Abstract
Headwater streams generally comprise the majority of stream area in a watershed and can have a strong influence on downstream food webs. Our objective was to determine the effect of altering streamside management zone (SMZ) configurations on headwater aquatic insect communities. Timber harvests were implemented within six watersheds in eastern Kentucky. The SMZ configurations varied in width, canopy retention and best management practice (BMP) utilization at the watershed scale. Benthic macroinvertebrate samples collected one year before and four years after harvest indicated few differences among treatments, although post-treatment abundance was elevated in some of the treatment streams relative to the unharvested controls. Jaccard index values were similar across SMZ treatments after logging, indicating strong community overlap. These findings suggest that stream invertebrate communities did respond to the timber harvest, though not negatively. Results also suggest that SMZ criteria for aquatic habitats in steeply sloping topography, including at least 50 percent canopy retention and widths of at least 16.8 m, appear to be adequate for protecting benthic macroinvertebrate communities from logging impacts.
1. Introduction
Headwater streams represent the majority of stream area in a watershed and strongly influence downstream conditions, including water levels, nutrients, prey subsidies, and both coarse and fine woody debris [1,2,3]. Because of their smaller size, headwater stream watersheds are readily influenced by localized disturbances [4,5]. Riparian zones are areas of reciprocal influence between aquatic and terrestrial components of the landscape. These interfaces play a key role in maintaining stream temperatures, nutrients, and food resources within aquatic systems [6,7,8,9,10].
Changes to the vegetative composition of riparian zones associated with headwater streams can have negative implications for the ecological health of adjacent streams and the overall watershed [1,5,8]. In temperate forests, headwater streams are often deeply shaded by surrounding canopies, resulting in systems of low primary productivity that is sustained by allochthonous energy sources in the form of coarse particulate organic matter (CPOM) [1,11,12]. This CPOM is converted to fine particulate organic matter (FPOM) via several pathways and is utilized as a food resource by benthic macroinvertebrates [13]. Benthic macroinvertebrate communities can be altered by subtle changes in stream characteristics [14,15,16] and changes to riparian forest composition [13]. Thus, local disturbances in the riparian zone can influence the composition and biological diversity of benthic macroinvertebrate communities [17].
Timber harvesting (logging) is one example of a forest disturbance that can alter stream conditions and thereby impact aquatic biota. Effects of timber harvesting on streams can vary spatially and temporally, thus it is important to evaluate the value of a variety of mitigation or prevention strategies across locations to successfully implement conservation management plans. Stream parameters such as total suspended solids, flow, and sediment loading can be affected by logging [18,19,20]. Loss of streamside vegetation can cause increased water temperatures, which in turn can contribute to reduced dissolved oxygen concentrations [10,21]. Benthic macroinvertebrates are closely associated with localized land cover patterns [17,22] and are influenced by timber harvesting in adjacent forests [23,24,25]. The effects of timber removal on benthic macroinvertebrate communities can be observed for extended periods of time [26].
Impacts to stream conditions from timber harvesting can be minimized through the use of streamside management zones (SMZs). SMZ’s are forested buffer strips left intact between stream channels and timber harvesting area which can help to reduce sedimentation, maintain stream temperatures and flow, and regulate dissolved oxygen levels and nutrient loading [27,28,29,30]. Streams adjacent to timber harvests with no SMZ can have greater sediment and nutrient loading than streams protected by SMZs [31,32]. Implementation of an SMZ can yield improved protection of native biota. For example, greater numbers of reptiles and amphibians have been observed in SMZs with 30–95 m of undisturbed buffer than in SMZ’s <25 m in width [33]. Streamside buffers with various widths (15, 30, and 45 m) provided adequate habitat for avifauna in Georgia, though some forest interior species found in the unharvested control areas were not present in the SMZs [34]. In a study that evaluated 10.6 m SMZs in Florida, Vowell [35] found no differences in benthic macroinvertebrate community composition or stream biomonitoring indices between unharvested controls and SMZ reaches. In a similar study conducted in northern California, Newbold et al. [23] found no difference in benthic macroinvertebrate communities in streams adjacent to unharvested control sites and corresponding streams with a 30 m SMZ.
Best management practices to protect stream quality from potential degradation related to logging practices vary regionally. In Kentucky and throughout Appalachia, the SMZ width is dependent on the slope of the terrain, with steeper slopes requiring wider SMZs [36] with the potential for up to 50% overstory removal within the SMZ buffer. Further, Kentucky requires no riparian zone protection along ephemeral channels, although improved stream crossings must be used whenever feasible [36]. Historically, the guidelines for SMZs from other states have varied widely and are based less on experimental results than anecdotal observations and compromises [37].
Given the need for increased rigor for forestry best management practices (BMPs) to test the effect of harvesting and SMZ designs on macroinvertebrate communities, the objective of our study was to determine the appropriate SMZ width for protecting benthic macroinvertebrates within headwater streams in eastern Kentucky forests. We compared those communities across streams with varying SMZ width and proportion of canopy retention to control streams with no timber harvesting adjacent one year prior to the timber harvest and four years post-harvest. Other studies have shown a marked increase in overall benthic macroinvertebrate abundance and biomass production in harvested streams relative to unharvested references, perhaps due to increased light and primary productivity, or increases in the organic material from the riparian zone [26]. As a result, we hypothesized that there would be elevated overall abundances and greater biodiversity of benthic macroinvertebrate communities within streams adjacent to our least intensive SMZs relative to the streams protected by more intensive SMZs.
2. Materials and Methods
2.1. Study Area
Our study was conducted at the University of Kentucky’s Robinson Forest (37°27′ N and 83°08′ W), a 6000 ha research forest located in the Cumberland Plateau region of southeastern Kentucky. The terrain in this area is rugged and highly dissected, with underlying geologic material comprised of shale and sandstone with abundant coal seams and elevations ranging from 600 to 1261 m [38,39]. Annual precipitation ranges from 106 to 139 cm, and temperatures vary from −6.2° C to 8.3° C in January and from 16.6° C to 31.6° C in July [39]. The forest was harvested during the early 20th century. The regenerated forest is categorized as mixed mesophytic forest dominated by maples (Acer spp.), oaks (Quercus spp.), hickories (Carya spp.), and yellow-poplar (Liriodendron tulipifera). In the riparian zones of headwater streams, eastern hemlock (Tsuga canadensis) is also prevalent [30].
