Taxonomy of Means and Ends in Aquaculture Production—Part 3: The Technical Solutions of Controlling N Compounds, Organic Matter, P Compounds, Metals, Temperature and Preventing Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Scope
2.2. Literature Review
2.3. Synthesis
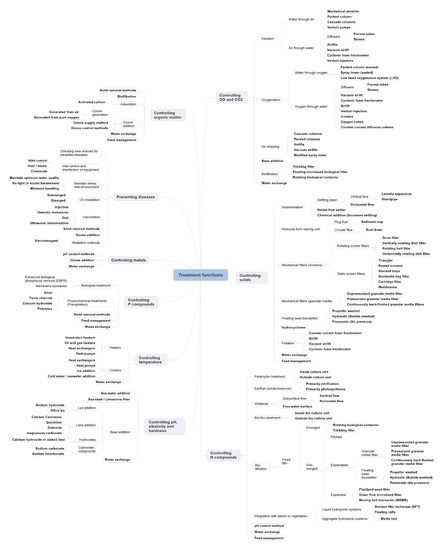
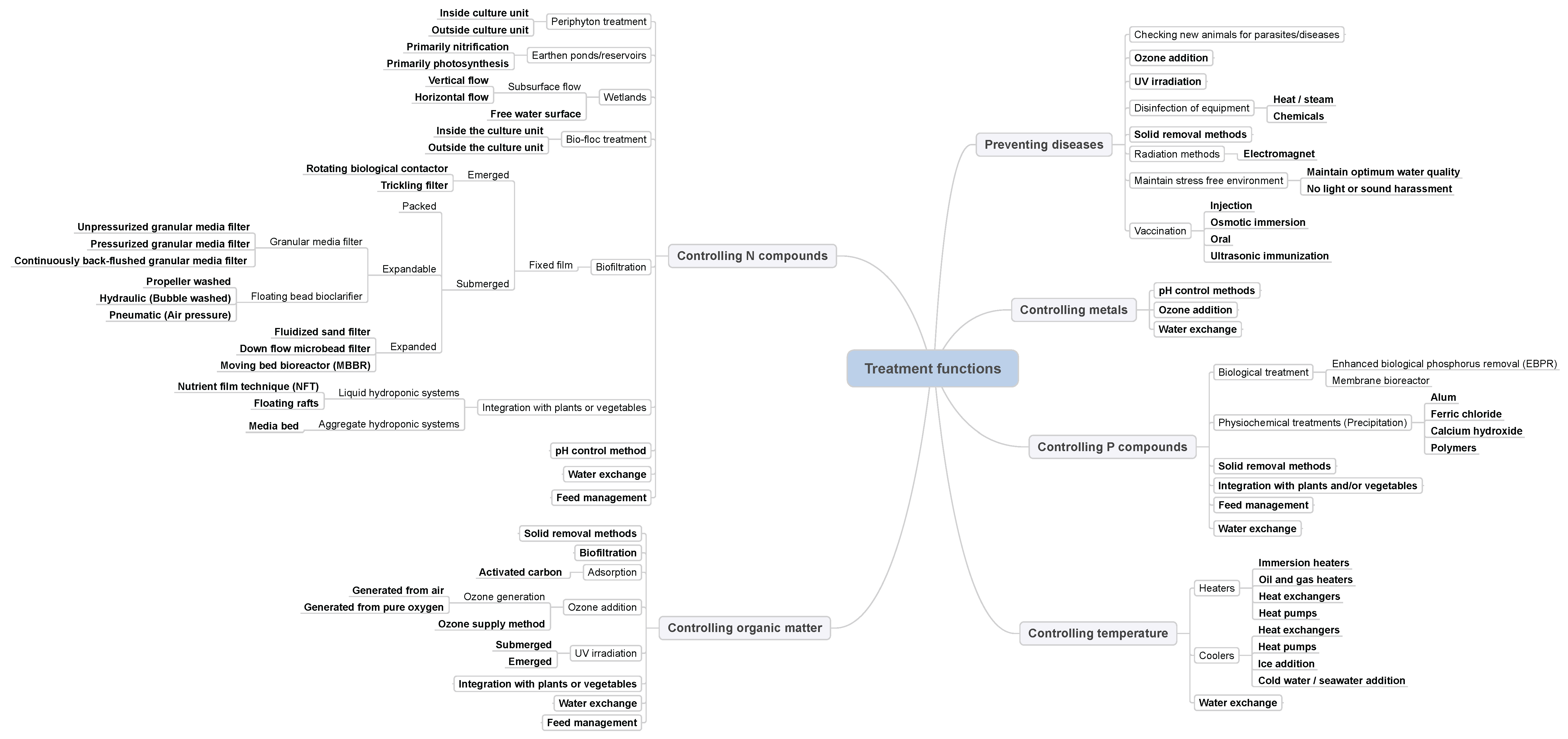
3. Resolving the Treatment Functions of Controlling N Compounds, Organic Matter, P Compounds, Metals, Temperature and Preventing Disease
3.1. Preventing Diseases
3.1.1. Inlet Control and Disinfection of Equipment
3.1.2. Maintain Stress-Free Environment
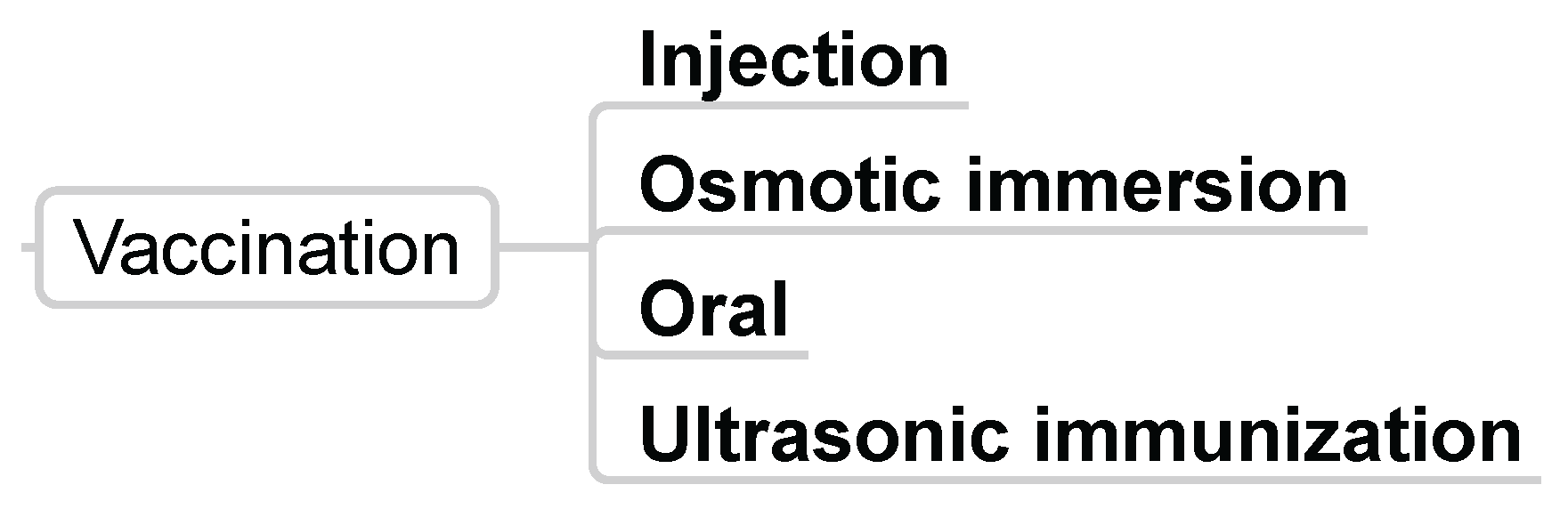
3.1.3. Vaccination
3.1.4. Solid Removal Methods
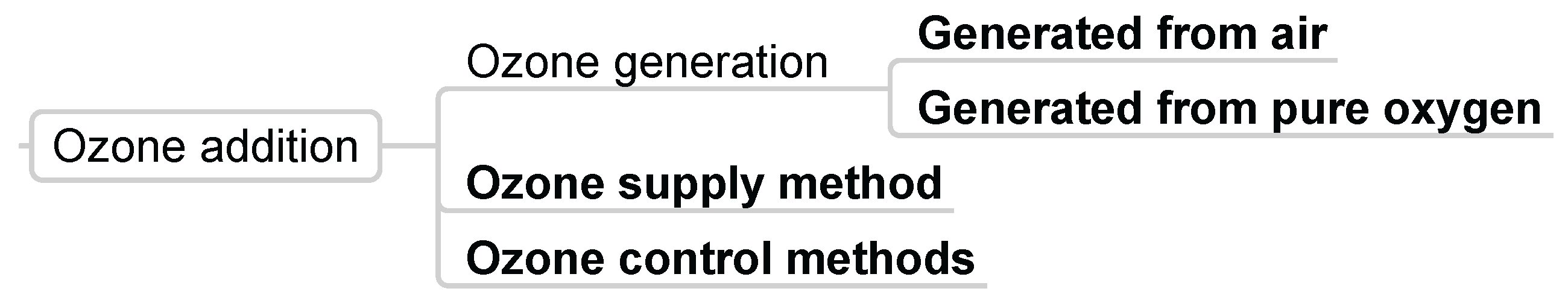
3.1.5. Ozone Addition
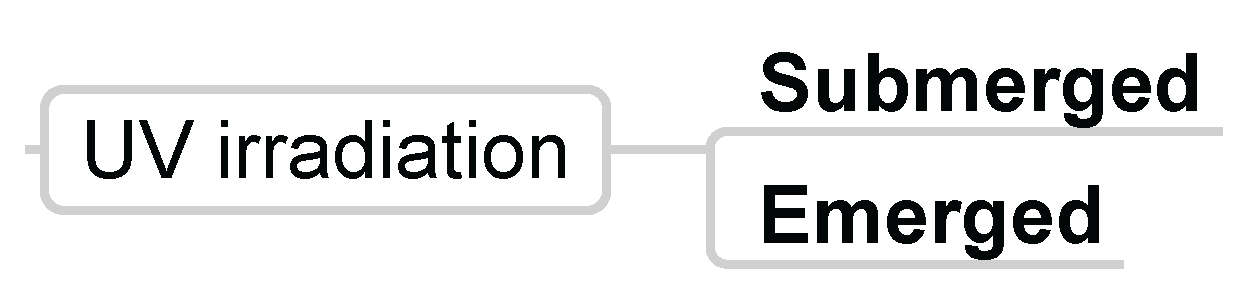
3.1.6. UV Irradiation
3.1.7. Radiation Methods
3.2. Controlling Temperature
3.2.1. Heaters
3.2.2. Coolers
3.3. Controlling N Compounds
Nitrification
3.3.1. pH Control Methods
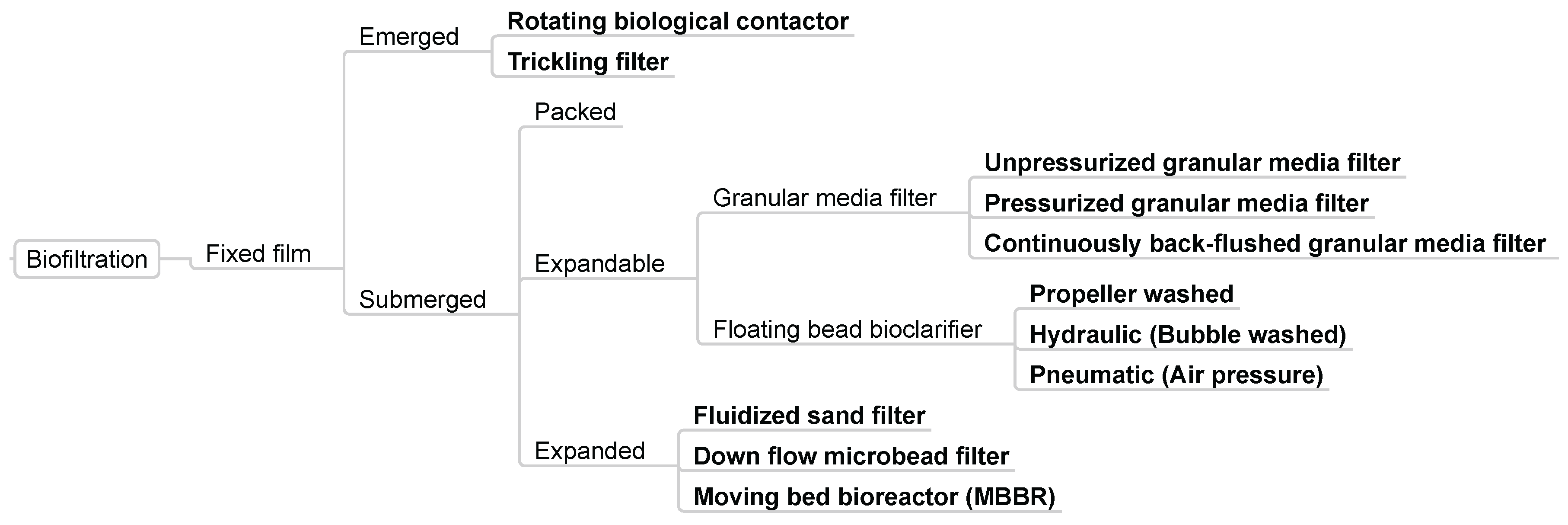
3.3.2. Biofiltration
Emerged Filters
Submerged Filters
3.3.3. Integration with Plants/Vegetables
Liquid Hydroponic Systems
Aggregated Hydroponic Systems
3.3.4. Wetlands
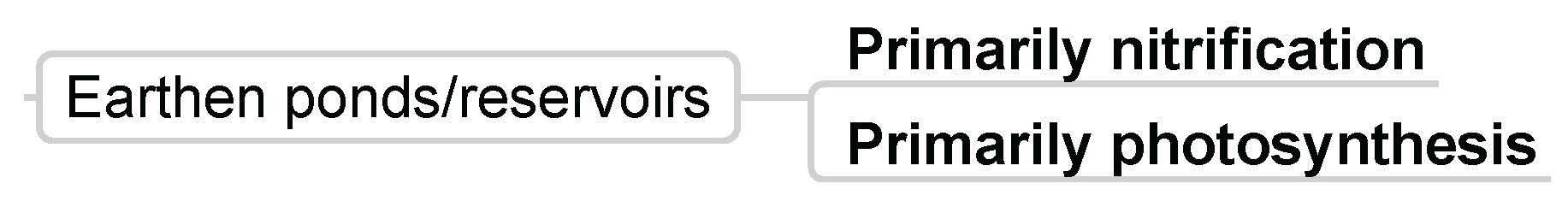
3.3.5. Earthen-Ponds/Reservoirs
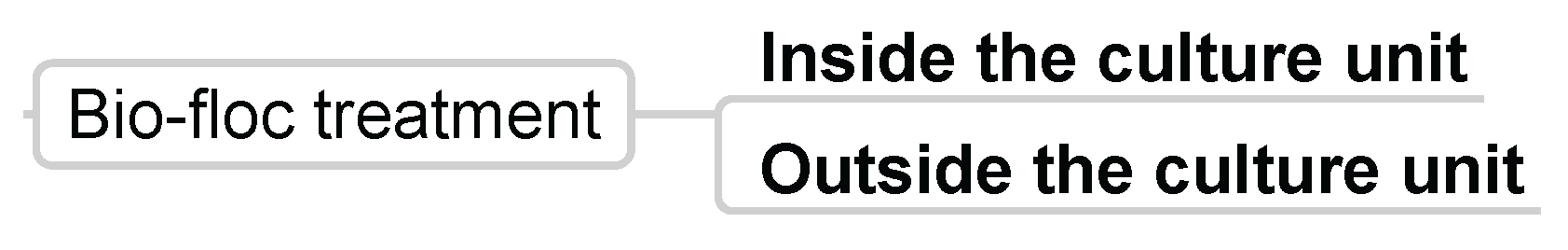
3.3.6. Bio-Floc Treatment
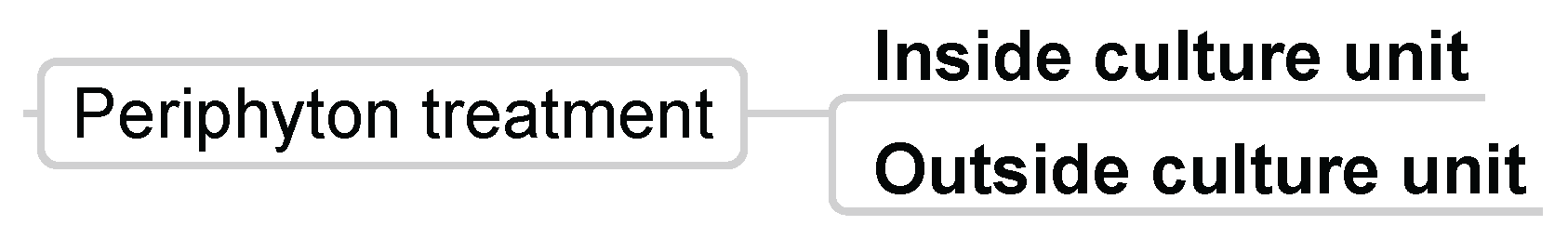
3.3.7. Periphyton Treatment
3.4. Controlling Organic Matter
3.4.1. Solid Removal Methods
3.4.2. Ozone Addition
3.4.3. Adsorption Process
3.4.4. Biofiltration
3.5. Controlling P Compounds
3.5.1. Solid Removal Methods
3.5.2. Physicochemical Treatments
3.6. Controlling Metals
3.6.1. pH Control Methods
3.6.2. Ozone Addition
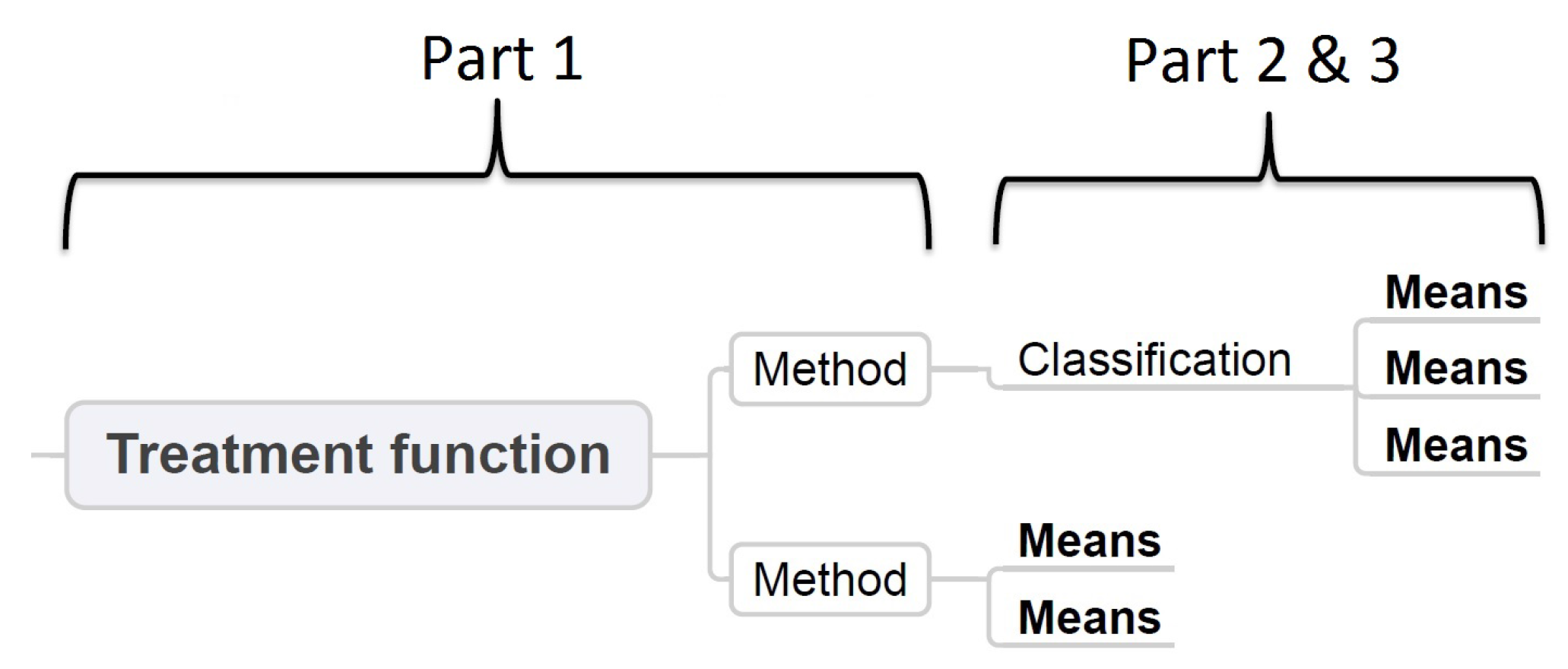
4. Taxonomy of the Technical Solutions of the Treatment Functions
5. Discussion
Supplementary Files
Supplementary File 1Author Contributions
Conflicts of Interest
References
- Björnsdóttir, R.; Oddsson, G.V.; Thorarinsdottir, R.; Unnthorsson, R. Taxonomy of means and ends in aquaculture production—Part 1: The functions. Water 2016, 8, 319. [Google Scholar] [CrossRef]
- Vilbergsson, B.; Oddsson, G.V.; Unnthorsson, R. Taxonomy of means and ends in aquaculture production—Part 2: The technical solutions of controlling solids, disolved gasses and pH. Water 2016, 8, 387. [Google Scholar] [CrossRef]
