Allelopathy Inhibitory Effects of Hydrodictyon reticulatum on Chlorella pyrenoidosa under Co-Culture and Liquor-Cultured Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
+ 0.1 × (OD630 − OD750)) × (V1/V)
2.3. Experimental Design
2.3.1. Co-Culture Experiment between Hydrodictyon reticulatum and Chlorella pyrenoidosa
2.3.2. Liquor-Cultured Experiment of Chlorella pyrenoidosa
3. Results and Discussion
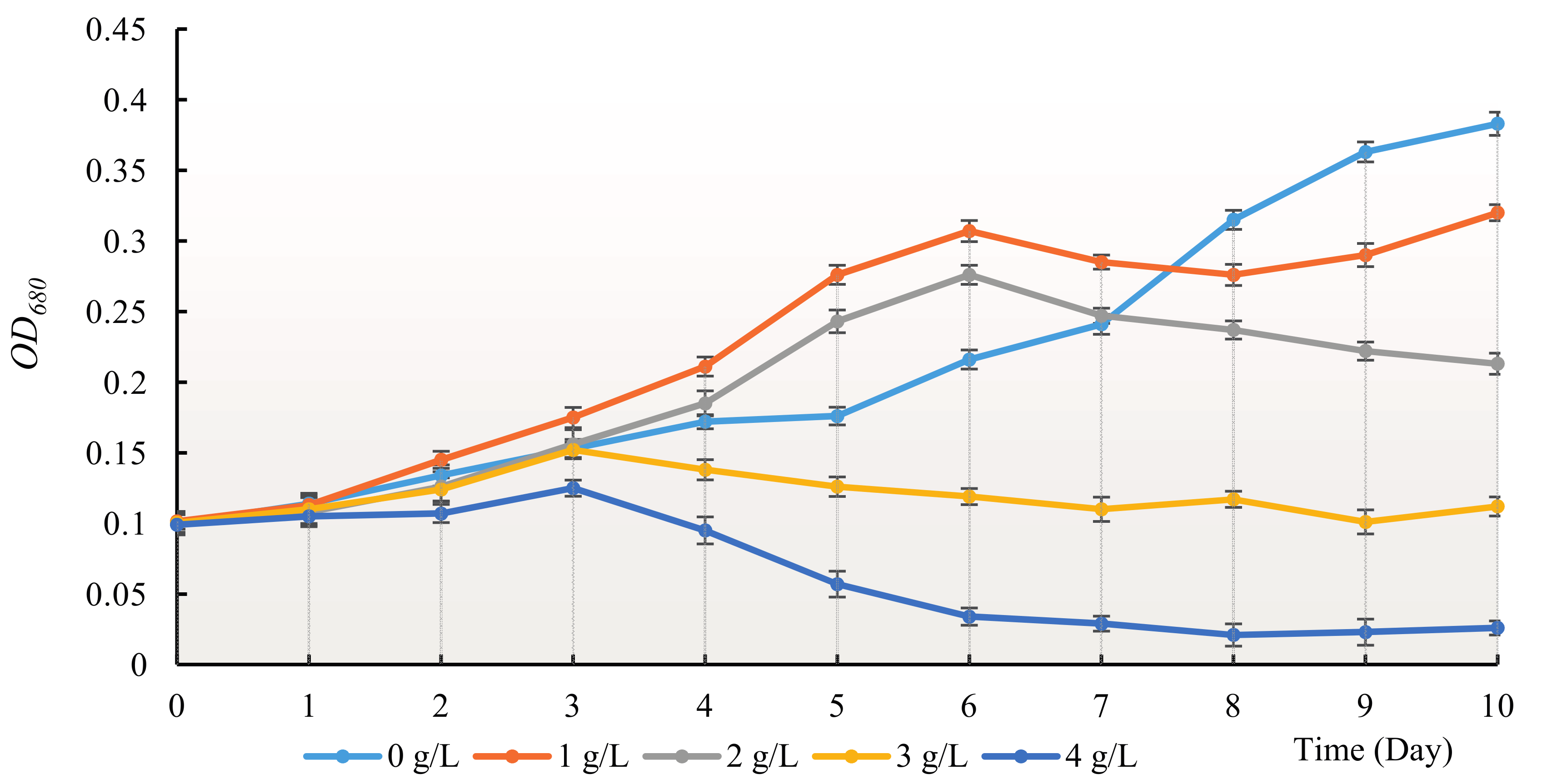
3.1. The Allelopathy Inhibitory Effects of Hydrodictyon reticulatum on Chlorella pyrenoidosa under Co-Culture Condition
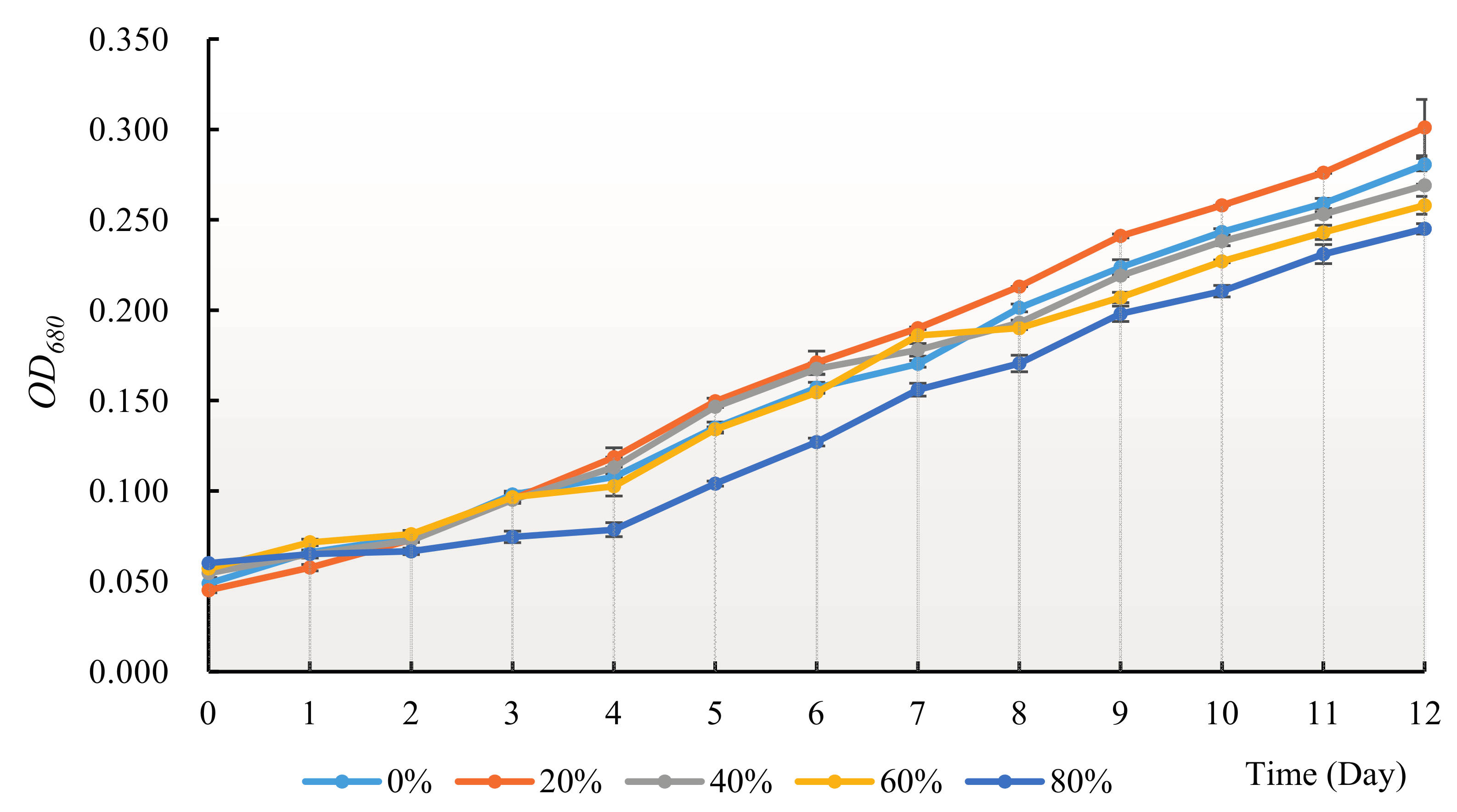
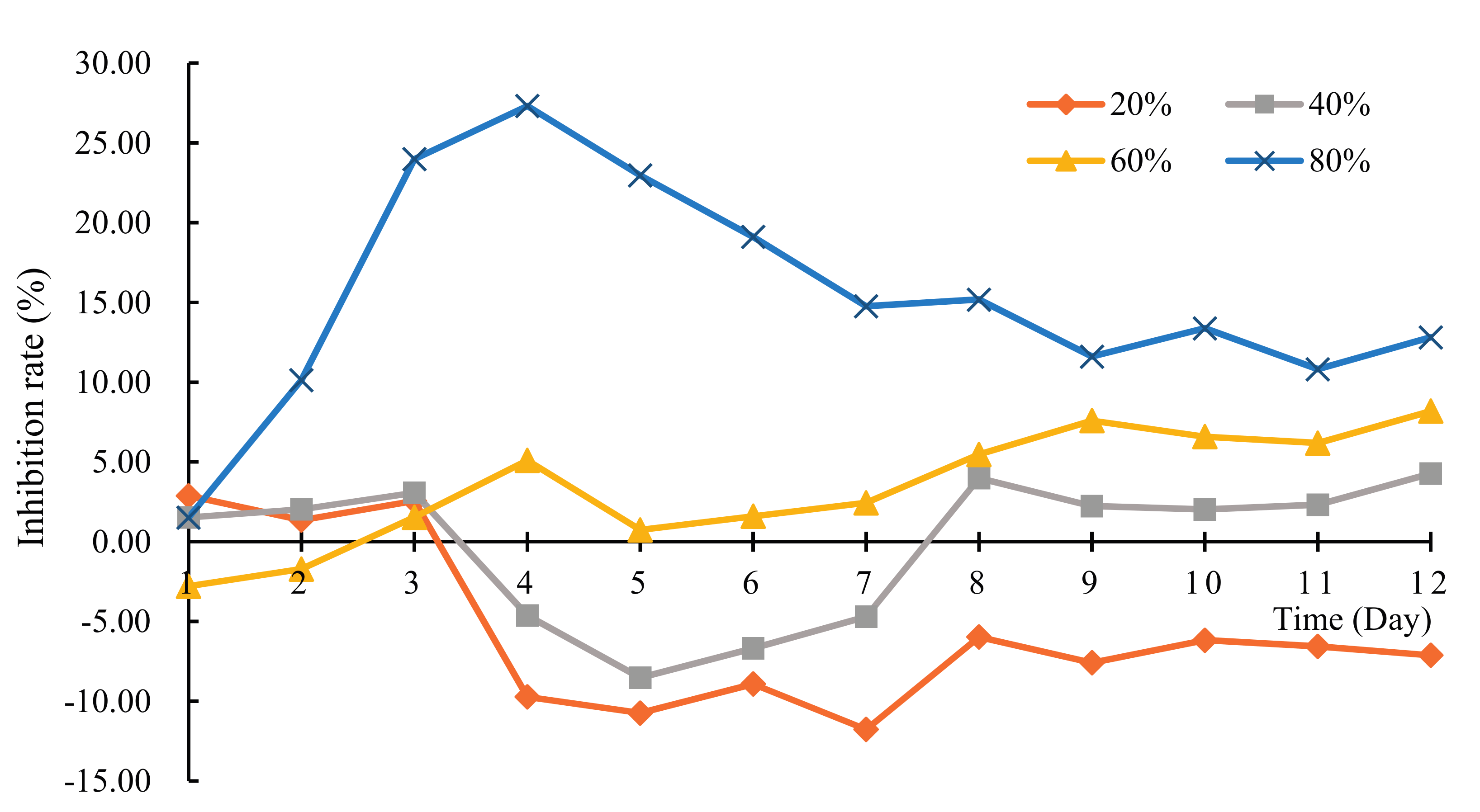
3.2. Effect of H. reticulatum Growing Water on the Growth of C. pyrenoidosa
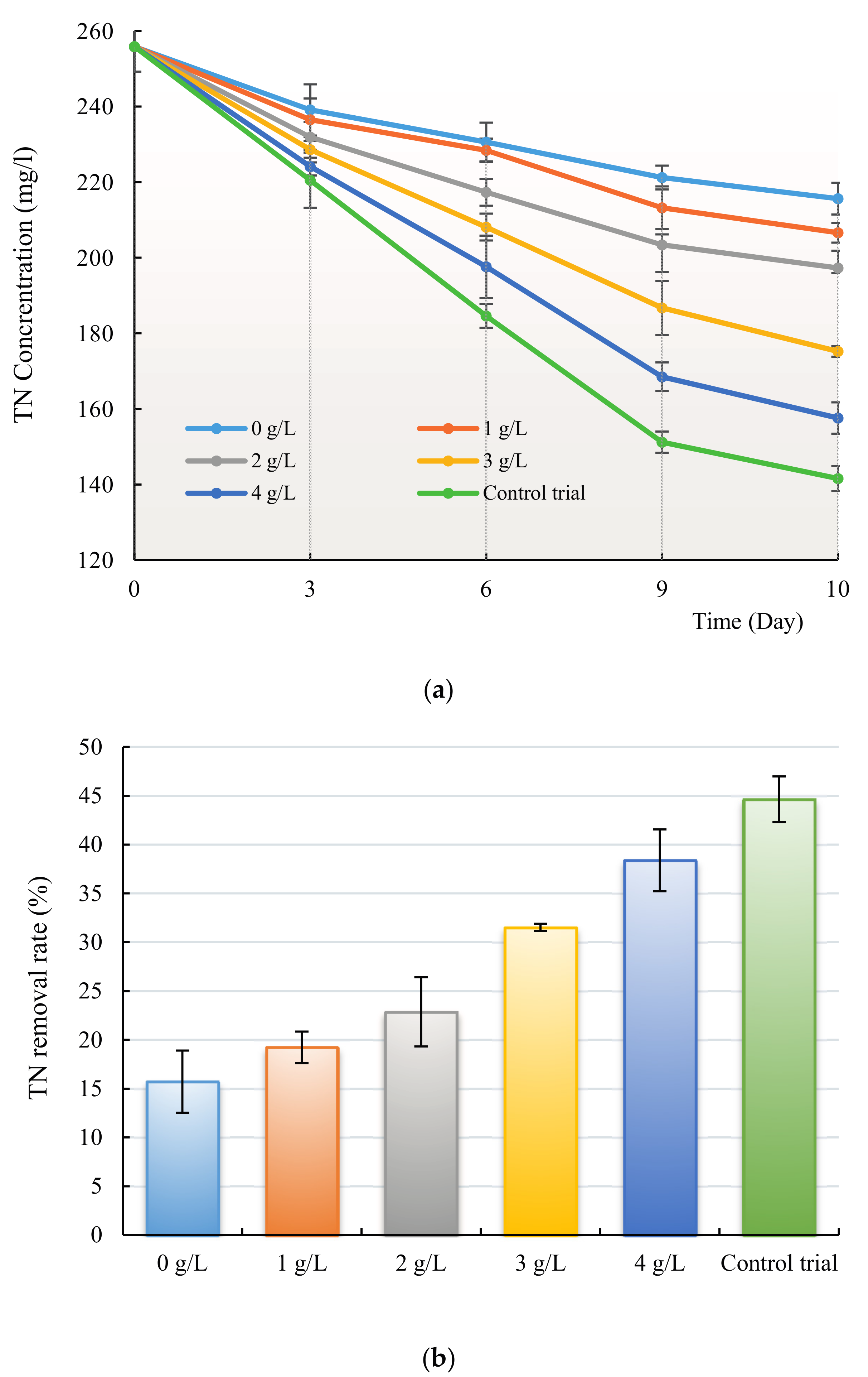
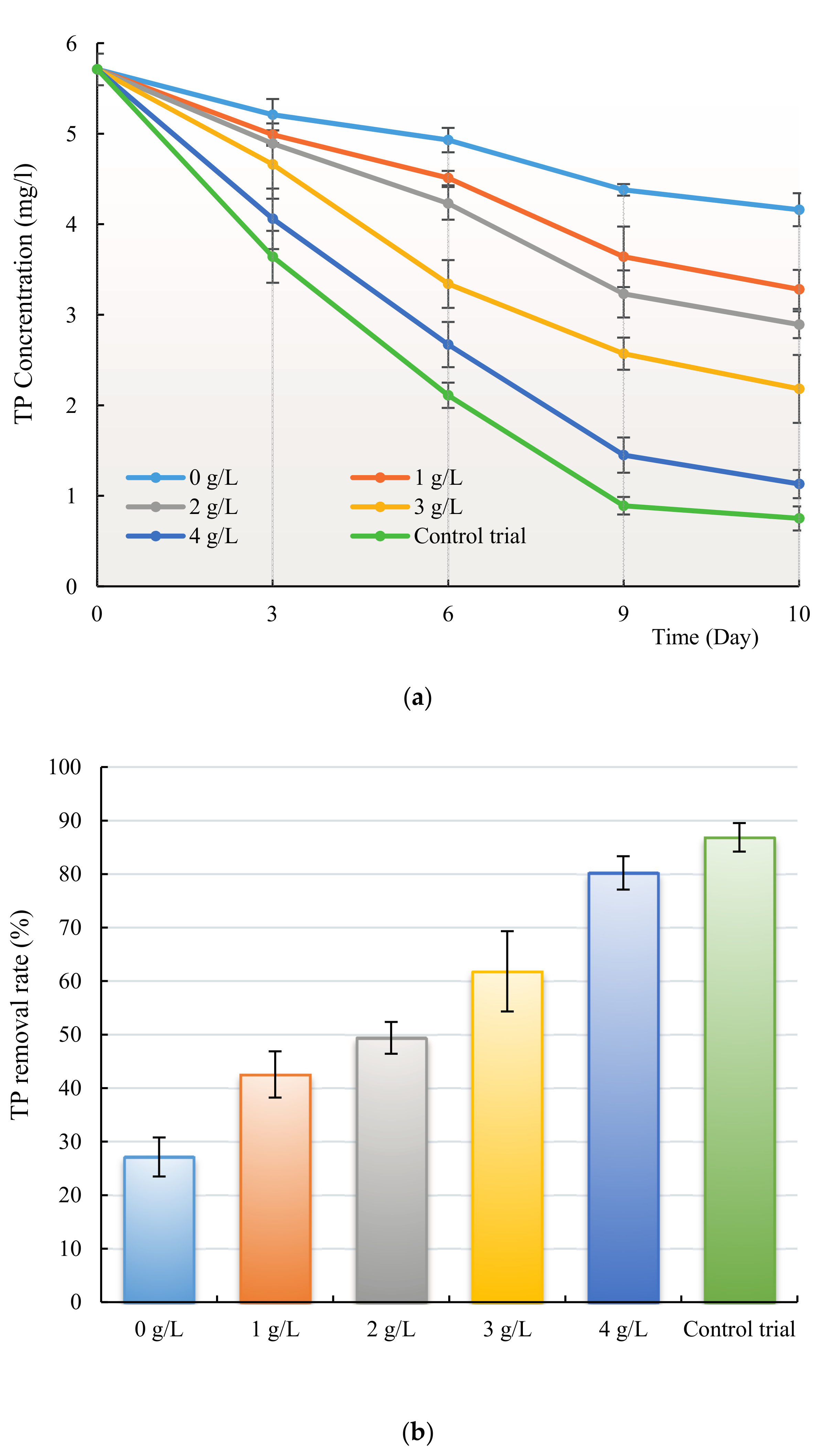
3.3. Removal of Nitrogen and Phosphorus by H. reticulatum under Co-Culture Condition
4. Conclusions
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Oliveira, M.; Machado, A. The role of phosphorus on eutrophication: A historical review and future perspectives. Environ. Technol. Rev. 2013, 2, 117–127. [Google Scholar] [CrossRef]
- Schoumans, O.; Chardon, W.; Bechmann, M.; Gascuel-Odoux, C.; Hofman, G.; Kronvang, B.; Rubæk, G.H.; Ulén, B.; Dorioz, J.-M. Mitigation options to reduce phosphorus losses from the agricultural sector and improve surface water quality: A review. Sci. Total Environ. 2014, 468, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Men, L.; Chen, Q. Ocean Observation with Opto-Microfluidic Devices. J. Environ. Inform. 2016, 28, 120–125. [Google Scholar]