The SMZ evaluation took place in eight first-order watersheds in the Clemons Fork watershed (Figure 1). These watersheds ranged from 27 to 112 ha, with drainage densities ranging from 0.0023 to 0.0106 m/m2 (Table 1). Six watersheds were harvested from June 2008 to October 2009 using a shelterwood with reserves or a two-aged deferment harvest [40,41,42] with a target post-harvest basal area of approximately 3.4 m2·ha−1. The remaining two watersheds served as controls with no harvesting.
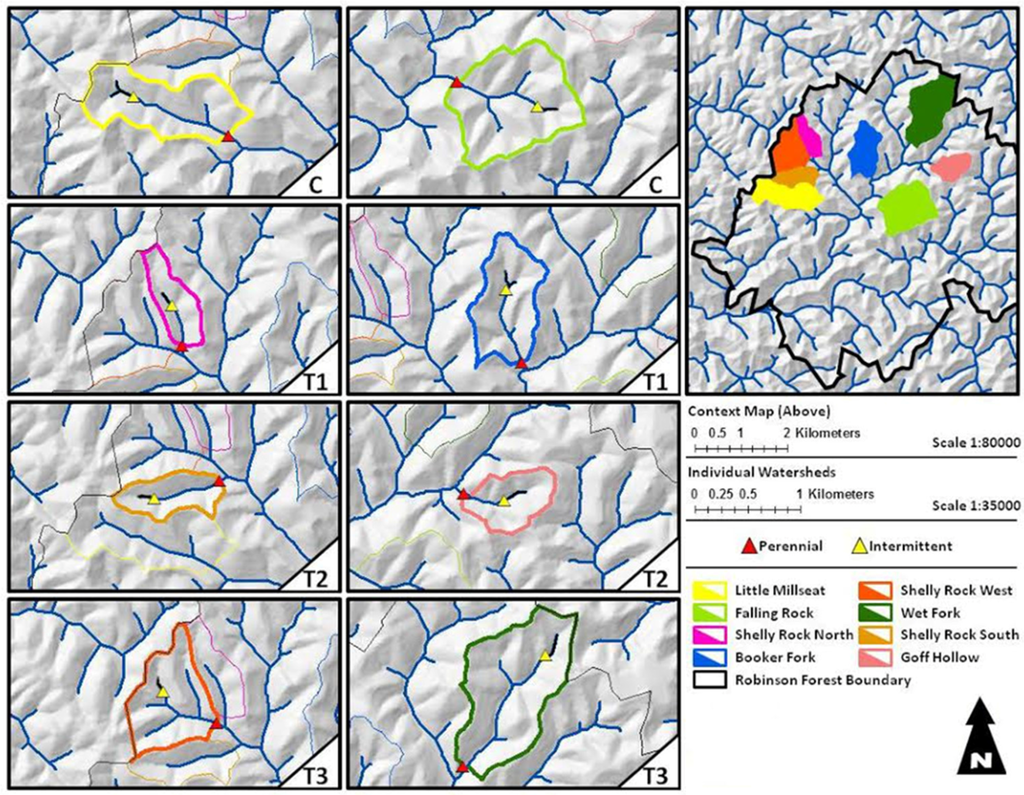
Figure 1.
Robinson Forest is an approximately 6000 ha experimental forest located in portions of Breathitt, Perry and Knott counties, Kentucky. This study was conducted on the main block (outlined in black) in the 1538 ha Clemons Fork watershed. Falling Rock and Little Millseat branches represent the unharvested control (C) watersheds. Shelly Rock North and Booker Fork are treatment 1 (T1) watersheds. Shelly Rock South and Goff Hollow are treatment 2 (T2) watersheds. Shelly Rock West and Wet Fork represent treatment 3 (T3) watersheds. Perennial (blue line) and intermittent (black line) stream segments were created in ArcMap using a 10 m Digital Elevation Model and the Hydrology tools in Spatial Analyst. Adapted from Witt 2012 [30].

Table 1.
Characteristics of the intermittent and perennial channels within each treatment watershed at Robinson Forest, Kentucky (USA). Adapted from Witt 2012 [30].
Timber removals for sawtimber and pulpwood >20 cm in diameter at breast height (dbh) were completed using a mix of mechanical (Timbco 445 EXL tracked feller buncher) and manual felling. Uphill skidding with Caterpillar 525 cable and 545 wheeled skidders on constructed skid trails to landings located along higher elevations of the watersheds avoided haul road and skidder crossing of intermittent and perennial streams. All skid trails were constructed and retirement work completed with John Deere 650, 700 and 850 bulldozers. All skidder and dozer activity was restricted to constructed skid trails as was the tracked feller buncher with the exception of limited operations on slopes <30 percent outside of the SMZs. Retirement work using best management practices included construction of water bars and other cross-drained structures as well as re-vegetation of the trail system.
2.2. Treatments
Three SMZ treatment configurations were applied twice each to a total of six watersheds along with the two unharvested, control watersheds (Figure 1). Both control watersheds (Falling Rock Branch and Little Millseat Branch) are listed as exceptional waters by the state of Kentucky. Treatment 1 (T1) was based on the current Kentucky best management practices and included a 16.8 m (55 ft.) perennial SMZ with 50% overstory retention and a 7.6 m (25 ft.) intermittent SMZ with no overstory retention requirement. SMZ overstory buffers were not used along ephemeral streams, and for the purpose of this study non-elevated crossings (fords) were used. Treatment 2 (T2) maintained the 16.8 m (55 ft.) perennial SMZ but required 100% canopy retention, as well as 25% canopy retention in the 7.6 m (25 ft.) intermittent SMZ. Additionally, elevated stream crossings (temporary skidder bridges and culverts) were used to cross ephemeral channels. Treatment 3 (T3) increased the perennial SMZ width to 33.5 m (110 ft.) with 100% canopy retention, and the intermittent SMZ width to 15.2 m (50 ft.) with 25% canopy retention and included a 7.6 m (25 ft.) SMZ around ephemeral streams, and elevated crossings were used to traverse ephemeral streams. Pre- and post-harvest assessments of canopy cover were performed within intermittent and perennial stream reaches using a spherical crown densiometer (www.forestry-suppliers.com).
