kolka, R.K.; Lehto, L.L.P.; Sebestyen, S.D.; Seifert-Monson, L.R. Ecoenzymatic stoichiometry and microbial processing of organic matter in northern bogs and fens reveals a cmmon P-limitation between peatland types. Biogeochemistry 2014, 120, 203–224. [Google Scholar] [CrossRef]
- Becerra-Castro, C.; Lopes, A.R.; Vaz-Moreira, I.; Silva, E.F.; Manaia, C.M.; Nunes, O.C. Wastewater reuse in irrigation: A microbiological perspective on implications in soil fertility and human and environmental health. Environ. Int. 2015, 75, 117–135. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 232—Collembolan Reproduction Test in Soil. In Guidelines for Testing Chemicals; OECD: Paris, France, 2009. [Google Scholar]
- Jänsch, S.; Römbke, J.; Schallnaß, H.-J.; Terytze, K. Derivation of soil values for the path ‚soil—Soil organisms‘ for metals and selected organic compounds using species sensitivity distributions. Environ. Sci. Pollut. Res. 2007, 14, 308–318. [Google Scholar] [CrossRef]
- Lock, K.; Janssen, C.R. Toxicity of arsenate to the compost worm Eisenia fetida, the Potworm Enchytraeus Albidus and the Springtail Folsomia Candida. Bull. Environ. Contam. Toxicol. 