Nutrient Monitoring and Comparison of On-Site Community Science Data Collection Methods for Indigenous Water Protection
Abstract
1. Introduction
2. Approach, Materials, and Methods
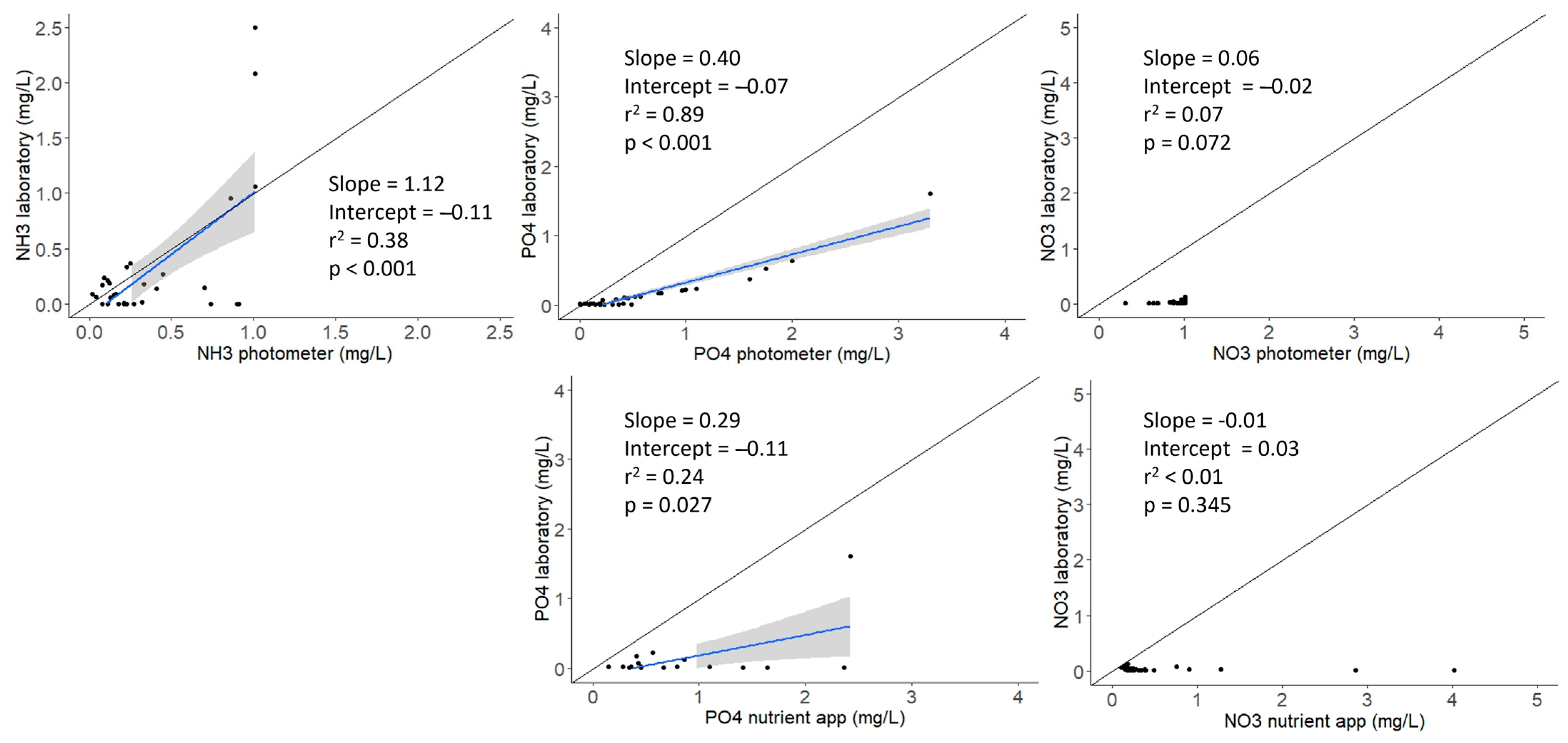
2.1. Assessing Community Science Device Accuracy
2.2. Identifying Nutrient Hotspot Identification
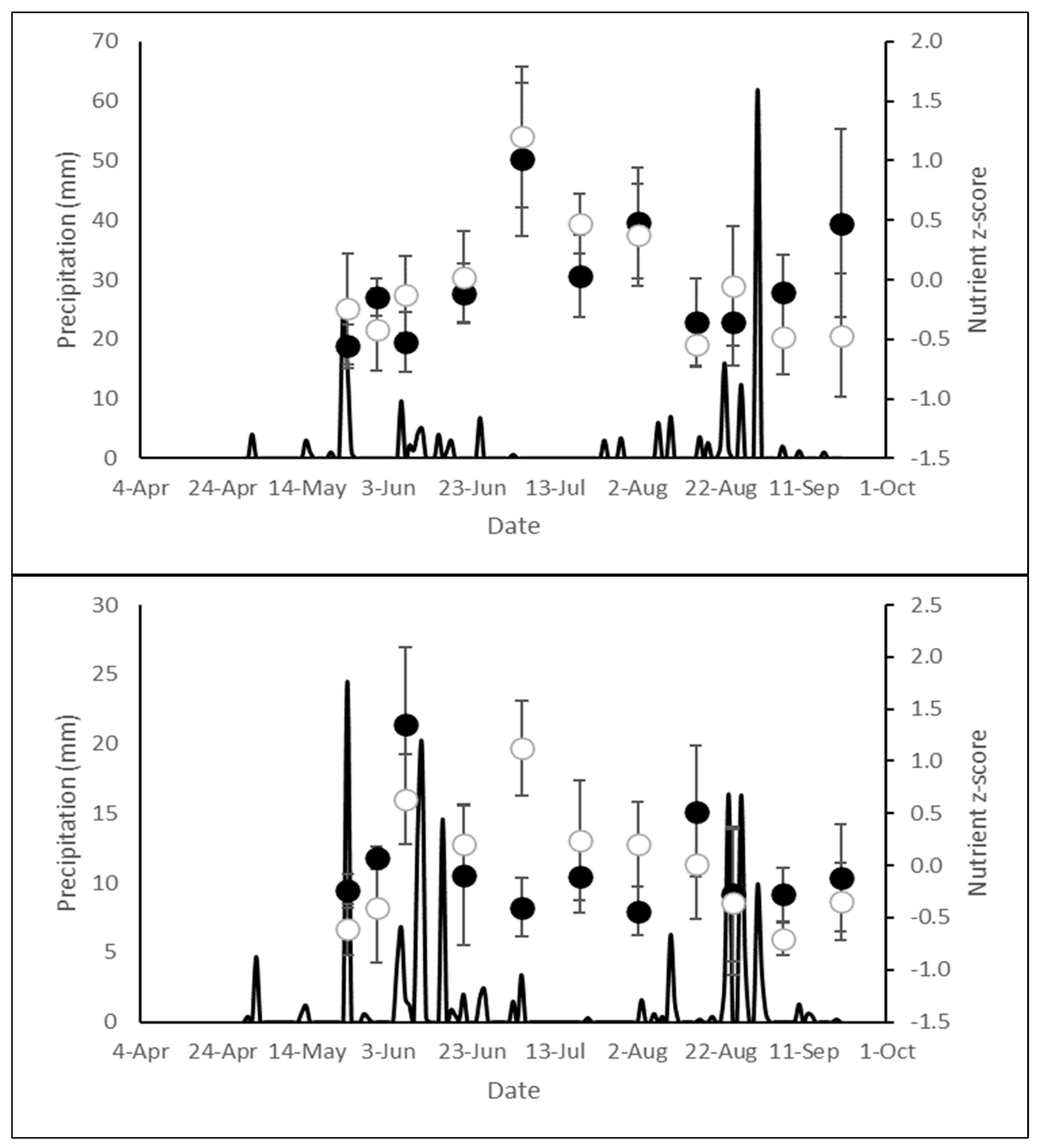
2.3. Nutrient Concentrations in Normal and Low Precipitation Years
3. Results
3.1. Community Science Device Accuracy
3.2. Nutrient Hotspot Identification
3.3. Nutrient Concentration Within and Between Years of Normal and Low Precipitation
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suresh, K.; Tang, T.; Van Vliet, M.T.; Bierkens, M.F.; Strokal, M.; Sorger-Domenigg, F.; Wada, Y. Recent advancement in water quality indicators for eutrophication in global freshwater lakes. Environ. Res. Lett. 2023, 18, 063004. [Google Scholar] [CrossRef]
- du Plessis, A. Persistent degradation: Global water quality challenges and required actions. One Earth 2022, 5, 129–131. [Google Scholar] [CrossRef]
- Beusen, A.H.; Bouwman, A.F. Future projections of river nutrient export to the global coastal ocean show persisting nitrogen and phosphorus distortion. Front. Water 2022, 4, 893585. [Google Scholar] [CrossRef]
- Ginger, L.J.; Zimmer, K.D.; Herwig, B.R.; Hanson, M.A.; Hobbs, W.O.; Small, G.E.; Cotner, J.B. Watershed vs. within-lake drivers of nitrogen: Phosphorus dynamics in shallow lakes. Ecol. Appl. 2017, 27, 2155–2169. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2011, 46, 1349–1363. [Google Scholar] [CrossRef]
- Kling, H.J.; Watson, S.B.; McCullough, G.K.; Stainton, M.P. Bloom development and phytoplankton succession in Lake Winnipeg: A comparison of historical records with recent data. Aquat. Ecosyst. Health Manag. 2011, 14, 219–224. [Google Scholar] [CrossRef]
- McKindles, K.M.; Zimba, P.V.; Chiu, A.S.; Watson, S.B.; Gutierrez, D.B.; Westrick, J.; Davis, T.W. A multiplex analysis of potentially toxic cyanobacteria in Lake Winnipeg during the 2013 bloom season. Toxins 2019, 11, 587. [Google Scholar] [CrossRef]
- Akhtar, M.K.; Simonovic, S.P.; Wibe, J.; MacGee, J. Future realities of climate change impacts: An integrated assessment study of Canada. Int. J. Glob. Warm. 2019, 17, 59–88. Available online: https://globalchange-uwo.ca/manuals/J226_ANEMI2_CDN_IJGW_2018.pdf (accessed on 10 April 2025). [CrossRef]
- Gmitrowicz-Iwan, J.; Ligęza, S.; Pranagal, J.; Smal, H.; Olenderek, H. Small floodplain reservoirs in the face of climate change—Sink or source of nutrients? Water 2020, 12, 3423. [Google Scholar] [CrossRef]
