Prospect of Conductive Materials in the Anaerobic Digester Matrix for Methane Production: Electron Transfer and Microbial Communication
Abstract
1. Introduction
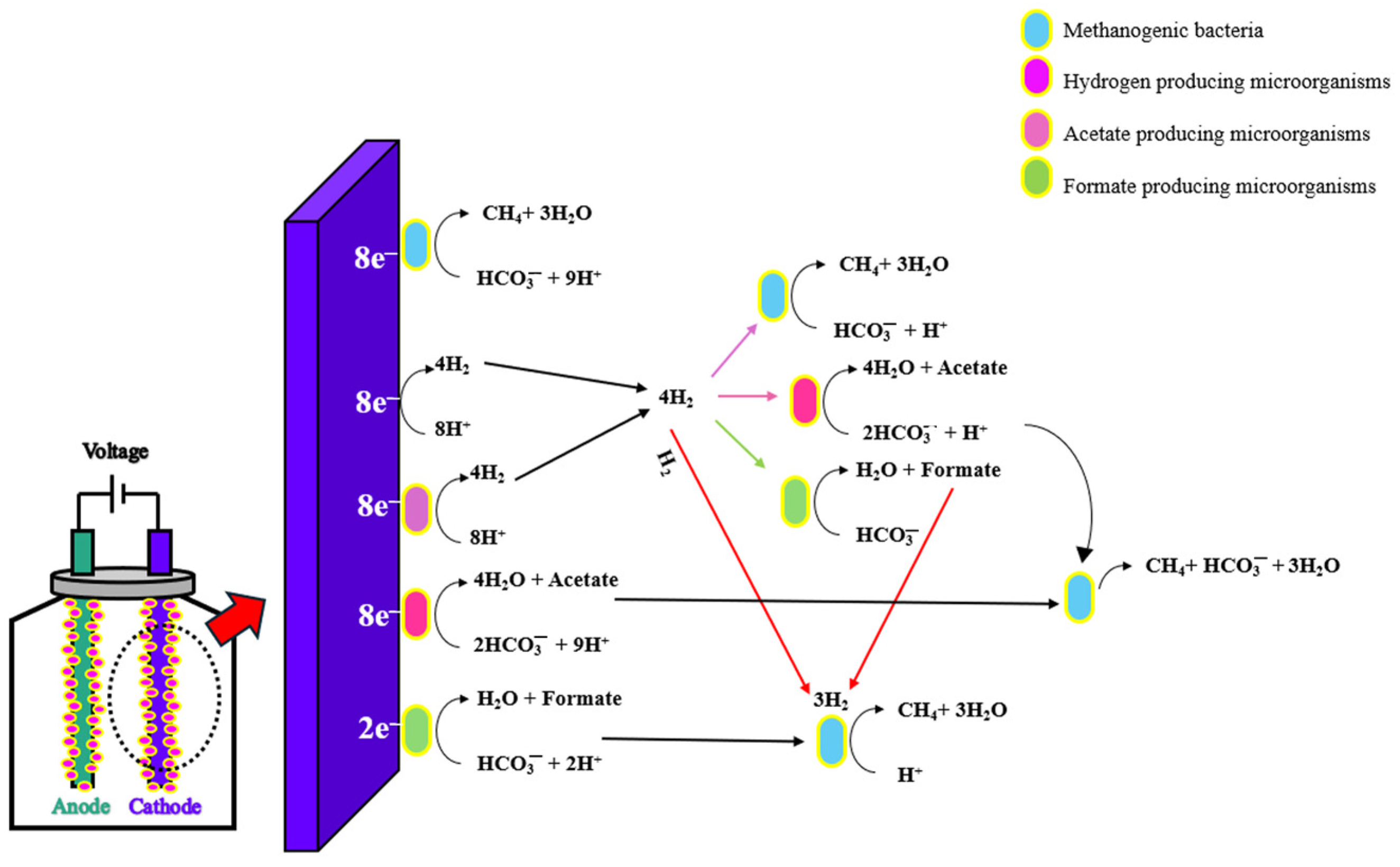
2. Role of Conductive Materials for Electron Transfer
2.1. Indirect Interspecies Electron Transfer
2.2. Direct Interspecies Electron Transfer
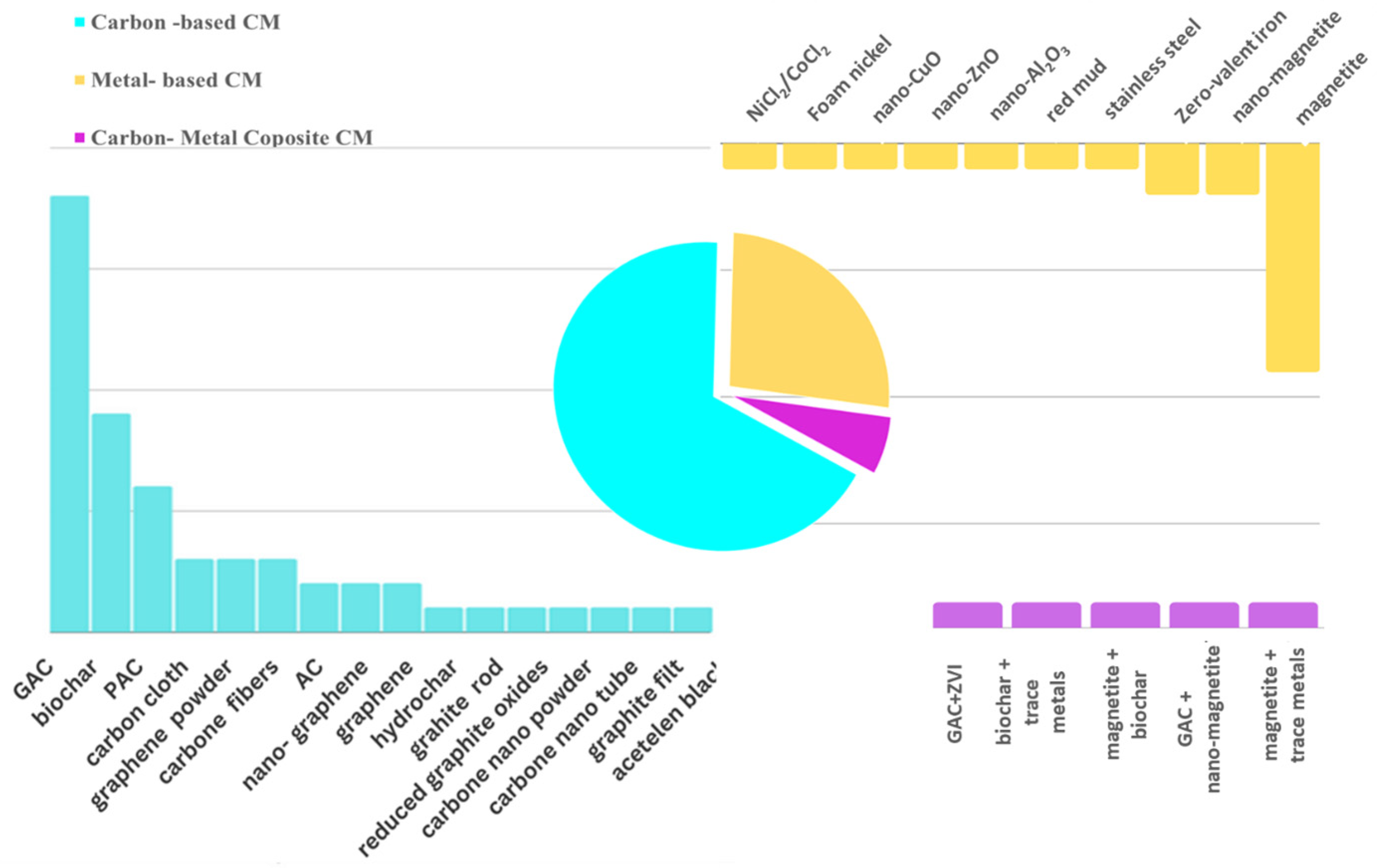
3. Types of Conductive Materials Applied in Anaerobic Digestion
3.1. Carbon-Based Materials
| Material | Reactor | Substrate | Methane CH4 Production | % CH4 Increased | Ref. | |
|---|---|---|---|---|---|---|
| 1 | GAC | UASB | Synthetic brewery wastewater | 60% | 60 | [12] |
| PAC | 70% | 70 | ||||
| 2 | Graphite rods | UASB | Artificial wastewater | 23 mL | 10 | [65] |
| Carbon cloth | 30 mL | 43 | ||||
| Biochar | 28 mL | 33 | ||||
| 3 | GAC | Continuous-flow AD | Synthetic wastewater | 35.7 mL | 78 | [72] |
| 4 | GAC | TAD | Artificial dairy wastewater | 1232.5 ± 27.8 mL | 6 | [79] |
| 5 | Carbon nanotube | Batch | Glucose | 0.48 mL/g VSS | 44 | [80] |
| GAC | 0.67 mL/g VSS. | 56 | ||||
| 6 | Graphene | Batch | Ethanol | 695.0 ± 9.1 mL/g | 25 | [41] |
| 7 | Biochar | Batch | Food waste | 92–110% of initial VFA | 12.8 | [69] |
| 8 | GAC | Batch | Nejayote wastewater | 26 L/kg VS | 34 | [70] |
| 9 | Biochar | Batch | Swine manure | 593.1 ± 50.4 mL | 39 | [81] |
| 10 | Carbon fibers | Batch | Propionate and butyrate | 800 mL | 100 | [82] |
| 11 | GAC | Batch | Acetic acid | 176.7 (±1.4) mL | 31 | [83] |
| Ethanol | 168.9 (±1.6) mL | |||||
| 12 | GAC | Batch | Fat, oil, and grease | Max: 108 ± 11 L/kg VS | 50–80 | [71] |
| 13 | Graphite felt | ASBRs | Artificial wastewater | 537.1 ± 6.4 mL/d | 16.7 | [25] |
| 14 | Carbon fibers | Batch | Ethanol | 205 ± 32 mL/g sCOD | 50 | [64] |
| 15 | GAC | UASB | Raw incineration leachate | 0.27 m3/kg COD | - | [84] |
| 16 | PAC | Batch | Brewery spent yeast | 675 L/kg VS | 69 | [74] |
| 17 | Nano-carbon powder | Batch | Sewage sludge | 593.94 mL/g VS | 16.9 | [85] |
| 18 | AC | Pilot-scale reactor | Food waste | 413 ± 25 mL/g VS | 88 | [86] |
| 19 | Graphite powder | Batch | Glucose | 750.9 mL | −4 | [87] |
| AC | 740.1 mL | −5 | ||||
| 20 | GAC | Batch | Kitchen waste lipid–rapeseed oil | 3300.6 nmol/L | 10 | [77] |
| 21 | GAC | Batch | Liquid swine manure | 23.6 ± 0.7 mL | 33 | [88] |
| Raw swine manure | 165.7 ± 6.4 mL | 10.8 | ||||
| 22 | GAC | Batch | Rural wastewater | 16.7 mL | 23.4 | [89] |
| 23 | Acetylene black | Batch | Vinegar Residue | 94.0 ± 20 mL/g VS | 232 | [90] |
| Hydrochar | 50.3 ± 18.5 mL/g VS | 76.8 | ||||
| 24 | Nano-graphite | Batch | Waste fat, oil, and grease | 168 mL | 14 | [78] |
| GAC | 167.3 mL | 9 | ||||
| Carbon cloth | 179.3 mL | 22 | ||||
| 25 | Biochar | Batch | Sewage sludge and food waste | 335.7 ± 7.1 mL/g VS | 23 | [91] |
| 26 | Carbon fiber | Batch | Synthetic glucose | 83 ± 3 mL/g COD | - | [92] |
| 27 | Biochar | Semi-continuous | Kitchen wastes | Max: 956.1 ± 65.7 mL | 42 | [93] |
| 28 | GAC | Batch | Synthesized blackwater | Max: 318 ± 28 mL/g COD | 8 | [94] |
| PAC | Max: 229 mL/g COD | −1 | ||||
| 29 | Biochar | Batch | Chicken manure | 260 mL/g VS | 31 | [95] |
| 30 | Graphene oxide | Semi-continuous | MSW and sewage sludge | 0.211 NL/gVS | 13.4 | [46] |
| Carbonnanotubes | 0.206 NL/gVS | 10.7 |
3.2. Metal-Based Materials
| Material | Reactor | Substrate | Methane Production | % CH4 Increased | Ref. | |
|---|---|---|---|---|---|---|
| 1 | Magnetite nanoparticles | Batch | Propionate | - | 12 | [109] |
| CSTR | Butyrate | - | 22 | |||
| 2 | Magnetite (Fe3O4) | TAD | Artificial dairy wastewater | 939.6 ± 73.2 mL | 38 | [79] |
| 3 | Magnetite (Fe3O4) | ASBR | Tryptone-based high-strength wastewater | 70.8 ± 7.6 mL | 12.2 | [99] |
| 4 | Magnetite | ASBR | Fischer–Tropsch wastewater | 7.46 ± 0.24 L | [110] | |
| 5 | Red mud with 45.46% hematite | Batch | Waste activated sludge | 1.41 ± 0.02 mmoL/g VSS | 35.52 ± 2.6 | [111] |
| 6 | Magnetite | Batch | Fat, oil, and grease | Max: 72 ± 9 L/kg VS | [71] | |
| 7 | Nano-Al2O3 | Batch | Sewage sludge | 627.11 mL/g VS | 23.40 | [85] |
| Nano-ZnO | 49.57 mL/g VS | −90.20 | ||||
| Nano-CuO | 420.03 mL/g VS | −17.30 | ||||
| 8 | Foam nickel | Batch | Ethanol | Max: 94.5 mL/g | 14.50 | [27] |
| 9 | Zero-valent iron | Batch | Food waste | Max: 778.2 mL/g VS | [101] | |
| 10 | Magnetite | Batch | Glucose | 786.5 mL | 1 | [92] |
| Iron(II) sulfate | 760.5 mL | −2 | ||||
| 12 | Fe3O4 | Batch | Antibiotic fermentation residue | 280 mL/g VS | 48 | [112] |
| 13 | Zero-valent iron | Batch | Sewage sludge and food waste | 272.6 ±11.0 mL/gVS | 45 | [91] |
| Magnetite (Fe3O4) | 394.0 ± 6.3 mL/g VS | 16 | ||||
| 14 | Nano zero-valent iron | Batch | Artificial wastewater | 309.89 mL/g COD | 24 | [113] |
| 15 | Micron zero-valent Iron | Batch | Chicken manure | 276 mL/g VS | 31 | [95] |
| Micron-magnetite | 288 mL/g VS | 37 | ||||
| 16 | Red mud | Batch | Kitchen waste | 75.31 mL/g VS | 201 | [14] |
3.3. Modified Conductive Materials
| Material | Reactor | Substrate | Methane Production Without Modifications | Methane Production with Modification | % CH4 Increased | Ref. | |
|---|---|---|---|---|---|---|---|
| 1 | GAC with nano-Fe3O4 (magnetic granular activated carbon) | Batch | Low-strength wastewater | 4.7 ± 0.2 mL | 57 | [119] | |
| 3.0 ± 0.4 mL, over a cycle | |||||||
| 2 | Biochar without trace metals | Batch | Food waste | 358.5 ± 21.2 mL/g VS | 8 | [102] | |
| Biochar + trace metals | 386.6 ± 16.8 mL/g VS | 23 | |||||
| 3 | GAC and nZVI combined | Batch | Synthetic brewery water | __ | Cum: 122.16 mL/g COD | 14.29 | [121] |
| 4 | Magnetite—biochar | Batch | Artificial dairy wastewater | No biochar (54.4 mg/day) | 66.7 mL/day | 23 | [122] |
| 5 | ZVI/AC | Batch | Dichlorophen synthetic wastewater | 20 mL | 253.41 mL | 1167 | [15] |
| 6 | Biochar/ZVI | Batch | Chicken manure | 210 mL/g VS | 314 mL/g VS | 50 | [95] |
| 7 | g-C3N4/polyaniline * | Batch | Wastewater | 60.5 mL | 110 mL | 82 | [123] |
4. Operation Conditions Affecting Conductive Materials Performance
4.1. Effects of Sizes and Concentrations of Added Conductive Materials
