Illuminating the Impact of a Floating Photovoltaic System on a Shallow Drinking Water Reservoir: The Emergence of Benthic Cyanobacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Local Kralingen Reservoir (Shallow Reservoir)
2.2. Sun-Tracking Floating Photovoltaic System
2.3. Diving Inspections
2.4. Nutrients, Phytoplankton, and Zooplankton
2.5. Field Measurements: Temperature, Oxygen, Secchi Disk Depth, and Light
2.6. Calculations of Light at Bottom of Reservoir and Euphotic Zone
2.7. Metals and Metalloids
2.8. Birds, Bird Droppings, and Faecal Bacteria
2.9. Data Analysis
3. Results and Discussion
3.1. Shift from Stonewort and Macrophytes to Benthic Cyanobacteria
3.2. Biofouling of the FPV System: Massive Development of Dreissena Mussels
3.3. Metals and Metalloids: No Exceedance of Quality Standards
3.4. Phytoplankton Reduced in Reservoir, Differences in Zooplankton Composition
3.5. Temperature and Oxygen Profiles Very Similar
3.6. No Significant Changes in the Number of Birds or Concentrations of Faecal Bacteria
3.7. Mitigating Negative Effects and Future Directions of FPV Systems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Where Sun Meets Water: Floating Solar Market Report. Washington, DC. Available online: http://hdl.handle.net/10986/31880 (accessed on 1 December 2024).
- Spencer, R.S.; Macknick, J.; Aznar, A.; Warren, A.; Reese, M.O. Floating photovoltaic systems: Assessing the technical potential of photovoltaic systems on man-made water bodies in the continental United States. Environ. Sci. Technol. 2019, 53, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
- Roadmap PV Systemen en Toepassingen. Available online: https://www.uu.nl/sites/default/files/roadmap-pv-systemen-en-toepassingen-final.pdf (accessed on 1 December 2024). (In Dutch).
- Floating Solar PV Gains Global Momentum. PV Magazine. Available online: https://www.pv-magazine.com/2020/09/22/floating-solar-pv-gains-global-momentum/ (accessed on 1 December 2024).
- Nain, P.; Kumar, A. Understanding the possibility of material release from end-of-life solar modules: A study based on literature review and survey analysis. Renew. Energy 2020, 160, 903–918. [Google Scholar] [CrossRef]
- Cazzaniga, R.; Cicu, M.; Rosa-Clot, M.; Rosa-Clot, P.; Tina, G.M.; Ventura, C. Floating PV plants: Performance analysis and design solutions. Renew. Sustain. Energy Rev. 2018, 81, 1730–1741. [Google Scholar] [CrossRef]
- Dörenkämper, M.; Wahed, A.; Kumar, A.; De Jong, M.; Kroon, J.; Reindl, T. The cooling effect of floating PV in two different climate zones: A comparison of field test data from the Netherlands and Singapore. Sol. Energy 2021, 214, 239–247. [Google Scholar] [CrossRef]
- Ziar, H.; Prudon, B.; Lin, F.-Y.; Roeffen, B.; Heijkoop, F.; Stark, T.; Teurlincx, S.; De Senerpont Domis, L.; Garcia Goma, E.; Garro Extebarria, J.; et al. Innovative floating bifacial photovoltaic solutions for inland water areas. Progr. Photovolt. Res. Appl. 2021, 29, 725–743. [Google Scholar] [CrossRef]
- Redón Santafé, M.; Torregrosa Soler, J.B.; Sánchez Romero, F.J.; Ferrer Gisbert, P.S.; Ferrán Gozálvez, J.J.; Ferrer Gisbert, C.M. Theoretical and experimental analysis of a floating photovoltaic cover for water irrigation reservoirs. Energy 2014, 67, 246–255. [Google Scholar] [CrossRef]
- Haas, J.; Khalighi, J.; De la Fuente, A.; Gerbersdorf, S.U.; Nowak, W.; Chen, P.W. Floating photovoltaic plants: Ecological impacts versus hydropower operation flexibility. Energy Conv. Manag. 2020, 206, 112414. [Google Scholar] [CrossRef]
- Exley, G.; Page, T.; Thackeray, S.J.; Folkard, A.M.; Couture, R.M.; Hernandez, R.R.; Cagle, A.E.; Salk, K.R.; Clous, L.; Whittaker, P.; et al. Floating solar panels on reservoirs impact phytoplankton populations: A modelling experiment. J. Environ. Manag. 2022, 324, 116410. [Google Scholar] [CrossRef]
