Abstract
Universities and other institutes of higher education could be considered as key actors in the implementation of sustainability pillars, such as the adoption of sustainable practices in wastewater management. However, the adoption of such practices is still an emerging issue. This paper discusses the design and operation of the first combined Oxylag and high rate algal pond (COHRAP) constructed at the university campus in Tunisia for irrigation. Performance was evaluated based on the removal efficiencies of nutrients, chemical oxygen demand (COD), biochemical oxygen demand (BOD), heavy metals, coliforms, and biomass productivity. The potential reuse of sludge and algal biomass is discussed based on the Tunisian national standard regulation for sludge reuse in agriculture (NT 106.20) and the European regulation (EC, 2019/1009) for fertilizer products. Effluent phytotoxicity is tested on the germination and growth on Zea mays L. The results indicate that the COHRAP performance was globally satisfactory; however, biomass productivity (1.4 g m−2d−1) was low, indicating the need for adjustments in the operational parameters. Despite the effluent limitations for TSS and Hg, no phytotoxic effect was observed. Regarding the heavy metal content in sludge and algal biomass, the results obtained were in compliance with NT 106.20 and EC, 2019/1009), respectively. The energy consumption of COHRAP is 1.05 kWh/m3 resulting in operational costs of 0.29 euros/m3. This study revealed that COHRAP could be a sustainable option to treat wastewater from university campuses with resource recovery. Such a choice can be improved by the implementation of an algae recovery step.
1. Introduction
Tunisia is affected by arid to semi-arid climate conditions and has limited water resources, especially as growing population needs and recent economic development have led to a rapid increase of water demand, resulting in high levels of water stress. In addition, water scarcity is accentuated by factors such as climate change and inefficient water management. In this context, advancing wastewater management and sanitation, including water reuse, is recognized as a key solution for developing countries, as it reduces the water deficit [1]. Environmental engagement must be integrated across various sectors (e.g., education, agriculture, industry) and sustainable innovative solutions have to be promoted. University campuses can significantly contribute to water conservation efforts because of their high-water consumption. Implementing water treatment and reuse systems reduces demand and promotes environmental responsibility.
Sustainable wastewater treatment technology has become a fashionable trend in recent years due to its economic, social and environmental advantages. These systems could be an interesting alternative in rural areas or in small communities such as university campuses, where traditional treatment plants cannot be installed [2]. Different and extensive technologies exist for wastewater treatment, each with its benefits and drawbacks. Traditional waste stabilization ponds, for example, provide a cost effective and efficient degradation of organic matter but have disadvantages, including large land requirements, variable effluent quality and a deficient nutrient and pathogen removal. More than that, these systems are not intended to recover resources from wastewater. The treatment of domestic wastewater by the aerated lagoon system remains among the most used techniques in countries with arid to semi-arid hot climates. Aerobic bacteria in the aerated lagoon consume dissolved oxygen to oxidize organic matter in the wastewater. Oxygenation is provided by a surface aerator or air insufflation. The operating principle differs from activated sludge in the absence of continuous sludge recycling or removal [3,4]. The research into aerated lagoons has led to the development of a new aerated lagoon system, marketed under the name Oxylag. In this new type of reactor, oxygen transfer and biodegradation volume are separated in order to reduce oxygen supply cost and land requirement [5]. This system includes an oxygen transfer pit, separated into two parts, ensuring both aeration and circulation of water. Several Oxylag municipal wastewater treatment plants have been installed in Morocco and Algeria for populations ranging from 1000 to 15,000 inhabitants.
The use of alternative and sustainable treatment methods (such as algae-based systems) is of interest. These systems, such as high rate algal ponds (HRAP), typically allow for high levels of resource recovery, promote circular economy concepts and reduce greenhouse gas emissions. HRAP is an advanced pond system suitable for arid and semi-arid areas [6] and has many advantages, such as a low land occupation and a low cost investment [7]. Furthermore, HRAP is a suitable solution for wastewater treatment, particularly in small communities [8]. These natural systems provide cost-effective and efficient wastewater treatment where weather conditions are favorable for microalgae growth [9,10,11]. The latter assimilates nutrients and produces oxygen, which is then consumed by bacteria for the oxidation of biodegradable organic matter [12,13].
HRAP overcomes many of the disadvantages of conventional ponds, including the limited nutrient and pathogen removal. It also provides significant nitrogen and phosphorus removal through assimilation into algae biomass and stripping at high pH [14]. Microalgae are not only restricted to nutrient removal from wastewater, they are also efficient at removing pharmaceutical and pesticide substances [15,16] and metal pollutants [17]. For microalgal cell development, several positive heavy metals, such as Fe, Cu, Mn, Co, Zn, and Mo, are necessary and may be adsorbed [18]. However, negatively charged exopolysaccharides (EPSs) may be released by a number of microalgae [19] and are able to adsorb metals [20]. In addition, HRAP provides significant bacteria removal. Pathogen inactivation within the HRAP is influenced by high pH, dissolved oxygen concentration and UV radiation exposure. These factors damage pathogens’ RNA and DNA [21] and promote the photo-oxidation of dissolved organic contaminants [22].
In addition, HRAP technology has been shown to provide more effective wastewater treatment for use in agriculture applications [12,23], with the additional benefit of recovering wastewater nutrients as harvestable algal/bacterial biomass. HRAP has a notable algal productivity, with a mean of 45 g m−2 d−1, and can thus serve as a source of animal protein [7]. In addition, microalgal biomass allows CO2 capture while treating wastewater. Indeed, producing 1 kg of microalgal biomass would require 1.83 kg of carbon dioxide [24].
In terms of potential applications, algal biomass from wastewater can be used as biofertilizers, animal feed and valuable compounds such as pigments and lipids [25,26,27]. However, there is still a lack of knowledge regarding the accumulation of several contaminants into biomass such as heavy metals, which could impair its safe use as biofertilizer [28]. Moreover, algal biomass from wastewater can also be recovered for bioenergy through conversion into biogas, bioethanol, bio-crude oil or biodiesel via lipid trans-esterification [29]. However, recovery and harvest algae meet technical and economic difficulties [30].
In HRAP, more than 3000 microalgae strains have been identified [31], with varying features and capacities to remediate wastewater. Environmental factors like temperature, light, and nutrients can cause temporal shifts in dominant species or community structures.
Several studies have been conducted to optimize HRAP operating conditions, including depth, hydraulic retention time (HRT), and dynamics [32,33,34]. However, the role and effect of the primary treatment upstream of HRAP remains controversial. Posadas et al. [35] suggest that primary suspended solids removal is probably not an essential step prior to HRAP systems but further research is needed to prove that this configuration is feasible. Tyagi and Couillard [36] have reported that wastewater may include a number of substances that could inhibit microalgal growth and suggest that the pretreatment of wastewater is necessary. According to Couto et al. [37], the HRAP system could be combined with other ponds, such as maturation ponds or anaerobic digestion, to meet effluent requirements prior to effluent discharge.
Despite the operational and environmental benefits of HRAP, as well as its effectiveness in treating domestic or university wastewater, the large-scale deployment with on-site reuse of treated wastewater in agriculture is still relatively limited [38]. Most research studies have been conducted in laboratory-scale systems operating under constant conditions, which will never accurately describe system performance under real conditions. In addition, few studies have focused on the treatment of wastewater generated on university campuses using the HRAP treatment system. These effluents are characterized by their heterogeneity and are generally of an industrial nature [39].
In December 2023, a demonstration plant of a combined Oxylag–HRAP system, pioneered in Tunisia, was constructed at the campus of the High Agronomic Institute of Chott Mariem, to contribute to water conservation efforts, to increase knowledge on this technology among the local community and authorities and to promote sustainable practices and a culture of environmental responsibility among students.
The objectives of this study were to (1) demonstrate the feasibility of sustainable wastewater management practices on a university campus, (2) evaluate the performance of a COHRAP system and verify its compliance with current standards, (3) identify the main limitations and provide possible alternatives and solutions and (4) discuss the potential reuse of treated wastewater, algal biomass and sludge.
2. Materials and Methods
2.1. Study Site
The wastewater treatment demonstration pilot plant (WWTDPP) was built on the campus of the High Agronomic Institute of Chott Mariem (ISA-CM) located in the Sousse region of Tunisia. The plant was part of a research project funded by Wallonie Bruxelles International. The institute is built on a 54-hectare site with 450 people, including researchers, students, administrative staff and workers. The operation of the plant started in January 2023. In this region, the climate is semi-arid, the average rainfall is less than 361 mm per year and the average temperature is around 18 °C. Prior to the construction of the plant, the raw wastewater was sampled from the general sewage network of the High Agronomic Institute of Chott Mariem and analyzed weekly for physicochemical characteristics for one month. The daily flow rate of raw wastewater was monitored for one week. Wastewater corresponding to 40 m3 d−1 is produced by laboratories, lecture halls libraries, housing services, restaurant, and academic dormitory, and is discharged into the municipal sewerage system through a unified sewage network.
2.2. Design Criteria
The Oxylag and HRAP designs are based on the raw wastewater characteristics (Table 1), the organic loading rate of the influent was estimated as 100 population equivalents (PE) considering a specific load of 60 g BOD per person per day and a specific flow of 90 L per person per day. Tunisian standards for reuse (NT 106.03) [40], desired BOD percentage of 95% removal, climate (solar irradiation, temperature and precipitation) and available area have also been taken into consideration in the pilot design. Biological and aeration sizing was carried out to estimate the volume of the two ponds, the surface area needed, the amount of dissolved oxygen per hour required and the water velocity in the HRAP. The biological sizing of the Oxylag and HRAP is based on flow rate and BOD concentration of the raw wastewater.

Table 1.
Raw wastewater characteristics.
