Toxicological Efficiency Evaluation of the ASEC Technology for Contaminated Mining Water Using Lemna minor
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and ASEC Treatment
2.2. Toxicological Bioassays with Lemna minor
2.3. Statistical Analysis
3. Results
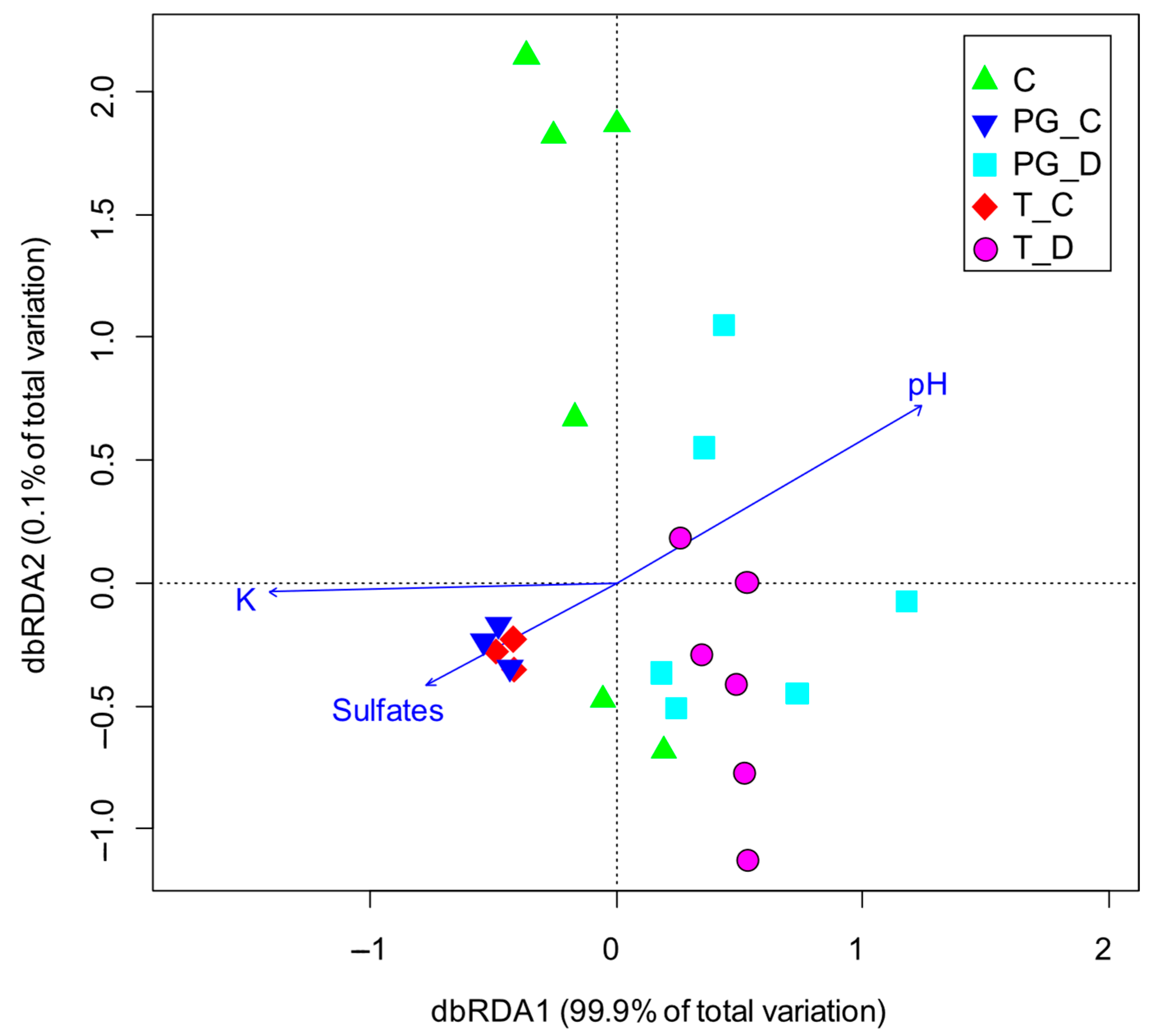
3.1. Physicochemical Characterization
3.2. Biological Responses of Lemna minor
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nieto, J.M.; Sarmiento, A.M.; Canovas, C.R.; Olías, M.; Ayora, C. Acid mine drainage in the Iberian Pyrite Belt: 1. Hydrochemical characteristics and pollutant load of the Tinto and Odiel rivers. Environ. Sci. Pollut. Res. 2013, 20, 7509–7519. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, A.M. Study of the Pollution by Acid Mine Drainage of the Surface Waters in the Odiel Basin (SW Spain). Ph.D. Thesis, University of Huelva, Huelva, Spain, 2007. Available online: https://produccioncientifica.uhu.es/documentos/60379bf0a28f0c5681f27e65?lang=en (accessed on 26 February 2025).
