Enhanced Electrocatalytic Degradation of Phenol by Mn-MIL-100-Derived Carbon Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Mn-MIL-100 Materials
2.2. Fabrication of Mn-MOF-Modified CP Electrodes
2.3. Characterization and Analysis
3. Results and Discussion
3.1. Microstructure Characterization
3.2. Surface Morphology
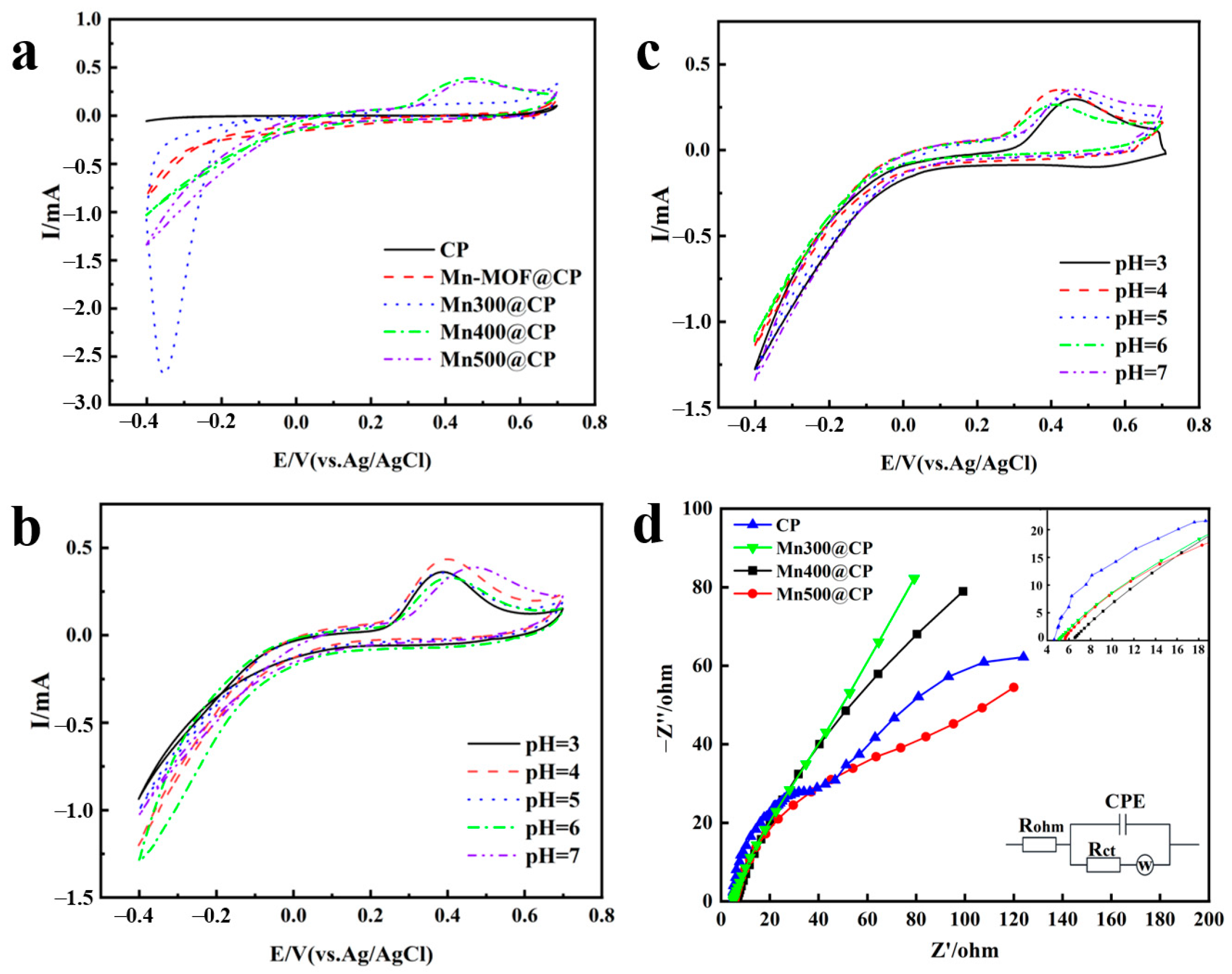
3.3. Analysis of Electrochemical Activity
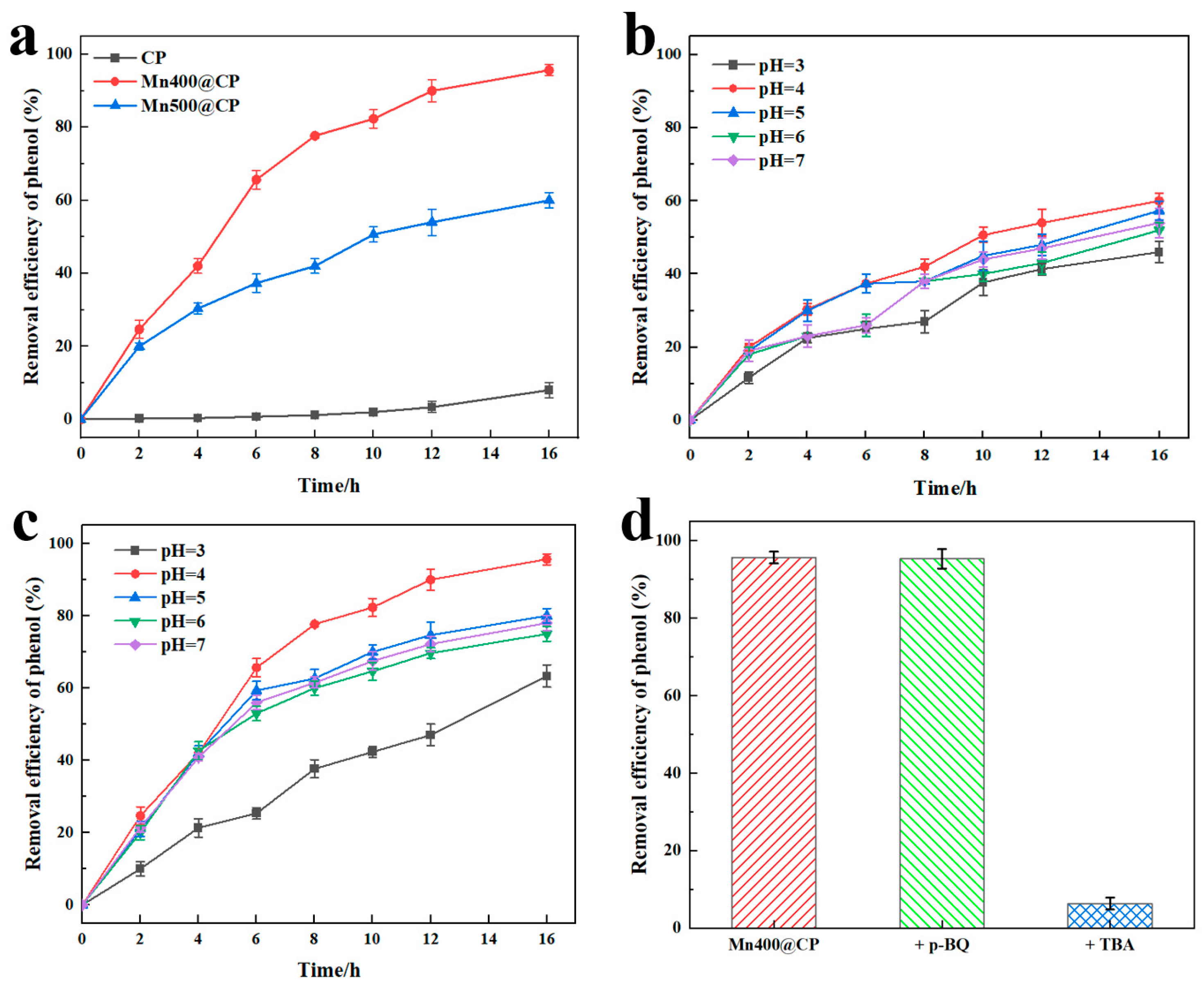
3.4. Application of the Modified Eelectrodes in Phenol Degradation
3.5. Assessment of Stability
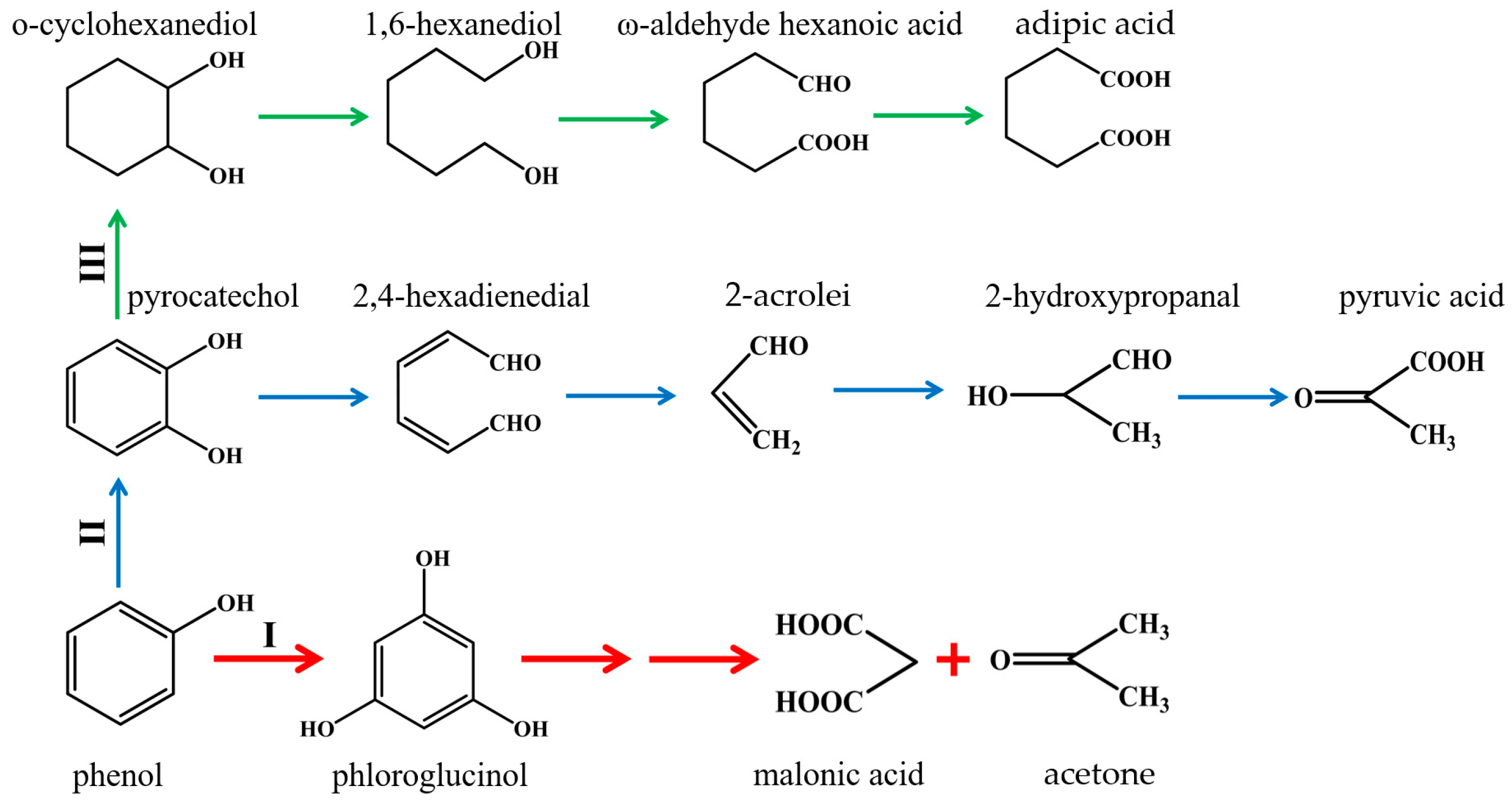
3.6. Possible Degradation Mechanism Involved in Phenol Oxidation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meng, H.; Zhou, J.; Nie, C.; Li, W.; Li, D.; Zhang, Y.; Ao, Z. Insights into Mn-doped biochar induce peroxymonosulfate activation for phenol degradation: The overlooked significance of C-O-Mn. J. Hazard. Mater. 2025, 492, 138031. [Google Scholar] [CrossRef]
- Wang, Y.; Song, J.; Zhao, W.; He, X.; Chen, J.; Xiao, M. In situ degradation of phenol and promotion of plant growth in contaminated environments by a single Pseudomonas aeruginosa strain. J. Hazard. Mater. 2011, 192, 354–360. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, S.; Zhu, B.; Shen, W.; She, R. Persulfate activation by nanoscale zero-valent iron supported by modified blast furnace slag for degradation of phenol wastewater. Environ. Res. 2024, 260, 119434. [Google Scholar] [CrossRef]
- Nadavala, S.K.; Swayampakula, K.; Boddu, V.M.; Abburi, K. Biosorption of phenol and o-chlorophenol from aqueous solutions on to chitosan–calcium alginate blended beads. J. Hazard. Mater. 2009, 162, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Nikbakht, R.; Sadrzadeh, M.; Mohammadi, T. Effect of operating parameters on concentration of citric acid using electrodialysis. J. Food Eng. 2007, 83, 596–604. [Google Scholar] [CrossRef]
- Busca, G.; Berardinelli, S.; Resini, C.; Arrighi, L. Technologies for the removal of phenol from fluid streams: A short review of recent developments. J. Hazard. Mater. 2008, 160, 265–288. [Google Scholar] [CrossRef]