- Cagle, A.E.; Armstrong, A.; Exley, G.; Grodsky, S.M.; Macknick, J.; Sherwin, J.; Hernandez, R.R. The land sparing, water surface use efficiency, and water surface transformation of floating photovoltaic solar energy installations. Sustainability 2020, 12, 8154. [Google Scholar] [CrossRef]
- Nugent, D.; Sovacool, B.K. Assessing the lifecycle greenhouse gas emissions from solar PV and wind energy: A critical meta-survey. Energy Policy 2014, 65, 229–244. [Google Scholar] [CrossRef]
- Sahu, A.; Yadav, N.; Sudhakar, K. Floating photovoltaic power plant: A review. Renew. Sustain. Energy Rev. 2016, 66, 815–824. [Google Scholar] [CrossRef]
- Nain, P.; Kumar, A. Metal dissolution from end-of-life solar photovoltaics in real landfill leachate versus synthetic solutions: One-year study. Waste Manag. 2020, 1, 351–361. [Google Scholar] [CrossRef]
- Nover, J.; Zapf-Gottwick, R.; Feifel, C.; Koch, M.; Werner, J.H. Leaching via weak spots in photovoltaic modules. Energies 2021, 14, 692. [Google Scholar] [CrossRef]
- Panthi, G.; Bajagain, R.; An, Y.; Jeong, S. Leaching potential of chemical species from real perovskite and silicon solar cells. Process Safety Environ. Protect. 2021, 149, 115–122. [Google Scholar] [CrossRef]
- Armstrong, A.; Page, T.; Thackeray, S.J.; Hernandez, R.R.; Jones, I.D. Integrating environmental understanding into freshwater floatovoltaic deployment using an effects hierarchy and decision trees. Environ. Res. Lett. 2020, 15, 114055. [Google Scholar] [CrossRef]
- De Lima, R.L.P.; Paxinou, K.; Boogaard, F.C.; Akkerman, O.; Lin, F. In-Situ water quality observations under a large-scale floating solar farm using sensors and underwater drones. Sustainability 2021, 13, 6421. [Google Scholar] [CrossRef]
- Exley, G.; Armstrong, A.; Page, T.; Jones, I.D. Floating photovoltaics could mitigate climate change impacts on water body temperature and stratification. Sol. Energy 2021, 219, 24–33. [Google Scholar] [CrossRef]
- Wagner, C.; Adrian, R. Cyanobacteria dominance: Quantifying the effects of climate change. Limnol. Ocean. 2009, 54, 2460–2468. [Google Scholar] [CrossRef]
- Marsden, M.W. Lake restoration by reducing external phosphorus loading: The influence of sediment phosphorus release. Freshw. Biol. 1989, 21, 139–162. [Google Scholar] [CrossRef]
- Visser, P.M.; Verspagen, J.M.H.; Sandrini, G.; Stal, L.J.; Matthijs, H.C.P.; Davis, T.W.; Paerl, H.W.; Huisman, J. How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae 2016, 54, 145–159. [Google Scholar] [CrossRef]
- Van Westreenen, A.; Zhang, N.; Kaiser, E.; Morales, A.; Evers, J.; Anten, N.; Marcelis, L. Rapid irradiance fluctuations occur in a greenhouse: Quantification and implication. Biosyst. Eng. 2023, 235, 215–229. [Google Scholar] [CrossRef]
- Poole, H.H.; Atkins, W.R.G. Photo-electric measurement of submarine illumination throughout the year. J. Mar. Biol. Assoc. U.K. 1929, 16, 297–324. [Google Scholar] [CrossRef]
- Middelboe, A.L.; Markager, S. Depth limits and minimum light requirements of freshwater macrophytes. Freshw. Biol. 1997, 37, 553–568. [Google Scholar] [CrossRef]
- Mathijssen, D.; Hofs, B.; Spierenburg-Sack, E.; Van Asperen, R.; Van der Wal, A.; Vreeburg, J.; Ketelaars, H. Potential impact of floating solar panels on water quality in reservoirs; pathogens and leaching. Water Pract. Technol. 2020, 15, 807–811. [Google Scholar] [CrossRef]
- NEN-EN-ISO 7899-2:2000; Water Quality-Detection and Enumeration of Intestinal Enterococci-Part 2: Membrane Filtration Method. Royal Netherlands Standardization Institute: Delft, The Netherlands, 2000. Available online: https://www.nen.nl/en/nen-en-iso-7899-2-2000-en-58860 (accessed on 1 December 2024).
- NEN-EN-ISO 9308-1:2014; Water Quality-Enumeration of Escherichia coli and Coliform Bacteria-Part 1: Membrane Filtration Method for Waters with Low Bacterial Background Flora. Royal Netherlands Standardization Institute: Delft, The Netherlands, 2014. Available online: https://www.nen.nl/en/nen-en-iso-9308-1-2014-en-199719 (accessed on 1 December 2024).