| Material | Dosage (mg/L) | Reactor | Substrate | Methane Production | % CH4 Increased | Ref. | |
|---|---|---|---|---|---|---|---|
| 1 | Nano-graphene | 30 | Continuous-flow AD | Synthetic wastewater | 12.8 ± 0.4 mL/g VSS/d | 17 | [130] |
| 120 * | 51.4 | ||||||
| 2 | Powder activated carbon | 1000 | Batch | Primary sludge | 150.6 ± 1.3 mL/g VS | 10.8 | [106] |
| 15,000 * | 151.6 ± 1.3 mL/g VS | ||||||
| 20,000 | 146.9 ± 1.2 mL/g VS | ||||||
| Graphite powder | 200 | 150.7 ± 1.5 mL/g VS | 13.70 | ||||
| 100 | 150.7 ± 1.4 mL/g VS | ||||||
| 500 | 149.0 ± 1.3 mL/g VS | ||||||
| Magnetite | 50 * | ||||||
| 100 | 145.7 ± 1.3 mL/g VS | 9.7 | |||||
| 200 | 145.1 ± 1.3 mL/g VS | ||||||
| 140.4 ± 1.3 mL/g VS | |||||||
| NiCl2/CoCl2 | 10/10 * | 137.2 ± 1.3 mL/g VS | −4 | ||||
| 100/100 | 102.8 ± 0.8 mL/g VS | ||||||
| 3 | Powder activated carbon | 2240 | Batch | Sewage sludge | 211 mL/g VS | 49 | [131] |
| 4480 | |||||||
| 11,210 * | |||||||
| Powder graphene | 2240 | 195.7 mL/g VS | 7.80 | ||||
| 4480 | |||||||
| 11,210 * | |||||||
| 4 | Granular activated carbon | 10,000 20,000 * 30,000 40,000 50,000 | Batch | Wheat husk and sewage sludge | 263 mL/g VS | 22 | [59] |
| GBC | 273 mL/g VS | 27 | |||||
| 5 | Granular activated carbon | 0/0.5/2 */4/ 8/16/25/33 | Batch | Lipid-rich wastewater (oleate) | 2980.7 ± 185.5 mg CH4 COD/L | 31 | [52] |
| 6 | Reduced graphene oxide | 10 20 * 30 | Batch | Municipal organic solid waste | Max: 816 ± 14 mL/gVS | 50 | [132] |
| 7 | Nano-sized magnetite particles | 4600 18,500 37,000 74,000 | TAD | Acetate | 0.96 mol CH4/mol acetate | 80 | [100] |
| 8 | Stainless steel | 200 500 * 800 | UASB | Artificial wastewater | 159.9 mL/d | 7.5 24.6 10.8 | [133] |
4.2. External Voltage Supply in Conductive Matrix and Methane Production Potential
4.2.1. Optimal Voltage Supply
4.2.2. Cathode Potential
| BEAD (Process Type) | Feedstock | Operation Condition | Anode Material | Cathode Material | T (°C) | Power Mode (V) | Methane Content in Biogas | Ref. |
|---|---|---|---|---|---|---|---|---|
| Direct biochemical methanation Hydrogenotrophic/electromethanogenesis Hydrogenotrophic/electromethanogenesis | Synthetic substrate | (1) Single large brush without electrodes (FB) (2) Half large brush with 2 electrodes operated in a closed circuit (HB-CC) (3) Half large brush with 2 electrodes operated in an open circuit (HB-OC) (4) Two electrodes with a closed circuit and no large brush (NB-CC) | Carbon fiber brush | Stainless-steel brush | 35 | 0.8 | 253 ± 16 mL 240 ± 22 248 ± 15 232 ± 63 | [34] |
| DIET DIET Hydrogenotrophic methanogenesis | Food waste Acetate H2/CO2 | Testing for SMA of AD, BEAD, with voltage and without voltage | Graphite carbon mesh coated with Ni | Graphite carbon mesh (metal catalyst) | 0.4 | 0.325 L/g 0.335 0.328 0.345 0.345 | [35] | |
| Hydrogenotrophic methanogenesis | Swine manure | V (0.1–0.9), and opt is (0.7), then opt (0.7) with different temperatures (25–45) | Graphite felt | Graphite felt | 35 25 35 45 | - 0.7 0.7 0.7 | 2197 mL/L 2229 2993 3691 | [36] |
| Acetate methanogenesis | Wastewater+ wheat straw | Different voltage supplies 0.02–0.12 V | Graphite | Graphite | 37 | 0.02 0.04 0.08 0.12 | 8270.28 ± 163.2 362.07 ± 480.2 16,349.17 ± 742.9 12,314.29 ± 626. 11,054.6 ± 480.6 | [38] |
| Indirect methanogenesis via hydrogen and acetate | Mixed culture | Different cathode potentials −0.7 V and −0.9 V vs. NHE | Platinum-coated titanium mesh | Graphite felt | 31 ± 1 | −0.7 −0.9 | 5200 mL | [7] |
| Hydrogenotrophic methanogen Acetate methanogenesis | Synthetic wastewater | R1 (control) R2 (graphene/PPy) R3 (MnO2 nanoparticles/PPy) at 3 phases P1 (0 V/20 C) (0.4 V/20 C) (0.4 V/12 C) | Graphite rod (Gr) | _ (Gr)/(PPy) MnO2 NPs/PPy | 20 20 12 | - 0.4 0.4 | R1 (10.2 ± 0.8) R2 (13.0 ± 1.8) R3 (14.3 ± 1.4) R1 (21.7 ± 0.5) R2 (27.2 ± 1.8) R3 (30.0 ± 1.1) R1 (12.3 ± 1.1) R2 (27.2 ± 1.8) R3 (17.1 ± 0.8) | [144] |
| Hydrogenotrophic and H2-dependent methylotrophic methanogens | Food waste | R1 (1–364 d), OLR (2–3) R2 (365–598 d), OLR (4.0) R3 (599–795 d), OLR (6.0) R4 (796–950 d), OLR (8.0) R5 (951–1086 d) OLR (10) kg/m3·d | Graphite carbon mesh coated with Ni | Graphite carbon mesh coated with Ni (metal catalyst) | 35 | 0.5 | 18.6 ± 0.9 L/d 35.0 ± 2.6 52.6 ± 4.3 65.0 ± 4.3 75.8 ± 3.2 | [145] |
| H2-dependent methylotrophic methanogens. Hydrogenotrophic methanogens. | Food waste | R1 (electrodes w/biofilm) R2 (electrodes w/biofilm) R3 (electrodes w/biofilm) R4 (electrodes w/obiofilm) | Graphite | Graphite | 35 | 0.3 | 62.1 ± 2.1 L/d 18.5 ± 2.8 13.0 ± 0.4 not produced | [146] |
| H2-dependent methylotrophic and hydrogenotrophic methanogens | Food waste | R1 (2.0) kg-COD/m3. d R2 (3.0) R3 (4.5) R4 (6.0) | Stainless-steel SUS304 | Stainless-steel SUS304. | 19.8 ± 2.9 | 0.3 | 0.24 ± 0.07 L/d 0.34 ± 0.07 0.42 ± 0.12 0.15 L/d | [147] |
| Indirect methanogenesis | Food waste | R1 (2) R2 (4) R3 (6) OLR (kg COD/m3. d) | GC coated with Ni | GC coated with Ni, Fe, and Cu | 35–37 | 0.5 | 16 ± 4.59 35 ± 3.87 53 ± 6.32 | [148] |
5. Critical Analysis of Conductive Materials Applications
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nanda, S.; Berruti, F. A technical review of bioenergy and resource recovery from municipal solid waste. J. Hazard. Mater. 2021, 403, 123970. [Google Scholar] [CrossRef] [PubMed]
- Moya, D.; Aldás, C.; Jaramillo, D.; Játiva, E.; Kaparaju, P. Waste-To-Energy Technologies: An opportunity of energy recovery from Municipal Solid Waste, using Quito—Ecuador as case study. Energy Procedia 2017, 134, 327–336. [Google Scholar] [CrossRef]
- Yong, Z.J.; Bashir, M.J.; Ng, C.A.; Sethupathi, S.; Lim, J.W.; Show, P.L. Sustainable Waste-to-Energy Development in Malaysia: Appraisal of Environmental, Financial, and Public Issues Related with Energy Recovery from Municipal Solid Waste. Processes 2019, 7, 676. [Google Scholar] [CrossRef]
- Feng, Y.; Rosa, L. Global biomethane and carbon dioxide removal potential through anaerobic digestion of waste biomass. Environ. Res. Lett. 2024, 19, 024024. [Google Scholar] [CrossRef]
- Abu Hasan, M.; Aqsha; Putra, Z.A.; Bilad, M.R.; Sapiaa, N.A.H.; Wirzal, M.D.H.; Tijani, M.M. Biogas production from chicken food waste and cow manure via multi-stages anaerobic digestion. AIP Conf. Proc. 2018, 2016, 020011. [Google Scholar] [CrossRef]
