Synthesis of Hydroxyapatite Mulberry Stem Biochar Composites for Efficient Pb(II) Adsorption from Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MBC, HMp, and Mg0.1-HMp
2.3. Characterization
2.4. Batch Experiments
2.5. Regeneration and Recycling of Adsorbents
3. Results and Discussion
3.1. Characterization of HMp and Mg0.1-HMp
3.1.1. FT-IR Analysis
3.1.2. XRD Analysis
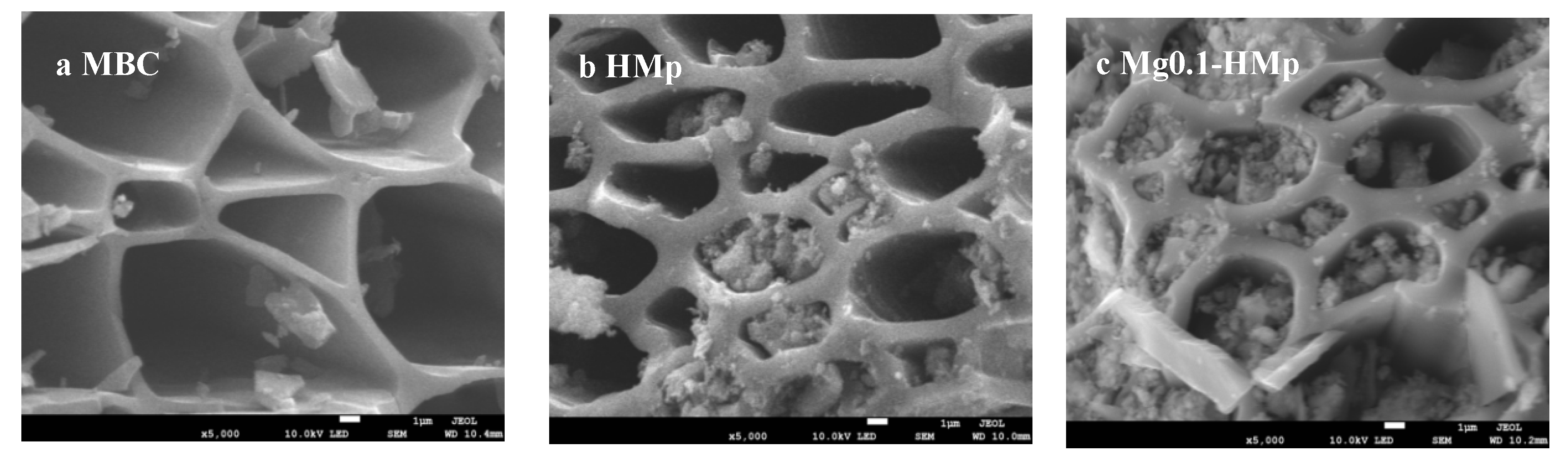
3.1.3. SEM-EDS Analysis
Analysis of Specific Surface Area
3.2. Effects of Reaction Conditions on Pb(II) Adsorption by HMp and Mg0.1-HMp
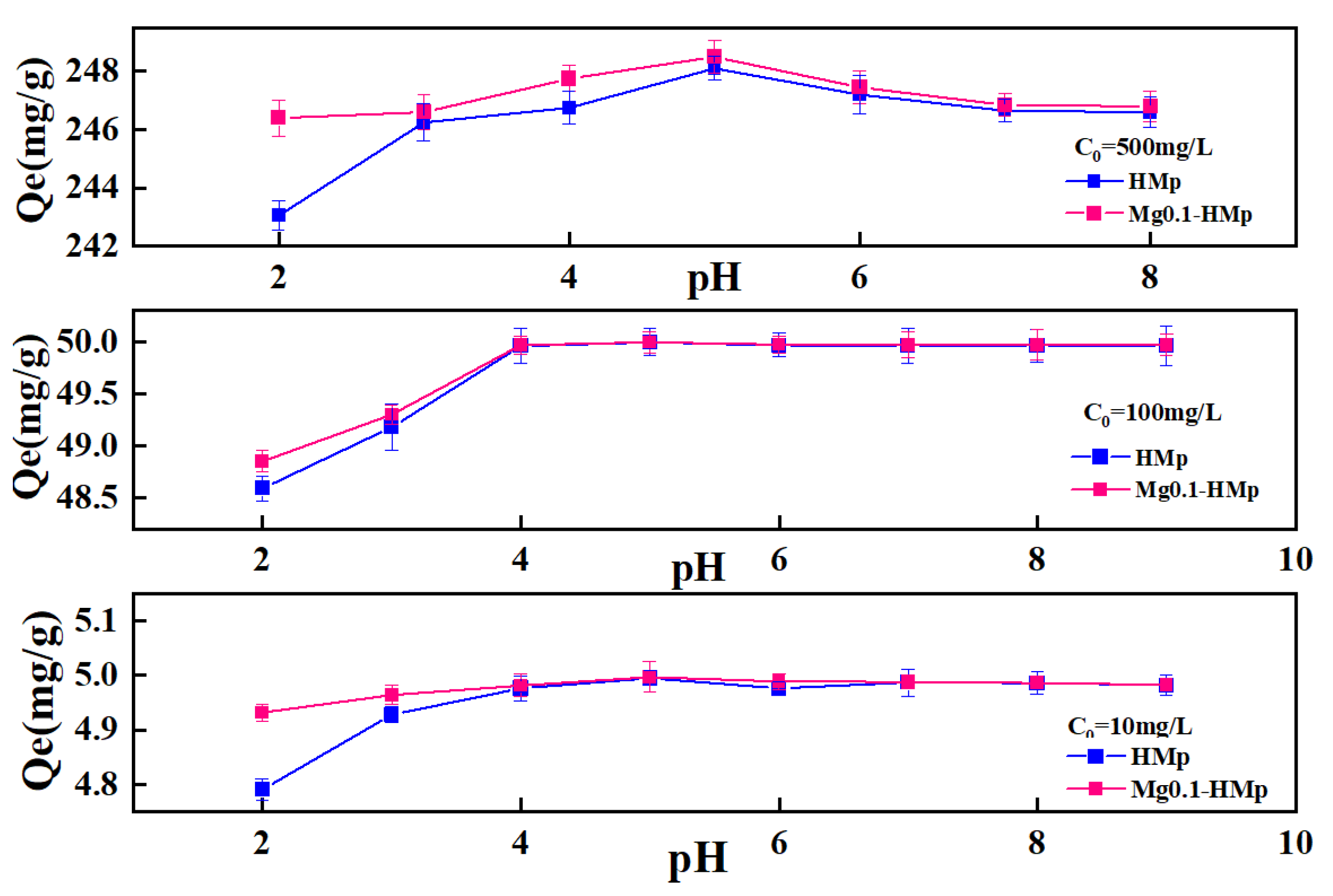
3.2.1. Effect of pH
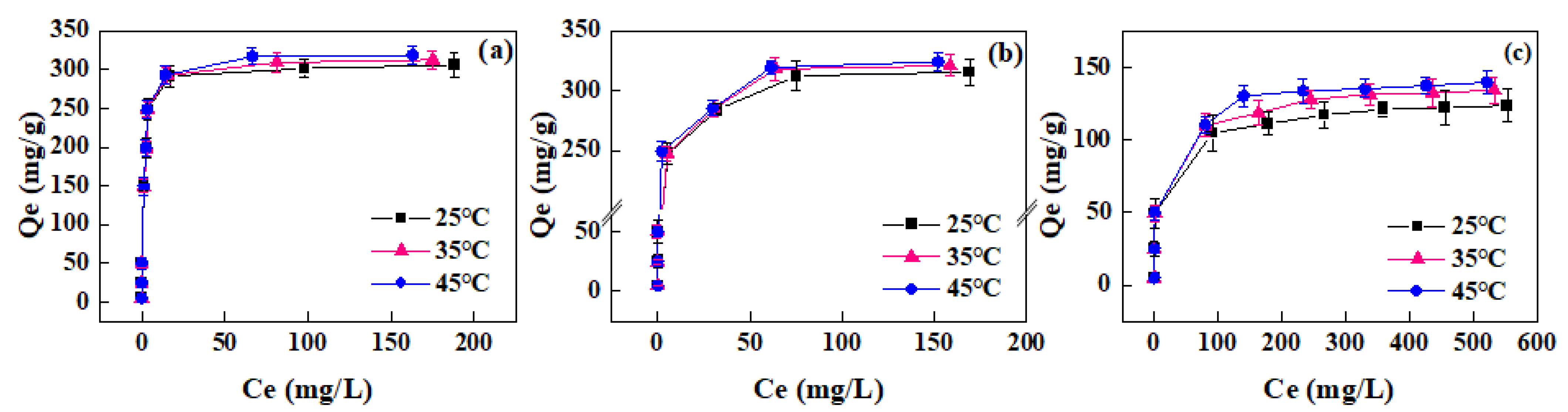
3.2.2. Adsorption Isotherms
3.2.3. Adsorption Kinetics
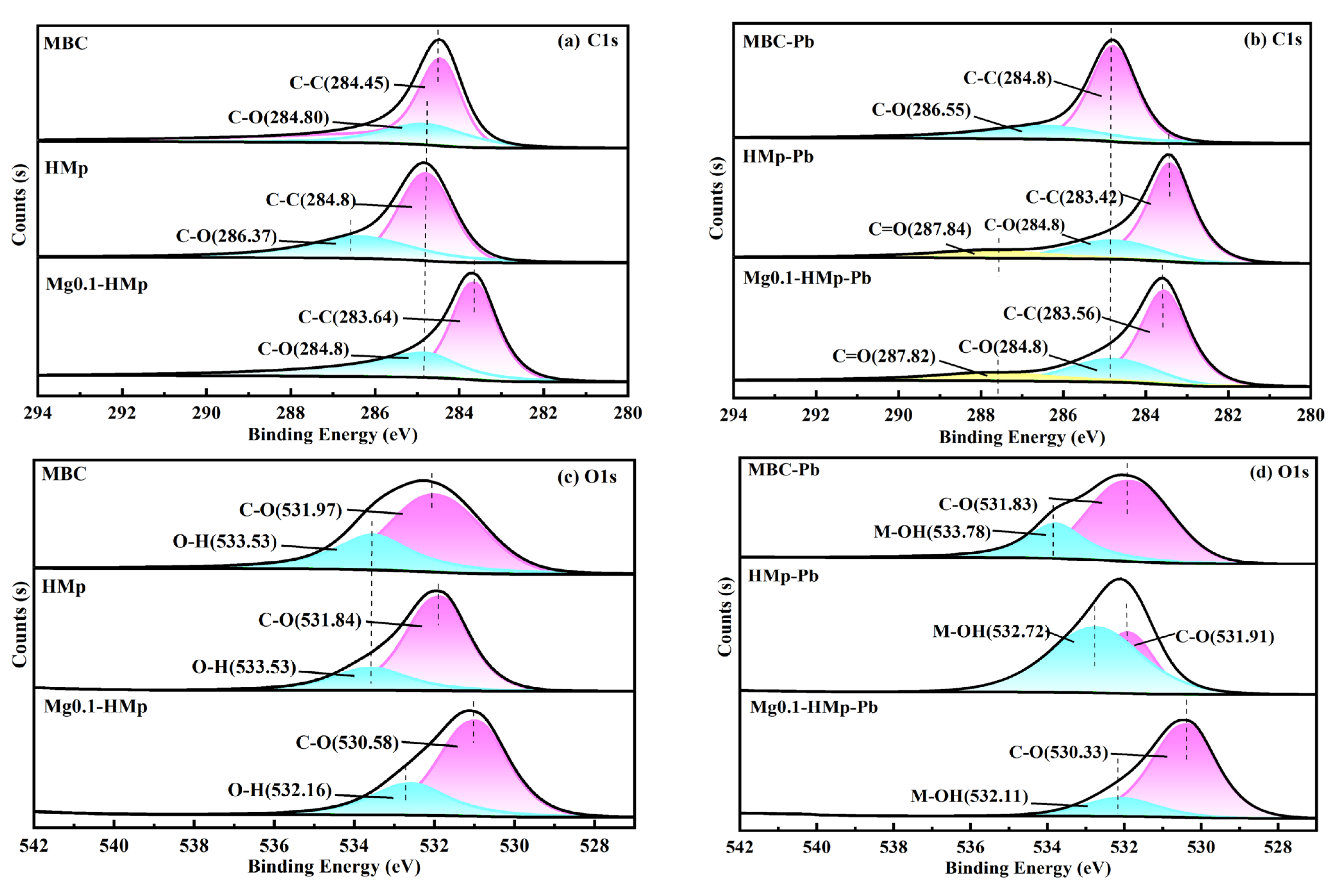
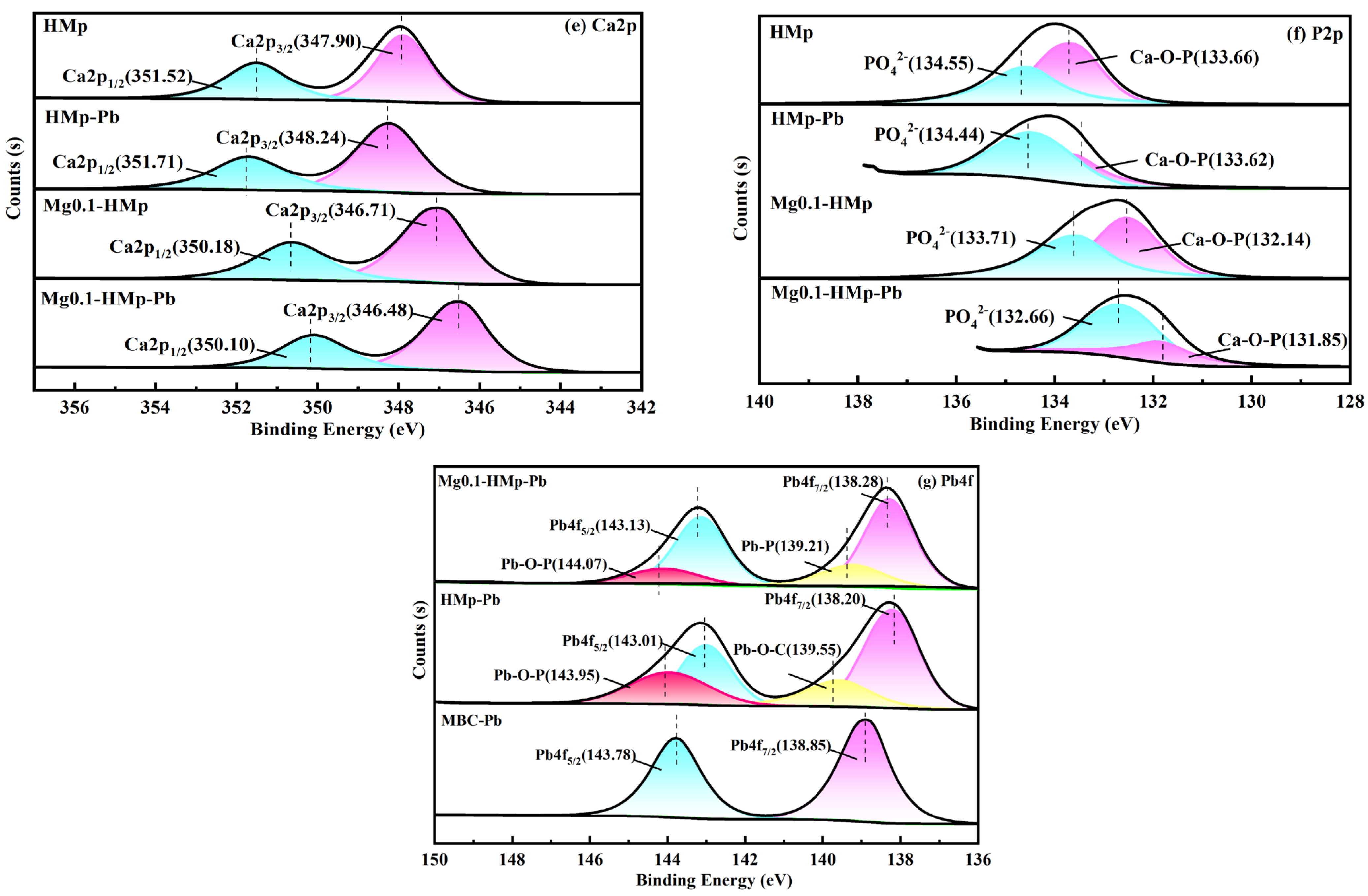
3.2.4. Possible Mechanisms Pb(II) Adsorption onto HMp and Mg0.1-HMp
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Beiyuan, J.; Awad, Y.M.; Beckers, F.; Tsang, D.C.W.; Ok, Y.S.; Rinklebe, J. Mobility and phytoavailability of As and Pb in a contaminated soil using pine sawdust biochar under systematic change of redox conditions. Chemosphere 2017, 178, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Lapwanit, S.; Trakulsujaritchok, T.; Nongkhai, P.N. Chelating magnetic copolymer composite modified by click reaction for removal of heavy metal ions from aqueous solution. Chem. Eng. J. 2016, 289, 286–295. [Google Scholar] [CrossRef]
- Ma, Y.-X.; Kou, Y.-L.; Xing, D.; Jin, P.-S.; Shao, W.-J.; Li, X.; Du, X.-Y.; La, P.-Q. Synthesis of magnetic graphene oxide grafted polymaleicamide dendrimer nanohybrids for adsorption of Pb(II) in aqueous solution. J. Hazard. Mater. 2017, 340, 407–416. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; van der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468–469, 843–853. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, Z.; Zhang, X.; Chen, X.; Yue, D.; Yin, Q.; Xiao, L.; Yang, L. Biochar from Alternanthera philoxeroides could remove Pb(II) efficiently. Bioresour. Technol. 2014, 171, 227–232. [Google Scholar] [CrossRef]
- Cruz-Olivares, J.; Pérez-Alonso, C.; Barrera-Díaz, C.; Ureña-Nuñez, F.; Chaparro-Mercado, M.C.; Bilyeu, B. Modeling of lead (II) biosorption by residue of allspice in a fixed-bed column. Chem. Eng. J. 2013, 228, 21–27. [Google Scholar] [CrossRef]
