Retention of Silica Nanoparticles in a Lab-Scale Membrane Bioreactor: Implications for Process Performance and Membrane Fouling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
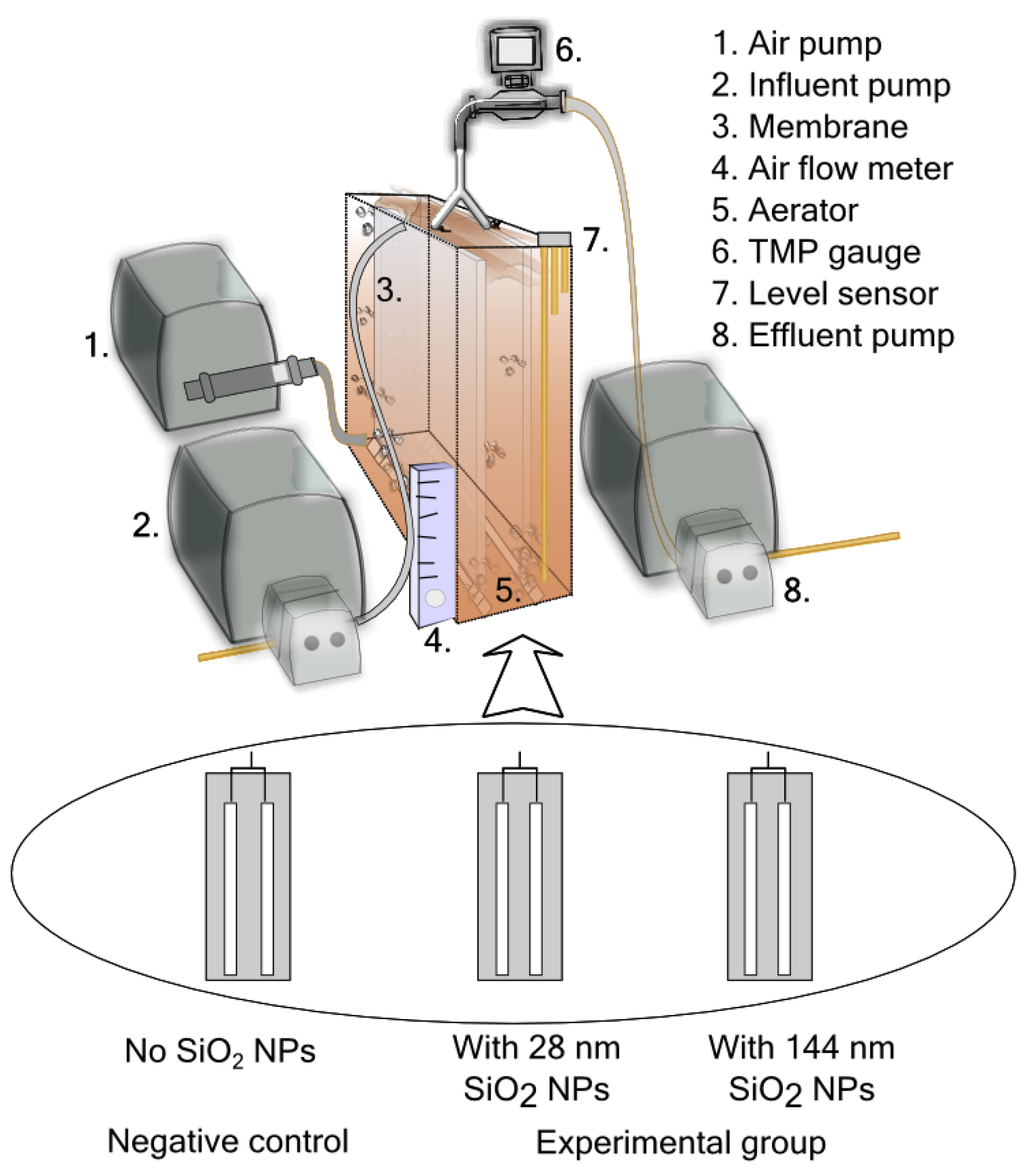
2.2. Continuous Lab-Scale MBR Experiments
2.2.1. Operating Conditions
2.2.2. Characterization of Activated Sludge
2.2.3. Activated Sludge Respiration Inhibition Test
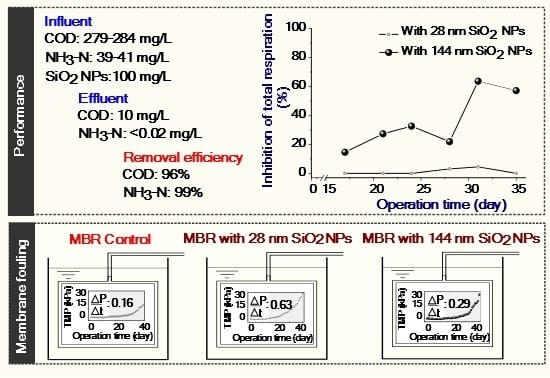
2.2.4. Performance Evaluation
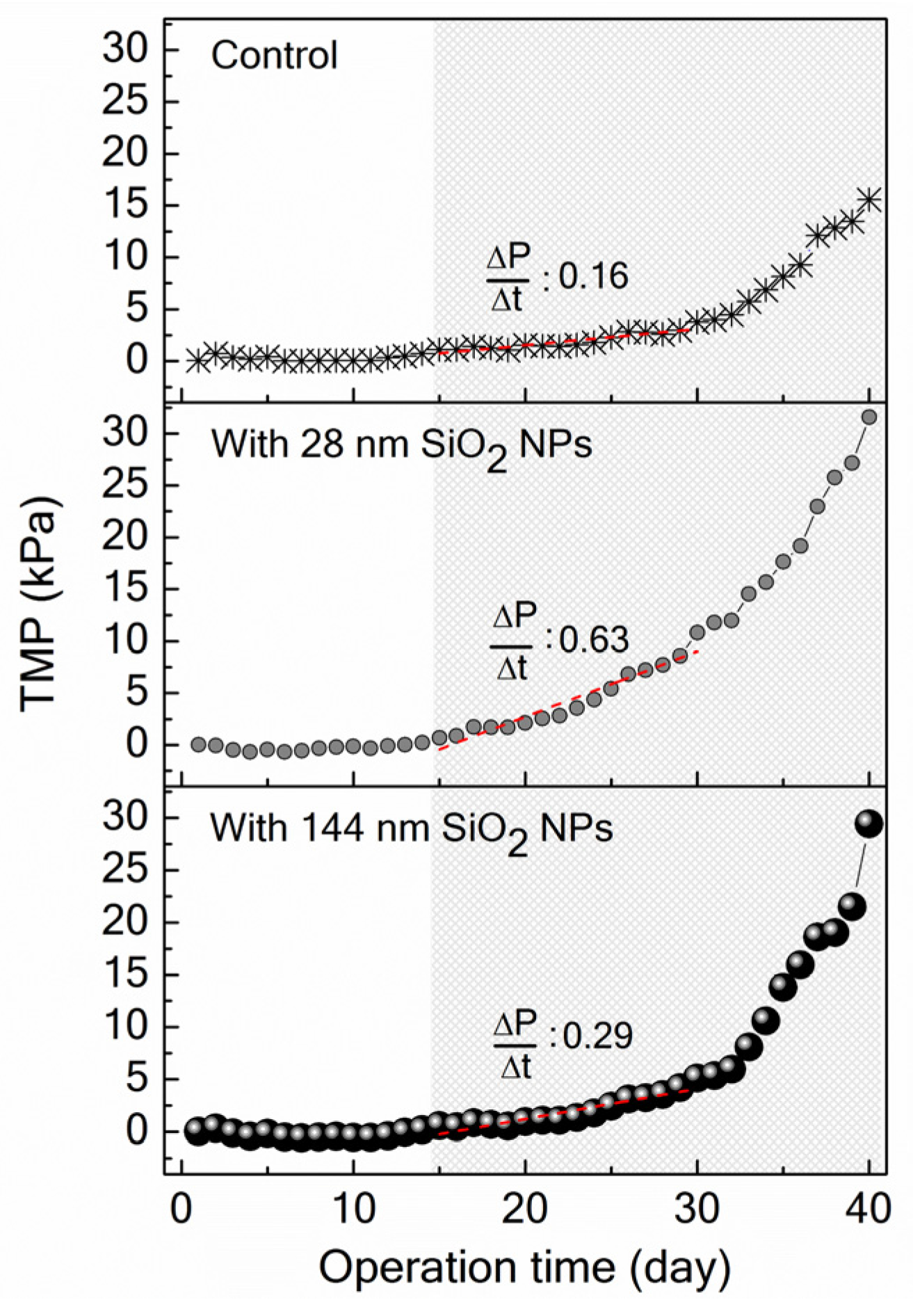
2.2.5. Transmembrane Pressure Monitoring
2.3. Batch Experiments
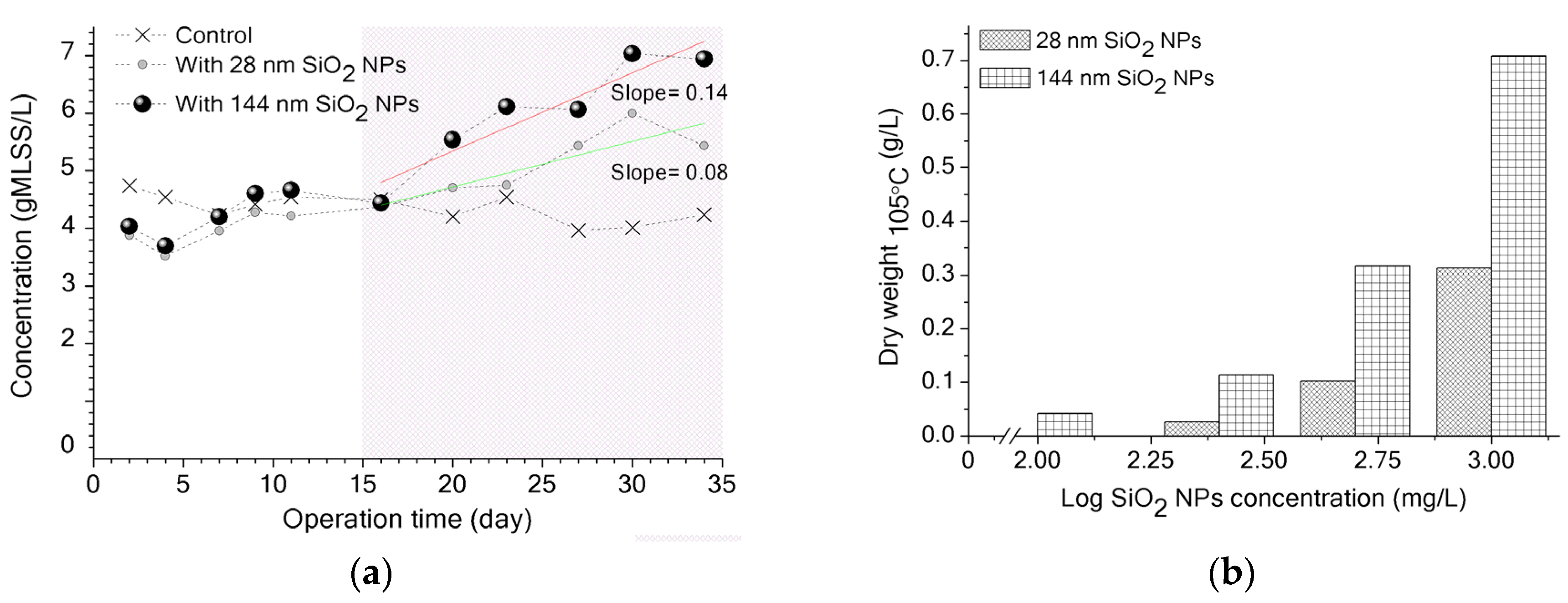
2.3.1. Influence of SiO2 NPs on MLSS Measurement
2.3.2. SiO2 NPs Settling Experiments and Dead-End Filtration Tests
3. Results and Discussion
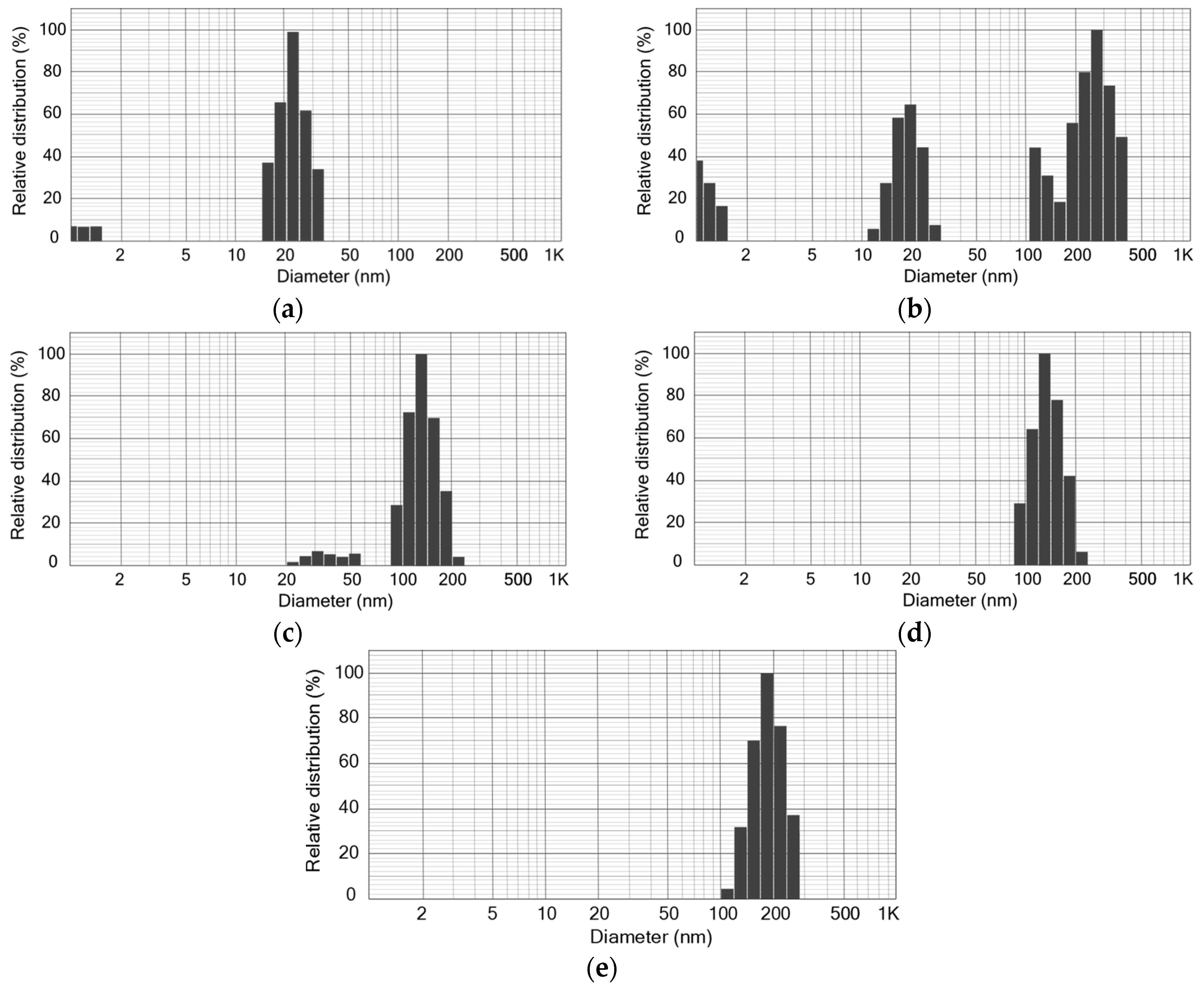
3.1. Retention of SiO2 NPs in MBR
3.2. Potential Biological Effect of SiO2 NPs on Activated Sludge and MBR Performance
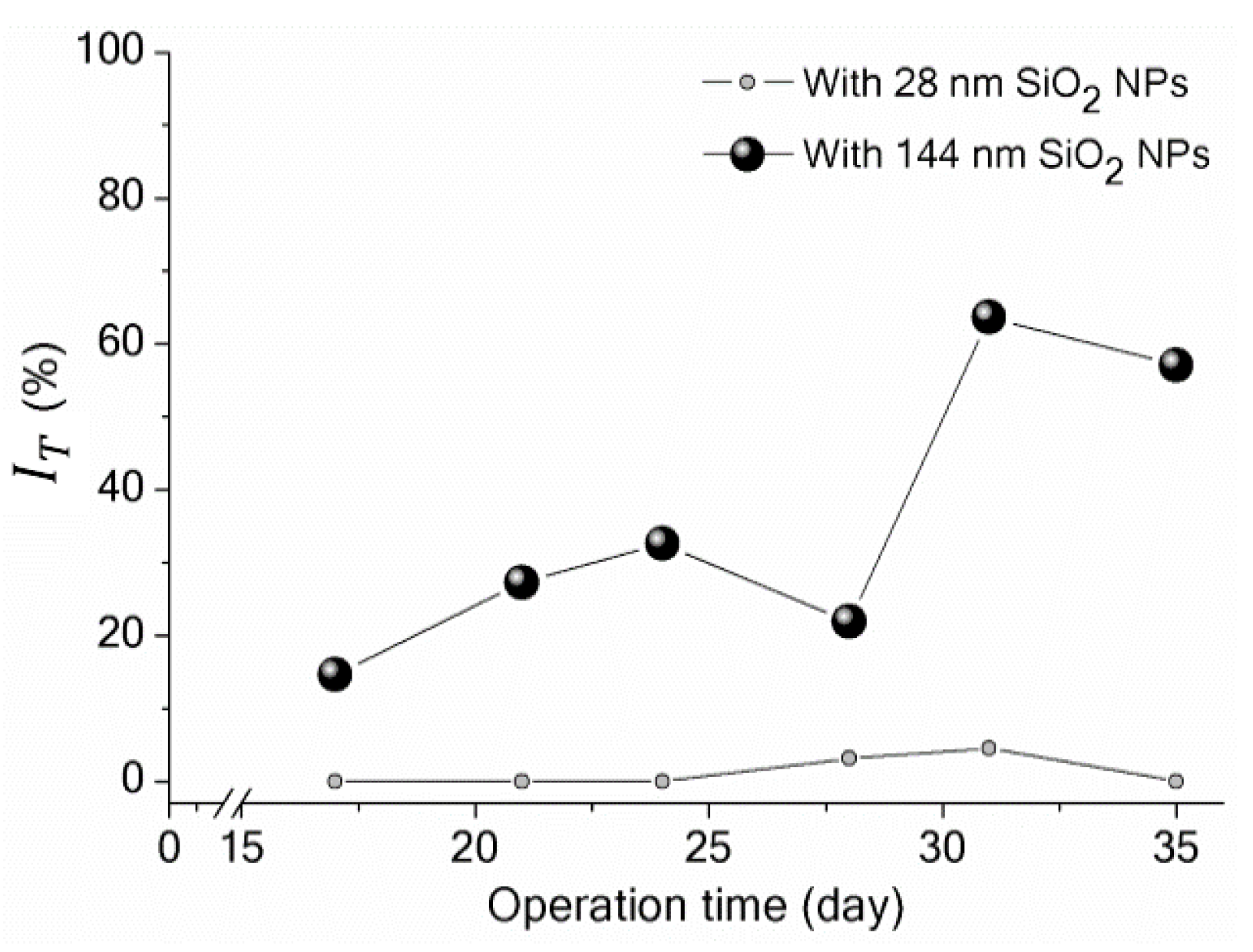
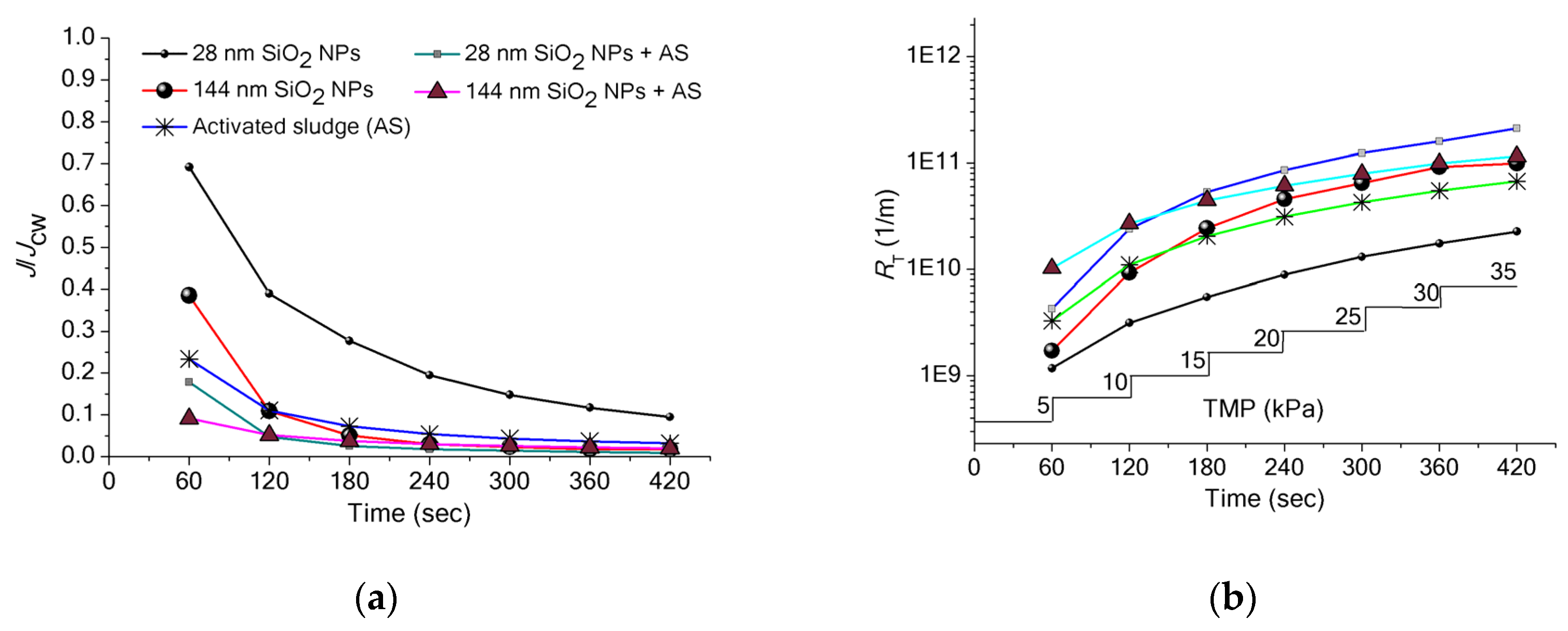
3.3. Implications of SiO2 NPs Retention for Membrane Fouling in an MBR
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CAS | Conventional activated sludge |
| COD | Chemical oxygen demand |
| DLS | Dynamic light scattering |
| TMP | Transmembrane pressure |
| MBR | Membrane bioreactor |
| C-PVC | Chlorinated polyvinyl chloride |
| DO | Dissolved oxygen |
| MLSS | Mixed liquor suspended solids |