- Muthu Kumara Pandian, A.; Rajasimman, M.; Rajamohan, N.; Varjani, S.; Karthikeyan, C. Anaerobic mixed consortium (AMC) mediated enhanced biosynthesis of silver nano particles (AgNPs) and its application for the removal of phenol. J. Hazard. Mater. 2021, 416, 125717. [Google Scholar] [CrossRef]
- Lin, K.; Pan, J.; Chen, Y.; Cheng, R.; Xu, X. Study the adsorption of phenol from aqueous solution on hydroxyapatite nanopowders. J. Hazard. Mater. 2009, 161, 231–240. [Google Scholar] [CrossRef]
- Cavalcante, P.R.M.; Melo, R.P.F.; Castro Dantas, T.N.; Dantas Neto, A.A.; Barros Neto, E.L.; Moura, M.C.P.A. Removal of phenol from aqueous medium using micellar solubilization followed by ionic flocculation. J. Environ. Chem. Eng. 2018, 6, 2778–2784. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, A.; Li, P.; Héroux, P.; Zhang, H.; Yu, X.; Liu, Y. Degradation of aqueous atrazine using persulfate activated by electrochemical plasma coupling with microbubbles: Removal mechanisms and potential applications. J. Hazard. Mater. 2021, 403, 124087. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, H.; Liang, J.; Yue, L.; Li, T.; Luo, Y.; Liu, Q.; Lu, S.; Asiri, A.M.; Gong, Z.; et al. Anodic oxidation for the degradation of organic pollutants: Anode materials, operating conditions and mechanisms. A mini review. Electrochem. Commun. 2021, 123, 106912. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Rodrigo, M.A.; Sirés, I.; Scialdone, O. Single and Coupled Electrochemical Processes and Reactors for the Abatement of Organic Water Pollutants: A Critical Review. Chem. Rev. 2015, 115, 13362–13407. [Google Scholar] [CrossRef]
- Mousset, E.; Wang, Z.; Hammaker, J.; Lefebvre, O. Electrocatalytic phenol degradation by a novel nanostructured carbon fiber brush cathode coated with graphene ink. Electrochim. Acta 2017, 258, 607–617. [Google Scholar] [CrossRef]
- Xuan, W.; Zhu, C.; Liu, Y.; Cui, Y. Mesoporous metal–organic framework materials. Chem. Soc. Rev. 2012, 41, 1677–1695. [Google Scholar] [CrossRef] [PubMed]
