Abstract
Textile dyeing wastewater is one of the most challenging industrial effluents to treat due to its high concentrations of persistent organic compounds and nitrogenous substances. Conventional treatment methods often fall short in achieving both sufficient removal efficiency and environmental safety. In this study, we aimed to remove the total nitrogen (T-N) and total organic carbon (TOC) of dyeing wastewater from an industrial complex in D City, Korea, by applying bipolar and packed bipolar electrolysis using aluminum (Al) electrodes and activated carbon (AC). The system was operated for 60 min under varying conditions of applied voltage (5–15 V), electrolyte type and concentration (non-addition, NaCl 5 mM, NaCl 10 mM, Na2SO4 5 mM, Na2SO4 10 mM), and AC packing amount (non-addition or 100 g/L). The highest T-N and TOC removal efficiencies were observed at 15 V, reaching 69.53% and 63.68%, respectively. Electrolyte addition significantly improved initial treatment performance, with NaCl 10 mM showing the best results. However, Al leaching also increased, from 549.83 mg/L (non-addition) to 623.06 mg/L (NaCl 10 mM). When AC was used without electrolysis (control experiment), the T-N and TOC removal efficiencies were limited to 30.24% and 29.86%, respectively. In contrast, AC packing combined with 15 V electrolysis under non-addition achieved 86.04% T-N and 77.98% TOC removal, while also reducing Al leaching by 40.12%. These results suggested that electrochemical treatment with AC packing under non-addition conditions offers the best balance between high treatment efficiency and low environmental impact. These findings demonstrate that the synergistic use of packed activated carbon and electrochemical treatment under additive-free conditions can overcome the limitations of conventional methods. This study contributes to the development of more sustainable and effective technologies for treating high-strength industrial wastewater.
Keywords:
dyeing wastewater; electrolysis system; T-N; TOC; aluminum; voltage; electrolyte; activated carbon 1. Introduction
Today, color functions not merely as a visual element but also as a medium for expressing personal identity and individuality. In everyday consumer goods such as clothing and furniture, design and color trends continuously evolve, stimulating consumer demand and consequently driving increased product manufacturing. As production increases, the use of dyeing processes expands, leading to the generation of dyeing wastewater, which poses significant environmental concerns. According to KOSIS [1], as of 2021, 1412 out of 56,430 industrial facilities across Korea discharged dyeing wastewater, accounting for a daily discharge volume of 283,009 m3/day out of the total 5,005,861 m3/day. This indicates that the dyeing facilities produce disproportionately high volumes of wastewater relative to their number. Furthermore, the European Parliament reported that approximately 20% of the world’s clean water is polluted by dyeing wastewater from textile production and that approximately 87.00% of clothing is either incinerated or landfilled, thereby causing additional environmental problems [2].
Wastewater containing dyes typically exhibits high color intensity, elevated pH, chemical oxygen demand (COD), biochemical oxygen demand (BOD), suspended solids (SS), and salinity. Even within the same dyeing process, these characteristics can vary significantly due to external factors such as seasonal changes [3]. Among the various types of dyes, azo dyes are particularly prevalent, accounting for approximately 60% of all dyes used worldwide [4]. The discharge of highly colored effluents not only causes visual pollution but also inhibits the growth of aquatic plants. Moreover, harmful substances contained in effluents can accumulate in aquatic organisms or crops grown in contaminated soils, ultimately entering the human body through the food chain and posing potential health risks [5]. Due to these concerns, dyeing wastewater remains an unresolved environmental issue, and ongoing research continues to focus on developing more effective treatment technologies.
Various treatment methods for dyeing wastewater have been studied, most of which fall into three main categories: physical, chemical, and biological [6]. Physical treatment methods primarily include membrane separation, dissolved air flotation (DAF), and adsorption. According to [7], when treating textile wastewater using nanofiltration under membrane processes, a removal efficiency of 57% for COD and 100% for color was achieved at 10 bar and 40 °C, demonstrating high performance and spatial efficiency. However, challenges such as membrane fouling, cost of pretreatment, and high energy requirements remain, necessitating further research for large-scale applications [8].
DAF processes have also shown high efficiency in color removal. As reported in [9], the removal efficiencies of reactive dyes using DAF reached 97.00% for Reactive Red, 96.00% for Reactive Blue, and 92.00% for Reactive Yellow, with advantages such as short processing time and compact system footprint. However, due to its low COD removal efficiency, DAF is often applied in combination with other processes or at the pretreatment stage.
Chemical treatment methods include the Fenton process, coagulation–flocculation, and photocatalytic oxidation. According to [10], the combination of Fenton oxidation and ion exchange achieved COD removal exceeding 45.00% at an FeSO4 concentration of 100 mg/L. These methods are generally simple and effective for removing refractory organic compounds. However, maintaining low-pH conditions for optimal reactions can be problematic when treating high-pH dyeing wastewater because it requires substantial amounts of chemical reagents, raising concerns over treatment costs and potential secondary pollution.
Biological treatment methods are typically classified into aerobic and anaerobic processes. As noted in [11], biological treatment is more cost-effective and environmentally friendly than physicochemical methods. However, it faces difficulties in handling high concentrations of pollutants and recalcitrant compounds, and the overall treatment time is relatively long [11,12].
In contrast, electrochemical treatment offers a more controllable and rapid alternative, with proven effectiveness in removing both refractory organic and nitrogenous compounds without the need for excessive chemical additives or prolonged retention times [13]. The electrochemical treatment method applied in this study removes pollutants through a combination of electro-oxidation, coagulation, flotation, surface adsorption, and chemical interactions, all of which are induced by applying an electric current to the dyeing wastewater containing electrolyte. Electrodes used in electrochemical processes are generally classified as either soluble or insoluble and are further categorized based on their material composition [14]. Among them, soluble electrodes, such as Al and iron (Fe) are widely used in electrocoagulation processes and have shown higher effectiveness in color removal [15].
