N-Doped Modified MoS2 for Piezoelectric–Photocatalytic Removal of Tetracycline: Simultaneous Improvement of Photocatalytic and Piezoelectric Properties
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Piezo-Photocatalysts
2.3. Characterization and Analysis Methods
2.4. Evaluation of the Synergistic Degradation Performance
3. Results and Discussion
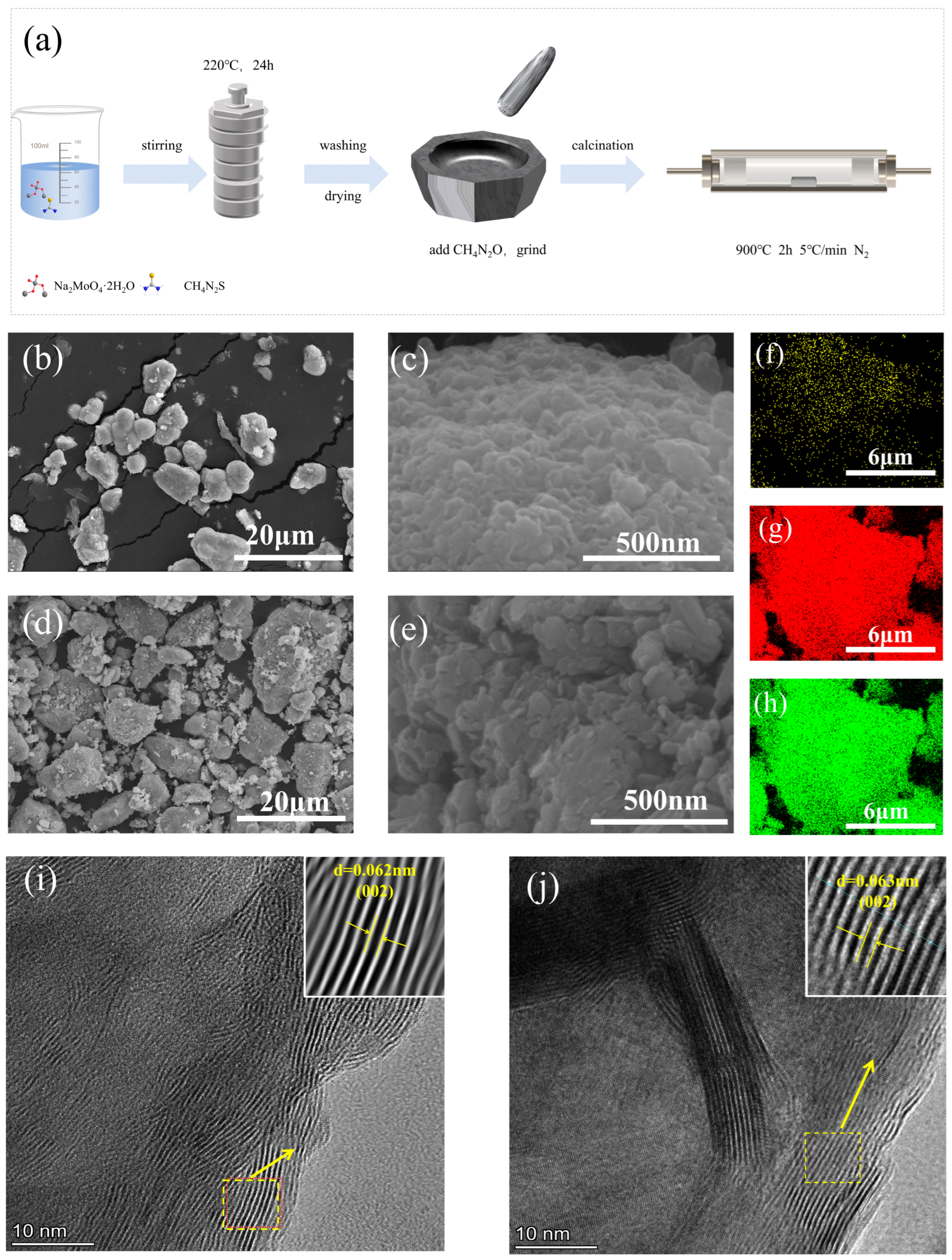
3.1. Characterizations of Catalysts
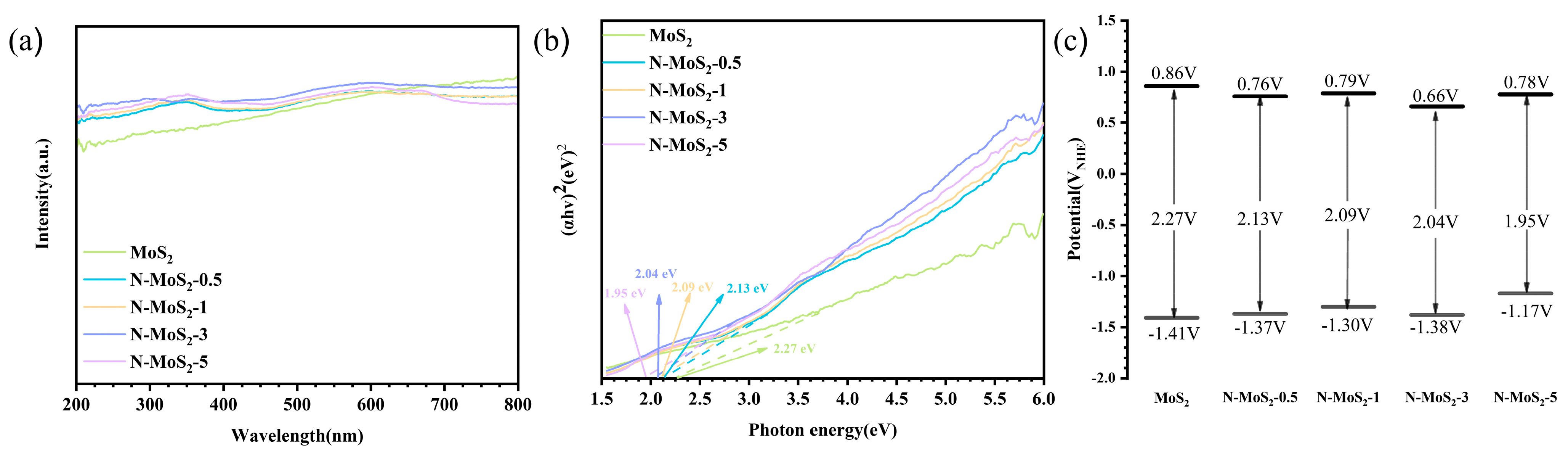
3.2. Photocatalytic Activity
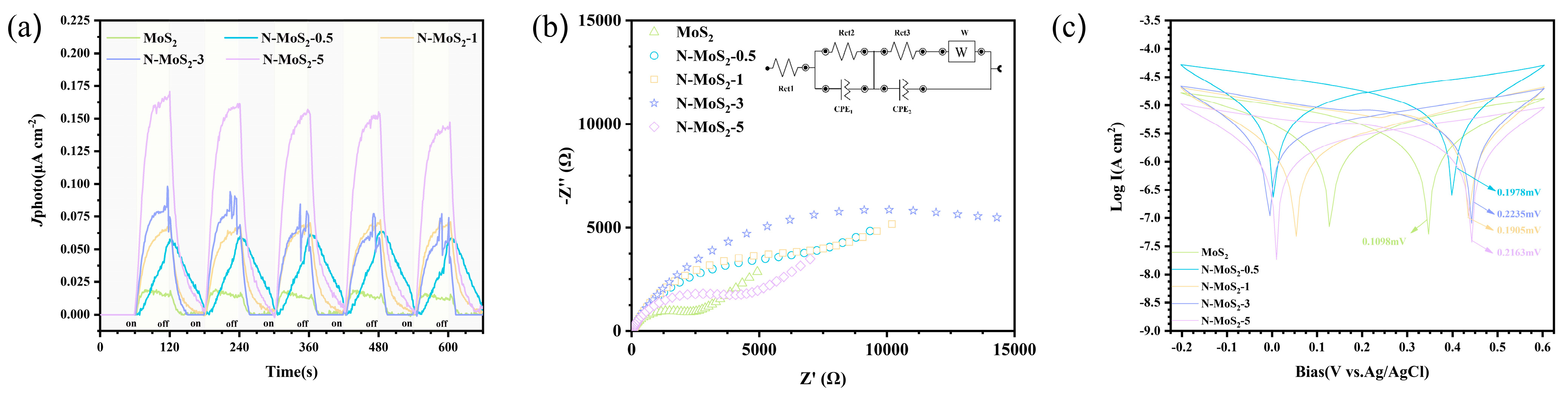
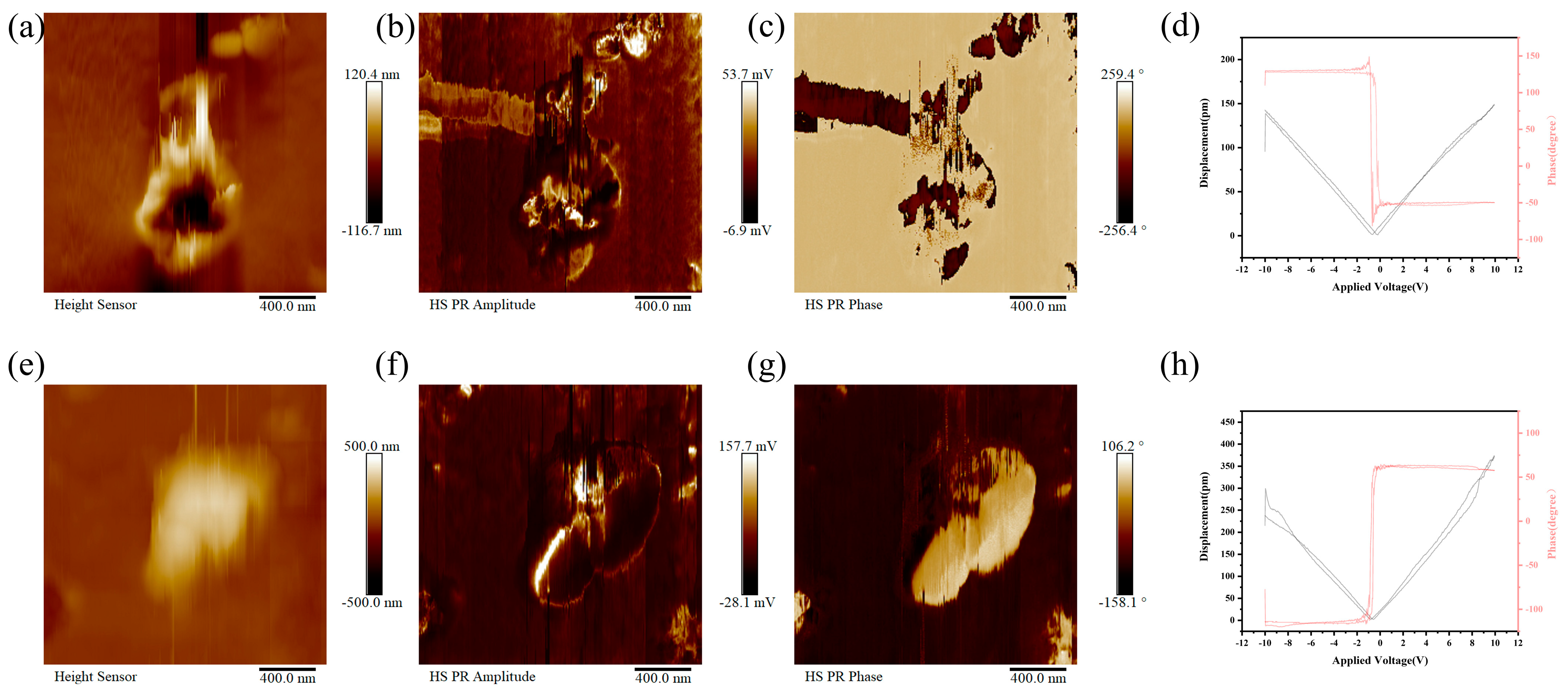
3.3. Piezoelectric Performances
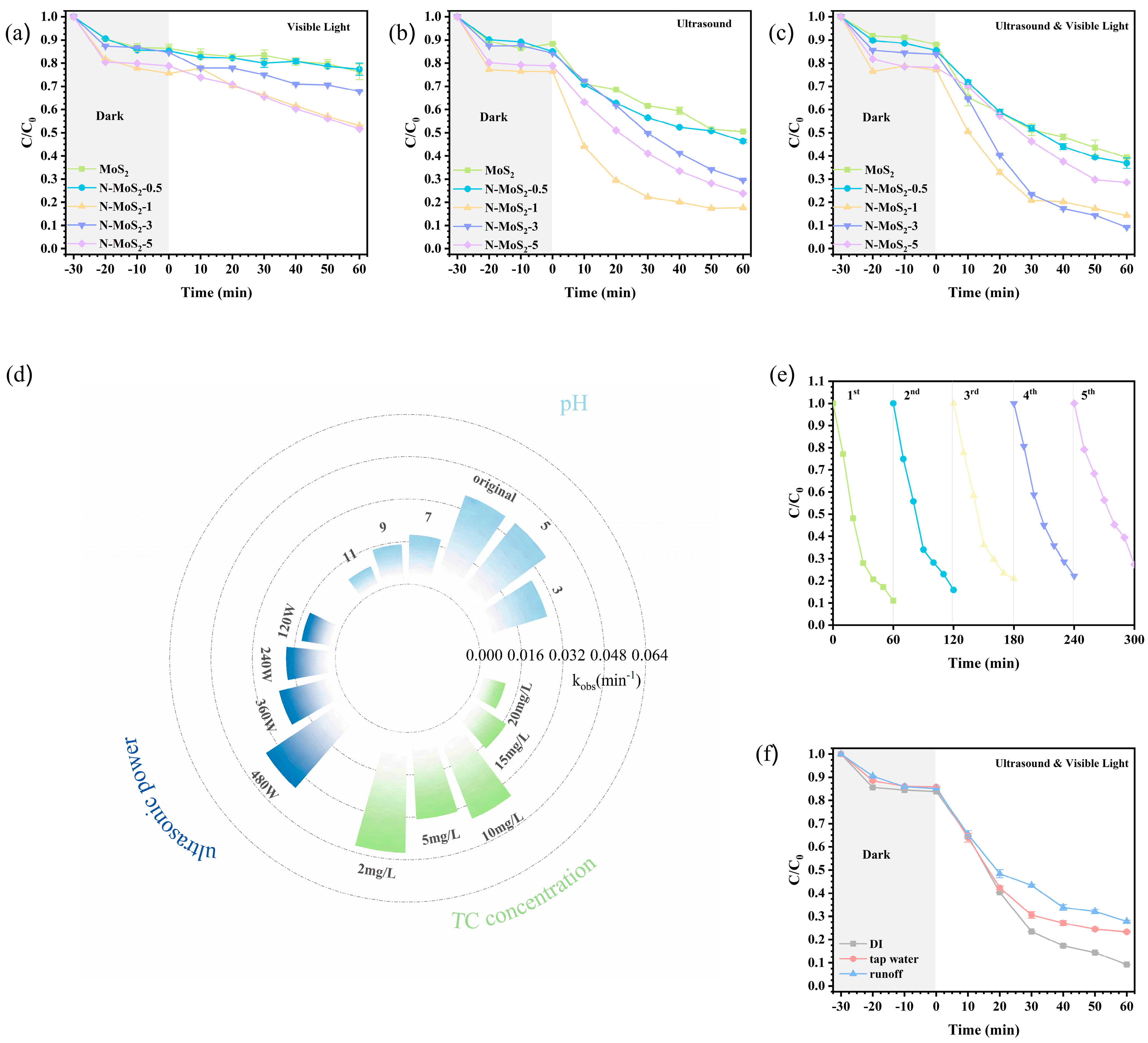
3.4. Performance Evaluation of Piezoelectric–Photocatalytic Degradation of Tetracycline
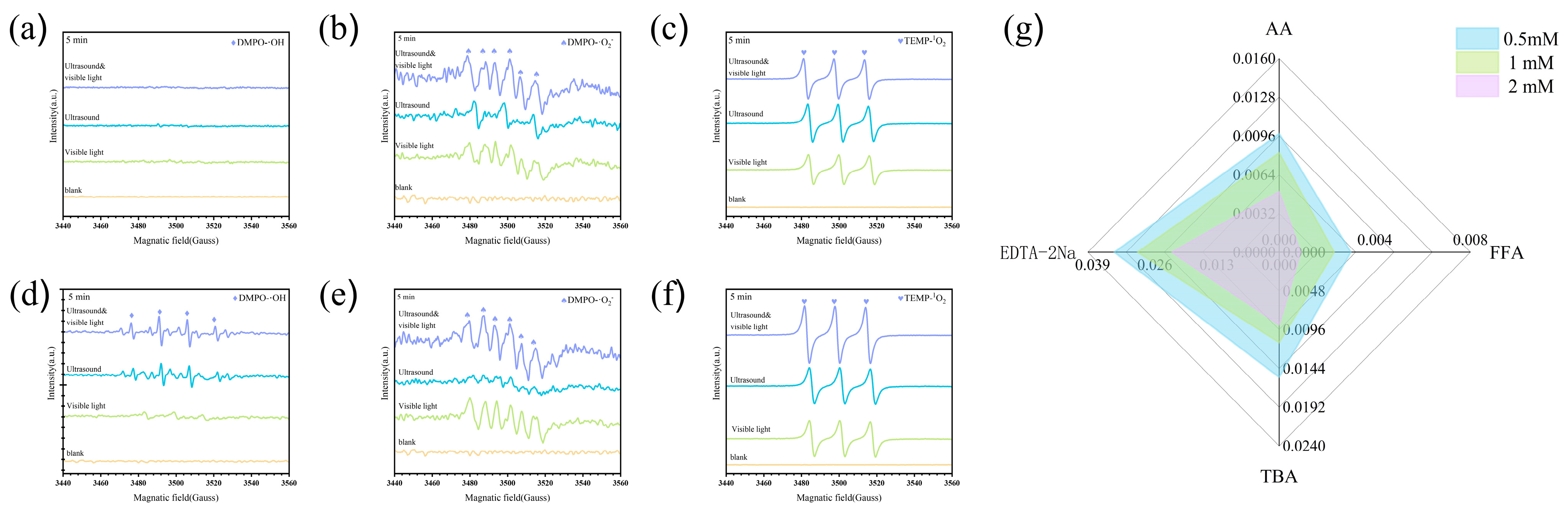
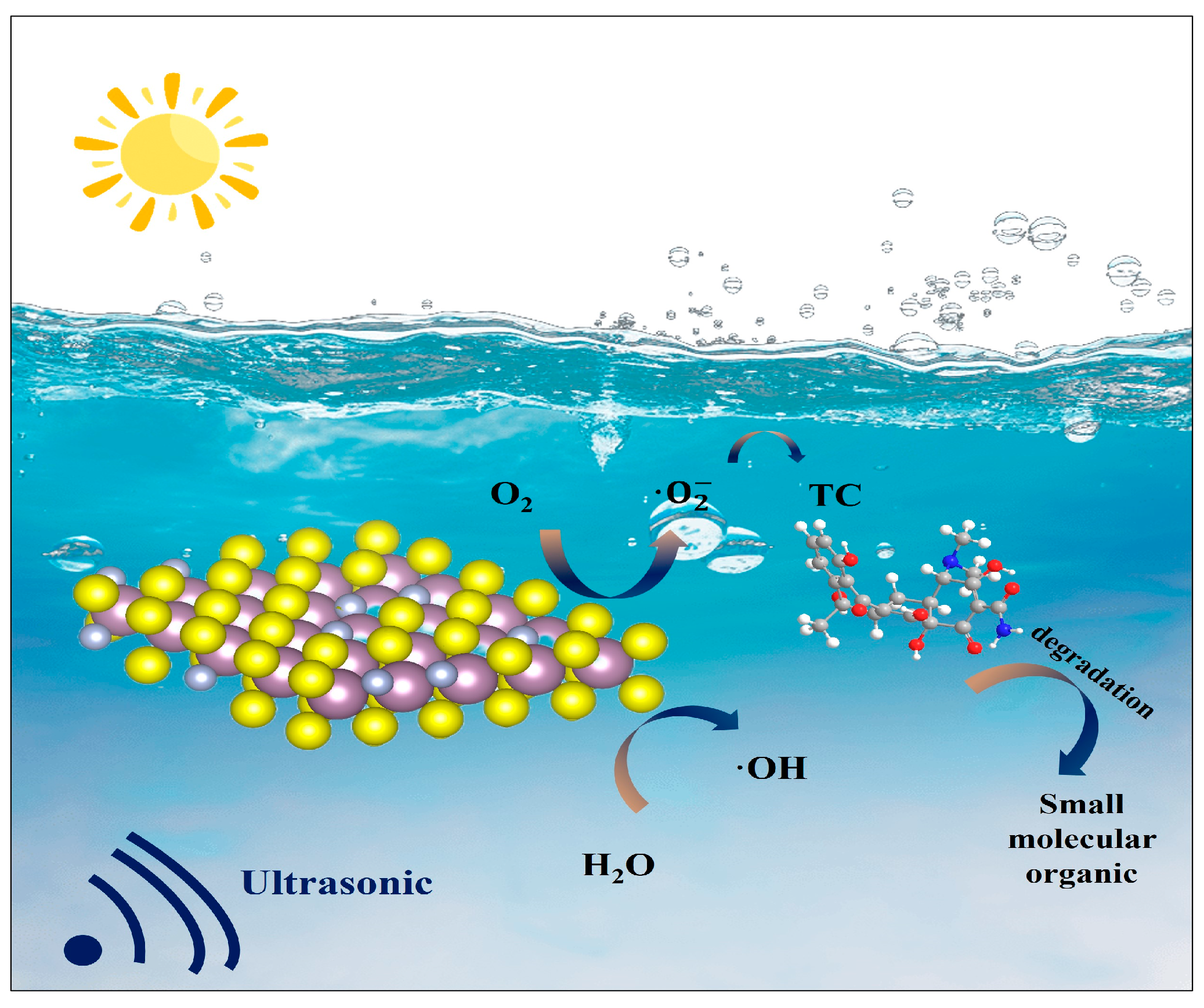
3.5. Piezocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, W.; Qi, Y.; Han, Y.; Ge, J.; Dong, Y.; Wang, J.; Yi, Y.; Volmer, D.A.; Li, S.L.; Fu, P. Seasonal variation and dissolved organic matter influence on the distribution, transformation, and environmental risk of pharmaceuticals and personal care products in coastal zone: A case study of Tianjin, China. Water Res. 2024, 249, 120881. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Nisar, B.; Mohd Yatoo, A.; Sehar, N.; Tomar, R.; Tariq, L.; Ali, S.; Ali, A.; Mudasir Rashid, S.; Bilal Ahmad, S.; et al. After effects of Pharmaceuticals and Personal Care Products (PPCPs) on the biosphere and their counteractive ways. Sep. Purif. Technol. 2024, 342, 126921. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, X.M.; Lai, R.W.S.; Liu, Y.; Liu, S.; Jin, X.; Zhou, G.J. Decoding Adverse Effects of Organic Contaminants in the Aquatic Environment: A Meta-analysis of Species Sensitivity, Hazard Prediction, and Ecological Risk Assessment. Environ. Sci. Technol. 2024, 58, 18122–18132. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.C.; Yi, X.H.; Chu, H.Y.; Wang, C.C.; Gao, Y.; Wang, F.; Wang, F.X.; Wang, P.; Wang, J.F. Floating MIL-88A(Fe)@expanded perlites catalyst for continuous photo-Fenton degradation toward tetracyclines under artificial light and real solar light. J. Hazard. Mater. 2024, 472, 134420. [Google Scholar] [CrossRef]