- Cánovas, C.R.; Olías, M.; Nieto, J.M.; Sarmiento, A.M.; Cerón, J.C. Influence of AMD pollution on water quality and metal bioaccumulation in a Mediterranean estuary: The Odiel–Tinto Estuary (SW Spain). Mar. Pollut. Bull. 2016, 104, 263–279. [Google Scholar] [CrossRef]
- Nieto, J.M.; Sarmiento, A.M.; Olías, M.; Cánovas, C.R.; Riba, I.; Kalman, J.; Delvalls, T.A. Acid mine drainage pollution in the Tinto and Odiel rivers (Iberian Pyrite Belt, SW Spain) and bioavailability of the transported metals to the Huelva Estuary. Environ. Int. 2007, 33, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Olías, M.; Cánovas, C.R.; Nieto, J.M.; Sarmiento, A.M. Evaluation of the dissolved contaminant load transported by the Tinto and Odiel rivers (South West Spain). Appl. Geochem. 2006, 21, 1733–1749. [Google Scholar] [CrossRef]
- Sarmiento, A.M.; Nieto, J.M.; Olías, M.; Cánovas, C.R. Hydrochemical characteristics and seasonal influence on the pollution by acid mine drainage in the Odiel river Basin (SW Spain). Appl. Geochem. 2009, 24, 697–714. [Google Scholar] [CrossRef]
- European Union’s Water Framework Directive (2000/60/EC). Available online: https://www.gov.ie/en/department-of-housing-local-government-and-heritage/publications/water-framework-directive/#:~:text=The%20EU%20Water%20Framework%20Directive%20(2000%2F60%2FEC),Water%20Policy)%20Regulations%202003%20(S.I (accessed on 15 May 2025).
- Sheoran, A.S.; Sheoran, V. Heavy metal removal mechanism of acid mine drainage in wetlands: A critical review. Miner. Eng. 2006, 19, 105–116. [Google Scholar] [CrossRef]
- Younger, P.L.; Banwart, S.A.; Hedin, R.S.; Younger, P.L.; Banwart, S.A.; Hedin, R.S. Mine water hydrology. In Mine Water: Hydrology, Pollution, Remediation; Springer: Heidelberg, The Netherlands, 2002; pp. 127–270. [Google Scholar]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Bonnail, E.; Vera, S.; Blasco, J.; Conradi, M.; DelValls, T.Á. Metal Pollution and Mining in the Iberian Pyrite Belt: New Remediation Technologies to Improve the Ecosystem Services of the River Basins. Water 2023, 15, 1302. [Google Scholar] [CrossRef]
- DelValls, T.Á.; Blasco, J.; Vera, S.; Núñez, N.O.; Bonnail, E. Decontamination and Circular Economy of Dredged Material and Mining Waters Using Adiabatic Sonic Evaporation and Crystallization (ASEC) Technology. Appl. Sci. 2024, 14, 11593. [Google Scholar] [CrossRef]
- Sarmiento, A.M.; DelValls, A.; Nieto, J.M.; Salamanca, M.J.; Caraballo, M.A. Toxicity and potential risk assessment of a river polluted by acid mine drainage in the Iberian Pyrite Belt (SW Spain). Sci. Total Environ. 2011, 409, 4763–4771. [Google Scholar] [CrossRef] [PubMed]
- Bonnail, E.; Sarmiento, A.M.; DelValls, T.A.; Nieto, J.M.; Riba, I. Assessment of metal contamination, bioavailability, toxicity and bioaccumulation in extreme metallic environments (Iberian Pyrite Belt) using Corbicula fluminea. Sci. Total Environ. 2016, 544, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Bonnail, E.; Lima, R.C.; Bautista–Chamizo, E.; Salamanca, M.J.; Cruz-Hernández, P. Biomarker responses of the freshwater clam Corbicula fluminea in acid mine drainage polluted systems. Environ. Pollut. 2018, 242, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Bonnail, E.; Macías, F.; Osta, V. Ecological improvement assessment of a passive remediation technology for acid mine drainage: Water quality biomonitoring using bivalves. Chemosphere 2019, 219, 695–703. [Google Scholar] [CrossRef] [PubMed]
- OECD 2002, Guideline 221. Available online: https://www.oecd.org/en/publications/test-no-221-lemna-sp-growth-inhabition-test_9789264016194-en.html (accessed on 11 June 2025).
