Successes of Restoration and Its Effect on the Fish Community in a Freshwater Tidal Embayment of the Potomac River, USA
Abstract
:1. Introduction
2. Materials and Methods
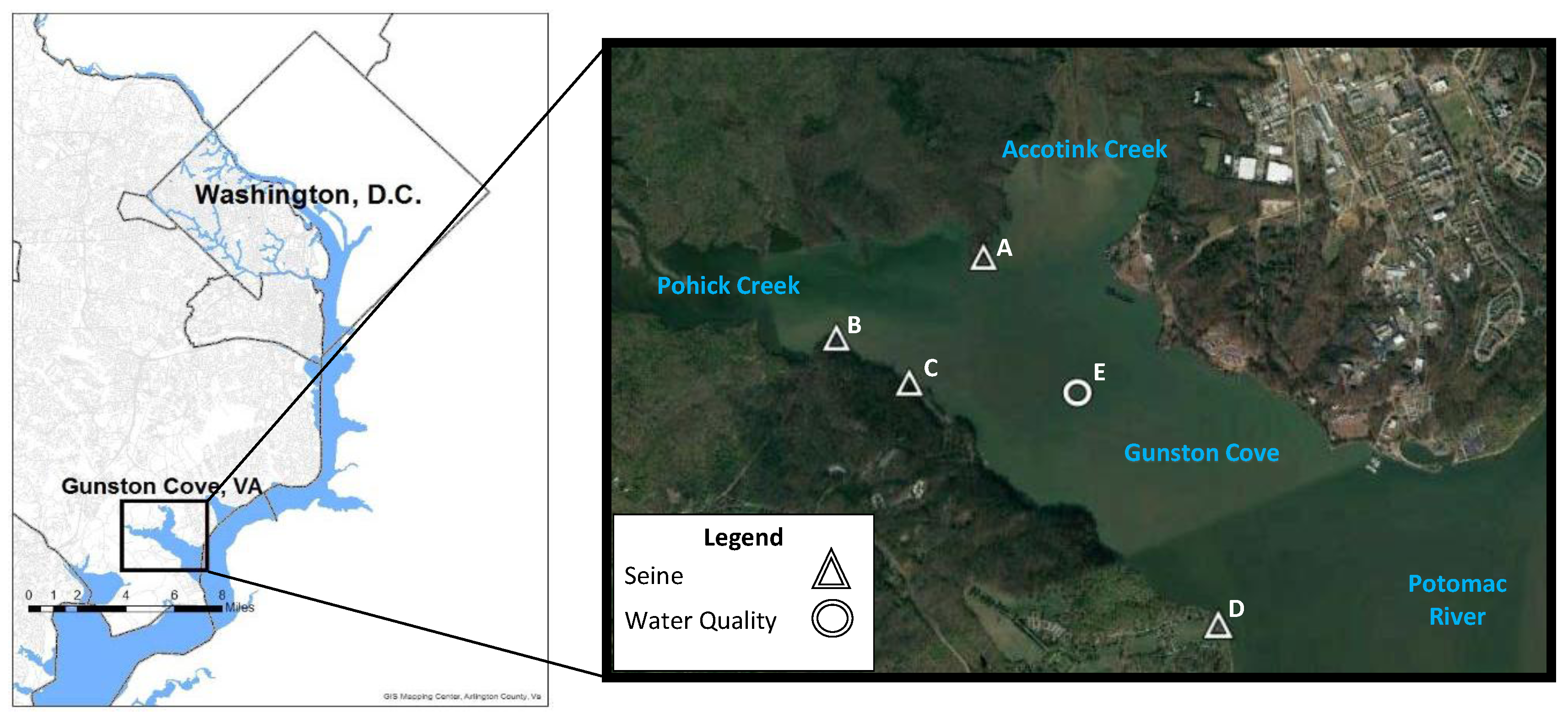
2.1. Description of the Study Site
2.2. Methods
2.3. Analyses
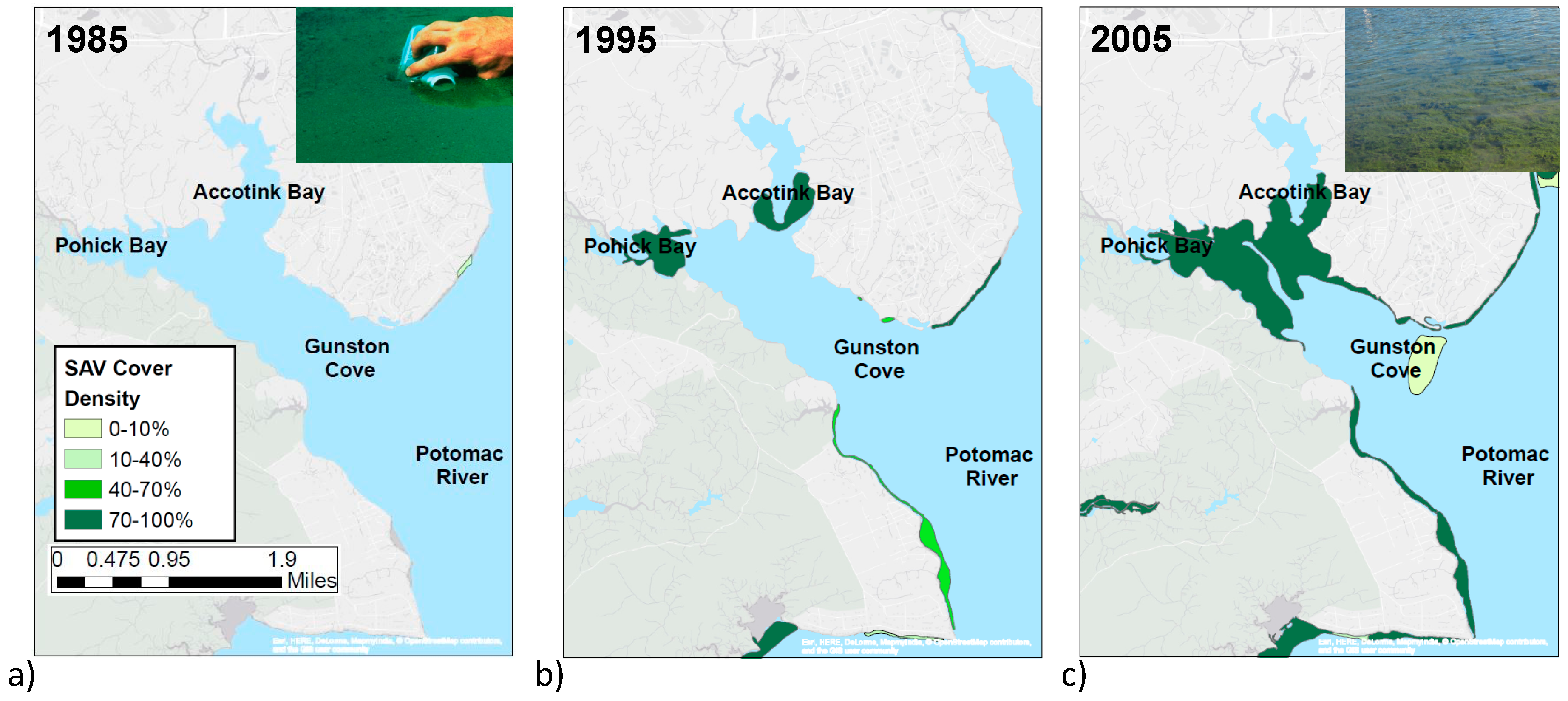