According to [16], under conditions of pH 5.5 and a current density of 15 mA/cm2, operating an electrochemical reactor equipped with Al electrodes for 23 min resulted in 97.00% color removal and 40.01% COD removal. These findings underscore the benefits of soluble electrodes, including ease of operation and rapid achievement of high removal efficiencies. However, the repeated use of soluble electrodes leads to their gradual consumption, and the dissolution of electrode material may cause residual metal ions to remain in the treated water, potentially posing challenges for downstream processes [17,18]. Therefore, it is essential to identify optimal operating conditions and consider supplementary measures to mitigate these limitations during system operation.
Among the physical treatment methods, adsorption is a surface-based process in which molecules or ions adhere to the surfaces of solid adsorbents. Zeolites and activated carbon are commonly used as adsorbents due to their high efficiency in dye removal and their regenerability [5]. According to [19], when dyeing wastewater was treated using zeolite and granular activated carbon, removal efficiencies of 58.40% for color, 60.82% for ammonia, and 59.46% for COD were achieved, demonstrating strong treatment performance. Furthermore, [20] reported that when adsorption was combined with an advanced oxidation process (AOP), the removal of dyes and organic substances was significantly enhanced. These findings suggest that coupling physical adsorption with other treatment technologies can produce synergistic effects for the removal of complex pollutants from dyeing wastewater.
With the revision of the Water Environment Conservation Act in October 2018, South Korea officially replaced COD with TOC as the regulatory organic indicator for effluents from wastewater discharge facilities starting in October 2019 [21]. Accordingly, this study aimed to evaluate the treatment performance of a bipolar electrolysis system by comparing the removal efficiencies of T-N and TOC as well as the amount of Al leached, under different applied voltages and electrolyte addition conditions. Furthermore, under the same operational settings, the effect of packing activated carbon was assessed to determine the extent of improvement in T-N and TOC removal, and the reduction rate of Al leaching.
2. Materials and Methods
2.1. Experimental Components
2.1.1. Activated Carbon
The granular activated carbon (GAC) used as the packing material in this experiment was supplied by Company S (Republic of Korea), a domestic supplier. It had a mesh size of 8 × 30, indicating a particle size range suitable for packed-bed applications. GAC was selected due to its high surface area and proven effectiveness in adsorbing organic and inorganic contaminants during the electrochemical treatment processes.
2.1.2. Test Sample
In this study, dyeing wastewater samples were collected from an industrial complex located in D City, Korea. Owing to the nature of the electrochemical treatment, electrical conductivity (EC) can significantly influence the system performance. According to [22], the EC of industrial wastewater increased from 0.92 mS/cm at 9.3 °C to 1.01 mS/cm at 41.7 °C. Similarly, as shown in Table 1, the EC of the dyeing wastewater used in this study also varied with temperature in the range of 5–30 °C. To minimize experimental errors caused by temperature fluctuations, the wastewater was pre-heated to 20 °C using a hotplate prior to system operation. The representative physicochemical properties of the dyeing wastewater at this temperature are summarized in Table 2.

Table 1.
Temperature-dependent electrical conductivity of dyeing wastewater.

Table 2.
Characteristics of dyeing wastewater (at 20 °C).
2.1.3. Electrolysis Apparatus
A schematic diagram of the experimental setup used in this study is presented in Figure 1. A DC power supply was used to provide voltage-based current to the system, and an automatic voltage regulator (AVR) was installed to stabilize the input and prevent fluctuations in the AC power source.
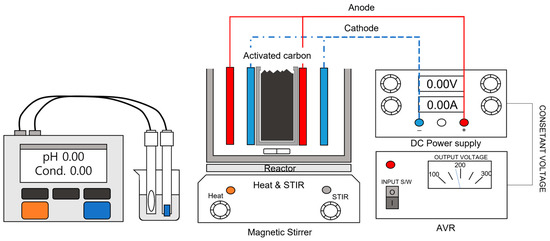
Figure 1.
Schematic diagram of the electrolysis apparatus.
The reactor vessel used for sample containment was constructed from 10T-thick acrylic, with internal dimensions of 113 mm (W) × 117 mm (L) × 150 mm (H). A stainless-steel mesh compartment was installed at the center of the reactor to hold the activated carbon medium. This compartment was designed with internal dimensions of 40 mm (W) × 117 mm (L) × 110 mm (H) and was made to be removable for flexibility during operation.
A magnetic stir bar was placed inside the reactor to ensure uniform mixing of the sample during operation, and a stirrer (MTOPS Technology Co., Shenzhen, China) device was used to maintain a consistent concentration throughout the solution.
2.1.4. Electrode
The electrodes used in this study were made of soluble aluminum with dimensions of 105 mm (W) × 110 mm (L) × 5 mm (H). To minimize the dead space during operation and promote effective sample circulation, the electrodes were fabricated in a porous form. To reduce errors caused by surface contaminants or impurities, the aluminum electrodes underwent a pretreatment process, as illustrated in Figure 2. The electrodes were first installed in a reactor containing a 10 mM NaCl solution and subjected to electrochemical treatment for 1 h. The samples were then immersed in a mixed acid solution containing more than 10% sulfuric and nitric acids for 8 h, followed by thorough rinsing with distilled water and drying.
