Integrated Assessment of Groundwater Quality for Water-Saving Irrigation Technology (Western Kazakhstan)
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Area Background and Geographical Setting
2.2. Field Measurements, Sampling, and Laboratory Analyses
2.3. Main Concepts and Methodology
- -
- Crop yields in terms of gross yield and cultivation intensity;
- -
- The agricultural products’ quality, in terms of worth and safety;
- -
- Maintaining soil vitality and increasing fertility and also preventing the salinization, alkalinization, soda formation, compaction, and violation of the biological regime.
- -
- The average weighted content of all ions (in the 0–100 cm layer) was assessed. If this value was less than 0.1%, there was no salinization in the 0–100 cm layer for all types of salinization. In this case, the type of salinization chemistry is not determined, and the soils were classified as non-saline. If more than 0.1% and if the sum of CO3 + HCO3 was greater than the content of Ca, the type of chemistry was determined as soda.
- -
- In the absence of soda salinization, the ratio of the contents of chloride ions and sulfate ions was considered. If sulfate ions prevailed, then the salinization chemistry was determined as sulfate or chloride–sulfate (the predominant ion was put in last place).
- -
- If the content of chlorine ions was greater than the content of sulfate ions, then the type of salinization was determined to be either chloride or sulfate–chloride.
2.4. Remote Sensing Data Processing
2.5. Correlation Multivariate Analysis
3. Results
3.1. Irrigation Groundwater Chemistry
3.2. Irrigation Water Quality Assessment According to Degree of Soil Salinity and Soil Granulometric Composition
3.3. The Groundwater Quality Assessment of the Explored Deposits, i.e., the Soil Sodic or Dispersive Soil Development Danger Is Based on the Values of the Exchangeable Sodium Percentage and Water Salinity
3.4. Agricultural Crop Salt Tolerance Dependence on Irrigation Groundwater Quality and Western Kazakhstan’s Irrigated Soils
3.5. Assessment of Groundwater Suitability According to Its Impact on Sprinkler and Drip Irrigation Systems
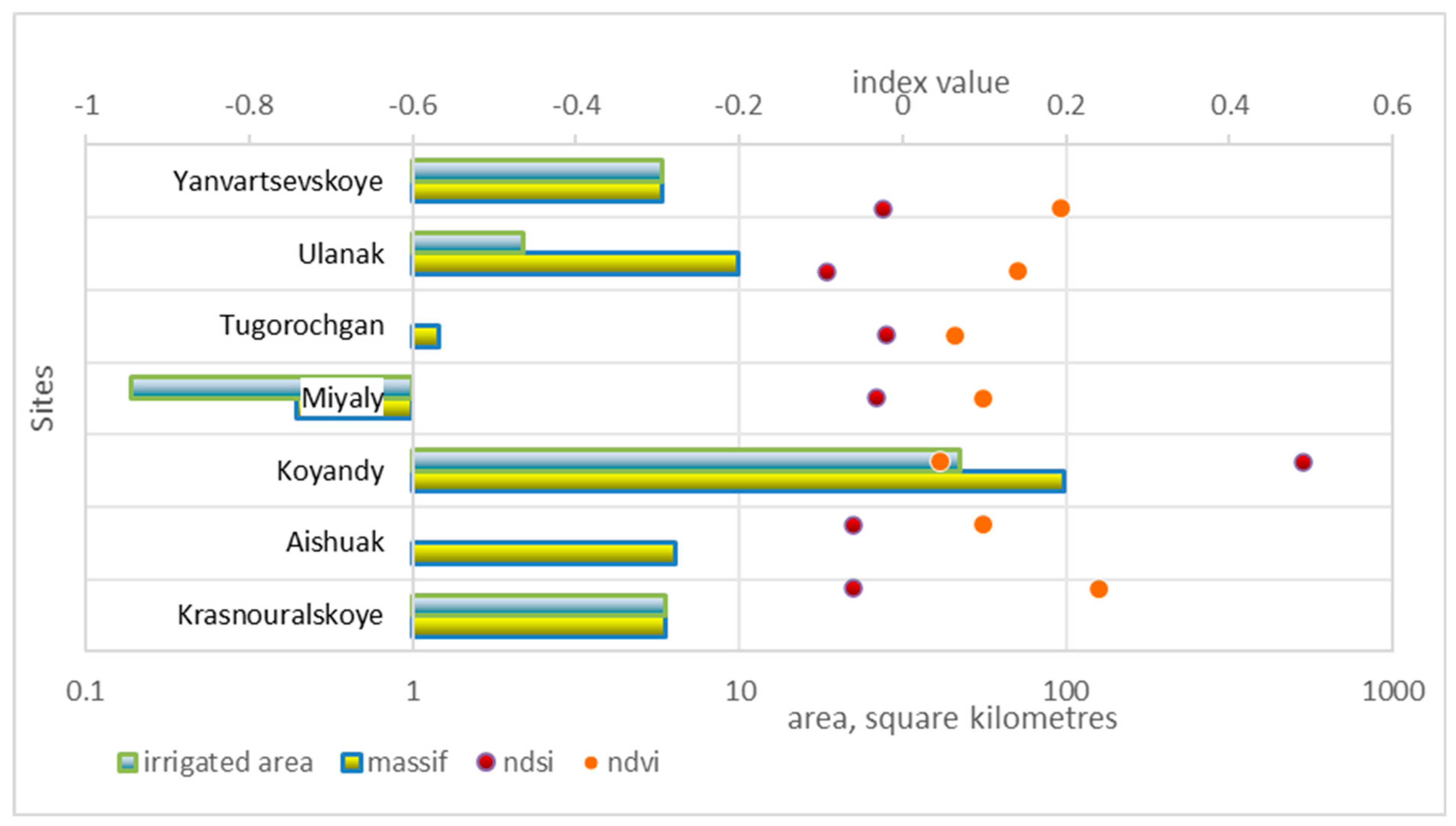
3.6. Assessment of Groundwater Suitability via Remote Sensing Methods
3.7. Results from Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Groundwater Deposit | Well No | Water Quality Class | Groundwater Quality Assessment According to: | |||||
|---|---|---|---|---|---|---|---|---|
| TDS, g/L | pH | Total Hardness, mg-eq/L | Ka by Staebler | SAR | Ka by Antipov-Karataev–Kader | |||
| North Aishuak | 9 | II | 0.83 (F) | 7.5 (N) | 7.05 (MH) | 36.5 (VG) | 10.0 (L) | C > Tк 1.1 > 0.19 (SU) |
| 10 | I | 0.42 (UF) | 7.29 (N) | 3.4 (SO) | 6.78 (G) | 13.4 (CM) | C > Tк 5.4 > 0.09 (SU) | |
| 12 | I | 0.29 (UF) | 7.29 (N) | 2.2 (SO) | 0.56 (G) | 13.9 (CM) | C > Tк 2.15 > 0.07 (SU) | |
| Aishuak | 13 | III | 1.52 (SB) | 7.36 (N) | 2.8 (SO) | 3.84 (SAT) | 14.83 (CM) | C < Tк 0.095 < 0.35 (SU) |
| 15 | III | 2.36 (SB) | 7.37 (N) | 12.65 (VH) | 1.2 (UNS) | 14.19 (CM) | C < Tк 0.36 < 0.54 (SU) | |
| 16 | III | 2.52 (SB) | 7.41 (N) | 12.3 (VH) | 2.11 (SAT) | 15.39 (CM) | C < Tк 0.36 < 0.58 (SU) | |
| Mataykum | 1 | III | 2.09 (SB) | 7.82 (SA) | 5.75 (MH) | 3.49 (SAT) | 18.97 (CM) | C < Tк 0.14 < 0.49 (SU) |
| 6 | III | 2.41 (SB) | 7.91 (SA) | 6.9 (MH) | 3.67 (SAT) | 16.3 (CM) | C < Tк 0.16 < 0.55 (SU) | |
| Myngyr | 2 | III | 1.62 (SB) | 8.32 (SA) | 2.8 (SO) | 2.7 (SAT) | 38.25 (HI) | C < Tк 0.026 < 0.37 (SU) |
| 3 | III | 2.27 (SB) | 7.96 (SA) | 3.4 (SO) | 3.16 (SAT) | 12.3 (L) | C < Tк 0.09 < 0.52 (SU) | |
| 4 | III | 2.2 (SB) | 8.14 (SA) | 2.0 (SO) | 3.32 (SAT) | 21.31 (M) | C < Tк 0.05 < 0.51 (SU) | |
| 6 | III | 1.87 (SB) | 7.9 (SA) | 1.5 (SO) | 1.25 (SAT) | 31.9 (HI) | C < Tк 0.04 < 0.43 (SU) | |
| Yanvartsevskoye | 2118 | I | 0.26 (UF) | 7.85 (SA) | 1.85 (SO) | 6.45 (G) | 2.62 (L) | C > Tк 0.91 > 0.21 (SU) |
| 2114 | I | 0.26 (UF) | 7.85(SA) | 2.95 (SO) | 6.72 (G) | 3.26 (L) | C > Tк 0.86 > 0.20 (SU) | |
| 2110 | I | 0.31 (UF) | 8.2 (SA) | 2.25 (SO) | 7.13 (G) | 3.4 (L) | C > Tк 0.71 > 0.16 (SU) | |
| Krasnouralskoye | 4 | I | 1.23 (SB) | 7.44 (N) | 10.2 (H) | 6.89 (G) | 6.77 (L) | C > Tк 0.93 > 0.28 (SU) |
| 5 | I | 1.1 (SB) | 7.2 (N) | 5.2 (MH) | 6.9 (G) | 9.47 (L) | C > Tк 0.31 > 0.25 (SU) | |
| Ulanak | No | IV | 6.63 (S) | 7.71 (SA) | 47 (SA) | 0.9 (UNS) | 19.71 (CM) | C < Tк 0.56 < 1.52 (SU) |
| Ulanak, Zhyngyldy | No | III | 1.4 (SB) | 7.96 (SA) | 2.7 (SO) | 0.56 (UNS) | 15 (CM) | C < Tк 0.11 < 0.32 (SU) |
| Ulanak, Tugorochgan | 1 | IV | 4.44 (B) | 7.99 (SA) | 2.1 (SO) | 0.87 (UNS) | 173.9 (HI) | C < Tк 0.02 < 1.02 (SU) |
| 2 | IV | 4.32 (B) | 7.99 (SA) | 1.55 (SO) | 0.45 (UNS) | 73.38 (HI) | C < Tк 0.02 < 0.99 (SU) | |
| Koyandy | 2301 | III | 2.4 (SB) | 6.9 (N) | 12.6 (VH) | 2.4 (SAT) | 0.09 (L) | C < Tк 0.35 < 0.55 (SU) |
| 2302 | II | 1.1 (SB) | 7.89 (SA) | 3.2 (SO) | 8.3 (SAT) | 2.13 (L) | C < Tк 0.17 < 0.25 (SU) | |
| 2303 | II | 1.31 (SB) | 7.78 (SA) | 4.6 (MH) | 12.15 (G) | 1.87 (L) | C < Tк 0.23 < 0.30 (SU) | |
| 2304 | II | 0.7 (F) | 7.45 (N) | 4.5 (MH) | 32 (G) | 0.67 (L) | C > Tк 0.59 > 0.16 (CU) | |
| 2305 | III | 1.2 (SB) | 7.8 (SA) | 5.8 (MH) | 9.26 (SAT) | 5.07 (L) | C > Tк 0.38 > 0.27 (CU) | |
| 2306 | II | 0.9 (F) | 7.84 (SA) | 3.3 (SO) | 17.67 (G) | 0.83 (L) | C > Tк 0.26 > 0.21 (CU) | |
Appendix B
| Aquifer | Depth Interval from Soil Surface, m | Soil Type | Soil Characteristics and Land Assessment According to the Soil Salinity | pH | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| By Salt Content, % | Soil Salinity Type by Cation Ratio, mg-eq | Soil Salinity Type by Anion Ratio, mg-eq | ||||||||||
| Ca/Mg | Ca/Na | Na/Mg | Major Actions | HCO3 + CO3/SO4 | HCO3 + CO3/Cl | SO4/Cl | Salinity Type | |||||
| Mataykum | 1/0.0–0.20 | Heavy silty sandy loam | 1.04 (MS) | 3.33 | 0.34 | 9.96 | Na, Mg | 14.49 | 10 | 0.69 | Sodas | 8.11 |
| 1/0.0–0.50 | Silty light loam | 2.5 (HS) | 4 | 0.08 | 45.44 | Na | 1.28 | 2.06 | 1.6 | Soda sulfate | 8.37 | |
| 2/0.0–0.20 | Heavy silty sandy loam | 1.8 (HS) | 22.4 | 0.05 | 400.8 | Na | 59 | 0.65 | 1.09 | Chloride-sulfate | 7.35 | |
| 2/0.0–0.50 | Heavy silty sandy loam | 2.5 (HS) | 0.63 | 0.04 | 14.1 | Na | 0.44 | 0.33 | 0.76 | Chloride-sulfate | 7.88 | |
| 3/0.0–0.20 | silty heavy loam | 1.3 (MS) | 2.09 | 0.41 | 5.11 | Na | 11.2 | 8.3 | 0.74 | Chloride sodas | 8.16 | |
| 3/0.0–0.50 | silty heavy loam | 2.7 (HS) | 1.17 | 0.04 | 30.48 | Na | 0.4 | 0.27 | 0.68 | Sulfate chloride | 7.7 | |
| Myngyr | 4/0.0–0.20 | Silty light loam | 6.2 (VHS) | 23 | 0.032 | 708.2 | Na | 0.28 | 0.4 | 1.62 | Soda chloride sulfate | 8.76 |
| 4/0.0–0.50 | Heavy silty sandy loam | 6.1 (VHS) | 38.5 | 0.05 | 749.5 | Na | 0.34 | 0.35 | 1.03 | Soda chloride sulfate | 8.38 | |