2.3. Invertebrate Sampling
Benthic macroinvertebrate sampling took place in February and April of 2004, and again in March and April in 2013. Stream community assessment was conducted in the intermittent and perennial segments of each of the eight watersheds. Samples were collected using a 0.09 m2 Surber sampler and a 60 second sampling interval from three riffles in the intermittent and perennial sections of each watershed, for a total of six samples per watershed. Benthic macroinvertebrate samples were sorted and identified in the laboratory to genus or the lowest practical taxonomic level [43]. Further, each specimen was assigned to a functional feeding group based on the most common feeding mode in each family (collector–gatherer, collector–filterer, scraper, shredder, or predator) [43]. Family and genus names were checked for recent taxonomic revisions via BugGuide (http://bugguide.net).
2.4. Data Treamtent
We used the EPT Index [44,45] and the Hilsenhoff Biotic Index [14,46] as measures of biotic integrity within these headwater stream communities. The EPT Index was calculated based on the proportion of the sum of Ephemeroptera, Plecoptera, and Trichoptera richness to the richness of other taxa. The Hilsenhoff Biotic Index was calculated as HBI = (Σ niTVi)/N where ni is the number of individuals of each taxon, TVi is the tolerance value associated with that taxon, and N is the total number of benthic invertebrates in the sample [14,46]. Modifications of the Hilsenhoff Biotic Index have been widely used for stream assessment in Kentucky and throughout the southeastern United States [47]. We used the Kentucky Division of Water Macroinvertebrate Bioassessment Index which utilizes a regional variant of the Hilsenhoff Index [48]. Simpson's index of diversity was calculated as D = 1 − [Σ (n/N)2] where n = the total number of organisms of a particular taxon and N = the total number of individuals [49]. Further, Jaccard Coefficients of Similarity were calculated and a matrix of pairwise community overlap comparisons was generated for the SMZ treatment streams [49].
2.5. Analysis
Functional feeding group abundance, bioassessment metrics, and population measures (abundance, richness) were analyzed using a two-way ANOVA (PROC GLM: SAS 9.3) with SMZ treatment and month of sample collection as the main effects. All abundance data were square root (1 + x) transformed and proportion data were arcsine transformed prior to the analysis to meet the assumptions of ANOVA. Tukey’s Honest Significant Difference (HSD) test was used as a post-hoc means separation procedure when appropriate. The initial analysis included year as a factor, but since we collected significantly greater abundances of benthic macroinvertebrates during 2004, pre-treatment and post-treatment data were analyzed separately. We also made comparisons by SMZ treatment prior to timber harvest to discern if there were major differences among watersheds that existed before treatment implementation. Further, because Grubbs found subtle differences in the density and composition of benthic macroinvertebrate communities between intermittent and perennial channels, the two channel types were treated separately. The intermittent and perennial pre- and post-harvest Jaccard values were compared using a non-parametric Mann-Whitney U test. Finally, nonmetric multidimensional scaling (NMDS) was used to explore the variation within our data [50]. To approximate original multivariate distances between sample points, NMDS projects distances across low dimensional space such that sites that are close in proximity on the graph are most similar in their overall composition, while sites located further away from one another on the graph have a more distinct community composition. We used a log transformation to normalize the insect abundance data prior to the analysis and conducted the NMDS using PCORD version 6 [51]. A Sorensen distance measure was used for the NMDS, alongside 250 runs with a randomized starting configuration and a dimensionality of three.
3. Results
We collected a total of 25,987 individuals across 71 aquatic insect genera in the 2004 and 2013 samples combined. Individuals in family Chironomidae were not identified below the level of family so the total number of genera that we recorded is conservative. We obtained 12,539 and 13,448 individuals from the intermittent and perennial channels, respectively. Prior to the timber harvest, a total of 16,420 individuals were captured. That number dropped to 9567 in the samples collected post-harvest. This difference in abundance between sample years was highly significant (F15,78 = 22.58, p < 0.01) and reductions were found in both harvested watersheds and unharvested controls. In both the pre- and post-treatment samples, the twenty most abundant taxa represented >90% of the abundance, and the 5 most abundant taxa made up >60%.
We captured 65 taxa in 2004, and 60 in 2013. Of those, 11 were unique to the 2004 samples, while 6 were captured only in the 2013 samples. Most of these unique genera were collected in low numbers. However, a few were common in the pre- or post-treatment samples and missing entirely from the other, including Agapetus (Trichoptera: Glossosomatidae, n = 432) and Yugus (Plecoptera: Perlodidae, n = 215), which was unique to the 2004 samples. Alternatively, Lype (Trichoptera: Psychomyiidae, n = 85) was found only in 2013. Because of these drastic pre- and post-harvest community level differences, the results from the two sampling intervals are presented separately.
Prior to harvest, canopy cover over the stream reaches was similar for all treatment and control watersheds at both intermittent and perennial locations (between 95 and 99% cover). After harvest, canopy cover remained the same in C, T2 and T3 stream sampling locations (96%–99% cover), but dropped in T1 watersheds due to timber removal within the SMZ. Post-harvest canopy cover was reduced to 79% in intermittent stream segments and 87% in perennial stream segments.
3.1. Pre-Treatment Aquatic Communities
Sixty-five taxa and a total of 16,420 benthic invertebrates were captured pre-harvest. Of these, 51.8%were found in the intermittent sections, and 48.2%were from the perennial sections. The most abundant taxon was family Chironomidae, which represented 20% of the total abundance. Following Chironomidae in descending order of abundance were Leuctra (Plecoptera: Leuctridae), Ephemerella (Ephemeroptera: Ephemerellidae), Paraleptophlebia (Ephemeroptera: Leptophblebiidae), and Baetis (Ephemeroptera: Baetidae). These five taxa represented 61% of the total catch in 2004. Of these, Paraleptophlebia was the only genus to differ significantly across intermittent SMZ treatments (Table 2), with elevated abundance in the T1 streams (Figure 2). In the perennial sections, only Ephemerella differed across SMZ treatments (Table 2), with greater abundance in the T2 streams (Figure 2). In terms of functional feeding group abundance, only scrapers varied across treatments in the intermittent streams (Table 2), with the lowest numbers collected in T3 streams and no differences between the other two SMZ configurations and the control (Figure 2). Genus richness did not vary across treatments in either stream type (Table 2). Further, no differences in functional feeding groups were detected among treatments within the perennial sections of the streams in 2004 (Table 2).