- Hu, H.; Hong, Y. Algal-bloom control by allelopathy of aquatic macrophytes—A review. Front. Environ. Sci. Eng. 2008, 2, 421–438. [Google Scholar] [CrossRef]
- Molot, L.A. The effectiveness of cyanobacteria nitrogen fixation: Review of bench top and pilot scale nitrogen removal studies and implications for nitrogen removal programs. Environ. Rev. 2017, 999, 1–4. [Google Scholar] [CrossRef]
- Graneli, E.; Weberg, M.; Salomon, P.S. Harmful algal blooms of allelopathic microalgal species: The role of eutrophication. Harmful Algae 2008, 8, 94–102. [Google Scholar] [CrossRef]
- An, C.; McBean, E.; Huang, G.; Yao, Y.; Zhang, P.; Chen, X.; Li, Y. Multi-Soil-Layering Systems for Wastewater Treatment in Small and Remote Communities. J. Environ. Inform. 2016, 27, 27. [Google Scholar] [CrossRef]
- Zhang, M.; Fu, H.; Tian, C.; Bin, Y.; Chen, X. A pilot-scale study on continuous self-cleaning microalgae-collection equipment. J. Environ. Eng. 2011, 5, 2018–2022. [Google Scholar]
- Wang, C.; Ao, Y.; Wang, P.; Hou, J.; Qian, J.; Zhang, S. Preparation, characterization, photocatalytic properties of titania hollow sphere doped with cerium. J. Hazard. Mater. 2010, 178, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ao, Y.; Wang, P.; Hou, J.; Qian, J. Preparation of cerium and nitrogen co-doped titania hollow spheres with enhanced visible light photocatalytic performance. Powder Technol. 2011, 210, 203–207. [Google Scholar] [CrossRef]
