Abstract
Hydrothermal liquefaction (HTL) is a thermochemical conversion process that converts wet biomass into biocrude oil, a gas phase, a solid phase, and an aqueous phase (HTL-AP). An obstacle to the development and scaling of HTL is the volume of HTL-AP produced during the process, which has high concentrations of nitrogen and carbon and cannot be disposed of in the environment without treatment. The HTL-AP is enriched with organic compounds, particularly light polar organics and nitrogenous compounds, which are inhibitory to microbial treatment in wastewater treatment plants. For this reason, the valorization of the HTL-AP is significant for the circular economy of HTL. This review synthesizes published findings on different types of treatment of the HTL-AP for the recovery of valuable nutrients and the removal of toxic compounds. This work outlines the trade-offs of the treatments to serve as a guide for future research to address these weaknesses and improve the valorization of the HTL-AP. Furthermore, this work uniquely focuses on HTL-AP treatment for recovering plant-available nitrogen, targeting its potential use as a fertilizer. The literature highlights the importance of increasing nitrogen bioavailability in HTL-AP through two-step treatments and by selecting HTL-AP derived from protein-rich feedstocks, which offer higher initial nitrogen content. According to the current state of research, further work is needed to optimize chemical and biological treatments for nutrient recovery from HTL-AP, particularly regarding treatment scale and duration. Additionally, economic analyses across different treatment types are currently lacking, but are essential to evaluate their feasibility and practicality.
1. Introduction
Biomass conversion methods are divided into thermochemical and biochemical processes. Thermochemical methods use heat, chemical reactions, and catalysts to break down biomass into fuels and valuable products, while biochemical methods rely on microorganisms or enzymes to convert biomass into biofuels and chemicals [1]. Thermochemical conversion of biomass includes combustion, torrefaction, liquefaction, and pyrolysis, each offering different ways to produce energy and fuels. Combustion burns biomass for heat and power but is limited by its low energy density and high moisture content. Torrefaction improves biomass properties by heating it in low-oxygen conditions, making it more coal-like, though it poses risks like self-heating and high ash content. Liquefaction uses solvents at high pressure and moderate temperatures to produce bio-oil, with yields depending on feedstock and process conditions. Pyrolysis, conducted in an oxygen-free environment, also produces bio-oil, but it requires upgrading due to poor fuel quality; co-pyrolysis with other materials may improve product outcomes, though the mechanisms are not yet fully understood. Liquefaction is identified as a key thermochemical process for producing liquid fuels, which are in high demand. Its importance is highlighted alongside pyrolysis, especially following the successful operation of a fast pyrolysis biorefinery pilot plant. Liquefaction is expected to be utilized as part of a broader strategy to displace fossil fuels and contribute to achieving net-zero emissions by 2050 [2].
Hydrothermal liquefaction (HTL) is suitable for wet feedstock conversion into fuel. HTL’s commercial interest has led to successful pilot projects and continuous reactor designs that aim to scale up production. Researchers have also highlighted HTL’s potential for integrated waste management and energy recovery, especially in urban areas where wet waste is abundant. Furthermore, HTL supports the development of algal biorefineries, which are especially sustainable due to algae’s high growth rates and CO2 sequestration potential. HTL aligns well with global decarbonization goals, offering a carbon-neutral or even carbon-negative pathway for producing renewable fuels. With continued research into reaction mechanisms, process optimization, and reactor scale-up, HTL is seen as a key thermochemical technology for achieving a more sustainable, circular bioeconomy and reducing dependence on fossil fuels [3].
HTL produces biochar, bio-oil, an aqueous phase, and gases under high temperature (200–550 °C) and pressure (5–25 MPa). Each of these products has potential applications: biochar can be used as a fuel, a pollutant adsorbent, or a soil amendment; bio-oil can serve as a light fuel or be upgraded into high-value chemicals; the aqueous phase can support microalgae cultivation or anaerobic digestion; and gases such as CO2, CH4, and H2 have niche energy or nutrient uses. However, despite these promising outputs, HTL faces significant limitations that hinder its scalability and competitiveness. The process is cost-intensive, requiring specialized equipment and energy for high-temperature and pressure operations. The bio-oil produced is often oxygen-rich, acidic, and unstable, necessitating further upgrading. The aqueous phase (HTL-AP) may contain toxic compounds that demand careful treatment before reuse. Additionally, solid residues and tar formation can reduce efficiency and complicate reactor performance. A major bottleneck remains the lack of a clear understanding of HTL reaction mechanisms, which are often studied under idealized lab conditions using model compounds that fail to reflect real feedstocks. Current kinetic models also often overlook critical secondary parameters (e.g., dry-to-wet ratio), making predictions less reliable. Overcoming these limitations will require advanced modeling tools (e.g., machine learning), process optimization, and economies of scale to move HTL closer to commercial viability in the renewable energy landscape [4].
While treatment of the HTL-AP at a wastewater treatment plant (WWTP) is technically possible, it presents significant challenges. The HTL-AP requires pH adjustment for downstream processes, which is costly and energy intensive. Additionally, the presence of phenolic compounds and other recalcitrant substances from the degradation of carbohydrates, lignin, proteins, and lipids during the HTL makes the mixture difficult to treat using conventional biological methods. Since WWTPs are not designed to handle complex and inhibitory chemical composition, the effectiveness of standard treatment processes is limited [5].
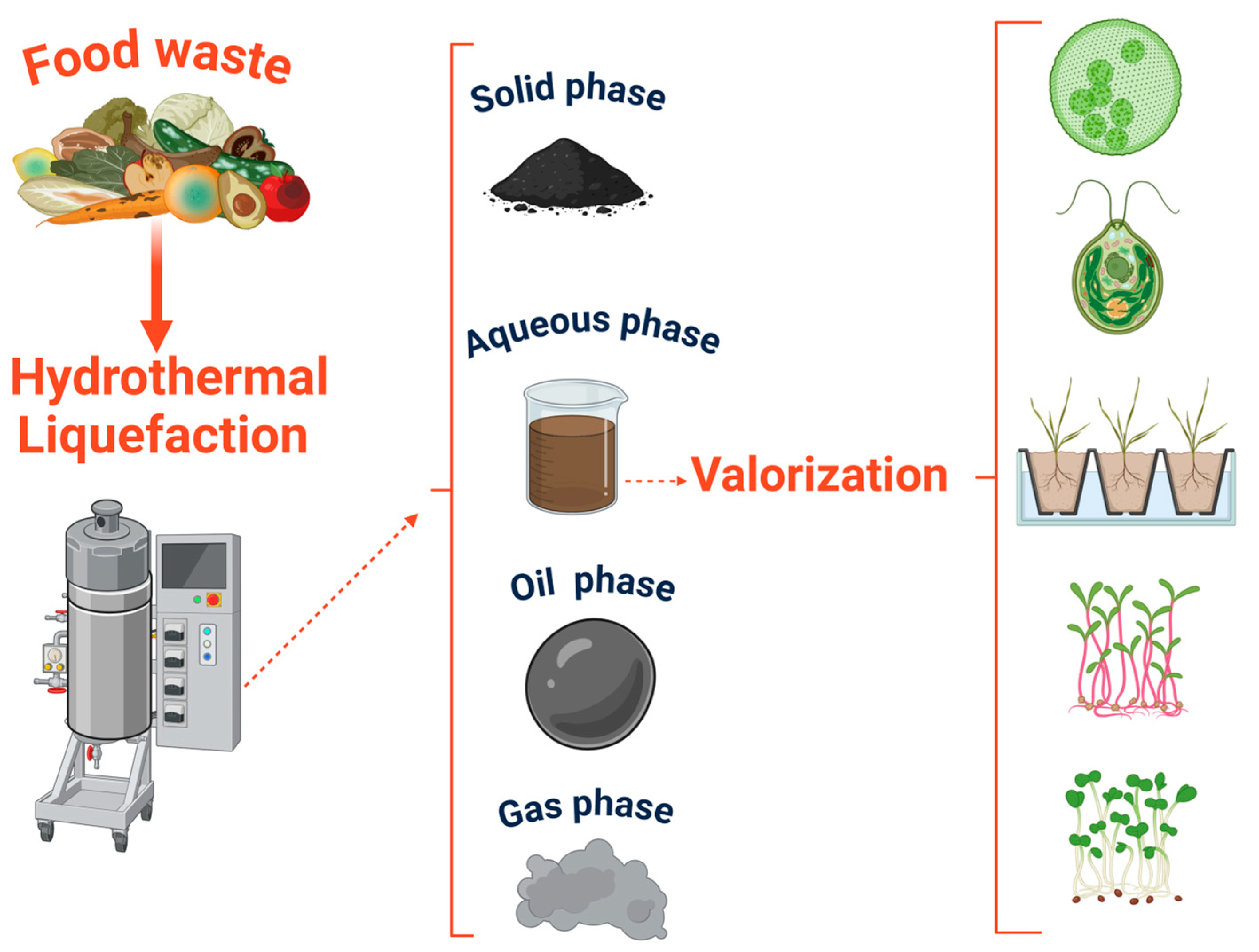
Furthermore, HTL-AP contains a high concentration of organic compounds, including acids, nitrogen-containing organic compounds, ammoniacal nitrogen, and phosphate. The HTL-AP, with appropriate treatment, may serve as a nutrient source for organisms, but compounds such as nitrogenous hydrocarbon compounds and heavy metals have been reported to inhibit microorganisms’ growth [6]. Additionally, the HTL-AP has been reported to lack enough available nutrients to sustain microorganisms’ growth [6] as well as plant growth (Figure 1) [7].