- Lan, J.; Liu, P.; Hu, X.; Zhu, S. Harmful algal blooms in eutrophic marine environments: Causes, monitoring, and treatment. Water 2024, 16, 2525. [Google Scholar] [CrossRef]
- Sutton, M.A.; Howard, C.M.; Brownlie, W.J.; Kanter, D.R.; de Vries, W.; Adhya, T.K.; Ometto, J.P.; Baron, J.S.; Winiwarter, W.; Ju, X.; et al. Global challenges for nitrogen science-policy interactions: Towards the international nitrogen management system (INMS) and improved coordination between multi-lateral environmental agreements. In Just Enough Nitrogen: Perspectives on How to Get There for Regions with too Much and too Little Nitrogen; Springer: Berlin/Heidelberg, Germany, 2020; pp. 517–560. [Google Scholar]
- Shaw, D.A.; Pietroniro, A.L.; Martz, L.W. Topographic analysis for the prairie pothole region of Western Canada. Hydrol. Process. 2013, 27, 3105–3114. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Boyer, G.L. Health impacts from cyanobacteria harmful algae blooms: Implications for the North American Great Lakes. Harmful Algae 2016, 54, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, H.; Baulch, H.; Gill, A.; Bharadwaj, L.; Bradford, L. Monitoring, managing, and communicating risk of harmful algal blooms (HABs) in recreational resources across Canada. Environ. Health Insights 2021, 15, 11786302211014401. [Google Scholar] [CrossRef]
- Brooks, B.W.; Lazorchak, J.M.; Howard, M.D.; Johnson, M.V.; Morton, S.L.; Perkins, D.A.; Reavie, E.D.; Scott, G.I.; Smith, S.A.; Steevens, J.A. Are harmful algal blooms becoming the greatest inland water quality threat to public health and aquatic ecosystems? Environ. Toxicol. Chem. 2016, 35, 6–13. [Google Scholar] [CrossRef]
- Collins, L.; McGregor, D.; Allen, S.; Murray, C.; Metcalfe, C. Source water protection planning for Ontario First Nations communities: Case studies identifying challenges and outcomes. Water 2017, 9, 550. [Google Scholar] [CrossRef]
- Galway, L.P. Boiling over: A descriptive analysis of drinking water advisories in First Nations communities in Ontario, Canada. Int. J. Environ. Res. Public Health 2016, 13, 505. [Google Scholar] [CrossRef]
- Patrick, R.J.; Grant, K.; Bharadwaj, L. Reclaiming indigenous planning as a pathway to local water security. Water 2019, 11, 936. [Google Scholar] [CrossRef]
- Lam, S.; Cunsolo, A.; Sawatzky, A.; Ford, J.; Harper, S.L. How does the media portray drinking water security in Indigenous communities in Canada? An analysis of Canadian newspaper coverage from 2000–2015. BMC Public Health 2017, 17, 282. [Google Scholar] [CrossRef]