Table 2.
Pre-harvest effects * of SMZ treatment, season, and their interaction on benthic macroinvertebrate abundance at Robinson Forest, Kentucky (USA).

Figure 2.
Pre-harvest differences in Paraleptophlebia and total Scraper abundance (intermittent stream segments) and Ephemerella (perennial stream segments) across streamside management zone (SMZ) treatments at Robinson Forest, Kentucky (USA). Columns within each response variable which share letters are not significantly different from one another (p < 0.05).
3.2. Post-Treatment Aquatic Communities
Four years after the SMZ treatments were implemented we captured a total of 9567 benthic invertebrates. Of these, a 42.2% were collected from the intermittent sections and 57.8% were from the perennial sections of the streams. These invertebrates represented 60 taxa, 53 were collected from the intermittent streams and 54 were found in the perennial streams. The five most abundant taxa collected in 2013 were the Paraleptophlebia, followed by Chironomidae, Ephemerella, Ameletus (Ephemeroptera: Ameletidae), and Hexatoma (Diptera: Limoniidae). These five taxa represented 60% of the overall abundance of the benthic invertebrates that we captured in 2013. There were differences in total abundances among treatments and both hydrologic regimes (Table 3). Total abundance was greatest in the T1 samples taken from intermittent streams, while T2 abundance was greatest in the perennial streams as compared to the control streams, where we found the lowest total abundances in both channel types.

Table 3.
Post-harvest effects * of SMZ treatment, season, and their interaction on benthic macroinvertebrate abundance at Robinson Forest, Kentucky (USA).
We found differences in functional feeding group abundances in both stream permanence categories during 2013 (Table 3). In the intermittent streams, gatherers were significantly more abundant in the T1 than in the C streams (Figure 3b). Alternatively, in the perennial streams we found significant differences for filterers and scrapers in regard to SMZ configuration (Table 3). The greatest abundances of filterers were found in T2, and scrapers were more abundant in T1 compared to T3 and C streams (Figure 3b).


Figure 3.
Post-treatment differences in benthic macroinvertebrate abundance across SMZ treatments. (a) Post-treatment differences in total abundance across SMZ treatments from intermittent and perennial stream segments at Robinson Forest, Kentucky (USA). Columns within each response variable which share letters are not significantly different from one another (p < 0.05); (b) Post-harvest differences in functional feeding group abundance across SMZ treatments at Robinson Forest, Kentucky (USA).Columns within each response variable which share letters are not significantly different from one another (p < 0.05).
3.3. Stream Community Metrics
We found no significant differences for %EPT index in either channel type in pre- and post-harvest samples (Table 2 and Table 3). In the post-harvest intermittent streams, EPT values ranged from 73% in C, T2 and T3 to 81% in T1. In the perennial streams we found similar values, with 82% EPT in the T1 streams, but these values did not differ from one another statistically.
We found a statistically significant difference for Hilsenhoff index values in the pre-treatment perennial samples (Table 2), with the greatest value (3.0) found in T2 streams. However, in the post-treatment samples we found no significant differences across treatments in either channel type (Table 3), with mean HBI values ranging from 2.5 to 3.0 across SMZ treatments.
We found no statistical differences in Simpson’s diversity index among SMZ treatments in the pre-treatment and post-treatment samples (Table 2 and Table 3). These values were >0.8 across both channel types, both before and after the timber harvest.
In 2004, the C streams were most similar in community composition to T1 in both channel types, and were least similar to T2 (Table 4). However, by 2013 the C streams were least similar to T1 in the intermittent streams. There were no significant differences between pre- and post-harvest Jaccard values in the intermittent streams (Mann-Whitney U = 9, n1 = n2 = 6, p < 0.05 two-tailed). In the post-treatment perennial streams the range of taxonomic similarity between the SMZ treatments and the control was narrow, from 64% (T2) to 66% (T3), while the overlap between the SMZ treatments ranged from 69% (T2–T3) to 77% (T1–T3) (Table 4). We found no significant difference between pre- and post-harvest Jaccard values in the perennial streams (Mann-Whitney U = 6.5, n1 = n2 = 6, p < 0.05 two-tailed).The NMDS plot (Figure 4) illustrated the variation within and among SMZ treatments. The close proximity and strong overlap of points within our graph reaffirms the Jaccard index of similarity results for the 2013 post-treatment communities (Table 4).

Table 4.
Jaccard similarity index for benthic macroinvertebrate communities across SMZ treatments at Robinson Forest, Kentucky (USA).