2002, 68, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Greenslade, P.; Vaughan, G.T. A comparison of collembola species for toxicity testing of Australian soils. Pedobiologia 2003, 47, 171–179. [Google Scholar] [CrossRef]
- Crouau, Y.; Moia, C. The relative sensitivity of growth and reproduction in the springtail, folsomia Candida, exposed to Xenobiotics in the laboratory: An indicator of soil toxicity. Ecotoxicol. Environ. Saf. 2006, 64, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Hallauer, J.; Geng, X.; Yang, H.-C.; Shen, J.; Tsai, K.-J.; Liu, Z. The effect of chronic arsenic exposure in zebrafish. Zebrafish 2016, 13, 405–412. [Google Scholar] [CrossRef] [PubMed]

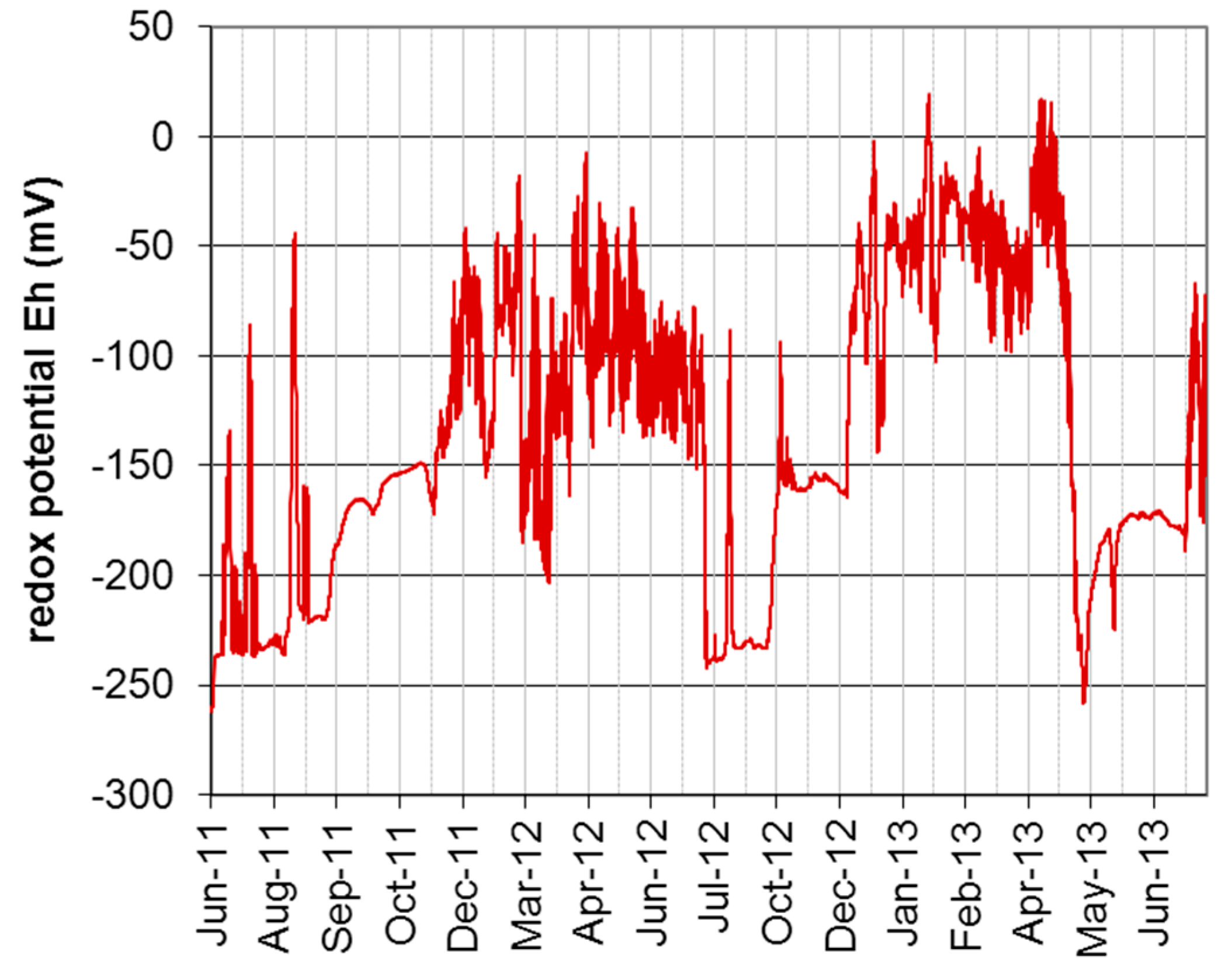
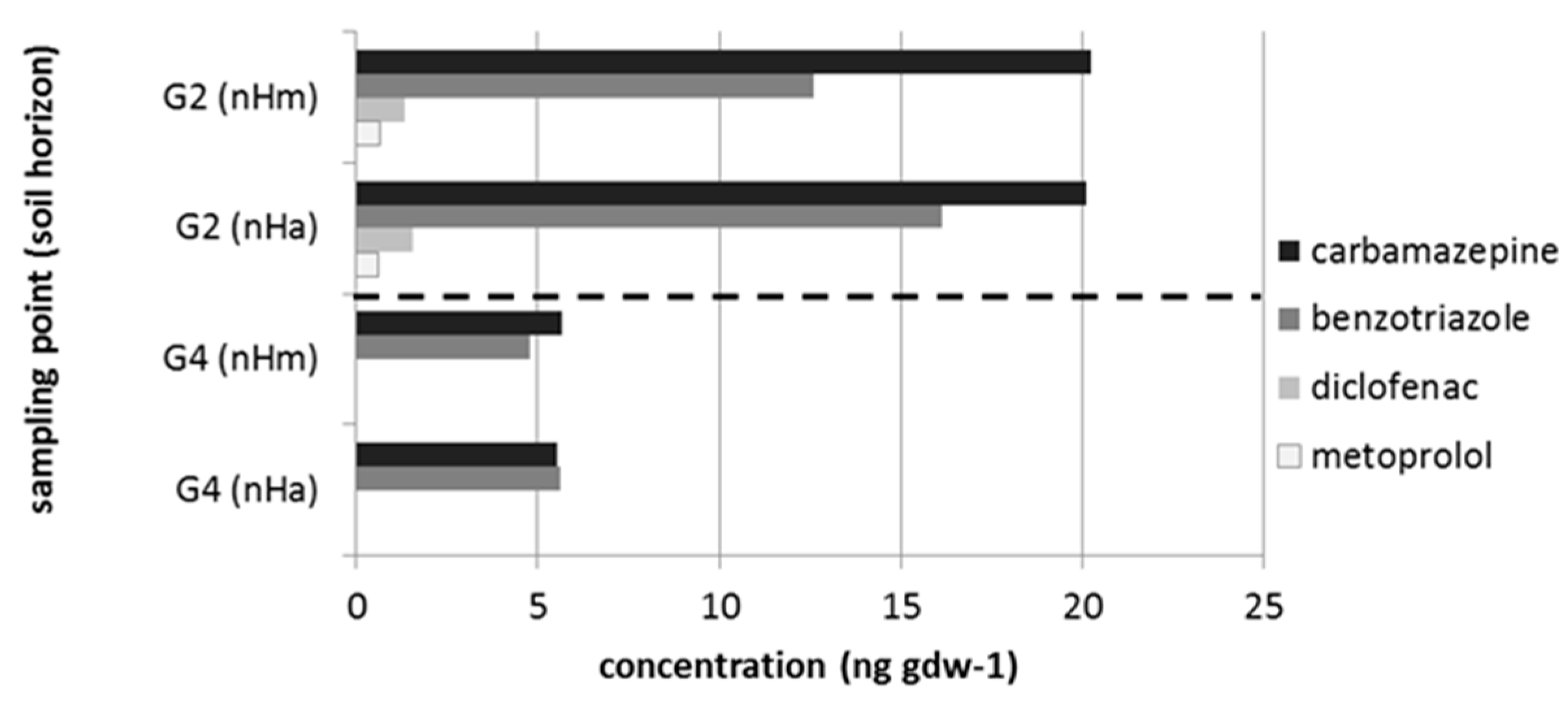
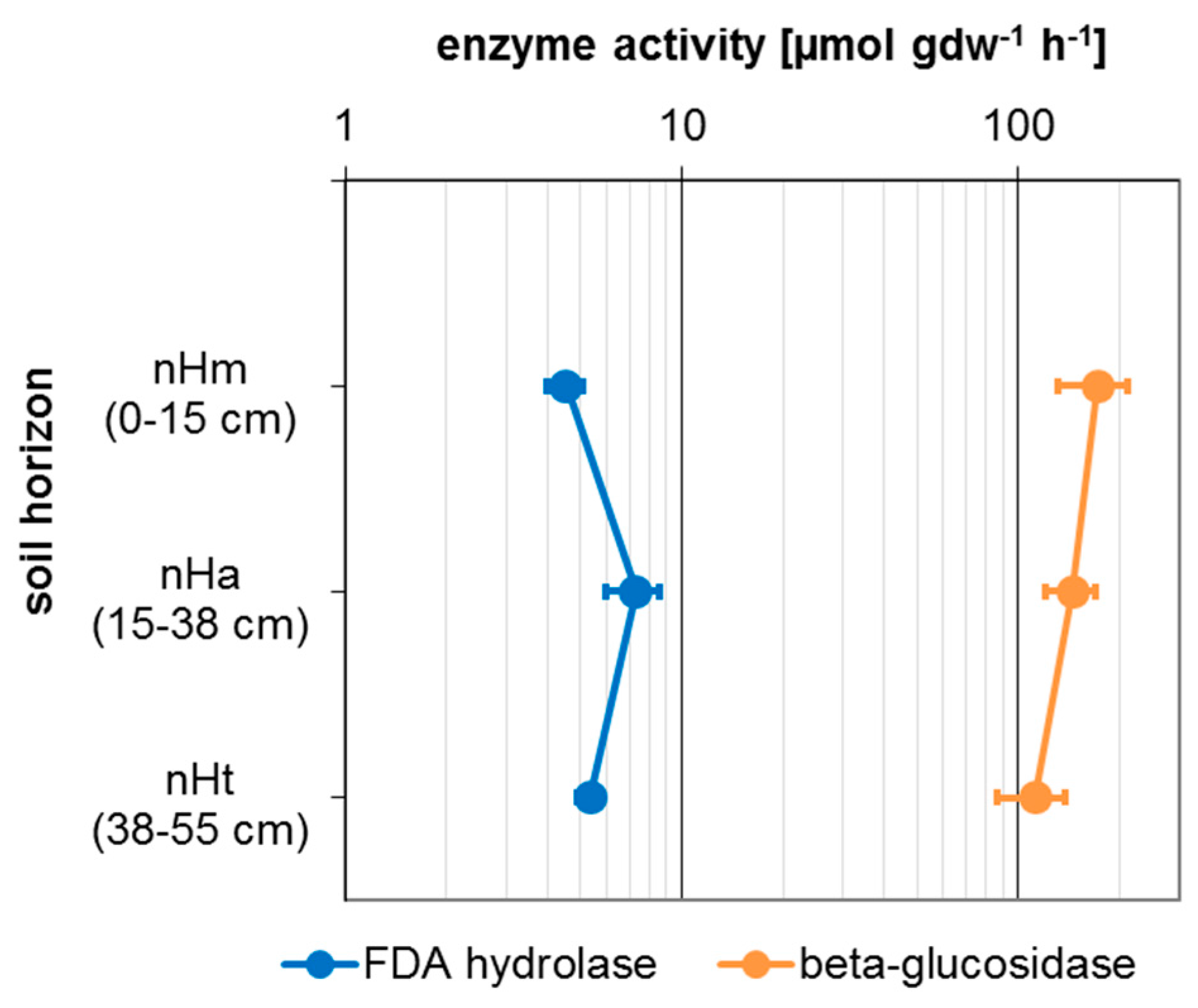
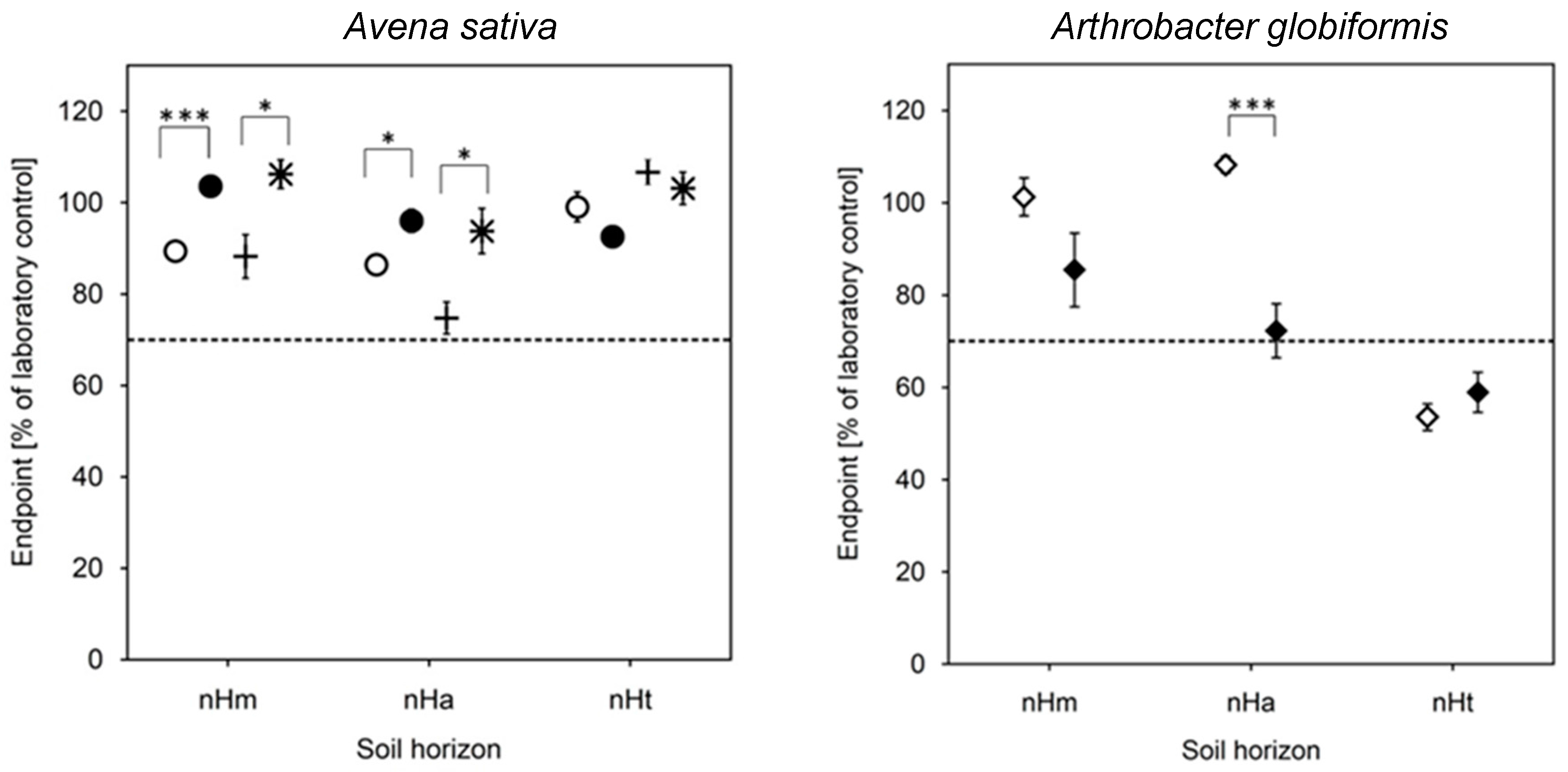

| Taxon | Species | Endpoint, Test Duration, Number of Replicates (n) | Test Guideline |
|---|---|---|---|
| Bacteria, micrococcaceae | Arthrobacter globiformis | inhibition of enzyme activity, 1 h (8) | ISO 18187 (2014) |
| Annelids, enchytreids | Enchytraeus crypticus | inhibition of reproduction, 28 d (8) | OECD 220 (2004a) |
| Arthropods, springtails | Folsomia candida | inhibition of reproduction, 28 d (7) | OECD 232 (2009) |