| MLVSS | Mixed liquor volatile suspended solids |
| OUR | Oxygen uptake rate |
| OECD | Organization for economic cooperation and development |
| TN | Total nitrogen |
| TP | Total phosphorus |
References
- Otero-González, L.; Barbero, I.; Field, J.A.; Shadman, F.; Sierra-Alvarez, R. Stability of alumina, ceria, and silica nanoparticles in municipal wastewater. Water Sci. Technol. 2014, 70, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Otero-González, L.; Field, J.A.; Calderon, I.A.; Aspinwall, C.A.; Shadman, F.; Zeng, C.; Sierra-Alvarez, R. Fate of fluorescent core-shell silica nanoparticles during simulated secondary wastewater treatment. Water Res. 2015, 77, 170–178. [Google Scholar]
- Brar, S.K.; Verma, M.; Tyagi, R.D.; Surampalli, R.Y. Engineered nanoparticles in wastewater and wastewater sludge—Evidence and impacts. Waste Manage. 2010, 30, 504–520. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.; Wu, Y.; Cao, B. Impacts of engineered nanomaterials on microbial community structure and function in natural and engineered ecosystems. Applied Microbiol. Biot. 2014, 98, 8457–8468. [Google Scholar] [CrossRef] [PubMed]
- Kiser, M.A.; Ryu, H.; Jang, H.; Hristovski, K.; Westerhoff, P. Biosorption of nanoparticles to heterotrophic wastewater biomass. Water Res. 2010, 44, 4105–4114. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, H.Y.; Cha, S.; Ahn, C.H.; Roh, J.; Park, S.; Kim, S.; Choi, K.; Yi, J.; Kim, Y.; Yoon, J. Removal characteristics of engineered nanoparticles by activated sludge. Chemosphere 2013, 92, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Rottman, J.; Shadman, F.; Sierra-Alvarez, R. Interactions of inorganic oxide nanoparticles with sewage biosolids. Water Sci. Technol. 2012, 66, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Su, Y.; Chen, Y. Acute and chronic responses of activated sludge viability and performance to silica nanoparticles. Environ. Sci. Technol. 2012, 46, 7182–7188. [Google Scholar] [CrossRef] [PubMed]
- Judd, S. The MBR Book: Principles and Applications of Membrane Bioreactors for Water and Wastewater Treatment; Elsevier: Oxford, UK, 2010; pp. 174–176. [Google Scholar]
- Auffan, M.; Rose, J.; Bottero, J.Y.; Lowry, G.V.; Jolivet, J.P.; Wiesner, M.R. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Tarabara, V.V. Pore blocking mechanisms during early stages of membrane fouling by colloids. J. Colloid Interf. Sci. 2008, 328, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Belfort, G.; Davis, R.H.; Zydney, A.L. The behavior of suspensions and macromolecular solutions in crossflow microfiltration. J. Membrane Sci. 1994, 96, 1–58. [Google Scholar] [CrossRef]
- Zhu, X.; Elimelech, M. Colloidal fouling of reverse osmosis membranes: measurements and fouling mechanisms. Environ. Sci. Technol. 1997, 31, 3654–3662. [Google Scholar] [CrossRef]