Figure 1.
Schematic overview of the hydrothermal liquefaction (HTL) process using wet feedstocks, highlighting the generation of bio-oil, biochar, gas, and the aqueous phase (HTL-AP), with a focus on the potential reuse of the HTL-AP as a nutrient solution for plant growth.
To valorize the HTL-AP, a treatment to remove or transform inorganic contaminants is needed to facilitate the recovery of valuable materials such as hydrogen or nitrogen and improve the recirculation of the HTL-AP back into the HTL system [8]. Operational parameters affect the yield and performance of the HTL. Therefore, it is necessary to understand the most effective treatments for decreasing the refractory compounds in the HTL-AP. The primary aim of this study is to provide a comprehensive bibliographical review of physical, chemical, and biological treatments of HTL-AP, drawing solely from existing sources, rather than engaging in the collection or analysis of primary data. This summary will also comment on the treatment’s effectiveness to help identify common challenges and limitations associated with these treatment methods. The key distinction of this study compared to previous work is its exclusive focus on the treatment of HTL-AP for plant-available nutrient recovery. While many studies explore HTL for bio-oil production or energy yield optimization, few, if any, have concentrated specifically on evaluating the HTL-AP as a source of nutrients, specifically nitrogen, for use in plant nutrient solutions. This work emphasizes the bioavailability of nitrogen in the HTL-AP and the potential to repurpose HTL-AP as a nutrient-rich input, rather than viewing it solely as a waste stream requiring disposal or general remediation.
2. HTL-AP Water Quality by Feedstock
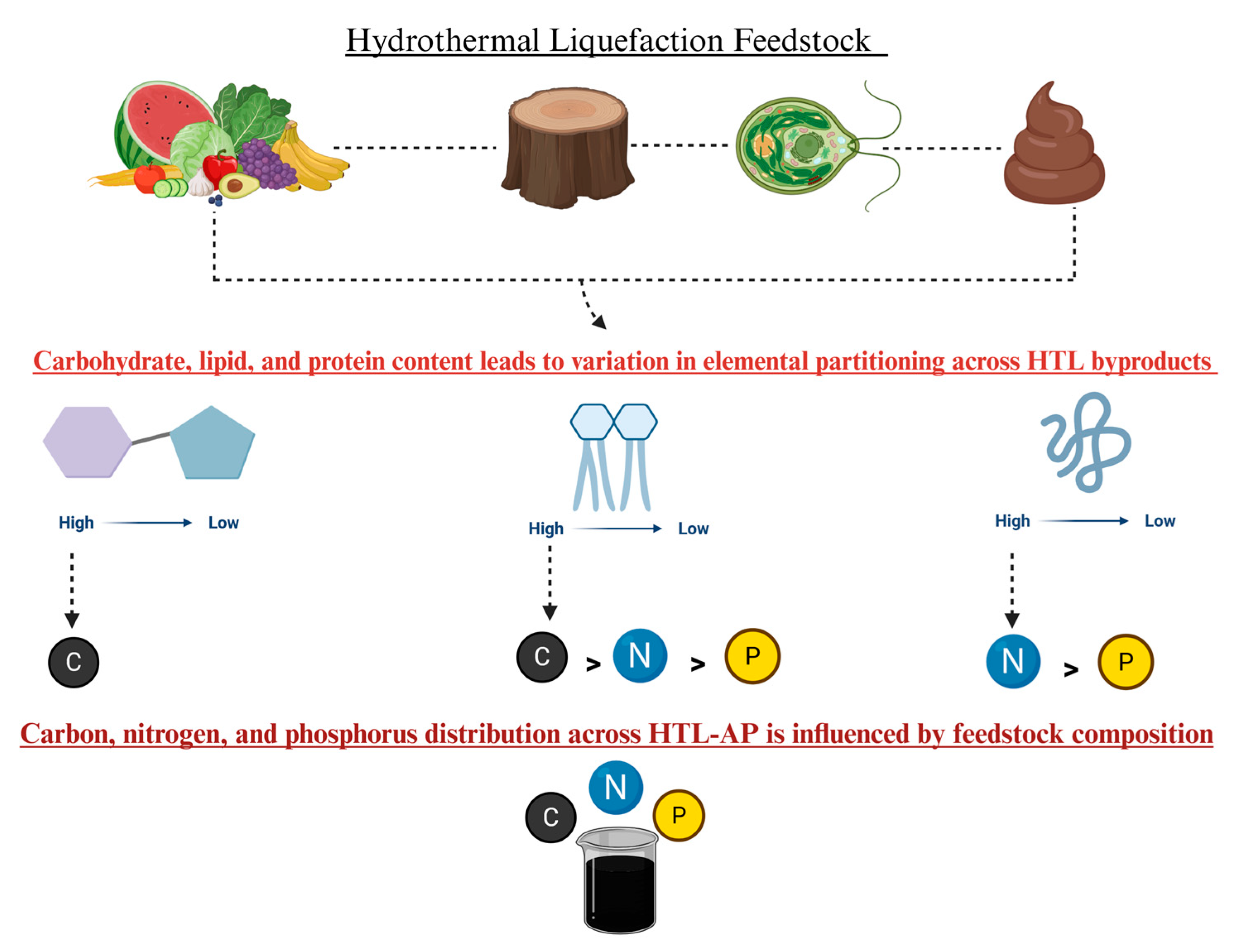
Biomass, a renewable energy source, varies mainly as lipid-rich, carbohydrate-rich, or lignocellulosic types, each showing different behaviors and bio-oil yields and byproducts during HTL. Lipid-rich biomass tends to produce more bio-oil due to the lipophilic nature of lipid hydrolysis products, whereas proteins and carbohydrates yield hydrophilic byproducts that mix with aqueous phases. HTL can process wet biomass directly, with optimal moisture improving reaction rates and product quality, though excess moisture may reduce bio-oil stability. Faster heating rates tend to increase bio-oil yield by minimizing char formation, while slower rates favor char due to repolymerization. The ideal temperature range is about 280–380 °C, balancing reaction speed and product yield, but overly high temperatures can reduce biocrude output. The residence time should be tailored to biomass type, too short causes incomplete conversion, while too long leads to more gas and char, lowering bio-oil yield. Pressure mainly keeps water liquid during HTL; it impacts biocrude yield at lower (subcritical) temperatures but has minimal effect at higher (supercritical) temperatures, with higher pressure potentially slightly enhancing conversion but also slowing pyrolysis by stabilizing molecular bonds [9]. The macromolecular composition of biomass strongly influences HTL bio-oil yield and characteristics. Optimizing HTL thus requires a detailed understanding of these compositional effects to maximize bio-oil and byproduct recovery [10]. This section focuses primarily on four feedstocks (Figure 2), which are the predominate feedstocks reported in the literature, to evaluate their influence on HTL-AP water quality characteristics under optimized hydrothermal liquefaction conditions. To compare diverse biochemical compositions on HTL-AP outcomes, this section offers a comprehensive perspective on the role of wet biomass on the HTL-AP variability.
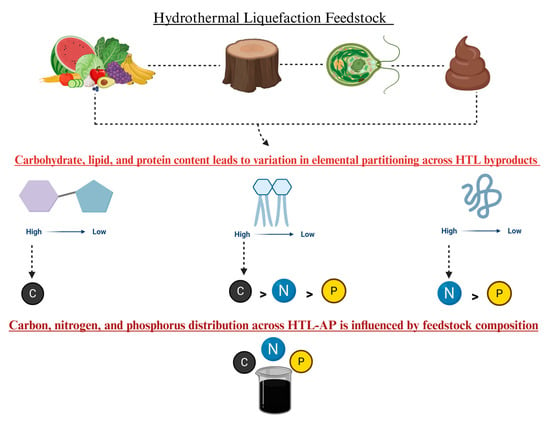
Figure 2.
Elemental distribution in HTL products varies with feedstock composition, with lipids enriching the oil, and proteins and carbohydrates contributing to the aqueous phase.
