Testing Macrophyte-Based Assessment Tools Developed Under the EU Water Framework Directive for Application in a Caucasus Region Country (Armenia)
Abstract
1. Introduction
2. Materials and Methods
| IBMR Score | MIR Score (Type RW_wap) | MIR Score (Type PNp) | Trophic Status |
|---|---|---|---|
| >14 | ≥64.2 | ≥46.7 | High (Very good) |
| >12 ≤14 | ≥49.7 | ≥36.8 | Good |
| >10 ≤12 | ≥35.2 | ≥27 | Moderate |
| >8 ≤10 | ≥23.7 | ≥16.3 | Poor |
| ≤8 | <23.7 | <16.3 | Bad |
3. Results
3.1. Gaps in the Reference Lists
3.2. Assignment of River Sections to River Types
3.3. Diversity of Macrophytes and Their Metrics
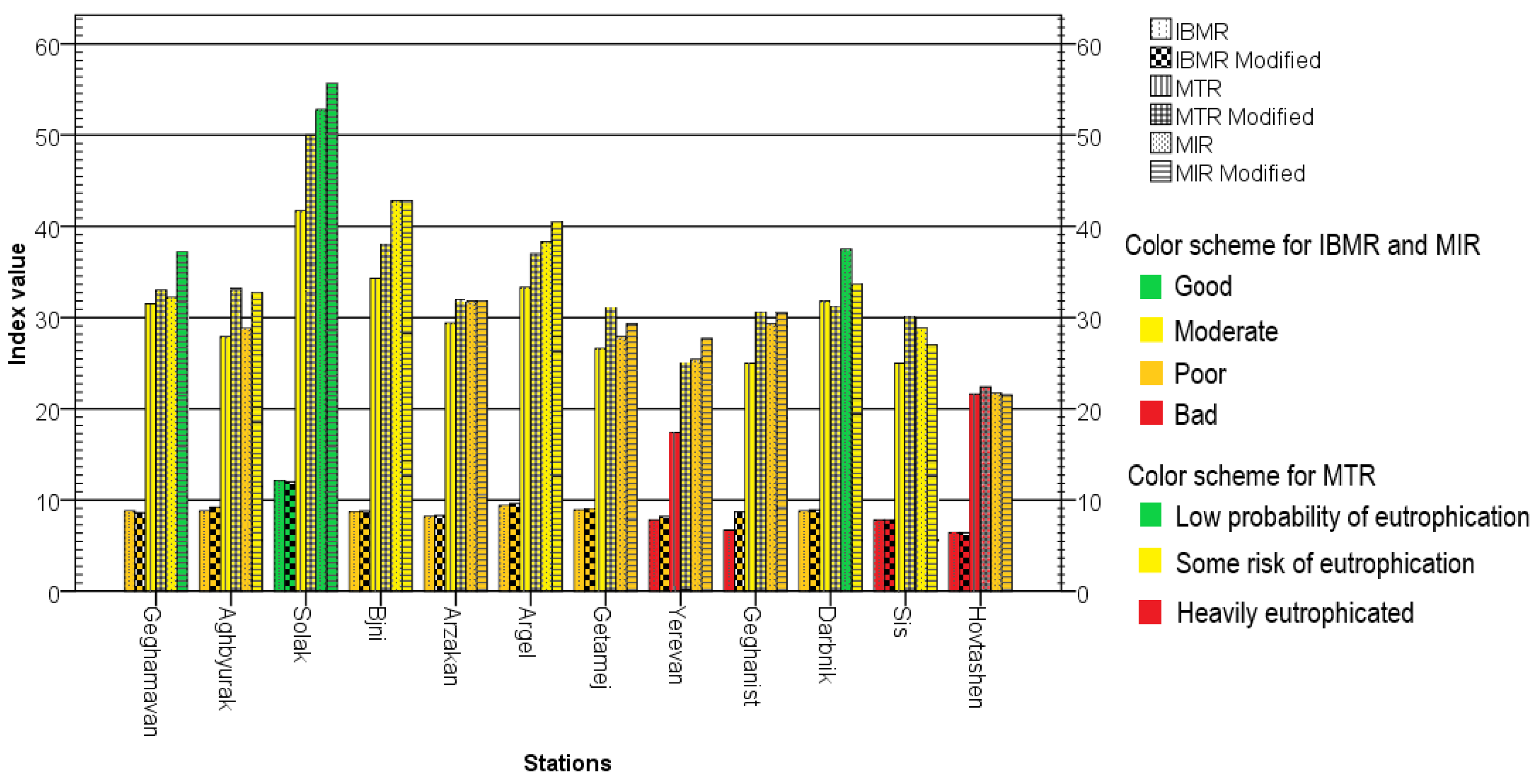
3.4. Assessment of Trophic Status
3.5. Concordance of Results
3.6. Validation of Results
4. Discussion
4.1. Limitations for the Use of EU WFD Macrophyte-Based Monitoring Tools in Mountain Regions Outside of the EU Countries
4.2. The Adaptation of EU-Based Indices for Armenia
4.3. The Reliability of Results
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Genus | Sampling Stations | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Ancylus | - | 1 | 62 | 4 | - | - | - | - | 1 | - | - | - |
| Apatania | - | - | 32 | - | - | - | 3 | - | - | - | - | - |
| Asellus | - | - | - | 9 | - | - | - | - | - | - | 1 | 2 |
| Baetis | 39 | 181 | 132 | 23 | 140 | 25 | 140 | - | - | - | 15 | - |
| Bithynia | - | - | - | 5 | - | - | 1 | 70 | 3 | - | - | - |
| Caenis | 8 | - | - | - | 9 | - | 2 | - | - | - | - | - |
| Calopteryx | - | - | - | - | 1 | - | - | - | - | - | - | - |
| Chaetopteryx | - | - | - | - | - | 12 | - | - | - | - | - | - |
| Chironomidae gen. | - | 208 | 208 | 5 | 8 | 0 | 15 | 105 | 108 | - | - | 136 |
| Corixa | - | - | - | - | - | - | - | - | - | - | 1 | 1 |
| Dicranota | - | - | - | - | - | 1 | - | - | - | - | - | - |
| Dugesia | - | 1 | 18 | 13 | - | - | 11 | 1 | - | - | - | - |
| Dytiscidae gen. | - | 2 | 2 | 1 | - | - | - | - | - | - | - | - |
| Ecdyonurus | - | - | - | 1 | - | 1 | - | - | - | - | - | - |
| Elmidae gen. ad | - | - | 13 | - | 2 | - | - | - | - | - | - | - |
| Elmidae gen. lv | 7 | - | 12 | 1 | 1 | 1 | - | - | - | - | - | - |
| Ephemerella | 2 | - | 20 | 9 | 4 | 18 | - | - | - | - | - | - |
| Eristalinus | - | - | - | - | - | - | - | - | - | 1 | - | - |
| Erpobdella | 1 | 3 | 3 | 31 | 1 | - | 2 | - | - | - | - | - |
| Gammarus | 16 | 18 | 1008 | 24 | 34 | - | 480 | 60 | - | - | 1 | 50 |
| Glossosoma | - | - | - | 1 | 1 | 3 | - | - | - | - | - | - |
| Haemopis | - | - | - | 1 | - | - | - | - | - | - | - | - |
| Halesus | - | - | - | 0 | 1 | - | - | - | - | - | - | - |
| Helobdella | - | 13 | - | 2 | - | - | 2 | 2 | - | - | - | - |
| Hydrachna | - | - | 1 | 2 | - | 5 | 1 | - | - | - | - | - |
| Hydropsyche | - | - | 6 | 2 | 1 | 22 | - | - | - | - | - | - |
| Hydroptila | 101 | 6 | 6 | 8 | 1 | - | - | - | - | - | - | - |
| Laccophilus | - | - | - | - | - | - | - | - | - | 1 | - | 1 |
| Habroleptoides | - | 2 | - | - | - | - | - | - | - | - | - | - |
| Limnephilidae gen. | - | - | - | 1 | - | - | - | - | - | - | - | - |
| Limnephilus | - | - | 1 | 1 | - | - | - | - | - | - | - | - |
| Lumbriculus | - | - | 2 | - | - | - | - | - | - | - | - | - |
| Lymnaea | - | 9 | - | - | - | - | - | 2 | - | - | - | - |
| Muscidae gen. | - | - | - | 1 | - | - | - | - | - | - | - | - |
| Naididae gen. | - | 24 | - | 1 | 2 | 1 | - | - | - | - | - | - |
| Tubifex | - | - | - | - | - | - | - | 7 | 12 | - | - | 37 |
| Perla | - | - | - | 1 | - | - | - | - | - | - | - | - |
| Physa | - | - | - | 5 | - | - | - | - | - | - | - | - |
| Physella | - | - | - | - | - | - | - | - | 1 | - | 2 | - |
| Planorbis | - | 1 | 1 | - | - | - | - | - | 2 | - | - | - |
| Plectrocnemia | - | - | 3 | - | - | - | - | - | - | - | - | - |
| Radix | - | - | - | - | - | - | - | - | 1 | - | - | - |
| Rhyacophila | - | - | 1 | - | 2 | 6 | 41 | - | - | - | - | - |
| Sigara | - | - | - | - | - | - | - | - | - | - | 1 | - |
| Simulium | 4 | 9 | 9 | 76 | 1 | 79 | 76 | 3 | 6 | - | - | - |
| Syrphidae gen. | - | - | - | - | - | - | - | - | - | 11 | - | - |
| Tabanus | - | - | - | 1 | - | - | 1 | - | - | - | - | - |
| Metrics | IBMR | Modified IBMR | MTR | Modified MTR_ | MIR | Modified MIR | |
|---|---|---|---|---|---|---|---|
| Number of taxa | Cor. Coeff. | 0.402 | 0.519 | 0.539 | 0.734 ** | 0.454 | 0.582 * |
| Sig. (2-tailed) | 0.195 | 0.084 | 0.071 | 0.007 | 0.138 | 0.047 | |
| BMWP score | Cor. Coeff. | 0.439 | 0.455 | 0.585 * | 0.755 ** | 0.455 | 0.594 * |
| Sig. (2-tailed) | 0.154 | 0.138 | 0.046 | 0.005 | 0.137 | 0.042 | |
| - Ntaxa | Cor. Coeff. | 0.424 | 0.519 | 0.534 | 0.737 ** | 0.424 | 0.586 * |
| Sig. (2-tailed) | 0.169 | 0.084 | 0.074 | 0.006 | 0.170 | 0.045 | |
| Average score per taxon | Cor. Coeff. | 0.702 * | 0.469 | 0.809 ** | 0.748 ** | 0.711 ** | 0.734 ** |
| Sig. (2-tailed) | 0.011 | 0.124 | 0.001 | 0.005 | 0.010 | 0.007 | |
| BMWP score (Czech version) | Cor. Coeff. | 0.432 | 0.434 | 0.579 * | 0.753 ** | 0.463 | 0.581 * |
| Sig. (2-tailed) | 0.160 | 0.158 | 0.049 | 0.005 | 0.129 | 0.047 | |
| - Ntaxa | Cor. Coeff. | 0.438 | 0.508 | 0.567 | 0.757 ** | 0.465 | 0.606 * |
| Sig. (2-tailed) | 0.155 | 0.092 | 0.055 | 0.004 | 0.128 | 0.037 | |
| Average score per taxon (Czech version) | Cor. Coeff. | 0.516 | 0.371 | 0.578 * | 0.699 * | 0.497 | 0.587 * |
| Sig. (2-tailed) | 0.086 | 0.236 | 0.049 | 0.011 | 0.100 | 0.045 | |
| IBE aqem | Cor. Coeff. | 0.527 | 0.567 | 0.547 | 0.766 ** | 0.394 | 0.575 |
| Sig. (2-tailed) | 0.079 | 0.054 | 0.066 | 0.004 | 0.205 | 0.051 | |
| Diversity (Shannon–Wiener Index) | Cor. Coeff. | 0.326 | 0.343 | 0.385 | 0.608 * | 0.315 | 0.510 |
| Sig. (2-tailed) | 0.301 | 0.276 | 0.216 | 0.036 | 0.318 | 0.090 | |
| Diversity (Margalef Index) | Cor. Coeff. | 0.337 | 0.441 | 0.543 | 0.713 ** | 0.487 | 0.517 |
| Sig. (2-tailed) | 0.284 | 0.152 | 0.068 | 0.009 | 0.108 | 0.085 | |
| Number of sensitive taxa (Austria) | Cor. Coeff. | 0.397 | 0.378 | 0.747 ** | 0.824 ** | 0.708 * | 0.757 ** |
| Sig. (2-tailed) | 0.202 | 0.225 | 0.005 | 0.001 | 0.010 | 0.004 | |
| - Heteroptera (%) | Cor. Coeff. | −0.537 | −0.640 * | −0.477 | −0.570 | −0.466 | −0.640 * |