- Atelge, M.R.; Krisa, D.; Kumar, G.; Eskicioglu, C.; Nguyen, D.D.; Chang, S.W.; Atabani, A.E.; Al-Muhtaseb, A.H.; Unalan, S. Biogas Production from Organic Waste: Recent Progress and Perspectives. Waste Biomass Valorization 2020, 11, 1019–1040. [Google Scholar] [CrossRef]
- Prajapati, K.B.; Singh, R. Enhancement of biogas production in bio-electrochemical digester from agricultural waste mixed with wastewater. Renew. Energy 2020, 146, 460–468. [Google Scholar] [CrossRef]
- Barrera, E.L.; Spanjers, H.; Romero, O.; Rosa, E.; Dewulf, J. Characterization of the sulfate reduction process in the anaerobic digestion of a very high strength and sulfate rich vinasse. Chem. Eng. J. 2014, 248, 383–393. [Google Scholar] [CrossRef]
- Fang, C.; Boe, K.; Angelidaki, I. Anaerobic co-digestion of by-products from sugar production with cow manure. Water Res. 2011, 45, 3473–3480. [Google Scholar] [CrossRef]
- Magdalena, J.A.; Greses, S.; González-Fernández, C. Impact of Organic Loading Rate in Volatile Fatty Acids Production and Population Dynamics Using Microalgae Biomass as Substrate. Sci. Rep. 2019, 9, 18374. [Google Scholar] [CrossRef]
- Abdelwahab, T.A.M.; Mohanty, M.K.; Sahoo, P.K.; Behera, D. Metal nanoparticle mixtures to improve the biogas yield of cattle manure. Biomass-Convers. Biorefinery 2021, 13, 2243–2254. [Google Scholar] [CrossRef]
- Xu, S.; He, C.; Luo, L.; Lü, F.; He, P.; Cui, L. Comparing activated carbon of different particle sizes on enhancing methane generation in upflow anaerobic digester. Bioresour. Technol. 2015, 196, 606–612. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Li, L.; Dai, X.; Dai, L. A review on application of single and composite conductive additives for anaerobic digestion: Advances, challenges and prospects. Resour. Conserv. Recycl. 2021, 174, 105844. [Google Scholar] [CrossRef]
- Hu, Y.; Wei, Q.; Wang, X.; Zhang, S.; Liu, S.; Fu, N.; Liu, Z.; Zou, Z.; Wu, J.; Wang, C. Enhancing High Solid Anaerobic Digestion of Kitchen Waste with Red Mud Addition: Performance and Microbial Community. Water Air Soil Pollut. 2024, 235, 34. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Li, Y.; Wang, R.; Zhou, M.; Zhao, L.; Pan, X.; Cai, G.; Lv, N.; Ning, J.; et al. Elucidation of high removal efficiency of dichlorophen wastewater in anaerobic treatment system with iron/carbon mediator. J. Clean. Prod. 2022, 330, 129854. [Google Scholar] [CrossRef]
- Cheng, Q.; Call, D.F. Hardwiring microbes via direct interspecies electron transfer: Mechanisms and applications. Environ. Sci. Process. Impacts 2016, 18, 968–980. [Google Scholar] [CrossRef]
- Viggi, C.C.; Rossetti, S.; Fazi, S.; Paiano, P.; Majone, M.; Aulenta, F. Magnetite particles triggering a faster and more robust syntrophic pathway of methanogenic propionate degradation. Environ. Sci. Technol. 2014, 48, 7536–7543. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Ueki, T.; Zhang, T.; Malvankar, N.S.; Shrestha, P.M.; Flanagan, K.A.; Aklujkar, M.; Butler, J.E.; Giloteaux, L.; Rotaru, A.-E.; et al. Geobacter: The Microbe Electric’s Physiology, Ecology, and Practical Applications. Adv. Microb. Physiol. 2011, 59, 1–100. [Google Scholar] [CrossRef]
- Reguera, G.; Nevin, K.P.; Nicoll, J.S.; Covalla, S.F.; Woodard, T.L.; Lovley, D.R. Biofilm and Nanowire Production Leads to Increased Current in Geobacter sulfurreducens Fuel Cells. Appl. Environ. Microbiol. 2006, 72, 7345–7348. [Google Scholar] [CrossRef]
- Stams, A.J.M.; Plugge, C.M. Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat. Rev. Microbiol. 2009, 7, 568–577. [Google Scholar] [CrossRef]
- An, J.; Li, N.; Wan, L.; Zhou, L.; Du, Q.; Li, T.; Wang, X. Electric field induced salt precipitation into activated carbon air-cathode causes power decay in microbial fuel cells. Water Res. 2017, 123, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Lü, F.; Shao, L.; He, P. Application of eco-compatible biochar in anaerobic digestion to relieve acid stress and promote the selective colonization of functional microbes. Water Res. 2015, 68, 710–718. [Google Scholar] [CrossRef]
- Wang, G.; Li, Q.; Gao, X.; Wang, X.C. Synergetic promotion of syntrophic methane production from anaerobic digestion of complex organic wastes by biochar: Performance and associated mechanisms. Bioresour. Technol. 2018, 250, 812–820. [Google Scholar] [CrossRef]
- Zhou, L.; Yan, X.; Pei, X.; Du, J.; Ma, R.; Qian, J. The role of NiFe2O4 nanoparticle in the anaerobic digestion (AD) of waste activated sludge (WAS). Chin. Chem. Lett. 2021, 33, 428–433. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, Y.; Ji, D.; Li, X.; Zhang, J.; Zang, L. Synergetic promotion of direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate with graphite felt in anaerobic digestion. Bioresour. Technol. 2019, 287, 121373. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Dhar, B.R. Advances towards understanding and engineering direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 244, 698–707. [Google Scholar] [CrossRef]
- Guo, X.; Sun, C.; Lin, R.; Xia, A.; Huang, Y.; Zhu, X.; Show, P.-L.; Murphy, J.D. Effects of foam nickel supplementation on anaerobic digestion: Direct interspecies electron transfer. J. Hazard. Mater. 2020, 399, 122830. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.I.; Marcus, A.K.; Rittmann, B.E. Kinetics of consumption of fermentation products by anode-respiring bacteria. Appl. Microbiol. Biotechnol. 2007, 77, 689–697. [Google Scholar] [CrossRef]
- Feng, Q.; Song, Y.-C.; Yoo, K.; Kuppanan, N.; Subudhi, S.; Lal, B. Influence of neutralization in acidic distillery wastewater on direct interspecies electron transfer for methane production in an upflow anaerobic bioelectrochemical reactor. Int. J. Hydrogen Energy 2017, 42, 27774–27783. [Google Scholar] [CrossRef]
- Villano, M.; Aulenta, F.; Ciucci, C.; Ferri, T.; Giuliano, A.; Majone, M. Bioelectrochemical reduction of CO2 to CH4 via direct and indirect extracellular electron transfer by a hydrogenophilic methanogenic culture. Bioresour. Technol. 2010, 101, 3085–3090. [Google Scholar] [CrossRef]