2.1. Food Waste-Derived HTL-AP
Food waste represents a major environmental, economic, and social challenge in the United States, where an estimated 30–40% of the food supply is wasted. Approximately 133 billion pounds, worth USD 161 billion, was lost in 2010. This discarded food not only contributes to food insecurity, as safe and edible items are often thrown away rather than redirected to those in need, but also wastes the land, water, labor, and energy used in its production. Organizations like Feeding America recover only a fraction of what could be salvaged. Economically, reducing food waste offers clear benefits, including cost savings for households and businesses, tax incentives for food donations, and lower waste treatment costs. Environmentally, it helps reduce methane emissions from landfills, where food is the single largest waste stream and a significant source of greenhouse gases [11]. HTL can leverage food waste into value-added chemicals. One of the key challenges in understanding HTL-AP lies in its variability, which is largely governed by the biochemical composition of the biomass feedstock. Due to its diverse and often unstandardized composition, food waste contributes an added layer of complexity to the already feedstock-dependent nature of HTL-AP. Quantitative data on the molecular composition of the HTL oil and HTL-AP showed variation depending on the feedstock type after the reaction (300 °C,150 bar, 120 min); protein (meat) and lipid-rich (cheese) feedstocks yielded more nitrogenous compounds, while carbohydrate-rich fruit produced bio-oil richer in oxygenated compounds. Authors attribute these results to the composition of meat (protein), cheese (lipids), and fruit carbohydrates to the availability of nitrogen-containing compounds in the HTL products [12]. Another study that explores the conversion of food waste mixture (52% carbohydrate, 20% lipid, 21% protein) into biocrude oil after the HTL (240–295 °C, 0–60 min) further highlights how the composition of food waste influences the migration of carbon and nitrogen into the HTL aqueous phase. The aqueous phase held up to 6.0 wt.% of the carbon, which decreased with temperature due to reduced presence of soluble organics, and the nitrogen recovery ranged from 4.5 to 14.8 wt.% (0.74–1.8 g/L TN), with no clear trends across conditions. Overall, the feedstock’s composition (notably its protein and carbohydrate content) and operating temperature had a strong influence on how carbon and nitrogen were partitioned into the different HTL products [13]. Other studies on catalytic HTL of food waste feedstock (40% carbohydrates, 9% protein, 3% lipid) found that catalytic HTL increased the carbon and nitrogen content in the HTL-AP with 96 g/L of carbon and 2.4 g/L of nitrogen in the HTL-AP compared to non-catalytic HTL products [14]. The use of catalytic-derived HTL-AP may offer a promising approach for nutrient recovery, particularly in agricultural applications. Given the elevated carbon and nitrogen migration into HTL products, catalytic-derived HTL could serve as a valuable source of plant-available nitrogen. Complementary studies have processed two types of food waste: grocery food waste (32% protein, 29% lipids, 30% carbohydrates) and salad dressing waste (2.3% protein, 62% lipid, 29% carbohydrate) using a pilot-scale HTL reactor (280 °C, 124 bar, 30 min) to evaluate their suitability as biofuel feedstock. The results showed significantly higher nitrogen presence downstream for the feedstock with higher initial protein concentration. The grocery food waste had retained more nitrogen- and carbon-based compounds in the HTL-AP, as seen in the total nitrogen (TN), ammonia-nitrogen (NH3-N), and chemical oxygen demand concentration; 101 g/L COD, 8.7 g/L TN, and 2.3 g/L NH3-N, respectively [15]. Based on the significantly higher nitrogen concentration observed in the HTL-AP for the protein-rich feedstock of the HTL, it is advisable to prioritize protein-rich food waste as feedstock for the HTL-AP reuse for agricultural applications due to its greater potential to produce higher total nitrogen and ammonia-nitrogen levels. In varying reaction conditions, carbon, nitrogen, and phosphorus distribution in the HTL-AP depends on the biochemical composition of food waste feedstocks (lipid, carbohydrate, and protein). High lipid feedstocks, such as salad dressing and cream cheese, have been reported to transfer less nitrogen than carbon and less phosphorus than nitrogen into the aqueous phase. Furthermore, carbon is the most abundant compound in high-protein feedstocks such as beef and chicken compared to nitrogen and phosphorus yields in the aqueous phase. The aqueous phase of high-protein feedstocks typically contains higher nitrogen than phosphorus. High-carbohydrate feedstocks such as burger buns, vegetables, and fruit peels have been shown to have a higher abundance of carbon compared to nitrogen and a higher abundance of nitrogen compared to phosphorus in the aqueous phase [16].
2.2. Algae-Derived HTL-AP
Algae, including both microalgae and macroalgae, are a diverse group of organisms with high-value biochemical components such as lipids, proteins, carbohydrates, nucleic acids, and pigments. Their high lipid yield is crucial for biofuel production. While algae are already successfully used in specialty markets like dietary supplements and food additives, the high cost of cultivation, harvesting, and processing make algae application as biofuels economically challenging. Furthermore, it is considered a third-generation energy source as it is useful for biofuel production without competing with food crops. A 2017 DOE analysis estimates the U.S. could sustainably produce 104–235 million metric tons of algal biomass, potentially generating 10–27 billion gallons of algal-based fuels per year, provided technological targets are met [17]. Microalgae with biochemical profiles rich in proteins and lipids can influence both the yield and composition of HTL products, including the HTL-AP. A study showed how the extraction of specific components, such as lipids and proteins, from Nannochloropsis gaditana (32% protein, 13% lipid) and Scenedesmus almeriensis (30% protein, 13% lipid) before HTL (300–375 °C, 5–15 min.) impacted biocrude oil quality and the nitrogen content of the products. Findings showed that the extraction of proteins before HTL reduced the nitrogen compounds in the HTL products; however, their recovery was not influenced by changes in temperature [18]. Therefore, to leverage the plant-available nutrients from the algae-derived HTL-AP, it can be advantageous to avoid protein–lipid extraction before HTL, which could be advantageous for downstream applications. Other studies utilized four Chlorella sorokiniana NIES 2173 feedstocks with distinct biochemical profiles to assess the impact of biomass composition on HTL outcomes. Feedstocks RAFCS0 and RAFCS1 were protein-rich, containing 45.4% and 43.9% protein, respectively, with moderate levels of lipids (18.8–23.2%) and carbohydrates (16.9–22.1%). In contrast, RAFCS2 and RAFCS3 shifted toward carbohydrate-dominant compositions, with 31.4% and 43.0% carbohydrates, lower protein contents (17.7–23.1%), and reduced lipid levels (13.3–14.0%). The mix of HTL-AP from the different algal feedstocks contained high concentrations of both carbon and nitrogen compounds (8.9 g/L total nitrogen and 5.6 g/L of organic nitrogen) with a predominance of organic compounds. Furthermore, the mixed algal HTL-AP also indicated the presence of phosphate (3.1 g/L). However, the mixture might include inhibitory compounds that could challenge its reuse as a nutrient solution for microalgae growth [19]. Using a mixture of algal-derived HTL-AP could enhance plant-available nutrient availability in terms of nitrogen and phosphorus for its use in agriculture. Another study used Chlorella sp. (SAG 211-11b) microalgae, cultivated using a sludge-derived HTL-AP as the feedstock for hydrothermal liquefaction (HTL). The experiments were performed using a fast HTL (300–500 °C and 20–1800 s). Results showed decreased total nitrogen in the HTL-AP with the increase in temperature and higher chemical oxygen demand after fast HTL [20]. Lower temperatures in fast HTL might be beneficial for the recovery of nitrogenous compounds such as amides in the HTL-AP since it improves the plant nutrient recovery potential of the HTL-AP.
Microalgae biochemical composition influences the nitrogen distribution in the HTL-AP as reported in the literature; high-carbohydrate microalgae transfer higher concentrations of oxygenated compounds to the aqueous phase compared to higher protein microalgae, which usually release higher nitrogenous compounds into the HTL-AP. High-protein and low-lipid microalgae have been shown to transfer more nitrogen into the HTL-AP, ranging between 40 and 70% of the elemental composition, with lower bioavailable nitrogen [21].
2.3. Manure-Derived HTL-AP
Manure can be effectively used in thermochemical conversion processes to produce syngas with energy recovery potential and reduce greenhouse gas emissions. Optimized gasification of manure, particularly with CO2, is a promising approach for sustainable waste-to-energy conversion [22].
Furthermore, studies have investigated the potential of livestock manures with different biochemical composition; swine manure (26% protein, 10% lipid), broiler and laying hen manures (25% and 23% protein, respectively), dairy cow and beef manures (14 and 18% protein, respectively, 5% and 4% lipid, respectively), and sheep manure (3% lipid) for HTL (310–340 °C, 30 min), and found that almost 40% of the feedstock nitrogen migrated to the HTL-AP with polar and oxidized nitrogen species in the HTL-AP. However, the variability in nitrogen species contributes to cytotoxicity; therefore, treatment is needed before safe reuse [23]. Since the total nitrogen concentration measured in the HTL-AP ranged between 1 and 5 g/L with substantial presence of organic nitrogen after HTL, the utilization of manure-derived nutrients could be valuable to recover for applications such as algal and plant cultivation.