| Sig. (2-tailed) | 0.072 | 0.025 | 0.117 | 0.053 | 0.127 | 0.025 | |
| - Trichoptera (%) | Cor. Coeff. | 0.649 * | 0.509 | 0.648 * | 0.712 ** | 0.520 | 0.657 * |
| Sig. (2-tailed) | 0.022 | 0.091 | 0.023 | 0.009 | 0.083 | 0.020 | |
| - Coleoptera (%) | Cor. Coeff. | 0.461 | 0.278 | 0.745 ** | 0.569 | 0.693 * | 0.683 * |
| Sig. (2-tailed) | 0.132 | 0.382 | 0.005 | 0.053 | 0.012 | 0.014 | |
| - Hydrachnidia (%) | Cor. Coeff. | 0.622 * | 0.628 * | 0.600 * | 0.599 * | 0.508 | 0.553 |
| Sig. (2-tailed) | 0.031 | 0.029 | 0.039 | 0.040 | 0.091 | 0.062 | |
| - EPT (%) (abundance classes) | Cor. Coeff. | 0.541 | 0.313 | 0.547 | 0.580 * | 0.496 | 0.481 |
| Sig. (2-tailed) | 0.069 | 0.322 | 0.066 | 0.048 | 0.101 | 0.114 | |
| - EPT taxa | Cor. Coeff. | 0.532 | 0.481 | 0.747 ** | 0.833 ** | 0.651 * | 0.693 * |
| Sig. (2-tailed) | 0.075 | 0.113 | 0.005 | 0.001 | 0.022 | 0.012 | |
| - EPT taxa (%) (Austria) | Cor. Coeff. | 0.598 * | 0.439 | 0.670 * | 0.735 ** | 0.586 * | 0.635 * |
| Sig. (2-tailed) | 0.040 | 0.154 | 0.017 | 0.007 | 0.045 | 0.027 | |
| - EP taxa | Cor. Coeff. | 0.428 | 0.389 | 0.628 * | 0.770 ** | 0.511 | 0.621 * |
| Sig. (2-tailed) | 0.165 | 0.212 | 0.029 | 0.003 | 0.089 | 0.031 | |
| - EPTCBO (Eph., Ple., Tri., Col., Bivalv., Odo.) | Cor. Coeff. | 0.570 | 0.491 | 0.804 ** | 0.851 ** | 0.676 * | 0.726 ** |
| Sig. (2-tailed) | 0.053 | 0.105 | 0.002 | 0.000 | 0.016 | 0.007 | |
| Number of families | Cor. Coeff. | 0.415 | 0.497 | 0.537 | 0.729 ** | 0.432 | 0.581 * |
| Sig. (2-tailed) | 0.180 | 0.100 | 0.072 | 0.007 | 0.161 | 0.047 | |
| Number of genera | Cor. Coeff. | 0.411 | 0.541 | 0.536 | 0.742 ** | 0.446 | 0.587 * |
| Sig. (2-tailed) | 0.184 | 0.070 | 0.072 | 0.006 | 0.146 | 0.045 | |
| - RETI | Cor. Coeff. | 0.481 | 0.476 | 0.497 | 0.685 * | 0.382 | 0.510 |
| Sig. (2-tailed) | 0.114 | 0.118 | 0.100 | 0.014 | 0.221 | 0.090 | |
| - (%) gatherers/collectors | Cor. Coeff. | −0.505 | −0.524 | −0.466 | −0.615 * | −0.431 | −0.524 |
| Sig. (2-tailed) | 0.094 | 0.080 | 0.127 | 0.033 | 0.162 | 0.080 | |
| - (%) shredders | Cor. Coeff. | −0.663 * | −0.395 | −0.303 | −0.174 | −0.185 | −0.267 |
| Sig. (2-tailed) | 0.019 | 0.204 | 0.338 | 0.588 | 0.564 | 0.402 | |
| - Trichoptera_taxa | Cor. Coeff. | 0.628 * | 0.590 * | 0.758 ** | 0.833 ** | 0.647 * | 0.717 ** |
| Sig. (2-tailed) | 0.029 | 0.044 | 0.004 | 0.001 | 0.023 | 0.009 | |
| Life Index | Cor. Coeff. | 0.575 | 0.434 | 0.536 | 0.622 * | 0.410 | 0.469 |
| Sig. (2-tailed) | 0.050 | 0.159 | 0.073 | 0.031 | 0.186 | 0.124 | |
| Total N of significant correlations | 6 | 3 | 13 | 24 | 7 | 18 |
| Metrics | IBMR | Adapted IBMR | MTR | Adapted MTR | MIR | Adapted MIR | |
|---|---|---|---|---|---|---|---|
| BOD5 mg/L | Cor. Coeff. | 0.636 | 0.467 | 0.700 * | 0.500 | 0.633 | 0.583 |
| Sig. (2-tailed) | 0.066 | 0.205 | 0.036 | 0.170 | 0.067 | 0.099 | |
| Nitrite ion mg/L | Cor. Coeff. | −0.686 * | −0.683 * | −0.717 * | −0.850 ** | −0.533 | −0.683 * |
| Sig. (2-tailed) | 0.041 | 0.042 | 0.030 | 0.004 | 0.139 | 0.042 | |
| Ammonium ion mg/L | Cor. Coeff. | −0.385 | −0.317 | −0.533 | −0.667 * | −0.450 | −0.433 |
| Sig. (2-tailed) | 0.306 | 0.406 | 0.139 | 0.050 | 0.224 | 0.244 | |
| Sulfate ion mg/L | Cor. Coeff. | −0.577 | −0.467 | −0.783 * | −0.817 ** | −0.683 * | −0.667 * |
| Sig. (2-tailed) | 0.104 | 0.205 | 0.013 | 0.007 | 0.042 | 0.050 | |
| Chloride ion mg/L | Cor. Coeff. | −0.762 * | −0.733 * | −0.333 | −0.500 | −0.167 | −0.267 |
| Sig. (2-tailed) | 0.017 | 0.025 | 0.381 | 0.170 | 0.668 | 0.488 | |
| Nitrate ion mg/L | Cor. Coeff. | −0.720 * | −0.717 * | −0.483 | −0.417 | −0.317 | −0.500 |
| Sig. (2-tailed) | 0.029 | 0.030 | 0.187 | 0.265 | 0.406 | 0.170 | |
| Hydrocarbonate ion mg/L | Cor. Coeff. | 0.502 | 0.576 | 0.780 * | 0.881 ** | 0.644 | 0.831 ** |
| Sig. (2-tailed) | 0.168 | 0.104 | 0.013 | 0.002 | 0.061 | 0.006 | |
| P mg/L | Cor. Coeff. | −0.603 | −0.667 * | −0.250 | −0.517 | −0.050 | −0.250 |
| Sig. (2-tailed) | 0.086 | 0.050 | 0.516 | 0.154 | 0.898 | 0.516 | |
| Ca mg/L | Cor. Coeff. | −0.695 * | −0.767 * | −0.150 | −0.250 | −0.017 | −0.200 |
| Sig. (2-tailed) | 0.038 | 0.016 | 0.700 | 0.516 | 0.966 | 0.606 | |
| Ti mg/L | Cor. Coeff. | −0.603 | −0.683 * | −0.067 | −0.267 | 0.017 | −0.100 |
| Sig. (2-tailed) | 0.086 | 0.042 | 0.865 | 0.488 | 0.966 | 0.798 | |
| V mg/L | Cor. Coeff. | −0.628 | −0.700 * | −0.667 * | −0.833 ** | −0.500 | −0.700 * |
| Sig. (2-tailed) | 0.070 | 0.036 | 0.050 | 0.005 | 0.170 | 0.036 | |
| Fe mg/L | Cor. Coeff. | −0.377 | −0.367 | −0.600 | −0.767 * | −0.517 | −0.550 |
| Sig. (2-tailed) | 0.318 | 0.332 | 0.088 | 0.016 | 0.154 | 0.125 | |
| Mn mg/L | Cor. Coeff. | −0.385 | −0.317 | −0.567 | −0.700 * | −0.450 | −0.467 |
| Sig. (2-tailed) | 0.306 | 0.406 | 0.112 | 0.036 | 0.224 | 0.205 | |
| Co mg/L | Cor. Coeff. | −0.628 | −0.667 * | −0.417 | −0.650 | −0.233 | −0.400 |
| Sig. (2-tailed) | 0.070 | 0.050 | 0.265 | 0.058 | 0.546 | 0.286 | |
| Ni mg/L | Cor. Coeff. | −0.669 * | −0.717 * | −0.200 | −0.400 | −0.117 | −0.200 |
| Sig. (2-tailed) | 0.049 | 0.030 | 0.606 | 0.286 | 0.765 | 0.606 | |
| Cu mg/L | Cor. Coeff. | −0.594 | −0.633 | −0.633 | −0.800 ** | −0.467 | −0.633 |
| Sig. (2-tailed) | 0.092 | 0.067 | 0.067 | 0.010 | 0.205 | 0.067 | |
| Mo mg/L | Cor. Coeff. | −0.368 | −0.233 | −0.767 * | −0.700 * | −0.650 | −0.650 |
| Sig. (2-tailed) | 0.330 | 0.546 | 0.016 | 0.036 | 0.058 | 0.058 | |
| Pb mg/L | Cor. Coeff. | −0.343 | −0.467 | −0.467 | −0.700 * | −0.400 | −0.533 |
| Sig. (2-tailed) | 0.366 | 0.205 | 0.205 | 0.036 | 0.286 | 0.139 | |
| Total N of significant correlations | 5 | 9 | 6 | 10 | 1 | 4 |
References
- UN-Water; Global Water Partnership (GWP). Roadmapping for Advancing Integrated Water Resources Management (IWRM) Processes; UN-Water: New York, NY, USA; GWP: Stockholm, Sweden, 2007. [Google Scholar]
- Hassing, J.; Ipsen, N.; Clausen, T.J.; Larsen, H.; Lindgaard-Jørgensen, P. Integrated Water Resources Management (IWRM) in Action; WWAP: Paris, France; DHI Water Policy: Copenhagen, Denmark; UNEP-DHI Centre for Water and Environment: Hørsholm, Denmark, 2009. [Google Scholar]