- de Bok, F.; Plugge, C.; Stams, A. Interspecies electron transfer in methanogenic propionate degrading consortia. Water Res. 2004, 38, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Greening, C.; Geier, R.; Wang, C.; Woods, L.C.; E Morales, S.; McDonald, M.J.; Rushton-Green, R.; Morgan, X.C.; Koike, S.; Leahy, S.C.; et al. Diverse hydrogen production and consumption pathways influence methane production in ruminants. ISME J. 2019, 13, 2617–2632. [Google Scholar] [CrossRef] [PubMed]
- Pyzik, A.; Ciezkowska, M.; Krawczyk, P.S.; Sobczak, A.; Drewniak, L.; Dziembowski, A.; Lipinski, L. Comparative analysis of deep sequenced methanogenic communities: Identification of microorganisms responsible for methane production. Microb. Cell Factories 2018, 17, 197. [Google Scholar] [CrossRef]
- Baek, G.; Saikaly, P.E.; Logan, B.E. Addition of a carbon fiber brush improves anaerobic digestion compared to external voltage application. Water Res. 2021, 188, 116575. [Google Scholar] [CrossRef]
- Park, J.-G.; Heo, T.-Y.; Kwon, H.-J.; Shi, W.-Q.; Jun, H.-B. Effects of voltage supply on the methane production rates and pathways in an anaerobic digestion reactor using different electron donors. Int. J. Hydrogen Energy 2020, 45, 9459–9468. [Google Scholar] [CrossRef]
- Yu, J.; Kim, S.; Kwon, O.-S. Effect of applied voltage and temperature on methane production and microbial community in microbial electrochemical anaerobic digestion systems treating swine manure. J. Ind. Microbiol. Biotechnol. 2019, 46, 911–923. [Google Scholar] [CrossRef]
- Kouzuma, A.; Kato, S.; Watanabe, K. Microbial interspecies interactions: Recent findings in syntrophic consortia. Front. Microbiol. 2015, 6, 477. [Google Scholar] [CrossRef]
- van Eerten-Jansen, M.C.A.A.; Jansen, N.C.; Plugge, C.M.; de Wilde, V.; Buisman, C.J.N.; ter Heijne, A. Analysis of the mechanisms of bioelectrochemical methane production by mixed cultures. J. Chem. Technol. Biotechnol. 2015, 90, 963–970. [Google Scholar] [CrossRef]
- Thiele, J.H.; Zeikus, J.G. Control of Interspecies Electron Flow during Anaerobic Digestion: Significance of Formate Transfer versus Hydrogen Transfer during Syntrophic Methanogenesis in Flocs. Appl. Environ. Microbiol. 1988, 54, 20–29. [Google Scholar] [CrossRef]
- Storck, T.; Virdis, B.; Batstone, D.J. Modelling extracellular limitations for mediated versus direct interspecies electron transfer. ISME J. 2015, 10, 621–631. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Zhang, J.; Zhou, J.; Cen, K.; Murphy, J.D. Boosting biomethane yield and production rate with graphene: The potential of direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 239, 345–352. [Google Scholar] [CrossRef]
- Guo, X.; Chen, H.; Zhu, X.; Xia, A.; Liao, Q.; Huang, Y.; Zhu, X. Revealing the role of conductive materials on facilitating direct interspecies electron transfer in syntrophic methanogenesis: A thermodynamic analysis. Energy 2021, 229, 120747. [Google Scholar] [CrossRef]
- Gahlot, P.; Aboudi, K.; Ahmed, B.; Tawfik, A.; Khan, A.A.; Khursheed, A.; Tyagi, V.K. Direct interspecies electron transfer (DIET) via conductive materials in anaerobic digestion of organic wastes. In Clean Energy and Resources Recovery: Biomass Waste Based Biorefineries, Volume 1; Elsevier: Amsterdam, The Netherlands, 2021; pp. 227–252. [Google Scholar] [CrossRef]
- Lovley, D.R.; Holmes, D.E. Electromicrobiology: The ecophysiology of phylogenetically diverse electroactive microorganisms. Nat. Rev. Microbiol. 2021, 20, 5–19. [Google Scholar] [CrossRef]
- Holmes, D.E.; Ueki, T.; Tang, H.-Y.; Zhou, J.; Smith, J.A.; Chaput, G.; Lovley, D.R. A Membrane-Bound Cytochrome Enables Methanosarcina acetivorans to Conserve Energy from Extracellular Electron Transfer. mBio 2019, 10, e00789-19. [Google Scholar] [CrossRef]
- Fazzino, F.; Frontera, P.; Malara, A.; Pedullà, A.; Calabrò, P.S. Effects of carbon-based conductive materials on semi-continuous anaerobic co-digestion of organic fraction of municipal solid waste and waste activated sludge. Chemosphere 2024, 357, 142077. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, L.; Gu, J.-D. Microbial electrocatalysis: Redox mediators responsible for extracellular electron transfer. Biotechnol. Adv. 2018, 36, 1815–1827. [Google Scholar] [CrossRef]
- Liu, X.; Zhuo, S.; Rensing, C.; Zhou, S. Syntrophic growth with direct interspecies electron transfer between pili-free Geobacter species. ISME J. 2018, 12, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, A.-E.; Shrestha, P.M.; Liu, F.; Markovaite, B.; Chen, S.; Nevin, K.P.; Lovley, D.R. Direct Interspecies Electron Transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl. Environ. Microbiol. 2014, 80, 4599–4605. [Google Scholar] [CrossRef]
- Shrestha, P.M.; Malvankar, N.S.; Werner, J.J.; Franks, A.E.; Elena-Rotaru, A.; Shrestha, M.; Liu, F.; Nevin, K.P.; Angenent, L.T.; Lovley, D.R. Correlation between microbial community and granule conductivity in anaerobic bioreactors for brewery wastewater treatment. Bioresour. Technol. 2014, 174, 306–310. [Google Scholar] [CrossRef]
- Liu, F.; Rotaru, A.-E.; Shrestha, P.M.; Malvankar, N.S.; Nevin, K.P.; Lovley, D.R. Promoting direct interspecies electron transfer with activated carbon. Energy Environ. Sci. 2012, 5, 8982–8989. [Google Scholar] [CrossRef]
- Tan, L.C.; Lin, R.; Murphy, J.D.; Lens, P.N. Granular activated carbon supplementation enhances anaerobic digestion of lipid-rich wastewaters. Renew. Energy 2021, 171, 958–970. [Google Scholar] [CrossRef]
- Zhang, T.; Nie, H.; Bain, T.S.; Lu, H.; Cui, M.; Snoeyenbos-West, O.L.; Franks, A.E.; Nevin, K.P.; Russell, T.P.; Lovley, D.R. Improved cathode materials for microbial electrosynthesis. Energy Environ. Sci. 2013, 6, 217–224. [Google Scholar] [CrossRef]
- Cuetos, M.J.; Martinez, E.J.; Moreno, R.; Gonzalez, R.; Otero, M.; Gomez, X. Enhancing anaerobic digestion of poultry blood using activated carbon. J. Adv. Res. 2017, 8, 297–307. [Google Scholar] [CrossRef]
- Boe, K.; Batstone, D.J.; Steyer, J.-P.; Angelidaki, I. State indicators for monitoring the anaerobic digestion process. Water Res. 2010, 44, 5973–5980. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, R.; Shi, X.; Wang, C.; Wang, L.; Dai, M. Magnetite nanoparticles enable a rapid conversion of volatile fatty acids to methane. RSC Adv. 2016, 6, 25662–25668. [Google Scholar] [CrossRef]
