Abstract
Carp (Cyprinus carpio) is one of the major farmed fish species in China. In recent years, the increasing water temperature caused by global warming has caused physiological stress in fish, which in turn affects the heart function and health of the fish. Therefore, we hypothesized that the electrocardiogram (ECG) parameters of carp could reflect the temperature-induced stress. To test this hypothesis, in this study, the real-time online cardiac function assessment system (OCFAS) was used to monitor the electrocardiogram signals (heart rate, P wave, R wave, T wave, P-R interval, QRS complex, Q-T interval) of carp at different temperatures. The results showed that the heart rate of the fish increased with the temperature within a certain range. However, when the temperature exceeded this range, the cardiac function of the fish was significantly impaired. The P-R interval was shortened with the increase in the body temperature, and there was a negative correlation between them. This study emphasizes the importance of using real-time online fish ECG assessments to evaluate cardiac health, and it further improves the evaluation index system for ECG in fish. At the same time, the temperature in aquaculture and water environments requires special attention to avoid its adverse effects on the health of aquatic populations.
1. Introduction
Today, global warming poses a serious ecological challenge [1], with ectothermic animals, such as fish, encountering significant challenges from fluctuating environmental temperatures, which directly impact their cardiovascular function [2]. Cardiac function, which can be defined as the heart’s ability to supply blood to other tissues, is a critical factor in determining the health of many animals [3]. It also plays a pivotal role in oxygen delivery, regulating the aerobic metabolic capacity and physical performance of fish [4]. Fish exhibit considerable variation in their cardiac function at different temperatures. This has led to increasing concern about the negative effects of global warming on fish due to increased water temperatures and an urgent need to understand how temperature affects heart function in fish. Under high-temperature conditions, the heart is often the first organ to fail in fish [5,6,7]. Changes in water temperature will directly affect the life activities of fish, including feeding, growth, gonadal development, spawning and reproduction, and their living environment, and they will also change their hydrological conditions and water environment [8]. Research indicates that the heart rate of ectothermic animals rises with increasing temperatures, but beyond a specific range, it slows or begins to decline. Continued water temperature increases lead to a decrease in heart rate, signaling arrhythmia and eventual cardiac failure [9,10].
Electrocardiography (ECG) captures real-time physiological changes in the heart caused by external factors, making it valuable for diagnosing and analyzing cardiac diseases [11]. ECG indexes are considered essential biomarkers in zoological experiments and are foundational for studying cardiovascular diseases [12]. The reason why the changes in a fish’s electrocardiogram are regarded as effective indicators to reflect the changes in the water environment is that the changes in water quality can affect the electrocardiogram activity of fish, mainly reflected in the changes in their electrocardiogram indicators. Routine monitoring of ECG indexes can assess an organism’s physical condition and indicate its stress levels [13]. These indexes include the heart rate, P wave, R-wave, T wave, QRS complex, P-R intervals, and Q-T intervals. Among these, the heart rate is a rapid indicator of abnormalities and is widely used as a stress marker in fish research [14,15]. Therefore, the heart rate is a critical physiological indicator for detecting the impact of environmental changes on fish [16,17,18].
In the context of biology, stress is defined as the non-specific response of the body to demands for change [19]. Conducting laboratory studies is fundamental for determining the relationship between stress and measurable physiological parameters. In recent years, advancements in fish ECG data collection technology have enabled a growing number of studies on real-time ECG measurements to assess stress sources. Fish, as organisms that live directly in the water environment, are highly sensitive to the changes in the water environment [20]. Cardiac electrical activity in fish may be altered due to changes in the water temperature, resulting in symptoms such as an irregular heartbeat, myocardial ischemia or injury [21]. Several advanced methods and technologies have emerged. For instance, Shen Y et al. used implantable ECG devices with biotelemetry technology to measure the heart rates in marine fish [22]. Svendsen E et al. measured the electrocardiograms and photoplethysmograms (PPGs) in Atlantic salmon using implanted electronic tags [23]. Le et al. developed a wireless ECG system, Zebra II, for measuring electrocardiograms in zebrafish [24]. Deng Y et al. proposed two non-invasive, user-friendly techniques utilizing photoplethysmography (PPG) and remote photoplethysmography (rPPG) to monitor the heart rate of large yellow croaker [25].
Fishes, as a fundamental component of aquatic ecosystems, have health conditions that not only affect their survival and reproductive capabilities but also directly influence the functioning of ecosystems, including nutrient cycling, energy flow, and habitat structure [26]. When ecosystems are under stress, the physiological and immune functions of fish may become suppressed, leading to increased susceptibility to diseases and a decline in population numbers [27]. The subject organism in this study was carp. Carp (Cyprinus carpio), the most extensively cultured and commercially valuable species in China’s aquaculture industry, is also the most widely distributed and diverse species in the Cyprinidae family [28]. In this experiment, carp was selected as the experimental object to study the effect of temperature on fish heart function, mainly because ornamental carp live in the artificial culture environment for a long time, and the hard bone tissue around the heart cavity disappears, which is convenient for experimental operation and easy to return to the original state after the experiment. In addition, during the long-term cultivation and domestication of carp, it has a diverse diet, strong adaptability, and low breeding cost [29]. Previous studies have indicated that the optimal growth temperature for crucian carp is between 21 °C and 25 °C [30]. Therefore, in this study, the control group for the temperature experiment was set at 22 °C. The water temperature for each experiment was set based on these considerations.
The device designed in this study consists of three components: monitoring the ECG, metabolism and behavior of fish. This study primarily utilizes the electrocardiography component to evaluate cardiac function in fish, analyzing the changes in various cardiac parameters to assess the impacts on heart health. Real-world conditions are simulated to examine the effects of temperature on fish cardiac function. The objective of this study is to quantify the real-time electrocardiographic signals without affecting normal fish behavior, using a custom-made IR-based ECG device [31,32], to analyze and evaluate the correlation between ECG parameters and temperature and to further refine the fish ECG assessment index system.
