Recovery of Effective Acid from Waste Generated in the Anodic Oxidation Polishing Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Properties of Raw Water
2.2. Acid Recovery from Chemical Polishing Waste Tank Solution
2.2.1. Extraction Capability of Single Solvents
2.2.2. Extraction Capability of Binary Solvent Systems
2.3. Acid Recovery from Chemical Polishing Cleaning Wastewater
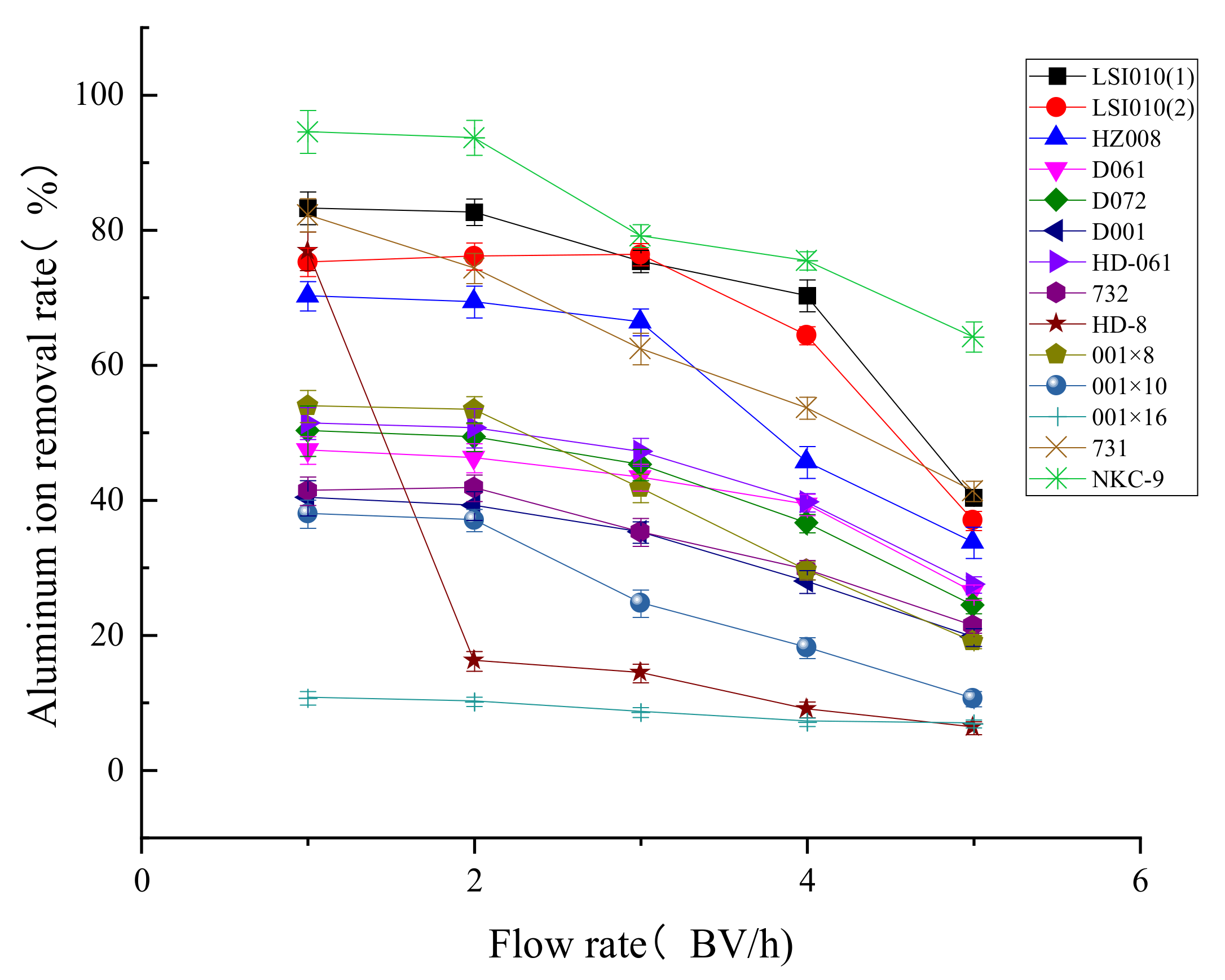
2.3.1. Dynamic Adsorption Capacity
2.3.2. Dynamic Desorption Capacity
2.3.3. Recovery of Desorbed Acid Solution
3. Results and Discussion
3.1. Acid Recovery from Chemical Polishing Waste Tank Solutions
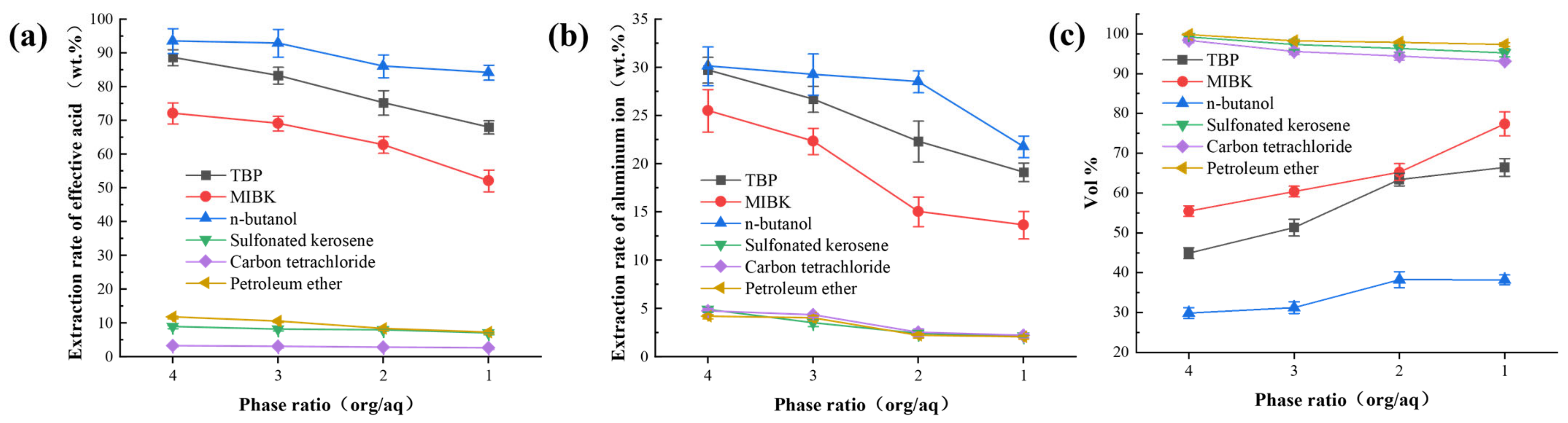
3.1.1. Extraction Capability of Single Solvents
3.1.2. Extraction Capability of Binary Solvent Systems
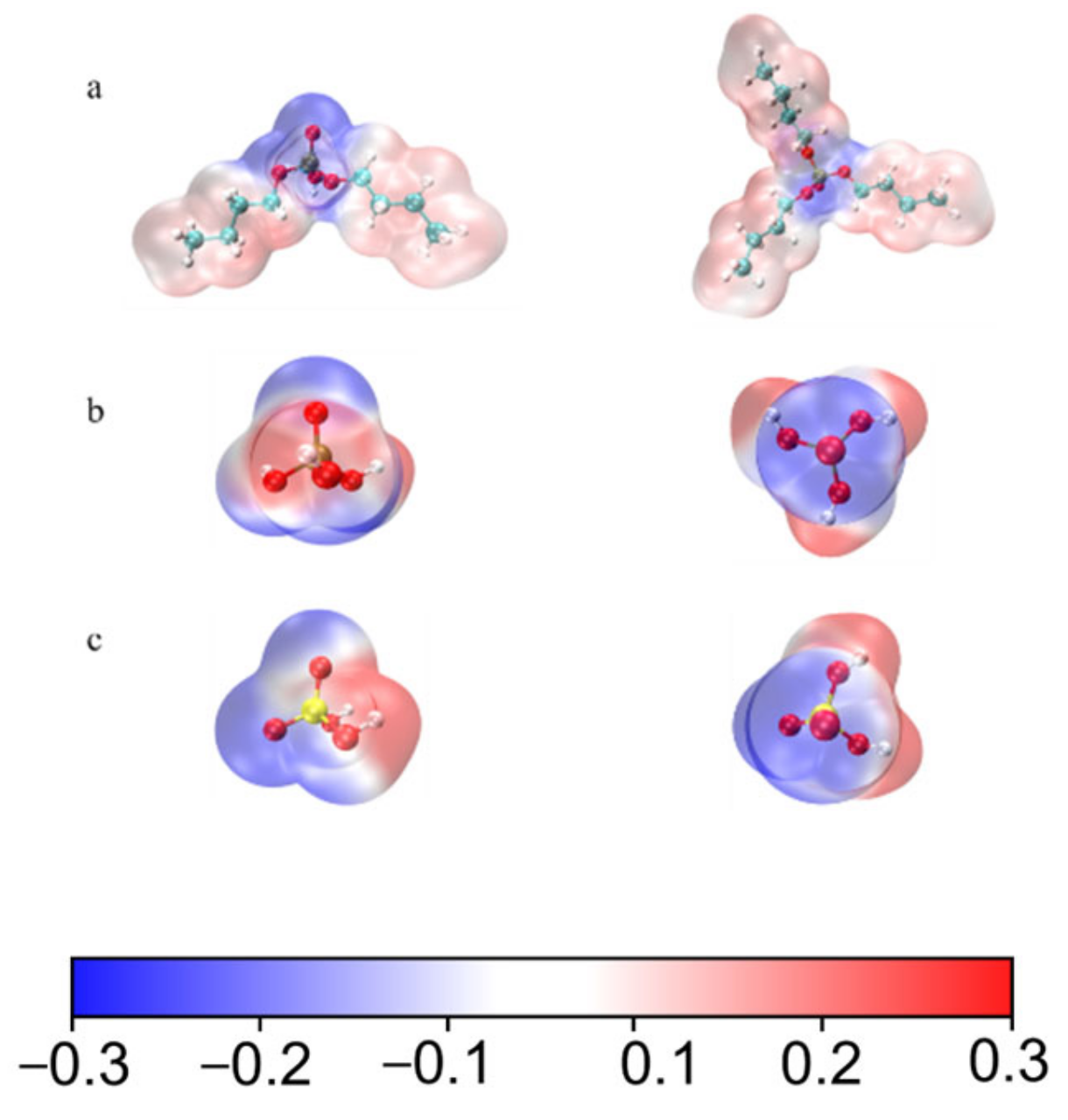
3.1.3. Electrostatic Potential and IRI Analysis
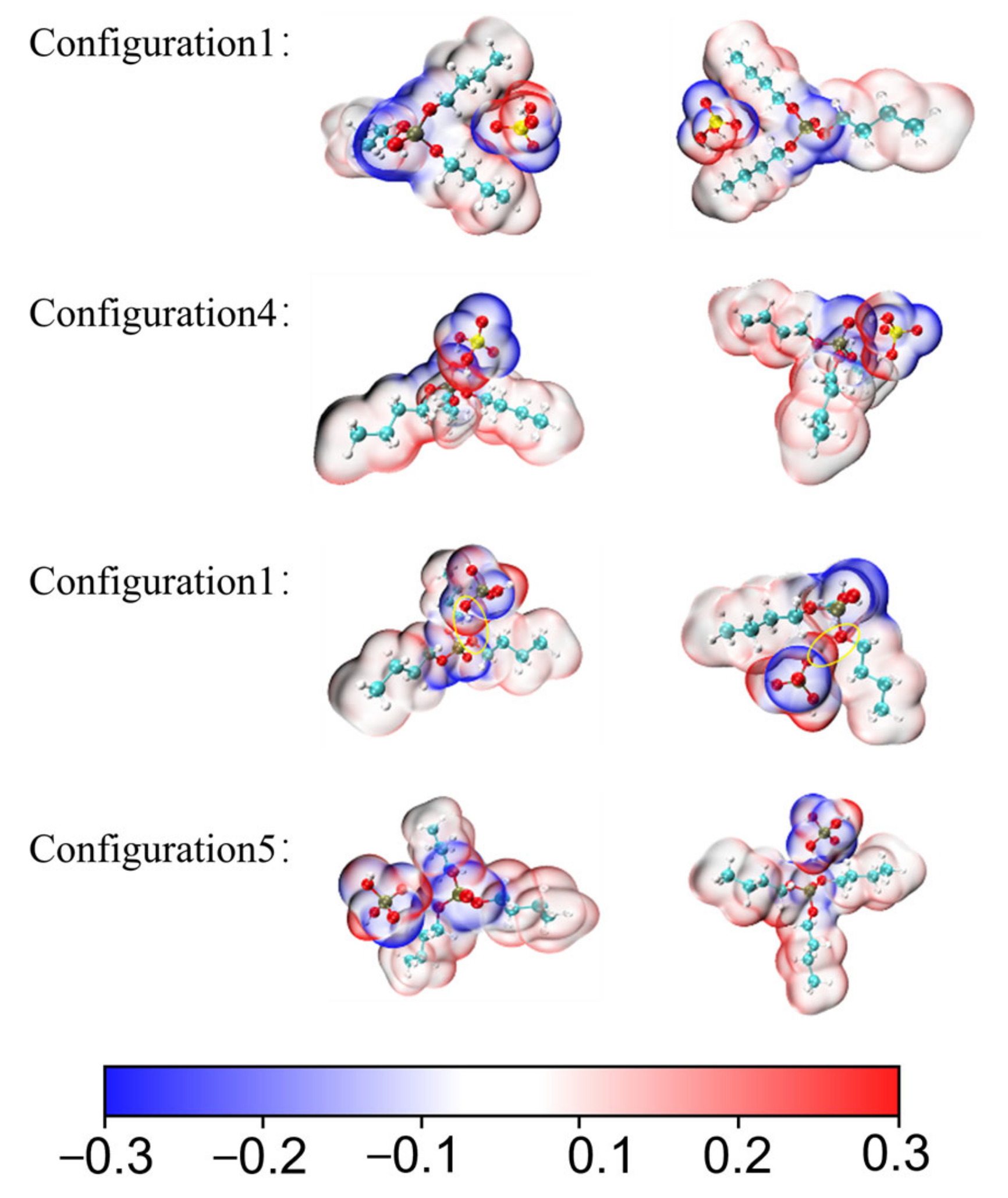
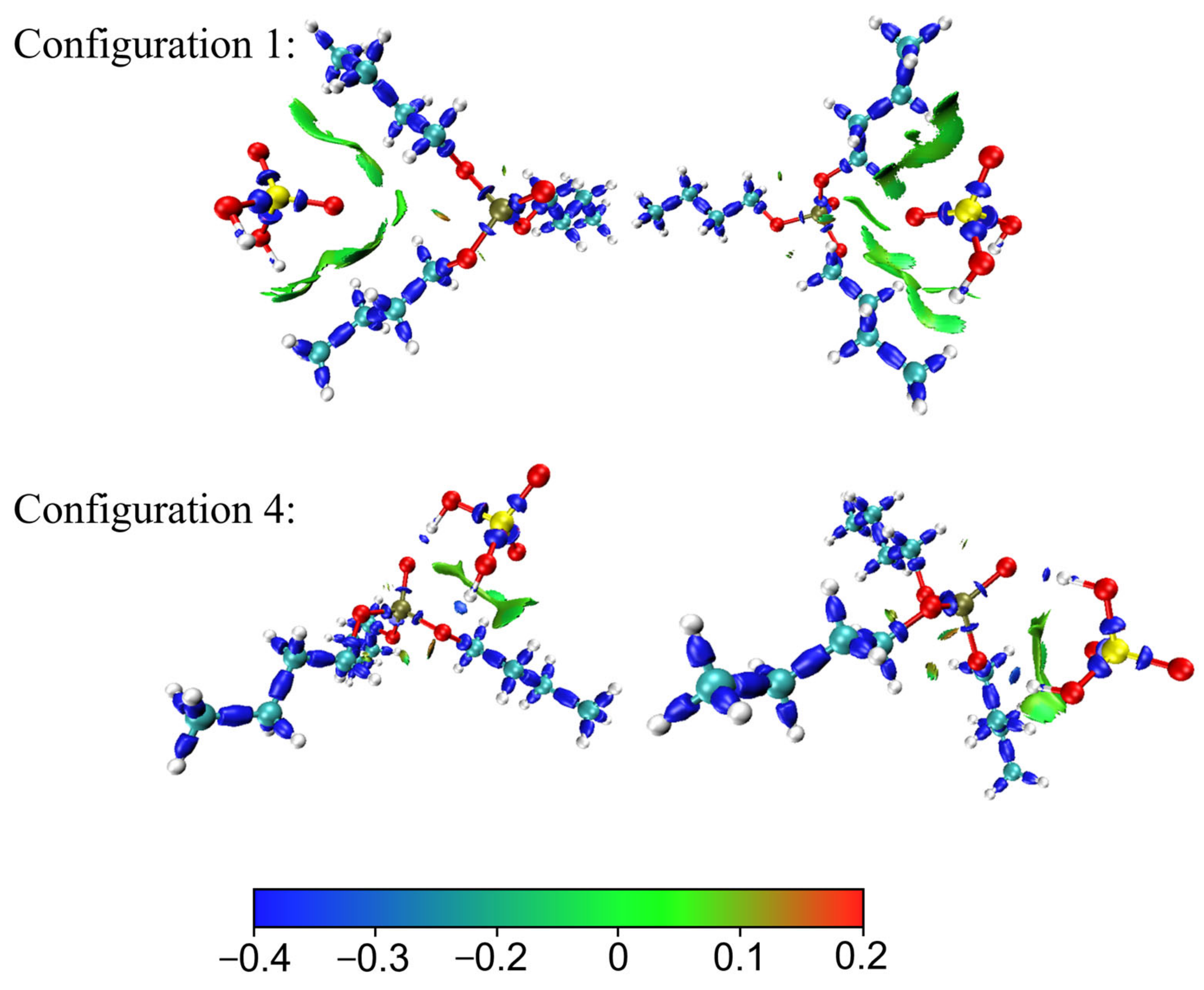

3.2. Acid Recovery from Chemical Polishing Cleaning Wastewater
3.2.1. Dynamic Adsorption Capacity
3.2.2. Dynamic Adsorption and Desorption Capacity
3.2.3. Recovery of Desorption Solution
3.3. Limitations of the Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y. Research on a New Chemical Polishing Process for Aluminum Products. Master’s Thesis, Shanxi University, Taiyuan, Shanxi, 2011. [Google Scholar]
- Chen, Z. Development Status of Chromic Acid Free Electrochemical Polishing Process for Aluminum Products in China. Mater. Prot. 2001, 34, 2. [Google Scholar]