| Monocotyledonous plants | Avena sativa | inhibition of growth, 14–21 d (5) | ISO 11269-2 (2012), OECD 208 (2006d) |
| Horizon | nHm | nHa | nHt | |||
|---|---|---|---|---|---|---|
| 2011 | 2014 | 2011 | 2014 | 2011 | 2014 | |
| Db (g cm–3) | 0.53 | 0.5 | 0.55 | 0.52 | 0.23 | 0.27 |
| Vp (%) | 73 | 72 | 87 | |||
| Corg (%) | 16 | 16 | 16 | 16 | 37 | 35 |
| Ct (%) | 16 | 16 | 17 | 16 | 38 | 36 |
| C/N | 12 | 12 | 13 | 12 | 19 | 18 |
| WHCmax (mm ) | 162 | 155 | 232 | |||
| Fe (g kg–1) | 79 | 81 | 85 | 81 | 27 | 29 |
| pH | 6.7 | 7 | 6.6 | 6.8 | 5.9 | 6.4 |
| As (mg kg–1) | 35 | 32 | 38 | 32 | 55 | 22 |
| Cd (mg kg–1) | 0.93 | 2 | 0.98 | 2 | 0.63 | 0.62 |
| Zn (mg kg–1) | 37 | 30 | 42 | 28 | 17 | 29 |
| Ni (mg kg–1) | 11 | 9.8 | 10 | 9.6 | 13 | 7.7 |
| Cu (mg kg–1) | 24 | 17 | 22 | 16 | 17 | 34 |
| Cr (mg kg–1) | 11 | 9.6 | 11 | 11 | 11 | 6.1 |
| Pb (mg kg–1) | 26 | 28 | 25 | 27 | 6.2 | 3.6 |
| Compound | LoQ | Treated Wastewater | TMC | Groundwater | ||||
|---|---|---|---|---|---|---|---|---|
| cmean | cmin/cmax | n | NoD | c | NoD | |||
| Pharmaceuticals | ||||||||
| Diclofenac | 0.03 | 7.94 | 2.0/13.0 | 7 | 7 | 0.33 | 0.30 | 1 (G4) |