- Vilbergsson, B.; Oddsson, G.V.; Unnthorsson, R. Taxonomy of means and ends in aquaculture production—Part 4: The mapping of technical solutions onto multiple treatment function. Water 2016, 8, 387. [Google Scholar] [CrossRef]
- Losordo, T.M.; Masser, M.P.; Rakocy, J. Recirculating Aquaculture Tank Production Systems—A Review of Component Options; SRAC Publication: Stoneville, MS, USA, 1999. [Google Scholar]
- Crab, R.; Avnimelech, Y.; Defoirdt, T.; Bossier, P.; Verstraete, W. Nitrogen removal techniques in aquaculture for a sustainable production. Aquaculture 2007, 270, 1–14. [Google Scholar] [CrossRef]
- Cripps, S.J.; Bergheim, A. Solids management and removal for intensive land-based aquaculture production systems. Aquac. Eng. 2000, 22, 33–56. [Google Scholar] [CrossRef]
- Malone, R.F.; Pfeiffer, T.J. Rating fixed film nitrifying biofilters used in recirculating aquaculture systems. Aquac. Eng. 2006, 34, 389–402. [Google Scholar] [CrossRef]
- Masser, M.; Rakocy, J.; Losordo, T. Recirculating Aquaculture Tank Production Systems—Management of Recirculating Systems; SRAC Publication: Stoneville, MS, USA, 1999. [Google Scholar]
- World Health Organization. Water Treatment and Pathogen Control: Process Efficiency in Achieving Safe Drinking Water; Technical Report; World Health Organization: London, UK, 2004. [Google Scholar]
- Lekang, O. Aquaculture Engineering; Blackwell Publishing Ltd.: Oxford, UK, 2007. [Google Scholar]
- Timmons, M.B.; Ebeling, J.M. Recirculating Aquaculture, 2nd ed.; Cayuga Aqua Ventures: Ithaca, NY, USA, 2010. [Google Scholar]
- Stickney, R.R. Aquaculture: An Introductory Text; CAB International: Cambridge, MA, USA, 2005. [Google Scholar]
- Dunn, E.J.; Polk, A.; Scarrett, D.J.; Olivier, G.; Lall, S.; Goosen, M.F. Vaccines in aquaculture: The search for an efficient delivery system. Aquac. Eng. 1990, 9, 23–32. [Google Scholar] [CrossRef]
- Zhou, Y.C.; Wang, J.; Zhang, B.; Su, Y.Q. Ultrasonic immunization of sea bream, Pagrus major (Temminck & Schlegel), with a mixed vaccine against Vibrio alginolyticus and V. anguillarum. J. Fish Dis. 2002, 25, 325–331. [Google Scholar]
- Bullock, G.L.; Summerfelt, S.T.; Noble, A.C.; Weber, A.L.; Durant, M.D.; Hankins, J.A. Ozonation of a recirculating rainbow trout culture system I. Effects on bacterial gill disease and heterotrophic bacteria. Aquaculture 1997, 158, 43–55. [Google Scholar] [CrossRef]
- Summerfelt, S.T.; Hankins, J.A.; Weber, A.L.; Durant, M.D. Ozonation of a recirculating rainbow trout culture system II. Effects on microscreen filtration and water quality. Aquaculture 1997, 158, 57–67. [Google Scholar] [CrossRef]