- Allen, P.T.; Stephenson, G.M. Inter-group understanding and size of organisation. Brit. J. Ind. Relat. 1983, 21, 312–329. [Google Scholar] [CrossRef]
- Hur, J.; Shin, J.; Kang, M.; Cho, J. Tracking variations in fluorescent-dissolved organic matter in an aerobic submerged membrane bioreactor using excitation-emission matrix spectra combined with parallel factor analysis. Bioprocess. Biosyst. Eng. 2014, 37, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater; American Public Health Association, American Water Works Association and Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- Organization for Economic Co-operation and Development (OECD). OECD 209 Guideline for the Testing of Chemicals, Activated Sludge Respiration Inhibition Test; OECD: Paris, France, 1993. [Google Scholar]
- Foley, G. Membrane Filtration: A Problem Solving Approach with MATLAB; Cambridge University Press: New York, NY, USA, 2013; pp. 16–45. [Google Scholar]
- Cho, J.; Song, K.G.; Ahn, K.H. The activated sludge and microbial substances influences on membrane fouling in submerged membrane bioreactor: unstirred batch cell test. Desalination 2005, 183, 425–429. [Google Scholar] [CrossRef]
- Ahmed, Z.; Cho, J.; Lim, B.R.; Song, K.G.; Ahn, K.H. Effects of sludge retention time on membrane fouling and microbial community structure in a membrane bioreactor. J. Membrane Sci. 2007, 287, 211–218. [Google Scholar] [CrossRef]