- Birk, S.; Schmedtje, U. Towards harmonization of water quality classification in the Danube River Basin: Overview of biological assessment methods for running waters. Arch. Hydrobiol. Suppl. Large Rivers 2005, 16, 171–196. [Google Scholar] [CrossRef]
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Official Journal L 327, 22/12/2000 P. 0001–0073. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32000L0060&from=DE (accessed on 14 June 2024).
- Karr, J.R.; Chu, E.W. Restoring Life in Running Waters; Island Press: Washington, DC, USA, 1999; 206p. [Google Scholar]
- Birk, S.; Bonne, W.; Borja, A.; Brucet, S.; Courrat, A.; Poikane, S.; Solimini, A.; van de Bund, W.; Zampoukas, N.; Hering, D. Three hundred ways to assess Europe’s surface waters: An almost complete overview of biological methods to implement the Water Framework Directive. Ecol. Indic. 2012, 18, 31–41. [Google Scholar] [CrossRef]
- Bytyqi, P.; Czikkely, M.; Shala-Abazi, A.; Fetoshi, O.; Ismaili, M.; Hyseni-Spahiu, M.; Prespa, Y.; Edona, K.-K.; Millaku, F. Macrophytes as biological indicators of organic pollution in the Lepenci River Basin in Kosovo. J. Freshw. Ecol. 2020, 35, 105–121. [Google Scholar] [CrossRef]
- Sentenac, H.; Loyau, A.; Leflaive, J.; Schmeller, D.S. The significance of biofilms to human, animal, plant and ecosystem health. Funct. Ecol. 2022, 36, 294–313. [Google Scholar] [CrossRef]
- Caraco, N.; Cole, J.; Findlay, S.; Wigand, C. Vascular plants as engineers of oxygen in aquatic systems. BioScience 2006, 56, 219–225. [Google Scholar] [CrossRef]
- Craig, J.F. A short review of pike ecology. Hydrobiologia 2008, 601, 5–16. [Google Scholar] [CrossRef]
- Bakker, E.S.; Wood, K.A.; Pagès, J.F.; Veen, G.F.C.; Christianen, M.J.A.; Santamaría, L.; Nolet, B.A.; Hilt, S. Herbivory on freshwater and marine macrophytes: A review and perspective. Aquat. Bot. 2006, 135, 18–36. [Google Scholar] [CrossRef]
- Janssen, A.B.G.; Hilt, S.; Kosten, S.; de Klein, J.J.M.; Paerl, H.W.; Van de Waal, D.B. Shifting states, shifting services: Linking regime shifts to changes in ecosystem services of shallow lakes. Freshw. Biol. 2020, 66, 1–12. [Google Scholar] [CrossRef]
- Aasim, M.; Bakhsh, A.; Sameeullah, M.; Karataş, M.; Khawar, K.M. Aquatic plants as human food. In Global Perspectives on Underutilized Crops; Ozturk, M., Hakeem, K.R., Ashraf, M., Ahmad, M.S.A., Eds.; Springer: Cham, Switzerland, 2018; pp. 165–187. [Google Scholar]
- Eliska, R. The role of macrophytes in wetland ecosystems. J. Ecol. Field Biol. 2011, 34, 333–345. [Google Scholar] [CrossRef]
- Costanza, R.; D’Arge, R.; de Groot, R.; Farber, S.; Grasso, M.; Hannon, B.; Limburg, K.; Naeem, S.; O’Neill, R.V.; Paruelo, J.; et al. The value of the world’s ecosystem services and natural capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- Veraart, A.J.; de Bruijne, W.J.J.; de Klein, J.J.M.; Peeters, E.T.H.M.; Scheffer, M. Effects of aquatic vegetation type on denitrification. Biogeochemistry 2011, 104, 267–274. [Google Scholar] [CrossRef]
- Cao, Q.; Wan, X.; Shu, X.; Xie, L. Bioaccumulation and detoxication of microcystin-LR in three submerged macrophytes: The important role of glutathione biosynthesis. Chemosphere 2019, 225, 935–942. [Google Scholar] [CrossRef]
- Santamaría, L. Why Are Most Aquatic Plants Widely Distributed? Dispersal, Clonal Growth and Small-Scale Heterogeneity in a Stressful Environment. Acta Oecologica 2002, 23, 137–154. [Google Scholar] [CrossRef]
- Szoszkiewicz, K.; Zbierska, J.; Staniszewski, R.; Jusik, S. The variability of macrophyte metrics used in river monitoring. Oceanol. Hydrobiol. Stud. 2009, XXXVIII, 117–126. [Google Scholar] [CrossRef]
- Pinto, P.; Morais, M.; Ilhe’u, M.; Sandin, L. Relationships among biological elements (macrophytes, macroinvertebrates and ichthyofauna) for different core river types across Europe at two different spatial scales. Hydrobiologia 2006, 566, 75–90. [Google Scholar] [CrossRef]
- Hering, D.; Feld, C.K.; Moog, O.; Ofenbock, T. Cook book for the development of a Multimetric Index for biological condition of aquatic ecosystems: Experiences from the European AQEM and STAR projects and related initiatives. Hydrobiologia 2006, 566, 311–324. [Google Scholar] [CrossRef]
- Szoszkiewicz, K.; Jusik, S.; Lewin, I.; Czerniawska-Kusza, I.; Kupiec, J.; Szostak, M. Macrophyte and macroinvertebrate patterns in unimpacted mountain rivers of two European ecoregions. Hydrobiologia 2018, 808, 327–342. [Google Scholar] [CrossRef]
- Gecheva, G.; Pall, K.; Todorov, M.; Traykov, I.; Gribacheva, N.; Stankova, S.; Birk, S. Anthropogenic Stressors in Upland Rivers: Aquatic Macrophyte Responses. A Case Study from Bulgaria. Plants 2021, 10, 2708. [Google Scholar] [CrossRef]
- Penning, W.; Mjelde, M.; Dudley, B.; Hellsten, S.; Hanganu, J.; Kolada, A.; Berg, M.; Poikane, S.; Phillips, G.; Willby, N.; et al. Classifying aquatic macrophytes as indicators of eutrophication in European lakes. Aquat. Ecol. 2008, 42, 237–251. [Google Scholar] [CrossRef]
- Alahuhta, J.; Rosbakh, S.; Chepinoga, V.; Heino, J. Environmental determinants of lake macrophyte communities in Baikal Siberia. Aquat. Sci. 2020, 82, 39. [Google Scholar] [CrossRef]
- Sinclair, J.S.; Mademann, J.A.; Haubrock, P.J.; Haase, P. Primarily neutral effects of river restoration on macroinvertebrates, macrophytes, and fishes after a decade of monitoring. Restor. Ecol. 2023, 31, e13840. [Google Scholar] [CrossRef]
- Hering, D.; Johnson, R.K.; Buffagni, A. Linking organism groups—Major results and conclusions from the STAR project. Hydrobiologia 2006, 566, 109–113. [Google Scholar] [CrossRef]
- Hering, D.; Johnson, R.K.; Kramm, S.; Szeszkiewicz, K.; Verdonschot, P.F.M. Assessment of European rivers with diatoms, macrophytes, invertebrates and fish: A comparative metric-based analysis of organism response to stress. Freshw. Biol. 2006, 51, 1757–1785. [Google Scholar] [CrossRef]
- Johnson, R.K.; Furse, M.T.; Hering, D.; Sandin, L. Ecological relationship between stream communities and spatial scale: Implication for designing catchment-level monitoring programs. Freshw. Biol. 2007, 52, 939–958. [Google Scholar] [CrossRef]