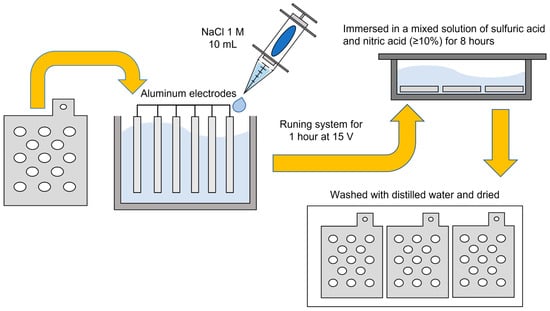
Figure 2.
Schematic diagram of electrode preparation and setup before use.
2.2. Experimental Conditions and Methods
2.2.1. Experimental Conditions
The fixed and variable parameters used in this study are listed in Table 3. During each run, 1000 mL of dyeing wastewater was introduced into the reactor, which was operated for 60 min. Samples (15 mL) were collected every 10 min for a total of six samples per experiment.

Table 3.
Constant and variable conditions.
Four aluminum electrodes were alternately arranged as anodes and cathodes. The spacing between each pair of electrodes was 10 mm. To minimize the experimental errors due to electrode contamination after each run, the electrodes were immersed in a mixed acid solution (containing more than 10% sulfuric and nitric acids) for at least 12 h, followed by rinsing with distilled water and drying prior to reuse.
Additionally, to evaluate the adsorption performance of activated carbon alone, a baseline adsorption experiment was conducted by adding activated carbon at a dosage of 100 g/L into the reactor without applying electrochemical treatment. The results were compared with those obtained from the electrochemical process under various conditions.
All experiments were conducted in triplicate, and the average values were reported. Additionally, error bars indicating the minimum and maximum values among the three trials were included in the figures to represent the data variability.
2.2.2. Measurement Instruments and Methods
The measurement parameters in this study included the removal efficiencies of T-N and TOC, as well as the Al concentration in the final treated water, under varying conditions of applied voltage, electrolyte addition, and activated carbon packing. An overview of the experimental setup and measurement instruments is presented in Figure 3, and the specific models and manufacturers of the equipment used are listed in Table 4.
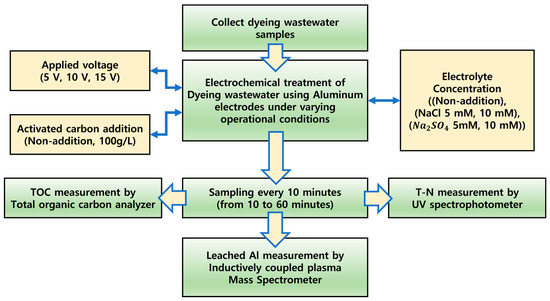
Figure 3.
Overview of experimental conditions and measurement instruments.

Table 4.
Apparatus and equipment used in the experiments.
T-N was measured using the Total Nitrogen–UV/Visible Spectrometry–Oxidation Method, in accordance with national water quality standards [22]. TOC was analyzed using the non-purgeable organic carbon (NPOC) method, and Al concentrations were determined via inductively coupled plasma mass spectrometry (ICP-MS) [23].
For both T-N and TOC, samples were collected every 10 min during the 60 min experiment, resulting in six data points per condition. The removal efficiency was calculated by subtracting each value from the initial concentration and the results were expressed as percentages. In the case of Al, only the final sample was analyzed at 60 min.
3. Results and Discussion
3.1. Effect of Applied Voltage
In this section, the removal efficiencies of T-N and TOC were evaluated under different applied voltages without the addition of electrolyte or packing of activated carbon. The bipolar electrolysis system was operated at 5, 10, and 15 V. Each experiment was conducted for 60 min and sampling was performed every 10 min. The time-dependent treatment efficiencies of the dyeing wastewater are presented in Figure 4, and the Al concentrations leached at the final treatment time are shown in Figure 5.
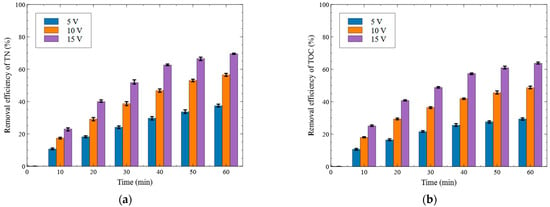
Figure 4.
Effect of voltage on the removal efficiency ((a) T-N removal, (b) TOC removal (Al electrode, no electrolyte addition)).
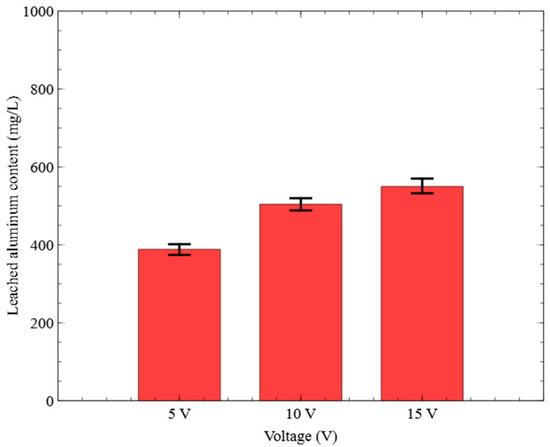
Figure 5.
Effect of voltage on the leached aluminum content (Al electrode, no electrolyte addition).
3.1.1. Effect of Applied Voltage on T-N Removal
As shown in Figure 4a, the T-N removal efficiencies of the samples collected at 10 min were 10.64%, 17.38%, and 23.03% under the applied voltages of 5, 10, and 15 V, respectively. This indicated that the removal efficiency increased by 12.39% at 15 V compared to that at 5 V. The difference in removal efficiency became more pronounced over time; at the final sampling point (60 min), the T-N removal efficiencies were 37.37%, 56.49%, and 69.53% under 5, 10, and 15 V, respectively, showing a 32.16%p increase at 15 V compared to 5 V.
