Plankton Community Stability and Its Relationship with Phytoplankton Species Richness in Lake Nansihu, China
Abstract
:1. Introduction
2. Materials and Methods
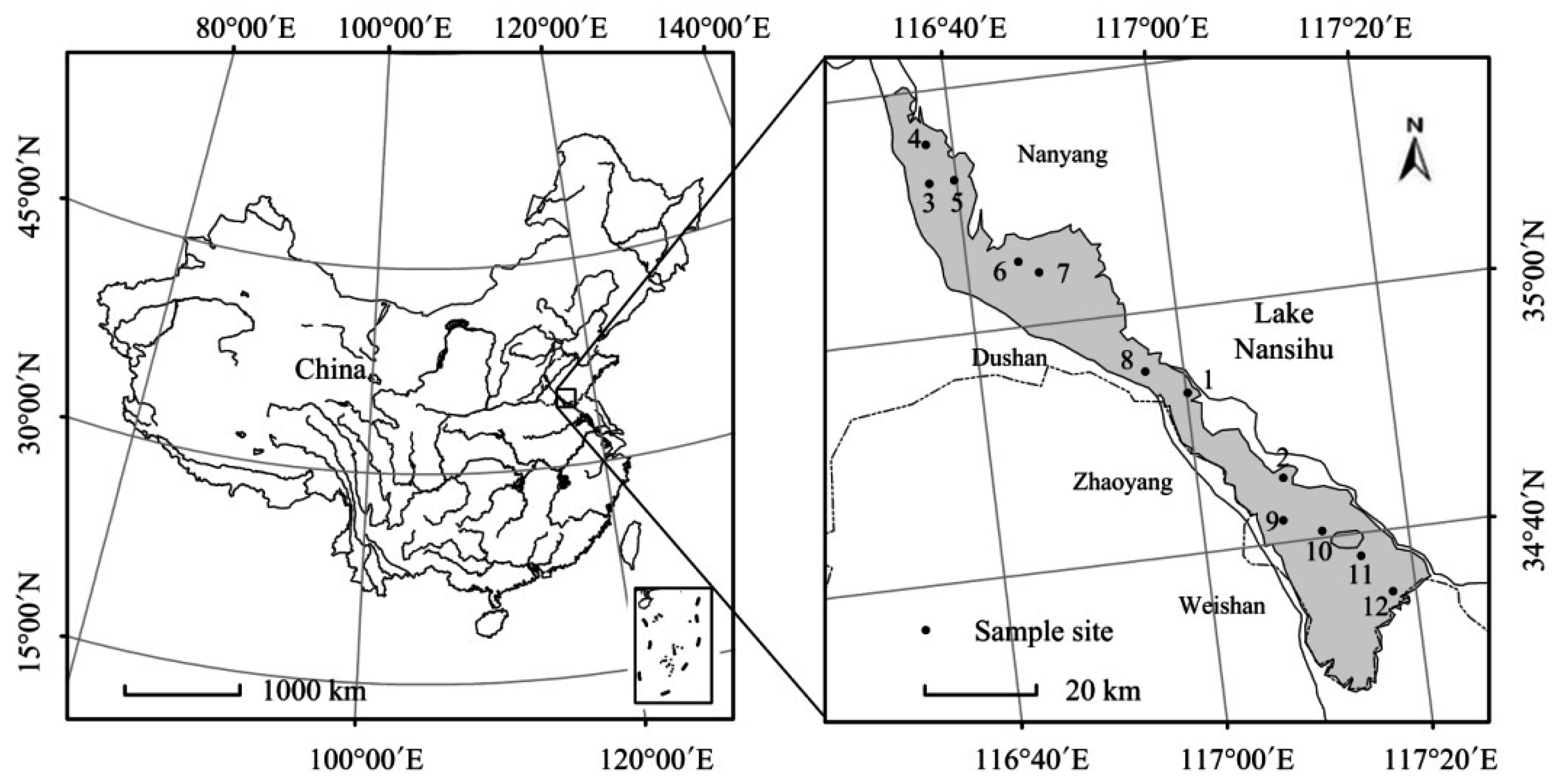
2.1. Study Area
2.2. Sampling and Measurements
2.3. Stability Index and Statistical Analyses