- Munir, A. Dead end membrane filtration, laboratory feasibility studies in environmental engineering. Available online: http://www.egr.msu.edu/~hashsham/courses/ene806/docs/Membrane%20Filtration.pdf (accessed on 27 June 2016).
- Simon-Deckers, A.; Loo, S.; Mayne-L’hermite, M.; Herlin-Boime, N.; Menguy, N.; Reynaud, C.; Gouget, B.; Cariere, M. Size-, composition-and shape-dependent toxicological impact of metal oxide nanoparticles and carbon nanotubes toward bacteria. Environ. Sci. Technol. 2009, 43, 8423–8429. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; de Aberasturi, D.J.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2010, 30, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Sibag, M.; Choi, B.G.; Suh, C.; Lee, K.H.; Lee, J.W.; Maeng, S.K.; Cho, J. Inhibition of total oxygen uptake by silica nanoparticles in activated sludge. J. Haz. Mat. 2015, 283, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Mashayekhi, H.; Xing, B. Bacterial toxicity comparison between nano-and micro-scaled oxide particles. Environ. Pollut. 2009, 157, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef] [PubMed]
- Bottero, J.Y. Environmental risks of nanotechnology: A new challenge? In Nanosciences and Nanotechnology; Springer International Publishing: Cham, Switzerland, 2016; pp. 287–311. [Google Scholar]