- Wang, P.; Ao, Y.; Wang, C.; Hou, J.; Qian, J. Enhanced photoelectrocatalytic activity for dye degradation by graphene-titania composite film electrodes. J. Hazard. Mater. 2012, 223, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ao, Y.; Wang, C.; Hou, J.; Qian, J. A one-pot method for the preparation of graphene–Bi2MoO6 hybrid photocatalysts that are responsive to visible-light and have excellent photocatalytic activity in the degradation of organic pollutants. Carbon 2012, 50, 5256–5264. [Google Scholar] [CrossRef]
- Nematian, J. An extended two-stage stochastic programming approach for water resources management under uncertainty. J. Environ. Inf. 2016, 27, 27. [Google Scholar] [CrossRef]
- Zhao, K.; Fu, H.; Chai, T.; Zhang, M.; Liu, Z.; Chen, X.; Hou, M.; Xu, P. Allelopathy of Hydrodictyon reticulatum on Microcystis aeruginosa and its removal capacity on nitrogen and phosphorus. Huan Jing Ke Xue 2011, 32, 2267–2272. (In Chinese) [Google Scholar] [PubMed]
- Zhao, S.; Huang, G.; Fu, H.; Wang, Y. Enhanced coagulation/flocculation by combining diatomite with synthetic polymers for oily wastewater treatment. Sep. Sci. Technol. 2014, 49, 999–1007. [Google Scholar] [CrossRef]
- Wang, P.; Cao, M.; Ao, Y.; Wang, C.; Hou, J.; Qian, J. Investigation on Ce-doped TiO2-coated BDD composite electrode with high photoelectrocatalytic activity under visible light irradiation. Electrochem. Commun. 2011, 13, 1423–1426. [Google Scholar] [CrossRef]