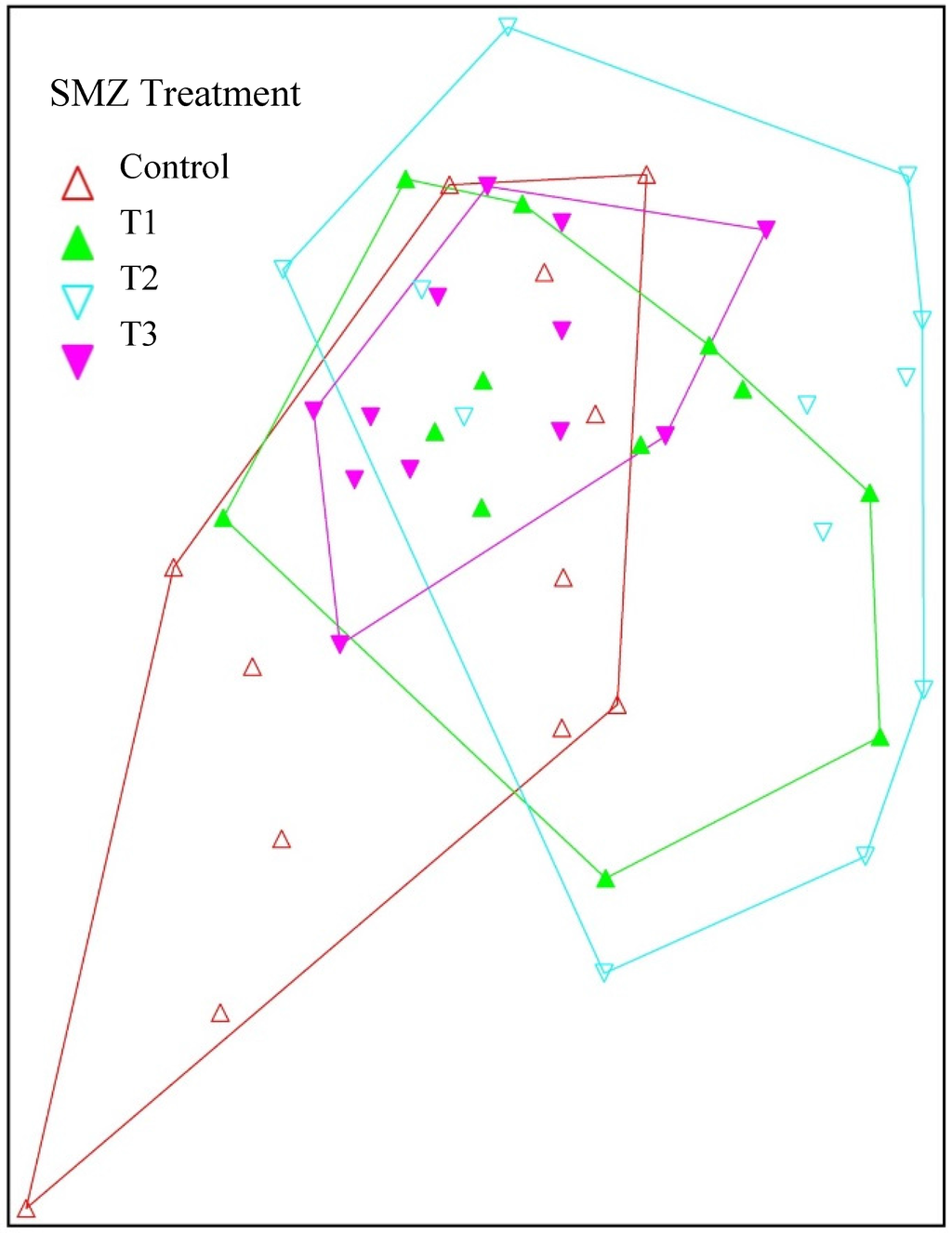
Figure 4.
A nonmetric multidimensional scaling (NMDS) plot of benthic macroinvertebrate communities collected from Robinson Forest SMZ streams which summarizes the variation between SMZ treatments. T1, T2, and T3 strongly overlap, while the Controls exhibit some outliers, reflecting lower overall abundances and weaker similarity in these streams relative to the SMZ treatments.
4. Discussion
While many SMZ studies, including ours, provide information on initial response one or two years after harvest, this study examined the longer-term response four years after the harvest treatments were applied. This allows us to glean the impacts of our SMZ treatments on benthic macroinvertebrate communities after the initial pulse of organic materials and dissolved nutrients have cycled through. More immediate impacts to the physical characteristics of the streams during and immediately following the timber harvest are presented in detail by Witt [30], Bowker, and others. While we found minor differences in the benthic macroinvertebrate community across these streams prior to the timber harvest, there was no discernible trend. Apart from the large numbers of Ephemerella captured in the T2 and T3 streams, significant differences between watersheds were not detected. Four years after the timber harvest was completed, we again found few differences in the benthic macroinvertebrate communities across the SMZ treatments at Robinson Forest. In the intermittent streams, total abundance was greater in T1 than C streams. T1 streams had 50% of its overstory removed in the SMZ, which approximates the relatively small amount of retention required by many states for intermittent streams [37].
In the perennial streams, the greatest abundance of benthic invertebrates was found in the T2 streams. Again we found that the other two SMZ treatments did not differ significantly from this value, but it was greater than the total number of invertebrates collected from the C streams. This elevated abundance in the treatment streams is not unique. Several studies have shown similar trends with elevated numbers of invertebrates associated with SMZ treatments [23,52,53]. Moldenke and Van Linden suggested that this increase in benthic macroinvertebrates in treatment streams relative to controls might be due to greater production of periphyton due to increased temperatures and light associated with canopy removal [53]. Witt found that T1 had a statistically higher maximum stream temperature and mean diurnal flux than the other treatment watersheds and the control [30] and our data showed a decrease in canopy cover in T1, though our benthic invertebrate data do not mirror this change in environmental conditions.
Other researchers have demonstrated that stream and terrestrial communities that might be altered by riparian zone disturbance via timber harvests are given adequate protection through the use of SMZs [54,55,56]. Further, several studies suggest that best management practices associated with timber harvest can be beneficial for conserving benthic macroinvertebrate communities as compared to harvesting timber without SMZ implementation [23,57,58]. In a study comparing benthic macroinvertebrate communities in streams pre- and post-logging SMZs, Newbold et al. found greater numbers of benthic invertebrates in harvested streams with SMZs relative to the unharvested control [23]. Further, Duncan and Brusven also found the greatest population densities in streams adjacent to harvested areas relative to the control, and that collector–gatherers were the dominant functional feeding group in all streams [59]. These findings closely corroborate the results of our study. We found elevated total abundances of benthic invertebrates in the SMZ treatment groups relative to the control. However, we found differences in the functional feeding group distribution across our SMZ treatments, with greater numbers of collector–filterers in the perennial segments of the T2 streams, and greater numbers of scrapers in T1 streams [60]. Additionally, collector–gatherers were more abundant in the T2 and T3 streams relative to the C and T1 streams, though they did not differ significantly from one another. The elevated abundance of scrapers in T1 may be explained by the relative openness of the canopy in this SMZ strategy as compared to the other treatments. Scrapers generally feed on algae [61], and there was a visually discernible increase in periphyton cover within these streams although no quantitative measures were made. However, collector–filterers are suspension feeders that utilize organic materials drifting in the water column as food [61]. Grubbs found significantly higher densities of collector–filterers in perennial reaches attributed to higher stream flows and greater availability of seston as a food source [60].