- Zanganeh, S.; Spitler, R.; Erfanzadeh, M.; Mahmoudi, M. Protein Corona: Opportunities and Challenges. Int. J. Biochem. Cell. Biol. 2016, 75, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Saptarshi, S.R.; Duschl, A.; Lopata, A.L. Interaction of nanoparticles with proteins: relation to bio-reactivity of the nanoparticle. J. Nanobiotechnol. 2013, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Lynch, I.; Cedervall, T.; Lundqvist, M.; Cabaleiro-Lago, C.; Linse, S.; Dawson, K.A. The nanoparticle-protein complex as a biological entity; a complex fluids and surface science challenge for the 21st century. Adv. Colloid Interface Sci. 2012, 134, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.R.; Huang, C.; Jiang, W.; Chen, C. Treatment of wastewater containing nano-scale silica particles by dead-end microfiltration: evaluation of pretreatment methods. Desalination 2005, 179, 31–40. [Google Scholar] [CrossRef]
- Singh, G.; Song, L. Quantifying the effect of ionic strength on colloidal fouling potential in membrane filtration. J. Colloid Interface Sci. 2005, 28, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Kissa, E. Dispersions: Characterization, Testing, and Measurement; CRC Press: Boca Raton, FL, USA, 1999; Volume 84. [Google Scholar]
- Trzaskus, K.W.; Zdeb, A.; de Vos, W.M.; Kemperman, A.; Nijmeijer, K. Fouling behavior during microfiltration of silica nanoparticles and polymeric stabilizers. J. Membrane Sci. 2016, 505, 205–215. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Boo, C.; Lee, S.; Elimelech, M.; Meng, Z.; Hong, S. Colloidal fouling in forward osmosis: Role of reverse salt diffusion. J. Membrane Sci. 2012, 390, 277–284. [Google Scholar] [CrossRef]