- Mamane, H.; Colorni, A.; Bar, I.; Ori, I.; Mozes, N. The use of an open channel, low pressure UV reactor for water treatment in low head recirculating aquaculture systems (LH-RAS). Aquac. Eng. 2010, 42, 103–111. [Google Scholar] [CrossRef]
- Summerfelt, S.T.; Sharrer, M.J.; Tsukuda, S.M.; Gearheart, M. Process requirements for achieving full-flow disinfection of recirculating water using ozonation and UV irradiation. Aquac. Eng. 2009, 40, 17–27. [Google Scholar] [CrossRef]
- Nofouzi, K.; Sheikhzadeh, N.; Mohamad-Zadeh Jassur, D.; Ashrafi-Helan, J. Influence of extremely low frequency electromagnetic fields on growth performance, innate immune response, biochemical parameters and disease resistance in rainbow trout, Oncorhynchus mykiss. Fish Physiol. Biochem. 2015, 41, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Eding, E.; Kamstra, A.; Verreth, J.; Huisman, E.; Klapwijk, A. Design and operation of nitrifying trickling filters in recirculating aquaculture: A review. Aquac. Eng. 2006, 34, 234–260. [Google Scholar] [CrossRef]
- Tidwell, J. Aquaculture Production Systems; Wiley-Blackwell: Oxford, UK, 2012. [Google Scholar]
- Hamlin, H.; Michaels, J.; Beaulaton, C.; Graham, W.; Dutt, W.; Steinbach, P.; Losordo, T.; Schrader, K.; Main, K. Comparing denitrification rates and carbon sources in commercial scale upflow denitrification biological filters in aquaculture. Aquac. Eng. 2008, 38, 79–92. [Google Scholar] [CrossRef]
- Davidson, J.; Good, C.; Welsh, C.; Summerfelt, S. The effects of ozone and water exchange rates on water quality and rainbow trout Oncorhynchus mykiss performance in replicated water recirculating systems. Aquac. Eng. 2011, 44, 80–96. [Google Scholar] [CrossRef]
- Loyless, J.; Malone, R. A sodium bicarbonate dosing methodology for pH management in freshwater-recirculating aquaculture systems. Prog. Fish Cult. 1997, 59, 198–205. [Google Scholar] [CrossRef]
- Summerfelt, S.T.; Sharrer, M.J. Design implication of carbon dioxide production within biofilters contained in recirculating salmonid culture systems. Aquac. Eng. 2004, 32, 171–182. [Google Scholar] [CrossRef]
- Brazil, B.L. Performance and operation of a rotating biological contactor in a tilapia recirculating aquaculture system. Aquac. Eng. 2006, 34, 261–274. [Google Scholar] [CrossRef]