The minimum surface area of the Oxylag pond was determined based on the maximum BOD loading rate commonly used for the design of an aerated lagoon while ensuring good performance. The BOD loading rate of 60 g m−2 d−1 should be supplied to the Oxylag pond to achieve 60% BOD removal. The water depth of 1.5 m was considered taking into account the power of the aeration system designed to supply the two ponds (Oxylag and HRAP) while ensuring correct mixing. The airlift design was based on the amount of oxygen required per day, expressed as (kgO2 d−1), allowing for the removal of 60% of the BOD loading rate and the performance of partial nitrification and in order to meet the sediment oxygen demand. The parameters used for the Oxylag aeration design are presented in Table 2. The amount of oxygen required per day was converted to standard conditions. Finally, the hourly air flow requirement, expressed as (Nm3 h−1) was determined by dividing the quantity of oxygen requirement, expressed as (kgO2 h−1), by the oxygenation capacity of perforated membrane air diffuser expressed as (g O2 Nm−3).

Table 2.
Parameters values used for airlift sizing of Oxylag.
For the HRAP, the minimum surface area was determined based on the BOD loading rate of 20 g m−2 d−1 [26,41] and the assumption that 40% of BOD loading rate supplied at the inlet of Oxylag would be treated in the HRAP. The total volume of the HRAP was calculated considering the water depth of 0.5 m and the ratio of length to width of the channel greater than 10. The selection of the BOD loading rate of 20 g m⁻2 d⁻1 builds on our previous studies carried out under climatic conditions similar to those in Tunisia.
Furthermore, the proposed HRT is a minimum of 4 days to allow for the removal of organic material and nutrients [42].
For HRAP airlift sizing, the desired velocity of 0.1 m s−1 [43] was assumed and the air flow requirement (m3 d−1) in the HRAP channel airlift was determined.
2.3. Wastewater Treatment Pilot Plant Description
The WWTDPP was established to treat a specific amount of the total wastewater generated by the campus. It was designed for 100 inhabitants with an average daily flow of 10 m3 d−1 and an organic loading rate of 2 kg BOD d−1.
The pilot plant consists of two wastewater treatment stages operated in series (Figure 1)—an aerated lagoon called Oxylag followed by a high rate algal pond (HRAP).

Figure 1.
Photograph of ISA-CM combined Oxylag and High rate algal pond wastewater treatment demonstration pilot plant (COHRAP/WWTDPP).
The plant was fed by raw wastewater taken from a 3.4 m3 storage tank equipped with an automated pumping system. The feeding rate was controlled by a Siemens LOGO programmable logic controller, by considering the desired hydraulic and organic load. The technical characteristics of the two ponds are shown in Table 3.

Table 3.
Dimensional specifications of the Oxylag and HRAP.
The Oxylag system has an area of 54.3 m2 and an operating depth of 1.5 m, with a capacity of 57.8 m3 due to the shape of the pond (45 degrees slope banks). Its main function is to reduce the organic loading rate of the HRAP feed. The aerated pond is equipped with an air lift aeration system to ensure both the circulation and homogenization of the effluent, in addition to enhancing biological degradation of the organic matter by bacteria. The airlift of the HRAP consists of a 13 m3 pit separated into two communicating parts (Figure 2). Air injection is ensured in the first part of this pit, where six perforated membrane air diffusers OTT 02-Ökonom Magnum Clip IN 1.000 Flexsil 2X500 lg 1200 D67 (OTT Group, Langenhagen, Germany) are installed at 25 cm above the bottom. Water flow velocities measured at the Oxylag and HRAP ponds are 7.86 and 13.14 cm s−1 respectively, ensuring circulation and homogenization of the water column.

Figure 2.
Photos of the Oxylag pit with air diffusers.
The high rate algal pond (45 degrees slope banks) has a serpentine shape with six channels of 12.7 m in length. It has a surface area of 86.7 m2 and an operating depth of 50 cm with an operational volume of 44.2 m3. It is fed from the Oxylag pond. It is equipped with an airlift aeration system to ensure the circulation and homogenization of the effluent, and to maintain the algal/bacterial flocs in suspension. The airlift of the HRAP consists of a 9 m3 pit separated into two communicating parts. Air supply is ensured in the first part where six perforated membrane air diffusers (OTT 02-Ökonom Magnum Clip IN 1.000 Flexsil 2X500 lg 1200 D67) are installed at 25 cm above the bottom of the pond (Figure 2).
The bottom and walls of the ponds (HRAP and Oxylag) are sealed using a membrane (high density polyethylene membrane, HDPE 1.5 mm) fitted on a geo-textile layer.
The two treatment stages (Oxylag and HRAP ponds) are supplied in the air by 4 KW Robuschi Robox type ES 15/1 P blower (Robuschi S.p.A, Parma, Italy) controlled by a frequency converter. The intermittent operation of the air supply system is performed by a Logo controller.
2.4. Hydraulic Flow and Operational Conditions
Figure 3 shows the flow diagram of the combined Oxylag and HRAP plant. The influent wastewater is taken from a storage tank and then passes through a first venturi device to measure the Oxylag’s influent flow rate. The defined influent flow rate is about 9.8 m3 d−1 and corresponds to 5.8 days hydraulic retention time (HRT) in the Oxylag and 4.5 days in the HRAP. After pretreatment within the Oxylag, the HRAP is gravity fed. Finally, the effluent passes through second counting equipment (venturi) before being discharged into a final tank equipped with a submersible pump for on-site water reuse if the water quality is suitable. Alternatively, in the event of unsatisfactory quality, treated water is discharged into the general sewage network of the campus.
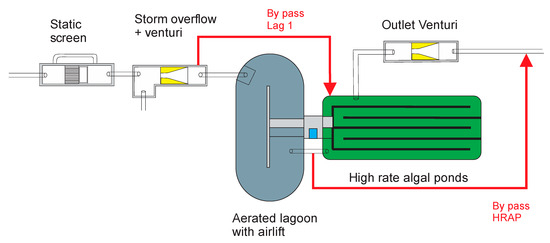
Figure 3.
Schematic diagram of a combined Oxylag–HRAP wastewater treatment demonstration pilot plant of the High Agronomic Institute of Chott Mariem.
During the observation period, air flow rates were 34 and 97 m3 h−1 at the Oxylag and HRAP, respectively, with a daily operating time of the blower air system of 6 h (5 min ON/15 min OFF). Air flow rate is determined by multiplying the air velocity by the cross-sectional area of air supply pipes. The air velocity was measured using a TROTEC TA400 pitot tube (TROTEC GmbH, Heinsberg, Germany).
2.5. Sampling and Monitoring
Climate monitoring was assessed by the weather station (WS-GP2, Delta-T Devices Ltd., Cambridge, UK) installed on the campus site, which continuously logged total solar radiation, air temperature, rainfall, humidity, wind speed and direction. During the observation period, the recorded average air temperature was 22 °C with a maximum of 48.6 °C in July 2023 and a minimum of 5.5 in January 2024, average light intensity during the sun rises was about 500 Wm−2.
Water quality parameters were monitored from July 2023 to December 2024. Samples were collected monthly from July to September 2023 for the first campaign and from March to December 2024 for the second campaign. These samples were collected at the inlet and outlet of each pond to assess the operating efficiency of the WWTDPP in the first-year operation period. Removal efficiency (R%) for water indicator parameters was calculated using Equation (1).
Continuous in-situ measurements of pH, temperature (T) and dissolved oxygen (DO) were performed using DO and pH probes Hach LDO 101 and Hach pH C 101 (Hach Company, Loveland, CO, USA) with data logging (Hach HQ40d).
For microalgal species identification, water samples (in triplicate) collected at HRAP were analyzed monthly during the observation period (May, August and November 2024) using a Leica DM 500 light microscope (Leica Microsystems GmbH, Wetzlar, Germany). Microalgae species were identified according to the identification guide books [44]. To determine algae and biomass productivity within the HRAP, water samples were collected weekly, within the pond, from March 2024 to May 2024 for chlorophyll-a and for total suspended solids (TSS) and volatile suspended solids (VSS) analysis. Algae biomass in HRAP was estimated from chlorophyll-a concentration according to Equation (2) [45]. The algal productivity was calculated based on Equation (3) [46].
Equation (2) assumes that the algal biomass has a chlorophyll-a content of 1.5% of dry weight [47]. This method provides a relative measure of algal biomass as chlorophyll content varies with algal species and growth condition. Using a constant ratio to convert chlorophyll a to algal biomass may not always give a correct estimate of algal biomass and may be subject to error (over or underestimation), which may be greater when samples are taken over a long period. Determining the relationship between the chlorophyll-a content and the concentration of algae species is part of our research.
where is algae productivity (g m−2 d−1); are the concentrations of dried algae biomass at , respectively; is the HRAP volume (44.17 m3); is hydraulic residence time; and is the surface area of the pond (86.6 m2).
In addition, water samples were taken from the HRAP and microalgae was extracted by centrifugation. The dried biomass was then analyzed for heavy metal (HM) content. Likewise, sludge samples were collected from Oxylag and analyzed for HM.
2.6. Analytical Procedures
Composite water samples were collected in 1000 mL polypropylene bottles for water quality analysis and 100 mL sterile bottles for microbiological examination at the following time range: 8 to 9 a.m., 12 to 14 p.m. and 17 to 19 p.m. All analysis were performed on the same day according to the standard methods for the examination of water and wastewater [48]. Physicochemical parameters, including pH, chemical oxygen demand (COD), biochemical oxygen demand (BOD), total suspended solids (TSS), volatile suspended solids (VSS) and total nitrogen (TN), were monitored. As HRAP effluent samples contained algae biomass, the BOD, COD and TN parameters were performed on filtered samples. Ammoniacal nitrogen (NH4-N), phosphate (PO4-P) and nitrate (NO3-N) were measured in filtered samples both at the inlet and outlet of the plant. All samples analyzed were performed in triplicate.
The determination of Chl a was performed using a hot-ethanol extraction technique (90%), as described in ISO 10260 (1992) [49].
The standard most probable number (MPN) method was used for the determination of total and fecal coliforms using McCrady’s probability tables.