2. Materials and Methods
2.1. Animal Husbandry
Freshwater fish, Cyprinus carpio (length: 30 ± 2 cm, weight: 300 ± 5 g, n = 27), were purchased from an aquarium and housed in the fish breeding laboratory of the Environmental and Ecological Research Institute at Shandong Normal University for 2 weeks for the experiment. The laboratory is equipped with comprehensive fish farming systems, including aeration and dichlorination water treatment systems, automatic lighting control systems, and water circulation systems. Impurities were primarily adsorbed and filtered through filter cotton, activated carbon, and ceramic rings arranged from top to bottom. Professional maintenance personnel regularly cleaned and maintained the aquariums, water tanks, and flow pipes.
The physicochemical conditions of the rearing system were consistently maintained throughout the experiment, including the temperature (22 ± 0.5 °C), water hardness (CaCO3: 250 ± 25 mg/L), pH (7.8 ± 0.2), and photoperiod cycles (16 h light and 8 h dark). These parameters remained consistent throughout the experiment. The fish were fed twice daily (7 am and 4 pm), but feeding was stopped 24 h before the experiment, and no feeding occurred during the experimental period.
2.2. Experimental Setup
The ECGs of the carp were measured using a custom-built platform for evaluating energy and material flow during fish passage, developed by the Environmental and Ecological Research Institute at Shandong Normal University. The water tank dimensions are 321 cm × 119 cm × 127 cm (L × W × H) (Figure 1). The apparatus mainly consists of a frequency converter, electric motor, and propeller. Temperature control within the tank was achieved using a heating rod and chiller, maintaining stable water temperatures throughout the experiment (fluctuations within 1 °C), monitored by a high-precision thermometer. The motor speed was regulated by the frequency converter, which in turn controlled the propeller to generate a steady water flow. This device includes three parts: ECG, metabolism and behavior. This study mainly evaluated the cardiac function of fish through the ECG part of the device.
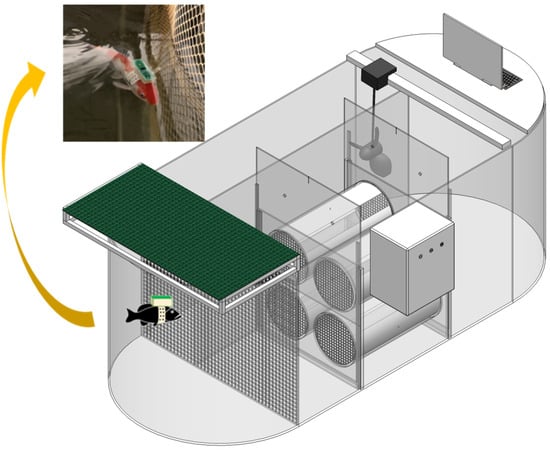
Figure 1.
Schematic of the experimental equipment and ECG monitoring.
The temperature experiment comprised four temperature gradients: 18 °C, 22 °C (control group), 26 °C, and 30 °C. The fish were divided into a heating group (26 °C, 30 °C) and a cooling group (18 °C). After adjusting to the experimental temperatures, one fish was randomly selected for testing. Each group was initiated simultaneously and performed three parallel experiments. The adaptation time of the fish in each experimental temperature was 48 h, and the adaptation time of all the temperature groups was the same. During the adaptation phase, we used physiological criteria to confirm that the fish had fully adapted to the temperature conditions. These criteria included monitoring the heart rate and general behavior of the fish to ensure that each fish was stable and without signs of acute stress or abnormal behavior. Only those fish that showed stable physiological parameters were used for the ECG recordings to ensure that the data collected represented the state after adaptation.
2.3. Assessment of Physiological Parameters in Cyprinus carpio
A randomly chosen carp was anesthetized using tricaine methane-sulfonate (MS-222). The Online Cardiac Function Assessment System (OCFAS, US Patent No: US 10,571,448 B2) was carefully attached to the carp’s dorsal surface. This system comprises an automated sampler along with two electrodes—positive and negative. Two silver needles, functioning as the electrodes, were inserted into the pericardial cavity at an approximate angle of 15° to establish reliable contact with the heart. Comprehensive descriptions and details about the OCFAS setup are available in earlier publications [32]. Once the system was securely installed, the carp was placed inside a water tank. The OCFAS continuously collected real-time electrocardiogram (ECG) signals, which were transmitted wirelessly to a connected computer via an infrared transceiver. The real-time ECG signals were organized and quantitatively analyzed using the Pclab-530C biomedical signal acquisition and processing system (Beijing Microsignal Star Technology Development Co., Ltd., Beijing, China). The experiment lasted for 48 h, and each group underwent three parallel experiments.
2.4. Statistical Analysis
The original ECG data were analyzed by correlation and variance after preliminary processing. Exce1 2021 and PowerPoint 2021 were used for chart making in this study, and IBM SPSS Statistics 19 was used for statistical analysis. To explore the effects of different temperatures on the heart function of carp, a one-way analysis of variance (ANOVA) was used for statistical analysis. Before the ANOVA, the data were first tested for homogeneity of variance. If the data met the hypothesis of homogeneity of variance, the LSD method was used for the post hoc multiple comparisons. The post hoc multiple comparisons were performed with the use of Tamhane’s T2 method if the assumption of homogeneity of variance was not met. Finally, using the HMM toolbox in the MATLAB environment (MATLAB 2009, © 1984–2009 The Math Works, Inc., Natick, MA, USA), we applied a 16 × 12 node size to pattern the ECG parameters influenced by the temperatures on a self-organizing map (SOM). During the data processing, no experimental data were excluded or corrected. The statistical analysis indicates the significance levels as follows: p < 0.05 is significant (*), p < 0.01 is highly significant (**), and p < 0.001 is extremely significant (***).