- Guo, P.; Liu, Y.; Liu, C. Effects of chitosan, gallic acid, and algicide on the physiological and biochemical properties of Microcystis flos-aquae. Environ. Sci. Pollut. Res. Int. 2015, 22, 13514–13521. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xiao, X.; Lin, F.; Grossart, H.-P.; Nie, Z.; Sun, L.; Xu, C.; Shi, J. Continuous-release beads of natural allelochemicals for the long-term control of cyanobacterial growth: Preparation, release dynamics and inhibitory effects. Water Res. 2016, 95, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Z.; Gobler, C.J. The green macroalga, Ulva lactuca, inhibits the growth of seven common harmful algal bloom species via allelopathy. Harmful Algae 2011, 10, 480–488. [Google Scholar] [CrossRef]
- Wang, C.; Lu, G.; Peifang, W.; Wu, H.; Qi, P.; Liang, Y. Assessment of environmental pollution of Taihu Lake by combining active biomonitoring and integrated biomarker response. Environ. Sci. Technol. 2011, 45, 3746–3752. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, S.H.; Wang, P.F.; Hou, J.; Zhang, W.J.; Li, W.; Lin, Z.P. The effect of excess Zn on mineral nutrition and antioxidative response in rapeseed seedlings. Chemosphere 2009, 75, 1468–1476. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, S.; Wang, C.; Lu, J. Effects of Pb on the oxidative stress and antioxidant response in a Pb bioaccumulator plant Vallisneria natans. Ecotoxicol. Environ. Saf. 2012, 78, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Yang, K.; Li, S.; Li, G.; Song, L. Submerged vegetation removal promotes shift of dominant phytoplankton functional groups in a eutrophic lake. J. Environ. Sci. 2014, 26, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Gross, E.M.; Hilt, S.; Lombardo, P.; Mulderij, G. Searching for allelopathic effects of submerged macrophytes on phytoplankton—State of the art and open questions. Hydrobiologia 2007, 584, 77–88. [Google Scholar] [CrossRef]