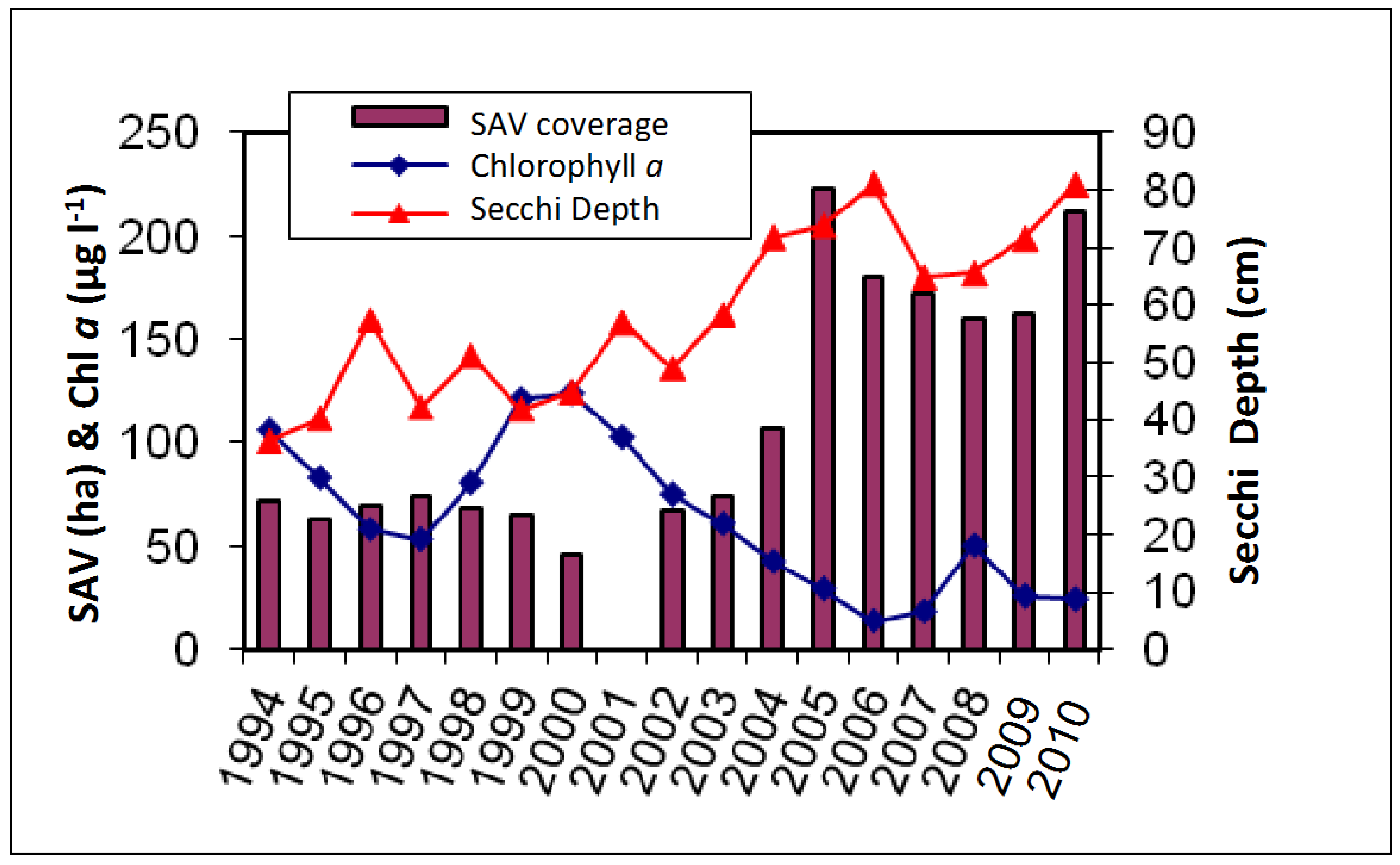
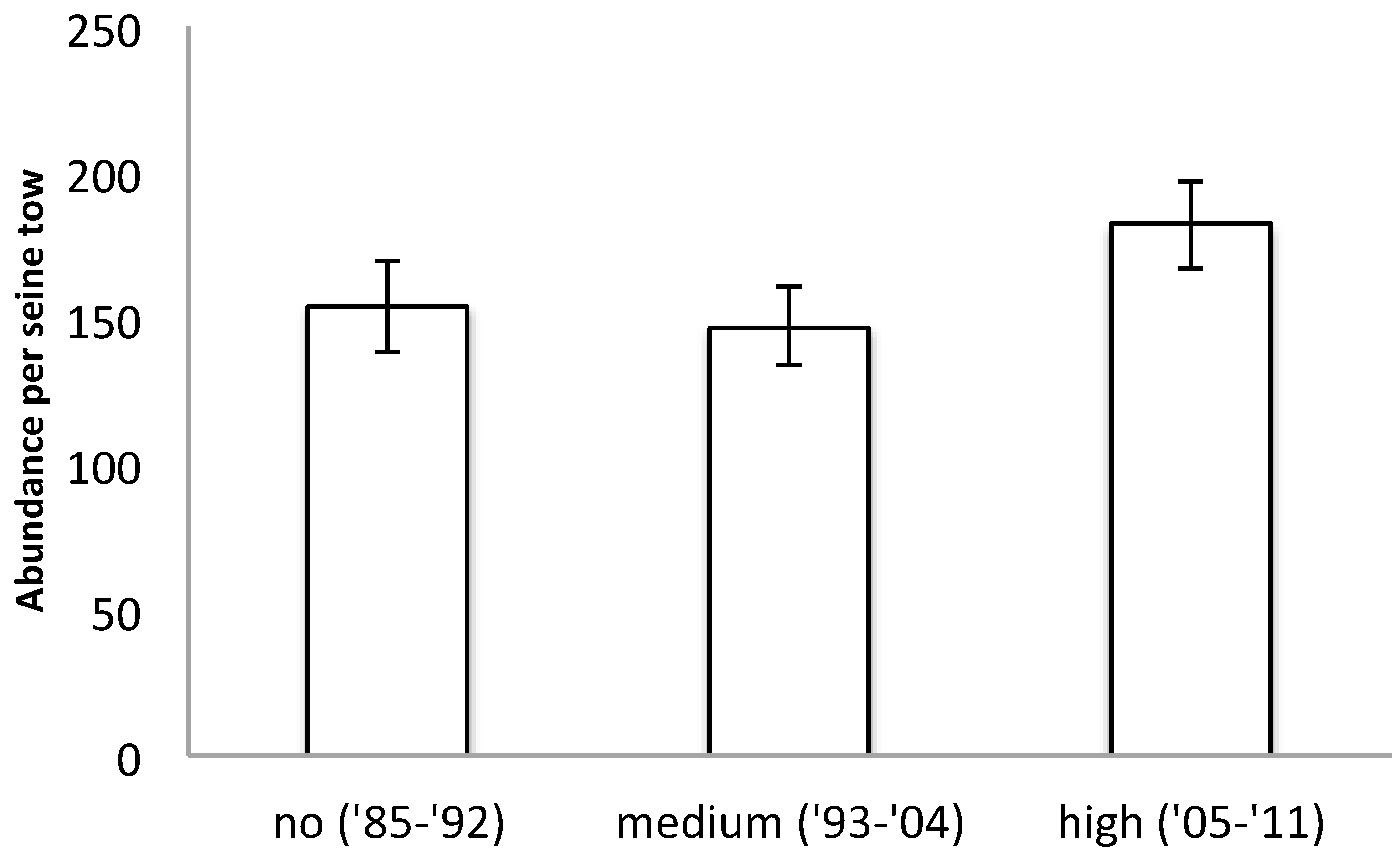
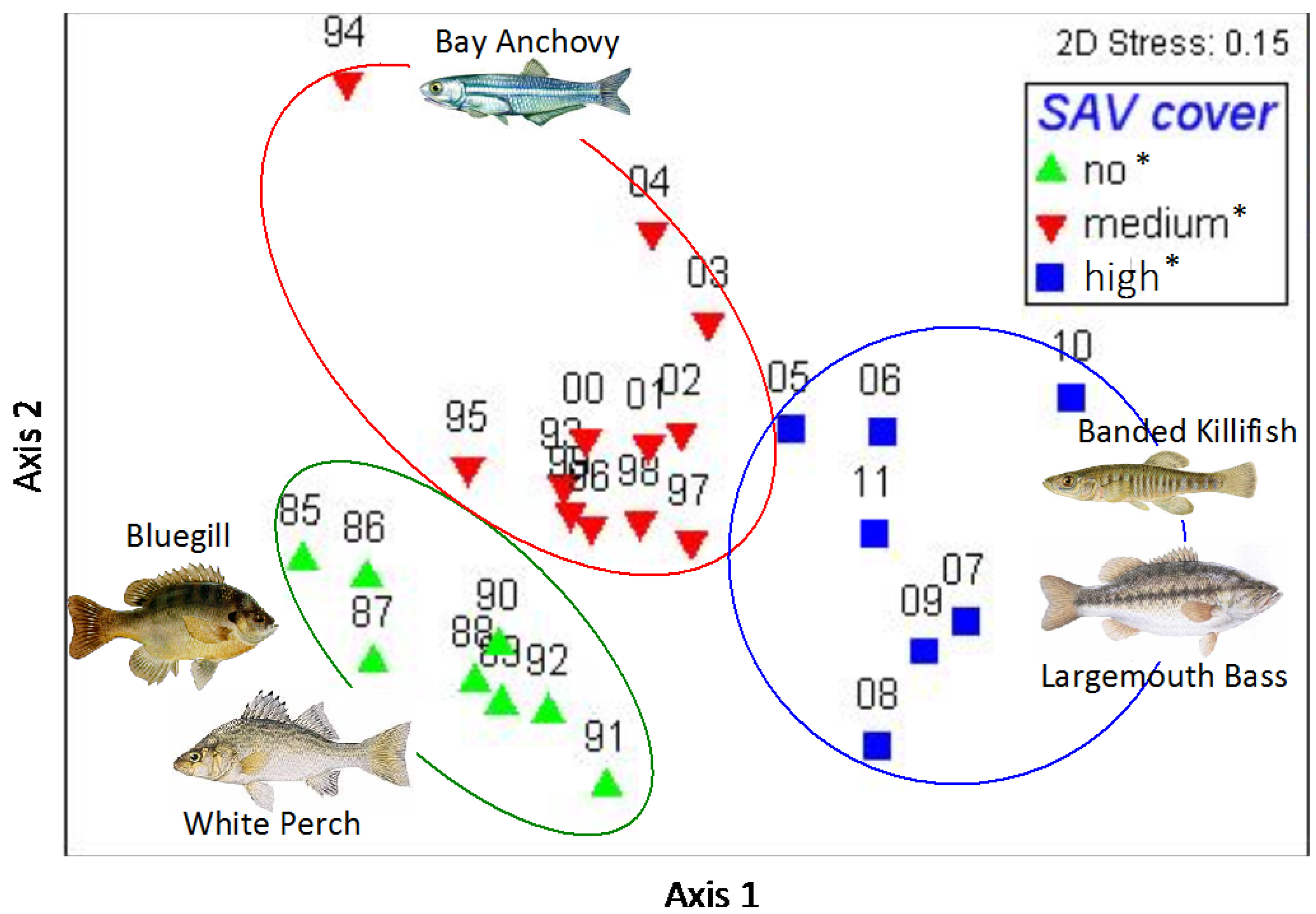
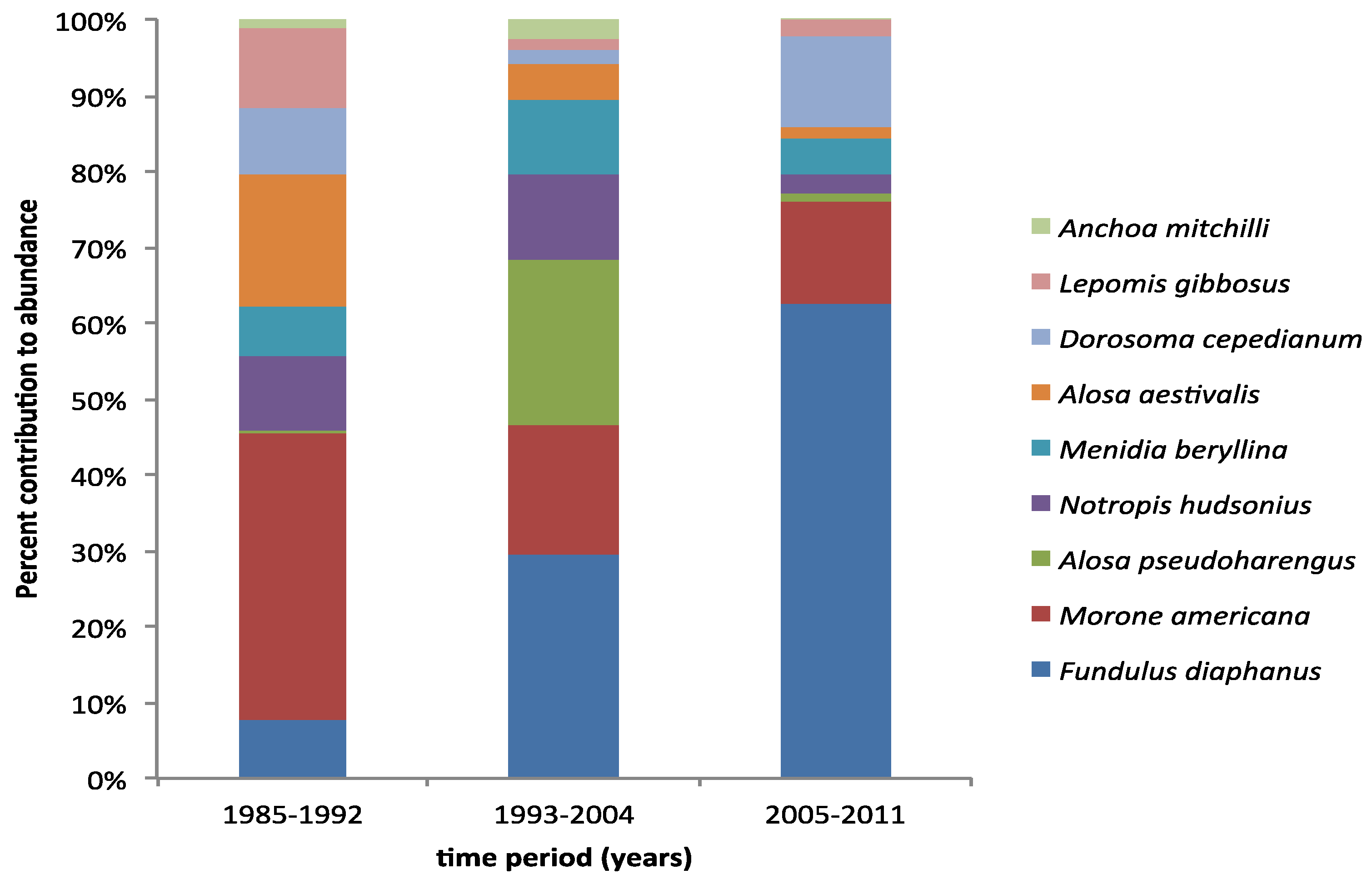
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smith, V.H. Eutrophication of freshwater and coastal marine ecosystems: A global problem. Environ. Sci. Pollut. Res. 2003, 10, 126–139. [Google Scholar] [CrossRef]