- Saleh, T.A.; Mustaqeem, M.; Khaled, M. Water treatment technologies in removing heavy metal ions from wastewater: A review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100617. [Google Scholar] [CrossRef]
- Krishnan, S.; Zulkapli, N.S.; Kamyab, H.; Taib, S.M.; Din, M.F.B.M.; Majid, Z.A.; Chaiprapat, S.; Kenzo, I.; Ichikawa, Y.; Nasrullah, M.; et al. Current technologies for recovery of metals from industrial wastes: An overview. Environ. Technol. Innov. 2021, 22, 101525. [Google Scholar] [CrossRef]
- Ramirez-Muñoz, A.; Pérez, S.; Muñoz-Saldaña, J.; Flórez, E.; Acelas, N. Eco-friendly materials obtained through a simple thermal transformation of water hyacinth (Eichhornia crassipes) for the removal and immobilization of Cd2+ and Cu2+ from aqueous solutions. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100574. [Google Scholar] [CrossRef]
- Xu, Z.; Lin, Y.; Lin, Y.; Yang, D.; Zheng, H. Adsorption behaviors of paper mill sludge biochar to remove Cu, Zn and As in wastewater. Environ. Technol. Innov. 2021, 23, 101616. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Amarasinghe, H.A.H.I.; Gunathilake, S.K.; Karunarathna, A.K. Ascertaining of Optimum Pyrolysis Conditions in Producing Refuse Tea Biochar as a Soil Amendment. Procedia Food Sci. 2016, 6, 97–102. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Denyes, M.J.; Langlois, V.S.; Rutter, A.; Zeeb, B.A. The use of biochar to reduce soil PCB bioavailability to Cucurbita pepo and Eisenia fetida. Sci. Total Environ. 2012, 437, 76–82. [Google Scholar] [CrossRef]
- Tan, M.; Li, Y.; Chi, D.; Wu, Q. Efficient removal of ammonium in aqueous solution by ultrasonic magnesium-modified biochar. Chem. Eng. J. 2023, 461, 142072. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, P.; Yang, Q.; Chen, L.; Wang, W.; Deng, W.; Wang, Y. Analysis of the Cd(II) Adsorption Performance and Mechanisms by Soybean Root Biochar: Effect of Pyrolysis Temperatures. Bull. Environ. Contam. Toxicol. 2021, 107, 553–558. [Google Scholar] [CrossRef]
- Thi Hanh, N.; Thi Huong, P.; Hong Tham Nguyen, T.; Thi Nham, N.; Minh-Viet, N.; Trinh Tran, D.; Minh Phuong, N.; Trung Quang, D.; Thao, P.; Thu Trang, H.; et al. Synthesis of Iron-Modified Biochar Derived from Rice Straw and Its Application to Arsenic Removal. J. Chem. 2019, 2019, 5295610. [Google Scholar] [CrossRef]
- Liang, M.; Xu, S.; Zhu, Y.; Chen, X.; Deng, Z.; Yan, L.; He, H. Preparation and Characterization of Fe-Mn Binary Oxide/Mulberry Stem Biochar Composite Adsorbent and Adsorption of Cr(VI) from Aqueous Solution. Int. J. Environ. Res. Public Health 2020, 17, 676. [Google Scholar] [CrossRef]