- Johnson, R.K.; Hering, D.; Furse, M.T.; Clarke, R.T. Detection of ecological change using multiple organism groups: Metrics and uncertainty. Hydrobiologia 2006, 566, 115–137. [Google Scholar] [CrossRef]
- Asatryan, V.; Dallakyan, M. Principles to develop a simplified multimetric index for the assessment of the ecological status of Armenian rivers on example of the Arpa River system. Environ. Monit. Assess. 2021, 193, 195. [Google Scholar] [CrossRef]
- Hlúbiková, D.; Wolfram, G.; Schaufler, K. Evaluation of the Diatom Analyses in Armenia and Moldova During the Survey 2019; Internal Report; European Union Water Initiative Plus for Eastern Partnership Countries (EUWI+): Results 2 and 3; Version AM_MD_Diatoms_02; May 2020; European Union Water Initiative: New York, NY, USA, 2020. [Google Scholar]
- Asatryan, V.L.; Dallakyan, M.R. Assessment of seasonal differences of ecological state of lotic ecosystems and applicability of some biotic indices in the basin of Lake Sevan (Armenia): Case study of Masrik River. Water Sci. Technol. Water Supply 2018, 19, 1238–1245. [Google Scholar] [CrossRef]
- Dallakyan, M.; Asatryan, V. Studying macrozoobenthos community and assessing the ecological status of the Tandzut River for improving hydrobiological monitoring system in Armenia. Ecosyst. Transform. 2021, 4, 24–31. [Google Scholar] [CrossRef]
- Barseghyan, A.M. Typical characteristics of wetland flora of Ararat valley. Ann. Bot. Inst. AS ArmSSR 1964, 14, 86–87. (In Russian) [Google Scholar]
- Barseghyan, A.M. Flora and vegetation of rivers and lakes of Armenia and their economic value. Ann. Bot. Inst. AS ArmSSR 1971, 17, 61. (In Russian) [Google Scholar]
- Yepremyan, H.V. Vascular Aquatic Plants of the Hrazdan and the Marmarik Rivers as Well as Their Role in Self-Purification Processes of the Hrazdan River. Ph.D. Dissertation, Scientific Center of Zoology and Hydroecology, Yerevan, Armenia, 2009; 127p. (In Armenian). [Google Scholar]
- Varadinova, E.; Gecheva, G.; Tyufekchieva, V.; Milkova, T. Macrophyte- and Macrozoobenthic-Based Assessment in Rivers: Specificity of the Response to Combined Physico-Chemical Stressors. Water 2023, 15, 2282. [Google Scholar] [CrossRef]
- Haury, J.; Peltre, M.C.; Tremolieres, M.; Barbe, J.; Thiebaut, G.; Bernez, I.; Daniel, H.; Chatenet, P.; Haan-Archipof, G.; Muller, S.; et al. A new method to assess water trophy and organic pollution—The Macrophyte Biological Index for Rivers (IBMR): Its application to different types of river and pollution. Hydrobiologia 2006, 570, 153–158. [Google Scholar] [CrossRef]
- Szoszkiewicz, K.; Zbierska, J.; Jusik, S.; Zgoła, T. Makrofitowa Metoda Oceny Rzek. Podręcznik Metodyczny do Oceny i Klasyfikacji Stanu Ekologicznego wód Płynących w Oparciu o Rośliny Wodne; Bogucki Wydawnictwo Naukowe: Poznań, Poland, 2010; p. 81. (In Polish) [Google Scholar]
- Holmes, N.T.H.; Newman, J.R.; Chadd, L.S.; Rouen, J.; Saint, L.; Dawson, F. Mean Trophic Rank: A User’s Manual; Environmental Agency: Bristol, UK, 1999; p. 134. [Google Scholar]
- Gecheva, G.; Cheshmedjiev, S.; Dimitrova, I.; Belkinova, D.; Mladenov, R. Implementation and Adaptation of Macrophyte Indication System: Assessment of Ecological Status of Rivers in Bulgaria According to the Water Framework Directive. Biotechnol. Biotechnol. Equip. 2014, 24, 171–180. [Google Scholar] [CrossRef]
- Haury, J.; Peltre, M.C.; Muller, S.; Tremolieres, M.; Barbe, J.; Dutatre, A.; Guerlesguim, M. Des indices macrophytiques pour estimer la qualite des cours d’eau francais: Premieres propositions. Ecologie 1996, 27, 233–244. Available online: https://hal.inrae.fr/hal-02575918 (accessed on 28 February 2025).
- Szoszkiewicz, K.; Jusik, S.; Pietruczuk, K.; Gebler, D. The Macrophyte Index for Rivers (MIR) as an Advantageous Approach to Running Water Assessment in Local Geographical Conditions. Water 2020, 12, 108. [Google Scholar] [CrossRef]
- Redakcja Zaktualizowanych Metodyk Monitoringu Biologicznych Elementów Oceny Stanu Ekologicznego wód Powierzchniowych Wraz z Recenzją Naukową i Publikacją. Załącznik do Sprawozdania: Anglojęzyczne Streszczenia Metodyk. 2020. Available online: https://www.gios.gov.pl/images/dokumenty/pms/monitoring_wod/Anglojezyczne_streszczenia_metodyk.pdf (accessed on 1 October 2021).
- Decree 1909-N. Decision of the Government of the Republic of Armenia on Approving the Management Plan for the 2022–2027 for the Hazdan Watershed Area. Available online: https://www.arlis.am/DocumentView.aspx?docid=171449 (accessed on 22 July 2024).
- Mnatsakanyan, B.P. Water Balance of Armenia; Zangak-97: Yerevan, Armenia, 2005; p. 16. (In Armenian) [Google Scholar]
- Asatryan, V.; Dallakyan, M. The changes of ecological status of the Hrazdan River under the impact of Aghbyurak dam. In Proceedings of the International Scientific Conference “Regularities of Formation and Impact of Marine and Atmospheric Hazardous Phenomena and Disasters on the Coastal Zone of the Russian Federation Under the Conditions of Global Climatic and Industrial Challenges, Rostov-on-Don, Russia, 21 June 2019. [Google Scholar]
- Yepremyan, H.; Asatryan, V.; Dallakyan, M. Structural changes of river ecosystems’ biological quality elements under conditions of mineral water flux. Electron. J. Nat. Sci. 2022, 38, 20–25. [Google Scholar] [CrossRef]
- Stepanyan, L.; Ghukasyan, E. Nutrient enrichment and its effect on the phytoplankton community of Hrazdan River in the Yerevan District, Republic of Armenia. Ecosyst. Transform. 2021, 4, 3–12. [Google Scholar] [CrossRef]
- Tepanosyan, G.; Harutyunyan, N.; Maghakyan, N.; Sahakyan, L. Potentially toxic elements contents and the associated potential ecological risk in the bottom sediments of Hrazdan river under the impact of Yerevan city (Armenia). Environ. Sci. Pollut. Res. 2022, 29, 36985–37003. [Google Scholar] [CrossRef]
- Asatryan, V.L.; Dallakyan, M.R.; Yepremyan, H.V.; Boshyan, T.V. The assessment of ecological state of the river Hrazdan by macrophytes and benthic fauna. Electron. J. Nat. Sci. 2012, 2, 51–56. [Google Scholar]
- EN ISO 10870:2012; Water Quality—Guidelines for the Selection of Sampling Methods and Devices for Benthic Macroinvertebrates in Fresh Waters. European Standard: Brussels, Belgium, 2012; pp. 1–36.