- Herb, W.R.; Stafan, H.G. Dynamics of vertical mixing in a shallow lake with submersed macrophytes. Water Resour. Res. 2005, 41, W02023. [Google Scholar] [CrossRef]
- Torma, P.; Wu, C.H. Temperature and circulation dynamics in a small and shallow lake: Effects of weak stratification and littoral submerged macrophytes. Water 2019, 11, 128. [Google Scholar] [CrossRef]
- Kovtun-Kante, A.; Torn, K.; Kotta, J. In situ production of charophyte communities under reduced light conditions in a brackish-water ecosystem. Est. J. Ecol. 2014, 63, 28–38. [Google Scholar] [CrossRef]
- Scheffer, M.; Rinaldi, S.; Gragnani, A.; Mur, L.R.; Van Nes, E.H. On the Dominance of filamentous cyanobacteria in shallow, turbid lakes. Ecology 1997, 78, 272–282. [Google Scholar] [CrossRef]
- Erratt, K.J.; Creed, I.F.; Freeman, E.C.; Trick, C.G.; Westrick, J.; Birbeck, J.A.; Watson, C.; Zastepa, A. Deep cyanobacteria layers: An overlooked aspect of managing risks of cyanobacteria. Environ. Sci. Technol. 2022, 56, 17902–17912. [Google Scholar] [CrossRef]
- Grossman, A.R.; Schaefer, M.R.; Chiang, G.G.; Collier, J.L. The phycobilisome, a light-harvesting complex responsive to environmental conditions. Microbiol. Rev. 1993, 57, 725–749. [Google Scholar] [CrossRef]
- Tandeau de Marsac, N. Phycobiliproteins and phycobilisomes: The early observations. Photosynth. Res. 2003, 76, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Gushulak, C.A.C.; Mezzini, S.; Moir, K.E.; Simpson, G.L.; Bunting, L.; Wissel, B.; Engstrom, D.R.; Laird, K.R.; St. Amand, A.; Cumming, B.F.; et al. Impacts of a century of land-use change on the eutrophication of large, shallow, prairie Lake Manitoba in relation to adjacent Lake Winnipeg (Manitoba, Canada). Freshwat. Biol. 2024, 69, 47–63. [Google Scholar] [CrossRef]
- Watermann, F.; Hillebrand, H.; Gerdes, G.; Krumbein, W.E.; Sommer, U. Competition between benthic cyanobacteria and diatoms as influenced by different grain sizes and temperatures. Mar. Ecol. Progr. Ser. 1999, 187, 77–87. [Google Scholar] [CrossRef]
- Søndergaard, M.; Phillips, G.; Hellsten, S.; Kolada, A.; Ecke, F.; Mäemets, H.; Mjelde, M.; Azzella, M.M.; Oggioni, A. Maximum growing depth of submerged macrophytes in European lakes. Hydrobiologia 2013, 704, 165–177. [Google Scholar] [CrossRef]
- Quiblier, C.; Wood, S.; Echenique-Subiabre, I.; Heath, M.; Villeneuve, A.; Humbert, J. A review of current knowledge on toxic benthic freshwater cyanobacteria–ecology, toxin production and risk management. Water Res. 2013, 47, 5464–5479. [Google Scholar] [CrossRef]
- Rangel, M.; Martins, J.C.G.; Garcia, N.A.; Conserva, G.A.A.; Costa-Neves, A.; Sant’Anna, C.L.; De Carvalho, L.R. Analysis of the toxicity and histopathology induced by the oral administration of Pseudanabaena galeata and Geitlerinema splendidum (cyanobacteria) extracts to mice. Mar. Drugs 2014, 12, 508–524. [Google Scholar] [CrossRef]
- Piriou, P.; Devesa, R.; De Lalande, M.; Glucina, K. European reassessment of MIB and geosmin perception in drinking water. European reassessment of MIB and geosmin perception in drinking water. J. Water Supply Res. Technol.-AQUA 2009, 58, 532–538. [Google Scholar] [CrossRef]
- Jüttner, F.; Watson, S.B. Biochemical and ecological control of geosmin and 2-methylisoborneol in source waters. Appl. Environ. Microbiol. 2007, 73, 4395–4406. [Google Scholar] [CrossRef]
- Slater, G.P.; Blok, V.C. Volatile compounds of the Cyanophyceae—A review. Water Sci. Technol. 1983, 15, 181–190. [Google Scholar] [CrossRef]
- Klatt, J.; Meyer, S.; Häusler, S.; Macalady, J.L.; De Beer, D.; Polerecky, L. Structure and function of natural sulphide-oxidizing microbial mats under dynamic input of light and chemical energy. ISME J. 2016, 10, 921–933. [Google Scholar] [CrossRef]
- Lichtenberg, M.; Cartaxana, P.; Kühl, M. Vertical migration optimizes photosynthetic efficiency of motile cyanobacteria in a coastal microbial mat. Front. Mar. Sci. 2020, 7, 359. [Google Scholar] [CrossRef]
- Jeppesen, E.; Jensen, J.P.; Søndergaard, M.; Lauridsen, T.; Pedersen, L.J.; Jensen, L. Top-down control in freshwater lakes: The role of nutrient state, submerged macrophytes and water depth. Hydrobiologia 1997, 342, 151–164. [Google Scholar] [CrossRef]
- Wetzel, R.G. Limnology: Lake and River Ecosystems, 3rd ed.; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- Kraak, M.H.S.; Lavy, D.; Peeters, W.H.M.; Davids, C. Chronic ecotoxicity of copper and cadmium to the zebra mussel Dreissena polymorpha. Arch. Environ. Contam. Toxicol. 1992, 23, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Gergs, R.; Karl-Otto, R. Effects of zebra mussels on a native amphipod and the invasive Dikerogammarus villosus: The influence of biodeposition and structural complexity. J. N. Am. Benthol. Soc. 2008, 27, 541–548. [Google Scholar] [CrossRef]