| Yanvartsevskoye | 5/0.0–0.20 | silty heavy loam | 0.65 (MS) | 2.26 | 2.43 | 0.93 | Mg, Ca | 13.8 | 9.28 | 0.67 | Sulfated sodas | 7.73 |
| 5/0.0–0.50 | clay | 0.78 (MS) | 13.8 | 8.96 | 0.71 | Mg, Ca | 11.41 | 24.3 | 2.13 | Sulfate chloride | 7.43 | |
| Krasnouralskoye | 6/0.0–0.20 | silty sand | 0.21 (US) | 0.83 | 11.36 | 0.07 | Ca, Mg | 0.26 | 1 | 3.78 | Chloride-sulfate | 6.7 |
| 6/0.0–0.50 | silty light sandy loam | 0.18 (US) | 1.2 | 7.36 | 0.16 | Na, Ca | 0.64 | 1 | 0.16 | Sulfate chloride | 6.7 | |
| Ulanak | 7/0.0–0.20 | Silty light loam | 0.79 (MS) | 4.99 | 1.76 | 2.8 | Ca, Na | 4.38 | 3.0 | 0.68 | Chloride sodas | 7.7 |
| 7/0.0–0.50 | 0.74 (MS) | 2.66 | 1.0 | 2.7 | Ca, Na | 20.97 | 3.60 | 0.17 | Sulfated sodas | 8.06 | ||
| Ulanak, Zhyngyldy site | 8/0.0–0.20 | Silty light loam | 0.78 (MS) | 1.38 | 1.44 | 26.9 | Ca, Mg | 7.98 | 7.5 | 0.94 | Sulfated sodas | 8.06 |
| 8/0.0–0.50 | 1.2 (MS) | 7.16 | 0.91 | 7.85 | Ca, Mg | 15.7 | 16.87 | 1.07 | Sulfated sodas | 8.22 | ||
| Ulanak, Tugorochgan site | 9/0.0–0.20 | Heavy silty sandy loam | 5.6 (VHS) | 4.2 | 0.03 | 55.3 | Ca, Mg | 3.03 | 11.13 | 0.37 | Sulfated sodas | 8.76 |
| North Aishuak | 10/0.0–0.20 | Light silty sandy loam | 0.17 (US) | 0.77 | 7.9 | 0.09 | Ca, Mg | 3.19 | 5 | 1.56 | Sodas | 6.86 |
| 10/0.0–0.50 | Silty light loam | 0.6 (MS) | 0.9 | 1.34 | 0.67 | Ca, Mg | 7.97 | 7.85 | 0.98 | Chloride-sulfate | 8.27 | |
| 11/0.0–0.20 | Heavy silty sandy loam | 0.37 (MS) | 1.67 | 6.4 | 0.26 | Na, Ca | 5.47 | 11.7 | 2.13 | Sulfated sodas | 7.43 | |
| 11/0.0–0.50 | Heavy silty sandy loam | 0.69 (MS) | 2.98 | 8.65 | 0.34 | Na, Ca | 8.5 | 10 | 1.2 | Sulfated sodas | 7.62 | |
| 12/0.0–0.20 | Heavy silty sandy loam | 0.37 (MS) | 1.67 | 6.4 | 0.26 | Na, Ca | 5.47 | 11.7 | 2.13 | Sulfated sodas | 7.41 | |
| 12/0.0–0.50 | silty sand | 0.94 (MS) | 2.87 | 11.55 | 0.24 | Na, Ca | 44.2 | 28.75 | 0.65 | Sulfated sodas | 8.04 | |
| Aishuak | 13/0.0–0.20 | Heavy silty sandy loam | 0.66 (MS) | 4.34 | 7.78 | 0.56 | Na, Ca | 17.9 | 14 | 0.78 | Sulfated sodas | 7.97 |
| 13/0.0–0.50 | Heavy silty sandy loam | 0.2 (US) | 0.66 | 0.53 | 1.24 | Na, Ca | 1.1 | 2.5 | 2.25 | Sulfated sodas | 6.92 | |
Appendix C
| Groundwater Deposit | Sampling Point Number/Depth Interval from Soil Surface, m | Soil Type | Characteristics and Assessment of Irrigated Sites According to the Level and Type of the Soil Salinity | Soil Granulometric Composition | Characteristics and Assessment of Irrigation Water Quality | ||||
|---|---|---|---|---|---|---|---|---|---|
| By Salt Content, % | Soil Salinity Type by Cation Ratio | Soil Salinity Type by Anion Ratio | TDS g/L | Irrigation Water Quality Class | Irrigation Water Quality Assessment | ||||
| North Aishuak | 10/0.0–0.20 | Silty light sandy loam | US | Ca, Mg | Sodas | light particle size | 0.42 | I | nonhazardous |
| 10/0.0–0.50 | Silty light loam | MS | Ca, Mg | Chloride-sulfate | light particle size | ||||
| 11/0.0–0.20 | Heavy silty sandy loam | MS | Mg, Ca | Sulfated sodas | heavy particle size | 0.29 | I | nonhazardous | |
| 11/0.0–0.50 | Heavy silty sandy loam | MS | Mg, Ca | Sulfated sodas | heavy particle size | ||||
| Aishuak | 13/0.0–0.20 | Heavy silty sandy loam | MS | Mg, Ca | Sulfated sodas | heavy particle size | 1.52 | IV | hazardous |
| 13/0.0–0.50 | US | Mg, Ca | Sulfated sodas | ||||||
| Mataykum | 1/0.0–0.20 | Heavy silty sandy loam | HS | Na, Mg | Sodas | heavy particle size | 1.52 | III | moderately hazardous |
| 1/0.0–0.50 | Silty light loam | MS | Na | Soda sulfate | light particle size | III | moderately hazardous | ||
| Myngyr | 4/0.0–0.20 | Silty light loam | VHS | Na | Soda chloride sulfate | light particle size | 2.7 | III | moderately hazardous |
| 4/0.0–0.50 | Heavy silty sandy loam | VHS | Na | Soda chloride sulfate | heavy particle size | IV | hazardous | ||
| Yanvartsevskoye | 5/0.0–0.20 | Silty heavy loam | MS | Mg, Ca | Sulfated sodas | heavy particle size | 0.27 | I | nonhazardous |
| 5/0.0–0.50 | Clay | MS | Mg, Ca | Sulfate chloride | heavy particle size | ||||
| Krasnouralskoye | 6/0.0–0.20 | Silty sand | US | Ca, Mg | Chloride–sulfate | light particle size | 1.11 | II | low-hazardous |
| 6/0.0–0.50 | Silty light sandy loam | US | Na, Ca | Sulfate chloride | light particle size | ||||
| Ulanak | 7/0.0–0.20 | Silty light loam | MS | Ca, Na | Chloride sodas | light particle size | 6.63 | IV | hazardous |
| 7/0.0–0.50 | MS | Sulfated sodas | |||||||
| Ulanak, Zhyngyldy site | 8/0.0–0.20 | Silty light loam | MS | Ca, Mg | Sulfated sodas | light particle size | 1.40 | III | moderately hazardous |
| 8/0.0–0.50 | MS | ||||||||
| Ulanak, Tugorochgan site | 0.0–0.20 | Heavy silty sandy loam | VHS | Ca, Na | Sulfated sodas | heavy particle size | 4.22 | IV | hazardous |
References
- Sandström, C.; Ring, I.; Olschewski, R.; Simoncini, R.; Albert, C.; Acar, S.; Adeishvili, M.; Allard, C.; Anker, Y.; Arlettaz, R.; et al. Mainstreaming Biodiversity and Nature’s Contributions to People in Europe and Central Asia: Insights from IPBES to Inform the CBD Post-2020 Agenda. Ecosyst. People 2023, 19, 2138553. [Google Scholar] [CrossRef]
- Margat, J.; van der Gun, J. Groundwater Around the World; Taylor & Francis Group: Boca Raton, FL, USA, 2013; ISBN 9788578110796. [Google Scholar]
- Katsanou, K.; Karapanagioti, H.K. Surface Water and Groundwater Sources for Drinking Water. Handb. Environ. Chem. 2019, 67, 1–19. [Google Scholar] [CrossRef]
- United Nations. Groundwater: Making the Invisible Visible; United Nations: New York, NY, USA, 2022; ISBN 978-92-3-100507-7. [Google Scholar]
- Asadi, E.; Isazadeh, M.; Samadianfard, S.; Ramli, M.F.; Mosavi, A.; Nabipour, N.; Shamshirband, S.; Hajnal, E.; Chau, K.W. Groundwater Quality Assessment for Sustainable Drinking and Irrigation. Sustainability 2020, 12, 177. [Google Scholar] [CrossRef]
- Venkateswaran, S.; Vijay Prabhu, M.; Mohammed Rafi, M.; Vallel, K.L.K. Assessment of Groundwater Quality for Irrigational Use in Cumbum Valley, Madurai District, Tamilnadu, India. Nat. Environ. Pollut. Technol. 2011, 10, 207–212. [Google Scholar]
- Nikolaou, G.; Neocleous, D.; Christou, A.; Kitta, E.; Katsoulas, N. Implementing Sustainable Irrigation in Water-Scarce Regions under the Impact of Climate Change. Agronomy 2020, 10, 1120. [Google Scholar] [CrossRef]
- Tomaz, A.; Palma, P.; Fialho, S.; Lima, A.; Alvarenga, P.; Potes, M.; Costa, M.J.; Salgado, R. Risk Assessment of Irrigation-Related Soil Salinization and Sodification in Mediterranean Areas. Water 2020, 12, 3569. [Google Scholar] [CrossRef]
- Minhas, P.S.; Qadir, M.; Yadav, R.K. Groundwater Irrigation Induced Soil Sodification and Response Options. Agric. Water Manag. 2019, 215, 74–85. [Google Scholar] [CrossRef]
- Strawn, D.; Bohn, H.L.; O’Connor, G.A. Soil Chemistry; John Wiley Blackwell: Hoboken, NJ, USA, 2020; 356p. [Google Scholar]