- EN 16150:2012; Water Quality—Guidance on Pro-Rata Multi-Habitat Sampling of Benthic Macro-Invertebrates from Wadeable Rivers. European Standard: Brussels, Belgium, 2012; pp. 1–16.
- Kutikova, L.A.; Starobogatov, Y.I. Key to Freshwater Invertebrates of European Part of USSR; Gidrometeoizdat: Leningrad, Russia, 1977. (In Russian) [Google Scholar]
- Ivanov, V.D.; Grigorenko, V.N.; Arefina, T.I. Trichoptera. In Key for Freshwater Invertebrates of Russia and Adjacent Territories; Tsalolokhina, S.Y., Ed.; Nauka: St. Petersbourg, Russia, 2001; pp. 1–825. (In Russian) [Google Scholar]
- LANUV. Bestimmungshilfen-Makrozoobenthos (1) 2010; LANUV Arbeitsblatt 14; Landesamt fur Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen: Duisburg, Germany, 2010. (In German) [Google Scholar]
- Waringer, J.; Graf, W. Atlas of Central European Trichoptera Larvae; Eric Mauch Verlag: Dinkelscherben, Germany, 2011; p. 468. [Google Scholar]
- Dallakyan, M.; Lipinskaya, T.; Asatryan, V.; Golovenchik, V.; Thormann, J.; von der Mark, L.; Astrin, J.J. Revealing Diversity in Gammarus (Amphipoda: Gammaridae) in the Freshwater Ecosystems of Armenia Using DNA Barcoding. Water 2023, 15, 3490. [Google Scholar] [CrossRef]
- Bain, M.B.; Finn, J.T.; Booke, H.E. Quantifying Stream Substrate for Habitat Analysis Studies. N. Am. J. Fish. Manag. 1985, 5, 499–500. [Google Scholar] [CrossRef]
- AQEM Consortium. Manual for the Application of the AQEM System. A Comprehensive Method to Assess European Streams Using Benthic Macro Invertebrates, Developed for the Purpose of the Water Framework Directive; AQEM Consortium, 2002. [Google Scholar]
- Ceschin, S.; Zuccarello, V.; Caneva, G. Role of macrophyte communities as bioindicators of water quality: Application on the Tiber River basin (Italy). Plant Biosyst. 2010, 144, 528–536. [Google Scholar] [CrossRef]
- Asatryan, V.; Keryan, T.; Radinger-Peer, V.; Dallakyan, M. Assessment of Cultural Ecosystem Services Potential in River Catchments in the Caucasus: Evidence from Dilijan National Park, Armenia. Mt. Res. Dev. 2024, 44, R1–R13. [Google Scholar] [CrossRef]
- Fayvush, G.; Aleksanyan, A.; Asatryan, V. Ecosystems of Armenia. In Biodiversity of Armenia; Fayvush, G., Ed.; Springer: Cham, Switzerland, 2023; pp. 19–92. [Google Scholar] [CrossRef]
- World Bank. South Caucasus in Motion. Poverty and Equity Global Practice. Europe and Central Asia; World Bank: Washington, DC, USA, 2019; p. 136. [Google Scholar]
- Szoszkiewicz, K.; Jusik, S.; Zgola, T.; Czechowska, M.; Hryc, B. Uncertainty of macrophyte-based monitoring for different types of lowland rivers. Belg. J. Bot. 2007, 140, 7–16. Available online: https://www.jstor.org/stable/20794619 (accessed on 28 February 2025).
- Kuljanishvili, T.; Epitashvili, G.; Freyhof, J.; Japoshvili, B.; Kalous, L.; Levin, B.; Mustafayev, N.; Ibrahimov, S.; Pipoyan, S.; Mumladze, L. Checklist of the freshwater fishes of Armenia, Azerbaijan and Georgia. J. Appl. Ichthyol. 2020, 36, 501–514. [Google Scholar] [CrossRef]
- Brabec, K.; Szoszkiewicz, K. Macrophytes and diatoms—Major results and conclusions from the STAR project. In The Ecological Status of European Rivers: Evaluation and Intercalibration of Assessment Methods. Developments in Hydrobiology; Furse, M.T., Hering, D., Brabec, K., Buffagni, A., Sandin, L., Verdonschot, P.F.M., Eds.; Springer: Dordrecht, The Netherlands, 2006; p. 188. [Google Scholar] [CrossRef]
- Birk, S.B.; Willby, N.J. Towards harmonization of ecological quality classification: Establishing common grounds in European macrophyte assessment for rivers. Hydrobiologia 2010, 652, 149–163. [Google Scholar] [CrossRef]
- Szoszkiewicz, K.; Karolewicz, K.; Ławniczak, A.; Dawson, F.H. An assessment of the MTR aquatic plant bioindication system for determining the trophic status of Polish rivers. Pol. J. Environ. Stud. 2002, 11, 421–427. [Google Scholar]
- Muratov, R.; Szoszkiewicz, K.; Zhamangara, A.; Jusik, S.; Gebler, D.; Beisenova, R.; Akbayeva, L. An attempt to prepare Macrophyte Index for Rivers for assessment watercourses in Kazakhstan. Meteorol. Hydrol. Water Manag. 2015, 3, 27–32. [Google Scholar] [CrossRef]
- Furse, M.; Hering, D.; Moog, O.; Verdonschot, P.; Johnson, R.; Brabec, K.; Gritzalis, K.; Buffagni, A.; Pinto, P.; Friberg, N.; et al. The STAR project: Context, objectives and approaches. In The Ecological Status of European Rivers: Evaluation and Intercalibration of Assessment Methods. Developments in Hydrobiology; Furse, M.T., Hering, D., Brabec, K., Buffagni, A., Sandin, L., Verdonschot, P.F.M., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 188, pp. 3–29. [Google Scholar] [CrossRef]
- Kobelyan, H.; Gevorgyan, G. Hydroecologica investigation of the Hrazdan River and “Yerevanyan lich” reservoir, Armenia. Biol. J. Armen. 2019, 4, 93–99. [Google Scholar]
- Yepremyan, H.; Kobelyan, H.; Mkrtchyan, Z.; Hakobyan, S.; Ghukasyan, E. Assessment of the ecological state of the Hrazdan River. Biol. J. Armen. 2022, 2, 71–75. [Google Scholar]
- Grinberga, L. Environmental factors influencing the species diversity of macrophytes in middle-sized streams in Latvia. Hydrobiologia 2010, 656, 233–241. [Google Scholar] [CrossRef]
- Statistical Committee of RA. RA Kotayk Marz in Figures. 2021. Available online: https://armstat.am/en/?nid=847 (accessed on 25 November 2021).