3. Results
3.1. Effects of Temperature on ECG Index of Cyprinus carpio
A comparison of the raw ECG data collected at various temperatures, as illustrated in Figure 2, reveals that the ECG of carp at 30 °C is relatively unstable. The heartbeats become irregular, the amplitude varies, and there is a degree of arrhythmia and disorder present. The primary focus of the analysis is on the heart rate, and compared to the control group (22 °C), the heart rate is lower in the low-temperature group (18 °C) and higher in the high-temperature groups (26 °C and 30 °C). Overall, from 18 °C to 26 °C, the heart rate increases significantly with the rising temperature (p < 0.01), but the increase is significantly limited when the temperature reaches 30 °C, as no further significant change is observed between 26 °C and 30 °C. The average heart rate of carp in the control group (22 °C) is 44 ± 4.65 (mean ± SD) beats min−1. In the 18 °C experimental group, it is 30 ± 3.07 beats min−1; in the 26 °C experimental group, it is 56 ± 7.39 beats min−1; and in the 30 °C experimental group, it is 60 ± 2.88 beats min−1. There is no significant difference in the average heart rate between 18 °C and 22 °C or between 26 °C and 30 °C (p > 0.05). However, there is a highly significant difference between 18 °C and 26 °C and between 18 °C and 30 °C (p < 0.01), and a significant difference between 22 °C and 30 °C (p < 0.05).
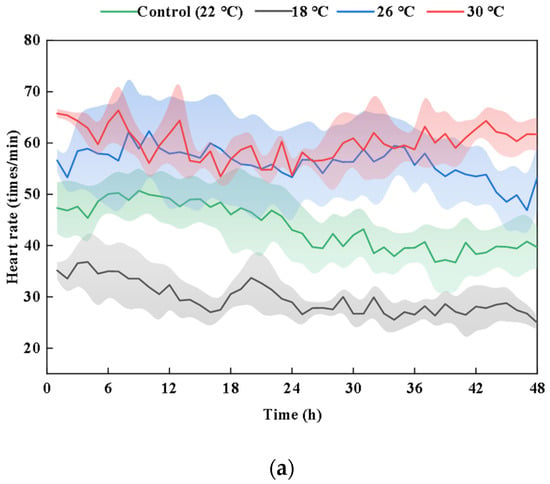
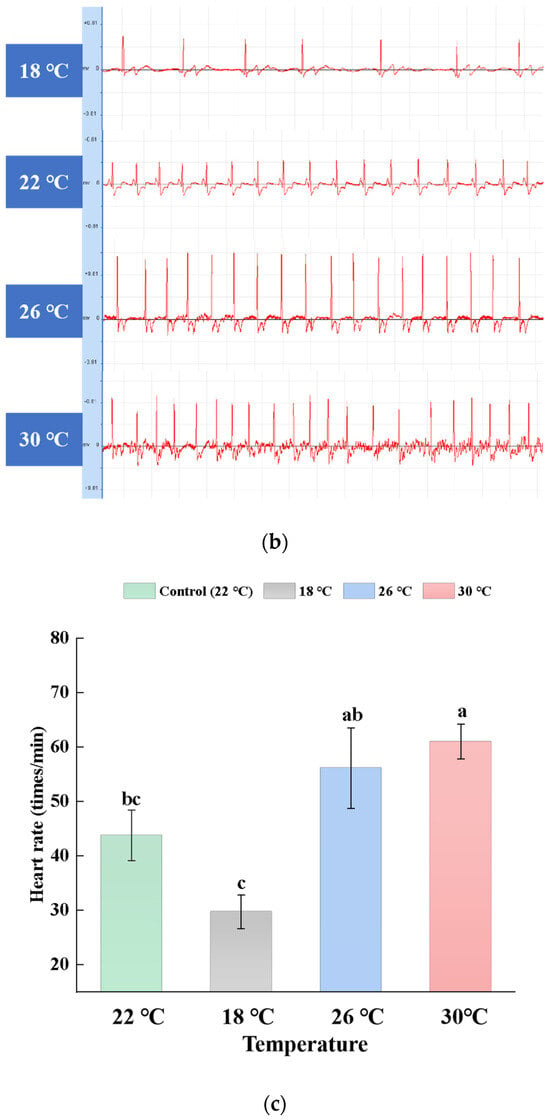
Figure 2.
Comparison of the carp heart rate at different temperatures. (a) Heart rate of carp at different temperatures for 48 h. (b) Representative recordings of the raw ECG data at different temperatures. (c) Significant difference between the heart rate at different temperatures. Results are means ± SD. The different lowercase letters indicate significant differences among samples (p < 0.05).
This study conducted a quantitative analysis of the ECG parameters of carp. The changes in the ECG indicators of carp within 48 h at different temperatures are, respectively, shown in Figure 3, revealing the amplitude of the three waves (P, R, and T) and the duration of the three intervals (P-R, QRS, and Q-T). There is a significant difference in the R wave amplitude between the control group and the 26 °C experimental group (p < 0.05). A clear pattern emerges when observing the P-R interval at different temperatures: the higher the temperature, the shorter the duration of the P-R interval (the time taken by impulse conduction from the beginning of atrial contraction to the beginning (peak) of ventricular contraction). There is a significant difference in the P-R interval between the 18 °C and 30 °C experimental groups (p < 0.05). The durations of the QRS complex (the time taken by impulse transmission through the ventricle) and the Q-T interval (the average duration of the ventricular action potential) also gradually shorten with the increasing temperature. Despite this trend, there are no statistically significant differences in the QRS complex and Q-T interval among the experimental groups (p > 0.05), indicating that these two intervals are relatively insensitive to temperature changes.