- Su, Z.; Wang, K.; Yang, F.; Zhuang, T. Antibiotic pollution of the Yellow River in China and its relationship with dissolved organic matter: Distribution and Source identification. Water Res. 2023, 235, 119867. [Google Scholar] [CrossRef]
- Zhu, Y.-M.; Chen, Y.; Lu, H.; Jin, K.; Lin, Y.; Ren, H.; Xu, K. Simultaneous efficient removal of tetracycline and mitigation of antibiotic resistance genes enrichment by a modified activated sludge process with static magnetic field. Water Res. 2024, 262, 122107. [Google Scholar] [CrossRef]
- Wang, G.; Huang, D.; Cheng, M.; Du, L.; Chen, S.; Zhou, W.; Li, R.; Li, S.; Huang, H.; Xu, W.; et al. The Surface Confinement of FeO Assists in the Generation of Singlet Oxygen and High-Valent Metal-Oxo Species for Enhanced Fenton-like Catalysis. Small 2024, 20, e2401970. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Wang, J.; Li, S. Tetracycline antibiotics in agricultural soil: Dissipation kinetics, transformation pathways, and structure-related toxicity. Sci. Total Environ. 2024, 949, 175126. [Google Scholar] [CrossRef]
- Xiang, W.; Liao, Y.; Cui, J.; Fang, Y.; Jiao, B.; Su, X. Room-temperature synthesis of Fe/Cu-BTC for effective removal of tetracycline antibiotic from aquatic environments. Inorg. Chem. Commun. 2024, 167, 112728. [Google Scholar] [CrossRef]
- Shen, Y.; Kang, J.; Guo, L.; Qiu, F.; Fan, Y.; Zhang, S. Recent progress on the application of MOFs and their derivatives in adsorbing emerging contaminants. Sep. Purif. Technol. 2024, 350, 127955. [Google Scholar] [CrossRef]