- Soufi, R.; Vidinova, Y.; Tyufekchieva, V.; Evtimova, V.; Stoianova, D.; Kerakova, M.; Georgieva, G.; Stoichev, S.; Dedov, I.; Wolfram, G. Intercalibration of macroinvertebrate-based method for status assessment of Bulgarian tributaries of the Danube River. Ecol. Balk. 2018, 10, 63–72. [Google Scholar]
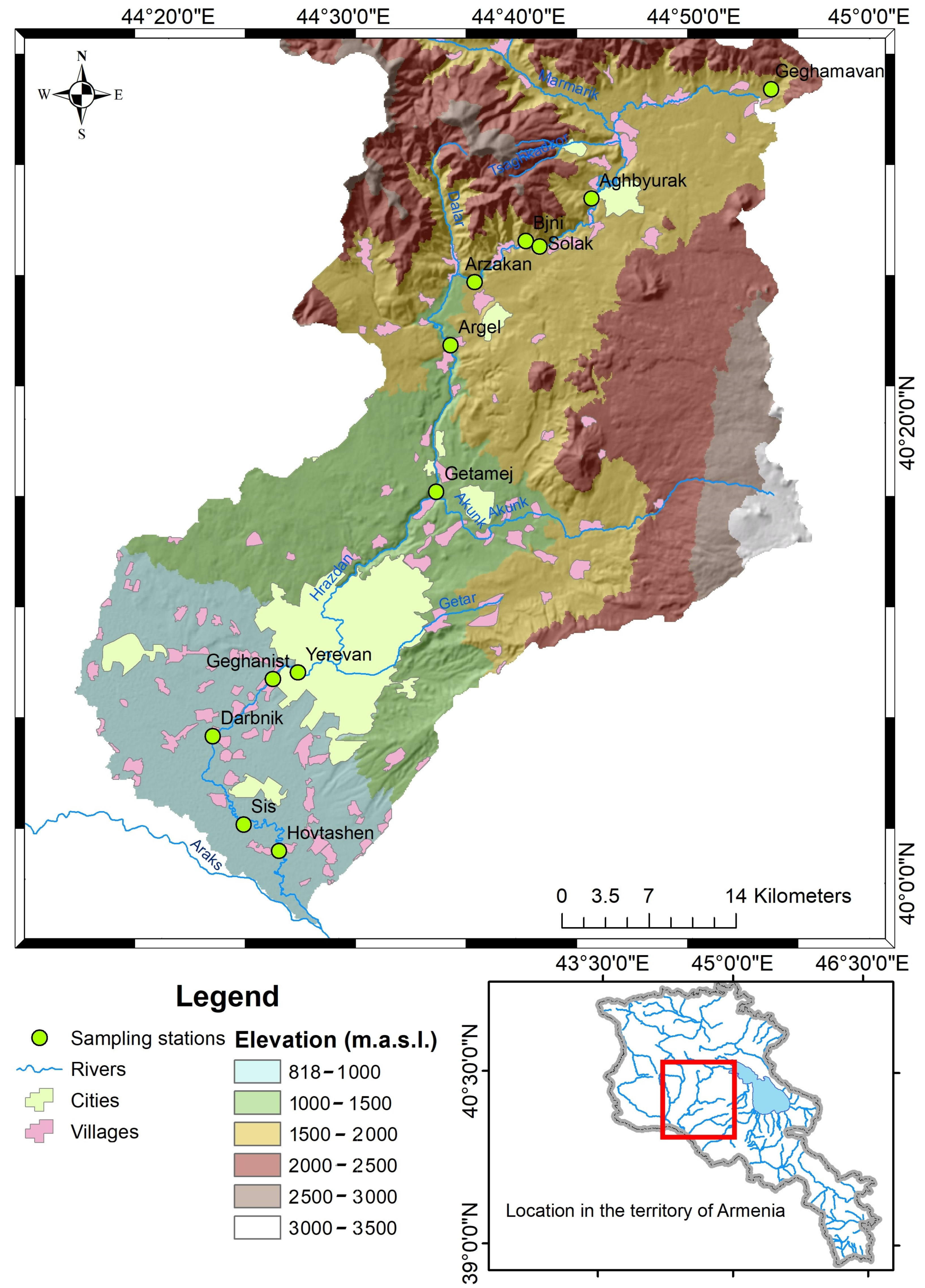
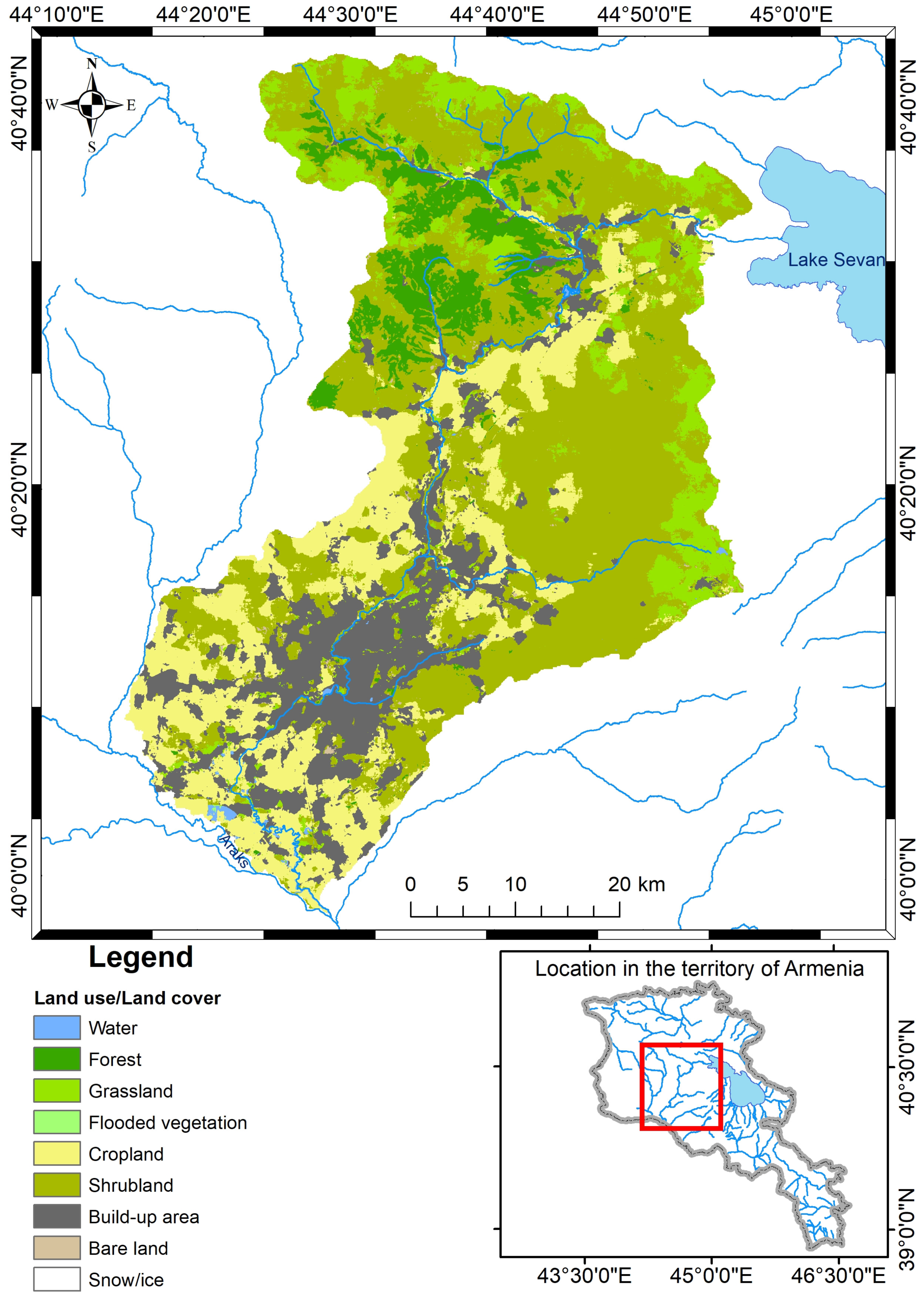
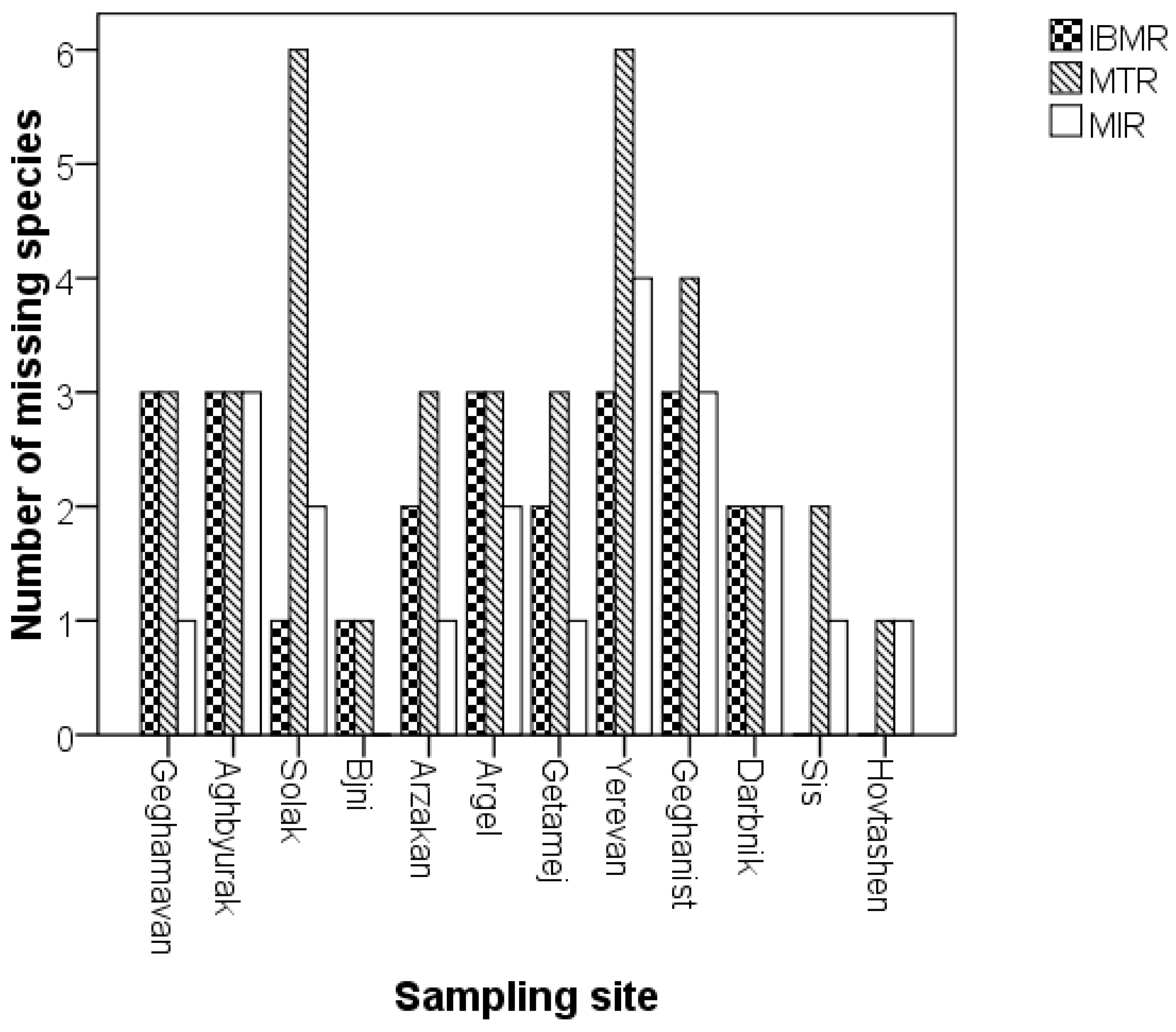
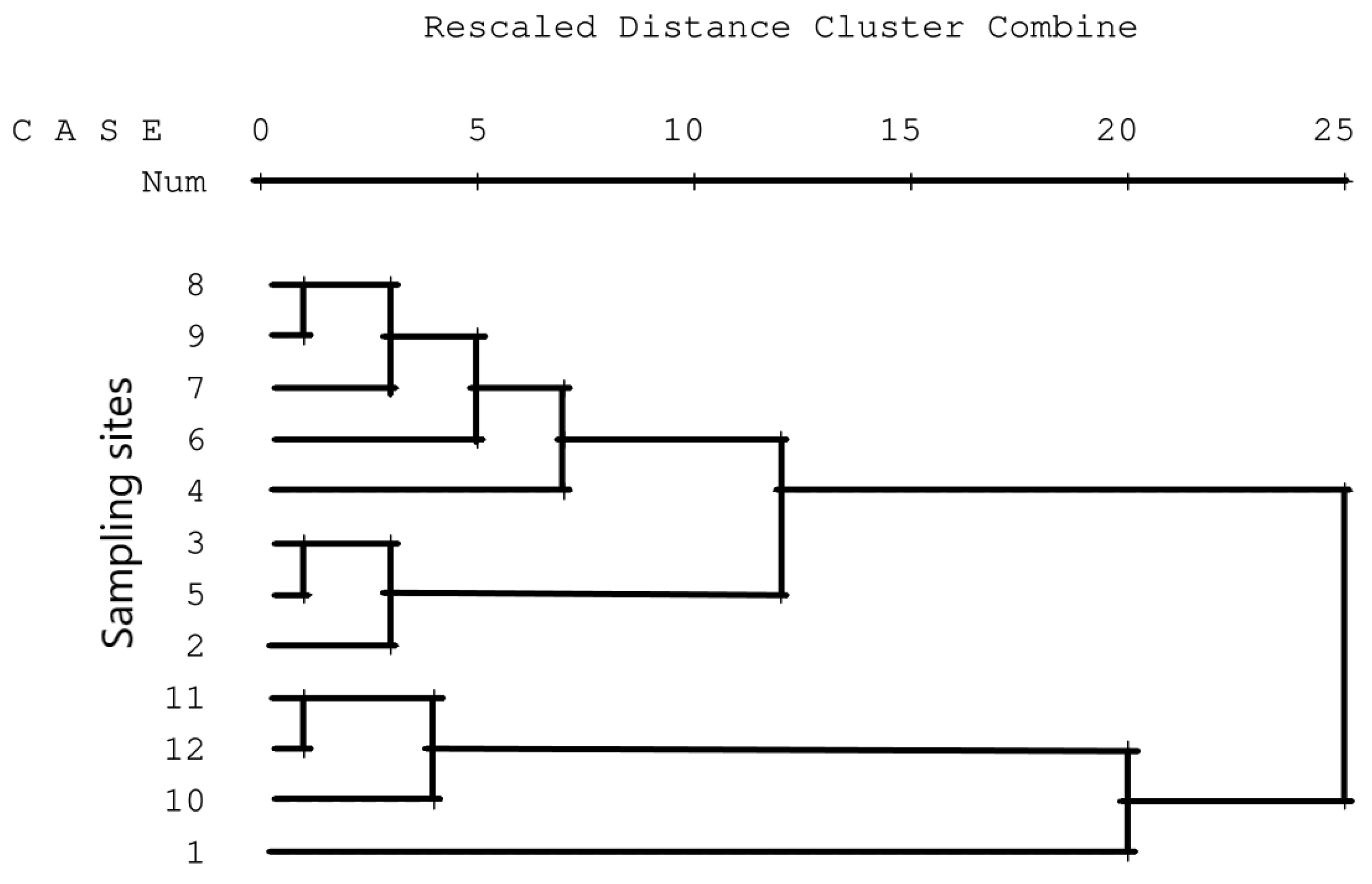
| Sampling Station ^ | Sampling Station Code | Description | Latitude | Longitude | Elevation (m.a.s.l.) |
|---|---|---|---|---|---|
| Geghamavan | 1 | Between Geghamavan and Tsaghkunq villages | 40.5698 | 44.8906 | 1835 |
| Aghbyurak | 2 | 0.5 km downstream of Aghbyurak dam | 40.4957 | 44.7360 | 1685 |
| Solak | 3 | 0.5 km downstream of Solak village | 40.4608 | 44.6872 | 1519 |
| Bjni | 4 | 0.5 km upstream of Bjni village | 40.4648 | 44.6737 | 1509 |
| Arzakan | 5 | In the territory of Arzakan village | 40.4347 | 44.6253 | 1460 |
| Argel | 6 | 0.5 km upstream of Argel HPP | 40.3868 | 44.6033 | 1384 |
| Getamej | 7 | 0.5 km upstream of Getamej village | 40.2829 | 44.5901 | 1225 |
| Yerevan | 8 | 0.5 km downstream of Yerevanyan Lich reservoir | 40.1512 | 44.4600 | 881 |
| Geghanist | 9 | 1.5 km upstream of Aeratsia water treatment plant | 40.1464 | 44.4363 | 871 |
| Darbnik | 10 | 4 km downstream of Aeratsia water treatment plant | 40.1050 | 44.3799 | 836 |
| Sis | 11 | 0.5 km downstream of Sis village | 40.0410 | 44.4096 | 829 |
| Hovtashen | 12 | Between Hovtashen and Noramarg villages | 40.0219 | 44.4431 | 829 |
| Genus | Species | Indication Tool | # of Sites with Records | ||
|---|---|---|---|---|---|
| IBMR | MTR | MIR | |||
| Nostoc | Nostoc sp. H | - | - | 1 | |
| Chara | Chara sp. H | - | 2 | ||
| Hygroamblystegium | Hygroamblystegium tenax | - | 1 | ||
| Myriophyllum | Myriophyllum spicatumH | - | 5 | ||
| Phragmites | Phragmites australis | - | 4 | ||
| Glyceria | Glyceria fluitans | - | 8 | ||
| Persicaria | Persicaria hydropiper | - | 7 | ||
| Eleocharis | Eleocharis palustris | - | 1 | ||
| Scirpus | Scirpus microcarpus | - | - | - | 3 |
| Carex | Carex acuta | - | 1 | ||
| Carex | Carex riparia | - | 1 | ||
| Lythrum | Lythrum salicaria | - | - | - | 3 |
| Epilobium | Epilobium hirsutum | - | - | - | 3 |
| Bidens | Bidens tripartita | - | - | - | 1 |
| Mentha | Mentha longifolia | - | - | - | 2 |
| Mentha | Mentha aquatica | - | 6 | ||
| Juncus | Juncus inflexus | - | - | - | 2 |
| Total | 10 | 12 | 8 | ||
| Sampling Station | Average Width (m) | Depth (m) | Velocity (m/sec) | Temperature (°C) | Mineral Substratum | Correspondence to Stream Type According to MIR System |
|---|---|---|---|---|---|---|
| Geghamavan | 2/1 | 0.2/0.2 | 0.3/0.3 | 13/15 | Sand and mud (80%), microlithal (10%), mesolithal (10%) | PNp |
| Aghbyurak | 3/2 | 0.15/0.15 | 0.2/0.2 | 17/26 | Mesolithal (40%), microlithal (30%), mud (20%) | RW_wap |
| Solak | 6/6 | 0.3/0.3 | 0.9/0.9 | 13/15 | Mesolithal (70%), microlithal (20%), macrolithal (10%) | RW_wap |
| Bjni | 13/13 | 0.7/0.7 | 0.6/0.5 | 15/19 | Sand and mud (40%), mesolithal (30%), macrolithal (30%) | RW_wap |
| Arzakan | 12/12 | 0.5/0.5 | 0.4/0.4 | 15/19 | Mesolithal (60%), microlithal (30%), sand, and mud (10%) | RW_wap |