- Anker, Y.; Mirlas, V.; Zilberbrand, M.; Oren, A. Chemical Reactivity: PH, Salinity and Sodicity Effects on Soil Health. In Laboratory Methods for Soil Health Analysis; Karlen, D.L., Stott, D.E., Mikha, M.M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; Volume 2, pp. 78–108. ISBN 9780891189831. [Google Scholar]
- Korolyuk, T.V. Specific Features of the Dynamics of Salts in Salt-Affected Soils Subjected to Long-Term Seasonal Freezing in the South Transbaikal Region. Eurasian Soil Sci. 2014, 47, 339–352. [Google Scholar] [CrossRef]
- Kostyakov, Alexey Nikolaevich—Osnovy Melioratsiy. In Russian/Basics of Reclamation. Available online: https://www.livre-rare-book.com/book/30378290/6244295 (accessed on 7 March 2025).
- Suarez, D.L. Irrigation water quality assessments. In Agricultural Salinity Assessment and Management; ASCE: Reston, VA, USA, 2012; pp. 343–370. [Google Scholar]
- Abbasnia, A.; Radfard, M.; Mahvi, A.H.; Nabizadeh, R.; Yousefi, M.; Soleimani, H.; Alimohammadi, M. Groundwater Quality Assessment for Irrigation Purposes Based on Irrigation Water Quality Index and Its Zoning with GIS in the Villages of Chabahar, Sistan and Baluchistan, Iran. Data Br. 2018, 19, 623–631. [Google Scholar] [CrossRef]
- Adimalla, N.; Qian, H. Groundwater Quality Evaluation Using Water Quality Index (WQI) for Drinking Purposes and Human Health Risk (HHR) Assessment in an Agricultural Region of Nanganur, South India. Ecotoxicol. Environ. Saf. 2019, 176, 153–161. [Google Scholar] [CrossRef]
- Batarseh, M.; Imreizeeq, E.; Tilev, S.; Al Alaween, M.; Suleiman, W.; Al Remeithi, A.M.; Al Tamimi, M.K.; Al Alawneh, M. Assessment of Groundwater Quality for Irrigation in the Arid Regions Using Irrigation Water Quality Index (IWQI) and GIS-Zoning Maps: Case Study from Abu Dhabi Emirate, UAE. Groundw. Sustain. Dev. 2021, 14, 100611. [Google Scholar] [CrossRef]
- Ravi, K.P.; Periasamy, S. Systematic Discrimination of Irrigation and Upheaval Associated Salinity Using Multitemporal SAR Data. Sci. Total Environ. 2021, 790, 148148. [Google Scholar] [CrossRef] [PubMed]
- Phogat, V.; Mallants, D.; Cox, J.W.; Šimůnek, J.; Oliver, D.P.; Awad, J. Management of Soil Salinity Associated with Irrigation of Protected Crops. Agric. Water Manag. 2020, 227, 105845. [Google Scholar] [CrossRef]
- Al-Aizari, H.S.; Aslaou, F.; Mohsen, O.; Al-Aizari, A.R.; Al-Odayni, A.B.; Abduh, N.A.Y.; Al-Aizari, A.J.M.; Taleb, E.A.B.O. Assessment of Groundwater Quality for Irrigation Purpose Using Irrigation Water Quality Index (Iwqi). J. Environ. Eng. Landsc. Manag. 2024, 32, 1–11. [Google Scholar] [CrossRef]
- Nagaiah, E.; Sonkamble, S.; Mondal, N.C.; Ahmed, S. Natural Zeolites Enhance Groundwater Quality: Evidences from Deccan Basalts in India. Environ. Earth Sci. 2017, 76, 536. [Google Scholar] [CrossRef]
- Çadraku, H.S. Groundwater Quality Assessment for Irrigation: Case Study in the Blinaja River Basin, Kosovo. Civ. Eng. J. 2021, 7, 1515–1528. [Google Scholar] [CrossRef]
- Gaagai, A.; Aouissi, H.A.; Bencedira, S.; Hinge, G.; Athamena, A.; Haddam, S.; Gad, M.; Elsherbiny, O.; Elsayed, S.; Eid, M.H.; et al. Application of Water Quality Indices, Machine Learning Approaches, and GIS to Identify Groundwater Quality for Irrigation Purposes: A Case Study of Sahara Aquifer, Doucen Plain, Algeria. Water 2023, 15, 289. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Thakral, N. Assessment of Groundwater Quality for Drinking and Irrigation Purposes Using Hydro-Chemical and Water Quality Index Studies in Kurukshetra (Haryana), India. Water. Air. Soil Pollut. 2024, 235, 351. [Google Scholar] [CrossRef]
- Anker, Y. Hydrogeochemistry of the Central Jordan Valley. Ph.D. Thesis, Tel Aviv University, Tel Aviv, Israel, 2007. [Google Scholar]
- Mirlas, V.; Anker, Y.; Aizenkod, A.; Goldshleger, N. Irrigation Quality and Management Determine Salinization in Israeli Olive Orchards. Geosci. Model Dev. 2022, 15, 129–143. [Google Scholar] [CrossRef]
- Mirlas, V.; Makyzhanova, A.; Kulagin, V.; Kuldeev, E.; Anker, Y. An Integrated Aquifer Management Approach for Aridification-Affected Agricultural Area, Shengeldy-Kazakhstan. Water 2021, 13, 2357. [Google Scholar] [CrossRef]
- Shaw, S.K.; Sharma, A. Assessment of Groundwater Quality and Suitability for Irrigation Purpose Using Irrigation Indices, Remote Sensing and GIS Approach. Groundw. Sustain. Dev. 2024, 26, 101297. [Google Scholar] [CrossRef]
- Nadun, S.N.E.M.; Maarof, I.; Ghazali, R.; Samad, A.M.; Adnan, R. Sustainable Groundwater Potential Zone Using Remote Sensing and GIS. In Proceedings of the 2010 6th International Colloquium on Signal Processing & Its Applications, Malacca, Malaysia, 21–23 May 2010; pp. 1–6. [Google Scholar] [CrossRef]
- Ni, B.; Wang, D.; Deng, Z.; Xu, H.; Wang, D.; Jiang, X. Review on the Groundwater Potential Evaluation Based on Remote Sensing Technology. IOP Conf. Ser. Mater. Sci. Eng. 2018, 394, 052038. [Google Scholar] [CrossRef]
- Gerardo, R.; de Lima, I.P. Sentinel-2 Satellite Imagery-Based Assessment of Soil Salinity in Irrigated Rice Fields in Portugal. Agriculture 2022, 12, 1490. [Google Scholar] [CrossRef]
- Fallatah, O.; Khattab, M.R. Evaluation of Groundwater Quality and Suitability for Irrigation Purposes and Human Consumption in Saudi Arabia. Water 2023, 15, 2352. [Google Scholar] [CrossRef]
- Taşan, M.; Demir, Y.; Taşan, S. Groundwater Quality Assessment Using Principal Component Analysis and Hierarchical Cluster Analysis in Alaçam, Turkey. Water Supply 2022, 22, 3431–3447. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, S.; Hu, C.; Zhao, Z.; Gao, Z.; Liu, J. Hydrochemical Assessment of Groundwater Utilizing Statistical Analysis, Integrated Geochemical Methods, and EWQI: A Case Study of Laiwu Region, North China. Environ. Monit. Assess. 2024, 196, 1222. [Google Scholar] [CrossRef]
- Kazakis, N.; Mattas, C.; Pavlou, A.; Patrikaki, O.; Voudouris, K. Multivariate Statistical Analysis for the Assessment of Groundwater Quality under Different Hydrogeological Regimes. Environ. Earth Sci. 2017, 76, 349. [Google Scholar] [CrossRef]
- Salnikov, V.; Talanov, Y.; Polyakova, S.; Assylbekova, A.; Kauazov, A.; Bultekov, N.; Musralinova, G.; Kissebayev, D.; Beldeubayev, Y. An Assessment of the Present Trends in Temperature and Precipitation Extremes in Kazakhstan. Climate 2023, 11, 33. [Google Scholar] [CrossRef]
- Tursunova, A.; Medeu, A.; Alimkulov, S.; Saparova, A.; Baspakova, G. Water Resources of Kazakhstan in Conditions of Uncertainty. J. Water L. Dev. 2022, 54, 138–149. [Google Scholar] [CrossRef]
- Smolyar, V.A.; Burov, B.V.; Mustafaev, S.T. Water Resources of Kazakhstan: Assessment, Forecast, Management. Volume XIX. Groundwater in Kazakhstan: Availability and Use; Almaty, Kazakhstan, 2012. Available online: http://e-lib.dulaty.kz/lib/document/TARGU/E457E399-FF49-4E92-8D47-C80EE7C7B205/ (accessed on 1 April 2025).