In the electrochemical treatment of nitrogen-containing wastewater using aluminum electrodes, nitrogen and ammonia can be removed through electrochemical reactions as described in reactions (1)–(14) [24,25,26]. The increase in T-N removal efficiency with higher applied voltage observed in this study is likely due to the increased release of Al3+ ions as the current rises, thereby promoting greater coagulation of nitrogenous compounds. In a related study [27], the ammonia nitrogen removal efficiency increased with current density. Similarly, another study reported that when insoluble titanium electrodes were used, increasing the current density from 10 to 50 mA/cm2 increased nitrate removal from 57.01% to 99.70%, suggesting that higher voltages enhanced nitrogen oxidation and removal [28].
3.1.2. Effect of Applied Voltage on TOC Removal
As shown in Figure 4b, the TOC removal efficiencies of the samples collected at 10 min were 10.64%, 17.97%, and 25.04% at the applied voltages of 5, 10, and 15 V, respectively. The removal efficiency at 15 V was 14.40%p higher than that at 5 V. This difference increased over time, and at 60 min, the TOC removal efficiencies reached 29.31%, 48.61%, and 63.68% at 5, 10, and 15 V, respectively, indicating a 34.37%p improvement under the 15 V condition compared to 5 V.
During electrolysis with aluminum electrodes, Al is oxidized into ionic forms such as Al2+ and Al3+. These aluminum ions react with OH− to form Al(OH)3, which acts as a flocculant that adsorbs TOC and facilitates its removal through precipitation [29]. In this study, the increase in TOC removal efficiency with higher applied voltage was likely due to the increased availability of Al ions, which promoted more effective coagulation of organic matter. A similar trend was also observed in a previous study [30], where the use of soluble Fe and Al electrodes in electrochemical treatment led to higher COD and TOC removal efficiencies as the current density increased.
[Anode] Al(metal) → Al2+ + 2e−
Al(metal) → Al3+ + 3e−
Al2+ → Al3+ + e−
4OH− → 2H2O + O2 + 4e−
[Cathode] H2O → H+ + 2OH−
Al2+ + 2OH− → Al(OH)2↓
Al(OH)2 + OH− → Al(OH)3↓
2H+ + 2e− → H2
NO3− + H2O + 2e− → NO2− + 2OH−
NO3− + 3H2O + 5e− → 1/2N2 + 6OH−
NO3− + 6H2O + 8e− → NH3 + 9OH−
NO2− + 2H2O + 3e− → 1/2N2 + 4OH−
NO2− + 5H2O + 6e− → NH3 + 7OH−
NO2− + 4H2O + 4e− → NH2OH + 5OH−
3.1.3. Effect of Applied Voltage on the Leached Aluminum Content
Figure 5 shows the concentration of Al leached in the final sample collected at the end of the 60 min treatment. The Al concentrations were measured as 388.39 mg/L at 5 V, 504.28 mg/L at 10 V, and 549.83 mg/L at 15 V. These results indicated that the amount of Al leached increased by 161.44 mg/L under the 15 V condition compared to 5 V.
3.2. Effect of Applied Addition of Electrolyte
In this section, the effects of electrolyte addition on the removal efficiencies of T-N and TOC were evaluated without activated carbon packing. The system was operated under a fixed voltage of 15 V, and a 1 M stock solution of either NaCl or Na2SO4 was added to adjust the electrolyte concentrations in the reactor to 0 mM (non-addition), 5 mM, and 10 mM. As described in Section 3.1, each condition was tested for 60 min, with sampling performed every 10 min. The time-dependent treatment efficiencies of the dyeing wastewater are shown in Figure 6, and the concentrations of the leached Al at the final treatment time are presented in Figure 7.
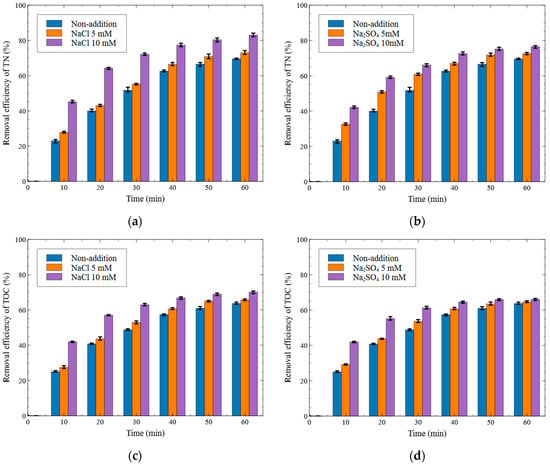
Figure 6.
Effect of electrolyte on the removal efficiency ((a) NaCl addition—T-N removal, (b) Na2SO4 addition—T-N removal, (c) NaCl addition—TOC removal, and (d) Na2SO4 addition—TOC removal (Al electrode, 15 V)).
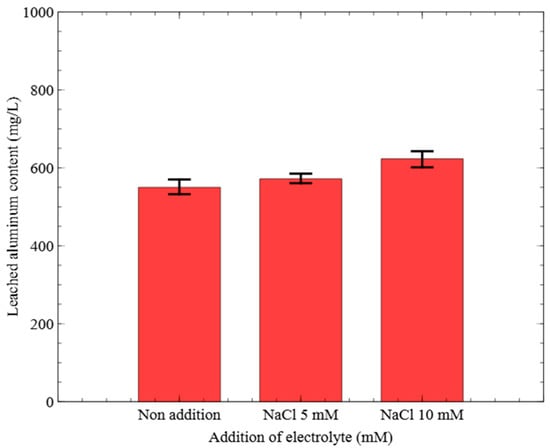
Figure 7.