- Lian, J.L. Chemical-mechanical polishing liquid used for polishing of aluminum alloy surface, comprises phosphoric acid, sulfuric acid, corrosion inhibitor, and thickener. China Patent CN103572296A, 12 February 2014. [Google Scholar]
- Liu, C.W.; Dai, B.T.; Yeh, C.F. Post cleaning of chemical mechanical polishing process. Appl. Surf. Sci. 1996, 92, 176–179. [Google Scholar] [CrossRef]
- Zhong, Z.W. Recent developments and applications of chemical mechanical polishing. Int. J. Adv. Manuf. Technol. 2020, 109, 1419–1430. [Google Scholar] [CrossRef]
- Mu, J.; Sun, T.; Leung, C.L.A.; Oliveira, J.P.; Wu, Y.; Wang, H.; Wang, H. Application of electrochemical polishing in surface treatment of additively manufactured structures: A review. Prog. Mater. Sci. 2023, 136, 101109. [Google Scholar] [CrossRef]
- Regel-Rosocka, M. A review on methods of regeneration of spent pickling solutions from steel processing. J. Hazard. Mater. 2010, 177, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.L.; Wang, S.F.; Qi, Z.Q.; Zhang, Y.S.; Wang, R.; Yuan, L.J. A deep treatment process for chemical polishing wastewater towards resource recovery: Optimization and performance. Sep. Purif. Technol. 2025, 358, 130285. [Google Scholar] [CrossRef]
- Huang, F.; Wu, B.; Chen, K.; Zhou, X. Ca4AlSiSO4F(13)·12H2O on the purification of wet process phosphoric acid. Chem. Miner. Process. 2019, 48, 46–51. [Google Scholar]
- Lian, P.; Meng, W.; Tang, D. Ion exchange technology for purifying magnesium ions in wet process phosphoric acid. J. Guizhou Univ. Technol. Nat. Sci. Ed. 2008, 37, 4. [Google Scholar]