- Shirazi, S.; Lin, C.J.; Chen, D. Inorganic fouling of pressure-driven membrane processes—A critical review. Desalination 2010, 250, 236–248. [Google Scholar] [CrossRef]
- Rosenberger, S.; Laabs, C.; Lesjean, B.; Gnirss, R.; Amy, G.; Jekel, M.; Schrotter, J.C. Impact of colloidal and soluble organic material on membrane performance in membrane bioreactors for municipal wastewater treatment. Water Res. 2006, 40, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Laabs, C.N.; Amy, G.L.; Jekel, M. Understanding the size and character of fouling-causing substances from effluent organic matter (EfOM) in low-pressure membrane filtration. Environ. Sci. Technol. 2006, 40, 4495–4499. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, X.Y. Investigation of the effect of nanoparticle exposure on the flocculability of activated sludge using particle image volecimetry in combination with the extended DLVO analysis. Colloids Surf. B Biointerfaces. 2016, 143, 382–389. [Google Scholar] [CrossRef] [PubMed]

| Treatment | COD (SD) mg/L | Removal % | NH3–N (SD) mg/L | Removal % | TN (SD) mg/L | Removal % | TP (SD) mg/L | Removal % | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Influent | Effluent | Influent | Effluent | Influent | Effluent | Influent | Effluent | |||||