Effect of electrolyte on the leached aluminum content (Al electrode, NaCl addition, 15 V).
3.2.1. Effect of Electrolyte on T-N Removal
As shown in Figure 6a, the T-N removal efficiencies at 10 min were 23.03%, 27.81%, and 45.19% with non-addition, NaCl 5 mM, and NaCl 10 mM, respectively. Under the NaCl 10 mM condition, the removal efficiency increased by 22.16% compared with the non-addition condition. However, this difference decreased over time. At 60 min, the T-N removal efficiencies were 69.53%, 73.01%, and 83.00% for non-addition, NaCl 5 mM, and NaCl 10 mM, respectively, showing a 13.47%p increase at NaCl 10 mM compared to non-addition.
As shown in Figure 6b, the T-N removal efficiencies at 10 min under the conditions of non-addition, Na2SO4 5 mM, and Na2SO4 10 mM were 23.03%, 28.59%, and 42.15%, respectively, indicating a 19.12%p improvement at Na2SO4 10 mM over non-addition. At 60 min, the removal efficiencies were 69.53%, 72.57%, and 76.48%, corresponding to a 6.95%p increase at Na2SO4 10 mM compared to non-addition.
These results confirmed that the addition of electrolyte enhanced the removal of T-N, with NaCl exhibiting a more pronounced effect. This may be attributed to the additional oxidation induced by the chloride ions present in the wastewater, which are released during electrolysis. A similar trend was reported in [31], where NaCl, Na2SO4, and NaClO4 were compared for nitrogen removal efficiency, and NaCl was found to yield the highest performance.
3.2.2. Effect of Addition of Electrolyte on TOC Removal
As shown in Figure 6c, the TOC removal efficiencies at 10 min under the conditions of non-addition, NaCl 5 mM, and NaCl 10 mM were 25.04%, 27.51%, and 41.94%, respectively. Under the presence of NaCl 10 mM, the removal efficiency increased by 16.90%p compared to that of non-addition. This difference decreased over time, and at 60 min, the removal efficiencies were 63.68%, 65.72%, and 70.13% for non-addition, NaCl 5 mM, and NaCl 10 mM, respectively, resulting in a 6.45% increase at NaCl 10 mM compared to non-addition.
As shown in Figure 6d, the TOC removal efficiencies at 10 min under the conditions of non-addition, Na2SO4 5 mM, and Na2SO4 10 mM were 25.04%, 29.18%, and 41.94%, respectively, again showing a 16.90%p increase at Na2SO4 10 mM over non-addition. However, this difference significantly decreased by the end of the experiment. At 60 min, the removal efficiencies were 63.68%, 64.62%, and 66.09% for non-addition, Na2SO4 5 mM, and Na2SO4 10 mM, respectively, indicating only a 2.41%p improvement at Na2SO4 10 mM compared to non-addition.
These results showed that, as with T-N, the use of NaCl as an electrolyte yielded higher TOC removal efficiency than Na2SO4. This is likely due to the increased conductivity and additional oxidative reactions induced by the chloride ions. A similar trend was observed in a previous study [30], where the addition of NaCl during electrochemical treatment using soluble Fe and Al electrodes led to enhanced COD and TOC removal owing to the increased conductivity.
3.2.3. Effect of Electrolyte on Leached Aluminum Content
Figure 7 presents the concentrations of Al leached in the final samples collected after 60 min under 15 V with and without NaCl electrolyte supplementation. The Al concentrations were 549.83 mg/L for the non-addition condition, 571.70 mg/L for NaCl 5 mM, and 623.06 mg/L for NaCl 10 mM. These results indicated that Al leaching increased by 73.23 mg/L under the NaCl 10 mM condition compared to non-addition.
3.3. Effect of Activated Carbon Packed
In this section, the difference in the removal efficiencies of T-N and TOC was investigated based on the presence or absence of AC packing, under electrochemical treatment without electrolyte addition. Three conditions were evaluated: (i) AC adsorption under stirring without electrochemical treatment, (ii) electrochemical treatment at 15 V without AC packing, and (iii) electrochemical treatment at 15 V with 100 g of AC packing. Each experiment was conducted for 60 min with sampling performed every 10 min. The time-dependent treatment efficiencies of dyeing wastewater under each condition are presented in Figure 8.
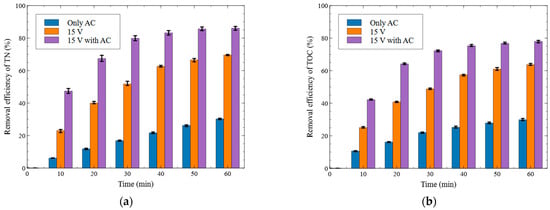
Figure 8.
Effect of activated carbon packing on the removal efficiency ((a) T-N removal, (b) TOC removal (Al electrode, no electrolyte addition, 15 V)).
3.3.1. Effect of Activated Carbon on T-N Removal
When dyeing wastewater was treated by adsorption using activated carbon, ammoniacal nitrogen was primarily removed through electrostatic attraction, as described by Equation (15) [32]. In contrast, organic nitrogen compounds were eliminated via physical adsorption and surface reactions, as described in Equation (16) [33].
ACsurface + NH4+ → ACsurface ∙ NH4+
ACsurface + R-NH2 → ACsurface ∙ R-NH2
As shown in Figure 8a, the T-N removal efficiencies at 10 min were 6.13% for the only AC conditions, 23.04% at 15 V, and 47.37% at 15 V with AC. Although the only AC condition showed a lower removal efficiency than the 15 V condition, the 15 V with AC condition exhibited a 24.33%p improvement compared to 15 V alone. At the final treatment time (60 min), the T-N removal efficiencies were 30.24% for only AC, 69.53% for 15 V, and 86.04% for 15 V with AC, indicating a 16.51%p increase in the 15 V with AC condition compared to 15 V.