- Mkandawire, M.; Dudel, E.G. Accumulation of arsenic in Lemna gibba L. (duckweed) in tailing waters of two abandoned uranium mining sites in Saxony, Germany. Sci. Total Environ. 2005, 336, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, P.; Sree, K.S.; Appenroth, K.J. Duckweeds for water remediation and toxicity testing. Toxicol. Environ. Chem. 2016, 98, 1127–1154. [Google Scholar] [CrossRef]
- Kindt, R.; Coe, R. Tree Diversity Analysis. A Manual and Software for Common Statistical Methods for Ecological and Biodiversity Studies; World Agroforestry Centre (ICRAF): Nairobi, Kenya, 2005. [Google Scholar]
- EMAESA, División de Calidad del Agua; Departamento de Control de Calidad. Valores Medios en la Red de Distribución (Sevilla Y Área Metropolitana). 2025. Available online: https://www.emasesa.com/wp-content/uploads/2025/01/Calidad-del-Agua.-Diciembre_2024.pdf (accessed on 22 May 2025).
- Landolt, E. The Family of Lemnaceae a Monographic Study, Vol. 1 of Biosystematic Investigations in the Family of Duckweeds (Lemnaceae); Veroffentlichungen des Geobotanischen, Instituts der ETH, Stiftung Rubel: Zurich, Switzerland, 1986. [Google Scholar]
- Landolt, E. Lemnaceae. In Flowering Plants· Monocotyledons: Alismatanae and Commelinanae (Except Gramineae); Springer: Berlin/Heidelberg, Germany, 1998; pp. 264–270. [Google Scholar]
- Tkalec, M.; Malari, K.; Pevalek-Kozlina, B. Influence of 400, 900, and 1900 MHz electromagnetic fields on Lemna minor growth and peroxidase activity. Bioelectromagnetics 2005, 26, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Ceschin, S.; Crescenzi, M.; Iannelli, M.A. Phytoremediation potential of the duckweeds Lemna minuta and Lemna minor to remove nutrients from treated waters. Environ. Sci. Pollut. Res. 2020, 27, 15806–15814. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Javed, A.; Baig, M.A. Growth and nutrient removal efficiency of duckweed (Lemna minor) from synthetic and dumpsite leachate under artificial and natural conditions. PLoS ONE 2019, 14, e0221755. [Google Scholar] [CrossRef] [PubMed]