In May of 2009, Robinson Forest experienced a catastrophic flood event which scoured stream substrates, resulted in mass bedload movement, and likely altered the benthic macroinvertebrate community composition and abundance. Thus, we chose to analyze the pre-treatment and post-treatment data separately to compare trends. Prior to the timber harvest, the benthic macroinvertebrate communities were similar across streams in terms of abundance, diversity measures, and stream health parameters. The Jaccard index values for these streams were very similar between pre- and post-treatment in either channel type. In the intermittent post-treatment samples, however, the C streams were more similar to the T2 and T3 streams than T1 (Table 4). The perennial SMZ treatment groups were more similar to one another than to the control watersheds in terms of taxa similarity (Table 4). We speculate that this is due to the treatment streams being affected less severely by the flood event as compared to the controls, likely due to logging debris in the harvested area and road BMPs that reduced the impact of rainwater flow within the treatment watersheds [62]. Additionally, the more open conditions in the SMZ treatment streams [30] could have resulted in greater algal growth, which might have served as a food resource for macroinvertebrates accelerating the re-colonization of benthic communities within the treatment watersheds after the flooding event.
Although the response of the arthropod community varied across the SMZs that we evaluated for this project, we saw few statistically significant differences in the benthic macroinvertebrate communities between SMZ treatments after the timber harvest. Our results corroborate trends found in similar lines of research that demonstrated either no effect of timber removal on benthic macroinvertebrate communities or an increase in benthic macroinvertebrate abundance following canopy reduction so long as SMZs are present [59,63]. Our study was comprised of samples collected in February and April 2004 and again in March and April 2013, four years after timber removal. It is possible that canopy reduction caused immature benthic invertebrates to develop earlier or more rapidly in the SMZ streams. Changes to benthic macroinvertebrate abundance can be rapid in response to environmental conditions [64,65], availability of food [66], and season [67,68]. Thus, sampling these streams over a longer period of time to elucidate long-term effects of canopy removal and to determine the most effective SMZ strategy to implement is imperative. Our post-treatment Jaccard index values comparing SMZ treatments are similar, and total abundance is greatest in the T2 streams. However, we detected few meaningful differences across the SMZ treatments that we implemented. As our NMDS revealed, some of the control sites are quite different, as represented by those points extending down and to the left on the graph (Figure 4). There was strong overlap between the three SMZ treatment groups, which corroborates the findings of our univariate statistical procedures. The final stress value of the 3-dimensional solution for the analysis was 17.21, which falls within the “useable” range of Clarke’s criteria [69], though caution is urged when interpreting the data using only the proximity of points in the graph [50]. Overall, our study demonstrates that the use of SMZs can preserve aquatic insect assemblages and should be included as a component of forestry best management practices. In the short term, our SMZ Treatment 2 and Treatment 3 could provide further protection for watersheds with sensitive species, given the slightly less dramatic community response detected in these streams as compared to Treatment 1.
Acknowledgments
This work was supported in part by the Kentucky Agricultural Experiment Station. A special thanks to Andrea Drayer for providing assistance in the field and laboratory.
Author Contributions
Barton, Kolka and Stringer conceived and designed the experiments and executed the forest harvest; Grubbs and Adkins collected the stream macroinvertebrates and analyzed the data; Adkins assembled the paper with input from all contributing authors.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| BMP | Best management practice |
| DBH | Diameter at breast height |
| NMDS | Non-metric multidimensional scaling |
| SMZ | Streamside management zone |
References
- Gomi, T.; Sidle, R.C.; Richardson, J.S. Understanding processes and downstream linkages of headwater systems. Bioscience 2002, 52, 905–916. [Google Scholar] [CrossRef]
- MacDonald, L.H.; Coe, D. Influence of headwater streams on downstream reaches in forested Areas. For. Sci. 2007, 53, 148–168. [Google Scholar]
- Alexander, L.C.; Hawthorne, D.J.; Palmer, M.A.; Lamp, W.O. Loss of genetic diversity in the North American mayfly Ephemerella invaria associated with deforestation of headwater streams. Freshw. Biol. 2011, 56, 1456–1467. [Google Scholar] [CrossRef]
- Lowe, W.H.; Likens, G.E. Moving headwater streams to the head of the class. Bioscience 2005, 55, 196–197. [Google Scholar] [CrossRef]
- Meyer, J.L.; Strayer, D.L.; Wallace, J.B.; Eggert, S.L.; Helfman, G.S.; Leonard, N.E. The Contribution of Headwater Streams to Biodiversity in River Networks. J. Am. Water Resour. Assoc. 2007, 43, 86–103. [Google Scholar] [CrossRef]
- Karr, J.R.; Schlosser, I.J. Water resources and the land-water interface. Science 1978, 201, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Gregory, S.V.; Swanson, F.J.; McKee, W.A.; Cummins, K.W. An ecosystem perspective of riparian zones. Bioscience 1991, 41, 540–551. [Google Scholar] [CrossRef]
- Naiman, R.J.; Décamps, H.; Henri, D. The ecology of interfaces: Riparian zones. Annu. Rev. Ecol. Syst. 1997, 28, 621–658. [Google Scholar] [CrossRef]