- Elleuch, M.; Amor, M.B.; Pourcelly, G. Phosphoric acid purification by a membrane process: Electrodeionization on ion-exchange textiles. Sep. Purif. Technol. 2006, 51, 285–290. [Google Scholar] [CrossRef]
- Zheng, X. Application of Membrane Separation Technology in Phosphoric Acid Purification. Phosphate Compd. Fertil. 2020, 35, 22–24. [Google Scholar]
- Bai, J.; Zhu, J.; Ye, S.; Xie, C.; Xin, Y. Experimental Study on Purification of Wet Process Phosphoric Acid by Membrane Dispersion Extraction. Henan Chem. Ind. 2013, 30, 38–40. [Google Scholar]
- Li, X.; Li, H.; Pan, L.; Deng, Y.; Li, H. Research on the application of hydrofluoric acid in the purification of wet process phosphoric acid. J. Guangxi Univ. (Nat. Sci. Ed.) 2009, 34, 53–56. [Google Scholar]
- Yang, J. Development of Wet Process Phosphoric Acid Purification Technology by Solvent Extraction. Master’s Thesis, China University of Petroleum (Beijing): Beijing, China, 2020. [Google Scholar]
- Huang, M.; Yang, S.; Li, J.; Zhong, B. Study on technology for purification of WPA by solvent extraction process . Phosphate Compd. Fertilizer. 2004, 19, 9–11. [Google Scholar]
- Ni, S.; Fang, X.; Wang, Z.; Chen, H. Purification of Wet Process Phosphoric Acid by Composite Solvent Extraction. Yunnan Chem. Ind. 2017, 44, 4. [Google Scholar]
- Lum, K.H.; Stevens, G.W.; Kentish, S.E. Development of a process for the recovery of zinc sulphate from hot-dip galvanizing spent pickling liquor via two solvent extraction steps. Hydrometallurgy 2014, 142, 108–115. [Google Scholar] [CrossRef]