| MBR control | 279 (60) | 10 (2) | 96 | 39 (9) | < 0.02 | 99 | 50 (5) | 47 (4) | 6 | 11 (2) | 9 (1) | 18 |
| MBR with 28 nm SiO2 NPs | 282 (54) | 10 (2) | 96 | 41 (7) | < 0.02 | 99 | 48 (3) | 44 (3) | 8 | 11 (1) | 9 (1) | 18 |
| MBR with 144 nm SiO2 NPs | 284 (105) | 11 (2) | 96 | 40 (9) | < 0.02 | 99 | 48 (4) | 45 (4) | 6 | 11 (1) | 9 (1) | 18 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sibag, M.L.; Lee, S.M.; Kim, H.J.; Cho, J. Retention of Silica Nanoparticles in a Lab-Scale Membrane Bioreactor: Implications for Process Performance and Membrane Fouling. Water 2016, 8, 277. https://doi.org/10.3390/w8070277
Sibag ML, Lee SM, Kim HJ, Cho J. Retention of Silica Nanoparticles in a Lab-Scale Membrane Bioreactor: Implications for Process Performance and Membrane Fouling. Water. 2016; 8(7):277. https://doi.org/10.3390/w8070277
Chicago/Turabian StyleSibag, Mark Larracas, Soo Mi Lee, Hee Jun Kim, and Jinwoo Cho. 2016. "Retention of Silica Nanoparticles in a Lab-Scale Membrane Bioreactor: Implications for Process Performance and Membrane Fouling" Water 8, no. 7: 277. https://doi.org/10.3390/w8070277
APA StyleSibag, M. L., Lee, S. M., Kim, H. J., & Cho, J. (2016). Retention of Silica Nanoparticles in a Lab-Scale Membrane Bioreactor: Implications for Process Performance and Membrane Fouling. Water, 8(7), 277. https://doi.org/10.3390/w8070277