- Van Hoeck, A.V.; Horemans, N.; Van Hees, M.; Nauts, R.; Knapen, D.; Vandenhove, H.; Blust, R. Radiation stress responses on growth and antioxidative defense system in plants: A study with strontium-90 in Lemna minor. Int. J. Mol. Sci. 2015, 16, 15309–15327. [Google Scholar] [CrossRef] [PubMed]
- Khellaf, N.; Zerdaoui, M. Growth response of the duckweed Lemna gibba L. to copper and nickel phytoaccumulation. Ecotoxicology 2010, 19, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Oros, V.; Toma, A. Ecotoxicological effects of heavy metals on duckweed plants (Lemna minor). II. Tests for growth rate reducing by the zinc. Sci. Bull. Ser. D Min. Miner. Process. Non-Ferr. Metall. Geol. Environ. Eng. 2012, 26, 15. [Google Scholar]
- Soucek, D.J.; Cherry, D.S.; Currie, R.J.; Latimer, H.A.; Trent, G.C. Laboratory to field validation in an integrative assessment of an acid mine drainage–impacted watershed. Environ. Toxicol. Chem. 2000, 19, 1036–1043. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, J. Toxicity of industrial wastewater to the aquatic plant Lemna minor L. J. Environ. Biol. 2006, 27, 385–390. [Google Scholar]
- Sasmaz Kislioglu, M. Removal of Ag, Au, and As from acid mine water using Lemna gibba and Lemna minor—A performance analysis. Water 2023, 15, 1293. [Google Scholar] [CrossRef]
- Gerhardt, A.; Janssens de Bisthoven, L.; Guhr, K.; Soares, A.M.V.M.; Pereira, M.J. Phytoecotoxicological assessment of acidic mining lakes: Bioassays with Lemna gibba and analysis of resident diatom communities. Ecotoxicology 2008, 17, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Abd