- Berends, A.M.; Witkamp, G.J.; Van Rosmalen, G.M. Extraction of aluminum from a pickling bath with supported liquid membrane extraction. Sep. Sci. Technol. 1999, 34, 1521–1543. [Google Scholar] [CrossRef]
- Ichlas, Z.T.; Ibana, D.C. Process development for the direct solvent extraction of nickel and cobalt from nitrate solution: Aluminum, cobalt, and nickel separation using Cyanex 272. Int. J. Miner. Metall. Mater. 2017, 24, 37–46. [Google Scholar] [CrossRef]
- Balinski, A.; Kelly, N.; Helbig, T.; Meskers, C.; Reuter, M.A. Separation of aluminum and iron from lanthanum-a comparative study of solvent extraction and hydrolysis-precipitation. Minerals 2020, 10, 556. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Y.; Zhang, T.; Lv, L.; Zheng, D.; Zhong, B.; Tang, S. Separation of H3PO4 from HCl-wet-processing phosphate rocks leach liquor by TBP: Extraction equilibria and mechanism study. Sep. Purif. Technol. 2020, 249, 117156. [Google Scholar] [CrossRef]
- Zhou, Z.; Qin, W.; Fei, W. Extraction equilibria of lithium with tributyl phosphate in three diluents. J. Chem. Eng. Data 2011, 56, 3518–3522. [Google Scholar] [CrossRef]
- Cao, J.; Liu, Y.; Zhao, F. Novel tributyl phosphate-based hydrophobic deep eutectic solvent: Application in simultaneous liquid–liquid microextraction of parabens and their metabolite in surface water samples. Green Chem. 2022, 24, 8005–8013. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Yang, X.; Hu, C.; Yang, L. A non-aqueous phase extraction system using tributyl phosphate for H3PO4 separation from wet-process superphosphoric acid: Extraction equilibrium and mechanism. Korean J. Chem. Eng. 2022, 39, 1659–1672. [Google Scholar] [CrossRef]