- Hilt, S. Allelopathic inhibition of epiphytes by submerged macrophytes. Aquat. Bot. 2006, 85, 252–256. [Google Scholar] [CrossRef]
- Do Carmo Bittencourt-Oliveira, M.; Chia, M.A.; de Oliveira, H.S.B.; Araújo, M.K.C.; Molica, R.J.R.; Dias, C.T.S. Allelopathic interactions between microcystin-producing and non-microcystin-producing cyanobacteria and green microalgae: Implications for microcystins production. J. Appl. Phycol. 2015, 27, 275–284. [Google Scholar] [CrossRef]
- Meng, P.; Pei, H.; Hu, W.; Liu, Z.; Li, X.; Xu, H. Allelopathic effects of Ailanthus altissima extracts on Microcystis aeruginosa growth, physiological changes and microcystins release. Chemosphere 2015, 141, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.L. Allelopathy; Harcourt Brace Jovanovich, Academic Press, INC.: Cambridge, MA, USA, 2012. [Google Scholar]
- Wang, C.; Zhang, S.H.; Wang, P.F.; Hou, J.; Li, W.; Zhang, W.J. Metabolic adaptations to ammonia-induced oxidative stress in leaves of the submerged macrophyte Vallisneria natans (Lour.) Hara. Aquat. Toxicol. 2008, 87, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yin, Y.; Guo, Y.; Wang, C. Removal of chlorpyrifos from waste water by wheat straw-derived biochar synthesized through oxygen-limited method. RSC Adv. 2015, 5, 72572–72578. [Google Scholar] [CrossRef]