- Irfan, M.; Ishaq, F.; Muhammad, D.; Khan, M.J.; Mian, I.A.; Dawar, K.M.; Muhammad, A.; Ahmad, M.; Anwar, S.; Ali, S.; et al. Effect of wheat straw derived biochar on the bioavailability of Pb, Cd and Cr using maize as test crop. J. Saudi Chem. Soc. 2021, 25, 101232. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, Y.; Zheng, Y.; Yang, Y.; Huang, J.; Chen, H.; Quan, G.; Gao, B. Potassium permanganate modification of hydrochar enhances sorption of Pb(II), Cu(II), and Cd(II). Bioresour. Technol. 2023, 386, 129482. [Google Scholar] [CrossRef]
- Somyanonthanakun, W.; Ahmed, R.; Krongtong, V.; Thongmee, S. Studies on the adsorption of Pb(II) from aqueous solutions using sugarcane bagasse-based modified activated carbon with nitric acid: Kinetic, isotherm and desorption. Chem. Phys. Impact 2023, 6, 100181. [Google Scholar] [CrossRef]
- Zhang, N.; Reguyal, F.; Praneeth, S.; Sarmah, A.K. A novel green synthesized magnetic biochar from white tea residue for the removal of Pb(II) and Cd(II) from aqueous solution: Regeneration and sorption mechanism. Environ. Pollut. 2023, 330, 121806. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-H.; Chen, Y.-d.; Yang, Z.-k.; Nagarajan, D.; Chang, J.-S.; Ren, N.-q. High-efficiency removal of lead from wastewater by biochar derived from anaerobic digestion sludge. Bioresour. Technol. 2017, 246, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Pan, J.; Chen, Y.; Cheng, R.; Xu, X. Study the adsorption of phenol from aqueous solution on hydroxyapatite nanopowders. J. Hazard. Mater. 2009, 161, 231–240. [Google Scholar] [CrossRef]
- Abdelmegeed, A.F.; Sayed, M.; Abbas, M.; Abdel Moniem, S.M.; Farag, R.S.; Sayed, A.Z.; Naga, S.M. Hydroxyapatite-magnetite nanocomposites: Synthesis and superior adsorption properties for lead ion removal, with insights into intraparticle diffusion, kinetic modeling, and phase dependency. Ceram. Int. 2024, 50, 36074–36087. [Google Scholar] [CrossRef]
- Biedrzycka, A.; Skwarek, E. Composites of hydroxyapatite and their application in adsorption, medicine and as catalysts. Adv. Colloid Interface Sci. 2024, 334, 103308. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, C.; Zhao, B.; Di, D.; Xiao, J.; Chen, G. One-pot synthesis of high-efficiency hydroxyapatite-hydrochar composites derived from phytoremediation biomass and bone meal: Heavy metal stabilization, adsorption performance and mechanism. J. Alloys Compd. 2025, 1022, 179859. [Google Scholar] [CrossRef]