- Allan, J.D.; Castillo, M.M. Stream Ecology: Structure and Function of Running Waters, 2nd ed.; Springer: Dordrecht, The Netherlands, 2007; ISBN 978-1-4020-5583-6. [Google Scholar]
- Bowen, J.L.; Valiela, I. The ecological effects of urbanization of coastal watersheds: Historical increases in nitrogen loads and eutrophication of Waquoit Bay estuaries. Can J. Fish. Aquat. Sci. 2001, 58, 1489–1500. [Google Scholar] [CrossRef]
- Paerl, H.W. Controlling eutrophication along the freshwater–marine continuum: Dual nutrient (N and P) reductions are essential. Estuar. Coasts 2009, 32, 593–601. [Google Scholar] [CrossRef]
- Kemp, W.M.; Boynton, W.R.; Adolf, J.E.; Boesch, D.F.; Bolcourt, W.C.; Brush, G.; Cornwell, J.C.; Fisher, T.R.; Gilbert, P.M.; Hagy, J.D.; et al. Eutrophication of Chesapeake Bay: Historical trends and ecological interactions. Mar. Ecol. Prog. Ser. 2005, 303, 1–29. [Google Scholar] [CrossRef]
- Paerl, H.W.; Xu, H.; McCarthy, M.J.; Zhu, G.; Qin, B.; Li, Y.; Gardner, W.S. Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): The need for a dual nutrient (N & P) management strategy. Water Res. 2011, 45, 1973–1983. [Google Scholar] [PubMed]
- Carter, V.; Gammon, P.T.; Bartow, N.C. Submersed Aquatic Plants of the Tidal Potomac River; U.S. Geological Survey: Alexandria, VA, USA, 1983.