- McLeod, L.; Bharadwaj, L.A.; Daigle, J.; Waldner, C.; Bradford, L.E. A quantitative analysis of drinking water advisories in Saskatchewan Indigenous and rural communities 2012–2016. Can. Water Resour. J. 2020, 45, 345–357. [Google Scholar] [CrossRef]
- De Loë, R.; Plummer, R. Climate Change, Adaptive Capacity, and Governance for Drinking Water in Canada. In Adaptive Capacity and Environmental Governance; Springer: Berlin/Heidelberg, Germany, 2010; pp. 157–178. [Google Scholar] [CrossRef]
- Kalcic, M.M.; Kirchhoff, C.; Bosch, N.; Muenich, R.L.; Murray, M.; Griffith Gardner, J.; Scavia, D. Engaging stakeholders to define feasible and desirable agricultural conservation in western Lake Erie watersheds. Environ. Sci. Technol. 2016, 50, 8135–8145. [Google Scholar] [CrossRef]
- de Figueiredo, D.R. Harmful Cyanobacterial Blooms: Going beyond the “Green” to Monitor and Predict HCBs. Hydrobiology 2024, 3, 11–30. [Google Scholar] [CrossRef]
- Leonard, K.; David-Chavez, D.; Smiles, D.; Jennings, L.; ‘Anolani Alegado, R.; Tsinnajinnie, L.; Manitowabi, J.; Arsenault, R.; Begay, R.L.; Kagawa-Viviani, A.; et al. Water Back: A review centering rematriation and Indigenous water research sovereignty. Water Altern. 2023, 16, 374–428. [Google Scholar]
- O’Donnell, E.; Kennedy, M.; Garrick, D.; Horne, A.; Woods, R. Cultural water and Indigenous water science. Science 2023, 381, 619–621. [Google Scholar] [CrossRef]
- Haque, M.I.; Rahman, M.T.; Nuruzzaman, M.; Prentice, C. Real-time water quality monitoring using IoT-based sensors: Current status and future prospects. Water 2022, 16, 1734. [Google Scholar] [CrossRef]
- MacDonald, R.J.; Jones, K.E.; Bradford, L. Indigenous water governance and community-based monitoring: Advancing self-determination in water security. Water 2023, 16, 934. [Google Scholar] [CrossRef]
- Capdevila, A.S.L.; Kokimova, A.; Ray, S.S.; Avellán, T.; Kim, J.; Kirschke, S. Success factors for citizen science projects in water quality monitoring. Sci. Total Environ. 2020, 728, 137843. [Google Scholar] [CrossRef]
- Conrad, C.C.; Hilchey, K.G. A review of citizen science and community-based environmental monitoring: Issues and opportunities. Environ. Monit. Assess. 2011, 176, 273–291. [Google Scholar] [CrossRef]
- Baijius, W.; Patrick, R.J. Planning around reserves: Probing the inclusion of First Nations in Saskatchewan’s watershed planning framework. Int. Indig. Policy J. 2019, 10, 5. [Google Scholar] [CrossRef]
- Pick, F.R. Blooming algae: A Canadian perspective on the rise of toxic cyanobacteria. Can. J. Fish. Aquat. Sci. 2016, 73, 1149–1158. [Google Scholar] [CrossRef]