- Huang, J.; Shen, X.; Lin, J.; Zhou, J.; Li, Y. Synergistic heavy metal sequestration from acidic metallurgical wastewater: Unraveling critical chemical bond-mediated adsorption mechanisms in vanadium slag-derived hydroxyapatite. J. Water Process Eng. 2025, 72, 107629. [Google Scholar] [CrossRef]
- Miyah, Y.; El Messaoudi, N.; Benjelloun, M.; Acikbas, Y.; Şenol, Z.M.; Ciğeroğlu, Z.; Lopez-Maldonado, E.A. Advanced applications of hydroxyapatite nanocomposite materials for heavy metals and organic pollutants removal by adsorption and photocatalytic degradation: A review. Chemosphere 2024, 358, 142236. [Google Scholar] [CrossRef]
- Pawar, S.; Theodore, T. Synthesis, characterization of hydroxyapatite from pomegranate fruit peel for Cr (VI) adsorption: Process modelling, kinetic and isotherm studies. Heliyon 2024, 10, e37540. [Google Scholar] [CrossRef]
- Wu, W.; Liu, Z.; Azeem, M.; Guo, Z.; Li, R.; Li, Y.; Peng, Y.; Ali, E.F.; Wang, H.; Wang, S.; et al. Hydroxyapatite tailored hierarchical porous biochar composite immobilized Cd(II) and Pb(II) and mitigated their hazardous effects in contaminated water and soil. J. Hazard. Mater. 2022, 437, 129330. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Sato, T. Removal of aqueous lead by poorly-crystalline hydroxyapatites. Chemosphere 2007, 69, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wu, J.; Wang, J.; Xu, M.; Zhou, W.; Du, Y.; Li, Y.; Li, H. Fabricating hydroxyapatite functionalized biochar composite using steel slag and Hami melon peel for Pb(II) and Cd(II) removal. Colloids Surf. A Physicochem. Eng. Asp. 2023, 666, 131310. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M.; Chen, T.; Tan, Y.; Yang, J. Safe utilization of heavy-metal-contaminated farmland by mulberry tree cultivation and silk production. Sci. Total Environ. 2017, 599–600, 1867–1873. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, H.; Ali, E.; Shahab, A.; Huang, H.; Ullah, H.; Zeng, H. Synthesis of novel magnetic activated carbon for effective Cr(VI) removal via synergistic adsorption and chemical reduction. Environ. Technol. Innov. 2023, 30, 103092. [Google Scholar] [CrossRef]