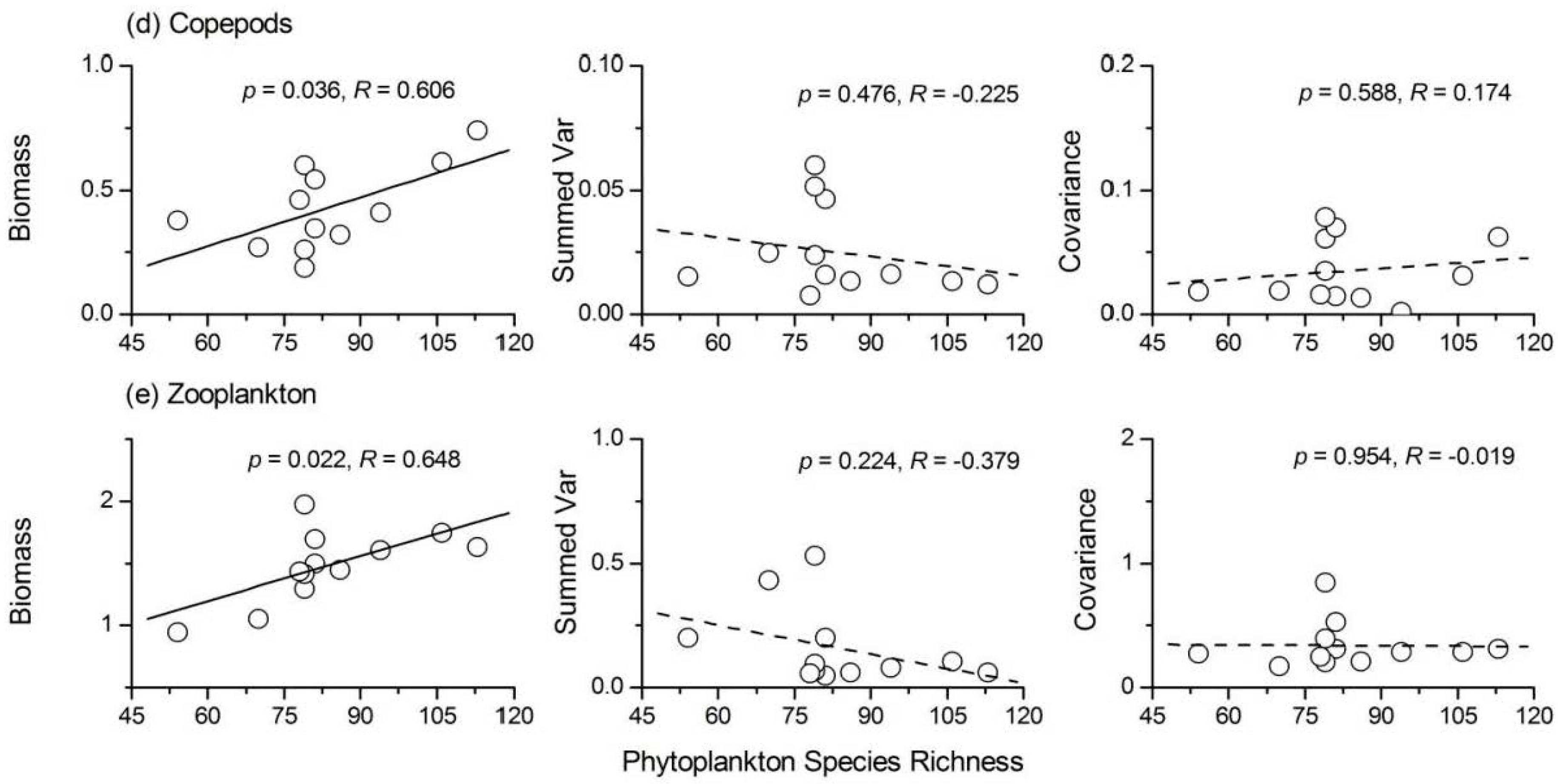
3. Results
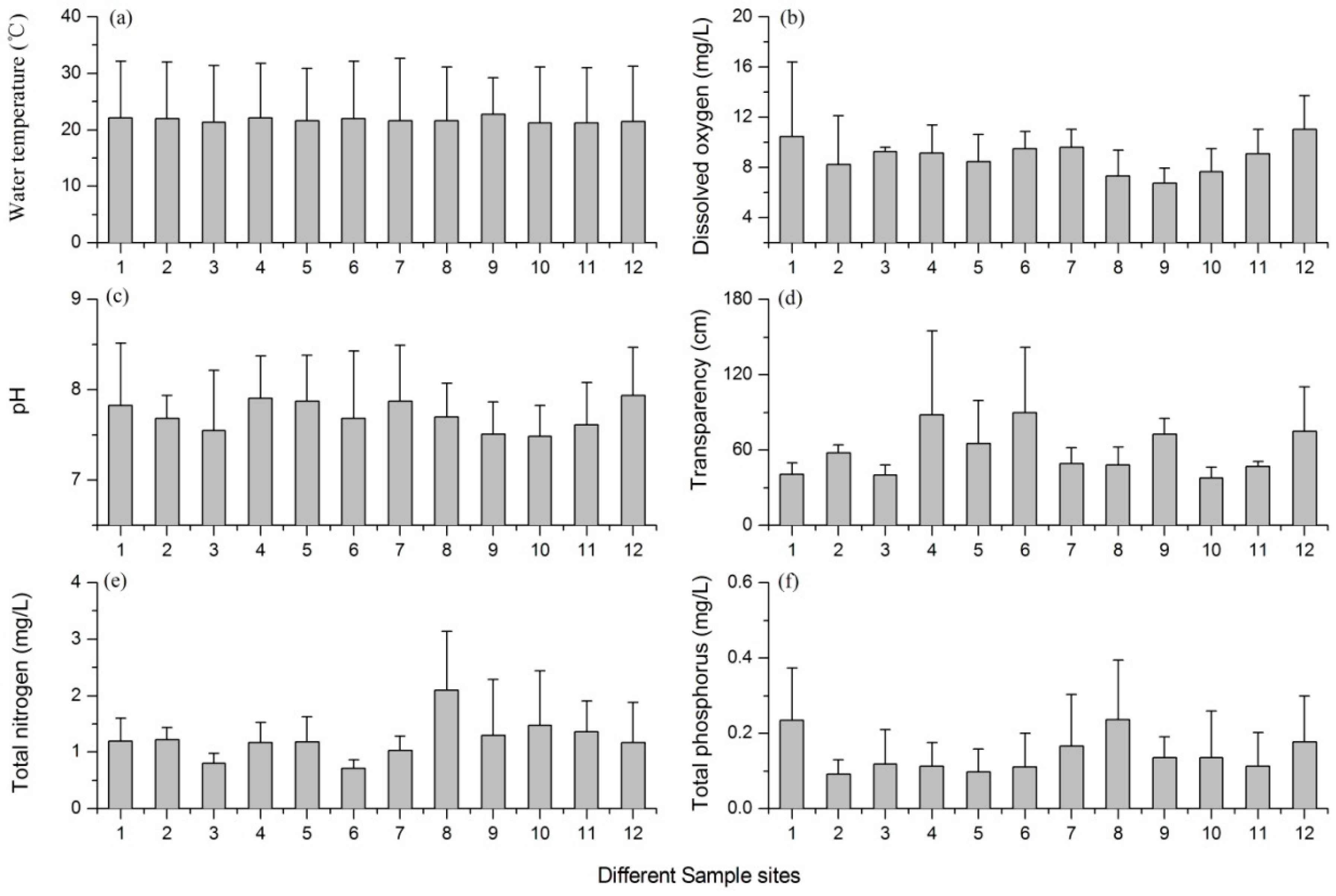
3.1. Variations of the Environmental Factors
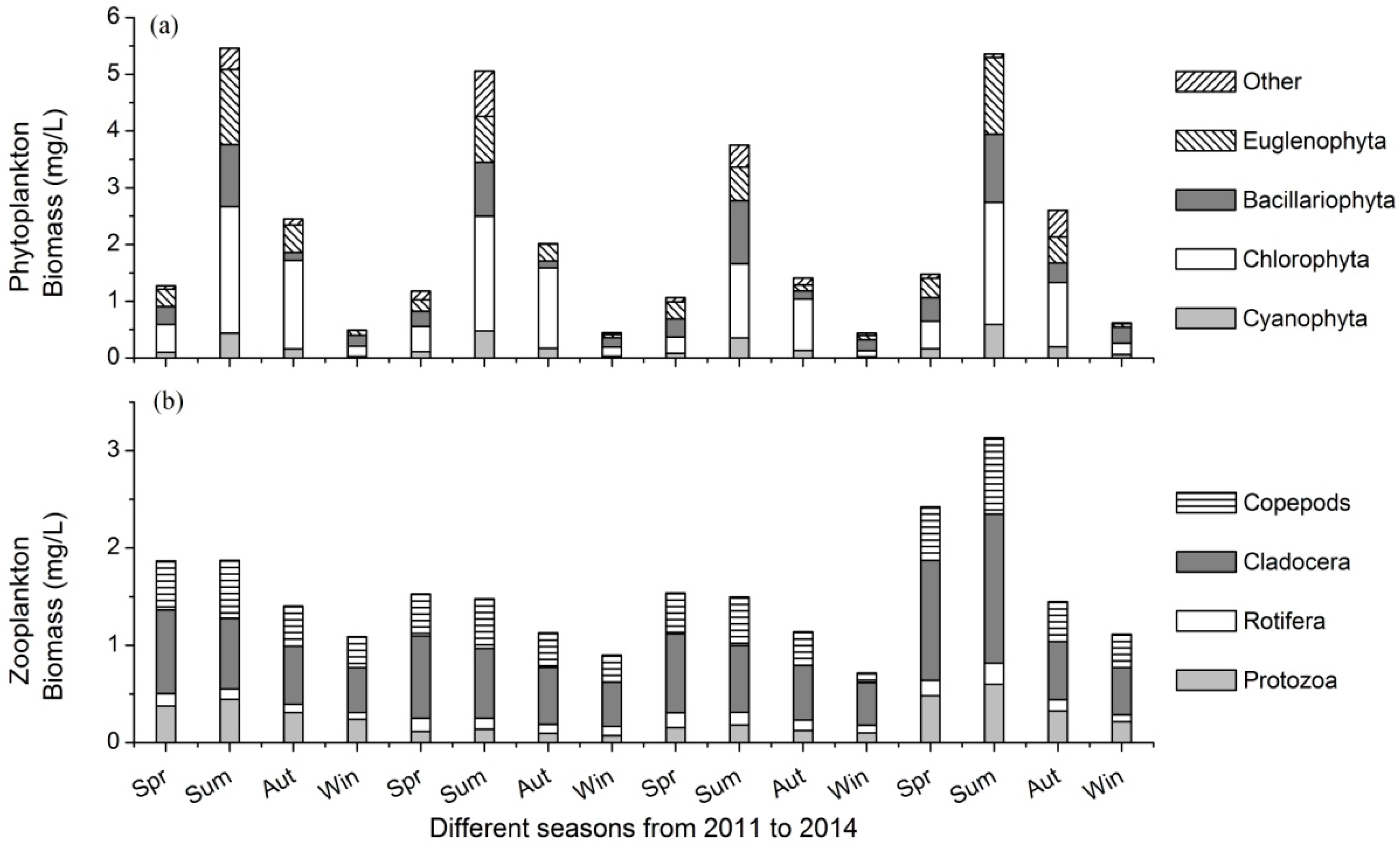
3.2. Variations of Plankton Community
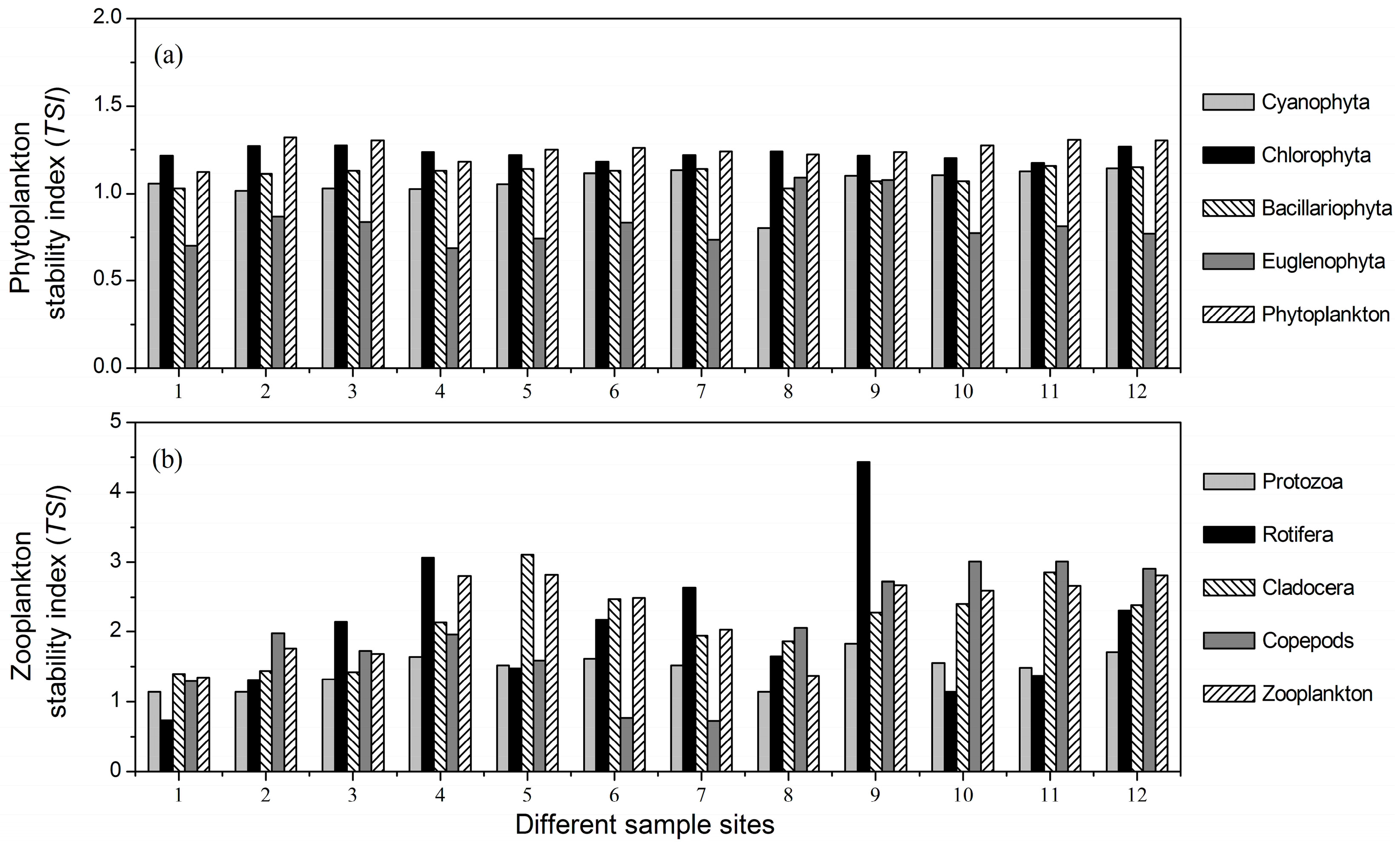
3.3. Stability Indices of Different Plankton Taxa