- Kobak, J.; Jermacz, Ł.; Płąchocki, D. Effectiveness of zebra mussels to act as shelters from fish predators differs between native and invasive amphipod prey. Aquat. Ecol. 2014, 48, 397–408. [Google Scholar] [CrossRef]
- Foster, K.W.; Smyth, R.D. Light antennas in phototactic algae. Microbiol. Rev. 1980, 44, 572–630. [Google Scholar] [CrossRef]
- Bastviken, D.T.E.; Caraco, N.F.; Cole, J.J. Experimental measurements of zebra mussel (Dreissena polymorpha) impacts on phytoplankton community composition. Freshwat. Biol. 1998, 39, 375–386. [Google Scholar] [CrossRef]
- Karpowicz, M.; Feniova, I.Y.; Sakharova, E.G.; Gorelysheva, Z.I.; Więcko, A.; Górniak, A.; Dzialowski, A.R. Top-down and bottom-up control of phytoplankton communities by zebra mussels Dreissena polymorpha (Pallas, 1771). Sci. Total Environ. 2023, 877, 162899. [Google Scholar] [CrossRef]
- Yamamichi, M.; Kazama, T.; Tokita, K.; Katano, I.; Doi, H.; Yoshida, T.; Hairston, N.G., Jr.; Urabe, J. A shady phytoplankton paradox: When phytoplankton increases under low light. Proc. R. Soc. B 2018, 285, 20181067. [Google Scholar] [CrossRef]
- Bramm, M.E.; Lassen, M.K.; Liboriussen, L.; Richardson, K.; Ventura, M.; Jeppesen, E. The role of light for fish-zooplankton-phytoplankton interactions during winter in shallow lakes—A climate change perspective. Freshw. Biol. 2009, 54, 1093–1109. [Google Scholar] [CrossRef]
- Ilgen, K.; Schindler, D.; Wieland, S.; Lange, J. The impact of floating photovoltaic power plants on lake water temperature and stratification. Sci. Rep. 2023, 13, 7932. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.D.; Stīpniece, A. Interactions between stoneworts (Charales) and waterbirds. Biol. Rev. Camb. Philos. Soc. 2024, 99, 390–408. [Google Scholar] [CrossRef] [PubMed]
- Van Breemen, L.W.C.A.; Ketelaars, H.A.M.; Visser, P.; Ebbeng, J.H. A new method to control growth of geosmin producing benthic cyanobacteria. Verh. Internat. Verein. Limnol. 1991, 24, 2168–2173. [Google Scholar] [CrossRef]
- Wood, S.A.; Kelly, L.; Bouma-Gregson, K.; Humbert, J.F.; Laughinghouse, H.D., 4th; Lazorchak, J.; McAllister, T.; McQueen, A.; Pokrzywinski, K.; Puddick, J.; et al. Toxic benthic freshwater cyanobacterial proliferations: Challenges and solutions for enhancing knowledge and improving monitoring and mitigation. Freshw. Biol. 2020, 65, 1824–1842. [Google Scholar] [CrossRef]
- Piel, T.; Sandrini, G.; Weenink, E.F.J.; Qin, H.; Herk, M.J.V.; Morales-Grooters, M.L.; Schuurmans, J.M.; Slot, P.C.; Wijn, G.; Arntz, J.; et al. Shifts in phytoplankton and zooplankton communities in three cyanobacteria-dominated lakes after treatment with hydrogen peroxide. Harmful Algae 2024, 133, 102585. [Google Scholar] [CrossRef]
- Geer, T.D.; Calomeni, A.J.; Kinley, C.M.; Iwinski, K.J.; Rodgers, J.H. Predicting in situ responses of taste-and odor-producing algae in a Southeastern US reservoir to a sodium carbonate peroxyhydrate algaecide using a laboratory exposure-response model. Water Air Soil Poll. 2017, 228, 53. [Google Scholar] [CrossRef]
- Calomeni, A.J.; Iwinski, K.J.; Kinley, C.M.; McQueen, A.; Rodgers, J.H., Jr. Responses of Lyngbya wollei to algaecide exposures and a risk characterization associated with their use. Ecotoxicol. Environm. Safety 2015, 116, 90–98. [Google Scholar] [CrossRef]
- McLaughlan, C.; Aldridge, D.C. Cultivation of zebra mussels (Dreissena polymorpha) within their invaded range to improve water quality in reservoirs. Water Res. 2013, 47, 4357–4369. [Google Scholar] [CrossRef]
- Mackie, G.L.; Claudi, R. Monitoring and Control of Macrofouling Mollusks in Fresh Water Systems, 2nd ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2010. [Google Scholar]
- Donskoy, D.M.; Ludyanskiy, M.; Wright, D.A. Effects of sound and ultrasound on Zebra Mussels. J. Acoust. Soc. Am. 1996, 99, 2577–2603. [Google Scholar] [CrossRef]
- Lürling, M.; Tolman, Y. Beating the blues: Is there any music in fighting cyanobacteria with ultrasound? Water Res. 2014, 66, 361–373. [Google Scholar] [CrossRef]
- NEN-EN 15204:2006; Water Quality-Guidance Standard on the Enumeration of Phytoplankton Using Inverted Microscopy (Utermöhl Technique). Royal Netherlands Standardization Institute: Delft, The Netherlands, 2006. Available online: https://www.nen.nl/en/nen-en-15204-2006-en-109691 (accessed on 1 December 2024).