- Ramos Guivar, J.A.; Sanches, E.A.; Bruns, F.; Sadrollahi, E.; Morales, M.A.; López, E.O.; Litterst, F.J. Vacancy ordered γ-Fe2O3 nanoparticles functionalized with nanohydroxyapatite: XRD, FTIR, TEM, XPS and Mössbauer studies. Appl. Surf. Sci. 2016, 389, 721–734. [Google Scholar] [CrossRef]
- Ramya, J.R.; Arul, K.T.; Elayaraja, K.; Kalkura, S.N. Physicochemical and biological properties of iron and zinc ions co-doped nanocrystalline hydroxyapatite, synthesized by ultrasonication. Ceram. Int. 2014, 40, 16707–16717. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Q.; Masse, S.; Zhang, H.; Li, L.; Coradin, T. Hierarchically-organized, well-dispersed hydroxyapatite-coated magnetic carbon with combined organics and inorganics removal properties. Chem. Eng. J. 2015, 275, 152–159. [Google Scholar] [CrossRef]
- Wei, Q.; Lu, J.; Ai, H.; Jiang, B. Novel method for the fabrication of multiscale structure collagen/hydroxyapatite-microsphere composites based on CaCO3 microparticle templates. Mater. Lett. 2012, 80, 91–94. [Google Scholar] [CrossRef]
- Tongamp, W.; Zhang, Q.; Saito, F. Preparation of meixnerite (Mg-Al-OH) type layered double hydroxide by a mechanochemical route. J. Mater. Sci. 2007, 42, 9210–9215. [Google Scholar] [CrossRef]
- Kumbhar, P.; Narale, D.; Bhosale, R.; Jambhale, C.; Kim, J.-H.; Kolekar, S. Synthesis of tea waste/Fe3O4 magnetic composite (TWMC) for efficient adsorption of crystal violet dye: Isotherm, kinetic and thermodynamic studies. J. Environ. Chem. Eng. 2022, 10, 107893. [Google Scholar] [CrossRef]
- Wang, H.; Yan, K.; Xing, H.; Chen, J.; Lu, R. Effective removal of Cu2+ from aqueous solution by synthetic abalone shell hydroxyapatite microspheres adsorbent. Environ. Technol. Innov. 2021, 23, 101663. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, Y.; Fan, Y.; Zhang, L.; Tang, S.; Zhu, Y.; Zhou, X. Strontium-doped hydroxyapatite as an efficient adsorbent for Cd(II) removal from wastewater: Performance, kinetics, and mechanism. Environ. Technol. Innov. 2022, 28, 102575. [Google Scholar] [CrossRef]
- Salehi, E.; Madaeni, S.S.; Vatanpour, V. Thermodynamic investigation and mathematical modeling of ion-imprinted membrane adsorption. J. Membr. Sci. 2012, 389, 334–342. [Google Scholar] [CrossRef]
- Ohtsu, N.; Hiromoto, S.; Yamane, M.; Satoh, K.; Tomozawa, M. Chemical and crystallographic characterizations of hydroxyapatite- and octacalcium phosphate-coatings on magnesium synthesized by chemical solution deposition using XPS and XRD. Surf. Coat. Technol. 2013, 218, 114–118. [Google Scholar] [CrossRef]