- Singh, V.; Suthar, S. Occurrence, seasonal variation, mass loading and fate of pharmaceuticals and personal care products (PPCPs) in sewage treatment plants in cities of upper Ganges bank, India. J. Water Process Eng. 2021, 44, 102399. [Google Scholar] [CrossRef]
- Zhou, X.; Shen, B.; Lyubartsev, A.; Zhai, J.; Hedin, N. Semiconducting piezoelectric heterostructures for piezo- and piezophotocatalysis. Nano Energy 2022, 96, 107141. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, Y.; Cai, C.; Nie, C.; Peng, H.; Ao, Z. Piezocatalysis for water treatment: Mechanisms, recent advances, and future prospects. Environ. Sci. Ecotechnol. 2025, 23, 100495. [Google Scholar] [CrossRef]
- Jia, P.; Li, J.; Huang, H. Piezocatalysts and Piezo-Photocatalysts: From Material Design to Diverse Applications. Adv. Funct. Mater. 2024, 34, 2407309. [Google Scholar] [CrossRef]
- Liu, J.; Jin, L.; Dai, Z.; Ji, Y.; Qian, J.; Lu, Y.; Wang, J.; Shen, B.; Li, Y.; Xu, R.; et al. Single Atom Embedding Enhanced Macroscopic Polarization in Carbon Nitride Nanosheets for pH-Universal Piezo-Photocatalytic Nitrate Reduction over a Wide Concentration Range. ACS Catal. 2025, 15, 4025–4038. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Wang, J.; Tian, C.; Hong, W.; Lv, C. Based on the internal electric field S scheme mesoporous g-C3N4 nanosheets supported 2H MoS2 heterostructures for HCHO degradation of interior decoration and organic dyes degradation. Inorg. Chem. Commun. 2024, 169, 113047. [Google Scholar] [CrossRef]
- Zeng, G.; Miao, H.; Wu, J.; Zhu, X.; Yi, j.; Zhu, X.; Qi, H.; Jiang, Z.; Mo, Z.; Liu, J.; et al. Ingenious regulation and activation of sites in the 2H-MoS2 basal planes by oxygen incorporation for enhanced photocatalytic hydrogen evolution of CdS. Chem. Eng. J. 2024, 499, 156367. [Google Scholar] [CrossRef]
- Li, H.; Tian, F.; Chen, D.; Wu, J. Preparation and electrochemical performance of sulfur-vacancy riched N-doped MoS2 for supercapacitors. J. Alloys Compd. 2025, 1016, 179027. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Song, X.; Jiang, H. Collaborative effect between single-atom Re and S vacancy on modulating localized electronic structure of MoS2 catalysts for alkaline hydrogen evolution. Nano Res. 2024, 17, 9507–9517. [Google Scholar] [CrossRef]
