Evaluation of Clogging during Sand-Filtered Surface Water Injection for Aquifer Storage and Recovery (ASR): Pilot Experiment in the Llobregat Delta (Barcelona, Spain)
Abstract
:1. Introduction
2. Background Information
3. Materials and Methods
3.1. Column Set-Up and Operation
3.2. Sampling and Analytical Methods
3.2.1. Pressure Head Loss Measurements
3.2.2. Determination of Organic Matter in Sediments Deposited on the Metallic Screen Pieces
3.2.3. Biofilm Growth Evolution Determination
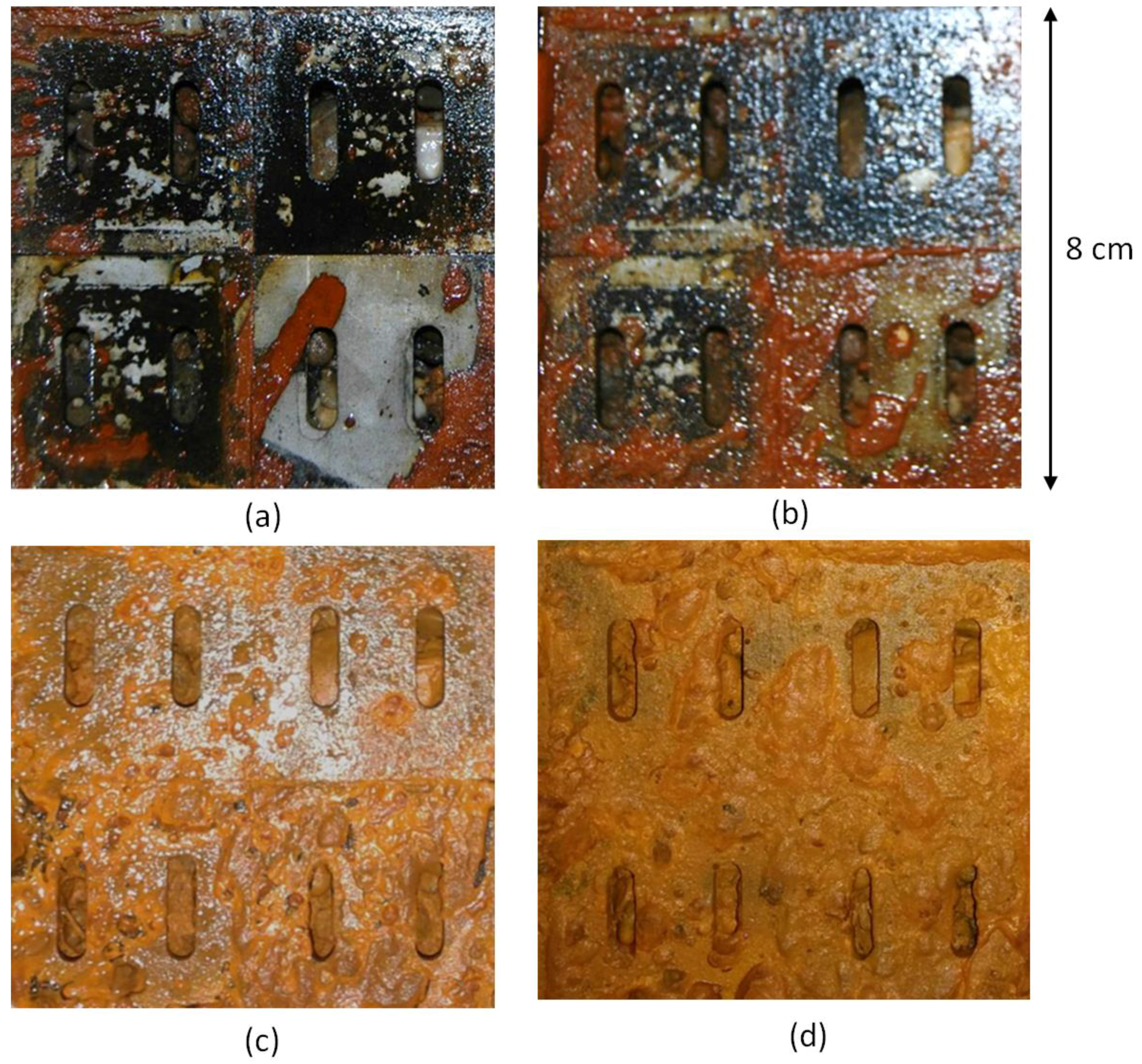
3.2.4. Visual Observation of the Metallic Screen Pieces
3.2.5. Examination of Sediment Morphology by Scanning Electron Microscopy with Energy Dispersive X-rays Analysis
4. Results
4.1. Well Screen Observations
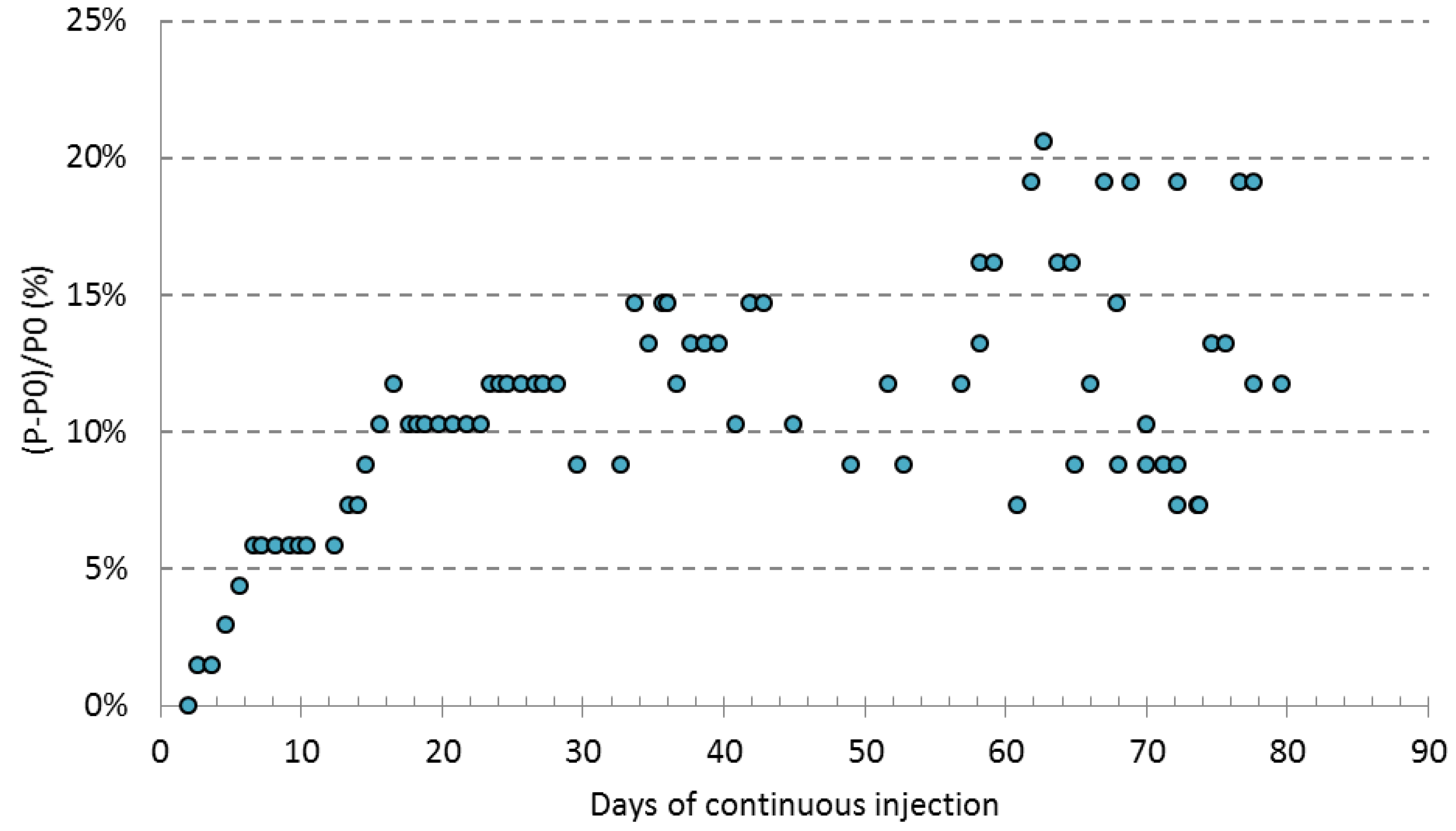
4.2. Preassure Head Loss
4.3. Determination of Organic Fraction of Biofilm
