Distribution and Potential Health Risks of Arsenic, Selenium, and Fluorine in Natural Waters in Tibet, China
Abstract
:1. Introduction
2. Materials and Methods
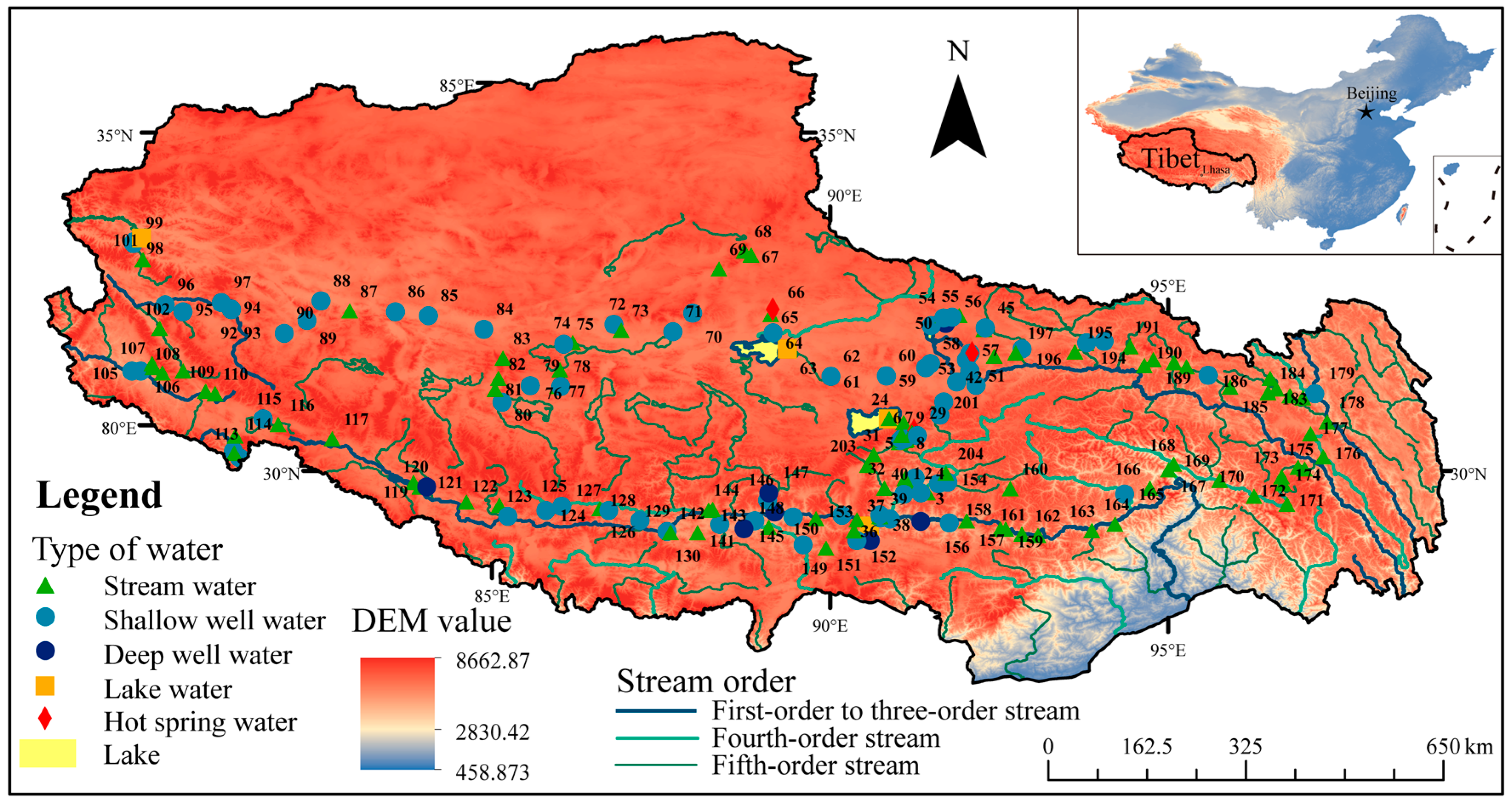
2.1. Study Area
2.2. Sampling and Field Measurement
2.3. Laboratory Analysis
3. Results and Discussion
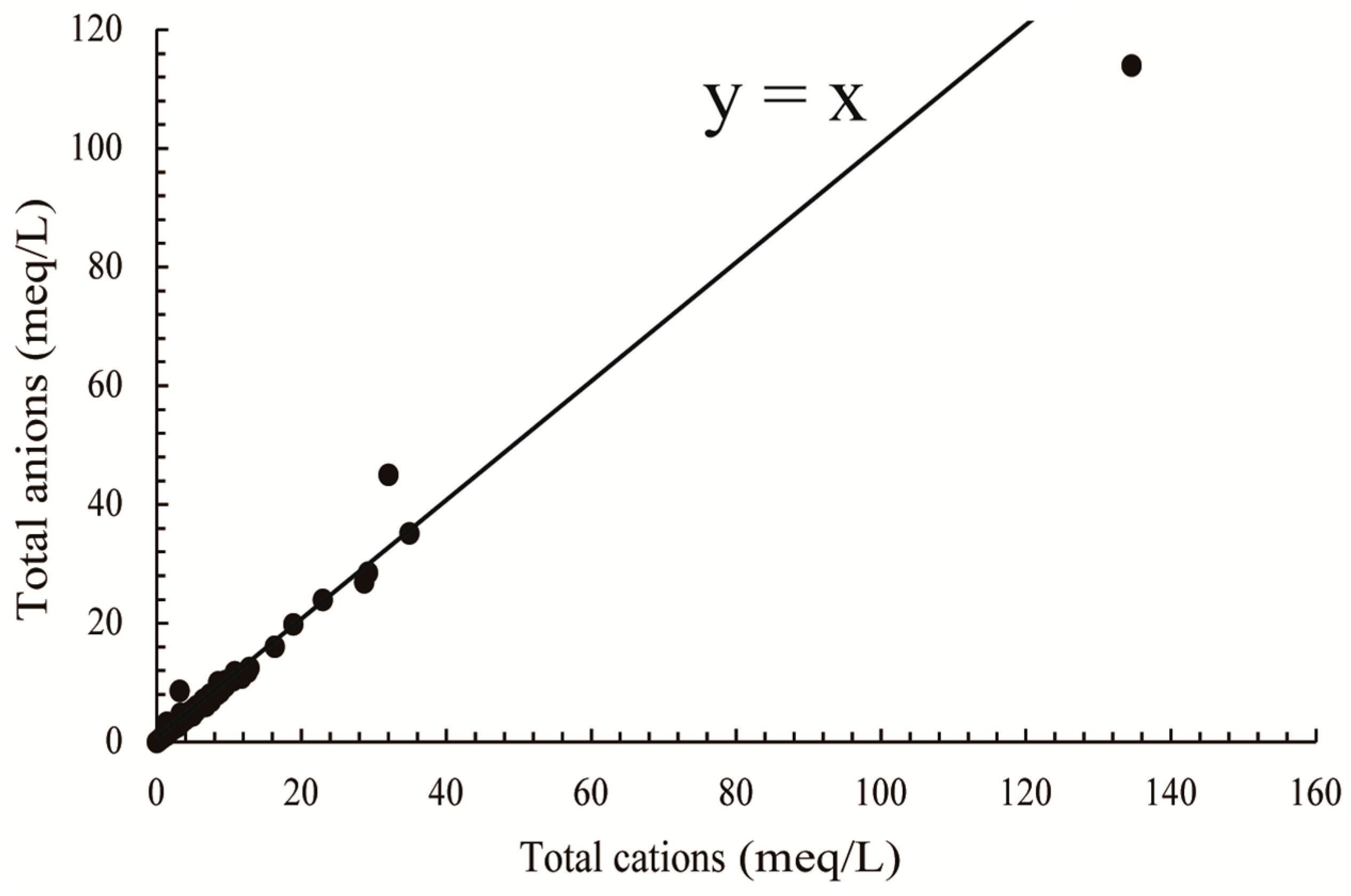