- Carter, V.; Rybicki, N. Resurgence of submersed aquatic macrophytes in the tidal Potomac River, Maryland. Estuaries 1986, 9, 368–375. [Google Scholar] [CrossRef]
- Jones, R.C. Long term reponse of water quality to changes in nutrient loading at a mainstem site in the tidal freshwater Potomac River. Verh. Int. Ver. Limnol. 2008, 30, 633–637. [Google Scholar]
- Van Nes, E.H.; Arani, B.M.S.; Staal, A.; van der Bolt, B.; Flores, B.M.; Bathiany, S.; Scheffer, M. What do you mean ‘tipping point’? TREE 2016, 31, 902–904. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.C.; de Mutsert, K. An Ecological Study of Gunston Cove 2012, Final Report; Potomac Environmental Research and Education Center (PEREC), George Mason University: Fairfax, VA, USA, 2013; 160p. [Google Scholar]
- Kraus, R.T.; Jones, R.C. Fish abundances in shoreline habitats and submerged aquatic vegetation in a tidal freshwater embayment of the Potomac River. Environ. Monit. Assess. 2012, 184, 3341–3357. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.C.; Buchanan, C.; Andrle, V. Spatial, seasonal, and interannual patterns in the phytoplankton communities of a tidal freshwater ecosystem. Va. J. Sci. 1992, 43, 25–40. [Google Scholar]
- Jones, R.C.; Kelso, D.P.; Schaeffer, E. Spatial and seasonal patterns in water quality in an embayment-mainstem reach of the tidal freshwater Potomac River, USA: A multiyear study. Environ. Monit. Assess. 2008, 147, 351–375. [Google Scholar] [CrossRef] [PubMed]
- Kelso, D.P.; Jones, R.C.; DeFur, P. An Ecological Study of Gunston Cove 1984, Final Report; Potomac Environmental Research and Education Center (PEREC), George Mason University: Fairfax, VA, USA, 1985. [Google Scholar]
- Jones, R.C.; de Mutsert, K. An Ecological Study of Gunston Cove 2011, Final Report; Potomac Environmental Research and Education Center (PEREC), George Mason University: Fairfax, VA, USA, 2012; 148p. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; PRIMER-E Ltd.: Plymouth, UK, 2001; ISBN 1855311402, 9781855311404. [Google Scholar]
- Scheffer, M. Multiplicity of stable states in freshwater systems. In Biomanipulation Tool for Water Management; Gulati, R.D., Lammens, E.H.R.R., Meijer, M.-L., van Donk, E., Eds.; Springer: Amsterdam, The Netherlands, 1990; ISBN 978-94-017-0924-8. [Google Scholar]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinfer, S.P.; Lancelot, C.; Likens, G.E. Controlling eutrophication: Nitrogen and phosphorous. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 2003, 506–509, 135–145. [Google Scholar] [CrossRef]
- Fisher, T.R.; Gustafson, A.B.; Sellner, K.; Lacouture, R.; Haas, L.W.; Wetzel, R.L.; Magnien, R.; Everitt, D.; Michaels, B.; Karrh, R. Spatial and temporal variation of resource limitation in Chesapeake Bay. Mar. Biol. 1999, 133, 763–778. [Google Scholar] [CrossRef]
- Wyda, J.C.; Deegan, L.A.; Hughes, J.E.; Weaver, M.J. The response of fishes to submerged aquatic vegetation complexity in two ecoregions of the Mid- Atlantic Bight: Buzzards Bay and Chesapeake Bay. Estuaries 2002, 25, 86–100. [Google Scholar] [CrossRef]
- Bayley, S.E.; Prather, C.M. Do wetland lakes exhibit alternative stable states? Submersed aquatic vegetation and chlorophyll in western boreal shallow lakes. Limnol. Oceanogr. 2003, 48, 2335–2345. [Google Scholar] [CrossRef]
- Jaureguizar, A.J.; Menni, R.; Guerrero, R.; Lasta, C. Environmental factors structuring fish communities of the Rı́o de la Plata estuary. Fish. Res. 2004, 66, 195–211. [Google Scholar] [CrossRef]
- Nobriga, M.L.; Feyrer, F.; Baxter, R.D.; Chotkowski, M. Fish community ecology in an altered river delta: Spatial patterns in species composition, life history strategies, and biomass. Estuaries 2005, 28, 776–785. [Google Scholar] [CrossRef]
- Tonn, W.M.; Magnuson, J.J. Patterns in the species composition and richness of fish assemblages in northern Wisconsin Lakes. Ecology 1982, 63, 1149–1166. [Google Scholar] [CrossRef]
- Kraft, C.E.; Carlson, D.M.; Carlson, M. Inland Fishes of New York (Online); Version 4.0; Department of Natural Resources, Cornell University, and the New York State Department of Environmental Conservation: Albany, NY, USA, 2006.