| Argel | 11/11 | 0.7/0.6 | 1.3/1 | 15/20 | Megalithal (40%), macrolithal (35%), mesolithal (10%), sand (10%) | RW_wap |
| Getamej | 11/10 | 0.5/0.4 | 0.4/0.3 | 12/17 | Macrolithal (50%), mesolithal (35%), megalithal (10%), sand (5%) | RW_wap |
| Yerevan | 15/15 | 0.5/0.5 | 1.1/1.1 | 13/21 | Macrolithal (40%), mesolithal (20%), microlithal (20%), megalithal (10%), sand (10%) | RW_wap |
| Geghanist | 12/12 | 0.5/0.5 | 0.9/0.9 | 13/19 | Macrolithal (35%), mesolithal (25%), microlithal (25%), megalithal (10%), sand (5%) | RW_wap |
| Darbnik | 20/20 | 0.6/0.6 | 0.3/0.3 | 24/19.5 | Mud (20%), sludge (80%) | PNp |
| Sis | 33/33 | 1.8/1.8 | 0.3/0.3 | 19/19.5 | Sludge (50%), sand (30%), mud (20%) | PNp |
| Hovtashen | 35/35 | 2/2 | 0.5/0.5 | 19/20 | Sludge (60%), mud (20%), sand (20%) | PNp |
| Cluster | Error | |||||
|---|---|---|---|---|---|---|
| Mean Square | df | Mean Square | df | F | Sig. | |
| Fine particulates | 10.212 | 1 | 0.079 | 10 | 129.643 | 0.000 |
| Stony | 10.059 | 1 | 0.094 | 10 | 106.879 | 0.000 |
| N | Species | Sampling Sites | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| 1 | Nostoc sp. H | - | - | 6/4 | - | - | - | - | - | - | - | - | - |
| 2 | Ulva intestinalis H | 5/4 | 2/2 | - | - | - | - | 6/4 | - | - | - | - | - |
| 3 | Cladophora glomerata H | 5/4 | 3/3 | 2/2 | 2/2 | 5/3 | 6/4 | 3/3 | 2/2 | 2/2 | |||
| 4 | Chara sp. H | - | - | 7/4 | 3/3 | - | - | - | - | - | - | - | - |
| 5 | Hygrohypnum ochraceum | - | - | 4/3 | - | - | - | - | - | - | - | - | - |
| 6 | Fontinalis antipyretica H | - | - | 1/1 | 5/3 | - | - | - | - | - | - | - | - |
| 7 | Stuckenia pectinata H | - | - | - | 7/4 | 6/4 | 2/2 | - | 8/5 | 9/5 | - | 2/2 | 3/3 |
| 8 | Zannichellia palustris H | - | - | - | 5/3 | 7/4 | 3/3 | 2/2 | - | - | - | - | - |
| 9 | Lemna minor H | - | 2/2 | - | 2/2 | 2/2 | 2/2 | - | 2/2 | - | 1/1 | - | 2/2 |
| 10 | Lemna gibba H | - | - | - | - | - | - | - | - | - | - | - | 1/1 |
| 11 | Lemna trisulca H | - | - | - | 2/2 | 2/2 | - | - | - | - | - | - | - |
| 12 | Ceratophyllum demersum H | 2/2 | - | - | - | - | - | - | - | - | - | 2/2 | 3/3 |
| 13 | Ranunculus trichophyllus H | 2/2 | - | - | 6/4 | 5/3 | 5/4 | 2/2 | - | - | - | - | - |
| 14 | Myriophyllum spicatum H | - | 8/5 | - | 4/3 | 6/4 | 4/3 | 2/2 | - | - | - | - | - |
| 15 | Phragmites australis | - | 2/2 | - | - | - | - | - | 2/2 | - | - | 2/2 | 2/2 |
| 16 | Glyceria fluitans | 2/2 | - | - | - | 2/2 | 2/2 | 2/2 | 2/2 | 2/2 | 2/2 | 2/2 | - |
| 17 | Catabrosa aquatica | 2/2 | 2/2 | - | - | 2/2 | - | 2/2 | - | 2/2 | - | - | - |
| 18 | Typha latifolia | 3/3 | - | - | - | - | - | 2/2 | - | - | - | 2/2 | 2/2 |
| 19 | Sparganium erectum | 4/3 | 4/4 | 1/1 | 2/2 | - | 2/2 | 2/2 | 2/2 | - | - | 2/2 | 2/2 |
| 20 | Persicaria hydropiper | 2/3 | - | 2/2 | - | - | - | - | 2/2 | 2/2 | 1/1 | 2/2 | 2/2 |
| 21 | Persicaria amphibia | - | - | 3/3 | - | - | - | - | - | - | - | - | - |
| 22 | Schoenoplectus tabernaemontani | 3/3 | 3/4 | - | - | - | - | - | 5/4 | - | 2/2 | - | 2/2 |
| 23 | Scirpus microcarpus | - | - | - | - | - | - | - | 2/2 | 2/2 | 2/2 | - | - |
| 24 | Carex vesicaria | 8/3 | 2/2 | - | - | 2/2 | - | - | 2/2 | - | 2/1 | - | - |
| 25 | Carex acuta | - | - | - | - | - | - | - | - | 4/3 | - | - | - |