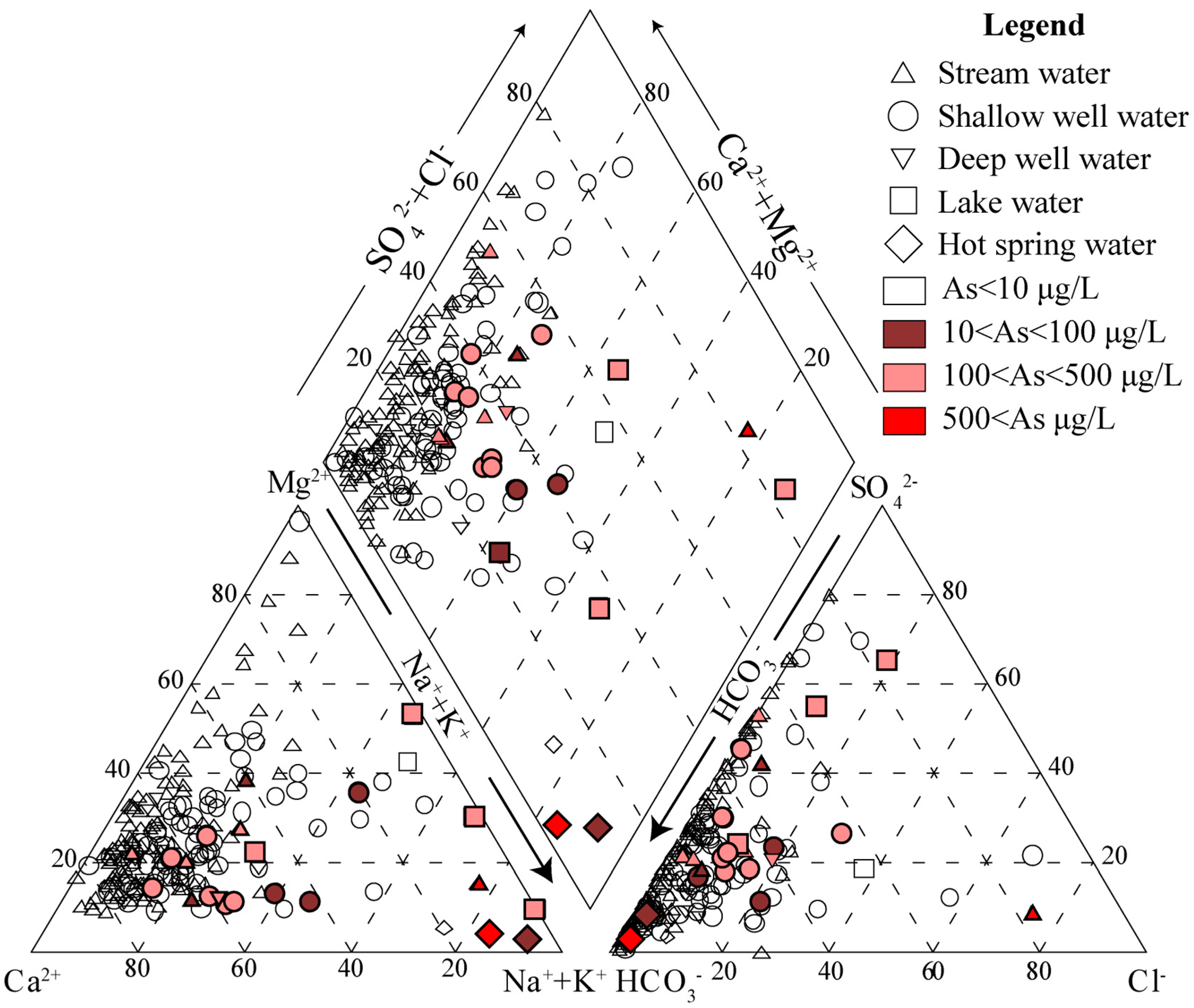
3.1. Water Chemistry
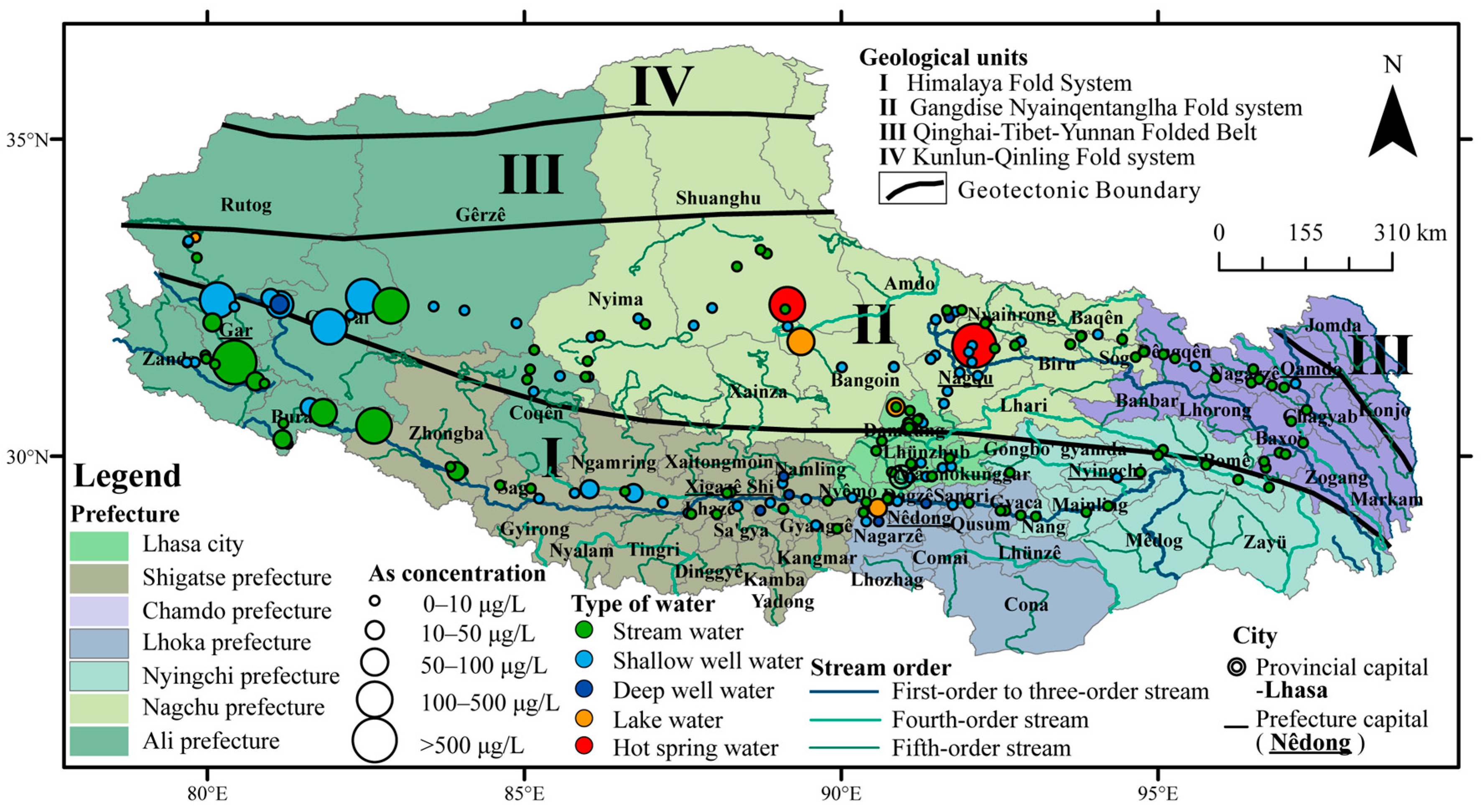
3.2. Concentrations of Arsenic in Different Types of Water and Different Prefectures
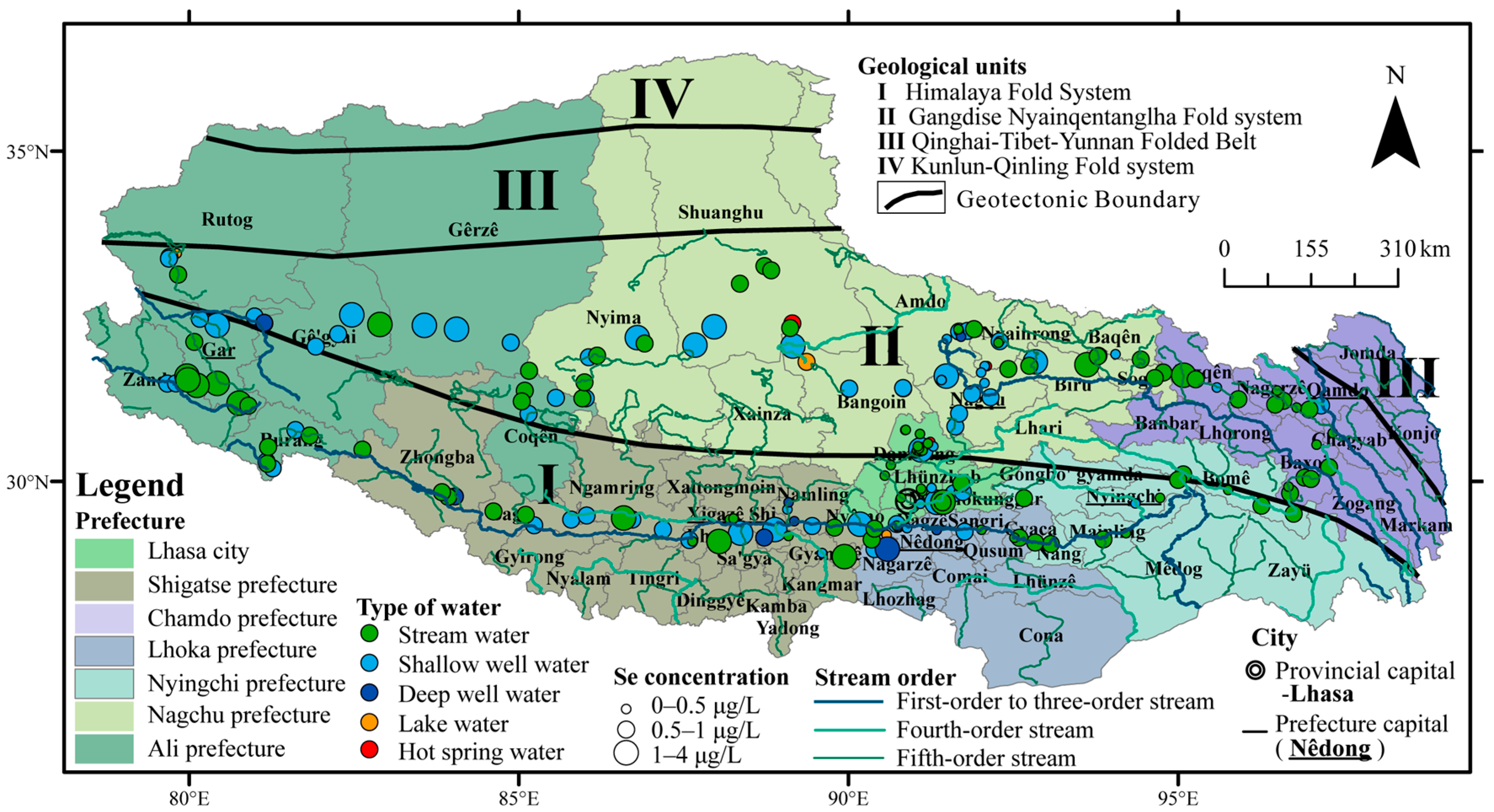
3.3. Concentrations of Selenium in Different Types of Water and Different Prefectures