- Feng, W.; Gao, K.; Cui, X.; Zhang, H. Study on the alkaline hydrolysis of tributyl phosphate. J. Radioanal. Nucl. Chem. 2024, 333, 1725–1732. [Google Scholar] [CrossRef]
- Hua, J.; He, J.; Pei, H.; Du, J.; Ma, X.; Li, J. A remarkable improved Li+/Mg2+ selectivity and Li+ recovery simultaneously by adding crown ether to tributyl phosphate-ionic liquid extraction system as co-extractant. Sep. Purif. Technol. 2024, 335, 126162. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Duan, W.; Du, C.; Tian, S.; Ren, Z.; Zhou, Z. Selective extraction of lithium ions from salt lake brines using a tributyl phosphate-sodium tetraphenyl boron-phenethyl isobutyrate system. Desalination 2023, 555, 116543. [Google Scholar] [CrossRef]
- Shakib, B.; Lee, J.Y.; Jyothi, R.K.; Kang, H.N.; Petranikova, M. Sustainable separation of molybdenum from mixed mineral acids generated as semiconductor industry waste streams using tributyl phosphate (TBP) by effects of hybrid machine learning models. J. Environ. Manag. 2024, 370, 122865. [Google Scholar] [CrossRef] [PubMed]
- Babain, V.; Alyapyshev, M.; Voronaev, I.; Tkachenko, L.; Kenf, E. Extraction of actinides with tributyl phosphate in carbonates of fluorinated alcohols. Solvent Extr. Ion Exch. 2021, 39, 255–270. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Chen, Z.; Zhang, Z.; Cheng, X.; Xu, C.; Chen, J.; Wang, J.; Hu, H.; Sun, T. Structure and aggregation behavior of pertechnetate/perrhenate in organic phase in the extraction by tributyl phosphate. J. Radioanal. Nucl. Chem. 2023, 332, 1723–1732. [Google Scholar] [CrossRef]
- Mouhib, M.; Kaddami, M.; Counioux, J.J.; Goutaudier, C. Three-liquid-phase behavior in the quaternary systems of water+ phosphoric acid+ diisopropyl ether+ tributyl phosphate/methyl isobutyl ketone at 298.2 K. J. Mol. Liq. 2024, 413, 126061. [Google Scholar] [CrossRef]
- Karim, H.; Castel, C.; Lelias, A.; Magnaldo, A.; Sarrat, P. Kinetic study of uranium (VI) extraction with tributyl-phosphate in a stratified flow microchannel. Sep. Purif. Technol. 2023, 314, 123489. [Google Scholar] [CrossRef]
- Blundell, R.J.; Lambert, H.; Holdsworth, A.F.; George, K.; Winterburn, J.; Livens, F.R.; Sharrad, C.A. Physicochemical properties of extraction solvents for the advanced recycling of spent nuclear fuel. Prog. Nucl. Energy 2024, 174, 105284. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, P.; Zhang, T.; Zhu, D.; Liu, Z. A novel solvent extraction system to recover germanium from H2SO4 leaching liquor of secondary zinc oxide: Extraction behavior and mechanism. J. Clean. Prod. 2023, 383, 13539. [Google Scholar] [CrossRef]
- Rao, M.D.; Singh, K.K.; Morrison, C.A.; Love, J.B. Recycling copper and gold from e-waste by a two-stage leaching and solvent extraction process. Sep. Purif. Technol. 2021, 263, 118400. [Google Scholar] [CrossRef]