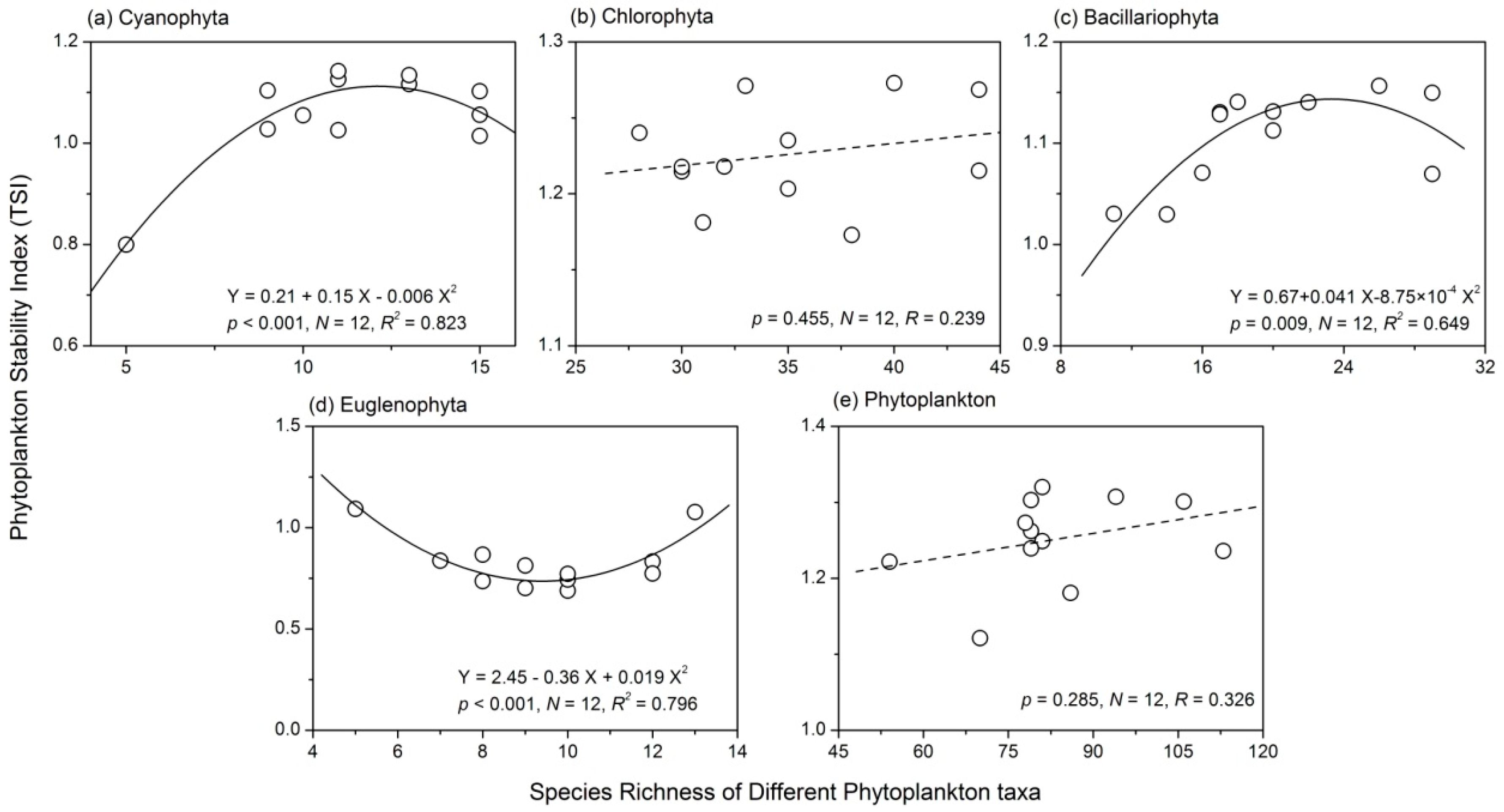
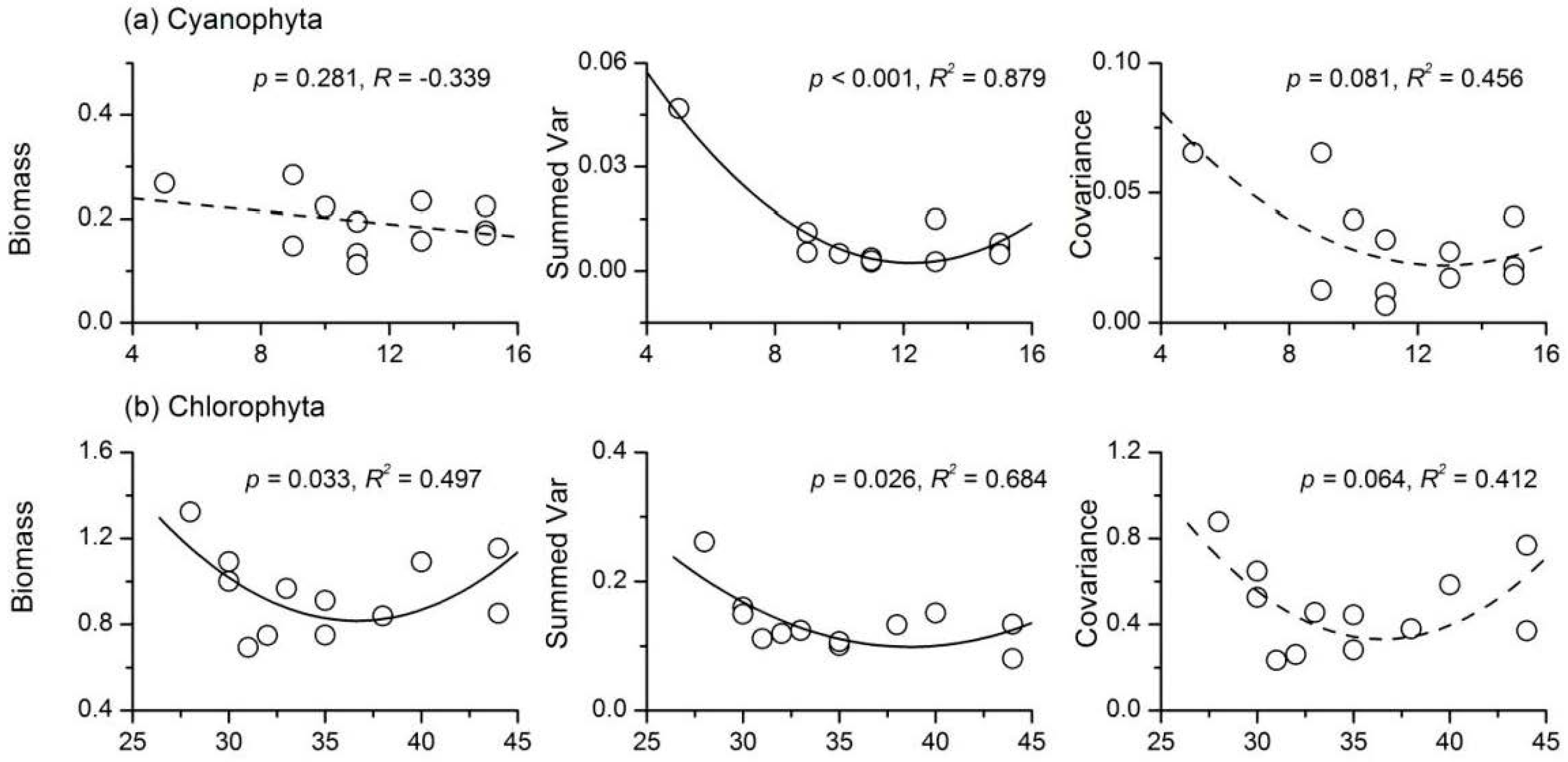
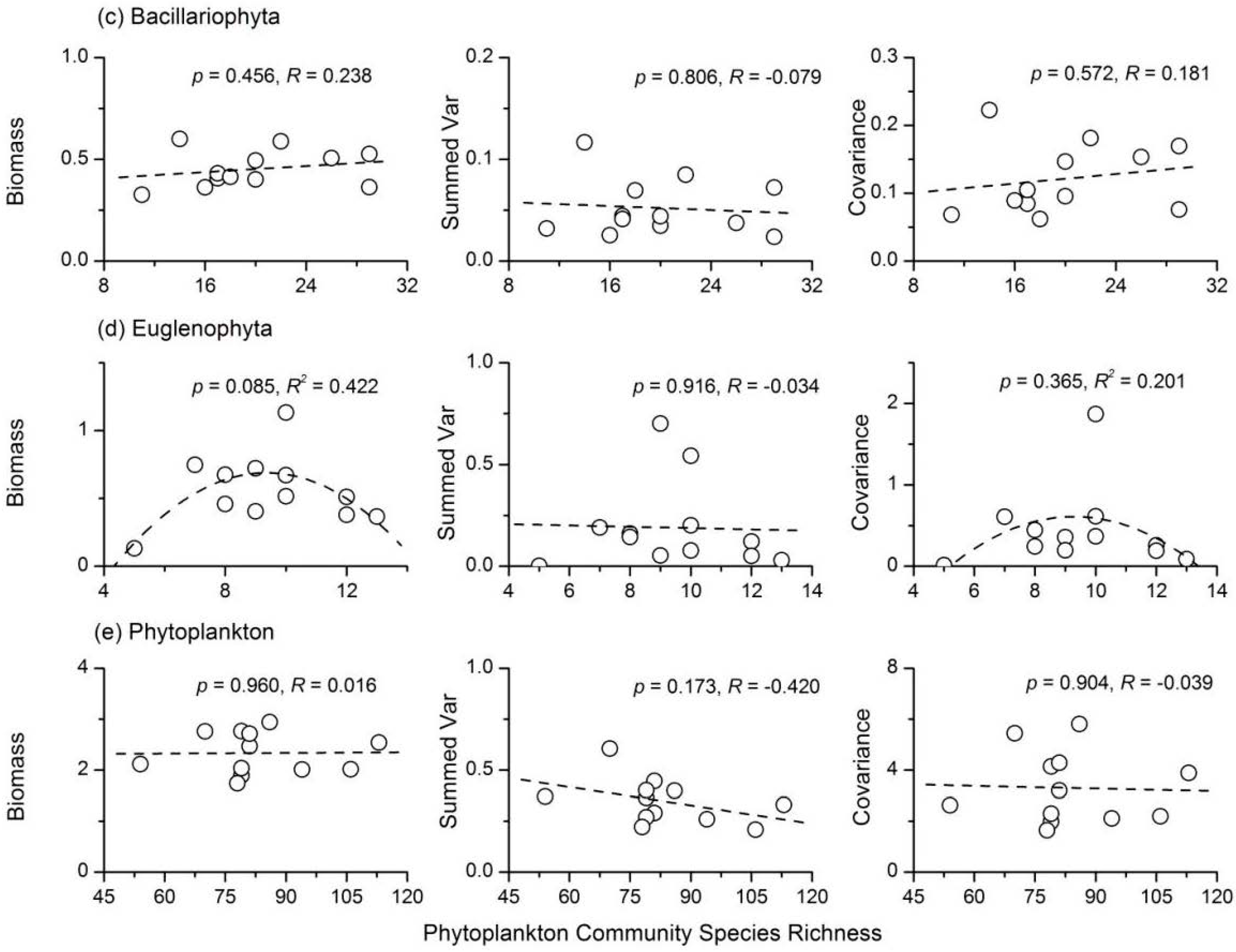
3.4. Relationship between Phytoplankton Diversity and Stability
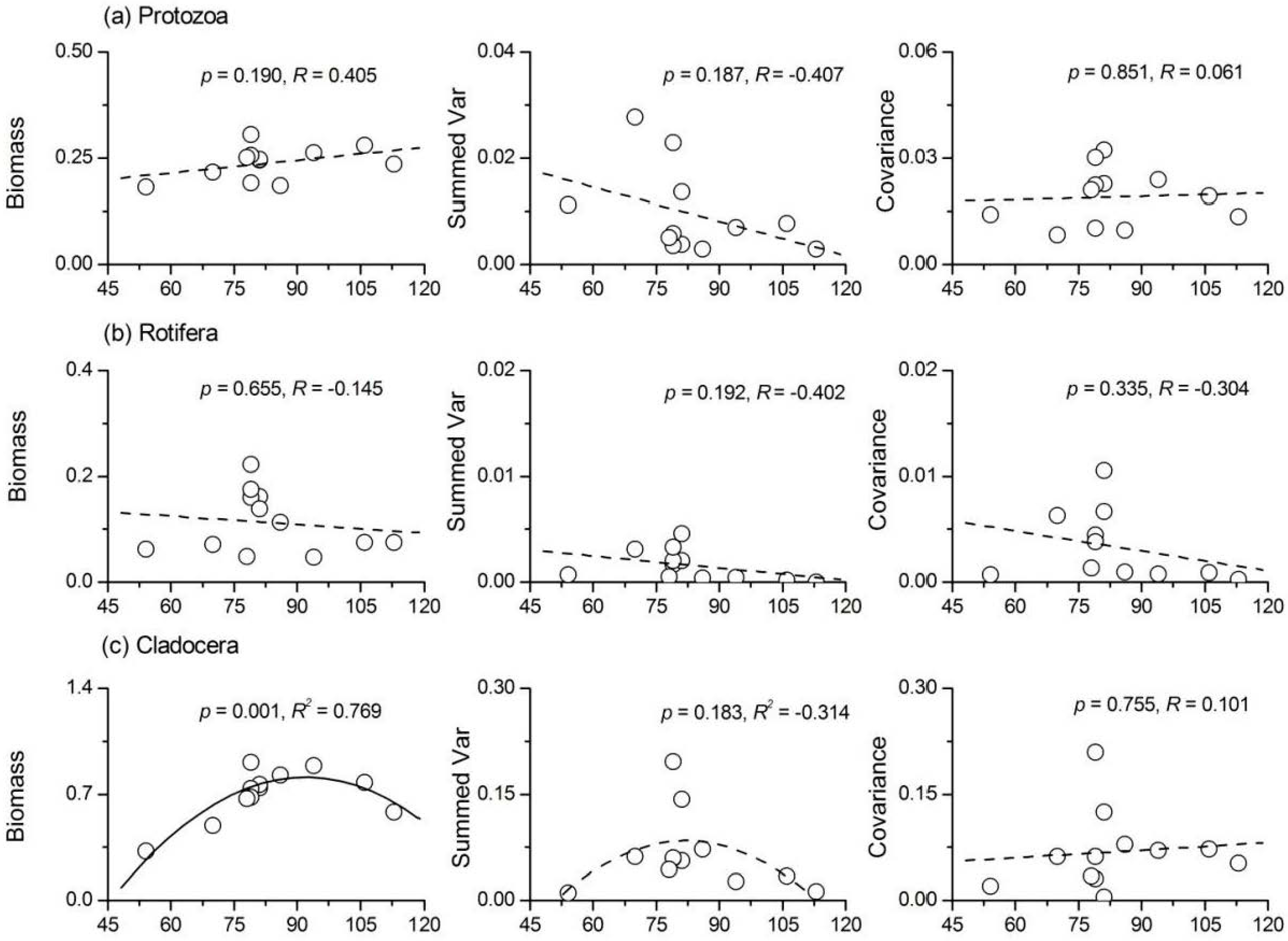
3.5. Relationship between Phytoplankton Diversity and Zooplankton Stability
4. Discussion
5. Conclusions
- (1)
- There were 138 phytoplankton and 76 zooplankton species in the lake. Phytoplankton biomass varied from 0.44 mg/L to 5.46 mg/L, and that of zooplankton ranged between 0.95 mg/L and 2.00 mg/L in different seasons.
- (2)
- There were significant differences among the mean temporal stability of different plankton taxa. Rotifera, Cladocera and copepods were the most stable taxa, followed by protozoa, and the stability of the phytoplankton taxa was the lowest.
- (3)
- There were complex relationships between species richness and temporal stability of different phytoplankton taxa: a unimodal relationship for Cyanophyta and Bacillariophyta; a strong concave relationship for Euglenophyta; and no apparent relationship for Chlorophyta and total phytoplankton. The portfolio effect was the dominant mechanism; while the effects of overyielding and species asynchrony were relatively weak.