- Graneli, E.; Hansen, P.J. Allelopathy in harmful algae: A mechanism to compete for resources? In Ecology of Harmful Algae; Springer: Berlin/Heidelberg, Germany, 2006; pp. 189–201. [Google Scholar]
- He, Y.; Zhou, Q.-H.; Liu, B.-Y.; Cheng, L.; Tian, Y.; Zhang, Y.-Y.; Wu, Z.-B. Programmed cell death in the cyanobacterium Microcystis aeruginosa induced by allelopathic effect of submerged macrophyte Myriophyllum spicatum in co-culture system. J. Appl. Phycol. 2016, 28, 2805–2814. [Google Scholar] [CrossRef]
- Rzymski, P.; Poniedziałek, B.; Kokociński, M.; Jurczak, T.; Lipski, D.; Wiktorowicz, K. Interspecific allelopathy in cyanobacteria: Cylindrospermopsin and Cylindrospermopsis raciborskii effect on the growth and metabolism of Microcystis aeruginosa. Harmful Algae 2014, 35, 1–8. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, X. Response of Chara globularis and Hydrodictyon reticulatum to lead pollution: Their survival, bioaccumulation, and defense. J. Appl. Phycol. 2012, 24, 245–251. [Google Scholar] [CrossRef]
- Ma, Z.; Fang, T.; Thring, R.W.; Li, Y.; Yu, H.; Zhou, Q.; Zhao, M. Toxic and non-toxic strains of Microcystis aeruginosa induce temperature dependent allelopathy toward growth and photosynthesis of Chlorella vulgaris. Harmful Algae 2015, 48, 21–29. [Google Scholar] [CrossRef]
- Ni, L.; Jie, X.; Wang, P.; Li, S.; Wang, G.; Li, Y.; Li, Y.; Acharya, K. Effect of linoleic acid sustained-release microspheres on Microcystis aeruginosa antioxidant enzymes activity and microcystins production and release. Chemosphere 2015, 121, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, B.; Xing, K.; Zhang, X.; Tian, X.; Dai, W. Inhibitory effects of golden thread (Coptis chinensis) and berberine on Microcystis aeruginosa. Water Sci. Technol. 