Another recent study compared two thermochemical strategies to convert chicken manure into bio-oil: direct HTL and a two-stage process combining hydrothermal carbonization (190–250 °C, 30 min) followed by HTL (250–350 °C, 30 min). The introduction of hydrothermal carbonization as a step before HTL enabled the migration of nitrogen from protein and amino acids into HTL-AP [24]. As hydrothermal carbonization reduced the carbon content in the HTL-AP, it may help lower the synergistic toxicity from organic compounds in the aqueous phase, thereby enhancing its potential use as a nutrient solution for algae cultivation and plant growth. The variation in manure composition influences the migration of organic carbon and nitrogen into the aqueous phase differently depending on the type of manure feedstock. For instance, human feces have been reported to release nitrogen than organic carbon into the aqueous phase. However, animal-derived manure has also been reported to migrate similarly high concentrations of nitrogen, but in ammonia form [25].
2.4. Wood-Derived HTL-AP
The USDA is actively investing in efforts, including developing agronomic best practices for lignocellulosic crops, to increase the utilization of agricultural wastes and food residuals, and improve access to woody biomass from forest management. The USDA is also supporting rural biofuel production capacity and advanced biorefineries, and incentivizing markets for biobased products such as sustainable aviation fuel and engineered wood [26]. HTL converts wet biomass like wood waste into HTL oil and HTL-AP, as well as other byproducts [27].
Results from studies focused on HTL of beech wood and soda lignin (300–350 °C, 20 min, 200–250 bar) suggested that samples with pulping processes yielded higher nitrogen in the HTL-AP compared to other samples, which yielded oxygenated organic compounds in the HTL-AP [28]. In the literature, the protein content is typically not reported for wood-based feedstocks, which limits the understanding of nitrogen behavior and nutrient recovery potential during HTL processing. Through the conversion of wood into biocrude oil, a significant amount of organic carbon migrates to the HTL-AP, and low concentrations of nitrogen, which makes it suitable for recirculation into the HTL process [29] but not ideal for its reuse as a nutrient solution for plant growth.
3. HTL-AP Treatment Methods
The HTL-AP contains a complex mixture of organic and inorganic compounds, including nitrogenous species, which presents both challenges and opportunities for treatment and recovery. Various treatment strategies have been explored to address its composition, aiming either to reduce environmental impact or to enable its reuse in circular systems such as nutrient solutions for crop production. This section summarizes the main HTL-AP treatment approaches reported in the literature, including physical, chemical, and biological processes, and evaluates them in terms of carbon removal, nitrogen recovery, treatment time, and scalability.
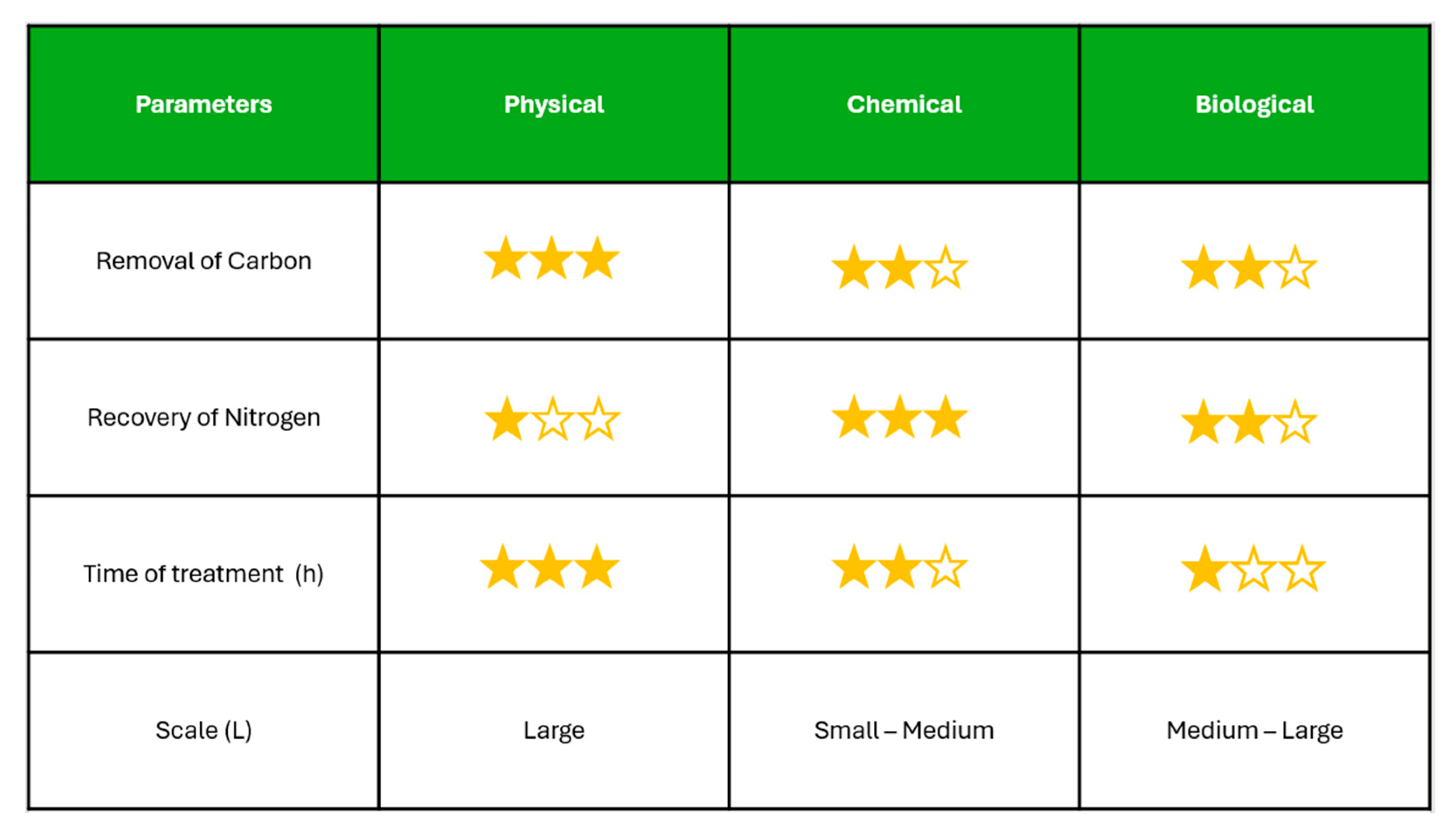
The types of treatment of the HTL-AP seen in Figure 3 help to assess how well each treatment performs in balancing environmental impact with operational feasibility, highlighting the trade-offs between fast, effective carbon removal and sustainable nutrient recovery for potential reuse.
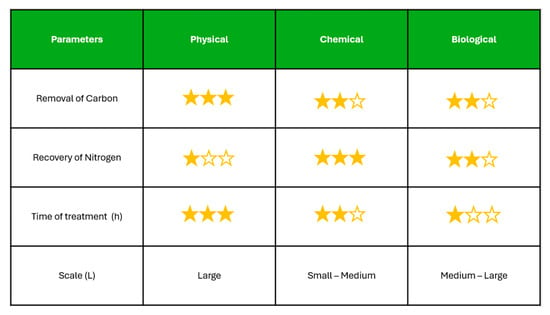
Figure 3.
The figure presents a comparative evaluation of different HTL-AP treatment strategies based on key performance indicators.
In terms of carbon removal from HTL-AP, physical treatments such as separation filtration have demonstrated higher efficiencies compared to chemical and biological methods, often achieving removal rates above 50%. This is likely due to their capacity to target a broad spectrum of organic compounds without relying on complex degradation pathways, which can be limited in chemical or microbial processes by the recalcitrant nature of the organics present in HTL-AP. Comparatively, chemical treatments have been reported to leverage the highest proportion of nitrogen from HTL-AP relative to biological and physical methods. This is attributed to their ability to break down complex organic nitrogen compounds into more bioavailable forms, facilitating greater nitrogen recovery for potential reuse. Despite the higher nitrogen recovery achieved through chemical treatments, their scalability remains a limiting factor, as most studies have only been conducted at lab or pilot scale. In contrast, physical and biological methods have demonstrated the capacity to handle larger volumes of HTL-AP, making them more feasible for scaled-up applications. The fastest treatment methods for HTL-AP generally follow the order: physical treatments being the quickest, followed by chemical treatments, and then biological treatments, which typically require longer retention times.
Below, additional examples from the literature highlighting the reported performance of physical, chemical, and biological treatment methods for carbon and nitrogen removal from HTL-AP are presented.
3.1. Physical Treatment Methods
Physical treatment methods are often able to handle high flow rates of wastewater; however, they may not be able to remove all compounds of interest in HTL-AP. Among some of the physical methods used to treat HTL-AP are as follows:
3.2. Sand Filtration
Sand filtration is a secondary treatment method that has been reported to help improve COD removal from effluents [30]. This method was previously applied to swine manure-derived HTL-AP to remove heavy metals from wastewater. Results showed 96% removal of mercury from diluted HTL-AP. Sand filtration achieved the lowest removal efficiency of lead and arsenic compared to the other filtration methods tested in a study. In addition to removing metals, this method showed evidence of decreasing the nutrient levels from the HTL-AP, and no significant increase in nutrient availability was observed when adding a nitrification step after sand filtration. Furthermore, the total nitrogen levels in the HTL-AP decreased at the lowest dilution rate tested in the study but slightly increased the nitrogen levels in the medium and high dilution rates evaluated [31]. Overall, this physical treatment method showed effectiveness for removing heavy metals and nitrogen from the HTL-AP and could be an alternative for treating HTL-AP before disposal into the environment. However, sand filtration was not effective in recovering sufficient levels of plant nutrients, with or without assistance from biological treatments.