- Kim, S.; Robson, C.; Zimmerman, T.; Pierce, J.; Haber, E.M. Creek watch: Pairing usefulness and usability for successful citizen science. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, Vancouver, BC, Canada, 7–12 May 2011; pp. 2125–2134. [Google Scholar] [CrossRef]
- Aceves-Bueno, E.; Adeleye, A.S.; Bradley, D.; Tyler Brandt, W.; Callery, P.; Feraud, M.; Garner, K.L.; Gentry, R.; Huang, Y.; McCullough, I.; et al. Citizen science as an approach for overcoming insufficient monitoring and inadequate stakeholder buy-in in adaptive management: Criteria and evidence. Ecosystems 2015, 18, 493–506. [Google Scholar] [CrossRef]
- Herman-Mercer, N.; Antweiler, R.; Wilson, N.; Mutter, E.; Toohey, R.; Schuster, P. Data Quality from a Community-Based, Water-Quality Monitoring Project in the Yukon River Basin. Citiz. Sci. Theory Pract. 2018, 3, 1. Available online: https://link.gale.com/apps/doc/A563813374/EAIM?u=usaskmain&sid=bookmark-EAIM&xid=c98b0fe2 (accessed on 10 April 2025). [CrossRef]
- Wilson, N.; Mutter, E.; Inkster, J.; Satterfield, T. Community-Based Monitoring as the practice of Indigenous governance: A case study of Indigenous-led water quality monitoring in the Yukon River Basin. J. Environ. Manag. 2018, 210, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Aziz, U.; Elliot, J.; Baulch, H.; Roy, B.; Schneider, K.; Pomeroy, J. The Nutrient App: Developing a smartphone application for on-site instantaneous community-based and monitoring. Environ. Model. Softw. Environ. Data News 2020, 133, 104829. [Google Scholar] [CrossRef]
- Newman, P.A.; Guta, A.; Black, T. Ethical considerations for qualitative research methods during the COVID-19 pandemic and other emergency situations: Navigating the virtual field. Int. J. Qual. Methods 2021, 20, 16094069211047823. [Google Scholar] [CrossRef]
- Waldner, C.L.; Alimezelli, H.T.; McLeod, L.; Zagozewski, R.; Bradford, L.E.; Bharadwaj, L.A. Self-reported effects of water on health in First Nations communities in Saskatchewan, Canada: Results from community-based participatory research. Environ. Health Insights 2017, 11, 1–13. [Google Scholar] [CrossRef]
- Wilderman, C.C.; McEver, C.; Bonney, R.; Dickinson, J.; Kelling, S.; Rosenberg, K. Models of community science: Design lessons from the field. In Proceedings of the Citizen Science Toolkit Conference, Cornell Laboratory of Ornithology, Ithaca, NY, USA, 20–23 June 2007; McEver, C., Bonney, R., Dickinson, J., Kelling, S., Rosenberg, K., Shirk, J.L., Eds.; National Science Foundation: Alexandria, VA, USA, 2007; Volume 1, pp. 83–96. Available online: https://www.researchgate.net/publication/283490639_Models_of_Community_Science_Design_Lessons_from_the_Field (accessed on 10 April 2025).