2010, 61, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jeppesen, E.; Wang, M.; Xu, X.; Wang, L. Allelopathic effect boosts Chrysosporum ovalisporum dominance in summer at the expense of Microcystis panniformis in a shallow coastal water body. Environ. Sci. Pollut. Res. 2017, 24, 4666–4675. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Xu, X.; Chen, W.; Jiang, H.; Jin, Y.; Liu, W.; Fu, Z. Allelochemical stress causes oxidative damage and inhibition of photosynthesis in Chlorella vulgaris. Chemosphere 2009, 75, 368–375. [Google Scholar] [CrossRef] [PubMed]
- State Environmental Protection Administration. Water and Wastewater Monitoring and Analysis Methods, 4th ed.; China Environmental Science Press: Beijing, China, 2002; pp. 244–248. [Google Scholar]
- Li, F.; Hu, H.; Men, Y.; Hong, Y.; Guo, M. Effects of allelochemicals on the activity of antioxidant enzymes in Chlorella vulgaris. Environ. Sci. 2006, 27, 89–92. [Google Scholar]
- Allen, R.D. Dissection of oxidative stress tolerance using transgenic plants. Plant Physiol. 1995, 107, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Geng, J.; Ren, H.; Xia, X.; Wang, X.; Yu, Y. Physiological and biochemical responses of Microcystis aeruginosa to glyphosate and its Roundup® formulation. J. Hazard. Mater. 2013, 248, 172–176. [Google Scholar] [CrossRef] [PubMed]


| Chemical | Dose |
|---|---|
| NaNO3 | 1.5 g |
| K2HPO4∙H2O | 0.04 g |