- French, J.R.P., III; Adams, J.V.; Craig, J.; Stickel, R.G.; Nichols, S.J.; Fleischer, G.W. Shell-free biomass and population dynamics of dreissenids in offshore Lake Michigan, 2001–2003. J. Great Lakes Res. 2007, 33, 536–545. [Google Scholar] [CrossRef]
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management, 2nd ed.; CRC Press: London, UK, 2021. [Google Scholar] [CrossRef]

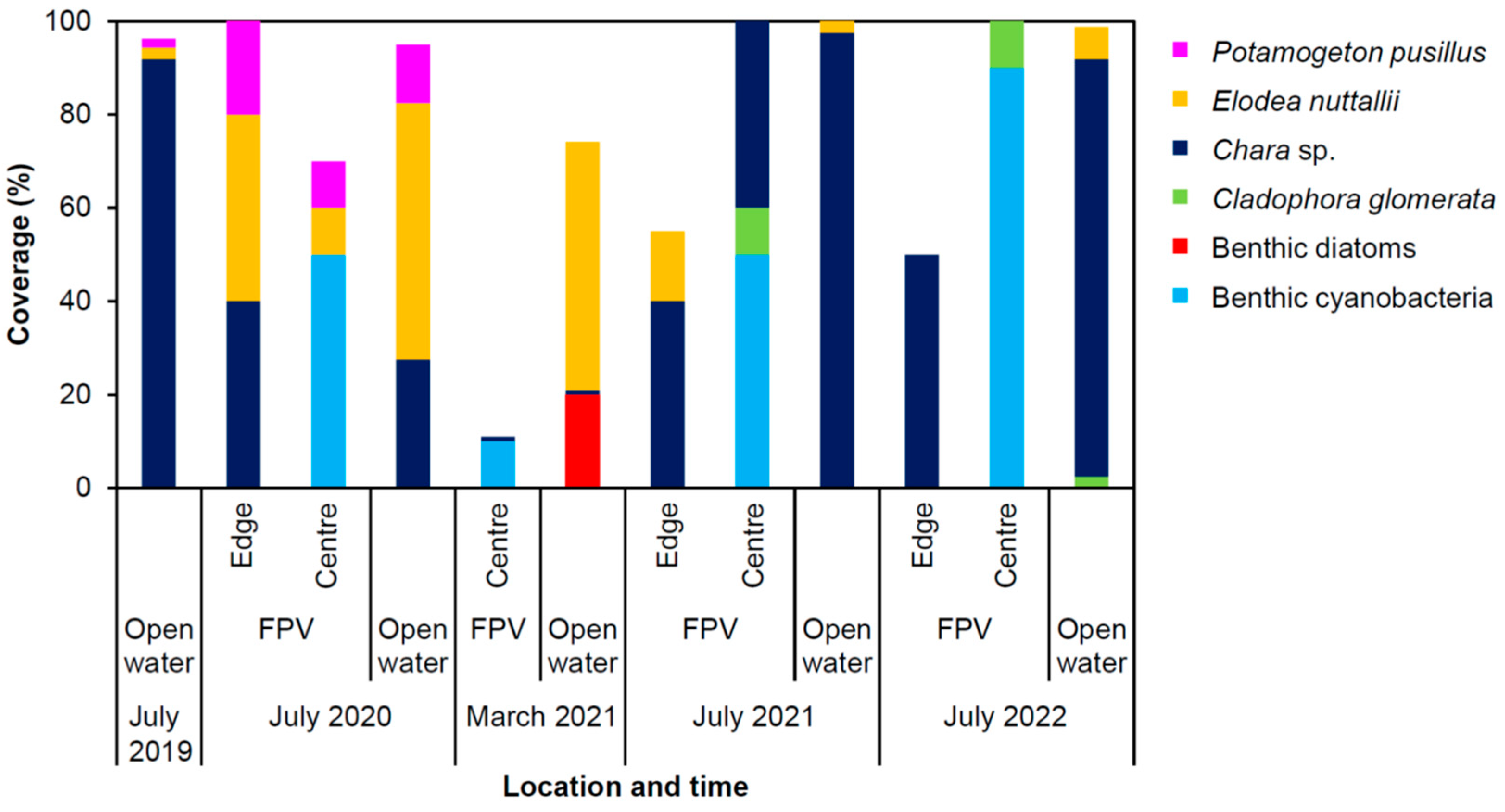
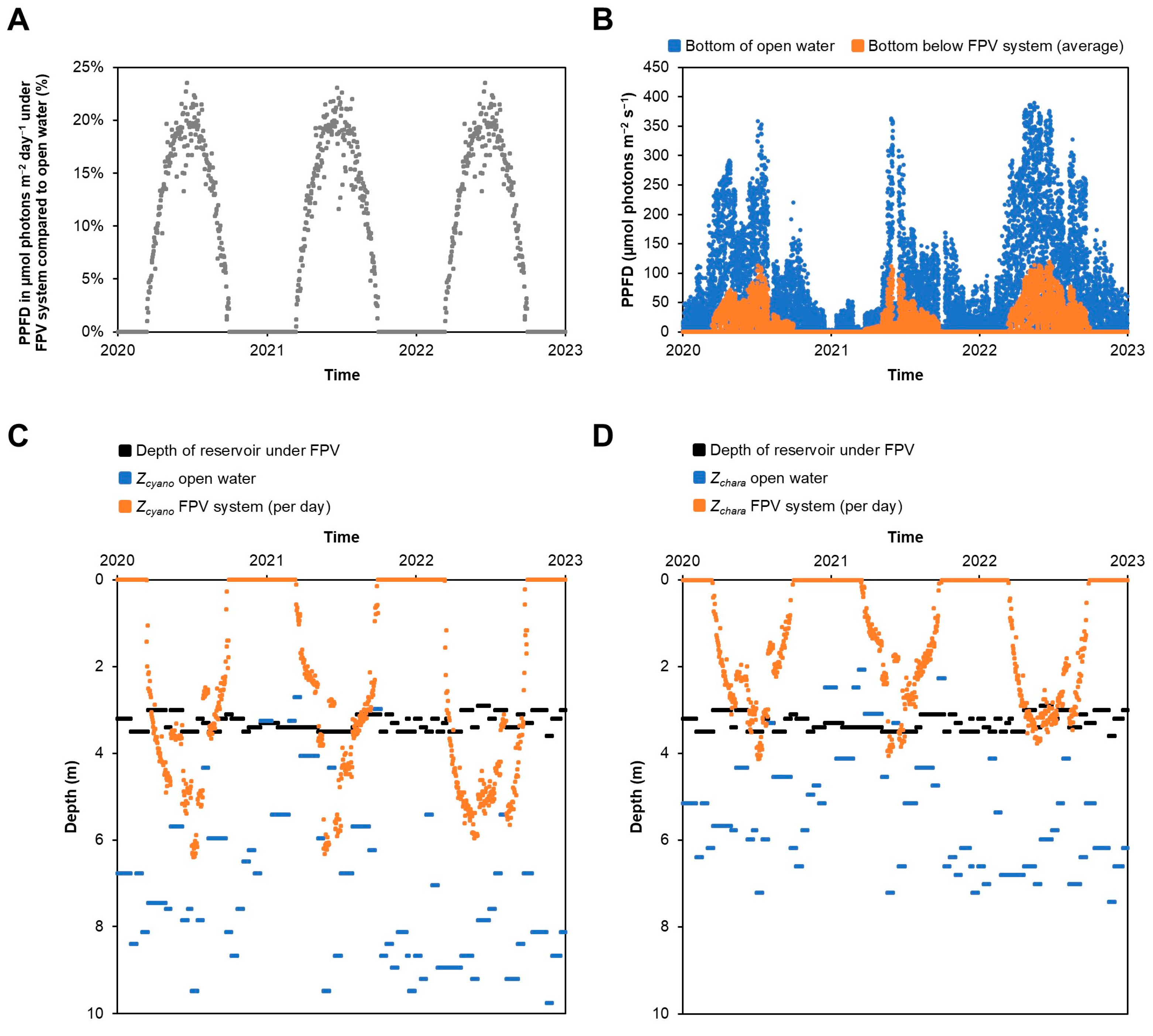


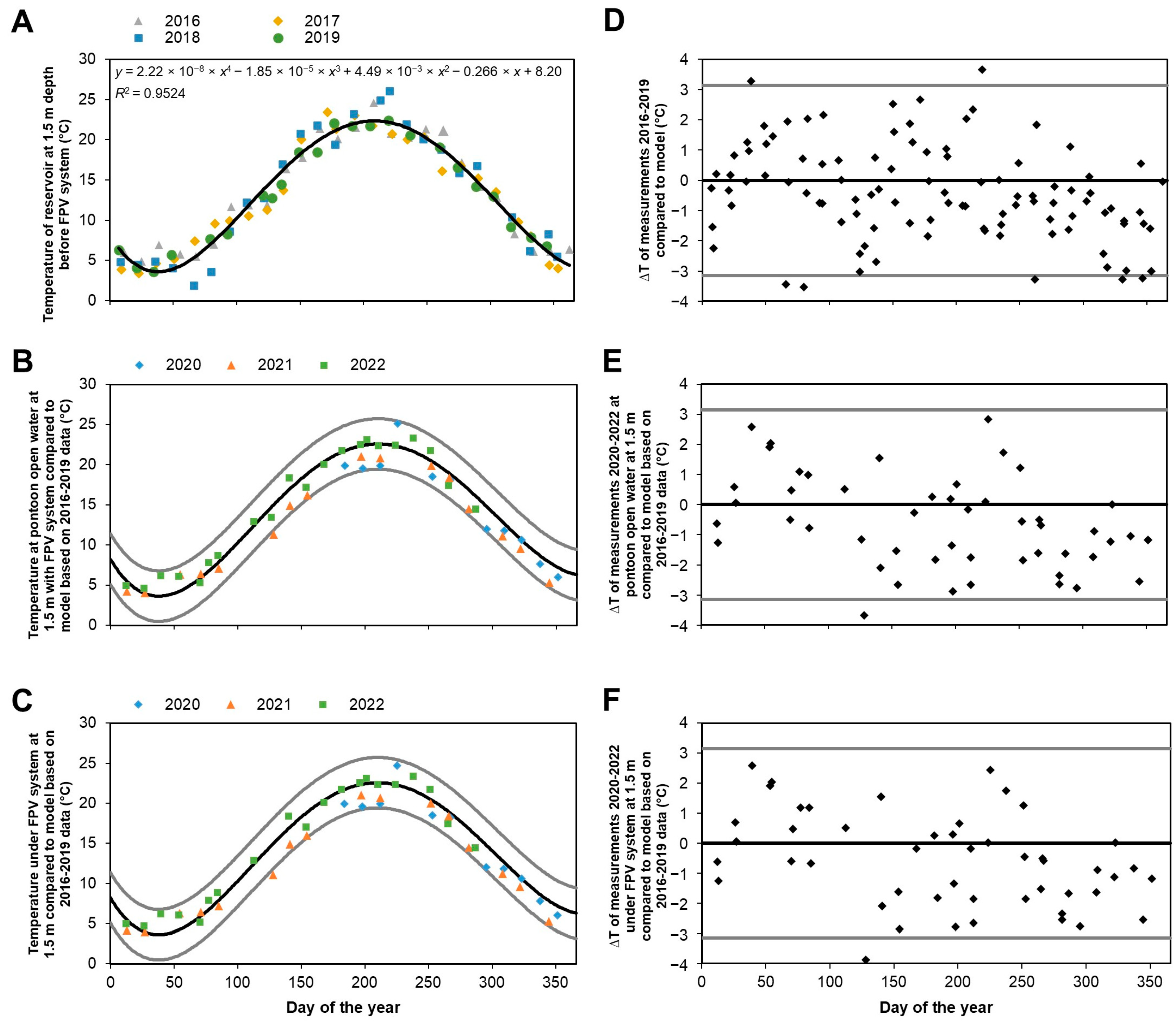
| Date | Benthic Cyanobacteria Identified by Microscopy (in Order of Decreasing Dominance) | Samples for Analyses of Compounds | Taste and Odour Compounds (ng L−1) | Cyanotoxins (µg g−1 Wet Weight) |
|---|---|---|---|---|
| 19 July 2019 | No benthic cyanobacterial mats detected (before FPV) | - | n.a. | n.a. |
| 30 July 2020 | Gomphosphaeria aponina | - | n.a. | n.a. |
| 10 March 2021 | Gomphosphaeria aponina | - | n.a. | n.a. |
| 29 July 2021 | Gomphosphaeria aponina; Phormidium cf. breve; Phormidium cf. tenue; Pseudanabaena sp. | Middle of water column under centre of FPV | 2.8 (geosmin) 2.7 (2-MIB) | n.a. |
| Benthic mat under FPV | n.a. | 0.014 (anatoxin-a) | ||
| 26 July 2022 | Phormidium breve, Phormidium sp., Microcystis aeruginosa *, Pseudanabaena sp. | Water under FPV just above benthic mat | 20 (geosmin) 0.8 (2-MIB) | n.a. |
| Benthic mat under FPV | n.a. | 8.8 (microcystins; 89% MC-LR) |
| Location | Date | Density (n m−2) | Average Condition Index 1 (n; s.d.) | Significant Differences in Condition Index 1 |
|---|---|---|---|---|
| Pontoon open water (shallow reservoir) | 30 July 2020 | 2250 | n.a. 2 | - |
| Pontoon open water (shallow reservoir) | 10 March 2021 | 240 | 207.7 (25; 35.5) | Group a |
| Pontoon open water (shallow reservoir) | 29 July 2021 | 104,000 | 89.2 (20; 22.5) | Group b |
| Pontoon open water (shallow reservoir) | 26 July 2022 | 23,000 | 61.8 (24; 11.0) | Group d |