3.3.2. Effect of Activated Carbon on TOC Removal
When dyeing wastewater is treated by adsorption using activated carbon, certain organic compounds are physically adsorbed onto the surface, according to Equation (17), whereas oxidized functional groups on the activated carbon surface enhance the removal efficiency through chemical interactions, as described in Equation (18) [34].
ACsurface + Organic Molecule → ACsurface ∙ Organic Molecule
ACsurface-COOH + Organic Molecule → ACsurface-COOH ∙ Organic Molecule
As shown in Figure 8b, the TOC removal efficiencies at 10 min were 25.04% for 15 V, 10.62% for only AC, and 42.19% for 15 V with AC. The 15 V AC condition exhibited a 17.15%p higher removal efficiency than the 15 V condition. At the final treatment time (60 min), the TOC removal efficiencies were 63.68% for 15 V, 29.86% for only AC, and 77.98% for 15 V with AC, representing a 14.30%p increase in the 15 V with AC condition compared to 15 V alone.
These results suggested that AC not only contributes to the physical adsorption of organic compounds but also enhances electrochemical reactions, thereby improving the removal efficiencies of both T-N and TOC.
3.4. Effect of AC Packed Under Added Electrolyte
In this section, the effects of AC packing on the removal efficiencies of T-N and TOC, as well as the removal of leached Al at the final treatment time, were evaluated under electrolyte-added conditions during electrochemical treatment. According to the results in Section 3.2 and Section 3.3, the addition of electrolyte and the presence of packed AC individually enhanced the treatment efficiency. Therefore, this experiment aimed to determine whether combining both optimal conditions—electrolyte addition and AC packing—would result in further improvement in removal performance. In addition, this section also assessed the extent to which the packed activated carbon could adsorb the Al ions leached from the electrode during electrochemical treatment. The system was operated at 15 V for 60 min under three electrolyte conditions: non-addition, NaCl 5 mM, and NaCl 10 mM, with 100 g of AC packed in each case. The time-dependent removal efficiencies of the dyeing wastewater under each condition are presented in Figure 9, and the concentrations and removal efficiencies of leached Al are shown in Figure 10.
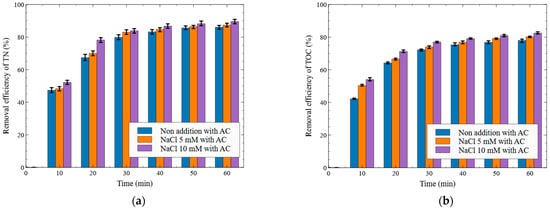
Figure 9.
Effect of activated carbon packing under electrolyte-added conditions on the removal efficiency ((a) T-N removal, (b) TOC removal (Al electrode, NaCl addition, 15 V)).
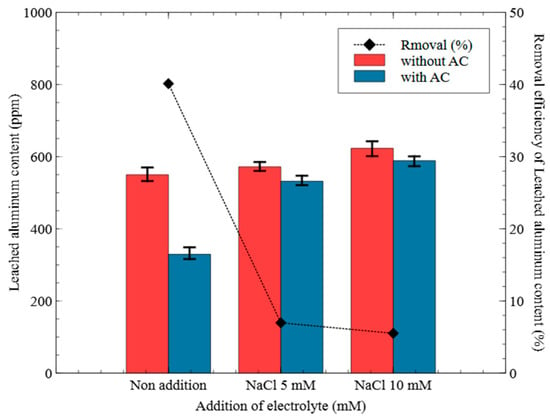
Figure 10.
Effect of activated carbon on the leached aluminum content (Al electrode, NaCl addition, 15 V).
3.4.1. Effect of Activated Carbon with Electrolyte on T-N Removal
As shown in Figure 9a, when the applied voltage was set to 15 V and 100 g of AC was packed at the center of the reactor, the T-N removal efficiencies at 10 min were 47.37% for the non-addition condition, 48.37% for NaCl 5 mM, and 52.15% for NaCl 10 mM. Under the NaCl 10 mM condition, the removal efficiency increased by 4.78%p compared to the non-addition. At the final sampling point (60 min), the T-N removal efficiencies were 86.04% for non-addition, 87.35% for NaCl 5 mM, and 89.52% for NaCl 10 mM, indicating a 3.48%p increase in the NaCl 10 mM condition compared to non-addition.
3.4.2. Effect of Activated Carbon with Electrolyte on TOC Removal
As shown in Figure 9b, when the applied voltage was set to 15 V and 100 g of AC was packed at the center of the reactor, the TOC removal efficiencies at 10 min were 42.19% for the non-addition condition, 50.37% for NaCl 5 mM, and 54.05% for NaCl 10 mM. The NaCl 10 mM condition resulted in an 11.86%p higher removal efficiency than the non-addition. At the final sampling point (60 min), the TOC removal efficiencies were 77.98% for non-addition, 80.14% for NaCl 5 mM, and 82.47% for NaCl 10 mM, showing a 4.49%p increase under the NaCl 10 mM condition compared to non-addition.
3.4.3. Effect of Activated Carbon with Electrolyte on the Leached Aluminum Content
As shown in Figure 10, under an applied voltage of 15 V, the Al concentrations in the final samples collected at 60 min were compared with and without 100 g of AC packing. Under the non-addition condition, Al leaching was reduced from 549.83 mg/L to 329.21 mg/L, corresponding to a decrease of approximately 40.12%. In contrast, under the NaCl 5 mM condition, the Al concentration decreased from 571.70 mg/L to 531.81 mg/L (a 6.97% reduction), and under the NaCl 10 mM condition, from 623.06 mg/L to 588.71 mg/L (a 5.51% reduction). These results indicated that the Al removal efficiency of AC decreased with the addition of electrolyte.