- Prosser, D.G. Air Driven Rotating Biological Contactor Apparatus. U.S. Patent 3,886,074 A, 27 May 1975. [Google Scholar]
- Cortez, S.; Teixeira, P.; Oliveira, R.; Mota, M. Rotating biological contactors: A review on main factors affecting performance. Rev. Environ. Sci. Biotechnol. 2008, 7, 155–172. [Google Scholar] [CrossRef]
- Ayoub, G.M.; Saikaly, P. The combined effect of step-feed and recycling on RBC performance. Water Res. 2004, 38, 3009–3016. [Google Scholar] [CrossRef] [PubMed]
- Kamstra, A.; van der Heul, J.; Nijhof, M. Performance and optimisation of trickling filters on eel farms. Aquac. Eng. 1998, 17, 175–192. [Google Scholar] [CrossRef]
- Malone, R.F.; Beecher, L.E. Use of floating bead filters to recondition recirculating waters in warmwater aquaculture production systems. Aquac. Eng. 2000, 22, 57–73. [Google Scholar] [CrossRef]
- Summerfelt, S.T. Design and management of conventional fluidized-sand biofilters. Aquac. Eng. 2006, 34, 275–302. [Google Scholar] [CrossRef]
- Tsukuda, S.; Christianson, L.; Kolb, A.; Saito, K.; Summerfelt, S. Heterotrophic denitrification of aquaculture effluent using fluidized sand biofilters. Aquac. Eng. 2015, 64, 49–59. [Google Scholar] [CrossRef]
- Timmons, M.B.; Holder, J.L.; Ebeling, J.M. Application of microbead biological filters. Aquac. Eng. 2006, 34, 332–343. [Google Scholar] [CrossRef]
- Greiner, A.D.; Timmons, M.B. Evaluation of the nitrification rates of microbead and trickling filters in an intensive recirculating tilapia production facility. Aquac. Eng. 1998, 18, 189–200. [Google Scholar] [CrossRef]
- Rusten, B.; Eikebrokk, B.; Ulgenes, Y.; Lygren, E. Design and operations of the Kaldnes moving bed biofilm reactors. Aquac. Eng. 2006, 34, 322–331. [Google Scholar] [CrossRef]
- Ödegaard, H. Method and Reactor for Purification of Water. Patent CA 2074470 C, 11 May 1999. [Google Scholar]
- Diver, S.; Rinehait, L. Aquaponics-Integration of Hydroponics with Aquaculture; NCAT Agriculture Specialist: Butte, MT, USA, 2010. [Google Scholar]
- Lennard, W.A.; Leonard, B.V. A Comparison of Three Different Hydroponic Sub-systems (gravel bed, floating and nutrient film technique) in an Aquaponic Test System. Aquac. Int. 2006, 14, 539–550. [Google Scholar] [CrossRef]
- Lam, S.S.; Ma, N.L.; Jusoh, A.; Ambak, M.A. Biological nutrient removal by recirculating aquaponic system: Optimization of the dimension ratio between the hydroponic & rearing tank components. Int. Biodeterior. Biodegrad. 2015, 102, 107–115. [Google Scholar]