- Shan, Y.; Liu, Y.; Feng, L.; Yang, S.; Tan, X.; Liu, Z. Magnetic Fe3O4-C@MoS2 composites coordinated with peroxymonosulfate catalysis for enhanced tetracycline degradation. J. Alloys Compd. 2024, 989, 174318. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, S.; Zhao, Y.; Chen, H.; Li, H.; Liu, H. Efficient degradation of sulfamethazine in aqueous solution by Co@MoS2-activated peroxomonosulfate: Performance and mechanism. J. Water Process Eng. 2024, 66, 105909. [Google Scholar] [CrossRef]
- Bai, H.; Yang, Y.; Dong, M.; Yuan, H.; Huang, Y.; Liu, X.; Ni, C. Structure and defect engineering synergistically boost Mo6+/Mo4+ circulation of Sv-MoS2 based photo-Fenton-like system for efficient levofloxacin degradation. Appl. Catal. B Environ. Energy 2024, 358, 124430. [Google Scholar] [CrossRef]
- Mano, P.; Jitwatanasirikul, T.; Roongcharoen, T.; Takahashi, K.; Namuangruk, S. Tuning covalent bonding of single transition metal atom doped in S vacant MoS2 for catalytic CO2 reduction reaction product selectivity. Appl. Surf. Sci. 2025, 688, 162339. [Google Scholar] [CrossRef]
- Sehrawat, P.; Mehta, S.K.; Kansal, S.K. Synergistic enhancement of photocatalytic activity in ZnS/P-doped-MoS2 composite for hydrogen generation simultaneously oxidation of benzyl alcohol through water splitting and dye degradation. Int. J. Hydrogen Energy 2024, 80, 573–585. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, F.; Lan, X.; Liu, Y.; Yang, W.; Zhang, J.; Yu, Y. Building P-doped MoS2/g-C3N4 layered heterojunction with a dual-internal electric field for efficient photocatalytic sterilization. Chem. Eng. J. 2022, 429, 132588. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Y.; Li, X.; Xiao, C.; Shi, Y.; Chen, Y.; Cheng, J.; Zhu, X.; Wang, G.; Xie, J. High-efficient degradation of chloroquine phosphate by oxygen doping MoS2 co-catalytic Fenton reaction. J. Hazard. Mater. 2023, 458, 131894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, T.; Li, J.; Zhang, Q.; Li, B.; Gao, M. Construction of Ru, O Co-Doping MoS2 for Hydrogen Evolution Reaction Electrocatalyst and Surface-Enhanced Raman Scattering Substrate: High-Performance, Recyclable, and Durability Improvement. Adv. Funct. Mater. 2022, 33, 2210939. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Huang, L.; Sun, T.; Kong, L.; Gao, Z.; Fu, R.; Xiao, Y. Transition metal doped MoS2 for piezoelectric Fenton-catalyzed degradation of norfloxacin: Rapid innergenerated-H2O2 conversion and dual pathways of non-radicals and radicals. Chem. Eng. J. 2025, 504, 159027. [Google Scholar] [CrossRef]
- Xie, L.; Lu, X.; Huang, W.; Jiang, G.; Liu, Y.; Fan, X. Local polarization electric field promoted the active sites formation on the piezo-photocatalytic BaCaBO3F containing OVs and N doping to effectively degradation of oxytetracycline in water. Sep. Purif. Technol. 2025, 361, 131637. [Google Scholar] [CrossRef]
- Zhao, E.; Yang, S.; Zhou, Z.; Deng, Y.; Qi, X.; Liu, Y.; Liu, L.; Yang, C.; Lan, Y.; Zhao, B.; et al. Oxygen vacancy-enhanced piezo-photocatalytic for tetracycline hydrochloride degradation in wastewater and H2 evolution. J. Colloid Interface Sci. 2025, 686, 359–366. [Google Scholar] [CrossRef]
- Wang, N.; Yang, W.H.; Wang, R.X.; Li, Z.R.; Xu, X.F.; Long, Y.Z.; Zhang, H.D. Oxygen Vacancy-Enhanced Centrosymmetric Breaking of SrFeO3−x for Piezoelectric-Catalyzed Synthesis of H2O2. Small 2023, 20, e2307291. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhou, W.; Shang, Q.; Guo, Y.; Hu, H.; Li, Z.; Zhang, Y.; Liu, L.; Wang, H.; Tan, X.; et al. Discerning the mechanism of expedited interfacial electron transformation boosting photocatalytic hydrogen evolution by metallic 1T-WS2-induced photothermal effect. Appl. Catal. B Environ. 2022, 310, 121295. [Google Scholar] [CrossRef]