| Carbamazepine | 0.02 | 4.85 | 2.7/7.0 | 6 | 6 | 0.20 | 0.03 | 1 (G2) |
| 0.04 | 1 (G4) | |||||||
| Metoprolol | 0.03 | 0.97 | <LoQ/1.8 | 7 | 7 | 0.041 | 0.07 | 1 (G2) |
| 0.09 | 1 (G4) | |||||||
| Bezafibrate | 0.01 | 0.75 | <LoQ/4.1 | 6 | 5 | 0.031 | 0.03 | 1 (G2) |
| 0.04 | 1 (G4) | |||||||
| Sotalol | 0.03 | 0.49 | 0.05/1.0 | 6 | 6 | 0.02 | <LoQ | 0 |
| Phenazone | 0.02 | 0.17 | 0.073/0.36 | 6 | 6 | 0.007 | <LoQ | 0 |
| Primidone | 0.05 | 0.067 | <LoQ/0.17 | 7 | 3 | 0.003 | <LoQ | 0 |
| Atenolol | 0.03 | 0.05 | <LoQ/0.19 | 7 | 2 | 0.002 | <LoQ | 0 |
| Oxazepan | 0.05 | 0.069 | <LoQ/0.14 | 7 | 4 | 0.003 | <LoQ | 0 |
| Clofibric acid | 0.01 | 0.043 | <LoQ/0.09 | 4 | 2 | 0.002 | <LoQ | 0 |
| Propranolol | 0.03 | 0.034 | <LoQ/0.09 | 6 | 2 | 0.001 | <LoQ | 0 |
| Diazepam | 0.05 | 0.029 | <LoQ/0.053 | 7 | 1 | 0.001 | <LoQ | 0 |
| Pharmaceutical Metabolites | ||||||||
| FAA | 0.02 | 7.54 | 3.2/15 | 7 | 7 | 0.32 | 0.03 | 1 (G2) |
| 0.07 | 1 (G4) | |||||||
| AAA | 0.02 | 2.42 | 0.47/5.8 | 7 | 7 | 0.10 | 0.02 | 1 (G2) |
| 0.02 | 1 (G4) | |||||||
| 4-Hydro-Diclofenac | 0.01 | 2.17 | 0.32/4.5 | 7 | 7 | 0.091 | <LoQ | 0 |
| AA | 0.03 | 1.3 | <LoQ/3.9 | 3 | 1 | 0.055 | 0.05 | 1 (G2) |
| 0.055 | 0.09 | 1 (G4) | ||||||