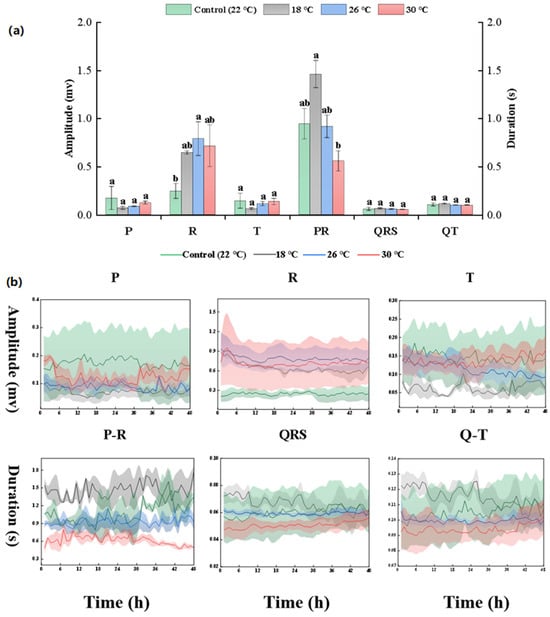
Figure 3.
Comparison of the carp heart ECG at different temperatures. (a) Amplitude and duration of the carp heart ECG indexes for 48 h. (b) Real-time heart ECG signal strength of carp. The units of amplitude (P, R, and T wave) and duration (P-R interval, QRS complex, and Q-T interval) are mv and s, respectively. Results are means ± SD. The different lowercase letters indicate significant differences among samples (p < 0.05).
By observing the relatively stable change curves of the three ECG intervals over 48 h, it is evident that the lengths of the P-R interval, QRS interval, and Q-T interval shorten as the temperature increases. There is a negative correlation between the rising temperature and the duration of these ECG intervals, demonstrating a shortening effect.
3.2. SOM Profiles
Figure 4 shows the SOM analysis results based on the seven ECG indexes of Cyprinus carpio under different temperature conditions, and the data are divided into six clusters. The vertical column color indicates the intensity, where red represents high intensity and blue represents low intensity, and the color from red to blue indicates the change of intensity from high to low. Clusters within the same period demonstrate greater closeness, reflecting the rhythmic phenomena of carp from the perspective of the clustering analysis. Clusters 3 and 5 correspond to the photoperiod range, which is consistent with the red high-intensity area, indicating that the intensity of the cardiac activity is high during the photoperiod. Clusters 2 and 4 correspond to the dark period range, which is consistent with the blue low-intensity area, indicating that the intensity of the cardiac activity is low during the dark period. The data of clusters 1 and 6 are more distributed, mainly located in the transition phase of the light–dark cycle.
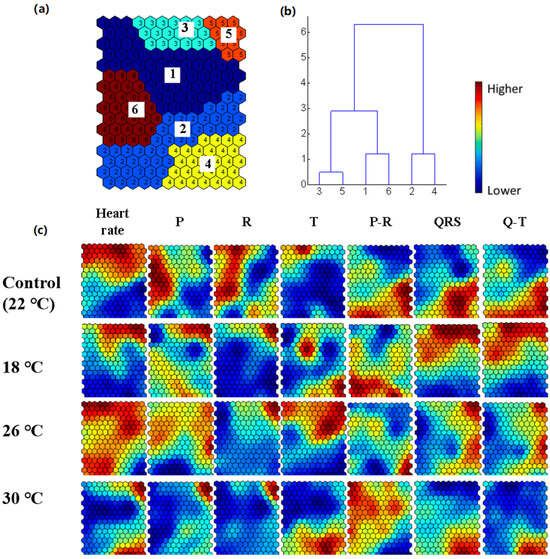
Figure 4.
SOM patterns of the carp heart ECG for 48 h. (a) Cluster map, (b) dendrogram of Ward’s linkage, and (c) SOM patterns. Dark colors represent the stress intensity, with blue indicating lower stress levels and red denoting higher stress levels.
Figure 4b represents the closeness between the clusters based on Ward’s linkage method. Cluster 3 is closely related to 5, cluster 1 to 6, and cluster 2 to 4, which is consistent with the temporal distribution of the light–dark cycle. In addition, clusters 3 and 5 are more closely related to clusters 1 and 6, but less closely related to clusters 2 and 4, indicating that the clusters within the same period are more closely related, further validating the rhythmic phenomenon in Cyprinus carpio.
The vertical rectangles in the figure indicate intensity changes, and the gradient from red to blue reflects the transition from high to low intensity. In the circadian rhythm analysis, in the SOM of the 18 °C experimental group, the low-intensity blue region extends to clusters 1 and 6, and the red region representing the higher intensity in the SOM of the 26 °C experimental group extends to clusters 1, 2, 3, 5, and 6, and the red region representing the higher intensity in the SOM of the 30 °C experimental group shows increased distribution randomness and decreased rhythmicity. This change may be due to the physiological stress caused by the high temperature, leading to the alteration of its circadian rhythm. Temperature stress disrupts the ability of Cyprinus carpio to regulate cardiac function, ultimately resulting in delayed cardiac function response in Cyprinus carpio.
3.3. Pearson Correlation Analysis
This study presents the results of the Pearson correlation analysis between the ECG indices and the temperatures in Table 1, highlighting the correlation coefficient (r) and significance (p) values. According to the analysis, the results show that the heart rate, P wave, R wave, and T wave were positively correlated with the temperature, while the P-R interval, QRS complex, and Q-T interval were negatively correlated with the temperature. Among these, the heart rate exhibited a strong correlation with the temperature, with a correlation coefficient of r = 0.844 and p < 0.01. The P-R interval showed an extremely strong correlation with the temperature, with a correlation coefficient of r = −0.860 and p < 0.001.