- Smith, V.H.; Tilman, G.D.; Nekola, J.C. Eutrophication: Impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 1999, 100, 179–196. [Google Scholar] [CrossRef]
- Allan, R.J.; Williams, J.D.H.; Joshi, S.R.; Warwick, W.F. Historical changes and relationship to internal loading of sediment phosphorus forms in hypertrophic prairie lakes. J. Environ. Qual. 1980, 9, 199–206. [Google Scholar] [CrossRef]
- Orihel, D.M.; Baulch, H.M.; Casson, N.J.; North, R.L.; Parsons, C.T.; Seckar, D.C.; Venkiteswaran, J.J. Internal phosphorus loading in Canadian fresh waters: A critical review and data analysis. Can. J. Fish. Aquat. Sci. 2017, 74, 2005–2029. [Google Scholar] [CrossRef]
- Hosseini, N.; Akomeah, E.; Davis, J.; Baulch, H.; Lindenschmidt, K. Water quality modelling of a prairie river-lake system. Environ. Sci. Pollut. Res. 2018, 25, 31190–31204. [Google Scholar] [CrossRef]
- Akomeah, E.; Chun, K.P.; Lindenschmidt, K. Dynamic water quality modelling and uncertainty analysis of phytoplankton and nutrient cycles for the upper South Saskatchewan River. Environ. Sci. Pollut. Res. 2015, 22, 18239–18251. [Google Scholar] [CrossRef]
- Haertel, L. Nutrient limitation of algal standing crops in shallow prairie lakes. Ecology 1976, 57, 664–678. [Google Scholar] [CrossRef]
- Haertel, L. A Long-Term Database for Plankton Populations and Nutrient Levels in Prairie Lakes; South Dakota Academy of Science: Madison, SD, USA, 2003; Volume 82, p. 169. [Google Scholar]
- Harris, T.D.; Smith, V.H.; Graham, J.L.; Van de Waal, D.B.; Tedesco, L.P.; Clercin, N. Combined effects of nitrogen to phosphorus and nitrate to ammonia ratios on cyanobacterial metabolite concentrations in eutrophic Midwestern USA reservoirs. Inland Waters 2016, 6, 199–210. [Google Scholar] [CrossRef]
- Crumpton, W.G.; Goldsborough, L.G. Nitrogen transformation and fate in prairie wetlands. Great Plains Res. 1998, 8, 57–72. [Google Scholar]
- Hergott, A.E. Prairie Potholes as Transformers on the Landscape: Exploring the Rates of Planktonic Nitrogen Uptake, DNRA, and Denitrification. Master’s Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2022. [Google Scholar]
- Kleinman, P.J.; Srinivasan, M.S.; Dell, C.J.; Schmidt, J.P.; Sharpley, A.N.; Bryant, R.B. Role of rainfall intensity and hydrology in nutrient transport via surface runoff. J. Environ. Qual. 2006, 35, 1248–1259. [Google Scholar] [CrossRef]