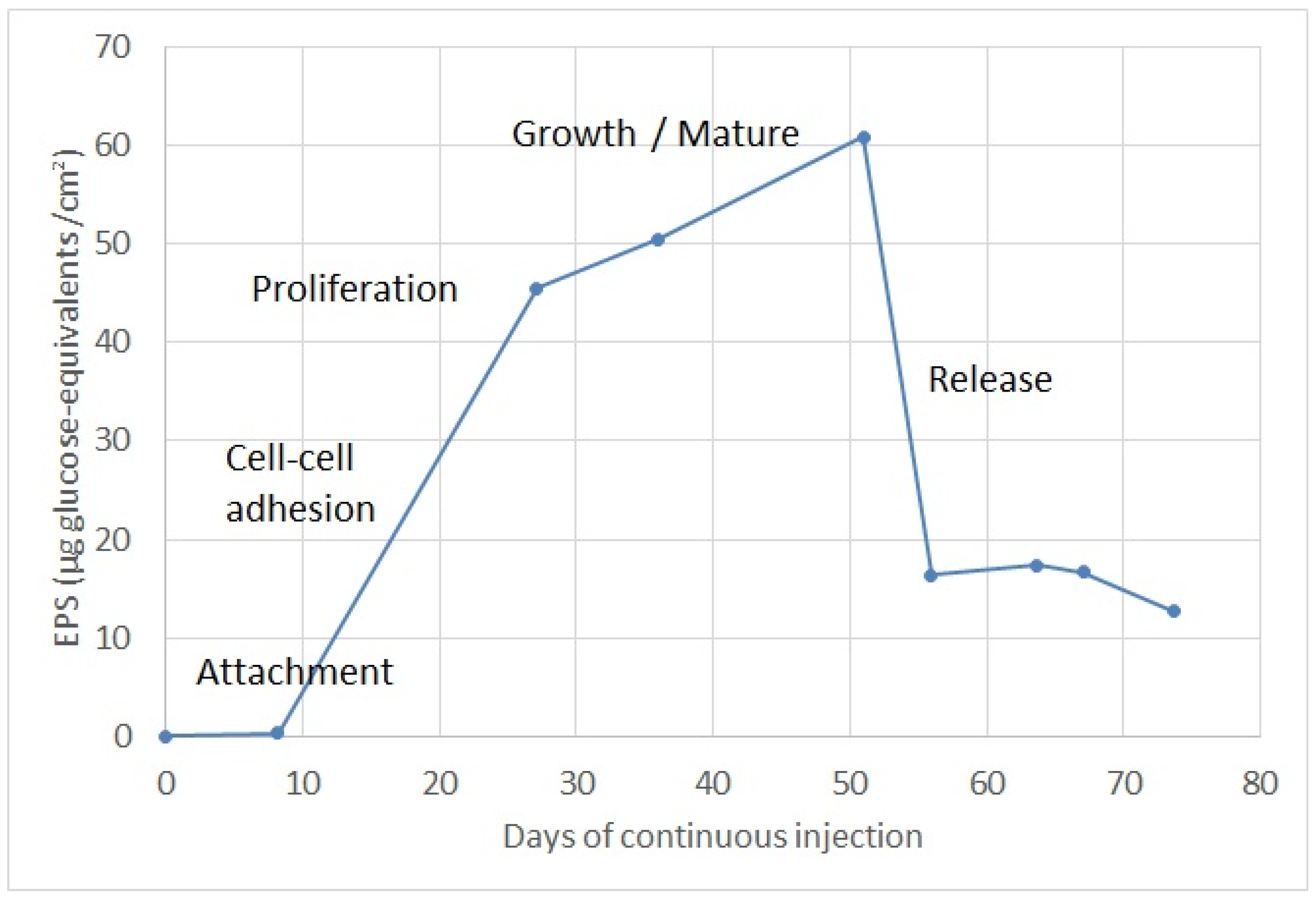
4.4. Biofilm Growth Evolution Determination
- Attachment: during the first ten days, EPS concentrations to the order of 1 µg glucose-eq/cm2 were observed, corresponding to the time at which planktonic bacteria adhere to the well screen surface. Previous research shows that a single bacterium will reach the surface through the liquid phase usually by swimming. The single bacterium will attach to surfaces using flagella and other surface appendages [21]. This attachment is generally reversible and it is largely mediated by van der Waals forces. However, early stages of biofilm development will depend on the specific strain. In nature biofilms, other eukaryotic organisms interact with the biofilm and thus form part of it, such as fungi, algae, yeasts, protozoa and other microorganisms.