- (4)
- Phytoplankton species richness had a significant positive influence on the temporal stability indices of protozoa, Rotifera and total zooplankton, while its influence on Cladocera and copepods was not significant. ‘Trophic overyielding’ and a weak ‘trophic portfolio effect’ were the main mechanisms involved, whereas the effect of ‘trophic species asynchrony’ was relatively weak.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Narwani, A.; Mazumder, A. Bottom-up effects of species diversity on the functioning and stability of food webs. J. Anim. Ecol. 2012, 81, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.U.; Adair, E.C.; Cardinale, B.J.; Byrnes, J.E.; Hungate, B.A.; Matulich, K.L.; Gonzalez, A.; Duffy, J.E.; Gamfeldt, L.; O’Connor, M.I. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 2012, 486, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.D.; Okabe, K.; Parrotta, J.A.; Brockerhoff, E.; Jactel, H.; Forrester, D.I.; Taki, H. Biodiversity and ecosystem services: Lessons from nature to improve management of planted forests for REDD-plus. Biodivers. Conserv. 2014, 23, 2613–2635. [Google Scholar] [CrossRef]
- Ptacnik, R.; Solimini, A.G.; Andersen, T.; Tamminen, T.; Brettum, P.; Lepistö, L.; Willén, E.; Rekolainen, S. Diversity predicts stability and resource use efficiency in natural phytoplankton communities. Proc. Natl. Acad. Sci. USA 2008, 105, 5134–5138. [Google Scholar] [CrossRef] [PubMed]
- Filstrup, C.T.; Hillebrand, H.; Heathcote, A.J.; Harpole, W.S.; Downing, J.A. Cyanobacteria dominance influences resource use efficiency and community turnover in phytoplankton and zooplankton communities. Ecol. Lett. 2014, 17, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Giller, P.S.; Hillebrand, H.; Berninger, U.G.; Gessner, M.O.; Hawkins, S.; Inchausti, P.; Inglis, C.; Leslie, H.; Malmqvist, B.; Monaghan, M.T.; et al. Biodiversity effects on ecosystem functioning: Emerging issues and their experimental test in aquatic environments. Oikos 2004, 104, 423–436. [Google Scholar] [CrossRef]
- Weyhenmeyer, G.A.; Peter, H.; Willen, E. Shifts in phytoplankton species richness and biomass along a latitudinal gradient-consequences for relationships between biodiversity and ecosystem functioning. Freshw. Biol. 2013, 58, 612–623. [Google Scholar] [CrossRef]
- Pimm, S.L. The complexity and stability of ecosystems. Nature 1984, 307, 321–326. [Google Scholar] [CrossRef]
- Ives, A.R.; Carpenter, S.R. Stability and diversity of ecosystems. Science 2007, 317, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Donohue, I.; Petchey, O.L.; Montoya, J.M.; Jackson, A.L.; McNally, L.; Viana, M.; Healy, K.; Lurgi, M.; O’Connor, N.E.; Emmerson, M.C. On the dimensionality of ecological stability. Ecol. Let. 2013, 16, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Reich, P.B.; Knops, J.M. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature 2006, 441, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D. The ecological consequences of changes in biodiversity: A search for general principles. Ecology 1999, 80, 1455–1474. [Google Scholar] [CrossRef]
- Venail, P.A.; Alexandrou, M.A.; Oakley, T.H.; Cardinale, B.J. Shared ancestry influences community stability by altering competitive interactions: Evidence from a laboratory microcosm experiment using freshwater green algae. Proc. Roy. Soc. B Biol. Sci. 2013, 280, 20131548. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, L.M.; Connolly, S.R. Understanding diversity-stability relationships: Towards a unified model of portfolio effects. Ecol. Lett. 2013, 16, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Descamps-Julien, B. Population and community variability in randomly fluctuating environments. Oikos 2004, 106, 105–116. [Google Scholar] [CrossRef]
- Sommer, U.; Gliwicz, Z.M.; Lampert, W.; Duncan, A. The PEG-model of seasonal succession of planktonic events in fresh waters. Arch. Hydrobiol. 1986, 106, 433–471. [Google Scholar]
- Litchman, E.; Klausmeier, C.A. Trait-based community ecology of phytoplankton. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 615–639. [Google Scholar] [CrossRef]
- Tilman, D.; Lehman, C.L.; Bristow, C.E. Diversity-stability relationships: Statistical inevitability or ecological consequence? Am. Nat. 1998, 9, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, B.J.; Wright, J.P.; Cadotte, M.W.; Carroll, I.T.; Hector, A.; Srivastava, D.S.; Loreau, M.; Weis, J.J. Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc. Natl. Acad. Sci. USA 2007, 104, 18123–18128. [Google Scholar] [CrossRef] [PubMed]
- Isbell, F.I.; Polley, H.W.; Wilsey, B.J. Biodiversity, productivity and the temporal stability of productivity: Patterns and processes. Ecol. Lett. 2009, 12, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Yachi, S.; Loreau, M. Biodiversity and ecosystem productivity in a fluctuating environment: The insurance hypothesis. Proc. Natl. Acad. Sci. USA 1999, 96, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Downing, A.L.; Leibold, M.A. Species richness facilitates ecosystem resilience in aquatic food webs. Freshw. Biol. 2010, 55, 2123–2137. [Google Scholar] [CrossRef]
- Tilman, D.; Downing, J.A. Biodiversity and stability in grasslands. Nature 1994, 367, 363–365. [Google Scholar] [CrossRef]
- Dodd, M.E.; Silvertown, J.; Mcconway, K.; Potts, J.; Crawley, M. Stability in the plant communities of the park grass experiment: The relationships between species richness, soil pH and biomass variability. Philos. Trans. R. Soc. B 1994, 346, 185–193. [Google Scholar] [CrossRef]
- Naeem, S.; Li, S. Biodiversity enhances ecosystem reliability. Nature 1997, 390, 507–509. [Google Scholar] [CrossRef]
- Corcoran, A.A.; Boeing, W.J. Biodiversity increases the productivity and stability of phytoplankton communities. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- McGrady-Steed, J.; Harris, P.M.; Morin, P.J. Biodiversity regulates ecosystem predictability. Nature 1997, 390, 162–165. [Google Scholar]