- Noori, T.; Vu, M.T.; Ali, R.B.; Min, B. Recent advances in cathode materials and configurations for upgrading methane in bioelectrochemical systems integrated with anaerobic digestion. Chem. Eng. J. 2020, 392, 123689. [Google Scholar] [CrossRef]
- Cazorla-Amorós, D. Grand challenges in carbon-based materials research. Front. Mater. 2014, 1–6. [Google Scholar] [CrossRef]
- Tiwari, S.B.; Dubey, M.; Ahmed, B.; Gahlot, P.; Khan, A.A.; Rajpal, A.; Kazmi, A.; Tyagi, V.K. Carbon-based conductive materials facilitated anaerobic co-digestion of agro waste under thermophilic conditions. Waste Manag. 2021, 124, 17–25. [Google Scholar] [CrossRef]
- Sanjinés, R.; Abad, M.D.; Vâju, C.; Smajda, R.; Mionić, M.; Magrez, A. Electrical properties and applications of carbon based nanocomposite materials: An overview. Surf. Coatings Technol. 2011, 206, 727–733. [Google Scholar] [CrossRef]
- Pötschke, L.; Huber, P.; Schriever, S.; Rizzotto, V.; Gries, T.; Blank, L.M.; Rosenbaum, M.A. Rational Selection of Carbon Fiber Properties for High-Performance Textile Electrodes in Bioelectrochemical Systems. Front. Energy Res. 2019, 7, 100. [Google Scholar] [CrossRef]
- De Vrieze, J.; Devooght, A.; Walraedt, D.; Boon, N. Enrichment of Methanosaetaceae on carbon felt and biochar during anaerobic digestion of a potassium-rich molasses stream. Appl. Microbiol. Biotechnol. 2016, 100, 5177–5187. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Xia, A.; Huang, Y.; Zhu, X.; Zhu, X.; Liao, Q. Effects of carbon cloth on anaerobic digestion of high concentration organic wastewater under various mixing conditions. J. Hazard. Mater. 2022, 423, 127100. [Google Scholar] [CrossRef]
- Barua, S.; Zakaria, B.S.; Lin, L.; Dhar, B.R. Shaping microbial communities with conductive carbon fibers to enhance methane productivity and kinetics. Bioresour. Technol. Rep. 2019, 5, 20–27. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Woodard, T.; Nevin, K.; Lovley, D. Enhancing syntrophic metabolism in up-flow anaerobic sludge blanket reactors with conductive carbon materials. Bioresour. Technol. 2015, 191, 140–145. [Google Scholar] [CrossRef]
- Oni, B.A.; Oziegbe, O.; Olawole, O.O. Significance of biochar application to the environment and economy. Ann. Agric. Sci. 2019, 64, 222–236. [Google Scholar] [CrossRef]
- Chen, S.; Rotaru, A.-E.; Shrestha, P.M.; Malvankar, N.S.; Liu, F.; Fan, W.; Nevin, K.P.; Lovley, D.R. Promoting Interspecies Electron Transfer with Biochar. Sci. Rep. 2014, 4, 5019. [Google Scholar] [CrossRef]
- Mašek, O.; Buss, W.; Roy-Poirier, A.; Lowe, W.; Peters, C.; Brownsort, P.; Mignard, D.; Pritchard, C.; Sohi, S. Consistency of biochar properties over time and production scales: A characterisation of standard materials. J. Anal. Appl. Pyrolysis 2018, 132, 200–210. [Google Scholar] [CrossRef]
- Viggi, C.C.; Simonetti, S.; Palma, E.; Pagliaccia, P.; Braguglia, C.; Fazi, S.; Baronti, S.; Navarra, M.A.; Pettiti, I.; Koch, C.; et al. Enhancing methane production from food waste fermentate using biochar: The added value of electrochemical testing in pre-selecting the most effective type of biochar. Biotechnol. Biofuels 2017, 10, 303. [Google Scholar] [CrossRef]
- Valero, D.; Rico, C.; Canto-Canché, B.; Domínguez-Maldonado, J.; Tapia-Tussell, R.; Cortes-Velazquez, A.; Alzate-Gaviria, L. Enhancing Biochemical Methane Potential and Enrichment of Specific Electroactive Communities from Nixtamalization Wastewater using Granular Activated Carbon as a Conductive Material. Energies 2018, 11, 2101. [Google Scholar] [CrossRef]
- Chowdhury, B.; Lin, L.; Dhar, B.R.; Islam, M.N.; McCartney, D.; Kumar, A. Enhanced biomethane recovery from fat, oil, and grease through co-digestion with food waste and addition of conductive materials. Chemosphere 2019, 236, 124362. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lee, S.-H.; Park, H.-D. Enrichment of specific electro-active microorganisms and enhancement of methane production by adding granular activated carbon in anaerobic reactors. Bioresour. Technol. 2016, 205, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Altamirano-Corona, M.F.; Anaya-Reza, O.; Durán-Moreno, A. Biostimulation of food waste anaerobic digestion supplemented with granular activated carbon, biochar and magnetite: A comparative analysis. Biomass-Bioenergy 2021, 149, 106105. [Google Scholar] [CrossRef]
- Valero, D.; Alzate-Gaviria, L.; Montes, J.A.; Rico, C. Influence of a Conductive Material and Different Anaerobic Inocula on Biochemical Methane Potential of Substrates from Alcoholic Beverage Production. Waste Biomass-Valorization 2020, 11, 5957–5964. [Google Scholar] [CrossRef]
- Long, J.H.; Aziz, T.N.; de los Reyes, F.L.; Ducoste, J.J. Anaerobic co-digestion of fat, oil, and grease (FOG): A review of gas production and process limitations. Process Saf. Environ. Prot. 2012, 90, 231–245. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Fazzino, F.; Limonti, C.; Siciliano, A. Enhancement of Anaerobic Digestion of Waste-Activated Sludge by Conductive Materials under High Volatile Fatty Acids-to-Alkalinity Ratios. Water 2021, 13, 391. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, R.; Wang, H.; Yang, K. Direct interspecies electron transfer stimulated by granular activated carbon enhances anaerobic methanation efficiency from typical kitchen waste lipid-rapeseed oil. Sci. Total Environ. 2019, 704, 135282. [Google Scholar] [CrossRef]
- He, X.; Guo, Z.; Lu, J.; Zhang, P. Carbon-based conductive materials accelerated methane production in anaerobic digestion of waste fat, oil and grease. Bioresour. Technol. 2021, 329, 124871. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Quan, X.; Zhang, Y. Towards engineering application: Potential mechanism for enhancing anaerobic digestion of complex organic waste with different types of conductive materials. Water Res. 2017, 115, 266–277. [Google Scholar] [CrossRef]
- Yan, W.; Shen, N.; Xiao, Y.; Chen, Y.; Sun, F.; Tyagi, V.K.; Zhou, Y. The role of conductive materials in the start-up period of thermophilic anaerobic system. Bioresour. Technol. 2017, 239, 336–344. [Google Scholar] [CrossRef]