Kadir, A.; Abdullah, S.R.S.; Othman, B.A.; Hasan, H.A.; Othman, A.R.; Imron, M.F.; Ismail, N.I.; Kurniawan, S.B. Dual function of Lemna minor and Azolla pinnata as phytoremediator for Palm Oil Mill Effluent and as feedstock. Chemosphere 2020, 259, 127468. [Google Scholar] [CrossRef] [PubMed]
- Abd Kadir, A.B.; Abdullah, S.R.S.; Hasan, H.A. Comparative phytotoxicity of Azolla pinnata and Lemna minor in treated palm oil mill effluent. Int. J. Eng. Technol. 2018, 7, 2499–2505. [Google Scholar] [CrossRef]
- Bokhari, S.H.; Ahmad, I.; Mahmood-ul-Hassan, M.; Mohammad, A. Phytoremediation potential of Lemna minor L. for heavy metals. Int. J. Phytoremed 2015, 18, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Ekperusi, A.O.; Sikoki, F.D.; Nwachukwu, E.O. Ecological remediation of heavy metals in crude oil polluted waters using duckweed. In SPE Nigeria Annual International Conference and Exhibition; SPE: Lagos, Nigeria, 2019; p. D023S004R002. [Google Scholar]
- Khellaf, N.; Djelal, H.; Amrane, A. An overview of the valorization of aquatic plants in effluent depuration through phytoremediation processes. Appl. Microbiol. 2022, 2, 309–318. [Google Scholar] [CrossRef]
- Li, J.; Yu, H.; Luan, Y. Meta-analysis of the copper, zinc, and cadmium absorption capacities of aquatic plants in heavy metalpolluted water. Int. J. Environ. Res. Public Health 2015, 12, 14958–14973. [Google Scholar] [CrossRef] [PubMed]
- Peeters, E.T.; Neefjes, R.E.; Zuidam, B.G.V. Competition between free-floating plants is strongly driven by previously experienced phosphorus concentrations in the water column. PLoS ONE 2016, 11, e0162780. [Google Scholar] [CrossRef] [PubMed]
- Jampeetong, A.; Sripakdee, T.; Khamphaya, T.; Chairuangsri, S. The Effects of Nitrogen as NO3− and NH4+ on the Growth and Symbiont (Anabaena azollae) of Azolla pinnata R. Brown. Chiang Mai Univ. J. Nat. Sci. 2016, 15, 11–20. [Google Scholar] [CrossRef]