- Kazhydromet. Overview of Climate Features in Kazakhstan; Kazhydromet: Astana, Kazakhstan, 2023. [Google Scholar]
- Alimkulov, S.; Tursunova, A.; Saparova, A.; Kulebaev, K.; Zagidullina, A.; Myrzahmetov, A. Resources of River Runoff of Kazakhstan. Int. J. Eng. Adv. Technol. 2019, 8, 2242–2250. [Google Scholar] [CrossRef]
- Eremkina, T.V.; Yarushina, M.I. Ural River Basin; Elsevier Ltd.: Amsterdam, The Netherlands, 2022; ISBN 9780081026120. [Google Scholar]
- Adenova, D.; Sapargaliyev, D.; Sagin, J.; Absametov, M.; Murtazin, Y.; Smolyar, V. Assessing Groundwater and Soil Quality in West Kazakhstan amid Climate Impacts and Oil Industry Contamination Risks. Sci. Rep. 2025, 15, 6663. [Google Scholar] [CrossRef] [PubMed]
- KMOA. Conducting Monitoring and Assessment of Meliorative Condition of Irrigated; KMOA: Astana, Kazakhstan, 2016. [Google Scholar]
- ST RK GOST R 51232; National Standard. Drinking Water General Requirements for the Organization and Methods of Quality Control. Committee for Standardization: Astana, Kazakhstan, 2003. Available online: https://waterservice.kz/downloads/rd/gst7.pdf?ysclid=ld3fpwacho899490449 (accessed on 2 April 2025).
- ISO 17294-2; International Standard. Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS), Part 2: Determination of 62 Elements. ISO: Geneva, Switzerland, 2003. Available online: https://cdn.standards.iteh.ai/samples/36127/5395f5b265b64455bb9890d9d161cf95/ISO-17294-2-2003.pdf (accessed on 2 April 2025).
- PND F 16.1:2:2.3:2.2.69-10; National Standard. Determination of Water-Soluble Forms of Inorganic and Organic Anions in Soils, Greenhouse Soils, Clays, Peat, Sewage Sludge, Activated Sludge, Bottom Sediments. Methodology M 03-06-2010. Lumex: Moscow, Russia, 2010. Available online: https://www.lumex.ru/metodics/19ARU03.01.12-1.pdf (accessed on 2 April 2025).
- Bezborodov, Y.G.; Khozhanov, N.N.; Mirdadayev, M.S.; Ustabaev, T.S. Methodology of Recycling of Drainage and Waste Water in Kazakhstan. Agrar. Sci. J. 2022, 11, 96–99. [Google Scholar] [CrossRef]
- Richards, L.A.; Allison, L.; Bernstein, C.A.; Bower, J.W.; Brown, M.; Fireman, J.T.; Hatcher, H.; Hayward, G.A.; Pearson, R.C.; Reeve, L.E.; et al. Diagnosis and improvement of saline and alkaline soils. Soil Sci. Soc. Am. J. 1954, 18, 348. [Google Scholar]
- Drovovozova, T.I.; Panenko, N.N.; Manzhina, S.A. Assessment of Water Availability From Open Collectors for Irrigation in Semikarakorsky District Rostov Region. Sci. J. Russ. Sci. Res. Inst. L. Improv. Probl. 2020, 3, 154–169. [Google Scholar] [CrossRef]
- Babaeva, K.S. Assessment of Irrigation Water Quality. 2017. Available online: https://direct.farm/content/257/2575d1f6d94d4a2cbce53b774b1c75531041898.pdf (accessed on 2 April 2025).
- Akhmedov, A.D.; Temerev, A.A.; Galliullina, E.Y. Reliability of Systems of Drip Irrigation. News Nizhnevolzhsky Agro-Univ. Complex 2010, 3, 83–88. [Google Scholar]
- Tucker, C.J. Technical Memorandum 80293 Radiometric Resolution For Monitoring Vegetation How Many Bits Are Needed? Goddard Space Flight Center: Greenbelt, MD, USA, 1979. [Google Scholar]
- Pettorelli, N.; Vik, J.O.; Mysterud, A.; Gaillard, J.M.; Tucker, C.J.; Stenseth, N.C. Using the Satellite-Derived NDVI to Assess Ecological Responses to Environmental Change. Trends Ecol. Evol. 2005, 20, 503–510. [Google Scholar] [CrossRef]
- Anker, Y.; Hershkovitz, Y.; Ben Dor, E.; Gasith, A. Application of Aerial Digital Photography for Macrophyte Cover and Composition Survey in Small Rural Streams. River Res. Appl. 2014, 30, 925–937. [Google Scholar] [CrossRef]
- Onglassynov, Z.A.; Akylbekova, A.Z.; Sotnikov, Y.V.; Rakhimov, T.A.; Kanafin, K.M.; Balla, D. Implementation of the Ers for Yield Analyzing of Irrigated Lands of South Kazakhstan. News Natl. Acad. Sci. Repub. Kazakhstan Ser. Geol. Tech. Sci. 2019, 4, 113–120. [Google Scholar] [CrossRef]
- Khan, N.M.; Rastoskuev, V.V.; Sato, Y.; Shiozawa, S. Assessment of Hydrosaline Land Degradation by Using a Simple Approach of Remote Sensing Indicators. Agric. Water Manag. 2005, 77, 96–109. [Google Scholar] [CrossRef]
- Allbed, A.; Kumar, L.; Aldakheel, Y.Y. Assessing Soil Salinity Using Soil Salinity and Vegetation Indices Derived from IKONOS High-Spatial Resolution Imageries: Applications in a Date Palm Dominated Region. Geoderma 2014, 230–231, 1–8. [Google Scholar] [CrossRef]
- Abuelgasim, A.; Ammad, R. Mapping Soil Salinity in Arid and Semi-Arid Regions Using Landsat 8 OLI Satellite Data. Remote Sens. Appl. Soc. Environ. 2019, 13, 415–425. [Google Scholar] [CrossRef]
- Bannari, A.; Guédon, A.M.; El-Ghmari, A. Mapping Slight and Moderate Saline Soils in Irrigated Agricultural Land Using Advanced Land Imager Sensor (EO-1) Data and Semi-Empirical Models. Commun. Soil Sci. Plant Anal. 2016, 47, 1883–1906. [Google Scholar] [CrossRef]
- Izenman, A.J. Modern Multivariate Statistical Techniques Regression, Classification, and Manifold Learning; Casella, G., Fienberg, S., Olkin, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 9780387781891. [Google Scholar]
- Thevapalan Correlation Matrix in Excel: A Complete Guide to Creating and Interpreting. Available online: https://www.datacamp.com/tutorial/correlation-matrix-excel (accessed on 7 March 2025).