| MgSO4∙7H2O | 0.075 g |
| CaCl2∙2H2O | 0.036 g |
| Citric acid | 0.006 g |
| Ferric ammonium citrate | 0.006 g |
| EDTA (dinatrium-salt) | 0.001 g |
| Na2CO3 | 0.02 g |
| A5 + Co Solution | 1 mL |
| Distilled water | 919 mL |
| Composition of the A5 + Co solution | |
| Distilled Water H3BO3 | 1000 mL |
| 2.86 g | |
| MnCl2∙H2O | 1.81 g |
| ZnSO4∙7H2O | 0.222 g |
| CuSO4∙5H2O | 0.079 g |
| Na2MO4∙2H2O | 0.39 g |
| Co(NO3)∙6H2O | 0.049 g |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Huang, G.; Fu, H.; An, C.; Yao, Y.; Cheng, G.; Suo, M. Allelopathy Inhibitory Effects of Hydrodictyon reticulatum on Chlorella pyrenoidosa under Co-Culture and Liquor-Cultured Conditions. Water 2017, 9, 416. https://doi.org/10.3390/w9060416
Chen X, Huang G, Fu H, An C, Yao Y, Cheng G, Suo M. Allelopathy Inhibitory Effects of Hydrodictyon reticulatum on Chlorella pyrenoidosa under Co-Culture and Liquor-Cultured Conditions. Water. 2017; 9(6):416. https://doi.org/10.3390/w9060416
Chicago/Turabian StyleChen, Xiujuan, Guohe Huang, Haiyan Fu, Chunjiang An, Yao Yao, Guanhui Cheng, and Meiqin Suo. 2017. "Allelopathy Inhibitory Effects of Hydrodictyon reticulatum on Chlorella pyrenoidosa under Co-Culture and Liquor-Cultured Conditions" Water 9, no. 6: 416. https://doi.org/10.3390/w9060416
APA StyleChen, X., Huang, G., Fu, H., An, C., Yao, Y., Cheng, G., & Suo, M. (2017). Allelopathy Inhibitory Effects of Hydrodictyon reticulatum on Chlorella pyrenoidosa under Co-Culture and Liquor-Cultured Conditions. Water, 9(6), 416. https://doi.org/10.3390/w9060416