- Buzby, K.M.; Lin, L.S. Scaling aquaponic systems: Balancing plant uptake with fish output. Aquac. Eng. 2014, 63, 39–44. [Google Scholar] [CrossRef]
- Endut, A.; Jusoh, A.; Ali, N.; Nik, W.B.W.; Hassan, A. A study on the optimal hydraulic loading rate and plant ratios in recirculation aquaponic system. Bioresour. Technol. 2010, 101, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.F.; Jing, S.R.; Lee, D.Y. The potential use of constructed wetlands in a recirculating aquaculture system for shrimp culture. Environ. Pollut. 2003, 123, 107–113. [Google Scholar] [CrossRef]
- Tilley, D.R.; Badrinarayanan, H.; Rosati, R.; Son, J. Constructed wetlands as recirculation filters in large-scale shrimp aquaculture. Aquac. Eng. 2002, 26, 81–109. [Google Scholar] [CrossRef]
- Kadlec, R.; Wallace, S. Treatment Wetlands, 2nd ed.; Taylor & Francis Group: New York, NY, USA, 2009. [Google Scholar]
- Zhang, S.; Zhou, Q.; Xu, D.; He, F.; Cheng, S.; Liang, W. Vertical-Flow Constructed Wetlands Applied in a Recirculating Aquaculture System for Channel Catfish Culture: Effects on Water Quality and Zooplankton. Pol. J. Environ. Stud. 2010, 19, 1063–1070. [Google Scholar]
- Zachritz, W.; Hanson, A.; Sauceda, J.; Fitzsimmons, K. Evaluation of submerged surface flow (SSF) constructed wetlands for recirculating tilapia production systems. Aquac. Eng. 2008, 39, 16–23. [Google Scholar] [CrossRef]
- Gál, D.; Pekár, F.; Kerepeczki, É.; Váradi, L. Experiments on the operation of a combined aquaculture-algae system. Aquac. Int. 2007, 15, 173–180. [Google Scholar] [CrossRef]
- Hargreaves, J.A. Photosynthetic suspended-growth systems in aquaculture. Aquac. Eng. 2006, 34, 344–363. [Google Scholar] [CrossRef]
- Azim, M.; Little, D. The biofloc technology (BFT) in indoor tanks: Water quality, biofloc composition, and growth and welfare of Nile tilapia (Oreochromis niloticus). Aquaculture 2008, 283, 29–35. [Google Scholar] [CrossRef]
- Silva, K. Nitrogen and phosphorus dynamics in the biofloc production of the pacific white shrimp, Litopenaeus vannamei. J. World Aquac. Soc. 2013, 44, 30–41. [Google Scholar] [CrossRef]
- De Schryver, P.; Crab, R.; Defoirdt, T.; Boon, N.; Verstraete, W. The basics of bio-flocs technology: The added value for aquaculture. Aquaculture 2008, 277, 125–137. [Google Scholar] [CrossRef]