| FPV system (shallow reservoir) | 30 July 2020 | n.d. | n.d. | - |
| FPV system (shallow reservoir) | 10 March 2021 | n.d. | n.d. | - |
| FPV system (shallow reservoir) | 29 July 2021 | 600 | n.a. | - |
| FPV system (shallow reservoir) | 26 July 2022 | 11,500 | 62.8 (24; 9.3) | Group d |
| Petrusplaat reservoir * | 9 March 2021 | n.a. | 37.0 (25; 8.2) | Group c |
| Petrusplaat reservoir * | 2 August 2021 | n.a. | 41.6 (25; 14.2) | Group c |
| Petrusplaat reservoir * | 26 July 2022 | n.a. | 50.0 (24; 16.4) | Group cd |
| Water Birds (Family) | A: November 2014–February 2015 (Before FPV System, n = 17) | B: November 2019–February 2020 (Before FPV System, n = 17) | C: November 2020–February 2021 (with FPV System, n = 17) | A Different from B 1 | B Different from C 1 | A Different from C 1 | |||
|---|---|---|---|---|---|---|---|---|---|
| Avg. (Min; Max) | Median (Quartile 1; Quartile 3) | Avg. (Min; Max) | Median (Quartile 1; Quartile 3) | Avg. (Min; Max) | Median (Quartile 1; Quartile 3) | ||||
| Rallidae | 29.8 (1; 64) | 29.0 (2; 55) | 99.8 (2; 339) | 6 (4; 267) | 63.2 (14; 139) | 64 (30; 85) | No (p = 0.448) | No (p = 0.241) | Yes (p = 0.008) |
| Anatidae | 197 (8; 462) | 178 (84; 270) | 111 (19; 320) | 71 (42.5; 154) | 91.4 (9; 237) | 75 (42.5; 153) | Yes (p = 0.048) | No (p = 0.679) | Yes (p = 0.016) |
| Lardidae | 21.6 (0; 81) | 8 (2.5; 44) | 24.6 (1; 71) | 20 (10.5; 33.5) | 23.2 (6; 75) | 15 (10; 27.5) | No (p = 0.190) | No (p = 0.629) | No (p = 0.196) |
| Phalacrocoracidae | 0.529 (0; 5) | 0 (0; 0.5) | 3.35 (1; 20) | 1 (1; 4) | 1.94 (0; 9) | 1 (0; 3.5) | Yes (p < 0.001) | No (p = 0.170) | Yes (p = 0.019) |
| Podicipedidae | 2.88 (1; 6) | 2 (2; 4.5) | 1.94 (0; 4) | 2 (1; 3) | 3.35 (0; 7) | 3 (1.5; 5.5) | No (p = 0.130) | No (p = 0.095) | No (p = 0.648) |
| Ardeidae | 0 (0; 0) | 0 (0; 0) | 0.353 (0; 2) | 0 (0; 1) | 0.294 (0; 1) | 0 (0; 1) | Yes (p = 0.019) | No (p = 0.931) | Yes (p = 0.018) |