3.4. Concentrations of Fluorine in Different Types of Water and Different Prefectures
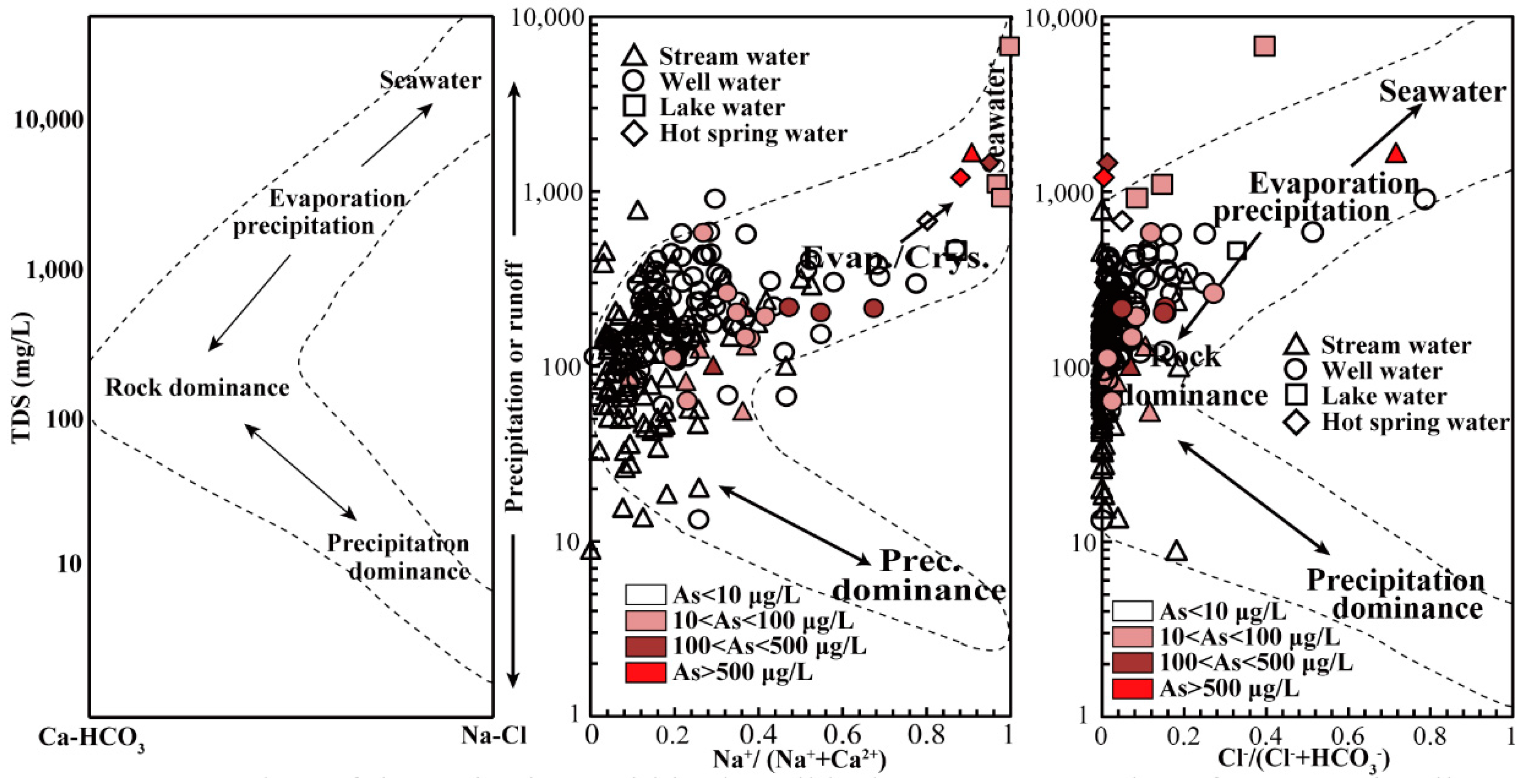
3.5. Elemental Concentration and Hydrochemical Characteristics of Tibet’s Natural Waters
3.6. Preliminary Discussion of the Causes of Variation in Tibetan Water Samples
3.7. Correlations of Elemental Concentrations
3.8. Assessment of Potential Health Risk in Tibet’s Natural Waters
3.8.1. Exposure Assessment
3.8.2. Non-Carcinogenic Risk
3.8.3. Carcinogenic Risk
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ministry of Water Resources of the People’s Republic of China (MWR). China’s Water Resource Report in 2007; MWR: Beijing, China, 2009.