- Reinsch, H.; Stock, N. Formation and characterisation of Mn-MIL-100. CrystEngComm 2013, 15, 544–550. [Google Scholar] [CrossRef]
- Qiu, P.; Liu, F.; Xu, C.; Chen, H.; Jiang, F.; Li, Y.; Guo, Z. Porous three-dimensional carbon foams with interconnected microchannels for high-efficiency solar-to-vapor conversion and desalination. J. Mater. Chem. A 2019, 7, 13036–13042. [Google Scholar] [CrossRef]
- Jin, J.; Cao, G.; Liu, Y.; Shu, Y.; Deng, Z.; Sun, W.; Yang, X. Metal-organic-frameworks passivated CuBi2O4 photocathodes boost CO2 reduction kinetics. Mater. Rep. Energy 2023, 3, 100229. [Google Scholar] [CrossRef]
- Song, G.; Wang, Z.; Wang, L.; Li, G.; Huang, M.; Yin, F. Preparation of MOF(Fe) and its catalytic activity for oxygen reduction reaction in an alkaline electrolyte. Chin. J. Catal. 2014, 35, 185–195. [Google Scholar] [CrossRef]
- Mao, J.; Yang, L.; Yu, P.; Wei, X.; Mao, L. Electrocatalytic four-electron reduction of oxygen with Copper (II)-based metal-organic frameworks. Electrochem. Commun. 2012, 19, 29–31. [Google Scholar] [CrossRef]
- Senthil Kumar, R.; Senthil Kumar, S.; Anbu Kulandainathan, M. Highly selective electrochemical reduction of carbon dioxide using Cu based metal organic framework as an electrocatalyst. Electrochem. Commun. 2012, 25, 70–73. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Wang, C.; Jia, H.; Shang, X.; Tang, J.; Sun, H. Metal-rich hyperaccumulator-derived biochar as an efficient persulfate activator: Role of intrinsic metals (Fe, Mn and Zn) in regulating characteristics, performance and reaction mechanisms. J. Hazard. Mater. 2022, 424, 127225. [Google Scholar] [CrossRef]
- Ha, Y.; Mu, M.; Liu, Q.; Ji, N.; Song, C.; Ma, D. Mn-MIL-100 heterogeneous catalyst for the selective oxidative cleavage of alkenes to aldehydes. Catal. Commun. 2018, 103, 51–55. [Google Scholar] [CrossRef]
- Bi, F.; Feng, X.; Zhou, Z.; Zhang, Y.; Wei, J.; Yuan, L.; Liu, B.; Huang, Y.; Zhang, X. Mn-based catalysts derived from the non-thermal treatment of Mn-MIL-100 to enhance its water-resistance for toluene oxidation: Mechanism study. Chem. Eng. J. 2024, 485, 149776. [Google Scholar] [CrossRef]