- Furtado, P.S.; Gaona, C.A.P.; Poersch, L.H.; Wasielesky, W. Application of different doses of calcium hydroxide in the farming shrimp Litopenaeus vannamei with the biofloc technology (BFT). Aquac. Int. 2013, 22, 1009–1023. [Google Scholar] [CrossRef]
- Beveridge, M.; Beveridge, M.; Verdegem, M.; Van Dam, A.A.; Azim, M.E. The potential of fish production based on periphyton. Rev. Fish Biol. Fish. 2002, 12, 1–31. [Google Scholar]
- Zhang, S.Y.; Li, G.; Wu, H.B.; Liu, X.G.; Yao, Y.H.; Tao, L.; Liu, H. An integrated recirculating aquaculture system (RAS) for land-based fish farming: The effects on water quality and fish production. Aquac. Eng. 2011, 45, 93–102. [Google Scholar] [CrossRef]
- Björnsdóttir, R. Mapping Aquaculture Production Systems: A Systematic Literature Review. Master’s Thesis, University of Iceland, Reykjavík, Iceland, 2015. [Google Scholar]
- Barrut, B.; Blancheton, J.P.; Callier, M.; Champagne, J.Y.; Grasmick, A. Foam fractionation efficiency of a vacuum airlift—Application to particulate matter removal in recirculating systems. Aquac. Eng. 2013, 54, 16–21. [Google Scholar] [CrossRef]
- Schroeder, J.; Croot, P.; Von Dewitz, B.; Waller, U.; Hanel, R. Potential and limitations of ozone for the removal of ammonia, nitrite, and yellow substances in marine recirculating aquaculture systems. Aquac. Eng. 2011, 45, 35–41. [Google Scholar] [CrossRef]
- Summerfelt, S.T.; Sharrer, M.J.; Hollis, J.; Gleason, L.E.; Summerfelt, S.R. Dissolved ozone destruction using ultraviolet irradiation in a recirculating salmonid culture system. Aquac. Eng. 2004, 32, 209–223. [Google Scholar] [CrossRef]
- Gonçalves, A.A.; Gagnon, G.A. Ozone Application in Recirculating Aquaculture System: An Overview. Ozone Sci. Eng. 2011, 33, 345–367. [Google Scholar] [CrossRef]
- Sharrer, M.J.; Summerfelt, S.T.; Bullock, G.L.; Gleason, L.E.; Taeuber, J. Inactivation of bacteria using ultraviolet irradiation in a recirculating salmonid culture system. Aquac. Eng. 2005, 33, 135–149. [Google Scholar] [CrossRef]
- Summerfelt, S.T. Ozonation and UV irradiation—An introduction and examples of current applications. Aquac. Eng. 2003, 28, 21–36. [Google Scholar] [CrossRef]
- Aitcheson, S.; Arnett, J.; Murray, K.; Zhang, J. Removal of aquaculture therapeutants by carbon adsorption. Aquaculture 2000, 183, 269–284. [Google Scholar] [CrossRef]
- Moreno-Castilla, C. Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon 2004, 42, 83–94. [Google Scholar] [CrossRef]