Table 1.
Correlation analysis between ECG and temperature in Cyprinus carpio.
4. Discussion
In a controlled laboratory environment, we effectively obtained electrocardiographic signals using either custom-made or commercially available ECG devices, such as the OCFAS [32]. Then, we compared the changes in the ECG indicators (heart rate, P wave, R wave, T wave, P-R interval, QRS complex, and Q-T interval) under different conditions. We have organized the experimental results of this study and the results of some previous fish studies into Table 2, and the conclusions are basically consistent.

Table 2.
Studies involving heart rate measurement of fish under different temperatures.
The responses of the heart rate of fishes under different temperatures are presented in Table 2, which provides the experience and basis for the study in this paper. In the temperature experiment, the average heart rate of the carp showed no significant difference between 18 °C and 22 °C but exhibited obvious differences compared to 26 °C and 30 °C. This finding is consistent with Kwon’s study on the heart rate of olive flounder [37]. Overall, from 18 °C to 30 °C, the heart rate of carp increases with rising temperatures. However, when the temperature reaches 30 °C, the increase in the heart rate is significantly limited. It can be concluded that within a certain temperature range, the higher the temperature, the higher the heart rate. But when the temperature exceeds a certain range, the heart rate of carp no longer increases with the rising temperatures. This phenomenon can be attributed to the temperature sensitivity of fish cardiac electrical excitability. Electrical excitability plays a pivotal role in initiating and regulating the rhythm and contraction speed of fish hearts, making it essential for temperature-related cardiac function adjustments [38]. Prolonged exposure to altered thermal environments triggers compensatory modifications in both ion channel expression and functionality, which can partially counterbalance the direct influence of temperature on the cardiac action potentials and heart rate [39]. Moreover, research indicates a significant positive correlation between the heart rate and oxygen consumption [40]. Since the cardiac output, defined as the blood volume pumped by the heart per unit time, is determined by the heart rate and stroke volume, any increase in the heart rate directly enhances the pumping performance of the heart [41,42]. Therefore, within a certain temperature scope, the higher the temperature, the higher the heart rate. This increase in the heart rate may be due to the elevated temperature leading to an increase in cardiac output to meet the higher oxygen demand [7,43].
Neither an increase nor a decrease in temperature affects the amplitude of the T wave in fish. This is consistent with the findings of Morita regarding goldfish at 10 °C and 25 °C [44]. The amplitudes of the R wave and T wave are determined by the projections of the depolarization and repolarization vectors on the ECG lead axis, respectively. Therefore, the ECG shows that, despite significant temperature changes, the directions of ventricular depolarization and repolarization remain unaltered. Simultaneously, observations reveal that the durations of the QRS complex and Q-T interval exhibit a negative correlation with the rising temperatures, showing a shortening effect. This is consistent with previous studies, indicating that these three intervals are not sensitive to temperature changes [37].
The heart rate is a reliable indicator of energy expenditure, showing a positive correlation with the metabolic rate [45]. Environmental-stress-induced increases in the heart rate elevate the total energy consumption, constrained by physical or physiological conditions [46]. Prolonged elevated heart rates due to physiological stress negatively impact individual health. Heart rate monitoring can be integrated into automated health monitoring across various environments [47,48]. Cardiovascular function and oxygen transport serve as critical connections between environmental factors and energy metabolism [49,50]. These physiological mechanisms at the individual level can collectively influence population-level responses to climate change. Among the abiotic factors, temperature plays a key role in determining the growth and survival of aquatic organisms. Extreme temperatures exceeding their thermal ranges often reduce their performance, health, and productivity. In fact, the heart rate is a major factor in the temperature-dependent regulation of blood circulation [7]. As the temperature increases, the increased heart rate leads to higher oxygen consumption, which increases the metabolic rate, creating more energy expenditure. The increase in the heart rate also prompts fish to surface for breathing, facilitating a rapid supply of oxygen [51]. Understanding the mechanisms of thermal response is crucial for predicting species and population vulnerability to climate change. Rising average temperatures associated with global climate change can significantly impact fish physiology, consequently affecting fish population health and piscine development.
In terms of aquaculture, it is recommended that the temperature should be strictly controlled within 22 °C–24 °C during aquaculture to prevent negative effects on the cardiac function of fish. At the same time, it is recommended to take temperature control measures in an environment with large temperature changes to avoid sudden temperature rises and drops (temperature changes do not exceed 2 °C/h). In addition, based on the high sensitivity of the heart rate to temperature changes, we suggest using ECG monitoring technology to track the effects of water temperature changes on cardiac function in real time and to adjust the temperature immediately when the heart rate is abnormal to reduce the physiological stress response of fish.
Many extrinsic and intrinsic factors can affect the ECG and heart rate of fish. From our laboratory and others’ research [52,53], it is evident that recording the amplitudes of various waves in fish electrocardiograms is quite challenging. This difficulty arises from the challenge of inserting two electrodes into relatively equal positions. Additionally, during the experiment, the behavior and activity of the fish can cause the electrodes to shift, which may contribute to significant experimental deviations in the ECG indicators of fish. In the future, our laboratory will continue to explore better methods for ECG monitoring. Although we currently have some understanding of how environmental temperature affects cardiac activity, much work remains to be done to elucidate the mechanisms underlying these patterns. Comparative studies on the ECG and heart rate of fish at different temperatures will enhance our understanding of the cardiac rhythms and activity patterns. Our laboratory will continue to conduct ECG recording experiments on carps at more temperatures to further explore the influence of temperature on the cardiac function of carps. Finally, it is urgent for governments and international organizations to raise awareness of the impacts of climate change among citizens to promote sustainable aquaculture productivity, thereby better protecting aquatic organisms under high-temperature stress.