3.3. Reverse Osmosis
Reverse osmosis is the separation of liquids through a semipermeable membrane [32]. This method allows the separation of the water from the contaminants through the membrane. During the process, the water moves freely through the membrane, and the solutes/contaminants are retained [33]. Reverse osmosis was applied in the Jesse and Davidson [31] study investigating the treatment of swine manure HTL-AP to be safely used for plant growth. Among the parameters evaluated in the study were fecal coliforms, heavy metals, and nutrient availability for plant growth. After the treatment with reverse osmosis of different dilutions of the HTL-AP, complete removal of cadmium and mercury was achieved with reverse osmosis at the low and medium dilutions tested, and 99% removal of lead and arsenic. An increase of 24% of nitrate-nitrite, 99% removal of ammonium-nitrogen, 33% removal of phosphate, and 58% removal of total phosphorus from HTL-AP was achieved at the lowest dilution tested in the study. Furthermore, at the medium dilution tested in the study, 81% removal of ammonium-nitrogen was achieved, as well as 85% removal of total nitrogen was obtained after ozone treatment.
3.4. Nanofiltration
Nanofiltration (NF) is a separation method that utilizes a nanomembrane with a charged surface. Nanofiltration has an application in wastewater treatment since high removal of COD and TSS can be achieved without major changes in the pH of the samples. The utilization of nano-filter membranes has been shown to help with the removal of COD to achieve 99% efficiency and 93% TSS [34]. As the HTL-AP is a mixture of phenol derivatives, acids, ketones, and saccharides, a synthetic HTL-AP based on the HTL of rice straw was tested for rejection of phenols and saccharides by nanofiltration. It was shown that it is feasible to separate phenols from sugars [35]. NF has the potential to recover compounds contained in the HTL-AP (Table 1).

Table 1.
Nanofiltration applications on the HTL-AP.
3.5. Activated Carbon Filtration
Activated carbon, also known as activated charcoal–graphite, is routinely used to remove pollutants from drinking water; however, its effectiveness in treating wastewater is less well known. Due to its properties, it can absorb heavy metals, such as arsenic and ammonia. Among the applications of activated carbon to treat wastewater is the removal of contaminants from agricultural wastewater [39]. Additionally, activated carbon has been used as a filter to remove compounds from the HTL-AP. The highlights of each published study, with the associated resulting pH, recovery efficiency of valuable compounds, and advantages/disadvantages, are presented in Table 2.

Table 2.
Activated carbon filtration studies on HTL-AP. “NA” indicates that the information was not available or not reported in the referenced source.
Even though physical treatments are selective, fast, and capable of handling larger volumes, currently, these treatments are not ideal for nitrogen recovery due to the risk of nitrogen loss through volatilization, particularly in the form of ammonia. It might also be worth exploring the development of a selective filtration system designed to retain and concentrate nitrogen compounds, enabling the production of a nitrogen-rich HTL-AP that is better suited for downstream nutrient recovery applications.
3.6. Chemical Treatment Methods
Precipitation, electrochemical oxidation, and ozone treatment are some of the chemical approaches applied to HTL-AP for the recovery of valuable materials as well as the removal of undesirable compounds. Below is the description of each treatment and its effectiveness in treating HTL-AP.
3.7. Struvite Precipitation
Struvite is a crystal of magnesium ammonium phosphate (MgNH4PO4·6H2O) that is produced through supersaturation because of the increase in ammonium, pH, magnesium, and orthophosphate [41]. Struvite precipitation is an effective treatment for the recovery of ammonium-nitrogen and phosphorus, as well as magnesium salts from influences that conventional biological treatment processes fail to address [42]. As this method offers an alternative to the recovery of valuable ions for plant growth, Table 3 lists published studies that attempted to recover nutrients from the HTL-AP:

Table 3.
Struvite precipitation studies on the HTL-AP. “NA” indicates that the information was not available or not reported in the referenced source.
3.8. Ozone
Ozone is an advanced oxidation process that utilizes ozone, which is an unstable gas, to oxidize organic pollutants through direct and indirect oxidation [46]. Ozonation, as a technology, can be used to improve the biodegradability of wastewater or in combination with biological treatments to eliminate persistent organic pollutants and personal care products through direct or radical ozone reactions. Ozone can mineralize organic matter, but it is an expensive oxidant, and the generation of toxic intermediates seems to be a limiting factor in its use [47]. Below are some applications of ozone treatment for HTL-AP (Table 4).

Table 4.
Ozone studies on the HTL-AP. “NA” indicate that the information was not available or not reported in the referenced source.
3.9. Electrochemical Oxidation
Electrochemical oxidation is an emerging technology that utilizes anodic oxidation as a method to perform selective oxidation of pollutants through conductive anodes, such as SnO2, PbO2, or boron-doped diamond (BDD), to convert organics to carbon dioxide [51]. Furthermore, wheat straw and sewage sludge-derived HTL-AP has been reported to have been subjected to electrochemical oxidation for hydrogen gas production utilizing boron-doped diamond as a conductive material for both anode and cathode. The study reported differences in the removal efficiency of COD depending on the initial concentration of organic matter. For instance, 80% removal of organic matter from the wheat straw-derived HTL-AP was achieved, and around 90% removal of COD from sewage sludge-derived HTL-AP with lower initial organic load under high current density applications. It is unclear how the differences in organic load influenced the COD removal efficiency of the treatment. As stated by the authors, differences in the presence of organic acids and aromatic species in the two types of wastewaters treated in the study might have influenced the removal of COD. Additionally, a similar pattern was observed in the production of ammoniacal nitrogen in the two types of HTL-AP. Higher initial organic load in the wheat straw-derived HTL-AP achieved higher production of ammonia-nitrogen at lower current densities compared to sewage sludge-derived HTL-AP with lower initial organic load that yielded higher ammonia-nitrogen at higher current levels. On the other hand, when the initial organic load was higher, the production of hydrogen gas was enhanced at higher current levels compared to the lower initial organic load that recovered most hydrogen gas at medium current levels [52].
Microalgae-derived HTL-AP has been recently reported to undergo an oxidation process to decrease chemical oxygen demand and increase the degradation of organic nitrogen in the HTL-AP. The process was carried out utilizing a system of two electrodes in which the boron-doped diamond was the anode and stainless steel the cathode. Different operational conditions to induce oxidation were tested and the treatment increased the availability of ammoniacal nitrogen and nitrate-nitrogen in the HTL-AP. Nitrogen heterocyclic compounds were converted into ammoniacal nitrogen species. The results show potential to treat HTL-AP for nutrient recovery. However, as the HTL-AP samples vary according to the feedstocks, the authors failed to comment if these results were to hold for different HTL-AP [53]. Further evidence of the ability of electrochemical techniques to convert organic nitrogen into nitrate-nitrogen and ammonia-nitrogen from HTL-AP food waste was reported recently [54].
Despite the advantages offered by chemical treatment for nutrient recovery, future work should focus on evaluating its cost-effectiveness and energy efficiency to ensure its practical viability at larger scales.
3.10. Biological Treatment Methods
Anaerobic digestion and microalgal cultivation are some of the most common methods of recovery of materials of interest of the HTL-AP. Fungal treatments have also been reported in 2024 to be applied to recover inorganic nitrogen for use in agriculture. A summary of the mentioned methods can be found below.
3.11. Anaerobic Digestion
Anaerobic digestion is a digestion process of four steps: hydrolysis, acidogenesis, acetogenesis, and methanogenesis, performed by anaerobic bacteria in which organic matter is broken down into methane and carbon dioxide that are stored along with the solid and liquid streams for future applications [55]. HTL-AP has been integrated into anaerobic digestion treatment to produce biogas in which the organic matter is valorized or converted into biogas and fertilizer for crop production. There is potential for HTL followed by anaerobic digestion after treatment of HTL-AP because the presence of recalcitrant compounds affects the efficacy of the recovery of valuable materials through anaerobic digestion [56]. Even though anaerobic digestion offers the possibility of recovery of methane and hydrogen gas, the removal of nutrients is restricted, and heavy dilutions of HTL-AP are needed, as well as longer retention times during digestion [57].