- Laeser, S.R.; Baxter, C.V.; Fausch, K.D. Riparian vegetation loss, stream channelization, and web-weaving spiders in northern Japan. Ecol. Res. 2005, 20, 646–651. [Google Scholar] [CrossRef]
- Richardson, J.S.; Danehy, R.J. A synthesis of the ecology of headwater streams and their riparian zones in temperate forests. For. Sci. 2007, 53, 131–147. [Google Scholar]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The river continuum concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Knight, A.W.; Bottorff, R.L. The importance of riparian vegetation to stream ecosystems. In California Riparian Systems: Ecology, Conservation, and Productive Management; University of California Press: Berkely, CA, USA, 1984; pp. 160–167. [Google Scholar]
- Wallace, J.B.; Eggert, S.L.; Meyer, J.L.; Webster, J.R. Effects of resource limitation on a detrital-based ecosystem. Ecol. Monogr. 1999, 69, 409–442. [Google Scholar] [CrossRef]
- Hilsenhoff, W. An improved biotic index of organic stream pollution. Gt. Lakes Entomol. 1987, 20, 31–40. [Google Scholar]
- Hilsenhoff, W.L. Rapid field assessment of organic pollution with a family-level biotic index. J. N. Am. Benthol. Soc. 1988, 7, 65–68. [Google Scholar] [CrossRef]
- Karr, J.R. Defining and measuring river health. Freshw. Biol. 1999, 41, 221–234. [Google Scholar] [CrossRef]
- Rios, S.L.; Bailey, R.C. Relationship between Riparian Vegetation and Stream Benthic Communities at Three Spatial Scales. Hydrobiologia 2006, 553, 153–160. [Google Scholar] [CrossRef]
- Bracken, L.J.; Croke, J. The concept of hydrological connectivity and its contribution to understanding runoff-dominated geomorphic systems. Hydrol. Process. 2007, 21, 1749–1763. [Google Scholar] [CrossRef]
- Litschert, S.E.; MacDonald, L.H. Frequency and characteristics of sediment delivery pathways from forest harvest units to streams. For. Ecol. Manag. 2009, 259, 143–150. [Google Scholar] [CrossRef]
- Kreutzweiser, D.; Capell, S.; Good, K.; Holmes, S. Sediment deposition in streams adjacent to upland clearcuts and partially harvested riparian buffers in boreal forest catchments. For. Ecol. Manag. 2009, 258, 1578–1585. [Google Scholar] [CrossRef]
- Lynch, J.A.; Rishel, G.B.; Corbett, E.S. Thermal alteration of streams draining clearcut watersheds: Quantification and biological implications. Hydrobiologia 1984, 111, 161–169. [Google Scholar] [CrossRef]
- Sponseller, R.A.; Benfield, E.F.; Valett, H.M.; Issues, A. Relationships between land use, spatial scale and stream macroinvertebrate communities. Freshw. Biol. 2001, 46, 1409–1424. [Google Scholar] [CrossRef]
- Newbold, J.D.; Erman, D.C.; Roby, K.B.; Ridge, O. Effects of Logging on Macroinvertebrates in Streams With and Without Buffer Strips. Can. J. Fish. Aquat. Sci. 1980, 37, 1076–1085. [Google Scholar] [CrossRef]
- Gurtz, M.E.; Wallace, J.B. Substrate-Mediated Response of Stream Invertebrates to Disturbance. Ecology 1984, 65, 1556–1559. [Google Scholar] [CrossRef]
- Noel, D.S.; Martin, C.W.; Federer, C.A. Effects of forest clearcutting in New England on stream macroinvertebrates and periphyton. Environ. Manag. 1986, 10, 661–670. [Google Scholar] [CrossRef]
- Stone, M.K.; Wallace, J.B. Long-term recovery of a mountain stream from clear-cut logging: The effects of forest succession on benthic invertebrate community structure. Freshw. Biol. 1998, 39, 151–169. [Google Scholar] [CrossRef]
- Lynch, J.A.; Corbett, E.S.; Mussallem, K. Best management practices for controlling nonpoint-source pollution on forested watersheds. J. Soil Water Conserv. 1985, 40, 164–167. [Google Scholar]
- Binkley, D.; Brown, T. Effects of Forest and Range Management on Water Quality; U.S. Government Publishing Office: Washington, DC, USA, 1993.
- Stringer, J.W.; Perkins, C. FOR-67: Kentucky Forest Practice Guidelines for Water Quality Management; University of Kentuck Cooperative Extension Service: Lexington, KY, USA, 2001. [Google Scholar]
- Witt, E. Evaluating Streamside Management Zone Effectiveness in Forested Watersheds of the Cumberland Plateau. Ph.D. Thesis, University of Kentucky, Lexington, KY, USA, 2012. [Google Scholar]
- Arthur, M.; Coltharp, G.B.; Brown, D.L. Effects of best management practices on forest stream water quality in Eastern Kentucky. J. Am. Water Resour. Assoc. 1998, 34, 481–495. [Google Scholar] [CrossRef]
- Clinton, B.D. Stream water responses to timber harvest: Riparian buffer width effectiveness. For. Ecol. Manag. 2011, 261, 979–988. [Google Scholar] [CrossRef]
- Rudolph, D.; Dickson, J. Streamside zone width and amphibian and reptile abundance. Southwest. Nat. 1990, 35, 472–476. [Google Scholar] [CrossRef]
- Thurmond, D. Effect of streamside management zone width on avifauna communities. South. J. Appl. For. 1995, 19, 166–169. [Google Scholar]
- Vowell, J.L. Using stream bioassessment to monitor best management practice effectiveness. For. Ecol. Manag. 2001, 143, 237–244. [Google Scholar] [CrossRef]
- Stringer, J.; Lowe, L.; Smidt, M.; Perkins, C. Field Guide to Best Management Practices for Timber Harvesting in Kentucky; University of Kentucky Cooperative Extension Service: Lexington, KY, USA, 1997. [Google Scholar]
- Stringer, J.; Thompson, A. Comparison of forestry best management practices, Part I: Streamside management zones. For. Landowner 2000, 59, 22–27. [Google Scholar]
- McDowell, R.C. The Geology of Kentucky—A Text to Accompany the Geologic Map of Kentucky; U.S.G.P.O.: Washington, DC, USA, 1986.
- Woods, A.; Omernik, J.; Martin, W.; Pond, G.; Andrews, W.; Call, S.; Comstock, J.; Taylor, D. Ecoregions of Kentucky (Color Poster with Map, Descriptive Text, Summary Tables, and Photographs); U.S. Geological Survey: Reston, VA, USA, 2002.