- Adhesion: the following ten days were observed as the concentration of EPS increased progressively, passing from 1 to 30 µg glucose-eq/cm2 in about 10 days. As observed by other researchers [22], during the second stage bacteria will slowly but tightly adhere to the surface via pili, proteins, polysaccharides and fimbriae. Filamentous fungi will carry out deposition of spores or other propagules, such as hyphal fragments or sporangia. Diatoms will attach to the substratum by the production of mucilage, which will encapsulate the cells.
- Proliferation: from day 20 to day 28 a concentration of EPS was observed around 45 µg glucose-eq/cm2, corresponding to this subsequent step of proliferation, which is characterized mainly by the proliferation and production of EPS. During this stage, cells lose their flagella-driven motility and the whole system becomes immobilized. EPS are not unique to bacteria; some of the most abundant EPS producers are microalgae (in particular, diatoms). Fungi (yeasts and molds) also produce EPS [23].
- Biofilm maturation: this phase is the most elongated in time, lasting approximately 25 days, and the maximum EPS formation occurs during the maturation phase, reaching a maximum value of 60 µg glucose-eq/cm2. During this step, microorganisms continue to proliferate and excrete large amounts of hydrated EPS consisting of polysaccharides, proteins, nucleic acids and lipids, which provide stability to the biofilm as a whole and additional shelter to individual microorganisms [24].
- Release or detachment: during this last stage, the EPS concentration decreased progressively, reducing from 60 to 12 µg glucose-eq/cm2 in about 5 days. This phase corresponds to the moment when motile cells may disperse from the film by diverse mechanisms. Cells from the biofilm will attach at other places and will promulgate the spreading of the film [25]. Dispersal of fungi involves spore dispersal or release of biofilm fragments.
4.5. Scanning Electron Microscopy Observations and Energy Dispersive X-ray Microanalysis