Our current study has certain limitations, including a small sample size, technical limitations, and possible environmental influences on the results. Future studies with larger sample sizes, different species, and diverse environmental conditions are needed to make the findings broadly applicable. In addition, attention will be paid to long-term monitoring and validation in different ecological settings to ensure the applicability of our findings to various contexts. This is essential for applying these research findings to real scenarios.
5. Conclusions
This study selects carps as the main experimental subjects and monitors their cardiac function in real time based on the water temperature. ECGs are observed and recorded at temperatures of 18 °C, 22 °C, 26 °C, and 30 °C. By comparing different ECG parameters (heart rate, P wave, Q wave, R wave, S wave, T wave, P-R interval, QRS complex, and Q-T interval) under different temperatures, this study analyzes and evaluates the correlation between these factors and fish cardiac indicators. The results indicate that temperature variations impose physiological stress on the cardiac functions of fish, with their heart rates proving to be a quantitative measure. This research provides a scientific basis for optimizing carp breeding environments and improving their overall health. It also demonstrates that the online fish health assessment system is highly effective and feasible.
Author Contributions
Conceptualization, Z.R.; methodology, M.Y.; software, M.Y.; validation, Z.R.; formal analysis, M.Y.; investigation, M.Y.; resources, Z.R.; data curation, Z.R.; writing—original draft preparation, M.Y.; writing—review and editing, Z.R.; visualization, M.Y.; supervision, Z.R.; project administration, Z.R.; funding acquisition, Z.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (42077224).
Institutional Review Board Statement
To ensure the ethical conduct and compliance of the experiment, it was officially approved by the Animal Ethics Committee of Shandong Normal University (Approving AEECSDNU2024143). Furthermore, all the procedures were strictly performed in accordance with current animal welfare policies in China.
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors thank the following funding agencies: the National Natural Science Foundation of China (42077224), the Natural Science Foundation of Shandong (ZR2020MD122) and the National Foreign Youth Talent Program, Foreign Youth Talent 2022 (QN2022024001L), Department of Science and Technology Bureau, China.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pearson, H. The rise of eco-anxiety: Scientists wake up to the mental-health toll of climate change. Nature 2024, 628, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Glass, M.L.; Wood, S.C. Cardio-Respiratory Control in Vertebrates; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Gradil, K.J.; Garner, S.R.; Wilson, C.C.; Farrell, A.P.; Neff, B.D. Relationship between cardiac performance and environment across populations of Atlantic salmon (Salmo salar): A common garden experiment implicates local adaptation. Evol. Ecol. 2016, 30, 877–886. [Google Scholar] [CrossRef]
- Kraskura, K.; Hardison, E.A.; Eliason, E.J. Body size and temperature affect metabolic and cardiac thermal tolerance in fish. Sci. Clentific Rep. 2023, 13, 17900. [Google Scholar] [CrossRef] [PubMed]
- Anttila, K.; Casselman, M.T.; Schulte, P.M.; Farrell, A.P. Optimum Temperature in Juvenile Salmonids: Connecting Subcellular Indicators to Tissue Function and Whole-Organism Thermal Optimum. Physiol. Biochem. Zoolocgy 2013, 86, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Eliason, E.J.; Clark, T.D.; Hinch, S.G.; Farrell, A.P. Cardiorespiratory collapse at high temperature in swimming adult sockeye salmon. Conserv. Physiol. 2013, 1, cot008. [Google Scholar] [CrossRef]
- Steinhausen, M.F.; Sandblom, E.; Eliason, E.J.; Verhille, C.; Farrell, A.P. The effect of acute temperature increases on the cardiorespiratory performance of resting and swimming sockeye salmon (Oncorhynchus nerka). J. Exp. Biol. 2008, 211, 3915–3926. [Google Scholar] [CrossRef]
- Haixiu, W. Study on the Ecological Flow of Coreius Pawning During the Integrated Water Temperature and High Flow Pulse Process; Changjiang River Scientific Research Institute: Wuhan, China, 2020. [Google Scholar]
- Schwieterman, G.D.; Hardison, E.A.; Cox, G.K.; Van Wert, J.C.; Birnie-Gauvin, K.; Eliason, E.J. Mechanisms of cardiac collapse at high temperature in a marine teleost (Girella nigrians). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2023, 286, 111512. [Google Scholar] [CrossRef]
- Casselman, M.T.; Anttila, K.; Farrell, A.P. Using maximum heart rate as a rapid screening tool to determine optimum temperature for aerobic scope in Pacific salmon Oncorhynchus spp. J. Fish Biol. 2012, 80, 358–377. [Google Scholar] [CrossRef]