- Arenas, C.B.; Meredith, W.; Snape, C.E.; Gómez, X.; González, J.F.; Martinez, E.J. Evaluating the effect of biochar addition on the anaerobic digestion of swine manure: Application of Py-GC/MS. Environ. Sci. Pollut. Res. 2018, 25, 25600–25611. [Google Scholar] [CrossRef]
- Barua, S.; Zakaria, B.S.; Dhar, B.R. Enhanced methanogenic co-degradation of propionate and butyrate by anaerobic microbiome enriched on conductive carbon fibers. Bioresour. Technol. 2018, 266, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Park, J.-H.; Seong, H.J.; Sul, W.J.; Jin, K.-H.; Park, H.-D. Metagenomic insight into methanogenic reactors promoting direct interspecies electron transfer via granular activated carbon. Bioresour. Technol. 2018, 259, 414–422. [Google Scholar] [CrossRef]
- Lei, Y.; Sun, D.; Dang, Y.; Feng, X.; Huo, D.; Liu, C.; Zheng, K.; Holmes, D.E. Metagenomic analysis reveals that activated carbon aids anaerobic digestion of raw incineration leachate by promoting direct interspecies electron transfer. Water Res. 2019, 161, 570–580. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Zhang, Y.; Xiang, Y.; Xu, R.; Jia, M.; Cao, J.; Xiong, W. Effects of different conductive nanomaterials on anaerobic digestion process and microbial community of sludge. Bioresour. Technol. 2020, 304, 123016. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, H.; Wang, X.; Tong, Y.W. Effects of activated carbon on mesophilic and thermophilic anaerobic digestion of food waste: Process performance and life cycle assessment. Chem. Eng. J. 2020, 399, 125757. [Google Scholar] [CrossRef]
- Namal, O.O. Investigation of the effects of different conductive materials on the anaerobic digestion. Int. J. Environ. Sci. Technol. 2020, 17, 473–482. [Google Scholar] [CrossRef]
- Romero, R.M.; Valenzuela, E.I.; Cervantes, F.J.; Garcia-Reyes, R.B.; Serrano, D.; Alvarez, L.H. Improved methane production from anaerobic digestion of liquid and raw fractions of swine manure effluent using activated carbon. J. Water Process. Eng. 2020, 38, 101576. [Google Scholar] [CrossRef]
- Park, J.-H.; Park, J.-H.; Lee, S.-H.; Jung, S.P.; Kim, S.-H. Enhancing anaerobic digestion for rural wastewater treatment with granular activated carbon (GAC) supplementation. Bioresour. Technol. 2020, 315, 123890. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Li, Y.; Zhao, K.; Xu, Q.; Jiang, H.; Zhou, H. Performance and Microbial Community Analysis of Anaerobic Digestion of Vinegar Residue with Adding of Acetylene Black or Hydrochar. Waste Biomass-Valorization 2020, 11, 3315–3325. [Google Scholar] [CrossRef]
- Liang, J.; Luo, L.; Li, D.; Varjani, S.; Xu, Y.; Wong, J.W. Promoting anaerobic co-digestion of sewage sludge and food waste with different types of conductive materials: Performance, stability, and underlying mechanism. Bioresour. Technol. 2021, 337, 125384. [Google Scholar] [CrossRef]
- Baek, G.; Rossi, R.; Saikaly, P.E.; Logan, B.E. The impact of different types of high surface area brush fibers with different electrical conductivity and biocompatibility on the rates of methane generation in anaerobic digestion. Sci. Total Environ. 2021, 787, 147683. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, Z.; Zhang, Y. Enhancing anaerobic digestion of kitchen wastes with biochar: Link between different properties and critical mechanisms of promoting interspecies electron transfer. Renew. Energy 2021, 167, 791–799. [Google Scholar] [CrossRef]
- Bose, R.S.; Chowdhury, B.; Zakaria, B.S.; Tiwari, M.K.; Dhar, B.R. Significance of different mixing conditions on performance and microbial communities in anaerobic digester amended with granular and powdered activated carbon. Bioresour. Technol. 2021, 341, 125768. [Google Scholar] [CrossRef]
- Ma, J.; Yao, Z.; Zhao, L. Comprehensive study of the combined effects of biochar and iron-based conductive materials on alleviating long chain fatty acids inhibition in anaerobic digestion. Environ. Res. 2023, 239, 117446. [Google Scholar] [CrossRef] [PubMed]
- Baudler, A.; Schmidt, I.; Langner, M.; Greiner, A.; Schröder, U. Does it have to be carbon? Metal anodes in microbial fuel cells and related bioelectrochemical systems. Energy Environ. Sci. 2015, 8, 2048–2055. [Google Scholar] [CrossRef]
- Kim, K.-R.; Kang, J.; Chae, K.-J. Improvement in methanogenesis by incorporating transition metal nanoparticles and granular activated carbon composites in microbial electrolysis cells. Int. J. Hydrogen Energy 2017, 42, 27623–27629. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.; Chen, Y.; Yang, D.; Cai, C.; Yuan, S.; Dai, X. Effect of Magnet-Fe3O4 composite structure on methane production during anaerobic sludge digestion: Establishment of direct interspecies electron transfer. Renew. Energy 2022, 188, 52–60. [Google Scholar] [CrossRef]
- Yin, Q.; He, K.; Liu, A.; Wu, G. Enhanced system performance by dosing ferroferric oxide during the anaerobic treatment of tryptone-based high-strength wastewater. Appl. Microbiol. Biotechnol. 2017, 101, 3929–3939. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, X.; Guo, R.; Fan, X.; Zhao, X. Accelerated methanogenesis from effluents of hydrogen-producing stage in anaerobic digestion by mixed cultures enriched with acetate and nano-sized magnetite particles. Bioresour. Technol. 2015, 190, 132–139. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Z.; Yang, Y.; Zhang, Y. Dual roles of zero-valent iron in dry anaerobic digestion: Enhancing interspecies hydrogen transfer and direct interspecies electron transfer. Waste Manag. 2020, 118, 481–490. [Google Scholar] [CrossRef]
- Akturk, A.S.; Demirer, G.N. Improved food waste stabilization and valorization by anaerobic digestion through supplementation of conductive materials and trace elements. Sustainability 2020, 12, 5222. [Google Scholar] [CrossRef]
- Zhuang, L.; Tang, J.; Wang, Y.; Hu, M.; Zhou, S. Conductive iron oxide minerals accelerate syntrophic cooperation in methanogenic benzoate degradation. J. Hazard. Mater. 2015, 293, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Tang, Z.; Ma, J.; Yu, Z.; Wang, Y.; Tang, J. Enhanced anaerobic biodegradation of benzoate under sulfate-reducing conditions with conductive iron-oxides in sediment of pearl river estuary. Front. Microbiol. 2019, 10, 374. [Google Scholar] [CrossRef]