| Charadriiformes | 0.412 (0; 4) | 0 (0; 0) | 0.529 (0; 3) | 0 (0; 1) | 0 (0; 0) | 0 (0; 0) | No (p = 0.487 | Yes (p = 0.019) | No (p = 0.080) |
| Alcedinidae | 0 (0; 0) | 0 (0; 0) | 0.353 (0; 1) | 0 (0; 1) | 0.176 (0; 1) | 0 (0; 0) | Yes (p = 0.008) | No (p = 0.260) | No (p = 0.080) |
| Bacterial Group | April 2016–March 2020 (Before FPV System) | April 2020–March 2022 (with FPV System) | Difference Between Two Periods 2 | ||||
|---|---|---|---|---|---|---|---|
| n | Average Concentration (Min; Max) 1 | Median Concentration (Quartile 1; Quartile 3) 1 | n | Average Concentration (Min; Max) 1 | Median Concentration (Quartile 1; Quartile 3) 1 | ||
| Bacteria of the coli group | 212 | 81.6 (0; 4100) | 10.9 (3.00; 32) | 102 | 23.7 (0; 275) | 13.7 (4.00; 29.0) | No (p = 0.359) |
| E. coli | 213 | 32.9 (0; 1700) | 8.00 (2.00; 26.5) | 102 | 19.2 (0; 275) | 10.8 (3.00; 25.6) | No (p = 0.256) |
| Enterococci | 213 | 25.5 (0; 450) | 6.00 (1.00; 19.5) | 102 | 36.8 (0; 610) | 13.5 (5.00; 29.8) | Yes (p < 0.001) |
| Intestinal enterococci | 213 | 8.84 (0; 264) | 2.00 (0; 5.65) | 102 | 11.5 (0; 312) | 4.80 (1.00; 10.8) | Yes (p = 0.004) |
| Parameter | Observed Effect |
|---|---|
| Macrophytes, benthic algae, and benthic cyanobacteria | Shift from macrophytes and benthic algae to toxin and taste-and-odour producing benthic cyanobacteria under the FPV system |
| Macroinvertebrates | Some groups absent under the FPV system |
| Biofouling | Massive development of Dreissena mussels, which also increases the number of Dreissena larvae; attachment of green algae and some sponges |
| Metals and metalloids | Increased concentrations of copper, manganese, and zinc, but well below threshold values |
| Light (PAR) | Lower and not homogeneous under FPV system; under FPV system insufficient light for Chara and macrophytes, but enough light for benthic cyanobacteria |
| Transparency | No difference with open water; overall increase by filtering effect of mussels attached to FPV system |