- Gutierrez-Wing, M.T.; Malone, R.F. Biological filters in aquaculture: Trends and research directions for freshwater and marine applications. Aquac. Eng. 2006, 34, 163–171. [Google Scholar] [CrossRef]
- Michaud, L.; Blancheton, J.; Bruni, V.; Piedrahita, R. Effect of particulate organic carbon on heterotrophic bacterial populations and nitrification efficiency in biological filters. Aquac. Eng. 2006, 34, 224–233. [Google Scholar] [CrossRef]
- True, B.; Johnson, W.; Chen, S. Reducing phosphorus discharge from flow-through aquaculture I: Facility and effluent characterization. Aquac. Eng. 2004, 32, 129–144. [Google Scholar] [CrossRef]
- Sharrer, M.J.; Rishel, K.; Summerfelt, S. Evaluation of geotextile filtration applying coagulant and flocculant amendments for aquaculture biosolids dewatering and phosphorus removal. Aquac. Eng. 2009, 40, 1–10. [Google Scholar] [CrossRef]
- Ebeling, J.; Welsh, C.; Rishel, K. Performance evaluation of an inclined belt filter using coagulation/flocculation aids for the removal of suspended solids and phosphorus from microscreen. Aquac. Eng. 2006, 35, 61–77. [Google Scholar] [CrossRef]
- Colt, J. Water quality requirements for reuse systems. Aquac. Eng. 2006, 34, 143–156. [Google Scholar] [CrossRef]
- van Bussel, C.G.; Schroeder, J.P.; Mahlmann, L.; Schulz, C. Aquatic accumulation of dietary metals (Fe, Zn, Cu, Co, Mn) in recirculating aquaculture systems (RAS) changes body composition but not performance and health of juvenile turbot (Psetta maxima). Aquac. Eng. 2014, 61, 35–42. [Google Scholar] [CrossRef]
- Rosseland, B.O.; Skogheim, O.K. Neutralization of acidic brook-water using a shell-sand filter or sea-water: Effects on eggs, alevins and smolts of salmonids. Aquaculture 1986, 58, 99–110. [Google Scholar] [CrossRef]






| Article | Description |
|---|---|
| Part 1: The functions [1] | The transformational view of aquaculture is introduced, and the functions are divided into input, treatment and output functions. There are 5 input functions, 10 treatment functions and 4 output functions. Key parameters used to control are identified and a nearly-exhaustive list of possible methods of technical solutions is provided. The results are presented as a map. |
| Part 2: Technical solutions for controlling solids, dissolved gasses and pH [2] | The map of aquaculture production is used to find all possible technical solutions for all of the methods in 3 treatment functions: controlling solids, dissolved gasses and pH functions. The result is a partial taxonomy of treatment functions from methods to technical solutions. |