Heavy metal (HM) analysis was performed on unfiltered samples for the inlet Oxylag and on filtered samples for the HRAP effluent. In the case of algal biomass and sludge, the samples were pretreated according to EPA 3051A method which involves digestion in a closed microwave oven system in the presence of concentrated HNO3 and HCl at an HNO3/HCl ratio of 3:1 [50]. Thereafter heavy metals (Cd, Cu, Ni, Pb, Cr, Zn, Fe, Hg, As, Se, Co, B) were analyzed using an inductively coupled plasma mass spectrometer (Thermo Scientific, iCAP RQ, Waltham, MA, USA) according to ISO 17294-2 [51]. All samples analyzed were performed in triplicate.
Heavy metal (HM) analysis was performed on unfiltered samples for raw effluent and filtered samples for the inlet and for water leaving the plant.
2.7. Phytotoxicity Tests of the HRAP Effluent
A growth test was carried out on pre-germinated seeds with the same radicle length (2 mm). These seeds were initially pre-germinated in distilled water. Afterward, they were placed in 9 cm Petri dishes lined with filter paper and exposed to 5 mL of HRAP effluent (treatment) or 5 mL of distilled water (control). The effect of the HRAP effluent on the growth of Zea mays seedlings was assessed by measuring the length of roots and shoots seven days after germination. Germination rate (GR) and germination indices (GIs) were calculated following Equations (4) and (5).
where are the proportions of seeds germinated in the first, second and days, expresses the delay in germination.
2.8. Energy Consumption and Operational Costs of WWTDPP
The power consumptions of the pump and air blower were measured and recorded using a KWh measurement device. The energy consumption is calculated based on operational time and KWh data recorded.
The annual operating costs of the WWTDPP include electricity, staff and maintenance expenditures. Staff costs were estimated by assuming a need of 100 working h/year with 3 EUR/h, this results in staff costs of 300 EUR/yr. A price of 0.156 EUR/kWh was assumed for the cost of electricity and a price of 16 EUR/m3 was considered for the cost of sewage sludge management.
2.9. Statistical Analysis
Mean values ± standard deviation (Std) are provided. Statistical analyses on measured physicochemical parameters of the effluents in the inlets and outlets points were performed using statistical software (SPSS: version 18.0). The significance between inlet and outlet series was made by paired t-test at the 5% level. The significance between the control and seeds irrigated with HRAP effluent was made by Duncan test.
3. Results and Discussion
3.1. Physicochemical Characteristics of Raw Wastewater
The monitored parameters of ISA-CM effluent have a high variability in terms of organic load. Physicochemical results (Table 4) show an average COD value of 886.5 ± 576 mg L−1, with a minimum value of 134 mg L−1 recorded in July 2023 and a maximum value of 1773 mg L−1 recorded in April 2024. The BOD value ranged from 21 and 594 mg L−1 with an average value of 284.7 ± 190 mg L−1. The minimum BOD value was recorded in July 2023. The organic pollution ratio BOD/COD is about 0.3 ± 0.1, allowing for a poor biodegradability of the effluent and acts as a limitation of the biological treatment. This could be related to the chemical solution discharged from laboratories and effluent from restaurant containing oils and fats.

Table 4.
Comparison of raw wastewater characteristics, with values from university campuses in Morocco [52] and Spain [53].
For particulate suspended solids (TSS), concentration values range between a minimum of 54 (July 2023) and a maximum of 330 mg L−1 (March 2023) with an average content of 178.1 ± 71 mg L−1. The nutrient concentration of ISA-CM effluent is characterized by a mean of total nitrogen (TN) contents of 91.2 ± 26 mg L−1, an average of ammonium ion (NH4+-N) of 42 ± 25 mg L−1, and an average value of nitrate 1.0 ± 0.7 mg L−1. For orthophosphate (P-PO4), the average content is about 6.5 ± 2 mg L−1.
Thus, the high variability of the physicochemical parameters is mainly related to the sampling period, which depends on the term time and vacations (July and August). Discharge of wastewater with high complexity due to different uses may also affect the wastewater characteristics, resulting in high variability. Previous studies have also shown a high variability in the characteristics of raw wastewater from university campuses. Melian et al. [53], studying stone filters in combination with a pond–wetland system treating raw wastewater from a university campus in Las Palmas de Gran Canaria in a pilot plant, obtained significant variability of BOD, COD, SS and NH4, with mean (±standard deviation) concentrations of 314 ± 92 mg L−1, 416 ± 215 mg L−1, 158 ± 100 mg L−1, and 139 ± 74 mg L−1, respectively. Grimah et al. [52] have also reported the same trend of variation in the quality of raw wastewater from the Faculty of Sciences Ben M’Sick (Casablanca, Morocco). Average values ranging between 7.18 and 8.18 for pH, 2.47 and 3.98 mS cm −1 for electrical conductivity, 967.4 and 1151 mg L−1 for COD, 70.5 and 119 mg L−1 for BOD, 223.6 and 1659.7 mg L−1 for TSS were obtained. The authors have also reported an orthophosphate (P-PO4) content varying between 0.7 and 2.7 mg L−1, while ammonium, nitrite and nitrate concentrations varied between 3.5 and 17.5 mg L−1; 0.17 and 3.6 mg L−1; 0.6 and 1.4 mg L−1 respectively. The authors suggest that the high variability may be slightly influenced by the rainwater when it is collected by the unitary sanitation system, resulting in a variation in pollutant concentration. As the daily climate data for the observation period of the present study indicates a severe rainfall deficit in the region of Sousse, and in the country as a whole, the high variability observed is not thus related to rainy periods.
3.2. Loading Rate, Efficiency and Effluent Quality
Oxylag pond and HRAP were fed with wastewater at 5.9 and 4.5 d HRT, respectively, to acclimate the algae and bacteria. These values are consistent with a typical range of 5–10 d for wastewater treatment in HRAP [29] and of 5–10 d for wastewater treatment by Oxylag [5].
Mean loading rates and effluent concentrations of the main parameters measured during the observation period for the two treatment stages are shown in Table 5.

Table 5.
Mean loading rates and effluent characteristics (n = 13) compared with limit values for wastewater reuse in Tunisia (NT 106.03) [40].
The chemical oxygen demand (COD), the biochemical oxygen demand (BOD) and total nitrogen (TN) loading rates applied to the Oxylag pond ranged from 24.3 to 321.2 g COD m−2 d−1, 3.8 to 107.6 g BOD m−2 d−1, and 12.1 to 32.9 g TN/m−2 d−1. Loading rates applied to the high-rate algal pond ranged from 3.9 to 38.6 g COD m−2 d−1, 0.5 to 15.7 g BOD m−2 d−1, and 0.6 to 16 g TN/m−2 d−1. The lowest COD and BOD loads applied to both systems were recorded in July and September 2023, while the highest were recorded in April 2024, which is linked to the campus activities, as explained earlier. It should be noted that, during vacation, the influent loads are reduced, while the influent volume remains constant following the data recorded by the pump’s daily operation time.
The loading factor is a ratio between the hydraulic and nominal surface loading rates. Considering the nominal surface loading rates of the Oxylag and HRAP, which are 60 g BOD m−2 d−1 and 20 g BOD m−2 d−1 [26], respectively, the load factors calculated varied between 0.02 and 0.78, with a mean of 0.31 for the HRAP. Additionally, the load factors calculated for the Oxylag varied between 0.06 and 1.79 with a mean of 0.86. This means that the inflow rate and hydraulic loads applied to the Oxylag pond were sometimes insufficient as a result of decreasing campus activities. On the other hand, the hydraulic loads applied to the Oxylag could sometimes reach twice the nominal loading rate. In the case of the HRAP, the hydraulic loads are insufficient because of overtreatment within the Oxylag, which was more efficient than specified in the design.
Therefore, maintaining an optimal hydraulic loading rate depending on the time periods and raw wastewater characteristics is crucial to ensure the efficiency of the WWTDPP and energy consumption. The modelling of the COHRAP could be of great interest to optimize the operating parameters and discover new improvements for this plant. This is part of our ongoing research activities. In addition, continuous assessment and control tools will be installed at the WWTDPP. An ultrasonic flow sensor equipped with a data logger is currently being implemented at the inlet of the Oxylag pond to ensure continuous monitoring of the volumetric flow and thus enables proactive operation of a WWTDPP. At the same time, and to overcome the high variability of the effluent, an automated sampler is intended to be installed to collect 24 h composite samples to cover the temporal variations associated with human activity, thus ensuring more representative and reliable monitoring.
The mean removal rates R (Figure 4) performed by the COHRAP during the monitoring period were 91.2% for BOD, 86.3% for COD, 81.4% for TN, 97.8% for N-NH4 and 75.5% for orthophosphate (P-PO4). BOD removal efficiency achieved in Oxylag is the result of organic biochemical oxidation by heterotrophic bacteria. Oxylag pond processes are similar to those of the aerated lagoon, where the conversion of organic matter, carbonaceous nitrogenous, into inorganic compounds occurs [54].
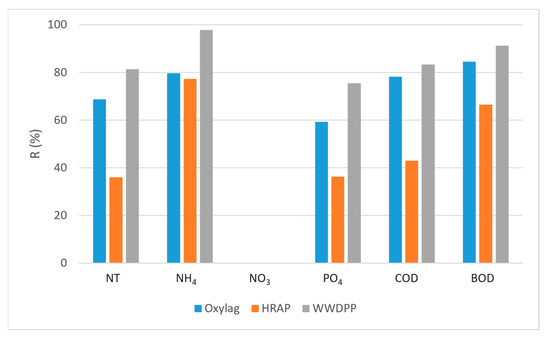
Figure 4.
Removal rates (R) of HRAP, Oxylag and WWTDPP.