Thus, under electrolyte-added conditions, the AC packing did not lead to a significant improvement in the dyeing wastewater treatment performance. Considering the required chemical input, treatment efficiency, and Al leaching, the most efficient approach appears to be electrochemical treatment without electrolyte addition but with AC packing.
4. Conclusions
In this study, dyeing wastewater generated from an industrial complex located in D City, Korea, was treated using bipolar and packed bipolar electrolysis systems equipped with aluminum electrodes and activated carbon. The system was operated for 60 min under varying conditions of applied voltage (5–15 V), electrolyte concentration (non-addition, NaCl 5 mM, NaCl 10 mM, Na2SO4 5 mM, and Na2SO4 10 mM), and activated carbon packing (with or without packing). The T-N and TOC removal efficiencies, along with Al leaching at the final treatment time under each condition, are summarized in Table 5. Based on the experimental results, the following conclusions were drawn:

Table 5.
Summary of experimental conditions and results.
The condition of 15 V with 100 g/L of activated carbon and the addition of 10 mM NaCl yielded the highest removal efficiencies, achieving 89.52% for T-N and 82.47% for TOC. However, under the same conditions without electrolyte addition, the removal efficiencies 86.04% for T-N and 77.98% for TOC were nearly equivalent. Notably, the concentration of leached aluminum increased significantly from 329.21 mg/L (no electrolyte condition) to 588.71 mg/L with the addition of 10 mM NaCl. Considering the combined factors of removal performance, aluminum leaching, and chemical input, it was determined that the most efficient condition is the one using 15 V with 100 g/L of activated carbon without electrolyte addition. This condition offered comparable pollutant removal performance to the electrolyte-added setups, while significantly reducing aluminum leaching and eliminating the need for additional chemical input. These findings indicate that the proposed system can reinforce the advantages of electrochemical treatment such as rapid reaction kinetics while addressing one of its key limitations, namely metal ion leaching from soluble electrodes. Overall, this study presents a promising strategy for achieving efficient and sustainable dyeing wastewater treatment.
Author Contributions
Conceptualization, H.-k.L. and S.-h.J.; methodology, H.-k.L. and G.-e.K.; validation, G.-e.K., S.-h.J. and Y.-c.S.; formal analysis, H.-k.L.; investigation, H.-k.L.; data curation, H.-k.L.; writing—original draft preparation, H.-k.L.; writing—review and editing, G.-e.K., S.-h.J. and Y.-c.S.; visualization, H.-k.L.; supervision, G.-e.K.; project administration, S.-h.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article material. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence this work.
Abbreviations
The following abbreviations are used in this manuscript:
| AC | Activated Carbon |
| Al | Aluminum |
| AOP | Advanced Oxidation Process |
| BOD | Biochemical Oxygen Demand |
| COD | Chemical Oxygen Demand |
| DAF | Dissolved Air Flotation |
| EC | Electrical Conductivity |
| Fe | Iron |
| GAC | Granular Activated Carbon |
| ICP-MS | Inductively Coupled Plasma Mass Spectrometry |
| NPOC | Non-Purgeable Organic Carbon |
| T-N | Total Nitrogen |
| TOC | Total Organic Carbon |
| SS | Suspended Solids |
| UV-Vis | Ultraviolet–Visible |
References
- Korea Ministry of Environment. Survey on Pollutant Emitting Facilities; KOSIS: Daejeon, Republic of Korea, 2024; Available online: https://kosis.kr/statHtml/statHtml.do?orgId=106&tblId=DT_106T_011815&conn_path=I2 (accessed on 2 September 2024).
- Anonymous. The Impact of Textile Production and Waste on the Environment. States News Service, 29 December 2020. [Google Scholar]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Ayed, L.; Mahdhi, A.; Cheref, A.; Bakhrouf, A. Decolorization and degradation of azo dye Methyl Red by an isolated Sphingomonas paucimobilis: Biotoxicity and metabolites characterization. Desalination 2011, 274, 272–277. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Ellouze, E.; Tahri, N.; Amar, R.B. Enhancement of textile wastewater treatment process using Nanofiltration. Desalination 2012, 286, 16–23. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Zainab, A.N.; Tlaiaa, Y.S.; Ali, A.H. Design of dissolved air flotation (DAF) process for treating dyes-contaminated wastewater. Casp. J. Environ. Sci. 2022, 20, 315–322. [Google Scholar] [CrossRef]
- Üstün, G.E.; Solmaz, S.K.A.; Birgül, A. Regeneration of industrial district wastewater using a combination of Fenton process and ion exchange—A case study. Resour. Conserv. Recycl. 2007, 52, 425–440. [Google Scholar] [CrossRef]
- Azimi, B.; Abdollahzadeh-Sharghi, E.; Bonakdarpour, B. Anaerobic-aerobic processes for the treatment of textile dyeing wastewater containing three commercial reactive azo dyes: Effect of number of stages and bioreactor type. Chin. J. Chem. Eng. 2021, 39, 228–239. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Duan, D.; Zhang, Z.; Liu, C.; Cai, W.; Zhao, Z. Environmental Impacts and Biological Technologies Toward Sustainable Treatment of Textile Dyeing Wastewater: A Review. Sustainability 2024, 16, 10867. [Google Scholar] [CrossRef]