- Chen, H.; Li, S.; Li, Y.; Du, Z.; Bin, L.; Li, W.; Tang, B. Cyclic utilization of waste aluminum polishing solution by an efficient co-extraction system: Influence factors and extraction mechanism. Process Saf. Environ. Prot. 2023, 178, 663–674. [Google Scholar] [CrossRef]
- Geow, C.H.; Tan, M.C.; Yeap, S.P.; Chin, N.L. A review on extraction techniques and its future applications in industry. Eur. J. Lipid Sci. Technol. 2021, 123, 2000302. [Google Scholar] [CrossRef]
- Raynie, D.E. Modern extraction techniques. Anal. Chem. 2006, 78, 3997–4004. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Q.; Fang, H.H. Applications of porous resin sorbents in industrial wastewater treatment and resource recovery. Crit. Rev. Environ. Sci. Technol. 2003, 33, 363–389. [Google Scholar] [CrossRef]



| Processing Methods | Key Achievements | Drawbacks |
|---|---|---|
| Chemical precipitation | Mature and reliable process, shorter process, wide range of applications | Low processing efficiency, high sludge volume, needs to be used with other processes |
| Ion exchange | High selectivity, good treatment results, high automation potential | High pre-treatment requirements, limited applicability, operating cost issues |
| Membrane separation | Acid reuse potential, high pollutant removal, low chemical consumption | Serious membrane contamination, poor adaptability to high-concentration waste liquids, concentrated liquid treatment problems |
| Regeneration | Efficient resource recovery, reduced hazardous waste disposal, and significant economic benefits | Stringent pre-treatment requirements, high technical complexity, recovery purity limitations |