- Smith, C.H.; Lamson, N.I.; Miller, G.W. An esthetic alternative to clearcutting? Deferment cutting in easter hardwoods. J. For. 1989, 87, 14–18. [Google Scholar]
- Miller, G.W.; Kochenderfer, J.N.; Fekedulegn, D.B. Influence of individual reserve trees on nearby reproduction in two-aged Appalachian hardwood stands. For. Ecol. Manag. 2006, 224, 241–251. [Google Scholar] [CrossRef]
- Dillaway, D.N.; Stringer, J.W.; Rieske, L.K. Light availability influences root carbohydrates, and potentially vigor, in white oak advance regeneration. For. Ecol. Manag. 2007, 250, 227–233. [Google Scholar] [CrossRef]
- Merritt, R.W.; Cummins, K.W. An Introduction to the Aquatic Insects of North America, 3rd ed.; Kendall Hunt Pub Co.: Dubuque, IA, USA, 1996. [Google Scholar]
- Kerans, B.; Karr, J.R. A benthic index of biotic integrity (B-IBI) for rivers of the Tennessee Valley. Ecol. Appl. 1994, 4, 768–785. [Google Scholar] [CrossRef]
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish; EPA 841-B-99-002; U.S. Environmental Protection Agency, Office of Water: Washington, DC, USA, 1999.
- Hilsenhoff, W.L. Using a Biotic Index to Evaluate Water Quality in Streams; Wisconsin Department of Natural Resources: Madison, WI, USA, 1982.
- Lenat, D.R. A biotic index for the southeastern United States: Derivation and list of tolerance values, with criteria for assigning water-quality ratings. J. N. Am. Benthol. Soc. 1993, 12, 279–290. [Google Scholar] [CrossRef]
- Pond, G.J.; Call, S.M.; Brumley, J.F.; Compton, M.C. The Kentucky Macroinvertebrate Bioassessment Index; Kentucky Department for Environmental Protection, Division of Water: Frankfort, KY, USA, 2003.
- Magurran, A.E. Ecological Diversity and Its Measure; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- McCune, B.; Medford, M.J. PC-ORD: Multivariate Analysis of Ecological Data; MjM Software Design: Gleneden Beach, OR, USA, 1999. [Google Scholar]
- Haggerty, S.M.; Batzer, D.P.; Jackson, C.R. Macroinvertebrate response to logging in coastal headwater streams of Washington, USA. Can. J. Fish. Aquat. Sci. 2004, 61, 529–537. [Google Scholar] [CrossRef]
- Moldenke, A.R.; Ver, L.C. Effects of clearcutting and riparian buffers on the yield of adult aquatic macroinvertebrates from headwater streams. For. Sci. 2007, 53, 308–319. [Google Scholar]
- Earson, S.C.F.P.; Pearson, S.F.; Manuwal, D.A. Breeding Bird Response To Riparian Buffer Width In Managed Pacific Northwest Douglas-Fir Forests. Ecol. Appl. 2001, 11, 840–853. [Google Scholar] [CrossRef]
- Miller, D.A.; Thill, R.E.; Melchiors, M.A.; Wigley, T.B.; Tappe, P.A. Small mammal communities of streamside management zones in intensively managed pine forests of Arkansas. For. Ecol. Manag. 2004, 203, 381–393. [Google Scholar] [CrossRef]
- Lorion, C.M.; Kennedy, B.P. Riparian forest buffers mitigate the effects of deforestation on fish assemblages in tropical headwater streams. Ecol. Appl. 2009, 19, 468–79. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.V.; Aguila, Y.; Brown, K.B.; Fowler, W.P. Responses of benthic macroinvertebrates in small intermittent streams to silvicultural practices. Hydrobiologia 1997, 347, 119–125. [Google Scholar] [CrossRef]
- Carroll, G.D.; Schoenholtz, S.H.; Young, B.W.; Dibble, E.D. Effectiveness of forestry streamside management zones in the sand-clay hills of Mississippi: Early indications. Water Air Soil Pollut. Focus 2004, 4, 275–296. [Google Scholar] [CrossRef]
- Duncan, W.F.A.; Brusven, M.A. Benthic macroinvertebrates in logged and unlogged low-order southeast Alaskan streams. Freshw. Invertebr. Biol. 1985, 4, 125–132. [Google Scholar]
- Grubbs, S. Influence of flow permanence on headwater macroinvertebrate communities in a Cumberland Plateau watershed, USA. Aquat. Ecol. 2011, 45, 185–195. [Google Scholar] [CrossRef]
- Wallace, J.B.; Webster, J.R. The role of macroinvertebrates in stream ecosystem function. Annu. Rev. Entomol. 1996, 41, 115–139. [Google Scholar] [CrossRef] [PubMed]
- Bowker, D.W. Forest Harvest Equipment Movement and Sediment Delivery to Streams. Master’s Thesis, University of Kentucky, Lexington, KY, USA, 2013. [Google Scholar]
- Carlson, J.Y.; Andrus, C.W.; Froehlich, H.A. Woody debris, channel features, and macroinvertebrates of streams with logged and undisturbed riparian timber in Northeastern Oregon, U.S.A. Can. J. Fish. Aquat. Sci. 1990, 47, 1103–1111. [Google Scholar] [CrossRef]
- Flecker, A.S.; Feifarek, B. Disturbance and the temporal variability of invertebrate assemblages in two Andean streams. Freshw. Biol. 1994, 31, 131–142. [Google Scholar] [CrossRef]
- Death, R.G. The effect of patch disturbance on stream invertebrate community structure: The influence of disturbance history. Oecologia 1996, 108, 567–576. [Google Scholar] [CrossRef]
- Richards, C.; Minshall, G. The influence of periphyton abundance on Baetis bicaudatus distribution and colonization in a small stream. J. N. Am. Benthol. Soc. 1988, 7, 77–86. [Google Scholar] [CrossRef]
- Boulton, A.J.; Peterson, C.G.; Grimm, N.B.; Fisher, S.G. Stability of an aquatic macroinvertebrate community in a multiyear hydrologic disturbance regime. Ecology 1992, 73, 2192–2207. [Google Scholar] [CrossRef]
- Ruse, L.P. Chironomid emergence from an English chalk stream during a 3 year study. Arch. Hydrobiol. 1995, 133, 223–244. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 1–26. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).