- Xiong, Y.; Che, L.; Fu, Z.; Ma, P. Preparation of CuxO/C composite derived from Cu-MOFs as Fenton-like catalyst by two-step calcination strategy. Adv. Powder Technol. 2018, 29, 1331–1338. [Google Scholar] [CrossRef]
- Zhang, B.; Hao, S.; Xiao, D.; Wu, J.; Huang, Y. Templated formation of porous Mn2O3 octahedra from Mn-MIL-100 for lithium-ion battery anode materials. Mater. Des. 2016, 98, 319–323. [Google Scholar] [CrossRef]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with atomic-level arranged perovskite and oxide layers for advanced oxidation with an enhanced non-free radical pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Zhang, X.; Xiang, S.; Du, Q.; Bi, F.; Xie, K.; Wang, L. Effect of calcination temperature on the structure and performance of rod-like MnCeOx derived from MOFs catalysts. Mol. Catal. 2022, 522, 112226. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, C.; Wang, H.; Zhang, M. Synthesis of highly efficient MnOx catalyst for low-temperature NH3-SCR prepared from Mn-MOF-74 template. Mater. Lett. 2016, 168, 17–19. [Google Scholar] [CrossRef]
- Lee, J.H.; Sa, Y.J.; Kim, T.K.; Moon, H.R.; Joo, S.H. A transformative route to nanoporous manganese oxides of controlled oxidation states with identical textural properties. J. Mater. Chem. A 2014, 2, 10435–10443. [Google Scholar] [CrossRef]
- Maiti, S.; Pramanik, A.; Manju, U.; Mahanty, S. Reversible Lithium Storage in Manganese 1,3,5-Benzenetricarboxylate Metal–Organic Framework with High Capacity and Rate Performance. ACS Appl. Mater. Interfaces 2015, 7, 16357–16363. [Google Scholar] [CrossRef]
- Guo, D.; Wu, Z.; An, Y.; Li, X.; Guo, X.; Chu, X.; Sun, C.; Lei, M.; Li, L.; Cao, L.; et al. Room temperature ferromagnetism in (Ga1−xMnx)2O3 epitaxial thin films. J. Mater. Chem. C 2015, 3, 1830–1834. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Fan, W.; Li, H.; Zhao, F.; Xiao, X.; Huang, Y.; Ji, H.; Tong, Y. Boosting the photocatalytic performance of (001) BiOI: Enhancing donor density and separation efficiency of photogenerated electrons and holes. Chem. Commun. 2016, 52, 5316–5319. [Google Scholar] [CrossRef] [PubMed]