Despite the large variability of the loading rate, these removals remain high, whatever the quality of the final effluent. Figure 4 shows that most of the COD and BOD reduction takes place in the first treatment stage, with removal efficiencies of 78.3 ± 15% for COD and 84.5 ± 6% for BOD in the Oxylag pond, whereas the COD and BOD removal rates in the HRAP are 48.5 ± 30% and 66.6 ± 33% respectively. The higher removal efficiency is probably due to the high mean DO concentration (8.2 mg/L) within this pond, in addition to the higher loading rate applied to this first stage, with a mean of 55.9 g BOD m−2 d−1 and 172 g COD m−2 d−1, compared with the second stage HRAP (5.9 g BOD m−2 d−1, 18.7 g COD m−2 d−1). This higher efficiency at higher loads is a known characteristic of extensive wastewater treatment systems [55].
Arashiro et al. [25] have reported an average COD removal efficiency between 60 and 67% in a HRAP pilot plant at the university of Catalunya in Spain, with an influent flow rate of 105 L/day and HRT of 4.5 days. In the present study, removal efficiencies were in accordance with Melian et al. [53] on a combined stone filter with a pond–wetland system treating wastewater from a university campus in Las Palmas (Spain), where removal efficiencies of 95% of BOD and 78% of COD were found.
The study of Abd-Elmaksoud, et al. [56] on an integrated pilot-scale system, including a facultative pond (FP), a rock filter (RF) and a high-rate algal pond (HRAP) with a 5-days hydraulic retention time and a volume 4.3 m3, reported removal rates of 89.39%, 89.26%, and 82.24% for NH4-N, BOD, and COD, respectively.
The NH4-N and TN concentrations in the effluent were significantly lower (p < 0.05) than the influent concentration with a removal rate of 79.7% and 68.7% in Oxylag, and 77.4% and 36.1% in the HRAP, respectively. Whereas NH4 and TN concentrations in the Oxylag and HRAP effluents were not significantly different. It is observed that nitrate concentration values in the raw wastewater (Table 4) varied between 0.1 and 2.2 mg L−1. In the subsequent treatment steps, these have higher concentration values that vary between 0.88 and 12 mg N-NO3 L−1 in the Oxylag effluent and 0.1 and 14.1 mg N-NO3 L−1 in the HRAP effluent (Table 5). However, nitrate concentration in the raw wastewater, Oxylag and HRAP effluents did not differ significantly (p > 0.05).
It seems that the nitrogen removal pathway within the Oxylag is nitrification, as indicated by the significantly lower ammonia and higher nitrate concentrations compared with the raw wastewater. However, nitrate concentrations found in the HRAP and Oxylag effluents are not significantly different compared with the inlet, suggesting that ammonia stripping occurs within the two ponds, where the pH varied between 7.4 and 9.3 in the Oxylag and from 7.6 to 10 in the HRAP system. Nitrifying bacteria have an optimum growth between pH 7.5 and 8.5 and are inhibited below pH 6.5 [57]. Sutherland et al. [58] found that nitrification rates were highest when cultures were maintained between pH 7.0–8.0. In wastewater treatment HRAP, N-NH4 can be removed by microalgae assimilation, nitrification or volatilization at pH > 9 [59]. In general, NH4+ is the preferred form of nitrogen uptake for most microalgal species, followed by NO3− [60]. In HRAP, algae biomass assimilation of ammonia and nitrate could occur. However, in this study, it is more plausible to assume that algal assimilation is low considering the low mean algal surface productivity obtained (1.4 g m−2 d−1), which favors the occurrence of ammonia stripping. In fact, when both nitrification and algal uptake are low. ammonia stripping is promoted [61]. As the present study is the first diagnosis of the WWTDPP’s operation, a more detailed analysis of the nitrogen removal processes needs to be performed in laboratory scale experiments using the plant effluent.
Likewise, the orthophosphate (P-PO4) concentrations in the Oxylag and HRAP effluents were significantly lower (p < 0.05) compared with the influent, with removal rates of 59.4% and 36.3%, respectively. However, P-PO4 concentrations in the Oxylag and HRAP effluents were not significantly different. The low P-PO4 removal efficiency recorded for HRAP could be related to low biomass productivity due to nitrogen and phosphorus limitation or probably to the mixing frequency of HRAP. In fact, the daily operating time of the blower air system is 6 h (5 min ON/15 min OFF). In this condition, the dark period could be increased and most of the algae will passively settle to the bottom of the pond, over time [62]. Conversely, as vertical mixing increases, algal biomass production is enhanced due to more favourable light conditions leading to higher net photosynthesis [58]. Cosgrove [63] suggests that extended darkness could leave algal cells in a chronic photoinhibition state, which may reduce photosynthesis and biomass production.
Finally, the TSS removal rate in the WWTDPP was unsatisfactory, with higher values in the effluent compared with the influent found in most cases. This result was expected, as a complementary treatment stage including a harvesting system was not yet installed. Ongoing laboratory research is underway to develop a low cost and sustainable harvesting method that could be effective on a semi-large-scale plant.
3.3. Microalgae Community
Based on three triplicate samples collected over the course of the study (May, August and November 2024), the microalgae group of Chlorophyta was identified. The predominant species were Scenedesmus, Chlorella and Closterium (Figure 5).
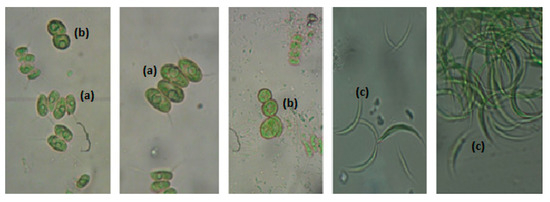
Figure 5.
Microalgae community in HRAP. (a) Scenedesmus, (b) Chlorella, (c) Closterium.
Recorded species are known to be commonly identified in the wastewater treatment HRAP with dominant relationships between species. In August, the prevailing microalgae specie is Closterium, while Chlorella and Scenedesmus dominate in November and May.
Previous studies of microalgal species in full-scale wastewater treatment in HRAP report that individual species become dominant when they develop adaptations under environmental conditions that give them an advantage over other species or due to biological interactions [64]. Some species are more competitive when water temperature or light are lower or when a major nutrient is poorly supplied. Refs. [30,65] report that influent ammonia concentration strongly influences the dominant species in HRAP. However, the authors note that nutrient removal efficiency in a full scale HRAP is not affected by microalgal species.
3.4. Microalgae Production, SS and VSS Variation in HRAP
During the experimental period, variation of VSS, TSS and algal biomass concentration within the HRAP were monitored from March to May 2024 and are shown in Figure 6. The mean VSS, TSS and algal biomass concentrations for the HRAP were 300.4 g m−3, 468.06 g m−3 and 237.52 g m−3 respectively. The mean proportion of algae in the volatile suspended solids of the HRAP is 80%. The mean algal productivity in the HRAP varied between 0.2 and 4.1 g m−2 d−1, with a mean of 1.4 ± 1 g m−2 d−1 (1.75 g VSS m−2 d−1), which is within the reported range of algal biomass in conventional wastewater treatment ponds, typically less than 2.5 g VSS m−2 d−1 [23]. However, maximum productivity was recorded in April with a value of 4.1 g m−2 d−1.
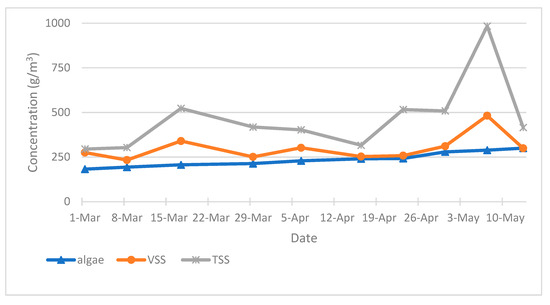
Figure 6.
Algal productivity, VSS and TSS variations in HRAP (March, April, May 2024 data).
Algal biomass productivity expressed as VSS (1.75 g VSS m−2 d−1), is lower than that measured in previous studies of HRAP. Craggs et al. [23] reported a value of mean algal productivity ranging from 4.4 to 11.5 g VSS m−2 day−1 in four full-scale replicate wastewater treatment HRAPs in New Zealand. Park and Craggs [47] have reported a mean algae biomass production of 20.7 g VSS m−2 d−1 on a HRAP with an HRT of 4 days and operated with CO2 addition. A contrast in algal biomass productivity has been observed across HRAP sizes in similar climates [66]. However, the main drivers for increasing algal biomass yields have been explored in the literature. Algal productivity is influenced by several parameters including nitrogen to phosphorus ratio (N:P). In HRAP influent, mean N:P ratio is about 2.6:1 during the observation period (March to May 2024), which is lower than the range reported in previous studies.
According to Klausmeier et al. [67], an N:P ratio between 5.5:1 and 17.4:1 was the optimal range. Otherwise, low N:P (<5.5:1) and high N:P (>30:1) imply the limitation of nitrogen and phosphorus, respectively.
Other parameters can affect algal productivity, such as increasing temperature beyond optimal growth conditions, which can reduce algal growth and productivity [35]. Long-term exposure to direct sunlight can also cause photoinhibition, leading to reduced growth cell death in algal cultures [68]. On the other hand, in warm climates, evaporation is high, leading to an increase in salinity that causes osmotic and cellular ionic stress [69].
The lowest algal biomass obtained in the present study could be related to the lack of nutrient in the HRAP. This is probably the reason why ammonia, nitrate and phosphate removals were low in this pond. To increase algal productivity, the operating conditions of Oxylag could be modified to limit the rate of nutrient removal in this pond, making more nutrients available in the HRAP influent. Another option that should be assessed is to discharge some of the raw effluent directly to the HRAP, bypassing the Oxylag.