- Bird, L.J.; Bonnefoy, V.; Newman, D.K. Bioenergetic challenges of microbial iron metabolisms. Trends Microbiol. 2011, 19, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Li, X.; Wang, C.; Kulandaivelu, J.; Jiang, G. Enhanced anaerobic digestion of primary sludge with additives: Performance and mechanisms. Bioresour. Technol. 2020, 316, 123970. [Google Scholar] [CrossRef]
- Mu, H.; Chen, Y.; Xiao, N. Effects of metal oxide nanoparticles (TiO2, Al2O3, SiO2 and ZnO) on waste activated sludge anaerobic digestion. Bioresour. Technol. 2011, 102, 10305–10311. [Google Scholar] [CrossRef]
- Ünşar, E.K.; Perendeci, N.A. What kind of effects do Fe2O3 and Al2O3 nanoparticles have on anaerobic digestion, inhibition or enhancement? Chemosphere 2018, 211, 726–735. [Google Scholar] [CrossRef]
- Vecchia, C.D.; Mattioli, A.; Bolzonella, D.; Palma, E.; Viggi, C.C.; Aulenta, F. Impact of magnetite nanoparticles supplementation on the anaerobic digestion of food wastes: Batch and continuous-flow investigations. Chem. Eng. Trans. 2016, 49, 1–6. [Google Scholar] [CrossRef]
- Wang, D.; Han, Y.; Han, H.; Li, K.; Xu, C.; Zhuang, H. New insights into enhanced anaerobic degradation of Fischer-Tropsch wastewater with the assistance of magnetite. Bioresour. Technol. 2018, 257, 147–156. [Google Scholar] [CrossRef]
- Ye, J.; Hu, A.; Ren, G.; Chen, M.; Tang, J.; Zhang, P.; Zhou, S.; He, Z. Enhancing sludge methanogenesis with improved redox activity of extracellular polymeric substances by hematite in red mud. Water Res. 2018, 134, 54–62. [Google Scholar] [CrossRef]
- Cai, C.; Li, L.; Hua, Y.; Liu, H.; Dai, X. Ferroferric oxide promotes metabolism in Anaerolineae other than microbial syntrophy in anaerobic methanogenesis of antibiotic fermentation residue. Sci. Total Environ. 2021, 758, 143601. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, C.; Lv, N.; Pan, X.; Cai, G.; Ning, J.; Zhu, G. Deeper insights into effect of activated carbon and nano-zero-valent iron addition on acidogenesis and whole anaerobic digestion. Bioresour. Technol. 2021, 324, 124671. [Google Scholar] [CrossRef]
- Guo, K.; Freguia, S.; Dennis, P.G.; Chen, X.; Donose, B.C.; Keller, J.; Gooding, J.J.; Rabaey, K. Effects of surface charge and hydrophobicity on anodic biofilm formation, community composition, and current generation in bioelectrochemical systems. Environ. Sci. Technol. 2013, 47, 7563–7570. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhang, F.; Liu, J.; Zhang, X.; Huang, X.; Logan, B.E. Methane production in microbial reverse-electrodialysis methanogenesis cells (MRMCS) using thermolytic solutions. Environ. Sci. Technol. 2014, 48, 8911–8918. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, Y.; Li, Y.; Quan, X.; Zhao, Z. Comparing the mechanisms of ZVI and Fe3O4 for promoting waste-activated sludge digestion. Water Res. 2018, 144, 126–133. [Google Scholar] [CrossRef]
- Zhang, G.; Shi, Y.; Zhao, Z.; Wang, X.; Dou, M. Enhanced two-phase anaerobic digestion of waste-activated sludge by combining magnetite and zero-valent iron. Bioresour. Technol. 2020, 306, 123122. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Quan, X.; Chen, S. Enhanced anaerobic digestion of waste activated sludge digestion by the addition of zero valent iron. Water Res. 2014, 52, 242–250. [Google Scholar] [CrossRef]
- Song, X.; Liu, J.; Jiang, Q.; Zhang, P.; Shao, Y.; He, W.; Feng, Y. Enhanced electron transfer and methane production from low-strength wastewater using a new granular activated carbon modified with nano-Fe3O4. Chem. Eng. J. 2019, 374, 1344–1352. [Google Scholar] [CrossRef]
- An, Z.; Feng, Q.; Zhao, R.; Wang, X. Bioelectrochemical methane production from food waste in anaerobic digestion using a carbon-modified copper foam electrode. Processes 2020, 8, 416. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, Z.; Liu, G.; Lv, M.; Feng, Y. Enhancing methane production of synthetic brewery water with granular activated carbon modified with nanoscale zero-valent iron (NZVI) in anaerobic system. Sci. Total Environ. 2021, 760, 143933. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, Z.; Zhang, Y. Magnetite-contained biochar derived from fenton sludge modulated electron transfer of microorganisms in anaerobic digestion. J. Hazard. Mater. 2021, 403, 123972. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Li, X.; Rene, E.R.; Hu, Q.; Qiu, B. Heterogeneous g-C3N4/polyaniline composites enhanced the conversion of organics into methane during anaerobic wastewater treatment. Environ. Res. 2024, 258, 119480. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Sun, S.; Zang, Z.; Li, X.; Sun, X.; Cao, F.; Wu, J. Effects of graphene with different sizes as conductive additives on the electrochemical performance of a LiFePO4cathode. RSC Adv. 2017, 7, 20882–20887. [Google Scholar] [CrossRef]
- Zhong, Y.; He, J.; Zhang, P.; Zou, X.; Pan, X.; Zhang, J. Effects of different particle size of zero-valent iron (ZVI) during anaerobic digestion: Performance and mechanism from genetic level. Chem. Eng. J. 2022, 435, 134977. [Google Scholar] [CrossRef]
- Kamar, E.; Khairy, M.; Mousa, M. Effect of morphology and particle size on the electrical properties of nano-nickel ferrite. J. Mater. Res. Technol. 2023, 24, 7381–7393. [Google Scholar] [CrossRef]
- Alrawashdeh, K.A.B.; A Al-Tabbal, J.; A Al-Samrraie, L.; Al Bsoul, A.; Al Zboon, K.K. Enhancing residue degradation and methane production from active sludge: The role of conductive nanoparticles in anaerobic digestion systems. Int. J. Low-Carbon Technol. 2023, 18, 1307–1317. [Google Scholar] [CrossRef]
- Florentino, A.P.; Xu, R.; Zhang, L.; Liu, Y. Anaerobic digestion of blackwater assisted by granular activated carbon: From digestion inhibition to methanogenesis enhancement. Chemosphere 2019, 233, 462–471. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, P.; Cheng, J.; Liu, G.; Zhang, X.; Feng, Y. Enhancing anaerobic digestion and methane production of tetracycline wastewater in EGSB reactor with GAC/NZVI mediator. Water Res. 2018, 136, 54–63. [Google Scholar] [CrossRef]