| Nutrients | No difference compared to open water |
| Phytoplankton | Overall, very similar to open water; overall decrease due to mussels attached to FPV system; increase in planktonic cyanobacteria |
| Zooplankton | Sometimes higher biovolume under FPV system or in open water |
| Temperature and oxygen profiles | No large differences to open water, except for on warm sunny days; small but not significant decrease in water temperature after installation of FPV system |
| Bird counts | No large changes |
| Bird droppings | Accumulation on cable pontoons, but hardly on FPV system |
| Faecal bacteria | No increase in E. coli, slight increase in intestinal enterococci |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandrini, G.; Wagenvoort, A.; van Asperen, R.; Hofs, B.; Mathijssen, D.; van der Wal, A. Illuminating the Impact of a Floating Photovoltaic System on a Shallow Drinking Water Reservoir: The Emergence of Benthic Cyanobacteria. Water 2025, 17, 1178. https://doi.org/10.3390/w17081178
Sandrini G, Wagenvoort A, van Asperen R, Hofs B, Mathijssen D, van der Wal A. Illuminating the Impact of a Floating Photovoltaic System on a Shallow Drinking Water Reservoir: The Emergence of Benthic Cyanobacteria. Water. 2025; 17(8):1178. https://doi.org/10.3390/w17081178
Chicago/Turabian StyleSandrini, Giovanni, Arco Wagenvoort, Roland van Asperen, Bas Hofs, Dirk Mathijssen, and Albert van der Wal. 2025. "Illuminating the Impact of a Floating Photovoltaic System on a Shallow Drinking Water Reservoir: The Emergence of Benthic Cyanobacteria" Water 17, no. 8: 1178. https://doi.org/10.3390/w17081178
APA StyleSandrini, G., Wagenvoort, A., van Asperen, R., Hofs, B., Mathijssen, D., & van der Wal, A. (2025). Illuminating the Impact of a Floating Photovoltaic System on a Shallow Drinking Water Reservoir: The Emergence of Benthic Cyanobacteria. Water, 17(8), 1178. https://doi.org/10.3390/w17081178