3.5. Heavy Metal (Hm) Content and Removal in WWTDPP
The concentrations of heavy metal (HM) at the inlet and outlet of the WWTDPP are shown in Table 6. The concentrations measured in the raw wastewater followed the following order: Cd < Hg < Co < Pb < Cr < Se < Ni < As < Cu < Zn < B < Fe. Those measured in the effluent at the outlet of the WWTDP were in the following order: Cd < Hg < Se < Pb < As < Cr < Cu < Ni < Co < Zn < Fe < B. No significant differences (p > 0.05) were observed between the concentrations monitored in the inlet and outlet. Nevertheless, HM concentrations are in accordance with the Tunisian standards (NT 106.03) for treated wastewater reuse, except for Hg content. The effluent Hg concentration varies between 0.001 and 3.71 μg L−1, with the highest concentrations of 1.08 and 3.71 μg L−1 observed during April and May 2024, respectively. Unlike the findings of previous studies on the efficiency of algae in removing metal pollutants [17,70], the removal efficiency of HM in the HRAP seems to be relatively low. The use of microalgae for heavy metal removal was first proposed by Oswald [71] and has been increasingly continued, with field experiments undertaken regarding the effect of factors affecting the efficiency of heavy metal removal, including pH, light regime, N:P mass ratio, algae biomass and species, contact time, temperature and ion strength [70]. The lowest HM removal could be linked to the low algal productivity in HRAP as a result of nutrient limitation. Wang et al. [70] suggest that the algal HM removal efficiency depends largely on nutrient availability. A positive correlation between HM removal and algal growth was noted [70]. The HM removal in wastewater treatment plants involves several mechanisms such as adsorption on algal cells and suspended particles, sedimentation and complexation with organic or mineral colloidal elements [72].

Table 6.
Heavy metal content in both influent and effluent compared with Tunisian standards (NT 106.03) [40].
In our case, the low HM removal in HRAP could also be attributed to the potential release of heavy metals from the sludge accumulated in the Oxylag, whose HM concentration is shown in Figure 7a. Indeed, the mixing conditions within the Oxylag pond results in sediment disturbance, which leads to a remobilization of HM as a result of change in the chemical proprieties of sediment [73]. Therefore, there is an urgent need to identify sources of Hg contamination and take suitable measurements to avoid their discharge in wastewater. The potential source of Hg in the raw wastewater could be related to the activities of the chemical laboratory, where chemical reagents containing mercury compounds are used in research and practical works. However, the full identification of the relationship between the mercury content of the wastewater and the potential source of this metal remains an unquantified assumption.
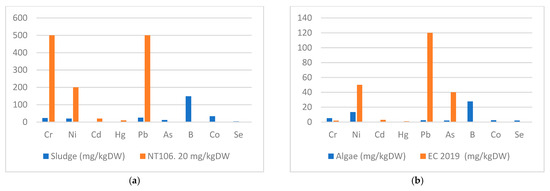
Figure 7.
Heavy metal content in sludge (a) and algae (b).
Previous studies have confirmed the high ability of several algae species such as Chlorella and Scenedesmus in heavy metal removal from wastewater [74,75,76]. However, most research studies have been conducted in lab-scale systems operated under constant temperature, pH, initial concentration of metal and contact time, which could not provide an accurate description of system performance under outdoors conditions [77].
Mean HM concentration measured on algae samples collected from HRAP showed the following order: Cd < Hg < As < Se < Pb < Co < Cr < Ni < Cu < B<Zn < Fe, whereas the mean HM concentrations measured on sludge samples collected from Oxylag pond followed the following trend: Cd < Hg < Se < As < Ni < Cr < Pb < Co < B<Cu < Zn < Fe. Iron is the most dominant heavy metal both in the effluent, algae biomass and the sludge with respective mean concentrations of 2206.96 ± 1853 μg L−1, 1858 ± 111 mg kgDW−1, and 28,356 ± 6805 mg kgDW−1.
The results suggest that HM adsorption on algal cells and suspended particles as well as sedimentation and complexation with organic or mineral colloidal elements occur during the treatment process.
An evaluation of heavy metal content of algae biomass was carried out to assess compliance with the European regulation (EC, 2019/1009) [78] on fertilizer products and to confirm their retention efficiency in the HRAP system. Although the regulation (EC, 2019/1009) does not cover algal biomass harvested from wastewater and used as biofertilizer, it could be an alternative to organo-mineral fertilizers due to its abundance in nitrogen, phosphorus and micronutrients [28]. The algae’s HM concentration is in accordance with the European regulation (EC, 2019/1009) for reuse, except for the chromium content of the algae (Figure 7b). Cr concentration varied between 4.6 and 5.8 mg kgDW−1 which exceeds the limit of the European regulation (2 mg kgDW−1). Heavy metal contents in wastewater-grown algae have been reported by Markou et al. [79]. The authors report that HM concentrations in algae using agro-industrial wastes were in the ranges of 13.5–44.5 mg kgDW−1, 226–333 mg kgDW−1 and 9.2–15.1 mg kgDW−1 for Cd, Cr and Hg, respectively. Alvarez-Gonzalez et al. [28] report heavy metal contents in wastewater-grown algae biomass of 3.10 mg kgDW−1 for Cd, 1.31 mg kgDW−1 for Cr, 46.5 mg kgDW−1 for Ni, 0.52 mg kgDW−1 for Hg, 46.5 mg kgDW−1 for Pb, 279 mg kgDW−1 for Cu and 437 mg kgDW−1 for Zn. The authors suggest that, despite these results on heavy metal concentrations, algae biomass could be used for agricultural purposes.
Regarding the sludge Hm content, the recorded concentrations are in accordance with the Tunisian standards (NT 106.20) [80] (Figure 7a) for reuse in agriculture as fertilizer, even for both Hg and Cd, with concentrations of 1.12 ± 0.02 and 0.63 ± 0.01 mg kgDW−1, respectively. The limit values of NT 106.20 for Hg and Cd are 10 and 20 mg kgDW−1, respectively. Therefore, this sludge can be reused as an organic fertilizer where the amount of organic matter in the soil is poor and the price of organic fertilizer is high. These results are consistent with the study reported by Marzougui et al. [81] on sludge produced by five Tunisian WWTPs to promote sustainable sludge use and management for agriculture. The authors report that Hg and Cd concentrations varied from 0.57 and 1.3 mg kgDW−1 for Cd and from 0.4 and 1.2 mg kgDW−1 for Hg, they have shown that, in terms of cadmium, chromium, copper, mercury, lead, zinc, and nickel, the results comply with Tunisian standards (NT 106.20) with an agricultural reuse for fodder, industrial and food crops.
In conclusion, the WWTDPP of the university campus of Chott Mariem is the only small scale combined HRAP and Oxylag system in Tunisia and is an opportunity of a practical case study of a real scenario where microalgae cultivation systems are exposed to the fluctuating environmental and physicochemical conditions. Therefore, additional monitoring studies with high sampling frequency approaches are needed and caution should be considered for the reuse of treated wastewater on the campus site. However, we did not directly test whether the sludge was a source and/or sink of heavy metals. In order to optimize the removal of heavy metals and to identify the factors affecting algae adsorption, metal sludge accumulation and release, further studies should be carried out under controlled environmental conditions. The investigation of the potential sources and sinks of harmful compounds remains of great interest.
3.6. Bacteria Removal
In this study, total coliform (TC) concentration ranged from 7.32 to 8.35 log10 in inlet samples and from 2.87 to 4.17 log unit in the effluent samples. The fecal coliform (FC) concentration ranged from 7.07 to 7.53 log units in inlet samples and from 2.65 to 3.07 log units in outlet samples. Furthermore, the average removal rates of TC and FC were 4.16 and 4.36 log units, respectively. It was observed that the removal rate of total and fecal coliforms was about 99.99%.
The reduction in coliform concentration in the effluent indicates the ability of the COHRAP to remove bacteria. HRAP is known for its efficiency in removing bacteria due to ultraviolet light. Algae are known to act as bactericides, thus reducing the ability of pathogenic bacteria to proliferate [82]. Pathogens could also be removed from the liquid fraction by sedimentation after adsorption to solid particles [83]. Pathogen removal in HRAP depends on several factors, such as sunlight, pH, dissolved oxygen (DO) and temperature, as well as predation [84]. However, Chambonniere et al. [85] have mentioned that the reduction of fecal coliforms does not necessarily imply the removal of various pathogens (e.g., viruses, helminth eggs or protozoa) and suggest that it is imperative to investigate the ability of the WWTP to remove different pathogens in order to avoid potential risk in water reuse.
In comparison with previous studies, the average removal rates obtained are similar to those reported by Albornoz et al. [86] for a compact wastewater treatment plant that included biological treatment, a secondary clarifier and a disinfection tank on a university campus in Brazil. The authors report a removal rate reaching 95%. Removal efficiencies of 5.66 and 5.63 log units for TC and FC, respectively, were reported by Abd-Elmaksoud et al. [56] in a wastewater treatment plant in Egypt, including three treatment stages (i.e., facultative pond, HRAP, and rock filter).
Considering the Tunisian standards (NT 106.03) for the reuse of treated wastewater, which defines the list of crops that can be irrigated with treated wastewater (e.g., industrial, cereal and fodder crops, fruit trees) and considers a limit for helminth eggs (<1 egg/L) and not for bacteria, the treated effluent provided by the WWTDPP and intended for irrigation of fruit trees, cereal crops and ornamental plants could be suitable (in terms of microbiological quality) for reuse at the Chott Mariem University campus. On the other hand, these promising results for bacterial removal need to be confirmed for other pathogens such as helminth eggs and E. coli pathogens. To prevent potential pathogen risk, selecting appropriate irrigation management (e.g., drip irrigation), regularly monitoring effluent and utilizing preventive measurements to protect workers from wastewater handling can help to minimize the risk. However, TSS compounds in the effluent need to be consolidated by algae recovery before drip irrigation can be implemented.