- Zhang, Q.G.; Zhang, D.Y. Species richness destabilizes ecosystem functioning in experimental aquatic microcosms. Oikos 2006, 112, 218–226. [Google Scholar] [CrossRef]
- Shurin, J.B.; Arnott, S.E.; Hillebrand, H.; Longmuir, A.; Pinel-Alloul, B.; Winder, M.; Yan, N.D. Diversity-stability relationship varies with latitude in zooplankton. Ecol. Lett. 2007, 10, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.E.; Cardinale, B.J.; France, K.E.; McIntyre, P.B.; Thébault, E.; Loreau, M. The functional role of biodiversity in ecosystems: Incorporating trophic complexity. Ecol. Lett. 2007, 10, 522–538. [Google Scholar] [CrossRef] [PubMed]
- Striebel, M.; Singer, G.; Stibor, H.; Andersen, T. “Trophic overyielding”: Phytoplankton diversity promotes zooplankton productivity. Ecology 2012, 93, 2719–2727. [Google Scholar] [CrossRef] [PubMed]
- Root, R.B. Organization of a plant-arthropod association in simple and diverse habitats: The fauna of collards (Brassica oleracea). Ecol. Monogr. 1973, 43, 95–124. [Google Scholar] [CrossRef]
- Leibold, M.A. Resource edibility and the effects of predators and productivity on the outcome of trophic interactions. Am. Nat. 1989, 134, 922–949. [Google Scholar] [CrossRef]
- Holt, R.D.; Loreau, M. Biodiversity and Ecosystem Functioning: The Role of Trophic Interactions and the Importance of System Openness. In The Functional Consequences of Biodiversity, Empirical Progress and Theoretical Expectations; Kinzig, A.P., Pacala, S.W., Tilman, D., Eds.; Princeton University Press: Princeton, NJ, USA, 2002; pp. 246–262. [Google Scholar]
- Hillebrand, H.; Cardinale, B.J. Consumer effects decline with prey diversity. Ecol. Let. 2004, 7, 192–201. [Google Scholar] [CrossRef]
- Ciros-Pérez, J.; Ortega-Mayagoitia, E.; Alcocer, J. The role of ecophysiological and behavioral traits in structuring the zooplankton assemblage in a deep, oligotrophic, tropical lake. Limnol. Oceanogr. 2015, 60, 2158–2172. [Google Scholar] [CrossRef]
- Shu, F.Y.; Liu, Y.P.; Zhao, Y.; Wu, Y.P.; Li, A.H. Spatio-temporal distribution of TN and TP in water and evaluation of eutrophic state of Lake Nansi. Environ. Sci. 2012, 33, 3748–3752. (In Chinese) [Google Scholar]
- Zhang, Z.L.; Xin, L.J.; Liang, C.L. The analysis of hydrological characteristics and processes of ecosystem in Lake Nansi during the past 50 years. Geogr. Res. 2007, 26, 957–966. (In Chinese) [Google Scholar]
- Gong, J.X.; Duan, D.X.; Wang, Z.Z.; Du, X.H.; Zhang, J.L.; Liu, H.C.; Chen, J.P.; Sun, D. Research and Analysis on the Plankton of Nansi Lake. J. Yangtze Univ. Nat. Sci. Ed. 2010, 7, 39–42. (In Chinese) [Google Scholar]
- Wu, Z.H.; Zhang, K.; Jin, L.R.; Yang, L.K.; Zhang, J. Analysis of spatial distribution of water quality and assessment of water quality improvement in Nansi Lake. Water Resour. Prot. 2012, 28, 1–7. (In Chinese) [Google Scholar]
- Feng, Y.W.; Hou, K.; Pei, H.Y.; Hu, W.R.; Ren, Y.; Wei, J.L. Desmid community characteristics and its relationship with environmental variables in Nansi Lake and Dongping Lake. Chin. J. Ecol. 2015, 34, 1738–1746. (In Chinese) [Google Scholar]
- Arhonditsis, G.B.; Winder, M.; Brett, M.T.; Schindler, D.E. Patterns and mechanisms of phytoplankton variability in Lake Washington (USA). Water Res. 2004, 38, 4013–4027. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Sun, J.; Liu, D.Y.; Qian, S.B. Study on phytoplankton biomass I. Phytoplankton measurement biomass from cell volume or plasma volume. Acta Oceanol. Sin. 1999, 21, 75–85. (In Chinese) [Google Scholar]
- Zhang, H.; Cui, B.; Zhang, Z.; Fan, X. Species diversity and distribution for zooplankton in the inter-tidal wetlands of the Pearl River estuary, China. Procedia Environ. Sci. 2012, 13, 2383–2393. [Google Scholar]
- O’neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Elser, J.J.; Goldman, C.R. Zooplankton effects on phytoplankton in lakes of contrasting trophic status. Limnol. Oceanogr. 1991, 36, 64–90. [Google Scholar] [CrossRef]
- Van Wichelen, J.; Van Gremberghe, I.; Vanormelingen, P.; Debeer, A.E.; Leporcq, B.; Menzel, D.; Codd, G.A.; Descy, J.P.; Vyverman, W. Strong effects of amoebae grazing on the biomass and genetic structure of a Microcystis bloom (Cyanobacteria). Environ. Microbiol. 2010, 12, 2797–2813. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.E. Biodiversity and ecosystem function: The consumer connection. Oikos 2002, 99, 201–219. [Google Scholar] [CrossRef]
- Jeppesen, E.; Jensen, J.P.; Søndergaard, M.; Lauridsen, T.; Landkildehuset, F. Trophic structure, species richness and biodiversity in Danish lakes: changes along a phosphorus gradient. Freshw. Boil. 2000, 45, 201–218. [Google Scholar] [CrossRef]
- Rodrigues, L.C.; Simões, N.R.; Bovo-Scomparin, V.M.; Jati, S.; Santana, N.F.; Roberto, M.C.; Train, S. Phytoplankton alpha diversity as an indicator of environmental changes in a neotropical floodplain. Ecol. Indic. 2015, 48, 334–341. [Google Scholar] [CrossRef]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, W.; Zhang, H.; Zhao, L.; Xu, X.; Huang, H. Plankton Community Stability and Its Relationship with Phytoplankton Species Richness in Lake Nansihu, China. Water 2016, 8, 454. https://doi.org/10.3390/w8100454
Tian W, Zhang H, Zhao L, Xu X, Huang H. Plankton Community Stability and Its Relationship with Phytoplankton Species Richness in Lake Nansihu, China. Water. 2016; 8(10):454. https://doi.org/10.3390/w8100454
Chicago/Turabian StyleTian, Wang, Huayong Zhang, Lei Zhao, Xiang Xu, and Hai Huang. 2016. "Plankton Community Stability and Its Relationship with Phytoplankton Species Richness in Lake Nansihu, China" Water 8, no. 10: 454. https://doi.org/10.3390/w8100454
APA StyleTian, W., Zhang, H., Zhao, L., Xu, X., & Huang, H. (2016). Plankton Community Stability and Its Relationship with Phytoplankton Species Richness in Lake Nansihu, China. Water, 8(10), 454. https://doi.org/10.3390/w8100454