- Sheng, Y.; Rui, Y.; Yu, Y. Heavy metal pollution in water of rivers and lake in Tibet by ICP-MS. Asian J. Chem. 2012, 24, 5403–5404. [Google Scholar]
- Tian, Y.; Yu, C.; Luo, K.; Zha, X.; Wu, J.; Zhang, X.; Ni, R. Hydrochemical characteristics and element contents of natural waters in Tibet, China. J. Geogr. Sci. 2015, 25, 669–686. [Google Scholar] [CrossRef]
- Wang, M.; Li, S.; Wang, H.; Xiao, T.; Zheng, B. Distribution of arsenic in surface water in Tibet. Environ. Sci. 2012, 33, 3411–3416. [Google Scholar]
- Li, S.; Wang, M.; Yang, Q.; Wang, H.; Zhu, J. Enrichment of arsenic in surface water, stream sediments and soils in Tibet. J. Geochem. Explor. 2012, 135, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xiao, T.; Zheng, B. Medical geology of arsenic, selenium and thallium in China. Sci. Total Environ. 2012, 421, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wang, Y. Hydrochemical anomaly of drinking waters in some endemic kashin-beck disease areas of Tibet, China. Environ. Earth Sci. 2012, 65, 659–667. [Google Scholar] [CrossRef]
- La Grange, M.; Mathieu, F.; Begaux, F.; Suetens, C.; Durand, M.-C. Kashin-beck disease and drinking water in central Tibet. Int. Orthop. 2001, 25, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Reyes, R.; Suetens, C.; Mathieu, F.; Begaux, F.; Zhu, D.; Rivera, M.T.; Boelaert, M.; Nève, J.; Perlmutter, N.; Vanderpas, J. Kashin–beck osteoarthropathy in rural tibet in relation to selenium and iodine status. N. Engl. J. Med. 1998, 339, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Study on the relationship between selenium concentrations in drinking water and kashin-beck disease in Tibet. Chin. J. Epidemiol. 2006, 25, 428–429. [Google Scholar]
- Cao, J.; Zhao, Y.; Liu, J.; Xirao, R.; Danzeng, S. Environmental fluoride content in Tibet. Environ. Res. 2000, 83, 333–337. [Google Scholar]
- Cao, J.; Zhao, Y.; Liu, J.; Xirao, R.; Danzeng, S. Fluoride concentrations of water sources in Tibet. Fluoride 2000, 33, 205–209. [Google Scholar]
- Cao, J.; Zhao, Y.; Liu, J. Brick tea consumption as the cause of dental fluorosis among children from Mongol, Kazak and Yugu populations in China. Food Chem. Toxicol. 1997, 35, 827–833. [Google Scholar] [CrossRef]
- Cao, J.; Zhao, Y.; Liu, J.; Bai, X.; Zhou, D.; Fang, S.; Jia, M.; Wu, J. Fluorine intake of a Tibetan population. Food Chem. Toxicol. 1996, 34, 755–757. [Google Scholar] [CrossRef]
- Cao, J.; Bai, X.; Zhao, Y.; Liu, J.; Zhou, D.; Fang, S.; Jia, M.; Wu, J. The relationship of fluorosis and brick tea drinking in Chinese Tibetans. Environ. Health Perspect. 1996, 104, 1340–1343. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.Y. Multiple criteria data envelopment analysis for full ranking units associated to environment impact assessment. Int. J. Environ. Pollut. 1998, 41, 2279–2280. [Google Scholar] [CrossRef]
- Xie, J.X. A hybrid adaptive time-delay neural network model for multi-step-ahead prediction of sunspot activity. Int. J. Environ. Pollut. 2006, 28, 364–381. [Google Scholar] [CrossRef]
- Wu, C.L.; Chau, K.W.; Li, Y.S. Methods to improve neural network performance in daily flows prediction. J. Hydrol. 2009, 372, 80–93. [Google Scholar] [CrossRef]
- Wang, W.C.; Xu, D.M.; Chau, K.W.; Lei, G.J. Assessment of river water quality based on theory of variable fuzzy sets and fuzzy binary comparison method. Water Resour. Manag. 2014, 28, 4183–4200. [Google Scholar] [CrossRef]
- Muttil, N.; Chau, K.W. Machine-learning paradigms for selecting ecologically significant input variables. Eng. Appl. Artif. Intell. 2007, 20, 735–744. [Google Scholar] [CrossRef] [Green Version]
- Chau, K.W.; Wu, C.L. A hybrid model coupled with singular spectrum analysis for daily rainfall prediction. J. Hydroinf. 2010, 12, 458–473. [Google Scholar] [CrossRef]
- GB/T 5750-2006 Standards Examination Methods for Drinking Water; China Standards Press: Beijing, China, 2007.
- Chinese SEPA. Methods for Chemical Analysis of Water and Waste Water, 4th ed.; China Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- U.S. Environmental Protection Agency (EPA). Determination of Inorganic Anions by Ion Chromatography; Environmental Protection Agency Environmental Monitoring Systems Laboratory Office of Research and Development: Cincinnati, OH, USA, 1993.
- Shen, Z.; Zhu, W.; Zhong, Z. Hydrogeochemical Basis; Geological Publishing House: Beijing, China, 1986. [Google Scholar]
- Ma, L.; Qiao, X.; Liu, N. Geological atlas of China; Geological Publishing House: Beijing, China, 2002. [Google Scholar]
- GB 5749-2006 Standards for Drinking Water Quality; China Standards Press: Beijing, China, 2007.
- World Health Organization. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water-analyses. Eos Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Chen, L.; Wang, G.; Hu, F.; Wang, Y.; Liu, L. Groundwater hydrochemistry and isotope geochemistry in the Turpan basin, northwestern China. J. Arid Land 2014, 6, 378–388. [Google Scholar] [CrossRef]
- Zhu, B.; Yang, X.; Rioual, P.; Qin, X.; Liu, Z.; Xiong, H.; Yu, J. Hydrogeochemistry of three watersheds (the Erlqis, Zhungarer and Yili) in northern Xinjiang, NW China. Appl. Geochem. 2011, 26, 1535–1548. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Mudelsee, M. Correlation. In Climate Time Series Analysis; Springer: Berlin, Germany, 2010; Volume 42, pp. 285–338. [Google Scholar]
- Wedepohl, K.H.; Correns, C.W. Handbook of Geochemistry; Elsevier: Amsterdam, The Netherlands, 1969. [Google Scholar]
- Peter, A.J.; Viraraghavan, T. Thallium: A review of public health and environmental concerns. Environ. Int. 2005, 31, 493–501. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency (EPA). Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment) Final; EPA/540/R/99/005 OSWER 9285.7-02EP PB99-963312; Office of Superfund Remediation and Technology Innovation U.S. Environmental Protection Agency: Washington, DC, USA, 2004.