- Sajeevanie, T.L. Testing and Interpreting Correlation, Moderation and Mediation Effects in Social Science Researches Testing and Interpreting Mediation Effect. Int. J. Res. Innov. Soc. Sci. 2020, IV, 789–795. [Google Scholar]
- Naily, W. Hendarmawan Chemical Water Types of Unconfined Groundwater in Southern Bandung. In Proceedings of the 7th Mathematics, Science, and Computer Science Education International Seminar, MSCEIS 2019, Bandung, Indonesia, 12 October 2019; pp. 1–7. [Google Scholar] [CrossRef]
- Piper, A.M. A Graphic Procedure in the Geochemical Interpretation of Water-analyses. Eos Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Durov, S.A. Classification of natural waters and graphic presentation of their composition. Dokl. Akad. Nauk 1948, 59, 87–90. [Google Scholar]
- Shokri, N.; Hassani, A.; Sahimi, M. Multi-Scale Soil Salinization Dynamics from Global to Pore Scale: A Review. Rev. Geophys. 2024, 62, e2023RG000804. [Google Scholar] [CrossRef]
- Wen, D.; Wang, J.; Ding, J.; Zhang, Z. Distribution Characteristics and Relationship Between Soil Salinity and Soil Particle Size in Ebinur Lake Wetland, Xinjiang. Land 2025, 14, 297. [Google Scholar] [CrossRef]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; FAO United Nations: Rome, Italy, 1985; p. 97. [Google Scholar]
- Paramonov, A.I. Current State and Prospects for the Development of Irrigated Agriculture in the Northern Region of the Republic of Kazakhstan; KazNIIVH: Taraz, Kazakhstan, 2019. [Google Scholar]
- Haman, D.Z. Causes and Prevention of Emitter Plugging in Microirrigation Systems. IFAS Ext. 2011, BUL 258, 1–11. [Google Scholar] [CrossRef]
- Lili, Z.; Yang, P.; Ren, S.; Li, Y.; Liu, Y.; Xia, Y. Chemical Clogging of Emitters and Evaluation of Their Suitability for Saline Water Drip Irrigation. Irrig. Drain. 2016, 65, 439–450. [Google Scholar] [CrossRef]
- Ramachandrula, V.R.; Kasa, R.R. Prevention and Treatment of Drip Emitter Clogging: A Review of Various Innovative Methods. Water Pract. Technol. 2022, 17, 2059–2070. [Google Scholar] [CrossRef]
- Robbins, C.W.; Meyer, W.S. Calculating Ph from Ec and Sar Values in Salinity Models and Sar from Soil and Bore Water Ph and Ec Data. Aust. J. Soil Res. 1990, 28, 1001–1011. [Google Scholar] [CrossRef]



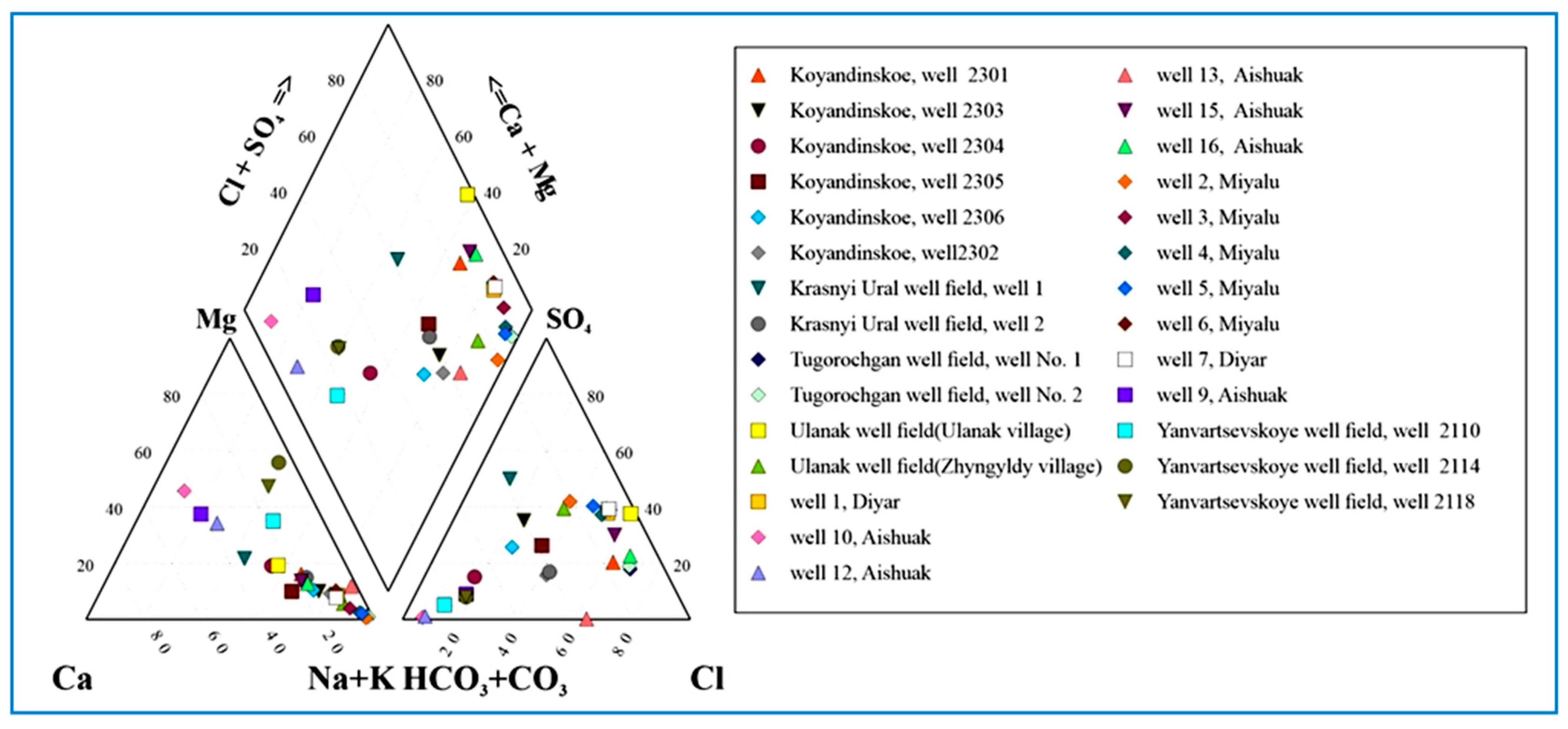

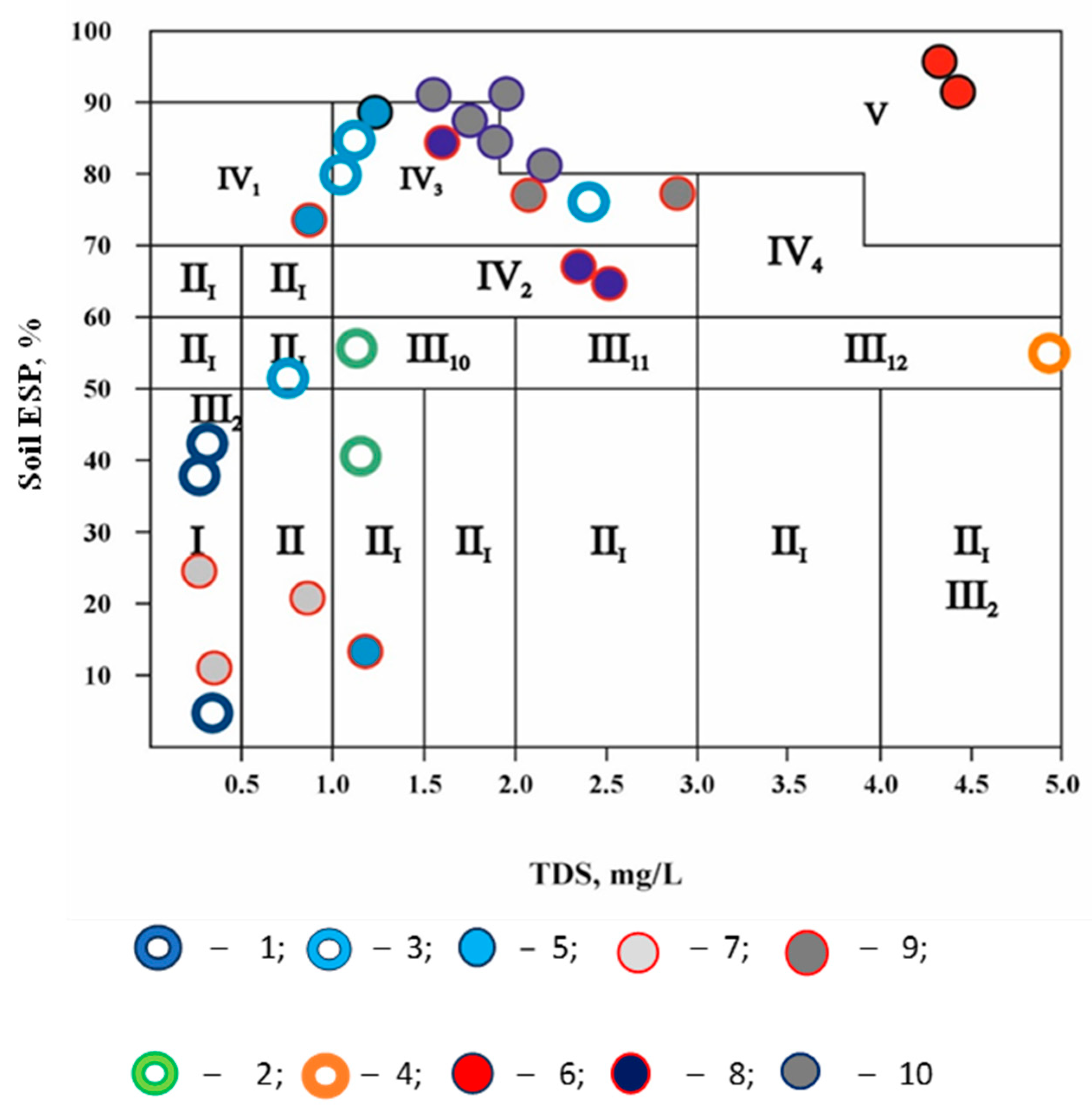
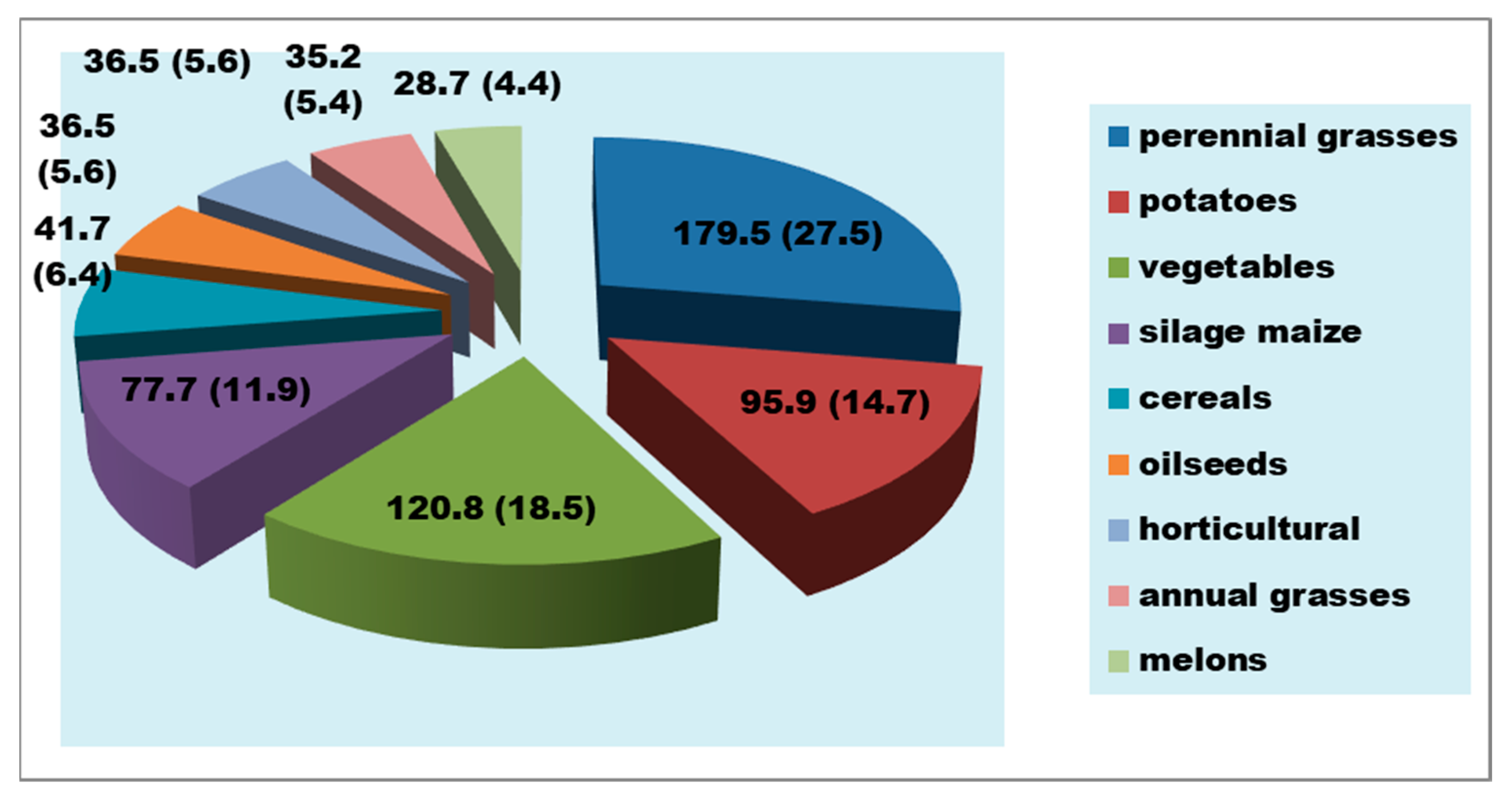
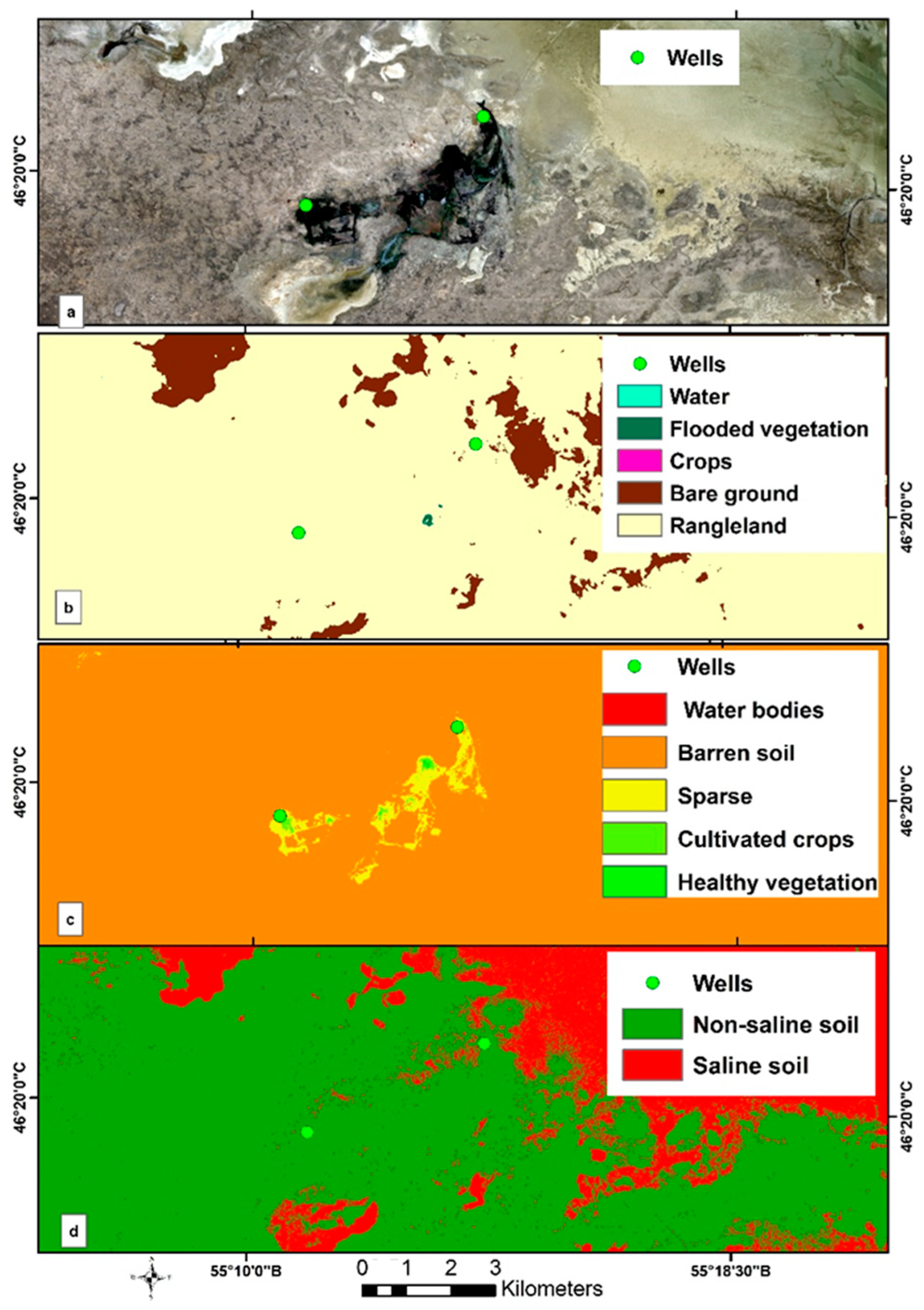
| Groundwater Well Field | Aishuak | North Aishuak | Mataykum | Myngyr | Ulanak | Yanvar Tsevskoye | Krasno Uralskoye | Koyandy |
|---|---|---|---|---|---|---|---|---|
| Cadaster code | 4708 | 5569 | 4954 | 4955 | 5098 | 5146 | 5220 | 5226 |
| Exploitable reserves, thousand m3/day | 84.7 | 372.7 | 88.1 | 34 | 4.8 | 54.5 | 12.9 | 7.17 |
| Designation | Irrigation | Drinking, Irrigation | Irrigation | Irrigation | Drinking, Irrigation | Irrigation | Irrigation | Drinking, Irrigation |
| Exploitation period | 27 | 25 | 27 | 25 | 5 | 27 | 27 | 27 |
| Start of exploitation | 1976 | 1980 | 1977 | 1977 | 1960 | 1982 | 1987 | 1988 |
| Parameter | Method | Error Rate | Equipment |
|---|---|---|---|
| Potential of hydrogen | electrometric | 0.1 pH units | SevenCompact liquid analyzer, Metter-Toledo Ltd., Leicester, UK |
| Dense residue | gravimetric | 5% | Electronic laboratory scales, OHAUS, Nanikon, Switzerland |
| Calcium | titrimetric | 5% | Burette, 100 mL, Eisco Labs, NY, USA |
| Magnesium | titrimetric | 5% | |
| Hydrocarbonates | titrimetric | 5% | |
| Chlorides | titrimetric | 5 | |
| Sodium | flame photometric | 7.5% | Flame Photometer PFP 7 Keison Products, Essex, UK |
| Potassium | flame photometric | 10% | |
| Sulfates | gravimetric | 5% | Electronic laboratory scales ‘OHAUS’ |
| Nitrates | photometric | 20% | UV spectrophotometer, Shimadzu Corporation, Kyoto, Japan |
| Fluorides | electrometric | 11% | SevenCompact liquid analyzer, Metter-Toledo Ltd., Leicester, UK |
| Petroleum products | fluorimetric | 17% | Fluorat liquid analyzer LUMEX-Marketing Ltd. Mission, Canada |
| Humus | photometric | 20% | UV spectrophotometer, Shimadzu Corporation, Kyoto, Japan |
| Particle size distribution | sieve | 1% | Laboratory sieve, Electronic laboratory scales OHAUS, Nanikon, Switzerland |
| Ka Value | The Suitability of Water for Irrigation |
|---|---|