| AMPH | 0.02 | 0.23 | 0.10/0.49 | 7 | 7 | 0.01 | 0.03 | 1 (G2) |
| 0.03 | 1 (G4) | |||||||
| PEMA | 0.05 | 0.051 | <LoQ/0.18 | 7 | 1 | 0.002 | 0.06 | 1 (G2) |
| Pesticides | ||||||||
| Desethylatrazine | 0.02 | 0.25 | <LoQ/1.2 | 5 | 1 | 0.01 | <LoQ | 0 |
| MCPA | 0.01 | 0.089 | <LoQ/0.31 | 4 | 2 | 0.004 | <LoQ | 0 |
| Isoproturon | 0.02 | 0.05 | <LoQ/0.16 | 6 | 4 | 0.002 | 0.02 | 1 (G2) |
| 0.02 | 1 (G4) | |||||||
| Mecoprop | 0.01 | 0.023 | 0.01/0.062 | 4 | 2 | 0.001 | <LoQ | 0 |
| Diuron | 0.02 | 0.029 | <LoQ/0.087 | 7 | 3 | 0.001 | <LoQ | 0 |
| Other Substances | ||||||||
| Acesulfame | 0.008 | 43.62 | 19.85/67.0 | 3 | 3 | 1.83 | 0.31 | 1 (G4) |
| Tolytriazol | 0.02 | 6.29 | 1.6/13 | 7 | 7 | 0.26 | 0.02 | 1 (G2) |
| Diatrizoate | 0.008 | 10.17 | 2.5/23.0 | 3 | 3 | 0.43 | 0.02 | 1 (G2) |
| Benzotriazole | 0.02 | 4.1 | 1.8/5.8 | 7 | 7 | 0.17 | <LoQ | 0 |
| p-TSA+o-TSA | 0.05 | 1.73 | <LoQ/5.0 | 6 | 5 | 0.073 | 0.2 | 1 (G2) |
| PBSA | 0.025 | 3.10 | 1.66/4.55 | 2 | 2 | 0.13 | 0.07 | 1 (G4) |
| TCEP | 0.03 | 0.081 | <LoQ/0.2 | 6 | 4 | 0.003 | <LoQ | 0 |