- Frankcombe, T.J.; Liu, Y. Interpretation of Oxygen 1s X-ray Photoelectron Spectroscopy of ZnO. Chem. Mater. 2023, 35, 5468–5474. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Hou, F.; Yang, Y.; Dong, H.; Liu, N.; Wang, Y.; Cui, L. Synthesis of highly efficient Mn2O3 catalysts for CO oxidation derived from Mn-MIL-100. Appl. Surf. Sci. 2017, 411, 27–33. [Google Scholar] [CrossRef]
- Pimentel, M.; Oturan, N.; Dezotti, M.; Oturan, M.A. Phenol degradation by advanced electrochemical oxidation process electro-Fenton using a carbon felt cathode. Appl. Catal. B Environ. 2008, 83, 140–149. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, X.; Fan, B.; Zhang, J.; Zhou, M.; Yang, W.; Hu, X.; Wang, H.; Pan, B.; Xie, Y. Ultrathin spinel-structured nanosheets rich in oxygen deficiencies for enhanced electrocatalytic water oxidation. Angew. Chem. Int. Ed. 2015, 54, 7399–7404. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, D.; Zhang, H.; Liu, G.; Hu, Y.; Xie, H.; Ma, J. Titanium oxide electrocatalytic membrane filtration: “two faces” of oxygen vacancies in generation and transformation of reactive oxygen species. Environ. Sci. Technol. 2023, 57, 13226–13235. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Hu, J.; Li, X.-y.; Zou, Y.; Li, Y.; Lin, L.; Li, B. Constructing zinc single-atom catalysts for the direct electron-transfer mechanism in peroxymonosulfate activation to degrade sulfamethoxazole efficiently. Chem. Eng. J. 2023, 474, 145973. [Google Scholar] [CrossRef]
- Pezzola, S.; Tarallo, S.; Iannini, A.; Venanzi, M.; Galloni, P.; Conte, V.; Sabuzi, F. An accurate approach forcomputational pKa determination of phenolic compounds. Molecules 2022, 27, 8590. [Google Scholar] [CrossRef]
- Wang, X.; Li, T.; Fan, Z.; Duan, P.; Wang, L.; Pan, J.; Gao, B. Redox potentials of sulfonamide antibiotics mediating the electron transfer process in single-atom Cu catalyst/peroxymonosulfate system: Selective removal mechanisms for sulfonamides. J. Hazard. Mater. 2025, 485, 136880. [Google Scholar] [CrossRef]