| More than 18 | Good: the water is suitable for irrigation purposes. |
| 6–18 | Satisfactory: the water may accumulate alkalis in the soil, but this is not a significant issue. |
| 1.2–5.9 | Unsatisfactory: artificial drainage is required for irrigation. |
| Less than 1.2 | Poor: the water is unsuitable for irrigation. |
| Water Salinity g/L | The Danger of Soil Alkalinization by SAR | |||
|---|---|---|---|---|
| Low | Medium | High | Very High | |
| less than 1 | 8–10 | 15–18 | 22–26 | Over 26 |
| 1–2 | 6–8 | 12–15 | 18–22 | Over 22 |
| 2–3 | 4–6 | 9–12 | 14–18 | Over 18 |
| more than 3 | 2–4 | 6–9 | 11–14 | Over 14 |
| Irrigation Water Quality Class | Water Quality According to Negative Water Impact on Soils | |||
|---|---|---|---|---|
| Chloride Salinization | Sodium Salinization | Magnesium Salinization | Sodic Processes | |
| Cl, mg-eq/L | Ca/Na, mg-eq/L | Ca/Mg, mg-eq/L | (CO32 + HCO3) − (Ca + Mg), mg-eq/L | |
| I | less than 2.0 | over 2.0 | over 1.0 | less than 1.0 |
| II | 2.0–4.0 | 2.0–1.0 | 1.0–0.7 | 1.0–1.25 |
| III | 4.0–10.0 | 1.0–0.5 | 0.7–0.4 | 1.25–2.5 |
| IV | over 10.0 | less than 0.5 | less than 0.4 | over 2.5 |
| Irrigation Water Quality Class | Permissible Salinity Irrigation Water Levels (g/L) for Soils Characterized: | ||
|---|---|---|---|
| Heavy Particle Size Distribution and/or SAC Greater than 30 | Medium Particle Size Distribution and/or a SAC from 30 to 15 | Light Particle Size Distribution and/or SAC Less than 15 | |
| I—non-hazardous | 0.2–0.5 | 0.2 | 0.2–0.7 |
| II—low-hazardous | 0.5–0.8 | 0.6 | 0.7–1.2 |
| III—moderately hazardous | 0.8–1.2 | 0.6–1.0 | 1.2–2.0 |
| IV—hazardous | over 1.2 | 1.0–1.5 | Over 2.0 |
| Water Chemical Component | Degree of Water Suitability for Drip Irrigation | ||
|---|---|---|---|
| Suitable | Conditionally Suitable | Not Suitable | |
| TDS, mg/L | 500 | 500–2000 | >2000 |
| HCO3, mg-eq/L | <2.0 | >2.0 | >2.5 |
| pH | 6–7 | 7–8 | >8 |
| Fe, mg/L | 0.2 | 0.2–1.5 | >1.5 |
| District | Aquifer | Chemical Groundwater Composition |
|---|---|---|
| Aktobe | Mataykum | |
| Myngyr | ||
| North Aishuak | ||
| Aishuak | ||
| West Kazakhstan | Yanvartsevskoye | |
| Krasnouralskoye | ||
| Mangistau | Ulanak | |
| Atyrau | Koyandy |
| Micro-Component | Groundwater Deposit | ||||||
|---|---|---|---|---|---|---|---|
| Mataykum and Myngyr | North Aishuak | Aishuak | Yanvartsevskoye | Krasnouralskoye | Ulanak | Koyandy | |
| Nitrate | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 |
| Nitrite | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Ammonium | 0.29–0.49 | 9.4–16.1 | 0.1–5.9 | up to 5.5 | Not measured | <0.05 | 0.18–0.19 |
| Silicon | 5.2–6.3 | 1.3–5.4 | 0.6–4.9 | 0.46–1.14 | 5.7–7.2 | 5.6–5.8 | 6.1–6.3 |
| Total iron | Up to 1.0 | up to 3.8 | 0.6–2.8 | up to 0.8 | 0.6–1.2 | 0.8–1.2 | 0.8–1.2 |
| Petroleum | Up to 0.002 | 0.06–0.18 | up to 0.27 | 0.016–0.097 | 0.047–0.056 | No | No |
| Lead | 0.03–0.07 | 0.01–0.08 | up to 0.03 | 0.01–0.02 | 0.05–0.09 | up to 0.03 | 0.006–0.04 |
| Copper | 0.01–0.03 | 0.004–0.015 | 0.01–0.02 | 0.005–0.01 | 0.009–0.01 | 0.01–0.06 | up to 0.01 |
| Zinc | 0.002–0.01 | 0.002–0.007 | 0.002–0.01 | Not measured | 0.003–0.009 | up to 0.016 | up to 0.03 |
| Groundwater Well Field | Well No. | Groundwater Classes According to the Risk of Developing in the Soils: | Integrated Groundwater Quality Assessment | |||
|---|---|---|---|---|---|---|
| Chloride Salinization | Sodic Salinization | Magnesium Salinization | Soda Formation | |||
| Cl, mg-eq/L | Ca/Na, mg-eq/L | Ca/Mg, mg-eq/L | (CO32 + HCO3) − (Ca + Mg), mg-eq/L | |||
| North Aishuak | 9 | 2.03 (I) * | 4.59 (I) | 1.10 (I) | 1.15 (II) | (I)—excellent |
| 10 | 0.37 (I) | 3.80 (I) | 0.94 (II) | 1.60 (III) | (II)—good | |
| 12 | 0.29 (I) | 1.32 (II) | 1.10 (I) | 1.20 (II) | (I)—excellent | |
| Aishuak | 13 | 15.00 (IV) | 0.02 (IV) | 0.17 (IV) | 5.40 (IV) | (IV)—unsatisfactory |
| 15 | 21.30 (IV) | 0.27 (IV) | 1.34 (I) | 0.80 (I) | (III)—satisfactory | |
| 16 | 27.29 (IV) | 0.23 (IV) | 1.30 (I) | 0.65 (I) | (III)—satisfactory | |
| Mataykum | 1 | 16.50 (IV) | 0.10 (IV) | 0.98 (II) | 5.05 (IV) | (III)—satisfactory |
| 6 | 19.70 (IV) | 0.10 (IV) | 0.80 (II) | 1.40 (III) | (III)—satisfactory | |
| Myngyr | 2 | 8.60 (III) | 0.02 (IV) | 4.00 (I) | 3.80 (IV) | (IV)—unsatisfactory |
| 3 | 3.10 (II) | 0.07 (IV) | 1.60 (I) | 3.30 (IV) | (III)—satisfactory | |