- Li, S.; Zhang, Q. Risk assessment and seasonal variations of dissolved trace elements and heavy metals in the upper Han River, China. J. Hazard. Mater. 2010, 181, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Miguel, E.D.; Iribarren, I.; Chacón, E.; Ordoñez, A.; Charlesworth, S. Risk-based evaluation of the exposure of children to trace elements in playgrounds in Madrid (Spain). Chemosphere 2007, 66, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zhao, D.Y.; Jia, H.Y.; Zhang, Y.; Zhang, X.X.; Cheng, S.P. Preliminary risk assessment of trace metal pollution in surface water from Yangtze River in Nanjing section, China. Bull. Environ. Contam. Toxicol. 2009, 82, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Pez, D.R.; Castro-Rojo, R. Science and transscience in carcinogen risk assessment—The European Union regulatory process for trichloroethylene. J. Toxicol. Environ. Health B 2003, 6, 257–277. [Google Scholar]
- Luo, K.; Zhang, S.; Yuan, T.; Gao, X. Arsenic distribution pattern in different sources of drinking water and their geological background in Guanzhong basin, Shaanxi, China. Acta Geol. Sin. 2014, 88, 984–994. [Google Scholar] [CrossRef]
- Liu, Y.L.; Luo, K.L.; Lin, X.X.; Gao, X.; Ni, R.X.; Wang, S.B.; Tian, X.L. Regional distribution of longevity population and chemical characteristics of natural water in Xinjiang, China. Sci. Total Environ. 2014, 473–474, 54–62. [Google Scholar] [CrossRef] [PubMed]

| Se | Cl− | F− | NO3− | SO42− | HCO3 | Ag | Al | Ba | Be | Cd | Co | Cr | |
| Pearson Correlation | 0.15 * | 0.73 ** | 0.00 | –0.02 | 0.06 | 0.27 ** | 0.27 ** | –0.01 | 0.13 | 0.45 ** | 0.12 | –0.01 | 0.14 * |
| Sig. (2-tailed) | 0.03 | 0.00 | 1.00 | 0.74 | 0.41 | 0.00 | 0.00 | 0.86 | 0.06 | 0.00 | 0.08 | 0.93 | 0.04 |
| N | 204 | 204 | 204 | 204 | 204 | 204 | 204 | 204 | 204 | 204 | 204 | 204 | 204 |
| Cu | Li | Mn | Ni | Pb | Tl | V | Cs | Ga | In | Rb | U | Bi | |
| Pearson Correlation | 0.28 ** | 0.83 ** | 0.02 | 0.14 * | –0.02 | 0.74 ** | 0.09 | 0.94 ** | 0.18 ** | 0.24 ** | 0.94 ** | –0.02 | –0.01 |
| Sig. (2-tailed) | 0.00 | 0.00 | 0.79 | 0.05 | 0.80 | 0.00 | 0.21 | 0.00 | 0.01 | 0.00 | 0.00 | 0.74 | 0.86 |
| N | 204 | 204 | 204 | 204 | 204 | 204 | 204 | 204 | 204 | 204 | 204 | 204 | 204 |
| Mo | Zn | Fe | Ti | Hg | B | Ca | K | Mg | Na | P | SiO2 | Sr | |
| Pearson Correlation | 0.03 | –0.03 | 0.00 | 0.06 | 0.95 ** | 0.93 ** | 0.03 | 0.26 ** | 0.13 | 0.26 ** | 0.05 | 0.52 ** | 0.18 ** |
| Sig. (2-tailed) | 0.70 | 0.70 | 0.96 | 0.42 | 0.00 | 0.00 | 0.71 | 0.00 | 0.06 | 0.00 | 0.52 | 0.00 | 0.01 |
| N | 204 | 204 | 204 | 204 | 204 | 204 | 204 | 204 | 204 | 204 | 204 | 204 | 204 |
| Elements | Al | As–NC | Ba | Cd-NC | Co | Cr-NC | Cu | Fe | Mn | Ni-NC | Pb | Se | Sr | V | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADD-Lhasa | ADD1 | 1.030 | 0.027 | 0.188 | 5.23 × 10−5 | 9.07 × 10−4 | 0.040 | 6.76 × 10−3 | 2.075 | 0.037 | 6.72 × 10−3 | 1.78 × 10−3 | 3.18 × 10−3 | 0.932 | 0.000 |
| ADD2 | 0.022 | 0.023 | 0.220 | 9.80 × 10−5 | 9.84 × 10−4 | 0.080 | 0.011 | 1.549 | 0.041 | 0.014 | 1.16 × 10−3 | 4.77 × 10−3 | 2.731 | 0.012 | |
| ADD-Shigatse | ADD1 | 1.319 | 0.040 | 0.098 | 3.01 × 10−5 | 9.98 × 10−4 | 0.038 | 4.06 × 10−3 | 2.034 | 0.018 | 8.03 × 10−3 | 1.29 × 10−3 | 0.010 | 1.968 | 0.039 |
| ADD2 | 0.027 | 0.066 | 0.349 | 9.22 × 10−5 | 1.07 × 10−3 | 0.080 | 4.96 × 10−3 | 1.465 | 1.07 × 10−3 | 0.013 | 1.31 × 10−5 | 9.21 × 10−3 | 2.857 | 0.020 | |
| ADD-Chamdo | ADD1 | 3.525 | 0.032 | 0.444 | 5.33 × 10−5 | 2.56 × 10−3 | 0.064 | 7.71 × 10−3 | 4.853 | 0.432 | 0.023 | 2.12 × 10−3 | 9.40 × 10−3 | 2.712 | 0.017 |