- Mirzaei Alavijeh, M.; Habibzadeh, S.; Roohi, K.; Keivanimehr, F.; Naji, L.; Ganjali, M.R. A selective and efficient precious metal-free electrocatalyst for chlorine evolution reaction: An experimental and computational study. Chem. Eng. J. 2021, 421, 127785. [Google Scholar] [CrossRef]
- Wei, W.; Cui, X.; Chen, W.; Ivey, D.G. Manganese oxide-based materials as electrochemical supercapacitor electrodes. Chem. Soc. Rev. 2011, 40, 1697–1721. [Google Scholar] [CrossRef]
- Kim, E.-J.; Oh, D.; Lee, C.-S.; Gong, J.; Kim, J.; Chang, Y.-S. Manganese oxide nanorods as a robust Fenton-like catalyst at neutral pH: Crystal phase-dependent behavior. Catal. Today 2017, 282, 71–76. [Google Scholar] [CrossRef]
- Li, D.; Tang, J.; Zhou, X.; Li, J.; Sun, X.; Shen, J.; Wang, L.; Han, W. Electrochemical degradation of pyridine by Ti/SnO2–Sb tubular porous electrode. Chemosphere 2016, 149, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Shen, J.; Jiang, X.; Mu, Y.; Ma, D.; Han, W.; Sun, X.; Li, J.; Wang, L. Fabrication of polypyrrole/β-MnO2 modified graphite felt anode for enhancing recalcitrant phenol degradation in a bioelectrochemical system. Electrochim. Acta 2017, 244, 119–128. [Google Scholar] [CrossRef]
- Yuan, K.; Song, T.; Wang, D.; Zou, Y.; Li, J.; Zhang, X.; Tang, Z.; Hu, W. Bimetal–organic frameworks for functionality optimization: MnFe-MOF-74 as a stable and efficient catalyst for the epoxidation of alkenes with H2O2. Nanoscale 2018, 10, 1591–1597. [Google Scholar] [CrossRef]
- Zhou, J.; Li, H.; Wei, C.; Kong, Y.; Li, Y.; Zhang, X.; Zhou, X. Significant improvement in the phenol degradation performance of Ti/PbO2 electrode through La and PVP co-doping strategy. J. Water Process Eng. 2025, 71, 107137. [Google Scholar] [CrossRef]
- Gao, S.; Liu, W.; Wang, M.; Fu, D.; Zhao, Z.; Liu, X. In situ self-grown synthesis of c-MOF@NiO heterostructure anchored to c-MOF/rGA particle electrode: Promoting sustained and efficient degradation of phenol in coking wastewater. Appl. Catal. B Environ. Energy 2025, 365, 124911. [Google Scholar] [CrossRef]