3.7. Phytotoxicity of Treated Wastewater and Potential Reuse for Agriculture Purposes
Preliminary trials were conducted in Petri dishes to evaluate the effect of treated wastewater irrigation on germination and growth of Zea mays L., aiming to ensure the safe use of treated wastewater. The results indicate that treated wastewater had no significant effect on germination, as both germination rate (GR) and germination index (GI) showed no significant differences between the control and seeds irrigated with HRAP effluent (p > 0.05, Duncan test). The GR was 100% for both groups. The GI, expressed as a percentage of the control, was 97.44 ± 0.16%, indicating that the treated seeds exhibited similar germination dynamics to the control. Similarly, no significant differences were observed in growth parameters between the control and the wastewater irrigated seeds. Specifically, the root and shoot lengths for the control were 7.73 ± 0.17 cm and 1.63 ± 0.08 cm, respectively, compared with 7.42 ± 0.21 cm and 1.57 ± 0.04 cm for the treated seeds. These results suggest that treated wastewater is not phytotoxic to the target species. Given the large area of forest plants, fruit trees and fodder crop as well as ornamental plants on the university campus of Chott Mariem, a possible reuse of the treated wastewater appears as an interesting alternative to address the water deficit across the campus area.
However, further experiments should be carried out to confirm this finding. In fact, the effect of the reuse of WWTDPP effluent on plant growth, in pots experiments, are part of our ongoing research. Furthermore, field trials could be conducted to assess the potential microbial risk and to ensure the benefits of reusing treated water for plants and soil. Improving the quality of treated water for further non-restrictive crop irrigation could be considered in future research.
Finally, considering the current regulation in Tunisia (NT 106.03) for the reuse of water in agriculture with restrictive crop irrigation, the results obtained in this study have limitations with regard to TSS and Hg, as the limit values are sometimes exceeded (Table 5). However, even if the limits for all parameters were respected, bioaccumulation of HM after long-term irrigation remains a challenge.
3.8. Operational Costs of WWTDPP
The annual operating costs of the WWTDPP include the costs of personal, electricity and sludge management (Table 7). The results indicate that energy and sludge management are the two units with the highest costs. The energy consumption expressed in KWh per 1 m3 of treated water is 1.05 KWh m−3, which corresponds to an average operating cost of 0.29 EUR m−3 of treated wastewater, assuming the price of 0.156 EUR/kWh. This energy consumption includes both air blower of Oxylag and HRAP and wastewater pumping.

Table 7.
Energy consumption and cost categories of WWTDPP.
In literature, limited studies dealing with energy consumption are available using a combined HRAP, particularly on small scale treatments plants. Nevertheless, the obtained results are higher than those reported by Kohelhb et al. [87] on a demonstration scale HRAP treating 300 m−3 d−1, including with an energy consumption of 0.10 kWh m−3 in the presence of submersed mixed technology. Whereas the energy consumption is about 0.17 kWh m−3 with a simple paddle wheel mixing system. Robles et al. [88] has reported an energy requirement of around 0.29 kWh per m3 of treated water on a combined membrane–HRAP system with a working volume range comparable to that of COHRAP.
It should be noted that the installed blower has a higher oxygenation capacity than the required oxygenation, as it is the smallest blower range available on the market. In our case, lower energy consumption could be achieved by reducing the frequency of blower operation. However, the oxygen concentration in the Oxylag should be maintained at 2.5 mg L−1 and the flow velocities in HRAP and Oxylag must be respected.
4. Conclusions
The implementation of WWTDPP on the university campus of Chott Mariem could provide an alternative water resource for irrigation. It would provide a concrete example of innovative water treatment and reuse of water and bio-products within the campus and the wider environmental community. In addition to promoting sustainability and resource recovery, the implementation of the demonstration plant will provide and disseminate scientific knowledge on HRAP and Oxylag technologies.
The studied WWTDPP presents a good performance. However, improvement measures are needed in terms of monitoring, regulatory compliance and energy performance in order to establish the groundwork for future optimization.
In terms of monitoring and control strategies, continuous flow measurement and automated sampling have been suggested and are currently being implemented to ensure reliable monitoring and greater accuracy of the results.
Considering the current regulation in Tunisia (NT 106.03) for wastewater reuse in agriculture with restrictive crop irrigation, the treated effluent provided by the WWTDPP have limitations with regard to TSS and Hg, as the limit values are sometimes exceeded. The implementation of an algae recovery system downstream of the HRAP is necessary to improve TSS and Hg removal. In addition, it is suggested that the frequency setting of the variable speed blower drive and the operating time should be changed in order to reduce energy consumption and the rate of oxygen supply to the Oxylag. Nitrogen and phosphorus removal occurring in the Oxylag should be minimized to make these elements more readily available in the HRAP influent. Thus, nutrients in the HRAP will improve algae productivity, which is a valuable resource for items such as biofertilizer. On the other hand, the operational strategy of flow and retention time adjustment must be integrated, taking into account the different periods of the academic year. A more detailed critical analysis of the effect of loading rate on efficiency removal will be undertaken to identify opportunities for improvement. Regarding heavy metal content in the effluent, there is an urgent need to identify the source of Hg contamination and take appropriate measures to prevent its discharge. In addition, further studies should be performed to investigate the factors affecting heavy metal algae adsorption and release.
Although the levels of TSS and Hg were occasionally higher than the Tunisian standards (NT 106.03), the phytotoxicity tests show no significant differences between the control and the seeds irrigated with HRAP effluent. Hence, reuse of effluent for the irrigation of fruit trees, cereal crops and ornamental plants could be appropriate on the campus site. In addition, the algae biomass and the sludge comply with the requirements of the European regulation on fertilizers (EC, 2019/1009) and the Tunisian national standard (NT 106.20) regarding sludge reuse in agriculture, except for the chromium content of the algae. However, even if the limits for all parameters were respected, the bioaccumulation, persistence, occurrence and toxicity of heavy metals and harmful pollutants after long-term irrigation and reuse of sludge and algae as biofertilizers remains a challenge.
In the context of sustainable development, the combined HRAP-Oxylag system is a viable and promising alternative for wastewater treatment with valuable by-products.
Author Contributions
Conceptualization, C.K., G.J., A.M., F.Z., N.D.L., B.T. and H.J.; methodology, C.K., G.J., A.M., F.Z. and H.J.; validation, C.K., G.J., A.M., F.Z. and H.J; formal analysis, C.K., G.J., A.M., F.Z., N.D.L. and H.J.; investigation, C.K., G.J., A.M. and H.J.; data curation, C.K. and H.J.; writing—original draft preparation, C.K. and H.J.; writing—review and editing, C.K., G.J., N.D.L., B.T. and H.J.; project administration, B.T. and H.J.; funding acquisition, C.K., F.Z., B.T. and H.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Wallonie Bruxelles International, Belgique through the project « Traitement des eaux usées II » ref 1.1.5, grant between Liège University and Higher Agronomic Institute of Chott Mariem.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Special thanks to the staff of Wallonie Bruxelles International for their cooperation and financial support during the field survey. Special thanks to the team involved in this project.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| COHRAP | Combined Oxylag and high rate algal pond |
| COD | Chemical oxygen demand |
| BOD | Biochemical oxygen demand |
| TSS | Total suspended solids |
| HRAP | High rate algal pond |
| PE | Population equivalents |
| WWTDPP | Wastewater treatment demonstration pilot plant |
| HDPE | High density polyethylene membrane |
| HRT | Hydraulic retention time |
| Algae productivity | |
| TN | Total nitrogen |
| HM | Heavy metal |
| GR | Germination rate |
| GI | Germination indices |
| ISA-CM | Higher agronomic institute of Chott Mariem |
| FP | Facultative pond |
| RF | Rock filter |
| DW | Dry weight |
| TC | Total coliforms |
| FC | Fecal coliforms |
References
- Nikuze, M.J.; Niyomukiza, J.B.; Nshimiyimana, A.; Kwizera, J.P. Assessment of the efficiency of the wastewater treatment plant: A case of Gacuriro Vision City. IOP Conf. Ser. Earth Environ. Sci 2020, 448, 012046. [Google Scholar] [CrossRef]
- UNESCO World Water Assessment Programme (WWAP). The United Nations World Water Development Report: Leaving No One Behind; UNESCO: Paris, France, 2019; p. 186. [Google Scholar]
- Pirsaheb, M.; Khamutian, R.; Khodadadian, M. A comparison between extended aeration sludge and conventional activated sludge treatment for removal of linear alkylbenzene sulfonates (Case study: Kermanshah and Paveh WWTP). Desalin. Water Treat. 2014, 52, 4673–4680. [Google Scholar] [CrossRef]
- Zobeidi, A.; Bebba, A.A.; Douadi, A. Effects of hydraulic retention time on organic loading rate in efficiency of aerated lagoons in treating rural domestic wastewater at El-Oued (South-East Algeria). Orient. J. Chem. 2017, 33, 1890–1898. [Google Scholar]