5. Discussion
6. Conclusions
- Maximum relative change in head loss was 20% after 75 days of continuous operation in the ARS simulation experiment. Although the clogging formation caused an increase in head loss within the column, this was not enough to limit the flow rate. This suggests that in a real ASR system, a limitation in the aquifer infiltration capacity is not expected (the gravel would still be able to accept this injection flow), but a rise in the piezometric level of the well would be expected.
- Based on the EPS formation, it was determined that the bio-clogging formation evolved with a rapid increase in the first 140 days and a subsequent stabilization and decrease in the following days. It was also assessed that the composition of the muddy sediment settled in the well screen simulation was mainly inorganic, but comprised 11% of organic content.
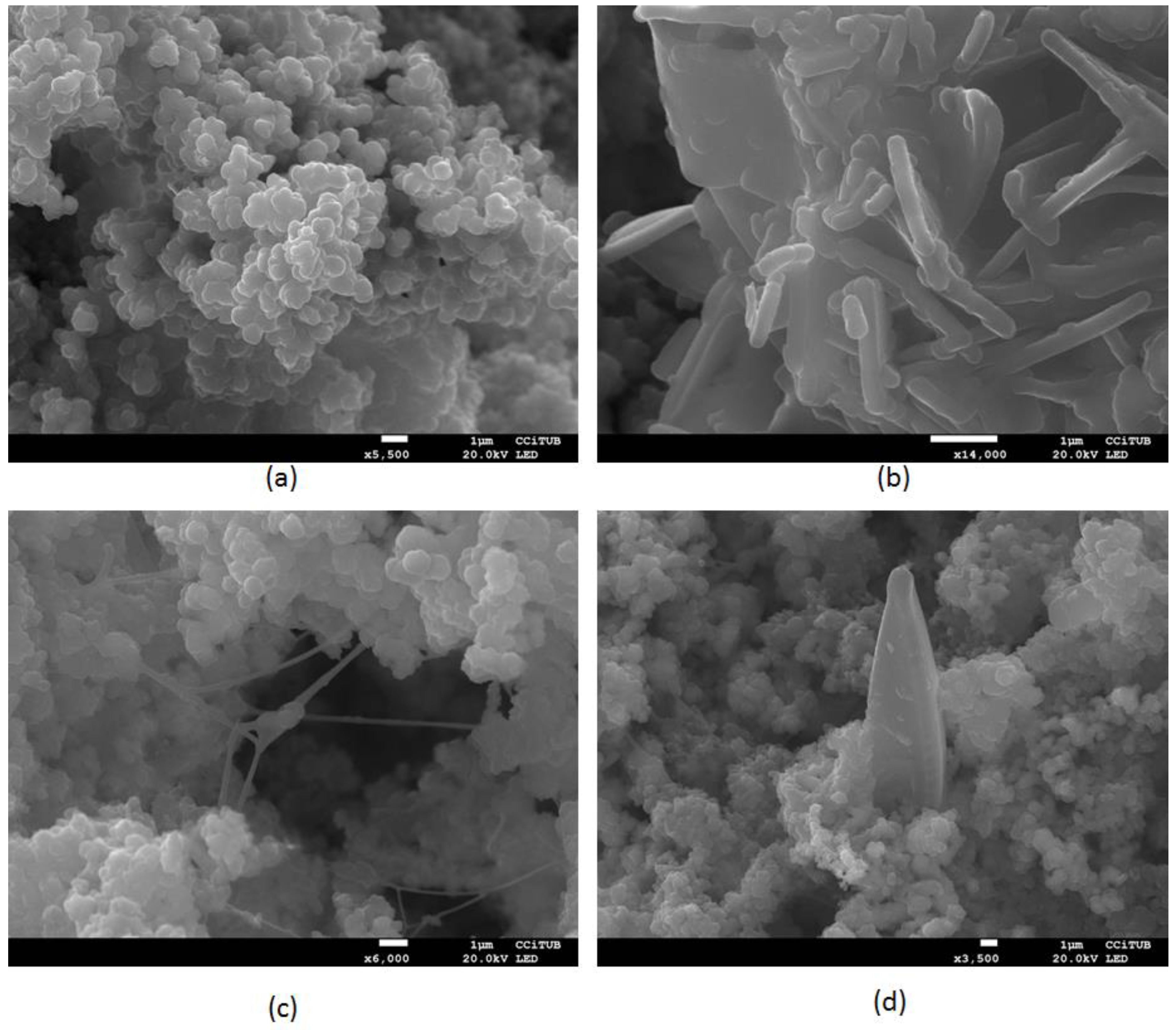
- Bio-clogging formation characterization by SEM photography and elemental components determination identified the presence of carbon, oxygen and hydrogen, corresponding to the organic fraction, and iron, calcium, magnesium, potassium, silica and aluminium, corresponding to the main fraction of inorganic material. The pictures have shown some isolated bacillus and hifas, while most of the ubiquitous material observed with the microscope corresponds to biological mass aggregates, presumably EPS.
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AOC | Assimilable Organic Carbon |
| ASR | Aquifer Storage and Recovery |
| DWTP | Drinking Water Treatment Plant |
| EPS | Extracellular Polymeric Substances |
| EDX | Energy dispersive X-ray |
| MAR | Managed Aquifer Recharge |
| MFI | Modified Fouling Index |
| SEM | Scanning Electron Microscopy |
| SFSW | Sand-filtered Surface Water |
| SJD | Sant Joan Despí Municipality |
| TOC | Total organic Carbon |
References
- DESSIN (Demonstrate Ecosystem Services Enabling Innovation in the Water Sector). Available online: https://dessin-project.eu/ (accessed on 10 November 2016).