| ADD2 | 0.029 | 0.030 | 0.678 | 6.16 × 10−5 | 1.79 × 10−3 | 0.101 | 7.94 × 10−3 | 2.112 | 7.56 × 10−3 | 0.020 | 1.22 × 10−4 | 8.51 × 10−3 | 5.012 | 0.011 | |
| ADD-Nagchu | ADD1 | 3.525 | 0.032 | 0.444 | 5.33 × 10−5 | 2.56 × 10−3 | 0.064 | 7.71 × 10−3 | 4.853 | 0.432 | 0.023 | 2.12 × 10−3 | 9.40 × 10−3 | 2.712 | 0.017 |
| ADD2 | 0.030 | 0.031 | 0.698 | 6.16 × 10−5 | 1.71 × 10−3 | 0.099 | 7.59 × 10−3 | 2.031 | 4.04 × 10−3 | 0.019 | 1.28 × 10−4 | 8.66 × 10−3 | 5.035 | 0.012 | |
| ADD-Lhoka | ADD1 | 0.026 | 0.015 | 0.063 | 6.33 × 10−5 | 3.88 × 10−4 | 0.028 | 1.34 × 10−3 | 1.477 | 2.22 × 10−3 | 3.53 × 10−3 | 2.45 × 10−5 | 5.38 × 10−3 | 1.291 | 0.056 |
| ADD2 | 0.014 | 0.025 | 0.176 | 4.37 × 10−5 | 5.67 × 10−4 | 0.044 | 4.34 × 10−4 | 1.200 | 0.027 | 7.57 × 10−3 | 0.000 | 0.011 | 3.036 | 0.023 | |
| ADD-Nyingchi | ADD1 | 0.134 | 0.020 | 0.063 | 4.08 × 10−5 | 4.00 × 10−4 | 0.023 | 0.000 | 0.747 | 5.86 × 10−4 | 0.012 | 0.000 | 6.00 × 10−3 | 4.164 | 0.024 |
| ADD2 | 0.057 | 6.50 × 10−3 | 0.020 | 1.22 × 10−4 | 9.80 × 10−5 | 0.012 | 7.71 × 10−4 | 0.149 | 7.35 × 10−5 | 9.43 × 10−4 | 0.000 | 2.14 × 10−3 | 0.186 | 0.022 | |
| ADD-Ali | ADD1 | 0.490 | 1.620 | 0.200 | 1.09 × 10−4 | 9.46 × 10−4 | 0.020 | 6.94 × 10−3 | 2.071 | 0.017 | 0.015 | 5.08 × 10−4 | 0.012 | 3.312 | 0.006 |
| ADD2 | 0.031 | 0.458 | 0.309 | 7.63 × 10−5 | 1.21 × 10−3 | 0.046 | 5.56 × 10−3 | 1.577 | 0.022 | 0.014 | 5.23 × 10−5 | 0.010 | 4.161 | 0.006 | |
| Element | Al | As-NC | Ba | Cd-NC | Co | Cr-NC | Cu | Fe | Mn | Ni-NC | Pb | Se | Sr | V | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RfD | 1000 | 0.3 | 200 | 0.5 | 0.3 | 3 | 40 | 700 | 24 | 20 | 1.4 | 5 | 600 | 5 | |
| HQ-Lhasa | HQ1 | 1.03× 10−3 | 0.091 | 9.38 × 10−4 | 1.05 × 10−4 | 3.02 × 10−3 | 0.013 | 1.69 × 10−4 | 2.96 × 10−3 | 1.52 × 10−3 | 3.36 × 10−4 | 1.27 × 10−3 | 6.36 × 10−4 | 1.55 × 10−3 | 0.000 |
| HQ2 | 2.15 × 10−5 | 0.077 | 1.10 × 10−3 | 1.96 × 10−4 | 3.28 × 10−3 | 0.027 | 2.77 × 10−4 | 2.21 × 10−3 | 1.73 × 10−3 | 7.23 × 10−4 | 8.29 × 10−4 | 9.55 × 10−4 | 4.55 × 10−3 | 2.47 × 10−3 | |
| HQ-Shigatse | HQ1 | 1.32 × 10−3 | 0.132 | 4.88 × 10−4 | 6.03 × 10−5 | 3.33 × 10−3 | 0.013 | 1.02 × 10−4 | 2.91 × 10−3 | 7.38 × 10−4 | 4.01 × 10−4 | 9.20 × 10−4 | 2.03 × 10−3 | 3.28 × 10−3 | 7.76 × 10−3 |
| HQ2 | 2.68 × 10−5 | 0.220 | 1.74 × 10−3 | 1.84 × 10−4 | 3.56 × 10−3 | 0.027 | 1.24 × 10−4 | 2.09 × 10−3 | 4.44 × 10−5 | 6.44 × 10−4 | 9.33 × 10−6 | 1.84 × 10−3 | 4.76 × 10−3 | 4.08 × 10−3 | |
| HQ-Chamdo | HQ1 | 4.37 × 10−5 | 0.041 | 1.51 × 10−3 | 1.47 × 10−4 | 2.07 × 10−3 | 7.57 × 10−3 | 9.49 × 10−6 | 1.02 × 10−3 | 1.55 × 10−4 | 4.49 × 10−4 | 2.73 × 10−5 | 1.64 × 10−3 | 2.93 × 10−3 | 2.21 × 10−3 |
| HQ2 | 1.47 × 10−5 | 0.106 | 1.68 × 10−3 | 0.000 | 1.45 × 10−3 | 8.97 × 10−3 | 3.90 × 10−5 | 1.12 × 10−3 | 0.000 | 2.04 × 10−4 | 1.31 × 10−5 | 1.48 × 10−3 | 1.25 × 10−2 | 2.05 × 10−3 | |
| HQ-Nagchu | HQ1 | 3.53 × 10−3 | 0.106 | 2.22 × 10−3 | 1.07 × 10−4 | 8.54 × 10−3 | 0.021 | 1.93 × 10−4 | 6.93 × 10−3 | 1.80 × 10−2 | 1.16 × 10−3 | 1.51 × 10−3 | 1.88 × 10−3 | 4.52 × 10−3 | 3.42 × 10−3 |
| HQ2 | 2.89 × 10−5 | 0.101 | 3.39 × 10−3 | 1.23 × 10−4 | 5.96 × 10−3 | 0.034 | 1.98 × 10−4 | 3.02 × 10−3 | 3.15 × 10−4 | 9.90 × 10−4 | 8.75 × 10−5 | 1.70 × 10−3 | 8.35 × 10−3 | 2.26 × 10−3 | |
| HQ-Lhoka | HQ1 | 2.58 × 10−5 | 0.051 | 3.13 × 10−4 | 1.27 × 10−4 | 1.29 × 10−3 | 9.38 × 10−3 | 3.36 × 10−5 | 2.11 × 10−3 | 9.26 × 10−5 | 1.76 × 10−4 | 1.75 × 10−5 | 1.08 × 10−3 | 2.15 × 10−3 | 1.12 × 10−2 |
| HQ2 | 1.36 × 10−5 | 0.084 | 8.79 × 10−4 | 8.75 × 10−5 | 1.89 × 10−3 | 0.015 | 1.08 × 10−5 | 1.71 × 10−3 | 1.12 × 10−3 | 3.79 × 10−4 | 0.000 | 2.30 × 10−3 | 5.06 × 10−3 | 4.56 × 10−3 | |