- Gong, Y.; Jia, W.; Zhou, B.; Zheng, K.; Gao, J.; Wu, Y.; Yu, S.; Xue, Y.; Wu, Y. Novel graphite-based boron-doped diamond coated electrodes with refractory metal interlayer for high-efficient electrochemical oxidation degradation of phenol. Sep. Purif. Technol. 2025, 355, 129550. [Google Scholar] [CrossRef]
- Yang, X.; Zou, R.; Huo, F.; Cai, D.; Xiao, D. Preparation and characterization of Ti/SnO2–Sb2O3–Nb2O5/PbO2 thin film as electrode material for the degradation of phenol. J. Hazard. Mater. 2009, 164, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Zhao, L.; Wu, C.; Lin, L.; Liu, X. Bi5+ doping improves the electrochemical properties of Ti/SnO2–Sb/PbO2 electrode and its electrocatalytic performance for phenol. J. Clean. Prod. 2022, 380, 135005. [Google Scholar] [CrossRef]
- Lv, T.; Liu, S.; Wang, T.; Wang, Z.; Liu, D. High-efficiency transformation of O2 into •OH by cooperative catalysis of double Ni-Ag codoped SiO2/ACF electrodes in membrane-less electrolyzer for phenol degradation. J. Environ. Chem. Eng. 2024, 12, 114050. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Liu, H.; Chen, D.; Zhang, Y.; Jiang, X.; Shen, J. Enhanced Electrocatalytic Degradation of Phenol by Mn-MIL-100-Derived Carbon Materials. Water 2025, 17, 1103. https://doi.org/10.3390/w17071103
Sun X, Liu H, Chen D, Zhang Y, Jiang X, Shen J. Enhanced Electrocatalytic Degradation of Phenol by Mn-MIL-100-Derived Carbon Materials. Water. 2025; 17(7):1103. https://doi.org/10.3390/w17071103
Chicago/Turabian StyleSun, Xueping, Haitao Liu, Dan Chen, Ya Zhang, Xinbai Jiang, and Jinyou Shen. 2025. "Enhanced Electrocatalytic Degradation of Phenol by Mn-MIL-100-Derived Carbon Materials" Water 17, no. 7: 1103. https://doi.org/10.3390/w17071103
APA StyleSun, X., Liu, H., Chen, D., Zhang, Y., Jiang, X., & Shen, J. (2025). Enhanced Electrocatalytic Degradation of Phenol by Mn-MIL-100-Derived Carbon Materials. Water, 17(7), 1103. https://doi.org/10.3390/w17071103