- Cockx, A.; Boumansour, B.E.; Line, A.; Vasel, J. Modeling the hydraulics and oxygen transfer in the “Oxylag” bioreactor. In Proceedings of the Conference: Second European Congress of Chemical Engineering (ECCE2), Montpellier, France, 5–7 October 1999. [Google Scholar]
- Bontoux, J.; Picot, B. Possibilites et limites des bassins lagunaires dans l’epuration des eaux usees. Water Pollut. Res. J. Can. 1994, 29, 545. [Google Scholar] [CrossRef]
- Abid, A.; Jellal, J.E.; Bamaarouf, M.; Ihmaine, E.G.H. Optimization of Kinetic Model for High Rate Algal Pond Design. IJEE 2015, 6, 134–140. [Google Scholar]
- Garfí, M.; Flores, L.; Ferrer, I. Life Cycle Assessment of wastewater treatment systems for small communities: Activated sludge, constructed wetlands and high rate algal ponds. J. Clean. Prod. 2017, 161, 211–219. [Google Scholar] [CrossRef]
- Heubeck, S.; Craggs, R.J.; Shilton, A. Influence of CO2 scrubbing from biogas on the treatment performance of a high rate algal pond. Water Sci. Technol. 2007, 55, 193. [Google Scholar] [CrossRef]
- Arbib, Z.; Ruiz, J.; Alvarez, P.; Garrido, C.; Barragan, J.; Perales, J.A. Long term outdoor operation of a tubular airlift pilot photobioreactor and a high rate algal pond as tertiary treatment of urban wastewater. Ecol. Eng. 2013, 52, 143–153. [Google Scholar] [CrossRef]
- Couto, E.A.; Calijuri, M.L.; Assemany, P.P.; Tango, M.D.; Santiago, A.F. Influence of solar radiation on nitrogen recovery by the biomass grown in high rate ponds. Ecol. Eng. 2015, 81, 140–145. [Google Scholar] [CrossRef]
- Craggs, R.; Park, J.; Heubeck, S.; Sutherland, D. High rate algal pond systems for low-energy wastewater treatment, nutrient recovery and energy production. N. Z. J. Bot. 2014, 52, 60–73. [Google Scholar] [CrossRef]
- Park, J.B.K.; Craggs, R.J.; Shilton, A.N. Recycling algae to improve species control and harvest efficiency from a high rate algal pond. Water Res. 2011, 45, 6637–6649. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, D.L.; Howard-Williams, C.; Turnbull, M.H.; Broady, P.A.; Craggs, R.J. Seasonal variation in light utilisation, biomass production and nutrient removal by wastewater microalgae in a full-scale high-rate algal pond. J. Appl. Phycol. 2014, 26, 1317–1329. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Kang, D.; Wu, C.; Wu, Y. Removal of pharmaceuticals and personal care products from wastewater using algae-based technologies: A review. Rev. Environ. Sci. Biotechnol. 2017, 16, 717–735. [Google Scholar] [CrossRef]
- Nie, J.; Sun, Y.; Zhou, Y.; Kumar, M.; Usman, M.; Li, J.; Shao, J.; Wang, L.; Tsang, D.C.W. Bioremediation of water containing pesticides by microalgae: Mechanisms, methods, and prospects for future research. Sci. Total Environ. 2020, 707, 136080. [Google Scholar] [CrossRef]
- Suresh Kumar, K.; Dahms, H.U.; Won, E.J.; Lee, J.S.; Shin, K.H. Microalgae—A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Gaignard, C.; Laroche, C.; Pierre, G.; Dubessay, P.; Delattre, C.; Gardarin, C.; Gourvil, P.; Probert, I.; Dubuffet, A.; Michaud, P. Screening of marine microalgae: Investigation of new exopolysaccharide producers. Algal Res. 2019, 44, 101711. [Google Scholar] [CrossRef]
- Yang, W.; Song, W.; Li, J.; Zhang, X. Bioleaching of heavy metals from wastewater sludge with the aim of land application. Chemosphere 2020, 249, 126134. [Google Scholar] [CrossRef]
- Park, J.B.; Weaver, L.; Davies-Colley, R.; Stott, R.; Williamson, W.; Mackenzie, M.; McGill, E.; Lin, S.; Webber, J.; Craggs, R.J. Comparison of faecal indicator and viral pathogen light and dark disinfection mechanisms in wastewater treatment pond mesocosms. J. Environ. Manag. 2021, 286, 112197. [Google Scholar] [CrossRef]
- Davies-Colley, R.J. Pond Disinfection in Pond Treatment Technology; Shilton, A., Ed.; IWA Scientific and Technical Report Series; IWA Publishing: London, UK, 2005; pp. 100–136. [Google Scholar]
- Craggs, R.; Sutherland, D.; Campbell, H. Hectare-scale demonstration of high rate algal ponds for enhanced wastewater treatment and biofuel production. J. Appl. Phycol. 2012, 24, 329–337. [Google Scholar] [CrossRef]
- Shahid, A.; Malik, S.; Zhu, H.; Xu, J.; Nawaz, M.Z.; Nawaz, S.; Asraful Alam, M.; Mehmood, M.A. Cultivating microalgae in wastewater for biomass production, pollutant removal, and atmospheric carbon mitigation: A review. Sci. Total Environ. 2020, 704, 135303. [Google Scholar] [CrossRef] [PubMed]
- Arashiro, L.T.; Ferrer, I.; Rousseau, D.P.; Van Hulle, S.W.; Garfí, M. The effect of primary treatment of wastewater in high rate algal pond systems: Biomass and bioenergy recovery. Bioresour. Technol. 2019, 280, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Craggs, R.J.; Heubeck, S.; Lundquist, T.J.; Benemann, J.R. Algal biofuels from wastewater treatment high rate algal ponds. Water Sci. Technol. 2011, 63, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Van Den Hende, S.; Beelen, V.; Julien, L.; Lefoulon, A.; Vanhoucke, T.; Coolsaet, C.; Sonnenholzner, S.; Vervaeren, H.; Rousseau, D.P.L. Technical potential of microalgal bacterial floc raceway ponds treating food-industry effluents while producing microalgal bacterial biomass: An outdoor pilot-scale study. Bioresour. Technol. 2016, 218, 969–979. [Google Scholar] [CrossRef]
- Álvarez-González, A.; Uggetti, E.; Serrano, L.; Gorchs, G.; Escolà Casas, M.; Matamoros, V.; Gonzalez-Flo, E.; Díez-Montero, R. The potential of wastewater grown microalgae for agricultural purposes: Contaminants of emerging concern, heavy metals and pathogens assessment. Environ. Pollut. 2023, 324, 121399. [Google Scholar] [CrossRef]
- Young, P.; Taylor, M.; Fallowfield, H.J. Mini-review: High rate algal ponds, flexible systems for sustainable wastewater treatment. World J. Microbiol. Biotechnol. 2017, 33, 117. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Turnbull, M.H.; Craggs, R.J. Environmental drivers that influence microalgal species in fullscale wastewater treatment High rate algal ponds. Water Res. 2017, 124, 504–512. [Google Scholar] [CrossRef]
- Sheehan, J.; Dunahay, T.; Benemann, J.; Roessler, P. A Look Back at the U.S. Department of Energy’s Aquatic Species Program—Biodiesel from Algae; National Renewable Energy Laboratory: Applewood, CO, USA, 1998; pp. 1–249. [Google Scholar]
- Sutherland, D.L.; Turnbull, M.H.; Craggs, R.J. Increased Pond depth improves algal productivity and nutrient removal in wastewater treatment high rate algal ponds. Water Res. 2014, 53, 271–281. [Google Scholar] [CrossRef]
- Amini, H.; Hashemisohi, A.; Wang, L.; Shahbazi, A.; Bikdash, M.; KC, D.; Yuan, W. Numerical and experimental investigation of hydrodynamics and light transfer in open raceway ponds at various algal cell concentrations and medium depths. Chem. Eng. Sci. 2016, 156, 11–23. [Google Scholar] [CrossRef]
- Buchanan, N.A.; Young, P.; Cromar, N.J.; Fallowfield, H.J. Performance of a high rate algal pond treating septic tank effluent from a community wastewater management scheme in rural South Australia. Algal Res. 2018, 35, 325–332. [Google Scholar] [CrossRef]
- Posadas, E.; Muñoz, R.; Guieysse, B. Integrating nutrient removal and solid management restricts the feasibility of algal biofuel generation via wastewater treatment. Algal Res. 2017, 22, 39–46. [Google Scholar] [CrossRef]
- Tyagi, R.D.; Couillard, D. Toxic effects of inhibitors in biological wastewater treatment processes. Can. J. Chem. Eng. 1988, 66, 97–106. [Google Scholar] [CrossRef]
- Couto, E.D.; Calijuri, M.L.; Assemany, P.P.; Cecon, P.R. Evaluation of high rate ponds operational and design strategies for algal biomass production and domestic wastewater treatment. Sci. Total Environ. 2021, 791, 148362. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.G.G.; Gómez, V.A.; Torre, R.M.; de Godos Crespo, I. Scale-down of high-rate algae ponds systems for urban wastewater reuse. J. Water Process Eng. 2023, 56, 104342. [Google Scholar] [CrossRef]
- Dahamsheh, A.; Wedyan, M. Evaluation and assessment of performance of Al-Hussein bin Talal University (AHU) wastewater treatment plants. Int. J. Adv. Appl. Sci. 2017, 4, 84–89. [Google Scholar] [CrossRef]
- Tunisian Standards NT 106.03; Reuse of Treated Wastewater in Agriculture—Physicochemical and Biological Specifications. National Institute for Standardization and Industrial Properties (INNORPI, Tunisia): Tunis, Tunisia, 1989. (In French)
- Cromar, N.J.; Fallowfield, H.J. Effect of nutrient loading and retention time on performance of high rate algal ponds. J. Appl. Phycol. 1997, 9, 301–309. [Google Scholar] [CrossRef]
- Solimeno, A.; García, J. Microalgae and bacteria dynamics in high rate algal ponds based on modelling results: Long-term application of BIO_ALGAE model. Sci. Total Environ. 2019, 650, 1818–1831. [Google Scholar] [CrossRef]
- Chisti, Y. Green Energy and Technology. In Algae Biotechnology: Products and Processes.; Bux, F., Chisti, Y., Eds.; Springer: Cham, Switzerland, 2016; pp. 1–344. [Google Scholar]
- John, D.M.; Whitton, B.A.; Brook, A.J. The Freshwater Algal Flora of the British Isles: An Identification Guide to Freshwater and Terrestrial Algae, 1st ed.; Cambridge University Press: Cambridge, UK, 2002; pp. 1–18. [Google Scholar]
- Raschke, R.L. Diatom community response to phosphorus in the Everglades National Park USA. Phycologia 1993, 32, 48–58. [Google Scholar] [CrossRef]
- Velásquez-Orta, S.B.; Yáñez-Noguez, I.; Ramírez, I.M.; Orta-Ledesma, M.T. Pilot-scale microalgae cultivation and wastewater treatment using high-rate ponds: A meta-analysis. Environ. Sci. Pollut. Res. 2024, 31, 46994–47021. [Google Scholar] [CrossRef]
- Park, J.B.; Craggs, R.J. Wastewater treatment and algal production in high rate algal ponds with carbon dioxide addition. Water Sci. Technol. 2010, 61, 633–639. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 15th ed.; American Public Health Association and Water Pollution Control Federation: Washington, DC, USA, 2000; pp. 12–56. [Google Scholar]
- ISO 10260; Water Quality, Measurement of Biochem. Parameters; Spectrometric Determination of the Chlorophyll-a Concentration. ISO: Geneva, Switzerland, 1992.