3.12. Fungal Treatment
Fungal treatment is an alternative biological treatment for wastewater as it can recover valuable materials from different types of wastewaters from food-processing industries and textile industries, since fungi have been demonstrated to remove organic compounds and degrade organic dyes. By applying fungal treatment, the degradation of complex organic compounds is achieved through enzymes such as manganese peroxidase (MnP) and lignin peroxidase (LiP), as well as the recovery of valuable products and simplification of the separation of the fungal biomass from the material of interest [58]. Fungal treatment has been previously used to attempt to valorize corn stover HTL-AP [59] and food-waste HTL-AP [60,61]. The work on the valorization of corn stover HTL-AP tried to compare the removal of COD and recovery of valuable products with three different strains of fungi: A. niger, T. versicolor, and P. chrysosporium. The study evaluated the effectiveness of coculture with a bacterium, R. jostii, with the three strains of fungi in the HTL-AP and assessed the COD removal and value-added product profile of the A. niger and R. jostii coculture with added diluted HTL-AP concentrations during fermentation. As observed in the study, the acidic pH of the diluted HTL-AP inhibited the fungal activity with all the strains tested in the study. Furthermore, the HTL-AP needed supplementation with ions and iron in order to remove up to 41% COD with T. versicolor and A. niger, and only 20% removal of COD was achieved with P. chyrsosporium. Supplementation of ions and iron was necessary to achieve higher recovery of oxalic acid with A. niger. Cocultured A. niger and R. jostii enabled the increase in removal of COD up to 63%. The highest removal of COD was achieved from the diluted HTL-AP with A. niger. The acidity, as well as the availability of ions and metals in the HTL-AP, might be a limiting factor for the valorization of the HTL-AP through bacterial and fungal growth. Another study of the valorization of diluted food-waste-HTL-AP with Trametes versicolor focused on increasing inorganic nitrogen (ammoniacal nitrogen, nitrate-nitrogen) for its future use as a nutrient solution. Additionally, the study evaluated the COD removal, the enzyme activity and growth of the fungus, and the feasibility of increasing nitrate-nitrogen concentration by adding nitrifying bacteria after fungal treatment in the HTL-AP. Two types of nitrifying bacteria were used for the study; Nitrosomas, an ammonia-oxidizing bacteria (AOB), and Nitrobacter, which is a nitrite-oxidizing bacteria (NOB). The main difference between these two types of bacteria is the enzymes that catalyze the conversion of the inorganic forms of nitrogen. For AOB, the conversion of ammonia-nitrogen to nitrite nitrogen is carried out by hydroxylamine oxidase and ammonia monooxygenase. On the other hand, for NOB, the conversion of nitrate to nitrite is performed by nitrite oxidoreductase [62]. The results of the study showed an increase in biomass after nine days of culture of T. versicolor in diluted HTL-AP, recovery of 50 mg/L of ammoniacal nitrogen after nine days of cultivation, and an increase in pH from 4 to 8. The addition of nitrifying bacteria was demonstrated to help increase the availability of nitrate-nitrogen in the HTL-AP after treatment with T. versicolor, achieving a yield of approximately 34 mg/L of nitrate-nitrogen. Continuous fungal removal of COD from diluted HTL-AP and the reason for the decline in removal efficiency of that treatment over time remains unclear. The pH of the HTL-AP seems to harm the microorganism’s growth and nitrifying activities [61].
3.13. Microalgae Treatment
Microalgae have been gaining attention as a tertiary treatment of wastewater since it can incorporate nutrients such as nitrogen and phosphorus while producing useful biomass for its use as a third-generation energy source. The use of microalgae as tertiary treatment has the potential to remove nutrients such as nitrate, phosphate, and ammonia while increasing the oxygen in the water [63]. Several microalgal species have been cultured in the HTL-AP, such as Phaeodactylum tricornutum, Desmodesmus sp., and Chlorella vulgaris, to valorize the HTL-AP through its use as a growth medium. However, the high concentration of nitrogen and absence of micronutrients necessary for microalgal growth might inhibit its growth in the HTL-AP [64]. Other studies highlight the ability of microalgae to capture carbon as biomass from the HTL-AP and serve as feedstock to produce fuels but emphasize the need for heavy dilutions and decreased heavy metals presence to achieve efficient growth and nutrient recovery from the HTL-AP [59]. Despite microalgae’s potential for recovery of nutrients from the HTL-AP, the tolerance to organic compounds as well as nitrogen concentrations depends on the strains, and several dilutions as well as supplementation are needed to enable algal cultivation in the HTL-AP [65].
Generally, biological treatment, even with acclimation and the use of multiple dilutions, requires a preliminary step to reduce the toxicity of the HTL-AP and enhance microbial growth and metabolic activity during the treatment process.
4. Future Directions and Challenges
In the literature, there is currently no standardized method for reporting feedstock composition, such as protein, lipid, and carbohydrate content, nor consistent reporting of operational conditions and aqueous phase yield in terms of water quality. Establishing such standardization would be highly beneficial, as it would allow researchers to identify common patterns and more effectively determine the optimal conditions for nutrient recovery from the HTL-AP.
HTL-AP derived from certain high-protein feedstocks such as algae, manure, and protein-rich food waste may be especially beneficial for enhancing circularity by enabling the reuse of HTL-AP as a nutrient source for crop production. Given the complexity of organic compounds present in HTL-AP derived from certain feedstocks, it is more advantageous to prioritize treatment strategies aimed at nutrient recovery rather than conventional disposal. This approach acknowledges that the characteristics of HTL-AP often render it unsuitable for treatment in standard wastewater treatment facilities.
So far, the only treatments that have shown the ability to work directly with raw HTL-AP, without prior detoxification or dilution, are physical and chemical. This demonstrates that the first step to valorize HTL-AP may be to apply treatments such as physical or chemical methods because they can work directly on the raw HTL-AP without modifications to increase its compatibility and potential for subsequent biological applications.
In future research, it could be valuable to explore a treatment system that first enhances the biodegradability of the organic compounds in HTL-AP through chemical treatment, followed by a biological treatment to further break down and recover nutrients efficiently. There is promising potential for chemical treatments to enhance the biodegradability of organic nitrogen in HTL aqueous phases, thereby enabling more effective subsequent biological treatment to recover nutrients for plant growth. However, current knowledge is largely limited to lab-scale exploration studies. Future efforts should focus on scaling up and optimizing integrated two-step processes combining chemical and biological treatments to maximize nutrient recovery.
A techno-economic analysis will be crucial for assessing the viability of HTL-AP treatment on a commercial scale. However, this information is currently too sparse to conduct any analysis or discussion in this review paper. This highlights the critical needed for further studies in this area.
5. Conclusions
Physical treatments such as sand filtration and reverse osmosis have been reported to effectively remove nitrogen compounds from the HTL-AP; therefore, these methods might not be ideal for the nutrient recovery of the HTL-AP for its use as a nutrient solution for plants. Sensitive methods such as nanofiltration might be adequate to retrieve nutrients such as ammonia-nitrogen from the HTL-AP for its use in agriculture; however, the pH and presence of organic acids in the HTL-AP might cause rejection of the desired chemicals. Chemical methods such as struvite precipitation have been shown to recover valuable nutrients such as phosphate and ammonia-nitrogen; however, their application is limited due to the increased chemical consumption. Furthermore, competitive reactions during nutrient precipitation of the HTL-AP happen and limit the recovery of phosphate and ammonium as well as magnesium. Other methods, such as ozone, have limitations in the removal of chemical oxygen demand as well as organic nitrogen compounds in the HTL-AP, which could be a limitation since the organic nitrogen needs to be leveraged, converted into inorganic forms that are more bioavailable for plants. Finally, electrochemical oxidation has been demonstrated to be able to leverage the organic nitrogen into bioavailable nitrogen forms through the oxidation of nitrogen cyclic compounds into ammonia-nitrogen and nitrate-nitrogen; however, due to the variability of the HTL-AP depending on the feedstock used for the HTL process, the reproducibility of this treatment might be difficult to achieve. Biological treatment, such as anaerobic digestion in HTL-AP, can recover methane and hydrogen gas but faces challenges with nutrient removal and microbial growth due to the pH levels. Similarly, microalgal treatment can help recover nutrients, but its effectiveness depends on the strain and requires dilution and supplementation. Overall, microbial activity in HTL-AP is limited by its pH and nutrient concentrations, affecting both digestion and algal cultivation.
The most effective strategy for valorizing HTL-AP appears to be selecting process water derived from protein-rich feedstocks, which inherently offers higher nitrogen content. Initial treatment should prioritize methods (physical or chemical processes) that can be applied directly to raw HTL-AP without requiring prior modifications. Once the toxicity and complexity are reduced, more sensitive approaches like biological treatments can be employed to further enhance nutrient recovery.
Author Contributions
Conceptualization, methodology, investigation, writing—original draft preparation, B.C.B.C.; writing—review and editing, Y.L. and P.K.; writing—review and editing, supervision, project administration, funding acquisition, P.D.; project administration, funding acquisition, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the United States Department of Agriculture (USDA) National Institute of Food and Agriculture’s (NIFA) Bioproduct Pilot Program (2023-79000-38974, 2023) and the Department of Agricultural and Biological Engineering, University of Illinois, Urbana-Champaign.
Data Availability Statement
This article is a review and does not include original data. All data discussed are available in the cited published sources.