| Diclofenac | Diatrizoate | Carbamazepine | Benzotriazole | Bezafibrate | Metoprolol | Primidone | Atenolol | Oxazepam | Isoproturone | Diurone | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Maximum Amount of Adsorption 1 | 11.4 | 7.2 | 7.0 | 5.9 | 1.6 | 1.4 | 0.17 | 0.17 | 0.14 | 0.1 | 0.04 |
| LoQ | 1.3 | 1.25 | 0.3 | 2.5 | 2.5 | 0.5 | 1.25 | 2.5 | 0.5 | 0.25 | 0.25 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maassen, S.; Richter, E.; Coors, A.; Guimarães, B.; Balla, D. Dissipation of Micropollutants in a Rewetted Fen Peatland: A Field Study Using Treated Wastewater. Water 2017, 9, 449. https://doi.org/10.3390/w9060449
Maassen S, Richter E, Coors A, Guimarães B, Balla D. Dissipation of Micropollutants in a Rewetted Fen Peatland: A Field Study Using Treated Wastewater. Water. 2017; 9(6):449. https://doi.org/10.3390/w9060449
Chicago/Turabian StyleMaassen, Sebastian, Elisabeth Richter, Anja Coors, Bruno Guimarães, and Dagmar Balla. 2017. "Dissipation of Micropollutants in a Rewetted Fen Peatland: A Field Study Using Treated Wastewater" Water 9, no. 6: 449. https://doi.org/10.3390/w9060449
APA StyleMaassen, S., Richter, E., Coors, A., Guimarães, B., & Balla, D. (2017). Dissipation of Micropollutants in a Rewetted Fen Peatland: A Field Study Using Treated Wastewater. Water, 9(6), 449. https://doi.org/10.3390/w9060449