- Sirés, I.; Brillas, E. Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: A review. Environ. Int. 2012, 40, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Dube, A.; Malode, S.J.; Alshehri, M.A.; Shetti, N.P. Electrochemical water treatment: Review of different approaches. J. Environ. Manag. 2025, 373, 123911. [Google Scholar] [CrossRef]
- El-Hefny, R.M.; Ali, M.N.; Ahmed, M.E. Application of iron and aluminum electrodes for wastewater treatment via electrocoagulation. Aust. J. Basic Appl. Sci. 2019, 13, 141–146. [Google Scholar] [CrossRef]
- Khorram, A.G.; Fallah, N. Treatment of textile dyeing factory wastewater by electrocoagulation with low sludge settling time: Optimization of operating parameters by RSM. J. Environ. Chem. Eng. 2018, 6, 635–642. [Google Scholar] [CrossRef]
- Cañizares, P.; Jiménez, C.; Martínez, F.; Sáez, C.; Rodrigo, M.A. Study of the Electrocoagulation Process Using Aluminum and Iron Electrodes. Ind. Eng. Chem. Res. 2007, 46, 6189–6195. [Google Scholar] [CrossRef]
- Arbabi, M.; Shafiei, S.; Mehraban, S.; Khodabakhshi, A.; Abdoli, A.; Arbabi, A. Electrocoagulation process using aluminum electrodes for treatment of baker’s yeast industry wastewater. Int. J. Environ. Health Eng. 2022, 11, 3. [Google Scholar] [CrossRef]
- St, S.; Abustan, I.; Dahlan, I.; Wah, C.K.; Umar, G. Treatment of Dye Wastewater Using Granular Activated Carbon and Zeolite Filter. Mod. Appl. Sci. 2012, 6, 37–51. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Singh, K.; Choudhary, A.; Brighu, U.; Singh, S.K.; Bhattacharya, S. Author Correction: Combined advanced oxidation dye-wastewater treatment plant: Design and development with data-driven predictive performance modeling. npj Clean Water 2024, 7, 15. [Google Scholar] [CrossRef]
- Nam, G.G.N. A Study on the Reduction of Non-Biodegradable Total Organic Carbon in Public Wastewater Treatment Facility Using Electrolysis: Focusing on A Industrial Complex in Chungcheong Area. Master’s Thesis, Koreatech University, Cheonan, Republic of Korea, 2020. [Google Scholar]
- Ministry of Environment, Republic of Korea. Total Nitrogen—UV/Visible Spectrometry—Oxidation Method; Notification No. 2024-72; Ministry of Environment: Sejong, Republic of Korea, 2011. [Google Scholar]
- Shimadzu Corporation. TOC Measurement Techniques: NPOC Method (Non-Purgeable Organic Carbon); Shimadzu TOC Application Note: Kyoto, Japan, 2018. [Google Scholar]
- Kim, S.K.; Park, S.W.; Hong, D.I. A Study on Dye Wastewater Treatment Using the Electrolysis. J. Environ. Sci. 1999, 8, 539–545. [Google Scholar]
- Kim, U.S. Study on the Treatment of Wastewater Containing Polyvinyl Alcohol by Electrolysis. Master’s Thesis, Kyunghee University, Seoul, Republic of Korea, 1992. [Google Scholar]
- Lee, Y.J. Nitrate Removal by Electrolysis Method. Master’s Thesis, Chonnam National University, Gwangju, Republic of Korea, 2010. [Google Scholar]
- Zuo, S.; Zhang, Y.; Guo, R.; Chen, J. Efficient Removal of Ammonia Nitrogen by an Electrochemical Process for Spent Caustic Wastewater Treatment. Catalysts 2022, 12, 1357. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Z.; Xu, J. Electrochemical Mechanisms and Optimization System of Nitrate Removal from Groundwater by Polymetallic Nanoelectrodes. Int. J. Environ. Res. Public Health 2023, 20, 1923. [Google Scholar] [CrossRef]
- Al-Marri, J.S.; Abouedwan, A.B.; Ahmad, M.I.; Bensalah, N. Electrocoagulation using aluminum electrodes as a sustainable and economic method for the removal of kinetic hydrate inhibitor (polyvinyl pyrrolidone) from produced wastewaters. Front. Water 2023, 5, 1305347. [Google Scholar] [CrossRef]
- Tanatti, N.P.; Sengil, I.A.; Özdemir, A. Optimizing TOC and COD removal for the biodiesel wastewater by electrocoagulation. Appl. Water Sci. 2018, 8, 58. [Google Scholar] [CrossRef]
- Iovino, P.; Fenti, A.; Galoppo, S.; Najafinejad, M.S.; Chianese, S.; Musmarra, D. Electrochemical Removal of Nitrogen Compounds from a Simulated Saline Wastewater. Molecules 2023, 28, 1306. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiang, C.; Nnorom, M.; Avignone-Rossa, C.; Yang, K.; Guo, B. Multi-faceted effects and mechanisms of granular activated carbon to enhance anaerobic ammonium oxidation (anammox) for nitrogen removal from wastewater. Bioresour. Technol. 2025, 418, 132001. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, F.; Shaban, M.; Zaki, S.K.; Abd-Elsamie, M.S.; Sayed, R.; Zayed, M.; Khalid, N.; Saad, S.; Omar, S.; Ahmed, A.M.; et al. Activated carbon derived from sugarcane and modified with natural zeolite for efficient adsorption of methylene blue dye: Experimentally and theoretically approaches. Sci. Rep. 2022, 12, 18031. [Google Scholar] [CrossRef]
- Karanfil, T.; Kilduff, J.E. Role of Granular Activated Carbon Surface Chemistry on the Adsorption of Organic Compounds. 1. Priority Pollutants. Environ. Sci. Technol. 1999, 33, 3217–3224. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).