- Parmar, A.; Nema, P.K.; Agarwal, T. Biochar production from agro-food industry residues: A sustainable approach for soil and environmental management. Curr. Sci. 2014, 107, 1673–1682. [Google Scholar]
- Lian, F.; Xing, B. Black Carbon (Biochar) In Water/Soil Environments: Molecular Structure, Sorption, Stability, and Potential Risk. Environ. Sci. Technol. 2017, 51, 13517–13532. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Li, Y.; Liu, T.; Ma, X.; Han, X.; Wang, Y. Aged biochar for simultaneous removal of Pb and Cd from aqueous solutions: Method and mechanism. Environ. Technol. Innov. 2023, 32, 103368. [Google Scholar] [CrossRef]
- Kumari, A.R.; Sobha, K. Removal of lead by adsorption with the renewable biopolymer composite of feather (Dromaius novaehollandiae) and chitosan (Agaricus bisporus). Environ. Technol. Innov. 2016, 6, 11–26. [Google Scholar] [CrossRef]
- Ain, Q.U.; Zhang, H.; Yaseen, M.; Rasheed, U.; Liu, K.; Subhan, S.; Tong, Z. Facile fabrication of hydroxyapatite-magnetite-bentonite composite for efficient adsorption of Pb(II), Cd(II), and crystal violet from aqueous solution. J. Clean. Prod. 2020, 247, 119088. [Google Scholar] [CrossRef]
- Mustapha, L.S.; Jacob-Oricha, S.O.; Yahya, M.D.; Lau, S.Y.; Yusuff, A.S.; Obayomi, K.S. Effective removal of Cr(VI) and Pb(II) ions from mining wastewater using eco-friendly synthesized magnesium oxide nanoparticles incorporated rice husk ash. Environ. Adv. 2024, 15, 100507. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, Z.; Yan, B.; Zhao, L.; Chen, T.; Yang, X. Effects of active silicon amendment on Pb(II)/Cd(II) adsorption: Performance evaluation and mechanism. J. Hazard. Mater. 2024, 478, 135614. [Google Scholar] [CrossRef] [PubMed]
- Gode, F.; Pehlivan, E. Removal of Cr(VI) from aqueous solution by two Lewatit-anion exchange resins. J. Hazard. Mater. 2005, 119, 175–182. [Google Scholar] [CrossRef]
- Zahedifar, M.; Seyedi, N.; Shafiei, S.; Basij, M. Surface-modified magnetic biochar: Highly efficient adsorbents for removal of Pb(11) and Cd(11). Mater. Chem. Phys. 2021, 271, 124860. [Google Scholar] [CrossRef]
- Yin, H.; Wang, B.; Zhang, M.; Zhang, F. Adsorption of Pb(II) in water by modified chitosan-based microspheres and the study of mechanism. Int. J. Biol. Macromol. 2024, 277, 134062. [Google Scholar] [CrossRef]