- Ren, M.; Zhao, P.; Fu, X.; Liu, M.; Ning, Y.; Zhang, Y.; Wang, C.; Lin, A.; Cui, J. Phase-modulated built-in electric field to boost photogenerated electron migration for efficient dehalogenation. Chem. Eng. J. 2023, 474, 145524. [Google Scholar] [CrossRef]
- Yin, M.; Wang, K.; Gao, C.; Yang, R.; Huang, Y.; Yu, L. Synthesis and insights into the gas sensing mechanisms of N-doped MoS2 hierarchical structures with superior gas sensing properties at room temperature. Mater. Res. Bull. 2024, 179, 112943. [Google Scholar] [CrossRef]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.; Hone, J.; Ryu, S. Anomalous Lattice Vibrations of Single- and Few-Layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef]
- Xu, X.; Robertson, S.J.; Yang, T.; Chen, F.; Geng, X.; Wang, Y.; Ji, F.; Sun, C.; Chen, S.; Shao, M.; et al. Interfacial space charge design with desired electron density to enhance sodium storage of MoS2@Nb2O5 anode. Nano Energy 2024, 127, 109739. [Google Scholar] [CrossRef]
- Meng, L.; Zhao, C.; Chu, H.; Li, Y.H.; Fu, H.; Wang, P.; Wang, C.C.; Huang, H. Synergetic piezo-photocatalysis of g-C3N4/PCN-224 core-shell heterojunctions for ultrahigh H2O2 generation. Chin. J. Catal. 2024, 59, 346–359. [Google Scholar] [CrossRef]
- Jang, E.; Ahn, J.; Yoon, S.; Cho, K. High Dielectric, Robust Composite Protective Layer for Dendrite-Free and LiPF6 Degradation-Free Lithium Metal Anode. Adv. Funct. Mater. 2019, 29, 1905078. [Google Scholar] [CrossRef]
- Su, Q.; Li, J.; Yuan, H.; Wang, B.; Wang, Y.; Li, Y.; Xing, Y. Visible-light-driven photocatalytic degradation of ofloxacin by g-C3N4/NH2-MIL-88B(Fe) heterostructure: Mechanisms, DFT calculation, degradation pathway and toxicity evolution. Chem. Eng. J. 2022, 427, 131594. [Google Scholar] [CrossRef]