| HQ-Nyingchi | HQ1 | 1.34 × 10−4 | 0.066 | 3.14 × 10−4 | 8.16 × 10−5 | 1.33 × 10−3 | 7.55 × 10−3 | 0.000 | 1.07 × 10−3 | 2.44 × 10−5 | 5.85 × 10−4 | 0.000 | 1.20 × 10−3 | 6.94 × 10−3 | 4.76 × 10−3 |
| HQ2 | 5.66 × 10−5 | 0.022 | 1.01 × 10−4 | 2.45 × 10−4 | 3.27 × 10−4 | 4.15 × 10−3 | 1.93 × 10−5 | 2.13 × 10−4 | 3.06 × 10−6 | 4.71 × 10−5 | 0.000 | 4.29 × 10−4 | 3.10 × 10−4 | 4.40 × 10−3 | |
| HQ-Ali | HQ1 | 4.90 × 10−4 | 5.400 | 9.98 × 10−4 | 2.19 × 10−4 | 3.15 × 10−3 | 6.79 × 10−3 | 1.73 × 10−4 | 2.96 × 10−3 | 7.23 × 10−4 | 7.44 × 10−4 | 3.63 × 10−4 | 2.35 × 10−3 | 5.52 × 10−3 | 1.28 × 10−3 |
| HQ2 | 3.14 × 10−5 | 1.526 | 1.55 × 10−3 | 1.53 × 10−4 | 4.03 × 10−3 | 0.015 | 1.39 × 10−4 | 2.25 × 10−3 | 9.29 × 10−4 | 7.18 × 10−4 | 3.74 × 10−5 | 2.02 × 10−3 | 6.94 × 10−3 | 1.26 × 10−3 | |
| HI-Lhasa | 1.05 × 10−3 | 0.168 | 2.04 × 10−3 | 3.00 × 10−4 | 6.30 × 10−3 | 0.040 | 4.46 × 10−4 | 5.18 × 10−3 | 3.25 × 10−3 | 1.06 × 10−3 | 2.10 × 10−3 | 1.59 × 10−3 | 6.11 × 10−3 | 2.47 × 10−3 | |
| HI-Shigatse | 1.35 × 10−3 | 0.352 | 2.23 × 10−3 | 2.45 × 10−4 | 6.89 × 10−3 | 0.039 | 2.26 × 10−4 | 5.00 × 10−3 | 7.82 × 10−4 | 1.05 × 10−3 | 9.29 × 10−4 | 3.88 × 10−3 | 8.04 × 10−3 | 0.012 | |
| HI-Chamdo | 5.84 × 10−5 | 0.146 | 3.19 × 10−3 | 1.47 × 10−4 | 3.52 × 10−3 | 0.017 | 4.85 × 10−5 | 2.13 × 10−3 | 1.55 × 10−4 | 6.53 × 10−4 | 4.04 × 10−5 | 3.12 × 10−3 | 0.015 | 4.25 × 10−3 | |
| HI-Nagchu | 3.55 × 10−3 | 0.207 | 5.61 × 10−3 | 2.30 × 10−4 | 0.015 | 0.055 | 3.91 × 10−4 | 9.95 × 10−3 | 0.018 | 2.15 × 10−3 | 1.60 × 10−3 | 3.58 × 10−3 | 0.013 | 5.69 × 10−3 | |
| HI-Lhoka | 3.94 × 10−5 | 0.135 | 1.19 × 10−3 | 2.14 × 10−4 | 3.18 × 10−3 | 0.024 | 4.45 × 10−5 | 3.82 × 10−3 | 1.21 × 10−3 | 5.55 × 10−4 | 1.75 × 10−5 | 3.37 × 10−3 | 7.21 × 10−3 | 0.016 | |
| HI-Nyingchi | 1.91× 10−4 | 0.088 | 4.15 × 10−4 | 3.27 × 10−4 | 1.66 × 10−3 | 0.012 | 1.93 × 10−5 | 1.28 × 10−3 | 2.75 × 10−5 | 6.33 × 10−4 | 0.000 | 1.63 × 10−3 | 7.25 × 10−3 | 9.16 × 10−3 | |
| HI-Ali | 5.21 × 10−4 | 6.926 | 2.54 × 10−3 | 3.71 × 10−4 | 7.18 × 10−3 | 0.022 | 3.12 × 10−4 | 5.21 × 10−3 | 1.65 × 10−3 | 1.46 × 10−3 | 4.00 × 10−4 | 4.36 × 10−3 | 1.25 × 10−2 | 2.54 × 10−3 | |
| Carcinogenic Risk | Classification | The Estimated Value of Cancer Risk |
|---|---|---|
| Carcinogenic risk-Lhasa | CR1 | 1.81 × 10−5 |
| CR2 | 1.55 × 10−5 | |
| Carcinogenic risk-Shigatse | CR1 | 2.64 × 10−5 |
| CR2 | 4.40 × 10−5 | |
| Carcinogenic risk-Chamdo | CR1 | 8.15 × 10−6 |
| CR2 | 2.11 × 10−5 | |
| Carcinogenic risk-Nagchu | CR1 | 2.12 × 10−5 |
| CR2 | 2.02 × 10−5 | |
| Carcinogenic risk-Lhoka | CR1 | 1.03 × 10−5 |
| CR2 | 1.67 × 10−5 | |
| Carcinogenic risk-Nyingchi | CR1 | 1.32 × 10−5 |
| CR2 | 4.33 × 10−6 | |
| Carcinogenic risk-Ali | CR1 | 1.08 × 10−3 |
| CR2 | 3.05 × 10−4 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Yu, C.; Zha, X.; Wu, J.; Gao, X.; Feng, C.; Luo, K. Distribution and Potential Health Risks of Arsenic, Selenium, and Fluorine in Natural Waters in Tibet, China. Water 2016, 8, 568. https://doi.org/10.3390/w8120568
Tian Y, Yu C, Zha X, Wu J, Gao X, Feng C, Luo K. Distribution and Potential Health Risks of Arsenic, Selenium, and Fluorine in Natural Waters in Tibet, China. Water. 2016; 8(12):568. https://doi.org/10.3390/w8120568
Chicago/Turabian StyleTian, Yuan, Chengqun Yu, Xinjie Zha, Jianshang Wu, Xing Gao, Chujian Feng, and Kunli Luo. 2016. "Distribution and Potential Health Risks of Arsenic, Selenium, and Fluorine in Natural Waters in Tibet, China" Water 8, no. 12: 568. https://doi.org/10.3390/w8120568
APA StyleTian, Y., Yu, C., Zha, X., Wu, J., Gao, X., Feng, C., & Luo, K. (2016). Distribution and Potential Health Risks of Arsenic, Selenium, and Fluorine in Natural Waters in Tibet, China. Water, 8(12), 568. https://doi.org/10.3390/w8120568