- Liao, J.; He, X.; Zhang, Y.; Zhang, L.; He, Z. The construction of magnetic hydroxyapatite-functionalized pig manure-derived biochar for the efficient uranium separation. Chem. Eng. J. 2023, 457, 141367. [Google Scholar] [CrossRef]
- Faheem; Yu, H.; Liu, J.; Shen, J.; Sun, X.; Li, J.; Wang, L. Preparation of MnOx-loaded biochar for Pb2+ removal: Adsorption performance and possible mechanism. J. Taiwan Inst. Chem. Eng. 2016, 66, 313–320. [Google Scholar] [CrossRef]
- Feng, G.; Zheng, E.; Jiang, F.; Hu, Z.; Fu, H.; Li, Y.; Meng, H.; Wu, Q.; Liu, J.; Yang, Q.; et al. Preparation of novel porous hydroxyapatite sheets with high Pb2+ adsorption properties by self-assembly non-aqueous precipitation method. Ceram. Int. 2023, 49, 30603–30612. [Google Scholar] [CrossRef]
- Mobasherpour, I.; Salahi, E.; Pazouki, M. Comparative of the removal of Pb2+, Cd2+ and Ni2+ by nano crystallite hydroxyapatite from aqueous solutions: Adsorption isotherm study. Arab. J. Chem. 2012, 5, 439–446. [Google Scholar] [CrossRef]
- Li, R.; Liang, W.; Wang, J.J.; Gaston, L.A.; Huang, D.; Huang, H.; Lei, S.; Awasthi, M.K.; Zhou, B.; Xiao, R.; et al. Facilitative capture of As(V), Pb(II) and methylene blue from aqueous solutions with MgO hybrid sponge-like carbonaceous composite derived from sugarcane leafy trash. J. Environ. Manag. 2018, 212, 77–87. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, H.; Hu, Y.; Hu, L.; Wei, Z.; Li, Y.Y.; Hu, X. Mn oxide-modified biochars with high adsorption capacity for Pb(II) in wastewater: Preparation and adsorption mechanisms. Environ. Res. 2025, 266, 120553. [Google Scholar] [CrossRef]




| Sample | SBET/(m2/g) | Vtotal/(cm3/g) | DBET/nm |
|---|---|---|---|
| MBC | 233.78 | 0.210 | 9.729 |
| HMp | 257.88 | 0.529 | 8.697 |
| Mg0.1-HMp | 316.324 | 0.551 | 7.549 |
| Sample | Temperature (°C) | Langmuir Equation | Freundlich Equation | ||||
|---|---|---|---|---|---|---|---|
| Qm(mg/g) | KL(L/mg) | R2 | KF(L/mg) | 1/n | R2 | ||
| MBC | 25 | 123.46 | 0.1321 | 0.9986 | 26.929 | 0.2692 | 0.8782 |
| 35 | 135.14 | 0.1276 | 0.9984 | 28.580 | 0.2750 | 0.8827 | |
| 45 | 138.89 | 0.1478 | 0.9987 | 30.009 | 0.2758 | 0.8820 | |
| HMp | 25 | 303.03 | 1.3750 | 0.9999 | 104.910 | 0.3126 | 0.8796 |
| 35 | 312.50 | 1.3333 | 0.9999 | 108.581 | 0.3149 | 0.8803 | |
| 45 | 322.58 | 1.4091 | 0.9999 | 113.466 | 0.3143 | 0.8785 | |
| Mg0.1-HMp | 25 | 312.50 | 0.9412 | 0.9996 | 34.522 | 0.4708 | 0.9425 |