| 4 | 17.25 (IV) | 0.04 (IV) | 1.50 (I) | 1.99 (III) | (III)—satisfactory | |
| 5 | 12.75 (IV) | 0.11 (IV) | 1.50 (I) | 2.00 (III) | (III)—satisfactory | |
| Yanvartsevskoye | 2118 | 0.70 (I) | 0.32 (IV) | 0.27 (IV) | 0.75 (I) | (III)—satisfactory |
| 2114 | 0.70 (I) | 0.50 (III) | 0.46 (III) | 3.55 (IV) | (III)—satisfactory | |
| Krasnouralskoye | 2110 | 0.50 (I) | 0.40 (IV) | 0.50 (III) | 0.95 (I) | (III)—satisfactory |
| - | 2.10 (II) | 0.80 (III) | 1.60 (I) | 0.10 (I) | (II)—good | |
| - | 6.70 (III) | 0.23 (IV) | 1.10 (I) | 0.80 (I) | (II)—good | |
| Ulanak | - | 65.61(IV) | 0.42 (III) | 1.20 (I) | 0.37 (I) | (III)—satisfactory |
| Ulanak, Zhyngyldy site | - | 0.70 (I) | 0.50 (III) | 0.27 (IV) | 0.76 (I) | (III)—satisfactory |
| Ulanak, Tugorochgan site | 1 | 47.51(IV) | 0.01 (IV) | 1.20 (I) | 5.60 IV | (V)—completely unsuitable |
| 2 | 7.50 (III) | 0.08 (IV) | 1.37 (I) | 2.05 (III) | (V)—completely unsuitable | |
| Koyandy | 2301 | 23.98(IV) | 0.30 (IV) | 1.00 (I) | 0.09 (I) | (III)—satisfactory |
| 2302 | 6.97 (III) | 0.13 (IV) | 1.10 (I) | 3.52 IV | (IV)—unsatisfactory | |
| 2303 | 4.74 (III) | 0.20 (IV) | 1.40 (I) | 2.41 (III) | (III)—satisfactory | |
| 2304 | 1.78 (I) | 0.50 (III) | 1.40 (I) | 2.12 (III) | (III)—satisfactory | |
| 2305 | 6.2 1(III) | 7.10 (I) | 47.40 (I) | 0.01 (I) | (II)—good | |
| 2306 | 3.27 (II) | 0.20 (IV) | 1.70 (I) | 2.48 (III) | (III)—satisfactory | |
| Groundwater Well Field | Well No. | Water Chemical Component/Water Suitability | |||
|---|---|---|---|---|---|
| TDS, mg/L | HCO3, mg-eq/L | pH | Fe, mg/L | ||
| North Aishuak | 9 | 2089 | 8.20 | 7.82 | 1.0 |
| Not suitable | Not suitable | Conditionally suitable | Conditionally suitable | ||
| 10 | 1616 | 5.00 | 8.32 | 0 | |
| Conditionally suitable | Not suitable | Not suitable | Suitable | ||
| Myngyr | 2 | 294 | 4.55 | 7.29 | 3.8 |
| Suitable | Not suitable | Conditionally suitable | Not suitable | ||
| 3 | 1515 | 18.20 | 7.37 | 2.8 | |
| Conditionally suitable | Not suitable | Conditionally suitable | Not suitable | ||
| 4 | 1515 | 18.20 | 7.37 | 2.8 | |
| Conditionally suitable | Not suitable | Conditionally suitable | Not suitable | ||
| 5 | 2518 | 3.50 | 7.41 | 0.6 | |
| Not suitable | Not suitable | Conditionally suitable | Conditionally suitable | ||
| Yanvartsevskoye | 2118 | 6630 | 2.60 | 7.11 | 1.2 |
| Not suitable | Not suitable | Conditionally suitable | Conditionally suitable | ||
| 2114 | 1402 | 2.60 | 7.96 | 0.8 | |
| Conditionally suitable | Not suitable | Conditionally suitable | Conditionally suitable | ||
| 2110 | 4444 | 3.20 | 7.99 | 0 | |
| Not suitable | Not suitable | Conditionally suitable | Suitable | ||
| Krasnouralskoye | - | 4316 | 6.20 | 8.03 | 0 |
| Not suitable | Not suitable | Not suitable | Suitable | ||
| - | 259 | 6.10 | 7.85 | 0 | |
| Suitable | Not suitable | Conditionally suitable | Suitable | ||
| Ulanak–Kuibyshevo | - | 281 | 1.80 | 8.40 | 0 |
| Suitable | Suitable | Not suitable | Suitable | ||
| Ulanak-Kuibyshevo | - | 306 | 4.75 | 8.20 | 0.8 |
| Suitable | Not suitable | Not suitable | Conditionally suitable | ||
| Ulanak | 1 | 1232 | 7.85 | 7.44 | 0.6 |
| Conditionally suitable | Not suitable | Conditionally suitable | Conditionally suitable | ||
| 2 | 1102 | 7.85 | 7.61 | 1.2 | |
| Conditionally suitable | Not suitable | Conditionally suitable | Conditionally suitable | ||
| Koyandy | 2301 | 2389 | 6.10 | 6.90 | 0 |
| Not suitable | Not suitable | Suitable | Suitable | ||
| 2302 | 1150 | 6.70 | 7.89 | 0.76 | |
| Conditionally suitable | Not suitable | Conditionally suitable | Conditionally suitable | ||
| 2303 | 1368 | 7.49 | 7.78 | 0 | |
| Conditionally suitable | Not suitable | Conditionally suitable | Suitable | ||
| 2304 | 754 | 6.60 | 7.45 | 0 | |
| Conditionally suitable | Not suitable | Conditionally suitable | Suitable | ||
| 2305 | 1218 | 6.51 | 7.80 | 1.22 | |
| Conditionally suitable | Not suitable | Conditionally suitable | Conditionally suitable | ||
| 2306 | 938 | 6.10 | 7.84 | 2.14 | |
| Conditionally suitable | Not suitable | Conditionally suitable | Not suitable | ||
| Water | Soil | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TDS | pH | Total Hardness | Cl | НСО3 | Fe | Ca/Na | Ca/Mg | (CO3 + HCO3) − (Ca + Mg) | SAR | Ka by Staebler | (Ca/Mg) | (Ca/Na) | (Na/Mg) | (HCO3 + CO3)/SO4) | (HCO3 + CO3/Cl) | SO4/Cl | pH | salt content, % | ||