Acknowledgments
The authors would like to acknowledge the support of the USDA NIFA BPP FORWARD research group for providing the materials to complete this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jha, S.; Okolie, J.A.; Nanda, S.; Dalai, A.K. A Review of Biomass Resources and Thermochemical Conversion Technologies. Chem. Eng. Technol. 2022, 45, 791–799. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.J. Thermochemical conversion of biomass: Potential future prospects. Renew. Sustain. Energy Rev. 2023, 187, 113754. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Elhassan, M.; Abdullah, R.; Kooh, M.R.R.; Chou Chau, Y.F. Hydrothermal liquefaction: A technological review on reactor design and operating parameters. Bioresour. Technol. Rep. 2023, 21, 101314. [Google Scholar] [CrossRef]
- Aktas, K.; Liu, H.; Eskicioglu, C. Treatment of aqueous phase from hydrothermal liquefaction of municipal sludge by adsorption: Comparison of biochar, hydrochar, and granular activated carbon. J. Environ. Manag. 2024, 356, 120619. [Google Scholar] [CrossRef]
- Couto, E.; Calijuri, M.L.; Assemany, P. Biomass production in high rate ponds and hydrothermal liquefaction: Wastewater treatment and bioenergy integration. Sci. Total Environ. 2020, 724, 138104. [Google Scholar] [CrossRef] [PubMed]
- Jesse, S.D.; Zhang, Y.; Margenot, A.J.; Davidson, P.C. Hydroponic Lettuce Production Using Treated Post-Hydrothermal Liquefaction Wastewater (PHW). Sustainability 2019, 11, 3605. [Google Scholar] [CrossRef]
- Davidson, S.D.; Lopez-Ruiz, J.A.; Zhu, Y.; Cooper, A.R.; Albrecht, K.O.; Dagle, R.A. Strategies to Valorize the Hydrothermal Liquefaction-Derived Aqueous Phase into Fuels and Chemicals. ACS Sustain. Chem. Eng. 2019, 7, 19889–19901. [Google Scholar] [CrossRef]
- Bao, R.; Wang, S.; Feng, J.; Duan, Y.; Liu, K.; Zhao, J.; Liu, H.; Yang, J. A Review of Hydrothermal Biomass Liquefaction: Operating Parameters, Reaction Mechanism, and Bio-Oil Yields and Compositions. Energy Fuels 2024, 38, 8437–8459. [Google Scholar] [CrossRef]
- Mansuri, S.Q.; Shekhawat, V.P.S. Hydrothermal liquefaction: Exploring feedstock for sustainable biofuel production. Environ. Exp. Biol. 2024, 22, 135–147. [Google Scholar] [CrossRef]
- Why Should We Care About Food Waste?|Home. Available online: https://www.usda.gov/about-food/food-safety/food-loss-and-waste/why-should-we-care-about-food-waste (accessed on 29 June 2025).
- Kostyukevich, Y.; Vlaskin, M.; Borisova, L.; Zherebker, A.; Perminova, I.; Kononikhin, A.; Popov, I.; Nikolaev, E. Investigation of bio-oil produced by hydrothermal liquefaction of food waste using ultrahigh resolution Fourier transform ion cyclotron resonance mass spectrometry. Eur. J. Mass Spectrom. 2018, 24, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Bayat, H.; Dehghanizadeh, M.; Jarvis, J.M.; Brewer, C.E.; Jena, U. Hydrothermal Liquefaction of Food Waste: Effect of Process Parameters on Product Yields and Chemistry. Front. Sustain. Food Syst. 2021, 5, 658592. [Google Scholar] [CrossRef]
- Biswas, B.; Rahman, T.; Adhikari, S. Mono-and bi-metal catalytic hydrothermal liquefaction of food waste: Screening the process parameter on product yield and characterizations. J. Clean. Prod. 2024, 471, 143398. [Google Scholar] [CrossRef]
- Summers, S.; Valentine, A.; Wang, Z.; Zhang, Y. Pilot-Scale Continuous Plug-Flow Hydrothermal Liquefaction of Food Waste for Biocrude Production. Ind. Eng. Chem. Res. 2023, 62, 12174–12182. [Google Scholar] [CrossRef]
- Aierzhati, A.; Stablein, M.J.; Wu, N.E.; Kuo, C.T.; Si, B.; Kang, X.; Zhang, Y. Experimental and model enhancement of food waste hydrothermal liquefaction with combined effects of biochemical composition and reaction conditions. Bioresour. Technol. 2019, 284, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Research, B.; Board, D. Federal Activities Report on the Bioeconomy: ALGAE BRD; US Department of Energy (USDOE): Washington DC, USA, 2020.
- Barreiro, D.L.; Samorì, C.; Terranella, G.; Hornung, U.; Kruse, A.; Prins, W. Assessing microalgae biorefinery routes for the production of biofuels via hydrothermal liquefaction. Bioresour. Technol. 2014, 174, 256–265. [Google Scholar] [CrossRef]
- Ramírez-Romero, A.; Martin, M.; Boyer, A.; Bolzoni, R.; Matricon, L.; Sassi, J.F.; Steyer, J.P.; Delrue, F. Microalgae adaptation as a strategy to recycle the aqueous phase from hydrothermal liquefaction. Bioresour. Technol. 2023, 371, 128631. [Google Scholar] [CrossRef]
- Yuan, C.; Zhao, S.; Ni, J.; He, Y.; Cao, B.; Hu, Y.; Wang, S.; Qian, L.; Abomohra, A. Integrated route of fast hydrothermal liquefaction of microalgae and sludge by recycling the waste aqueous phase for microalgal growth. Fuel 2023, 334, 126488. [Google Scholar] [CrossRef]
- Yin, S.; Shao, Y.; Bao, T.; Zhu, J. Review on Nitrogen Transformation during Microalgae Thermochemical Liquefaction: Recent Advances and Future Perspectives. Energy Fuels 2023, 37, 1525–1544. [Google Scholar] [CrossRef]
- Constantinescu, M.; Bucura, F.; Ionete, E.I.; Spiridon, Ş.I.; Ionete, R.E.; Zaharioiu, A.; Marin, F.; Ion-Ebrasu, D.; Botoran, O.R.; Roman, A. Cattle manure thermochemical conversion to hydrogen-rich syngas, through pyrolysis and gasification. Int. J. Hydrogen Energy 2024, 79, 1058–1070. [Google Scholar] [CrossRef]
- Lu, J.; Li, H.; Zhang, Y.; Liu, Z. Nitrogen Migration and Transformation during Hydrothermal Liquefaction of Livestock Manures. ACS Sustain. Chem. Eng. 2018, 6, 13570–13578. [Google Scholar] [CrossRef]
- Vadlamudi, D.P.; Pecchi, M.; Sudibyo, H.; Tester, J.W. Direct and Two-Stage Hydrothermal Liquefaction of Chicken Manure: Impact of Reaction Parameters on Biocrude Oil Upgradation. ACS Sustain. Chem. Eng. 2024, 12, 4300–4313. [Google Scholar] [CrossRef]
- Liu, Q.; Kong, G.; Zhang, G.; Cao, T.; Wang, K.; Zhang, X.; Han, L. Recent advances in hydrothermal liquefaction of manure wastes into value-added products. Energy Convers. Manag. 2023, 292, 117392. [Google Scholar] [CrossRef]
- USDA. USDA Implementation Framework for a Plan to Enable the Bioeconomy in America: Building a Resilient Biomass Supply A Message from Secretary Vilsack; USDA: Washington, DC, USA, 2022.