- Barry, H.; Iglesies-Grau, J.; Chaseling, G.K.; Paul, J.; Gosselin, C.; D’Oliviera-Sousa, C.; Juneau, M.; Harel, F.; Kaiser, D.; Pelletier-Galarneau, M.; et al. The Effect of Heat Exposure on Myocardial Blood Flow and Cardiovascular Function. Ann. Intern. Med. 2024, 177, 901–910. [Google Scholar] [CrossRef]
- Guo, J.; Xue, T.; Cao, M.; Han, X.Y.; Pan, Z.Y.; Huang, D.M.; Sun, W.; Mi, J.R.; Liu, Y.L.; Guan, T.J. Ambient temperature anomalies induce electrocardiogram abnormalities: Findings from a nationwide longitudinal study. Environ. Res. 2024, 246, 117996. [Google Scholar] [CrossRef]
- Svendsen, E.; Okland, F.; Fore, M.; Randeberg, L.L.; Finstad, B.; Olsen, R.E.; Alfredsen, J.A. Optical measurement of tissue perfusion changes as an alternative to electrocardiography for heart rate monitoring in Atlantic salmon (Salmo salar). Anim. Biotelemetry 2021, 9, 41. [Google Scholar] [CrossRef]
- Yousaf, M.N.; Ron, O.; Hagen, P.P.; McGurk, C. Monitoring fish welfare using heart rate bio-loggers in farmed Atlantic salmon (Salmo salar L.): An insight into the surgical recovery. Aquaculture 2022, 555, 738211. [Google Scholar] [CrossRef]
- Brijs, J.; Sandblom, E.; Axelsson, M.; Sundell, K.; Sundh, H.; Huyben, D.; Broström, R.; Kiessling, A.; Berg, C.; Gräns, A. The final countdown: Continuous physiological welfare evaluation of farmed fish during common aquaculture practices before and during harvest. Aquaculture 2018, 495, 903–911. [Google Scholar] [CrossRef]
- Lonthair, J.; Ern, R.; Esbaugh, A.J. The early life stages of an estuarine fish, the red drum (Sciaenops ocellatus), are tolerant to high pCO2. Ices J. Mar. Sci. 2017, 74, 1042–1050. [Google Scholar] [CrossRef]
- Campbell, H.A.; Egginton, S. The vagus nerve mediates cardio-respiratory coupling that changes with metabolic demand in a temperate nototheniod fish. J. Exp. Biol. 2007, 210, 2472–2480. [Google Scholar] [CrossRef]
- Lefrançois, C.; Claireaux, G. Influence of ambient oxygenation and temperature on metabolic scope and scope for heart rate in the common sole Solea solea. Mar. Ecol. Prog. Ser. 2003, 259, 273–284. [Google Scholar] [CrossRef]
- Selye, H. The Evolution of the Stress Concept: The originator of the concept traces its development from the discovery in 1936 of the alarm reaction to modern therapeutic applications of syntoxic and catatoxic hormones. Am. Sci. 1973, 61, 692–699. [Google Scholar] [PubMed]
- Wei, D.X. Studies on the Effects of Different Pollutants on ECG of Cyprinus Carpio. Master’s Thesis, Shandong Normal University, Jinan, China, 2024. [Google Scholar]
- Bakkers, J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc. Res. 2011, 91, 279–288. [Google Scholar] [CrossRef]
- Shen, Y.; Arablouei, R.; Hoog, F.d.; Malan, J.; Sharp, J.; Shouri, S.; Clark, T.D.; Lefevre, C.; Kroon, F.; Severati, A.; et al. Estimating Heart Rate and Detecting Feeding Events of Fish Using an Implantable Biologger. In Proceedings of the 2020 19th ACM/IEEE International Conference on Information Processing in Sensor Networks (IPSN), Sydney, NSW, Australia, 21–24 April 2020; pp. 37–48. [Google Scholar]
- Svendsen, E.; Føre, M.; Randeberg, L.L.; Olsen, R.E.; Finstad, B.; Remen, M.; Bloecher, N.; Alfredsen, J.A. ECG augmented pulse oximetry in Atlantic salmon (Salmo salar)—A pilot study. Comput. Electron. Agric. 2023, 212, 108081. [Google Scholar] [CrossRef]
- Le, T.; Zhang, J.; Nguyen, A.H.; Torres, R.S.T.; Vo, K.; Dutt, N.; Lee, J.; Ding, Y.H.; Xu, X.L.; Lau, M.P.H.; et al. A novel wireless ECG system for prolonged monitoring of multiple zebrafish for heart disease and drug screening studies. Biosens. Bioelectron. 2022, 197, 113808. [Google Scholar] [CrossRef]
- Deng, Y.C.; Hu, T.Y.; Chen, J.; Zeng, J.J.; Yang, J.Q.; Ke, Q.Z.; Miao, L.W.; Chen, Y.J.; Li, R.; Zhang, R.X.; et al. Non-invasive methods for heart rate measurement in fish based on photoplethysmography. J. Exp. Biol. 2024, 227, jeb246464. [Google Scholar] [CrossRef] [PubMed]
- Rabosky, D.L. Speciation rate and the diversity of fishes in freshwaters and the oceans. J. Biogeogr. 2020, 47, 1207–1217. [Google Scholar] [CrossRef]
- Winton, J.R. Fish health management. In Fish Hatchery Management, 2nd ed.; American Fisheries Society: Bethesda, MD, USA, 2001; pp. 559–640. [Google Scholar]
- Li, Y.; Chen, K.; Gui, B.; Yang, C.; He, L.; Liao, L.; Zhu, Z.; Wang, Y.; Huang, R. Effect of long-term temperature-controlled rearing on growth traits and sexual maturity of common carp (Cyprinus carpio). Aquaculture 2024, 593, 741271. [Google Scholar] [CrossRef]
- Ren, B. The Effect of Pollutants on the Behavior and Electrocardiogram of Some Cyprinidae Fishes. Master’s Thesis, Shandong Normal University, Jinan, China, 2021. [Google Scholar]
- Wang, B.; Ma, G.X.; Liu, Y.; Wang, Y.F.; Du, X.X.; Shi, Q.; Mao, H.P. Effects of Different Temperatures on the Antibacterial, Immune and Growth Performance of Crucian Carp Epidermal Mucus. Fishes 2021, 6, 66. [Google Scholar] [CrossRef]
- Wei, D.X.; Wang, L.; Poopal, R.K.; Ren, Z.M. IR-based device to acquire real-time online heart ECG signals of fish (Cyprinus carpio) to evaluate the water quality*. Environ. Pollut. 2023, 337. [Google Scholar] [CrossRef]