| Solvent extraction | High recycling efficiency, no secondary pollution to the environment, adaptable | High technical requirements, harsh requirements for the extractant, high operating costs, less industrialised domestic applications |
| Chemical Polishing of Waste Tank Liquid | |
|---|---|
| Density | 1.6037 g/mL |
| Total acid | 62.96 wt.% |
| Aluminium ion | 31,281 mg/L |
| Mass ratio of phosphoric acid to sulfuric acid in mixed acid | 4:1 |
| Chemical Polishing Cleaning Wastewater | |
| Density | 1.2097 g/mL |
| Total acid | 28.41 wt.% |
| Aluminium ion | 10,514 mg/L |
| Mass ratio of phosphoric acid to sulfuric acid in mixed acid | 4:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Cui, K.; Wu, W. Recovery of Effective Acid from Waste Generated in the Anodic Oxidation Polishing Process. Water 2025, 17, 1322. https://doi.org/10.3390/w17091322
Li H, Cui K, Wu W. Recovery of Effective Acid from Waste Generated in the Anodic Oxidation Polishing Process. Water. 2025; 17(9):1322. https://doi.org/10.3390/w17091322
Chicago/Turabian StyleLi, Haiyang, Kangping Cui, and Wenming Wu. 2025. "Recovery of Effective Acid from Waste Generated in the Anodic Oxidation Polishing Process" Water 17, no. 9: 1322. https://doi.org/10.3390/w17091322
APA StyleLi, H., Cui, K., & Wu, W. (2025). Recovery of Effective Acid from Waste Generated in the Anodic Oxidation Polishing Process. Water, 17(9), 1322. https://doi.org/10.3390/w17091322