- Rozas, L.P.; Odum, W.E. Occupation of submerged aquatic vegetation by fishes: Testing the roles of food and refuge. Oecologia 1988, 77, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Lippson, A.J.; Lippson, R.L. Life in the Chesapeake Bay; The Johns Hopkins University Press: Baltimore, MD, USA, 2006; p. 324. ISBN 9780801883385. [Google Scholar]
- De Robertis, A.; Ryer, C.H.; Veloza, A.; Brodeur, R.D. Differential effects of turbidity on prey consumption of piscivorous and planktivorous fish. Can J. Fish. Aquat. Sci. 2003, 60, 1517–1526. [Google Scholar] [CrossRef]
- Chen, R.J.; Hunt, K.M.; Ditton, R.B. Estimating the economic impacts of a trophy largemouth bass fishery: Issues and applications. N. Am. J. Fish. Man. 2003, 23, 835–844. [Google Scholar] [CrossRef]
- Oksanen, L.; Fretwell, S.D.; Arruda, J.; Niemela, P. Exploitation ecosystems in gradients of primary productivity. Am. Nat. 1981, 118, 240–261. [Google Scholar] [CrossRef]
- NOAA. Striped Bass. Available online: https://chesapeakebay.noaa.gov/fish-facts/striped-bass (accessed on 4 June 2017).
- ASMFC. River Herring Benchmark Assessment: Stock Assessment Report; No.12-02; Atlantic States Marine Fisheries Commission: Washington, DC, USA, 2012. [Google Scholar]
- Greene, K.E.; Zimmerman, J.L.; Laney, R.W.; Thomas-Blate, J.C. Atlantic Coast Diadromous Fish Habitat: A Review of Utilization, Threats, Recommendations for Conservation, and Research Needs; ScholarWorks@UMass Amherst: Washington, DC, USA, 2009. [Google Scholar]
- Hightower, J.E.; Wicker, A.M.; Endres, K.M. Historical trends in abundance of American shad and river herring in Albemarle Sound, North Carolina. N. Am. J. Fish. Man. 1996, 16, 257–271. [Google Scholar] [CrossRef]
- Schloesser, R.W.; Fabrizio, M.C.; Latour, R.J.; Garman, G.C.; Greenlee, B.; Groves, M.; Gartland, J. Ecological role of blue catfish in Chesapeake Bay communities and implications for management. Am. Fish. Soc. Sym. 2011, 77, 369–382. [Google Scholar]
- Odenkirk, J.; Owens, S. Northern snakeheads in the tidal Potomac River system. Trans. Am. Fish. Soc. 2005, 134, 1605–1609. [Google Scholar] [CrossRef]
- Gross, C.; Hagy, J.D., 3rd. Attributes of successful actions to restore lakes and estuaries degraded by nutrient pollution. J. Environ. Man. 2017, 187, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, M.; Hosper, S.H.; Meijer, M.-L.; Moss, B.; Jeppesen, E. Alternative equilibria in shallow lakes. TREE 1993, 8, 275–279. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Mutsert, K.; Sills, A.; Schlick, C.J.C.; Jones, R.C. Successes of Restoration and Its Effect on the Fish Community in a Freshwater Tidal Embayment of the Potomac River, USA. Water 2017, 9, 421. https://doi.org/10.3390/w9060421
De Mutsert K, Sills A, Schlick CJC, Jones RC. Successes of Restoration and Its Effect on the Fish Community in a Freshwater Tidal Embayment of the Potomac River, USA. Water. 2017; 9(6):421. https://doi.org/10.3390/w9060421
Chicago/Turabian StyleDe Mutsert, Kim, Amanda Sills, C. J. Carroll Schlick, and R. Christian Jones. 2017. "Successes of Restoration and Its Effect on the Fish Community in a Freshwater Tidal Embayment of the Potomac River, USA" Water 9, no. 6: 421. https://doi.org/10.3390/w9060421
APA StyleDe Mutsert, K., Sills, A., Schlick, C. J. C., & Jones, R. C. (2017). Successes of Restoration and Its Effect on the Fish Community in a Freshwater Tidal Embayment of the Potomac River, USA. Water, 9(6), 421. https://doi.org/10.3390/w9060421