- Wang, W.; Li, R.; Zhu, Q.; Tang, X.; Zhao, Q. Transcriptomic and physiological analysis of common duckweed Lemna minor responses to NH4+ toxicity. BMC Plant Biol. 2016, 16, 92. [Google Scholar] [CrossRef] [PubMed]
- Razinger, J.; Dermastia, M.; Koce, J.D.; Zrimec, A. Oxidative stress in duckweed (Lemna minor L.) caused by short-term cadmium exposure. Environ. Pollut. 2008, 153, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Radi, S.; Babi, M.; Skobić, D.; Roje, V.; Pevalek-Kozlina, B. Ecotoxicological effects of aluminium and zinc on growth and antioxidants in Lemna minor L. Ecotoxicol. Environ. Saf. 2010, 73, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Üçüncü, E.; Tunca, E.; Fikirdeşici, Ş.; Özkan, A.D.; Altındağ, A. Phytoremediation of Cu, Cr and Pb mixtures by Lemna minor. Bull. Environ. Contam. Toxicol. 2013, 91, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Üçüncü, E.; Tunca, E.; Fikirdeşici, Ş.; Altındağ, A. Decrease and increase profile of Cu, Cr and Pb during stable phase of removal by duckweed (Lemna minor L.). Int. J. Phytoremed. 2013, 15, 376–384. [Google Scholar] [CrossRef] [PubMed]
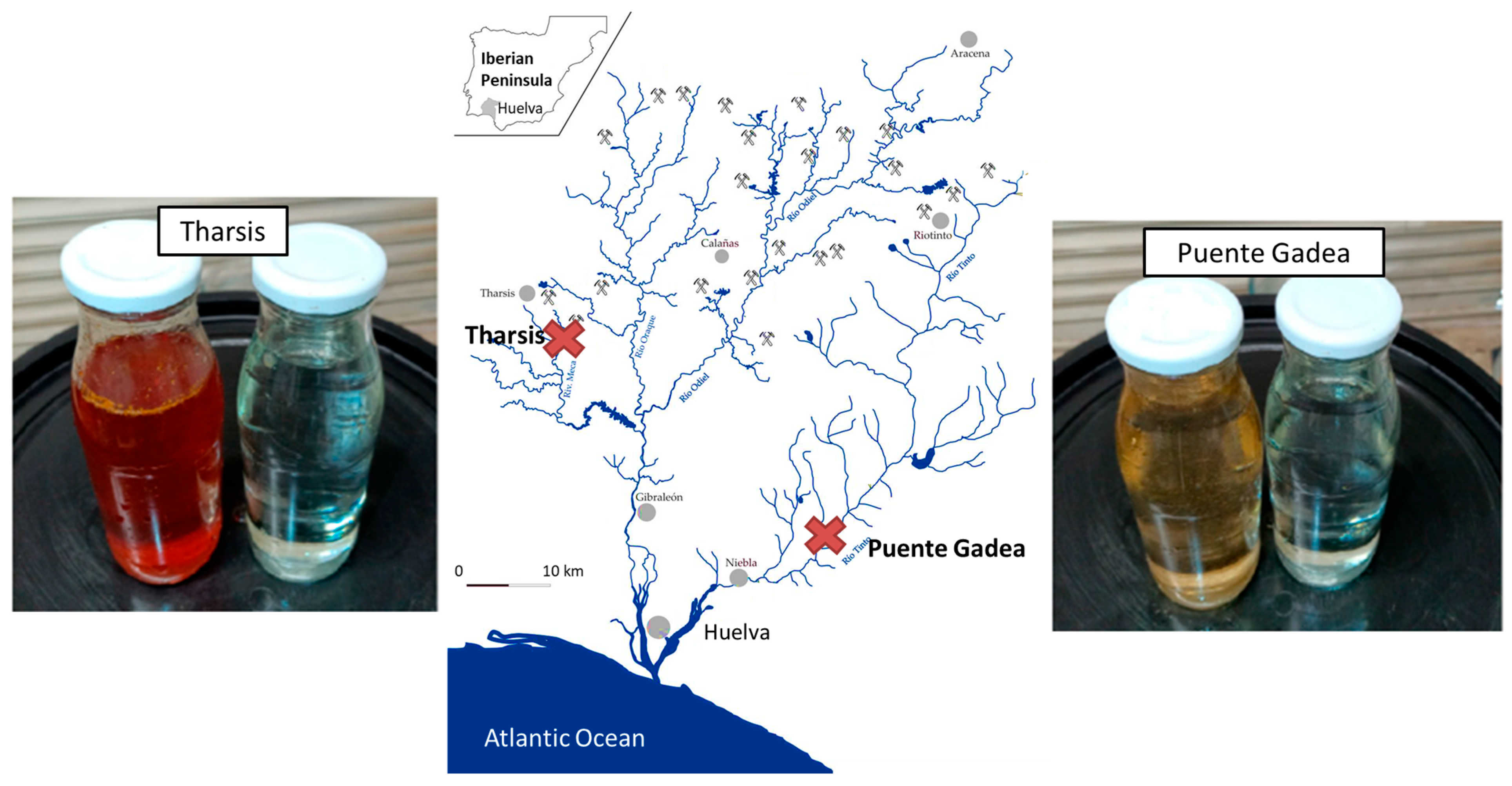

| Parameter | Control | Tharsis | Tharsis | Puente Gadea | Puente Gadea D |
|---|---|---|---|---|---|
| Type C | Type D | Type C | Type D | ||
| pHbioassays | 7.93 ± 0.17 | 2.32 ± 0.03 | 7.8 ± 0.09 | 2.57 ± 0.03 | 7.81 ± 0.09 |
| EC (µS/cm) | 269 | 12140 | 55.5 | 2450 | 35.8 |
| TDS (mg/L) | 55 | 7530 | 0.05 | 1516 | 0.05 |
| Fe (mg/L) | <DL | 2844.6 | <DL | 161.8 | <DL |
| Cu (mg/L) | <DL | 186.8 | <DL | 116.2 | <DL |
| Zn (mg/L) | <DL | 36.9 | <DL | 18.2 | <DL |
| As (mg/L) | <DL | 5.8 | <DL | 2.6 | <DL |
| Mg (mg/L) | 8.5 | 1.29 | <DL | 88.14 | <DL |