- Armenter, J.L. Artificial recharging of the aquifers in the joint management of the resources in the delta of the river Llobregat. Houill. Blanche-Rev. Int. Eau 2008. [Google Scholar] [CrossRef]
- Pérez-Paricio, A.; Carrera, J. Site Description Cornellà, Spain; Final Report, EU Project on Artificial Recharge of Groundwater 1999, Chapter 4.8. EC Project ENV4-CT95-0071; UPC: Barcelona, Spain, 1999. [Google Scholar]
- European Union Seventh Framework Programme DESSIN Website. Project Delivarbale D22.4 Evaluation of Pre-Potablewater for Injection Using ASR. Available online: https://dessin-project.eu/?wpdmpro=draft-d22–4-a-evaluation-of-pre-potablewater-for-injection-using-asr (accessed on 10 November 2016).
- Pérez-Paricio, A.; Carrera, J. EU Project on Artificial Recharge of Groundwater Contract ENV4-CT95-0071. In Clogging Handbook; UPC: Barcelona, Spain, 2001; Chapter 2. [Google Scholar]
- Hubbs, S.A. Riverbank Filtration Hydrology: Impacts on System Capacity and Water Quality. In Proceedings of the NATO Advanced Research Workshop on Riverbank Filtration Hydrology, Bratislava, Slovakia, 7–10 September 2004; Springer: Bratislava, Slovakia, 2006. [Google Scholar]
- Pyne, R.D.G. Aquifer Storage Recovery: A Guide to Groundwater Recharge through Wells, 2nd ed.; ASR Systems: Gainesville, FL, USA, 2005. [Google Scholar]
- Martin, R.; Dillon, P. Department of Water, Land and Biodiversity Conservation. In Aquifer Storage and Recovery, Future Directions for South Australia; Report DWLBC 2002/04; CSIRO: Adelaide, Australia, 2002. [Google Scholar]
- West Basin Municipal Water District. Recycled Water Quality Standards Study; Final Report; Carollo Engineers: Walnut Creek, CA, USA, 2008. [Google Scholar]
- Olsthoorn, T.N. The Clogging of Recharge Wells, Main Subjects; KIWA-Communications 72; UDC: Rijswijk, The Netherlands, 1982. [Google Scholar]
- Ribas, F.; Frias, J.; Huguet, J.M.; Lucena, F. Efficiency of various water treatment processes in the removal of biodegradable and refractory organic matter. Water Res. 1997, 31, 639–649. [Google Scholar] [CrossRef]