| Part 3: Technical solutions for controlling N compounds, organic matter, P compounds, metals, temperature and preventing disease (this article) | The map of aquaculture production is used to find all possible technical solutions for all of the methods in 6 treatment functions, the controlling N compounds, organic matter, P compounds, metals, temperature and preventing disease functions. The result is a partial taxonomy of treatment functions from methods to technical solutions. A complete taxonomy of technical solutions is presented. |
| Part 4: The mapping of technical solutions onto multiple treatment functions [3] | The one-to-one relation between the technical solution and treatment function is relaxed. The technical solutions from Parts 2 and 3 are analyzed, and all of their effects on treatment functions are mapped out. Each relation is put into one of three categories: intended, positive effect and negative effect. The result is a quality-function-deployment presentation of the interaction between solutions and functions. |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilbergsson, B.; Oddsson, G.V.; Unnthorsson, R. Taxonomy of Means and Ends in Aquaculture Production—Part 3: The Technical Solutions of Controlling N Compounds, Organic Matter, P Compounds, Metals, Temperature and Preventing Disease. Water 2016, 8, 506. https://doi.org/10.3390/w8110506
Vilbergsson B, Oddsson GV, Unnthorsson R. Taxonomy of Means and Ends in Aquaculture Production—Part 3: The Technical Solutions of Controlling N Compounds, Organic Matter, P Compounds, Metals, Temperature and Preventing Disease. Water. 2016; 8(11):506. https://doi.org/10.3390/w8110506
Chicago/Turabian StyleVilbergsson, Bjorgvin, Gudmundur V. Oddsson, and Runar Unnthorsson. 2016. "Taxonomy of Means and Ends in Aquaculture Production—Part 3: The Technical Solutions of Controlling N Compounds, Organic Matter, P Compounds, Metals, Temperature and Preventing Disease" Water 8, no. 11: 506. https://doi.org/10.3390/w8110506
APA StyleVilbergsson, B., Oddsson, G. V., & Unnthorsson, R. (2016). Taxonomy of Means and Ends in Aquaculture Production—Part 3: The Technical Solutions of Controlling N Compounds, Organic Matter, P Compounds, Metals, Temperature and Preventing Disease. Water, 8(11), 506. https://doi.org/10.3390/w8110506