- Moreira, G.D.O.; Costa, G.F.; Cavalcante, R.M.; Young, A.F. Process simulation and economic evaluation of pyrolysis and hydrothermal liquefaction as alternatives for the valorization of wood waste from the pulp and paper industry. Energy Convers. Manag. 2025, 325, 119387. [Google Scholar] [CrossRef]
- Wörner, M.; Hornung, U.; Karagöz, S.; Zevaco, T.; Dahmen, N. Focus on hydrochars produced from hydrothermal liquefaction of beech wood, soda lignin and black liquor. Eur. J. Wood Wood Prod. 2025, 83, 61. [Google Scholar] [CrossRef]
- Seehar, T.H.; Toor, S.S.; Shah, A.A.; Nielsen, A.H.; Pedersen, T.H.; Rosendahl, L.A. Catalytic hydrothermal liquefaction of contaminated construction wood waste for biocrude production and investigation of fate of heavy metals. Fuel Process. Technol. 2021, 212, 106621. [Google Scholar] [CrossRef]
- Hamoda, M.F.; Al-Ghusain, I.; AL-Mutairi, N.Z. Sand filtration of wastewater for tertiary treatment and water reuse. Desalination 2004, 164, 203–211. [Google Scholar] [CrossRef]
- Jesse, S.D.; Davidson, P.C. Treatment of Post-Hydrothermal Liquefaction Wastewater (PHWW) for Heavy Metals, Nutrients, and Indicator Pathogens. Water 2019, 11, 854. [Google Scholar] [CrossRef]
- Ibrahim, G.P.S.; Isloor, A.M.; Farnood, R. Fundamentals and basics of reverse osmosis. In Current Trends and Future Developments on (Bio-) Membranes: Reverse and Forward Osmosis: Principles, Applications, Advances; Elsevier: Amsterdam, The Netherlands, 2020; pp. 141–163. [Google Scholar] [CrossRef]
- Tufty, B. Reverse Osmosis Purifies. Sci. News-Lett. 1965, 88, 247. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Lyu, H.; Fang, Y.; Ren, S.; Chen, K.; Luo, G.; Zhang, S.; Chen, J. Monophenols separation from monosaccharides and acids by two-stage nanofiltration and reverse osmosis in hydrothermal liquefaction hydrolysates. J. Memb. Sci. 2016, 504, 141–152. [Google Scholar] [CrossRef]
- Kizza, R.; Eskicioglu, C. Ultrafiltration fractionation of potentially inhibitory substances of hydrothermal liquefaction aqueous phase derived from municipal sludge. Water Res. 2024, 257, 121703. [Google Scholar] [CrossRef]
- Sayegh, A.; Prakash, N.S.; Pedersen, T.H.; Horn, H.; Saravia, F. Treatment of hydrothermal liquefaction wastewater with ultrafiltration and air stripping for oil and particle removal and ammonia recovery. J. Water Process Eng. 2021, 44, 102427. [Google Scholar] [CrossRef]
- Zhang, X.; Scott, J.; Sharma, B.K.; Rajagopalan, N. Advanced treatment of hydrothermal liquefaction wastewater with nanofiltration to recover carboxylic acids. Environ. Sci. 2018, 4, 520–528. [Google Scholar] [CrossRef]
- Erkelens, M.; Ball, A.S.; Lewis, D.M. The application of activated carbon for the treatment and reuse of the aqueous phase derived from the hydrothermal liquefaction of a halophytic Tetraselmis sp. Bioresour. Technol. 2015, 182, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Huang, J.; Chen, H.; He, Z. Reducing nitrogen content in bio-oil from hydrothermal liquefaction of microalgae by using activated carbon-pretreated aqueous phase as the solvent. Biomass Bioenergy 2022, 167, 106638. [Google Scholar] [CrossRef]
- Pastor, L.; Mangin, D.; Barat, R.; Seco, A. A pilot-scale study of struvite precipitation in a stirred tank reactor: Conditions influencing the process. Bioresour. Technol. 2008, 99, 6285–6291. [Google Scholar] [CrossRef]
- Kumar, R.; Pal, P. Assessing the feasibility of N and P recovery by struvite precipitation from nutrient-rich wastewater: A review. Environ. Sci. Pollut. Res. 2015, 22, 17453–17464. [Google Scholar] [CrossRef]
- Shanmugam, S.R.; Adhikari, S.; Shakya, R. Nutrient removal and energy production from aqueous phase of bio-oil generated via hydrothermal liquefaction of algae. Bioresour. Technol. 2017, 230, 43–48. [Google Scholar] [CrossRef]
- Ovsyannikova, E.; Kruse, A.; Becker, G.C. Feedstock-Dependent Phosphate Recovery in a Pilot-Scale Hydrothermal Liquefaction Bio-Crude Production. Energies 2020, 13, 379. [Google Scholar] [CrossRef]
- Bauer, S.K.; Cheng, F.; Colosi, L.M. Evaluating the Impacts of ACP Management on the Energy Performance of Hydrothermal Liquefaction via Nutrient Recovery. Energies 2019, 12, 729. [Google Scholar] [CrossRef]
- Rekhate, C.V.; Srivastava, J.K. Recent advances in ozone-based advanced oxidation processes for treatment of wastewater—A review. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Rodríguez, A.; Rosal, R.; Perdigón-Melón, J.A.; Mezcua, M.; Agüera, A.; Hernando, M.D.; Letón, P.; Fernández-Alba, A.R.; García-Calvo, E. Ozone-Based Technologies in Water and Wastewater Treatment. Handb. Environ. Chem. 2008, 5, 127–175. [Google Scholar] [CrossRef]
- Yang, L.; Si, B.; Martins, M.A.; Watson, J.; Chu, H.; Zhang, Y.; Tan, X.; Zhou, X.; Zhang, Y. Improve the biodegradability of post-hydrothermal liquefaction wastewater with ozone: Conversion of phenols and N-heterocyclic compounds. Water Sci. Technol. 2018, 2017, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Si, B.; Tan, X.; Chu, H.; Zhou, X.; Zhang, Y.; Zhang, Y.; Zhao, F. Integrated anaerobic digestion and algae cultivation for energy recovery and nutrient supply from post-hydrothermal liquefaction wastewater. Bioresour. Technol. 2018, 266, 349–356. [Google Scholar] [CrossRef]
- Si, B.; Yang, L.; Zhou, X.; Watson, J.; Tommaso, G.; Chen, W.T.; Liao, Q.; Duan, N.; Liu, Z.; Zhang, Y. Anaerobic conversion of the hydrothermal liquefaction aqueous phase: Fate of organics and intensification with granule activated carbon/ozone pretreatment. Green Chem. 2019, 21, 1305–1318. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Panizza, M. Electrochemical oxidation of organic pollutants for wastewater treatment. Curr. Opin. Electrochem. 2018, 11, 62–71. [Google Scholar] [CrossRef]
- Matayeva, A.; Biller, P. Hydrothermal liquefaction aqueous phase treatment and hydrogen production using electro-oxidation. Energy Convers. Manag. 2021, 244, 114462. [Google Scholar] [CrossRef]
- Ciarlini, J.; Alves, L.; Rajarathnam, G.P.; Haynes, B.S.; Montoya, A. Electrochemical oxidation of nitrogen-rich post-hydrothermal liquefaction wastewater. Algal Res. 2020, 48, 101919. [Google Scholar] [CrossRef]
- Cantero, B.C.B.; Zhang, Y.; Davidson, P.C. Electrolysis of HTL-AP for nutrient recovery by converting cyclic nitrogen to nitrate-N fertilizer. Environ. Pollut. 2024, 363, 125069. [Google Scholar] [CrossRef]
- Uddin, M.M.; Wright, M.M. Anaerobic digestion fundamentals, challenges, and technological advances. Phys. Sci. Rev. 2023, 8, 2819–2837. [Google Scholar] [CrossRef]
- Tatla, H.K.; Ismail, S.; Khan, M.A.; Dhar, B.R.; Gupta, R. Coupling hydrothermal liquefaction and anaerobic digestion for waste biomass valorization: A review in context of circular economy. Chemosphere 2024, 361, 142419. [Google Scholar] [CrossRef]
- Swetha, A.; ShriVigneshwar, S.; Gopinath, K.P.; Sivaramakrishnan, R.; Shanmuganathan, R.; Arun, J. Review on hydrothermal liquefaction aqueous phase as a valuable resource for biofuels, bio-hydrogen and valuable bio-chemicals recovery. Chemosphere 2021, 283, 131248. [Google Scholar] [CrossRef]
- Sankaran, S.; Khanal, S.K.; Jasti, N.; Jin, B.; Pometto, A.L.; Van Leeuwen, J.H. Use of Filamentous Fungi for Wastewater Treatment and Production of High Value Fungal Byproducts: A Review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 400–449. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Umeda, I.; Wang, Z.; Kumar, S.; Zheng, Y. Harnessing filamentous fungi and fungal-bacterial co-culture for biological treatment and valorization of hydrothermal liquefaction aqueous phase from corn stover. Bioresour. Technol. 2024, 409, 131240. [Google Scholar] [CrossRef]
- Leme, V.F.C.; Lopez, K.; Costa, T.; Conerty, B.; Leonelli, L.B.; Zhang, Y.; Davidson, P.C. Hydrothermal liquefaction aqueous phase mycoremediation to increase inorganic nitrogen availability. Heliyon 2024, 10, e31992. [Google Scholar] [CrossRef]
- Lopez, K.; Leme, V.F.C.; Warzecha, M.; Davidson, P.C. Wastewater Nutrient Recovery via Fungal and Nitrifying Bacteria Treatment. Agriculture 2024, 14, 580. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Liu, Y.; Li, W. A review of partial nitrification in biological nitrogen removal processes: From development to application. Biodegradation 2021, 32, 229–249. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef]
- Rout, P.R.; Goel, M.; Mohanty, A.; Pandey, D.S.; Halder, N.; Mukherjee, S.; Bhatia, S.K.; Sahoo, N.K.; Varjani, S. Recent Advancements in Microalgal Mediated Valorisation of Wastewater from Hydrothermal Liquefaction of Biomass. BioEnergy Res. 2022, 16, 45–60. [Google Scholar] [CrossRef]
- Barreiro, D.L.; Bauer, M.; Hornung, U.; Posten, C.; Kruse, A.; Prins, W. Cultivation of microalgae with recovered nutrients after hydrothermal liquefaction. Algal Res. 2015, 9, 99–106. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).