- Liu, R.; Wang, J.; Shi, J.; Chen, Y.; Sun, C.; Zhang, P.; Shen, Z. Runoff characteristics and nutrient loss mechanism from plain farmland under simulated rainfall conditions. Sci. Total Environ. 2014, 468, 1069–1077. [Google Scholar] [CrossRef]
- Timmons, D.R.; Holt, R.F. Nutrient losses in surface runoff from a native prairie. J. Environ. Qual. 1977, 6, 369–373. [Google Scholar] [CrossRef]
- Bosch, N.S.; Evans, M.A.; Scavia, D.; Allan, J.D. Interacting effects of climate change and agricultural BMPs on nutrient runoff entering Lake Erie. J. Great Lakes Res. 2014, 40, 581–589. [Google Scholar] [CrossRef]
- Nanayakkara, L.; Wissel, B. Preliminary investigation of lake-use patterns in prairie lakes, stakeholder perceptions, and resulting management implications. Lake Reserv. Manag. 2017, 33, 49–61. [Google Scholar] [CrossRef]
- Datta, R.; Chapola, J.; Lewis, K. Rethinking Indigenous Community-Led Water Sustainability: Decolonial and Relational Approaches in Western Canada. Water 2025, 17, 334. [Google Scholar] [CrossRef]



| Year | 2019 | 2021 |
|---|---|---|
| Study Period | 29 April–20 September | 29 April–20 September |
| Days with Precipitation | 30 days | 31 days |
| Total Precipitation | 249 mm | 194 mm |
| Average Temperature | 7.9 °C–20.9 °C | 8.9 °C–20.0 °C |
| Days over 30 °C | 2 days | 20 days |
| Total Samples Taken at YQFN (sites) | * 180 samples (9 sites) | * 80 samples (5 sites) |
| Total Samples Taken at JSCN (sites) | None | * 64 samples (4 sites) |
| Total Photometer Tests (nitrate, ammonia, phosphate) | * 459 tests (153 nitrate, 153 ammonia, 153 phosphate) | * 285 tests (95 nitrate, 95 ammonia, 95 phosphate) |
| Total Nutrient App Tests (nitrate, phosphate) | * 360 tests (180 nitrate, 180 phosphate) | * 183 tests (135 nitrate, 48 phosphate) |
| Total Laboratory (SmartChem) Tests (nitrate, ammonia, phosphate) | No lab tests were conducted | * 102 tests (34 nitrate, 34 ammonia, 34 phosphate) |
| Sample Site | Location | Year |
Ammonia (, mg/L) (Measured by Photometer) |
Ammonia () (Estimated Mean Value) |
Phosphate () (Measured by Photometer) | Phosphate (PO4, mg/L) (Estimated Mean Value) |
|---|---|---|---|---|---|---|
| Southeast End of Little Nut Lake | YQFN | 2019 | 0.48 ± 0.36 (17) | 0.40 | 2.23 ± 0.87 (17) | 0.82 |
| Pond by 756 | YQFN | 2019 | 0.56 ± 0.25 (17) | 0.50 | 1.99 ± 0.76 (17) | 0.73 |
| Nut Lake South End | YQFN | 2019 | 0.71 ± 0.24 (17) | 0.66 | 2.29 ± 1.46 (17) | 0.85 |
| Middle of Nut Lake | YQFN | 2019 | 0.91 ± 0.10 (16) | 0.88 | 3.90 ± 0.26 (16) | 1.49 |