- Ren, B.X.; Yu, Y.X.; Poopal, R.K.; Qiao, L.L.; Ren, B.C.; Ren, Z.M. IR-Based Novel Device for Real-Time Online Acquisition of Fish Heart ECG Signals. Environ. Sci. Technol. 2022, 56, 4262–4271. [Google Scholar] [CrossRef]
- Anderson, W.G.; Booth, R.; Beddow, T.A.; McKinley, R.S.; Finstad, B.; Okland, F.; Scruton, D. Remote monitoring of heart rate as a measure of recovery in angled Atlantic salmon, Salmo salar (L.). Hydrobiologia 1998, 372, 233–240. [Google Scholar] [CrossRef]
- Nofrizal; Yanase, K.; Arimoto, T. Effect of temperature on the swimming endurance and post-exercise recovery of jack mackerel Trachurus japonicus as determined by ECG monitoring. Fish. Sci. 2009, 75, 1369–1375. [Google Scholar] [CrossRef]
- Nofrizal, N.; Arimoto, T. ECG monitoring on swimming endurance and heart rate of jack mackerel Trachurus japonicus during repeated exercise. Asian Fish. Sci. 2011, 20, 78–87. [Google Scholar]
- Riyanto, M.; Arimoto, T. Temperature effect on heart rate of jack mackerel Trachurus japonicus during swimming exercise. Fish. Sci. 2014, 80, 1241–1248. [Google Scholar] [CrossRef]
- Kwon, I.; Kim, T. Monitoring the effect of water temperature on the heart rate of olive flounder (Paralichthys olivaceus) using a bio-logger. Aquaculture 2023, 575, 739739. [Google Scholar] [CrossRef]
- Vornanen, M. The temperature dependence of electrical excitability in fish hearts. J. Exp. Biol. 2016, 219, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Ballesta, S.; Hanson, L.M.; Farrell, A.P. The effect of adrenaline on the temperature dependency of cardiac action potentials in pink salmon Oncorhynchus gorbuscha. J. Fish Biol. 2012, 80, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Hachim, M.; Rouyer, T.; Dutto, G.; Kerzerho, V.; Bernard, S.; Bourjea, J.; McKenzie, D.J. Oxygen uptake, heart rate and activities of locomotor muscles during a critical swimming speed protocol in the gilthead sea bream Sparus aurata. J. Fish Biol. 2021, 98, 886–890. [Google Scholar] [CrossRef]
- Kalinin, A.L.; Costa, M.J.; Rantin, F.T.; Glass, M.L. Effects of temperature on cardiac function in teleost fish. In Cardio-Respiratory Control in Vertebrates; Springer: Berlin/Heidelberg, Germany, 2009; pp. 121–160. [Google Scholar] [CrossRef]
- Haverinen, J.; Vornanen, M. Reduced ventricular excitability causes atrioventricular block and depression of heart rate in fish at critically high temperatures. J. Exp. Biol. 2020, 223, jeb225227. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, P.C.; Gamperl, A.K. The effects of acute changes in temperature and oxygen availability on cardiac performance in winter flounder (Pseudopleuronectes americanus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 155, 245–252. [Google Scholar] [CrossRef]
- Morita, A.; Tsukuda, H. The effect of thermal acclimation on the electrocardiogram of goldfish. J. Therm. Biol. 1994, 19, 343–348. [Google Scholar] [CrossRef]
- Halsey, L.G.; Green, J.A.; Twiss, S.D.; Arnold, W.; Burthe, S.J.; Butler, P.J.; Cooke, S.J.; Grémillet, D.; Ruf, T.; Hicks, O. Flexibility, variability and constraint in energy management patterns across vertebrate taxa revealed by long-term heart rate measurements. Funct. Ecol. 2019, 33, 260–272. [Google Scholar] [CrossRef]
- Wascher, C.A.F.; Kotrschal, K.; Arnold, W. Free-living greylag geese adjust their heart rates and body core temperatures to season and reproductive context. Sci. Rep. 2018, 8, 2142. [Google Scholar] [CrossRef]
- Berezowski, J.; Rüegg, S.R.; Faverjon, C. Complex system approaches for animal health surveillance. Front. Vet. Sci. 2019, 6, 153. [Google Scholar] [CrossRef]
- Bartlett, P.C.; Van Buren, J.W.; Neterer, M.; Zhou, C. Disease surveillance and referral bias in the veterinary medical database. Prev. Vet. Med. 2010, 94, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Wascher, C.A.F. Heart rate as a measure of emotional arousal in evolutionary biology. Philos. Trans. R. Soc. B 2021, 376, 20200479. [Google Scholar] [CrossRef] [PubMed]
- Little, A.G.; Loughland, I.; Seebacher, F. What do warming waters mean for fish physiology and fisheries? J. Fish Biol. 2020, 97, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.D.; Schneider, R.C.; Liggins, G.C.; Schuette, A.H.; Elliott, R.L.; Guppy, M.; Hochachka, P.W.; Qvist, J.; Falke, K.J.; Zapol, W.M. Heart-Rate and Body-Temperature during free diving of weddell seals. Am. J. Physiol. 1987, 253, R344–R351. [Google Scholar] [CrossRef]
- Arel, E.; Rolland, L.; Thireau, J.; Torrente, A.G.; Bechard, E.; Bride, J.; Jopling, C.; Demion, M.; Le Guennec, J.-Y. The effect of hypothermia and osmotic shock on the electrocardiogram of adult zebrafish. Biology 2022, 11, 603. [Google Scholar] [CrossRef]
- Le, T.; Lenning, M.; Clark, I.; Bhimani, I.; Fortunato, J.; Marsh, P.; Xu, X.L.; Cao, H. Acquisition, Processing and Analysis of Electrocardiogram in Awake Zebrafish. IEEE Sens. J. 2019, 19, 4283–4289. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).