| K (mg/L) | 3 | 4.6 | <DL | 6.0 | <DL |
| Mn (mg/L) | <DL | 134 | <DL | 8.4 | <DL |
| Na (mg/L) | 12 | 67.44 | <DL | 41.65 | <DL |
| Ca (mg/L) | 33.3 | 20.2 | <DL | 4.3 | <DL |
| Cd (mg/L) | <DL | 0.09 | <DL | 0.97 | <DL |
| B (mg/L) | 0.02 | 0.09 | <DL | 0.25 | <DL |
| Co (mg/L) | <DL | 9.35 | <DL | 0.55 | <DL |
| Sulfates (mg/L) | 55 | 7300 | <DL | 8266.6 | <DL |
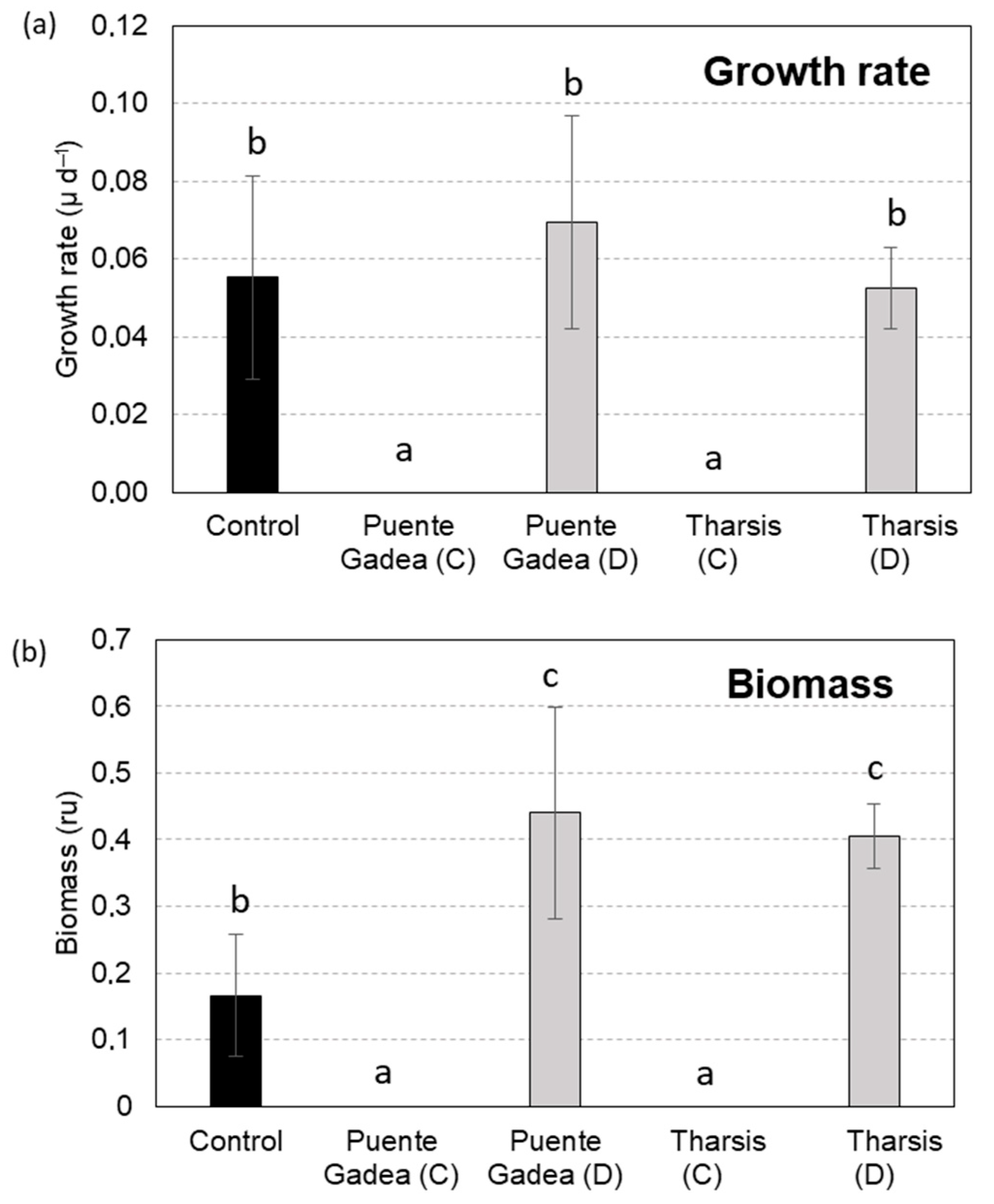
| Treatment | Growth Rate (µ, d−1) | %Inhibition Growth Rate | Biomass (Fronds) | %Inhibition Biomass |
|---|---|---|---|---|
| Control | 0.055 | - | 0.166 | - |
| Tharsis (Type C) | 0 | 100 | 0 | 100 |
| Tharsis (Type D) | 0.052 | 4.868 | 0.405 | 0 |
| Puente Gadea (C) | 0 | 100 | 0 | 100 |
| Puente Gadea (D) | 0.069 | 0 | 0.44 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conradi, M.; Sánchez-Moyano, J.E.; Bonnail, E.; DelValls, T.Á.; Riba, I. Toxicological Efficiency Evaluation of the ASEC Technology for Contaminated Mining Water Using Lemna minor. Water 2025, 17, 2175. https://doi.org/10.3390/w17152175
Conradi M, Sánchez-Moyano JE, Bonnail E, DelValls TÁ, Riba I. Toxicological Efficiency Evaluation of the ASEC Technology for Contaminated Mining Water Using Lemna minor. Water. 2025; 17(15):2175. https://doi.org/10.3390/w17152175
Chicago/Turabian StyleConradi, Mercedes, J. Emilio Sánchez-Moyano, Estefanía Bonnail, T. Ángel DelValls, and Inmaculada Riba. 2025. "Toxicological Efficiency Evaluation of the ASEC Technology for Contaminated Mining Water Using Lemna minor" Water 17, no. 15: 2175. https://doi.org/10.3390/w17152175
APA StyleConradi, M., Sánchez-Moyano, J. E., Bonnail, E., DelValls, T. Á., & Riba, I. (2025). Toxicological Efficiency Evaluation of the ASEC Technology for Contaminated Mining Water Using Lemna minor. Water, 17(15), 2175. https://doi.org/10.3390/w17152175