| Nut Lake Dam | YQFN | 2019 | 0.56 ± 0.26 (17) | 0.50 | 0.96 ± 0.41 (17) | 0.31 |
| Nut Lake Outlet | YQFN | 2019 | 0.48 ± 0.15 (17) | 0.41 | 0.58 ± 0.18 (17) | 0.16 |
| Nut Lake West End Inlet | YQFN | 2019 | 0.65 ± 0.15 (17) | 0.59 | 1.46 ± 0.42 (17) | 0.51 |
| Nut Lake (average of five sites) | YQFN | 2019 | 0.65 ± 0.24 (84) | 0.60 | 1.81 ± 1.40 (84) | 0.42 |
| Wetland on 756 | YQFN | 2019 | 0.77 ± 0.24 (17) | 0.74 | 3.45 ± 0.57 (17) | 1.31 |
| Wetland on 35 | YQFN | 2019 | 0.72 ± 0.14 (17) | 0.68 | 1.28 ± 0.73 (17) | 0.44 |
| Carps Lake Outlet | YQFN | 2021 | 0.24 ± 0.25 (10) | 0.14 | 0.08 ± 0.09 (10) | <0.13 |
| Nut Lake Outlet | YQFN | 2021 | 0.54 ± 0.28 (11) | 0.48 | 0.36 ± 0.36(11) | <0.13 |
| Little Nut Lake Inlet | YQFN | 2021 | 0.23 ± 0.17 (9) | 0.12 | 0.59 ± 0.27(9) | 0.17 |
| Little Nut Lake Outlet | YQFN | 2021 | 0.55 ± 0.44 (10) | 0.49 | 0.06 ± 0.15 (10) | <0.13 |
| Duck Creek | JSCN | 2021 | 0.30 ± 0.22 (11) | 0.19 | 0.36 ± 0.27 (11) | <0.13 |
| North Saskatchewan River | JSCN | 2021 | 0.17 ± 0.11 (11) | 0.06 | 0.14 ± 0.15 (11) | <0.13 |
| Pehanon | JSCN | 2021 | 0.51 ± 0.31 (11) | 0.40 | 1.83 ± 0.88 (11) | 0.66 |
| Carrot River | JSCN | 2021 | 0.28± 0.24 (11) | 0.28 | 1.04± 0.51 (11) | 0.35 |
| Goose Hunting Creek | JSCN | 2021 | 0.20± 0.12 (11) | 0.11 | 0.36± 0.23 (11) | <0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porter, J.D.; Bradford, L.; Jardine, T.D.; Neapetung, M.; Bharadwaj, L.A.; Strickert, G.; Burns, J. Nutrient Monitoring and Comparison of On-Site Community Science Data Collection Methods for Indigenous Water Protection. Water 2025, 17, 1386. https://doi.org/10.3390/w17091386
Porter JD, Bradford L, Jardine TD, Neapetung M, Bharadwaj LA, Strickert G, Burns J. Nutrient Monitoring and Comparison of On-Site Community Science Data Collection Methods for Indigenous Water Protection. Water. 2025; 17(9):1386. https://doi.org/10.3390/w17091386
Chicago/Turabian StylePorter, Jaclyn D., Lori Bradford, Tim D. Jardine, Myron Neapetung, Lalita A. Bharadwaj, Graham Strickert, and Justin Burns. 2025. "Nutrient Monitoring and Comparison of On-Site Community Science Data Collection Methods for Indigenous Water Protection" Water 17, no. 9: 1386. https://doi.org/10.3390/w17091386
APA StylePorter, J. D., Bradford, L., Jardine, T. D., Neapetung, M., Bharadwaj, L. A., Strickert, G., & Burns, J. (2025). Nutrient Monitoring and Comparison of On-Site Community Science Data Collection Methods for Indigenous Water Protection. Water, 17(9), 1386. https://doi.org/10.3390/w17091386