| 26 | Carex riparia | - | - | - | - | - | - | - | - | - | 1/2 | - | - |
| 27 | Lythrum salicaria | - | - | - | - | 2/2 | 2/2 | - | 2/2 | - | - | - | - |
| 28 | Veronica anagallis-aquatica | 2/2 | - | 4/3 | 2/2 | - | 1/1 | 6/4 | - | - | - | - | - |
| 29 | Alisma plantago-aquatica | 1/1 | - | - | - | - | - | - | - | - | - | - | - |
| 30 | Epilobium hirsutum | - | 5/3 | - | - | - | 2/2 | - | 3/3 | - | - | - | - |
| 31 | Bidens tripartita | - | - | - | - | - | - | - | - | 4/3 | - | - | - |
| 32 | Mentha longifolia | 2/2 | - | 3/2 | - | - | - | - | - | - | - | - | - |
| 33 | Mentha aquatica | - | 2/2 | 2/2 | - | 3/2 | 2/2 | 3/3 | 2/2 | - | - | - | - |
| 34 | Juncus inflexus | - | 2/2 | - | - | - | 2/2 | - | - | - | - | - | - |
| 35 | Eleocharis palustris | 3/2 | - | - | - | - | - | - | - | - | - | - | - |
| 36 | Equisetum palustre | 1/2 | - | - | - | - | - | - | - | - | - | - | - |
| 37 | Hygroamblystegium tenax | - | - | 2/2 | - | - | - | - | - | - | - | - | - |
| Sampling Stations | N Species Used in Calculation of Each Index Value | |||
|---|---|---|---|---|
| IBMR | MTR | MIR | Adapted Indices | |
| Geghamavan | 13 | 13 | 15 | 16 |
| Aghbyurak | 9 | 9 | 9 | 12 |
| Solak | 12 | 7 | 11 | 13 |
| Bjni | 9 | 9 | 10 | 10 |
| Arzakan | 10 | 9 | 11 | 12 |
| Argel | 8 | 8 | 9 | 11 |
| Getamej | 10 | 9 | 11 | 12 |
| Yerevan | 10 | 7 | 9 | 13 |
| Geghanist | 6 | 5 | 6 | 9 |
| Darbnik | 7 | 7 | 7 | 9 |
| Sis | 6 | 4 | 5 | 6 |
| Hovtashen | 10 | 9 | 9 | 10 |
| Indices | IBMR | MTR | MIR | Adapted IBMR | Adapted MTR | Adapted MIR |
|---|---|---|---|---|---|---|
| IBMR | 1.000 | 0.754 ** | 0.606 * | 0.878 ** | 0.765 ** | 0.730 ** |
| MTR | 1.000 | 0.946 ** | 0.715 ** | 0.928 ** | 0.949 ** | |
| MIR | 1.000 | 0.616 * | 0.862 ** | 0.935 ** | ||
| Adapted IBMR | 1.000 | 0.783 ** | 0.748 ** | |||
| Adapted MTR | 1.000 | 0.951 ** | ||||
| Adapted MIR | 1.000 |
| Pairs | t | df | Sig. (2-Tailed) |
|---|---|---|---|
| Adapted MIR—MIR (N = 12) | −1.526 | 11 | 0.155 |
| Adapted IBMR—IBMR (N = 11) | −1.629 | 10 | 0.134 |
| Adapted MTR—MTR (N = 11) | −5.127 | 10 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yepremyan, H.; Asatryan, V.; Dallakyan, M.; Shahnazaryan, G.; Pusch, M. Testing Macrophyte-Based Assessment Tools Developed Under the EU Water Framework Directive for Application in a Caucasus Region Country (Armenia). Water 2025, 17, 1352. https://doi.org/10.3390/w17091352
Yepremyan H, Asatryan V, Dallakyan M, Shahnazaryan G, Pusch M. Testing Macrophyte-Based Assessment Tools Developed Under the EU Water Framework Directive for Application in a Caucasus Region Country (Armenia). Water. 2025; 17(9):1352. https://doi.org/10.3390/w17091352
Chicago/Turabian StyleYepremyan, Hermine, Vardan Asatryan, Marine Dallakyan, Gayane Shahnazaryan, and Martin Pusch. 2025. "Testing Macrophyte-Based Assessment Tools Developed Under the EU Water Framework Directive for Application in a Caucasus Region Country (Armenia)" Water 17, no. 9: 1352. https://doi.org/10.3390/w17091352
APA StyleYepremyan, H., Asatryan, V., Dallakyan, M., Shahnazaryan, G., & Pusch, M. (2025). Testing Macrophyte-Based Assessment Tools Developed Under the EU Water Framework Directive for Application in a Caucasus Region Country (Armenia). Water, 17(9), 1352. https://doi.org/10.3390/w17091352