- Tu, S.; Wang, Y.; Huang, H.; Zhang, J.; Li, H.; Sun, J.; Chen, T.; Zhang, Y. Band shifting triggered ·OH evolution and charge separation enhanced H2O2 generation in piezo-photocatalysis of Silleń-Aurivillius-structured Bi4W0.5Ti0.5O8Cl. Chem. Eng. J. 2023, 465, 142777. [Google Scholar] [CrossRef]
- Liu, M.; Ning, Y.; Ren, M.; Fu, X.; Cui, X.; Hou, D.; Wang, Z.; Cui, J. Internal Electric Field-Modulated Charge Migration Behavior in MoS2/MIL-53(Fe) S-Scheme Heterojunction for Boosting Visible-Light-Driven Photocatalytic Chlorinated Antibiotics Degradation. Small 2023, 19, e2303876. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, X.; Chen, Y.; Zhang, Y. Internal Electric Field-Induced High-Efficiency Piezo-Photocatalytic Performance in Bimetal-Regulated Layered Perovskite SrBi2Nb2O9. Adv. Funct. Mater. 2024, 34, 2408838. [Google Scholar] [CrossRef]
- Zhang, C.; Lei, D.; Xie, C.; Hang, X.; He, C.; Jiang, H. Piezo-Photocatalysis over Metal-Organic Frameworks: Promoting Photocatalytic Activity by Piezoelectric Effect. Adv. Mater. 2021, 33, e2106308. [Google Scholar] [CrossRef]
- Lin, K.; Zhu, Z.; Ge, W.; Jiang, T.; Huang, H. Atomic-level unveiling secondary recrystallization enabled micro- and macroscopic polarization enhancement for piezo-photocatalytic oxygen activation. Nano Res. 2024, 17, 5040–5049. [Google Scholar] [CrossRef]
- Zhang, G.; Li, D.; Liu, M.; Wang, Y.; Zhang, J.; Zhang, Y.; Liu, H.; Li, W.; Li, Z.; Lv, W.; et al. Non-noble plasmonic TiN modified BiOBr for the piezo-photocatalytic removal of sulfisoxazole: Simultaneous improvement of photocatalytic and piezoelectric properties. Sep. Purif. Technol. 2024, 337, 126358. [Google Scholar] [CrossRef]
- Liang, Q.; Liu, X.; Shao, B.; Tang, L.; Liu, Z.; Zhang, W.; Gong, S.; Liu, Y.; He, Q.; Wu, T.; et al. Construction of fish-scale tubular carbon nitride-based heterojunction with boosting charge separation in photocatalytic tetracycline degradation and H2O2 production. Chem. Eng. J. 2021, 426, 130831. [Google Scholar] [CrossRef]