- Mills, W.R. The quest for water through artificial recharge and wastewater recycling. Management of Aquifer recharge for Sustainability. In Proceedings of the 4th International Symposium on Artificial Recharge of Groundwater, Adelaide, Australia, 22–26 September 2002; Balkema Publishers: Adelaide, Australia, 2002. [Google Scholar]
- Van der Kooij, D.; Hijnen, W.A.M. The effect of low concentrations of assimilable organic carbon in water on biological clogging of sand beds. Water Reg. 1992, 26, 963–972. [Google Scholar]
- Escalante, F. Técnicas de Tratamiento de Suelo y Acuífero (S.A.T) Aplicadas a la Gestión de la Recarga; Grafinat: Madrid, Spain, 2006. [Google Scholar]
- Dean, W.E., Jr. Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition: Comparison with other methods. J. Sediment. Petrol. 1974, 44, 242–248. [Google Scholar]
- Romaní, A.M.; Fund, K.; Artigas, J.; Schwartz, T.; Sabater, S.; Obst, U. Relevance of Polymeric Matrix Enzymes during Biofilm Formation. Microb. Ecol. 2008, 56, 427–436. [Google Scholar] [CrossRef] [PubMed]
- El Abed, S.; Ibnsouda, S.K.; Latrache, H.; Hamadi, F. Scanning Electron Microscopy (SEM) and Environmental SEM: Suitable Tools for Study of Adhesion Stage and Biofilm Formation, Scanning Electron Microscopy; Kazmiruk, V., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Else, T.A.; Pantle, C.R.; Amy, P.S. Boundaries for Biofilm Formation: Humidity and Temperature. Appl. Environ. Microbiol. 2003, 69, 5006–5010. [Google Scholar] [CrossRef] [PubMed]
- Bozzola, J.J.; Russell, L.D. Electron Microsscopy Principles and Techniques for Biologists; Jones and Bartlet Publishers: Sudbury, MA, USA, 1999. [Google Scholar]
- Flemming, H.-C.; Griegbe, W.J.; Mayer, C. Physico-chemical properties of biofilms. In Biofilms: Recent Advances in Their Study and Control; Evans, L.V., Ed.; Harwood Academic Publishers: Amsterdam, The Netherlands, 2000; pp. 19–34. [Google Scholar]