| 35 | 322.58 | 0.9118 | 0.9995 | 37.604 | 0.4781 | 0.9438 | |
| 45 | 322.58 | 0.7949 | 0.9967 | 41.022 | 0.4876 | 0.9448 | |
| Initial Pb Concentration (mg/L) | Pseudo-First-Order-Kinetics | Pseudo-Second-Order Kinetics | ||||||
|---|---|---|---|---|---|---|---|---|
| R2 | K1 (1/min) | Qmax (mg/g) | R2 | K2 (g∙mg/min) | Qmax (mg/g) | |||
| 10 | 0.8762 | 0.0021 | 4.9945 | 1.0000 | 0.0688 | 5 | ||
| 100 | 0.9693 | 0.0022 | 49.9955 | 1.0000 | 0.0800 | 50 | ||
| 500 | 0.9362 | 0.0023 | 248.483 | 1.0000 | 0.0013 | 250 | ||
| Initial Pb Concentration (mg/L) | Bangham kinetics model | Elovich kinetics model | ||||||
| R2 | K3 (g∙mg/min) | R2 | K4 (g∙mg/min) | |||||
| 10 | 0.8886 | 0.09 | 0.6568 | 0.0086 | ||||
| 100 | 0.8796 | 0.05 | 0.6420 | 0.0048 | ||||
| 500 | 0.9726 | 1.50 | 0.8706 | 0.1538 | ||||
| Initial Pb Concentration (mg/L) | Pseudo-First-Order Kinetics | Pseudo-Second-Order Kinetics | ||||||
|---|---|---|---|---|---|---|---|---|
| R2 | K1 (1/min) | Qmax (mg/g) | R2 | K2 (g∙mg/min) | Qmax (mg/g) | |||
| 10 | 0.8884 | 0.0015 | 4.996 | 1.0000 | 0.0833 | 5 | ||
| 100 | 0.9798 | 0.0018 | 49.997 | 1.0000 | 0.0434 | 50 | ||
| 500 | 0.9092 | 0.0031 | 248.62 | 1.0000 | 0.0011 | 250 | ||
| Initial Pb Concentration (mg/L) | Bangham kinetics model | Elovich kinetics model | ||||||
| R2 | K3 (g∙mg/min) | R2 | K4 (g∙mg/min) | |||||
| 10 | 0.9046 | 0.010 | 0.6230 | 0.0060 | ||||
| 100 | 0.9784 | 0.001 | 0.8873 | 0.0052 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Zhou, X.; Liang, M.; Wu, Z. Synthesis of Hydroxyapatite Mulberry Stem Biochar Composites for Efficient Pb(II) Adsorption from Aqueous Solutions. Water 2025, 17, 1389. https://doi.org/10.3390/w17091389
Wang D, Zhou X, Liang M, Wu Z. Synthesis of Hydroxyapatite Mulberry Stem Biochar Composites for Efficient Pb(II) Adsorption from Aqueous Solutions. Water. 2025; 17(9):1389. https://doi.org/10.3390/w17091389
Chicago/Turabian StyleWang, Dunqiu, Xinyu Zhou, Meina Liang, and Zimeng Wu. 2025. "Synthesis of Hydroxyapatite Mulberry Stem Biochar Composites for Efficient Pb(II) Adsorption from Aqueous Solutions" Water 17, no. 9: 1389. https://doi.org/10.3390/w17091389
APA StyleWang, D., Zhou, X., Liang, M., & Wu, Z. (2025). Synthesis of Hydroxyapatite Mulberry Stem Biochar Composites for Efficient Pb(II) Adsorption from Aqueous Solutions. Water, 17(9), 1389. https://doi.org/10.3390/w17091389