- Tian, T.; Qiao, S.; Li, X.; Zhang, M.; Zhou, J. Nano-graphene induced positive effects on methanogenesis in anaerobic digestion. Bioresour. Technol. 2016, 224, 41–47. [Google Scholar] [CrossRef]
- Pan, C.; Fu, X.; Lu, W.; Ye, R.; Guo, H.; Wang, H.; Chusov, A. Effects of conductive carbon materials on dry anaerobic digestion of sewage sludge: Process and mechanism. J. Hazard. Mater. 2020, 384, 121339. [Google Scholar] [CrossRef]
- Gökçek, Ö.B.; Muratçobanoğlu, F.; Muratçobanoğlu, H.; Uçar, D.; A Mert, R.; Yıldırım, B.; Zan, R.; Demirel, S. The effect of reduced graphene oxide addition on methane production from municipal organic solid waste. J. Chem. Technol. Biotechnol. 2021, 96, 2845–2851. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Yang, Y.; Quan, X.; Zhao, Z. Potentially direct interspecies electron transfer of methanogenesis for syntrophic metabolism under sulfate reducing conditions with stainless steel. Bioresour. Technol. 2017, 234, 303–309. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, W.; Yang, C.; Gao, L.; Thangavel, S.; Wang, L.; He, Z.; Cai, W.; Wang, A. Computational and experimental analysis of organic degradation positively regulated by bioelectrochemistry in an anaerobic bioreactor system. Water Res. 2017, 125, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Park, J.-H.; Park, H.-D. Effects of an applied voltage on direct interspecies electron transfer via conductive materials for methane production. Waste Manag. 2017, 68, 165–172. [Google Scholar] [CrossRef]
- Wang, B.; Liu, W.; Zhang, Y.; Wang, A. Bioenergy recovery from wastewater accelerated by solar power: Intermittent electro-driving regulation and capacitive storage in biomass. Water Res. 2020, 175, 115696. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Ren, H.-Y.; Pavlostathis, S.G.; Nan, J.; Ren, N.-Q.; Wang, A. Overview of value-added products bioelectrosynthesized from waste materials in microbial electrosynthesis systems. Renew. Sustain. Energy Rev. 2020, 125, 109816. [Google Scholar] [CrossRef]
- Cho, S.-K.; Lee, M.-E.; Lee, W.; Ahn, Y. Improved hydrogen recovery in microbial electrolysis cells using intermittent energy input. Int. J. Hydrogen Energy 2019, 44, 2253–2257. [Google Scholar] [CrossRef]
- Choi, K.-S.; Kondaveeti, S.; Min, B. Bioelectrochemical methane (CH4) production in anaerobic digestion at different supplemental voltages. Bioresour. Technol. 2017, 245, 826–832. [Google Scholar] [CrossRef]
- Dou, Z.; Dykstra, C.M.; Pavlostathis, S.G. Bioelectrochemically assisted anaerobic digestion system for biogas upgrading and enhanced methane production. Sci. Total Environ. 2018, 633, 1012–1021. [Google Scholar] [CrossRef]
- Cheng, S.; Xing, D.; Call, D.F.; Logan, B.E. Direct Biological Conversion of Electrical Current into Methane by Electromethanogenesis. Environ. Sci. Technol. 2009, 43, 3953–3958. [Google Scholar] [CrossRef]
- Marshall, C.W.; Ross, D.E.; Fichot, E.B.; Norman, R.S.; May, H.D. Electrosynthesis of Commodity Chemicals by an Autotrophic Microbial Community. Appl. Environ. Microbiol. 2012, 78, 8412–8420. [Google Scholar] [CrossRef] [PubMed]
- Van Eerten-Jansen, M.C.A.A.; Ter Heijne, A.; Buisman, C.J.N.; Hamelers, H.V.M. Microbial electrolysis cells for production of methane from CO2: Long-term performance and perspectives. Int. J. Energy Res. 2012, 36, 809–819. [Google Scholar] [CrossRef]
- Tian, T.; Qiao, S.; Yu, C.; Yang, Y.; Zhou, J. Low-temperature anaerobic digestion enhanced by bioelectrochemical systems equipped with graphene/PPy- and MnO2 nanoparticles/PPy-modified electrodes. Chemosphere 2019, 218, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-G.; Lee, B.; Park, H.-R.; Jun, H.-B. Long-term evaluation of methane production in a bio-electrochemical anaerobic digestion reactor according to the organic loading rate. Bioresour. Technol. 2019, 273, 478–486. [Google Scholar] [CrossRef]
- Park, J.-G.; Lee, B.; Kwon, H.-J.; Jun, H.-B. Contribution analysis of methane production from food waste in bulk solution and on bio-electrode in a bio-electrochemical anaerobic digestion reactor. Sci. Total Environ. 2019, 670, 741–751. [Google Scholar] [CrossRef]
- Park, J.; Lee, B.; Shin, W.; Jo, S.; Jun, H. Psychrophilic methanogenesis of food waste in a bio-electrochemical anaerobic digester with rotating impeller electrode. J. Clean. Prod. 2018, 188, 556–567. [Google Scholar] [CrossRef]
- Park, J.-G.; Shin, W.-B.; Shi, W.-Q.; Jun, H.-B. Changes of bacterial communities in an anaerobic digestion and a bio-electrochemical anaerobic digestion reactors according to organic load. Energies 2019, 12, 2958. [Google Scholar] [CrossRef]
- Lei, Y.; Sun, D.; Dang, Y.; Chen, H.; Zhao, Z.; Zhang, Y.; Holmes, D.E. Stimulation of methanogenesis in anaerobic digesters treating leachate from a municipal solid waste incineration plant with carbon cloth. Bioresour. Technol. 2016, 222, 270–276. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Hasani, Z.; Nayak, J.K.; Al Balushi, N.J.; Al-Mamun, A.; Samal, K. Prospect of Conductive Materials in the Anaerobic Digester Matrix for Methane Production: Electron Transfer and Microbial Communication. Water 2025, 17, 1321. https://doi.org/10.3390/w17091321
Al Hasani Z, Nayak JK, Al Balushi NJ, Al-Mamun A, Samal K. Prospect of Conductive Materials in the Anaerobic Digester Matrix for Methane Production: Electron Transfer and Microbial Communication. Water. 2025; 17(9):1321. https://doi.org/10.3390/w17091321
Chicago/Turabian StyleAl Hasani, Zahra, Jagdeep Kumar Nayak, Noor Juma Al Balushi, Abdullah Al-Mamun, and Kundan Samal. 2025. "Prospect of Conductive Materials in the Anaerobic Digester Matrix for Methane Production: Electron Transfer and Microbial Communication" Water 17, no. 9: 1321. https://doi.org/10.3390/w17091321
APA StyleAl Hasani, Z., Nayak, J. K., Al Balushi, N. J., Al-Mamun, A., & Samal, K. (2025). Prospect of Conductive Materials in the Anaerobic Digester Matrix for Methane Production: Electron Transfer and Microbial Communication. Water, 17(9), 1321. https://doi.org/10.3390/w17091321