- Kearns, D.B. A field guide to bacterial swarming motility. Nat. Rev. Microbiol. 2010, 8, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Monroe, D. Looking for Chinks in the Armor of Bacterial Biofilms. PLoS Biol. 2007, 5, e307. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [PubMed]
- Conrad, J.C. Physics of bacterial near-surface motility using flagella and type IV pili: Implications for biofilm formation. Res. Microbiol. 2012, 163, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.; Chen, Z.; Roos, D.S.; Pohlschröder, M. Identification of Surprisingly Diverse Type IV Pili, across a Broad Range of Gram-Positive Bacteria. PLoS ONE 2011, 6, e28919. [Google Scholar] [CrossRef] [PubMed]
- John, D.E.; Rose, J.B.; Kamarainen, A. Survival of Fecal Indicator Bacteria, Bacteriophage and Protozoa in Florida Surface and Ground Waters; Final Report of the Fate of Microorganisms in Aquifers Study; College of Marine Sciences, University of South Florida: Tampa, FL, USA, 2004. [Google Scholar]
- Freixa, A.; Rubol, S.; Brangarí, C.; Fernàndez-Garcia, D.; Sanchez-Vila, X.; Romaní, A. The effects of sediment depth and oxygen concentration on the use of organic matter: An experimental study using an infiltration tank. Sci. Total Environ. 2016, 540, 20–31. [Google Scholar] [CrossRef] [PubMed]
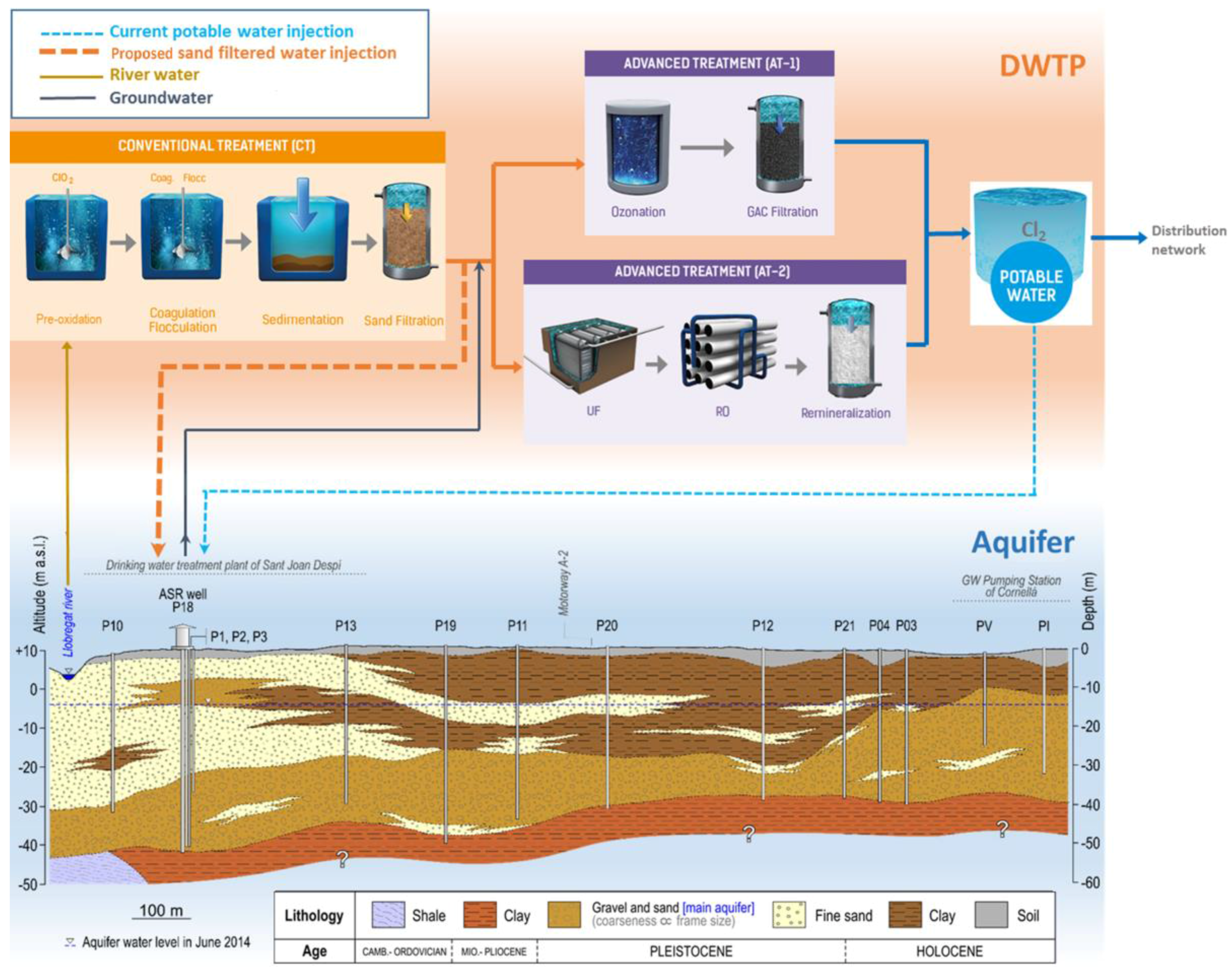
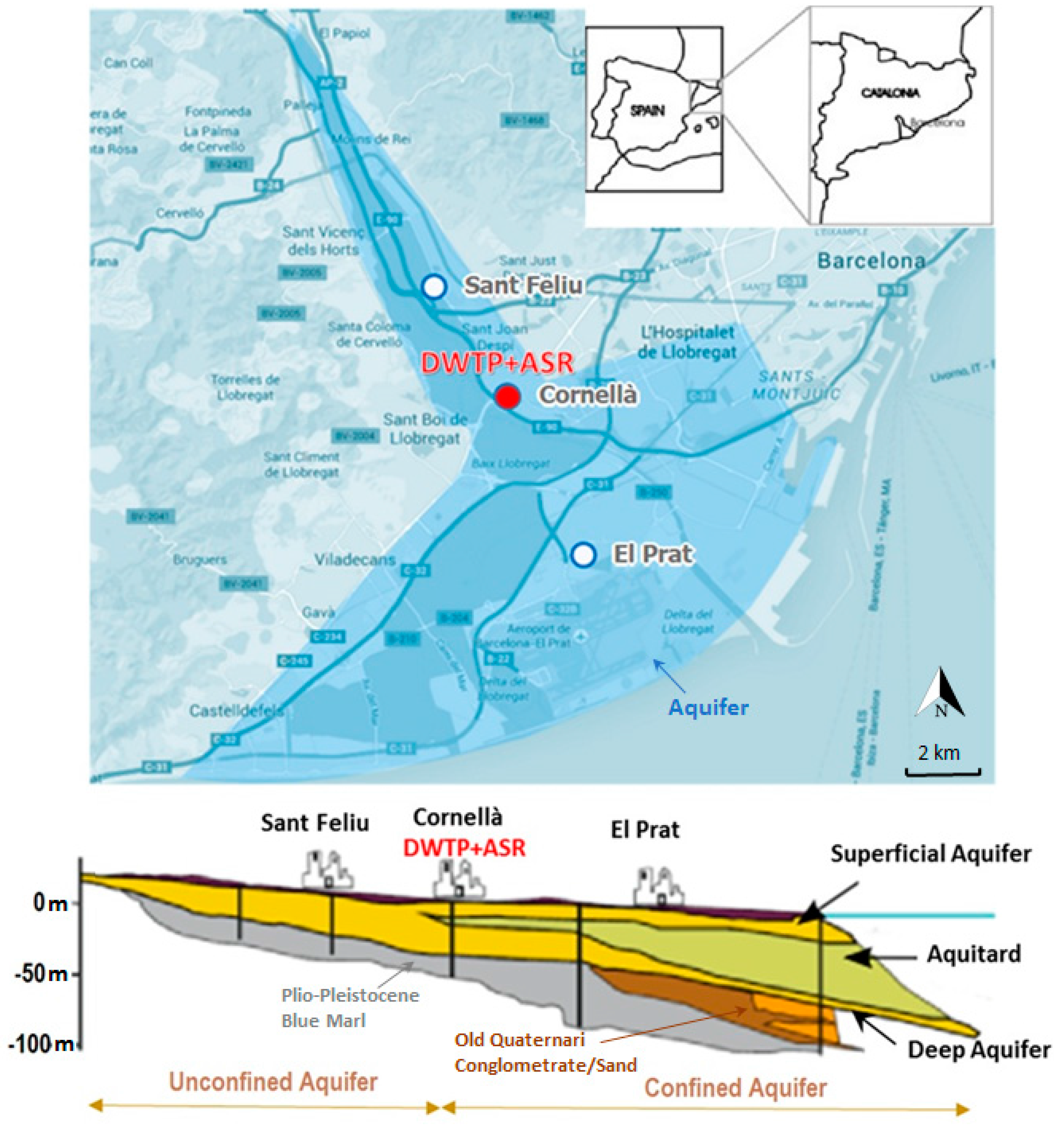
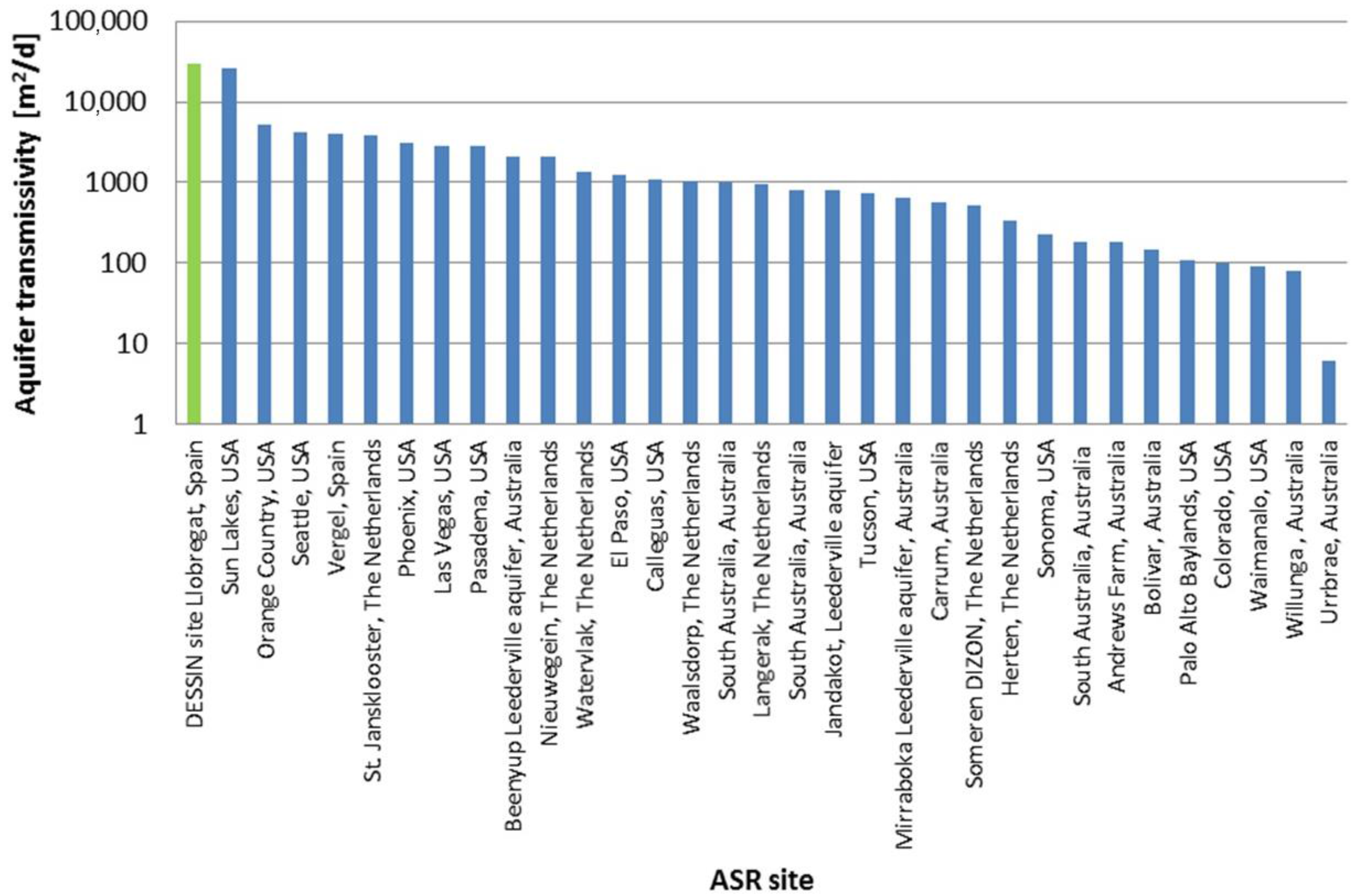
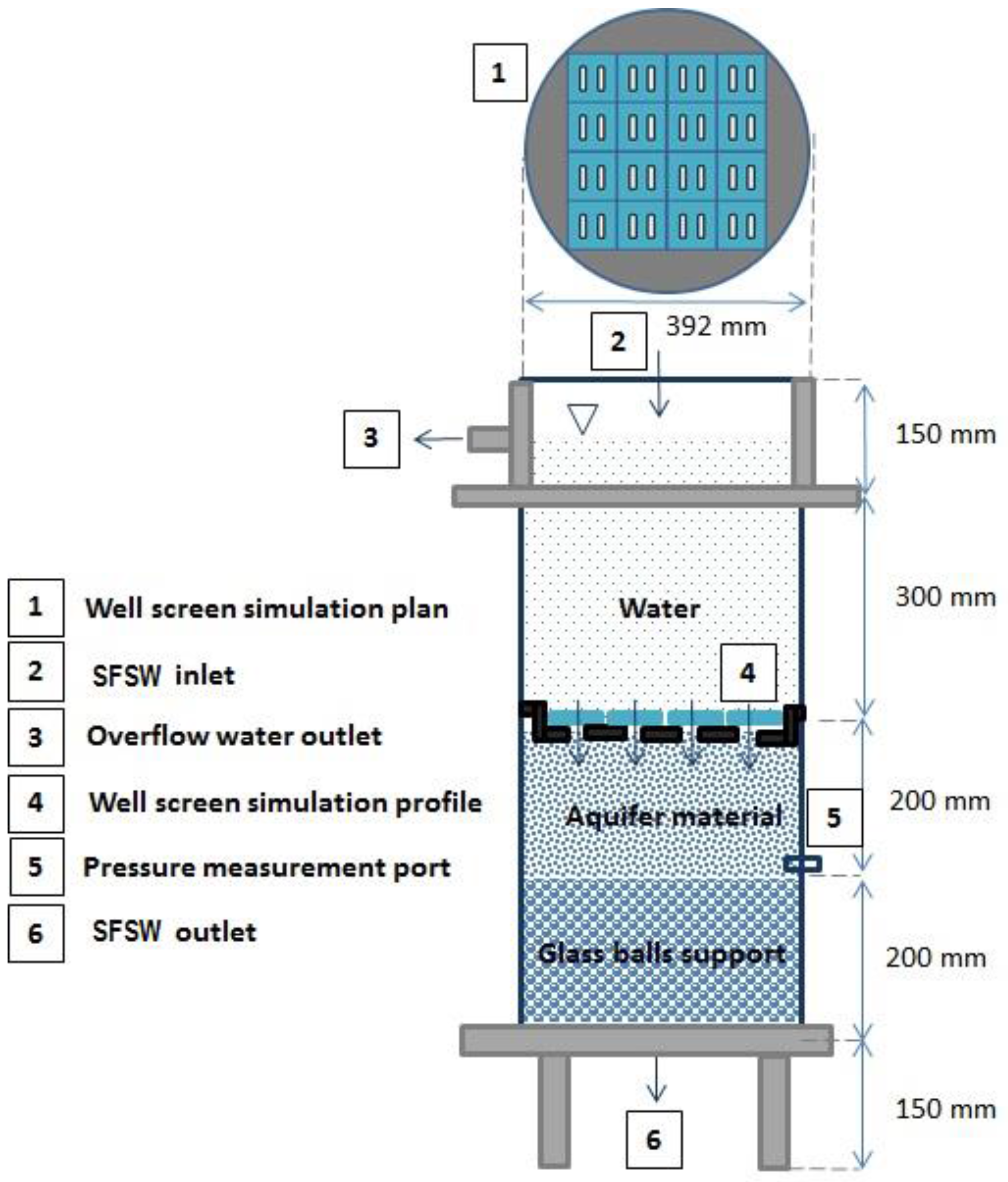
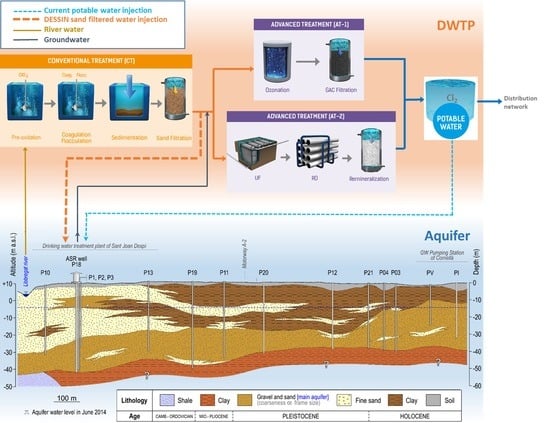
| Parameter (Units) | SFSW | Native Groundwater | Drinking Water Standards | Recommendations for ASR Injection Water [Reference] |
|---|---|---|---|---|
| Turbidity (NTU) | 0.24 | 0.13 | 1 | 1 [7], 0.2 [8] |
| MFI (s/L2) | 23.5 | NA | NR | 3–5 [5], 2 [8] |
| Colour (mg Pt/L) | 4.58 | 1.38 | 15 | NRF |
| Conductivity (µS/cm) | 1279 (*) | 1753 | 2500 | NRF |
| Chloride (mg/L) | 229 | 297 | 250 | NRF |
| TOC (mg/L) | 3.6 | 1.2 | 7 | 5 [9], 10 [5,10] |
| AOC (µg acetate-C/L) | 0.31 [11] (**) | NA | NR | 10 [12] |
| Calcium (mg/L) | 105 | 164 | NR | NRF |
| Magnesium (mg/L) | 26.7 | 48.1 | NR | NRF |
| Sodium (mg/L) | 116 | 173 | 200 | NRF |