- Du, S.; Sui, M.; Guo, Y.; Tian, Y.; Lv, X.; Qi, X. MoS2/SrTiO3 composite for piezocatalysis and piezocatalytic activation of peroxymonosulfate for efficient degradation of sulfamethoxazole. J. Water Process Eng. 2024, 60, 105120. [Google Scholar] [CrossRef]
- Wu, Z.; Song, W.; Xu, X.; Yuan, J.; Lv, W.; Yao, Y. High 1T phase and sulfur vacancies in C-MoS2@Fe induced by ascorbic acid for synergistically enhanced contaminants degradation. Sep. Purif. Technol. 2022, 286, 120511. [Google Scholar] [CrossRef]
- Ren, T.; Tian, W.; Shen, Q.; Yuan, Z.; Chen, D.; Li, N.; Lu, J. Enhanced piezocatalysis of polymorphic few-layered MoS2 nanosheets by phase engineering. Nano Energy 2021, 90, 106527. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, D.; Guo, C.; Ning, Y.; Fu, X.; Li, X.; Xu, X.; Wang, C.; Kou, Y.; Cui, J. N-Doped Modified MoS2 for Piezoelectric–Photocatalytic Removal of Tetracycline: Simultaneous Improvement of Photocatalytic and Piezoelectric Properties. Water 2025, 17, 1296. https://doi.org/10.3390/w17091296
Yuan D, Guo C, Ning Y, Fu X, Li X, Xu X, Wang C, Kou Y, Cui J. N-Doped Modified MoS2 for Piezoelectric–Photocatalytic Removal of Tetracycline: Simultaneous Improvement of Photocatalytic and Piezoelectric Properties. Water. 2025; 17(9):1296. https://doi.org/10.3390/w17091296
Chicago/Turabian StyleYuan, Donghai, Chao Guo, Yuting Ning, Xinping Fu, Xiuqing Li, Xueting Xu, Chen Wang, Yingying Kou, and Jun Cui. 2025. "N-Doped Modified MoS2 for Piezoelectric–Photocatalytic Removal of Tetracycline: Simultaneous Improvement of Photocatalytic and Piezoelectric Properties" Water 17, no. 9: 1296. https://doi.org/10.3390/w17091296
APA StyleYuan, D., Guo, C., Ning, Y., Fu, X., Li, X., Xu, X., Wang, C., Kou, Y., & Cui, J. (2025). N-Doped Modified MoS2 for Piezoelectric–Photocatalytic Removal of Tetracycline: Simultaneous Improvement of Photocatalytic and Piezoelectric Properties. Water, 17(9), 1296. https://doi.org/10.3390/w17091296