- USEPA. SW-846 Test Method 3051A. Microwave assisted acid digestion of sediments, sludges, soils and oils. In Test Methods for Evaluating Solid Waste, 2nd ed.; US Environmental Protection Agency: Washington, DC, USA, 1998; pp. 1–30. [Google Scholar]
- ISO 17294-2:2023; Water Quality, Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of Selected Elements Including Uranium Isotopes. ISO: Geneva, Switzerland, 2003.
- Grimah, K.; Abdelmottalib, N.; Lazrak, A.; Chlaida, M. Physicochemical characterization of university campus wastewater for internal treatment system installation (Casablanca, Morocco). ER&T 2024, 7, 13–26. [Google Scholar]
- Melián, J.H.; Araña, J.; Díaz, O.G.; Bujalance, M.A.; Rodríguez, J.D. Effect of stone filters in a pond–wetland system treating raw wastewater from a university campus. Desalination 2009, 237, 277–284. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Wastewater Engineering: Treatment and Reuse, 4th ed.; McGrawHill: New York, NY, USA, 2003; pp. 1–53. [Google Scholar]
- Molle, P.; Liénard, A.; Boutin, C.; Merlin, G.; Iwema, A. How to treat raw sewage with constructed wetlands: An overview of the French systems. Water Sci. Technol. 2005, 51, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elmaksoud, S.; Abdo, S.M.; Gad, M.; Hu, A.; El-Liethy, M.A.; Rizk, N.; Marouf, M.A.; Hamza, I.A.; Doma, H.S. Pathogens Removal in a Sustainable and Economic High-Rate Algal Pond Wastewater Treatment System. Sustainability 2021, 13, 13232. [Google Scholar] [CrossRef]
- Antoniou, P.; Hamilton, J.; Koopman, B.; Jain, R.; Holloway, B.; Lyberatos, G.; Svoronos, S.A. Effect of temperature and pH on the effective maximum specific growth rate of nitrifying bacteria. Water Res. 1990, 24, 97–101. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Howard-Williams, C.; Turnbull, M.H.; Broady, P.A.; Craggs, R.J. The effects of CO2 addition along a pH gradient on wastewater microalgal photo-physiology, biomass production and nutrient removal. Water Res. 2015, 70, 9–26. [Google Scholar] [CrossRef]
- Arcila, J.S.; Buitrón, G. Microalgae–bacteria aggregates: effect of the hydraulic retention time on the municipal wastewater treatment, biomass settleability and methane potential. J. Chem. Technol. Biotechnol. 2016, 91, 2862–2870. [Google Scholar] [CrossRef]
- Oliver, R.L.; Ganf, G.G. Freshwater Blooms. In Ecology of Cyanobacteria: Their Diversity in Time and Space; Whitton, B.A., Potts, M., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 149–194. [Google Scholar] [CrossRef]
- García, J.; Green, B.F.; Lundquist, T.; Mujeriego, R.; Hernández-Mariné, M.; Oswald, W.J. Long term diurnal variations in contaminant removal in high rate ponds treating urban wastewater. Bioresour. Technol. 2006, 97, 1709–1715. [Google Scholar] [CrossRef]
- Reynolds, C.S. The Ecology of Freshwater Phytoplankton; Cambridge University Press: Cambridge, UK, 1984; p. 384. [Google Scholar]
- Cosgrove, J.J. Marine Phytoplankton Primary Production and Ecophysiology Using Chlorophyll-A Fluorescence. Ph.D. Thesis in Philosophy, Murdoch University, Perth, Australia, 2007. [Google Scholar]
- Reynolds, C.S. Phytoplankton periodicity: The interactions of form, function and environmental variability. Freshw. Biol. 1984, 14, 111–142. [Google Scholar] [CrossRef]
- Reynolds, C.S. Physical determinants of phytoplankton succession. In Plankton Ecology; Sommer, U., Ed.; Springer: Berlin/Heidelberg, Germany, 1989; pp. 9–56. [Google Scholar]
- Sutherland, D.L.; Park, J.; Heubeck, S.; Ralph, P.J.; Craggs, R.J. Size matters—Microalgae production and nutrient removal in wastewater treatment high rate algal ponds of three different sizes. Algal Res. 2020, 45, 101734. [Google Scholar] [CrossRef]
- Klausmeier, C.A.; Litchman, E.; Daufresne, T.; Levin, S.A. Optimal nitrogen-to phosphorus stoichiometry of phytoplankton. Nature 2004, 429, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Christenson, L.; Sims, R. Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol. Adv. 2011, 29, 686–702. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Church, J.; Lee, S.J.; Park, J.; Lee, W.H. Use of microalgae for advanced wastewater treatment and sustainable bioenergy generation. Environ. Eng. Sci. 2016, 33, 882–897. [Google Scholar] [CrossRef]
- Wang, Y.; Song, X.; Li, H.; Ding, Y. Removal of metals from water using a novel high-rate algal pond and submerged macrophyte pond treatment reactor. Water Sci. Technol. 2019, 79, 1447–1457. [Google Scholar] [CrossRef]
- Oswald, W.J. The role of microalgae in liquid waste treatment and reclamation. In Micro-Algal Biotechnology; Borowitzka, M.A., Borowitzka, L.J., Eds.; Cambridge U.P.: Cambridge, UK, 1988; pp. 255–282. [Google Scholar]
- Malik, A. Metal bioremediation through growing cells. Environ. Int. 2004, 30, 261–278. [Google Scholar] [CrossRef]
- Eggleton, J.; Thomas, K.V. A Review of Factors Affecting the Release and Bioavailability of Contaminants during Sediment Disturbance Events. Environ. Int. 2004, 30, 973–980. [Google Scholar] [CrossRef]
- Khalil, Z.I.; Asker, M.M.; El-Sayed, S.; Kobbia, I.A. Effect of pH on growth and biochemical responses of Dunaliella bardawil and Chlorella ellipsoidea. World J. Microbiol. Biotechnol. 2010, 26, 1225–1231. [Google Scholar] [CrossRef]
- Aung, W.L.; Hlaing, N.N.; Aye, N.K. Biosorption of Lead (Pb2+) by using Chlorella vulgaris. IJCEBS 2013, 1, 2320–4087. [Google Scholar]
- Yang, J.; Cao, J.; Xing, G.; Yuan, H. Lipid production combined with biosorption and bioaccumulation of cadmium, copper, manganese and zinc by oleaginous microalgae Chlorella minutissima UTEX2341. Bioresour. Technol. 2015, 175, 537–544. [Google Scholar] [CrossRef]
- Yong, J.J.J.Y.; Chew, K.W.; Khoo, K.S.; Show, P.L.; Chang, J.S. Prospects and development of algal-bacterial biotechnology in environmental management and protection. Biotechnol. Adv. 2021, 47, 107684. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003; European Union: Luxembourg, 2019.
- Markou, G.; Wang, L.; Ye, J.; Unc, A. Using agro-industrial wastes for the cultivation of microalgae and duckweeds: Contamination risks and biomass safety concerns. Biotechnol. Adv. 2018, 36, 1238–1254. [Google Scholar] [CrossRef] [PubMed]
- Tunisian Standards NT 106.20; Fertilizers—Sludge from Urban Wastewater Treatment Works. National Institute of Standardization and Industrial Properties—INNORPI, Tunisia: Tunis, Tunisia, 2002. (In French)
- Marzougui, N.; Ounalli, N.; Sabbahi, S.; Fezzani, T.; Abidi, F.; Jebari, S.; Melki, S.; Berndtsson, R.; Oueslati, W. How Can Sewage Sludge Use in Sustainable Tunisian Agriculture Be Increased? Sustainability 2022, 14, 13722. [Google Scholar] [CrossRef]
- Wanjohi, L.; Mwasi, S.; Mwamburi, L.; Isaboke, J. Efficiency of university of Eldoret wastewater treatment plant, Kenya. AER J. 2019, 3, 99–109. [Google Scholar]
- Araki, S.; González, J.M.; De Luis, E.; Bécares, E. Viability of nematode eggs in high rate algal ponds: The effect of physico-chemical conditions. Water Sci. Technol. 2000, 42, 371–374. [Google Scholar] [CrossRef]
- Verbyla, M.E. Pathogen Removal in Natural Wastewater Treatment and Resource Recovery Systems: Solutions for Small Cities in an Urbanizing World. Ph.D. Thesis, Civil and Environmental Engineering, University of South Florida, Tampa, FL, USA, 2015. [Google Scholar]
- Chambonniere, P.; Bronlund, J.; Guieysse, B. Pathogen removal in high-rate algae pond: State of the art and opportunities. Appl. Phycol. 2021, 33, 1501–1511. [Google Scholar] [CrossRef]
- Albornoz, L.L.; Centurião, T.C.; Giacobbo, A.; Ferreira, J.Z.; Bernardes, A.M. Influence of rain events on the efficiency of a compact wastewater treatment plant: A case study on a university campus aiming water reuse for agriculture. Environ. Sci. Pollut. Res. 2020, 27, 41350–41360. [Google Scholar] [CrossRef]
- Kohlheb, N.; Affereden, M.V.; Lara, E.; Arbib, Z.; Conthe, M.; Poitzsch, C.; Marquartdt, T.; Becker, M.Y. Assessing the life-cycle sustainability of algae and bacteria-based wastewater treatment systems: High-rate algae pond and sequencing batch reactor. J. Environ. Manag. 2020, 264, 110459. [Google Scholar] [CrossRef]
- Robles, Á.; Capson-Tojo, G.; Gales, A.; Viruela, A.; Sialve, B.; Seco, A.; Steyer, J.P.; Ferrer, J. Performance of a membrane-coupled high-rate algal pond for urban wastewater treatment at demonstration scale. Bioresour. Technol. 2020, 301, 122672. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).