| Water | TDS | 1.00 | ||||||||||||||||||
| pH | 0.12 | 1.00 | ||||||||||||||||||
| Total hardness | 0.68 | −0.19 | 1.00 | |||||||||||||||||
| Cl | 0.87 | −0.03 | 0.75 | 1.00 | ||||||||||||||||
| НСО3 | −0.01 | −0.34 | 0.01 | 0.04 | 1.00 | |||||||||||||||
| Fe | 0.20 | −0.55 | 0.24 | 0.31 | 0.48 | 1.00 | ||||||||||||||
| Ca/Na | −0.25 | −0.18 | −0.04 | −0.22 | −0.05 | −0.03 | 1.00 | |||||||||||||
| Ca/Mg | −0.07 | 0.07 | −0.02 | −0.07 | −0.28 | −0.24 | 0.75 | 1.00 | ||||||||||||
| (CO3 + HCO3) − (Ca + Mg), mg-eq/L | 0.07 | 0.35 | −0.36 | 0.08 | 0.13 | 0.06 | −0.34 | −0.23 | 1.00 | |||||||||||
| SAR | 0.54 | 0.29 | −0.09 | 0.48 | −0.15 | 0.01 | −0.15 | −0.07 | 0.46 | 1.00 | ||||||||||
| Ka by Staeblerw | −0.40 | −0.15 | −0.14 | −0.36 | 0.04 | −0.16 | 0.38 | 0.05 | −0.07 | −0.29 | 1.00 | |||||||||
| Soil | Ca/Mg | 0.27 | 0.19 | 0.01 | 0.23 | −0.30 | −0.02 | −0.24 | −0.18 | 0.45 | 0.02 | −0.15 | 1.00 | |||||||
| Ca/Na | −0.70 | −0.06 | −0.67 | −0.60 | −0.25 | 0.02 | 0.44 | 0.28 | 0.00 | 0.11 | 0.15 | −0.36 | 1.00 | |||||||
| Na/Mg | 0.19 | 0.12 | 0.00 | 0.19 | −0.25 | 0.01 | −0.18 | −0.13 | 0.50 | 0.05 | −0.09 | 0.97 | −0.36 | 1.00 | ||||||
| (HCO3 + CO3)/SO4 | −0.27 | −0.32 | −0.18 | −0.28 | 0.05 | −0.19 | 0.39 | −0.39 | −0.26 | −0.49 | −0.02 | −0.32 | 0.32 | −0.41 | 1.00 | |||||
| (HCO3 + CO3)/Cl | −0.32 | −0.24 | −0.10 | −0.14 | −0.05 | −0.04 | 0.27 | −0.41 | −0.39 | −0.18 | −0.13 | −0.27 | 0.35 | −0.40 | 0.77 | 1.00 | ||||
| SO4/Cl | −0.48 | −0.08 | −0.46 | −0.22 | −0.04 | 0.33 | 0.19 | 0.20 | 0.23 | 0.11 | 0.09 | 0.02 | 0.76 | 0.04 | −0.05 | 0.03 | 1.00 | |||
| рН | 0.36 | −0.07 | 0.53 | 0.36 | 0.09 | −0.01 | −0.18 | −0.41 | −0.12 | −0.08 | −0.18 | 0.49 | −0.81 | 0.48 | −0.09 | 0.05 | −0.68 | 1.00 | ||
| Salt content, % | 0.32 | 0.14 | 0.29 | 0.32 | −0.13 | −0.06 | −0.30 | −0.11 | 0.22 | 0.30 | −0.23 | 0.71 | −0.62 | 0.74 | −0.46 | −0.22 | −0.42 | 0.82 | 1.00 | |
| X (Independent) | Y (Dependent) | R2 | a | b | Regression Equation | Correlation Type |
|---|---|---|---|---|---|---|
| TDSW | Clw | 0.7639 | 9.2323 | −4.4308 | Cl−w = 9.2323(TDSW) − 4.4308 | positive correlation |
| (Total hardness)w | Clw | 0.565 | 1.3256 | 2.8812 | Cl−w = 1.3256(Total hardness)w + 2.8812 | positive correlation |
| (Ca/Na)w | (Ca/Mg)w | 0.5605 | 4.0347 | −0.4548 | (Ca/Mg)w= 4.0347(Ca/Na)w − 0.4548 | positive correlation |
| Salt contents, % | (Ca/Mg)s | 0.5053 | 2.8785 | 0.5099 | (Ca/Mg)s= 2.8785(Salt contents) + 0.5099 | positive correlation |
| Salt contents, % | (Na/Mg)s | 0.5545 | 76.03 | −56.687 | (Na/Mg)s = 76.03(Salt contents) − 56.687 | positive correlation |
| Salt contents, % | (pH)s | 0.6644 | 0.2216 | 7.4552 | (pH)s = 0.2216(Salt contents) + 7.4552 | positive correlation |
| (pH)s | (Ca/Na)s | 0.6504 | −5.2398 | 44.628 | (Ca/Na)s = −5.2398(pH)s + 44.628 | negative correlation |
| (pH)s | (SO4/Cl)s | 0.4646 | −0.5956 | 5.8417 | (SO4/Cl)s = −0.5956(pH)s + 5.8417 | negative correlation |
| TDSW | (Ca/Na)s | 0.3778 | −1.1441 | 5.7651 | (Ca/Na)s = −1.1441(TDSW) + 5.7651 | negative correlation |
| TDS | Salt contents, % | 0.2204 | 0.3687 | 0.5522 | Salt contents = 0.3687(TDSW) + 0.5522 | positive correlation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murtazin, Y.; Kulagin, V.; Mirlas, V.; Anker, Y.; Rakhimov, T.; Onglassynov, Z.; Rakhimova, V. Integrated Assessment of Groundwater Quality for Water-Saving Irrigation Technology (Western Kazakhstan). Water 2025, 17, 1232. https://doi.org/10.3390/w17081232
Murtazin Y, Kulagin V, Mirlas V, Anker Y, Rakhimov T, Onglassynov Z, Rakhimova V. Integrated Assessment of Groundwater Quality for Water-Saving Irrigation Technology (Western Kazakhstan). Water. 2025; 17(8):1232. https://doi.org/10.3390/w17081232
Chicago/Turabian StyleMurtazin, Yermek, Vitaly Kulagin, Vladimir Mirlas, Yaakov Anker, Timur Rakhimov, Zhyldyzbek Onglassynov, and Valentina Rakhimova. 2025. "Integrated Assessment of Groundwater Quality for Water-Saving Irrigation Technology (Western Kazakhstan)" Water 17, no. 8: 1232. https://doi.org/10.3390/w17081232
APA StyleMurtazin, Y., Kulagin, V., Mirlas, V., Anker, Y., Rakhimov, T., Onglassynov, Z., & Rakhimova, V. (2025). Integrated Assessment of Groundwater Quality for Water-Saving Irrigation Technology (Western Kazakhstan). Water, 17(8), 1232. https://doi.org/10.3390/w17081232