| Sulphate (mg/L) | 160 (*) | 244 | 250 | NRF |
| Nitrate (mg/L) | 9.1 (*) | 10.3 | 50 | NRF |
| Ammonium (mg/L) | 1.11 (*) | 0.07 | 0.5 | 0.5 [6] |
| Total Iron (µg/L) | 10.1 | 96.4 | 200 | NRF |
| Aluminium (µg/L) | 178.8 | 12.5 | 200 | NRF |
| Nickel (µg/L) | 6.9 | 2.5 | 20 | NRF |
| Total manganese (µg/L) | 9.16 | 11.75 | 50 | NRF |
| Total phosphorus (µg/L) | 32 | 10 | NR | NRF |
| Total Trihalomethanes (µg/L) | <2 | <2 | 100 | NRF |
| E. coli (MPN/100 mL) | 120 | 0 | 0 | 10,000 [6] |
| Total coliforms (MPN/100 mL) | 731 | 0.5 | 0 | NRF |
| Enterococcus (CFU/100 mL) | 2 | 0 | 0 | NRF |
| C. perfringens (CFU/100 mL) | 35.7 | 0.1 | 0 | NRF |
| Colony count at 22 °C (CFU/100 mL) | 4024 | 31 | 100 | NRF |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camprovin, P.; Hernández, M.; Fernández, S.; Martín-Alonso, J.; Galofré, B.; Mesa, J. Evaluation of Clogging during Sand-Filtered Surface Water Injection for Aquifer Storage and Recovery (ASR): Pilot Experiment in the Llobregat Delta (Barcelona, Spain). Water 2017, 9, 263. https://doi.org/10.3390/w9040263
Camprovin P, Hernández M, Fernández S, Martín-Alonso J, Galofré B, Mesa J. Evaluation of Clogging during Sand-Filtered Surface Water Injection for Aquifer Storage and Recovery (ASR): Pilot Experiment in the Llobregat Delta (Barcelona, Spain). Water. 2017; 9(4):263. https://doi.org/10.3390/w9040263
Chicago/Turabian StyleCamprovin, Pere, Marta Hernández, Sonia Fernández, Jordi Martín-Alonso, Belén Galofré, and José Mesa. 2017. "Evaluation of Clogging during Sand-Filtered Surface Water Injection for Aquifer Storage and Recovery (ASR): Pilot Experiment in the Llobregat Delta (Barcelona, Spain)" Water 9, no. 4: 263. https://doi.org/10.3390/w9040263
APA StyleCamprovin, P., Hernández, M., Fernández, S., Martín-Alonso, J., Galofré, B., & Mesa, J. (2017). Evaluation of Clogging during Sand-Filtered Surface Water Injection for Aquifer Storage and Recovery (ASR): Pilot Experiment in the Llobregat Delta (Barcelona, Spain). Water, 9(4), 263. https://doi.org/10.3390/w9040263