Comparing Particulate Carbon Fluxes in Tropical Karst Lakes with Different Trophic Statuses
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Fluxes
2.3. Statistical Analyses
3. Results
3.1. Temperature and Dissolved Oxygen
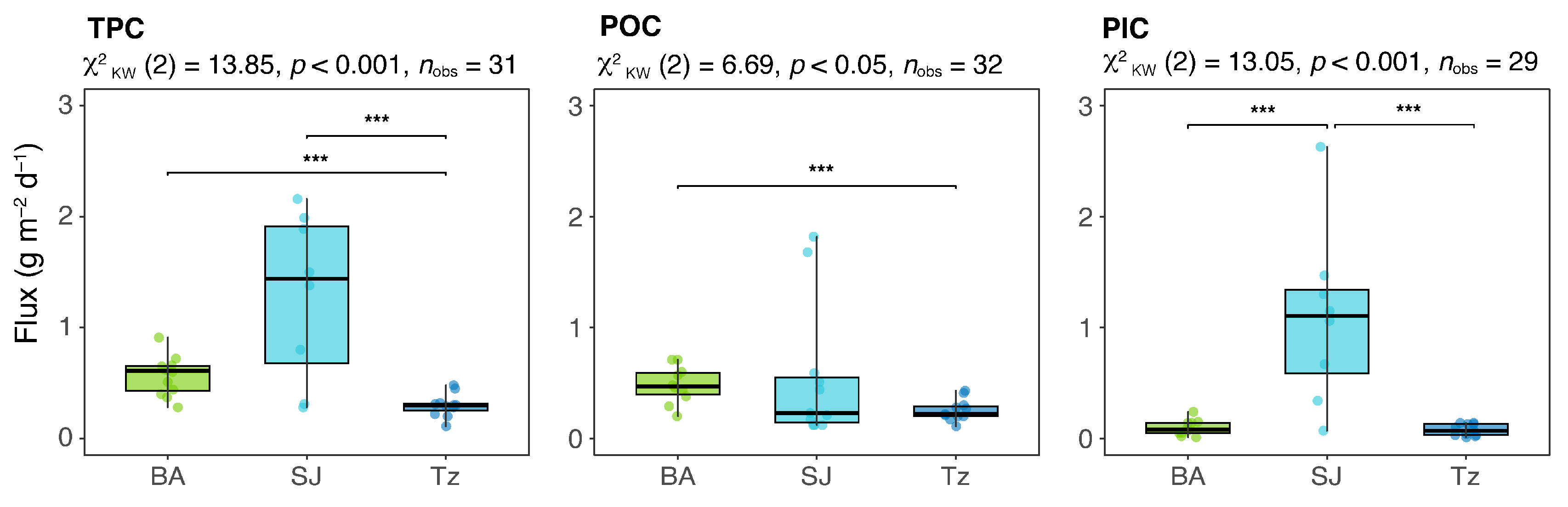
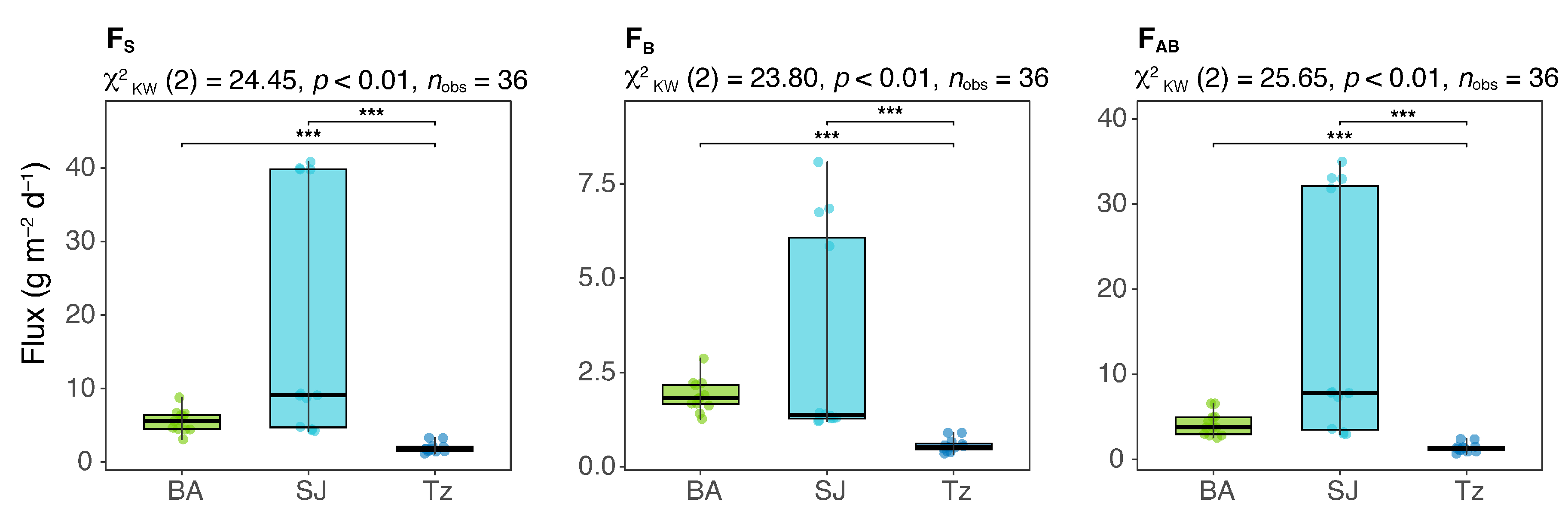
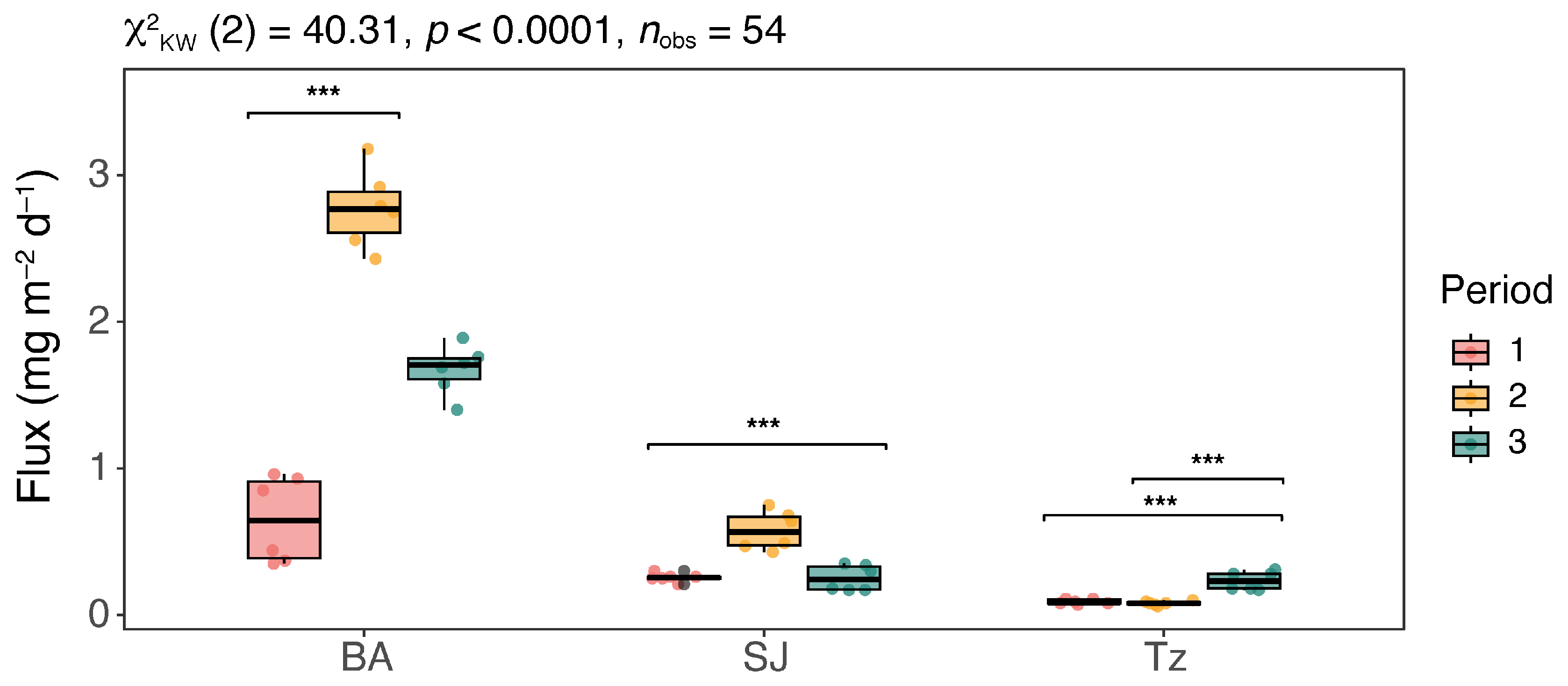
3.2. Fluxes
3.2.1. Carbon
3.2.2. Seston
3.2.3. Chlorophyll-a
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mendonça, R.; Müller, R.A.; Clow, D.; Verpoorter, C.; Raymond, P.; Tranvik, L.J.; Sobek, S. Organic Carbon Burial in Global Lakes and Reservoirs. Nat. Commun. 2017, 8, 1694. [Google Scholar] [CrossRef] [PubMed]
- Tranvik, L.J.; Cole, J.J.; Prairie, Y.T. The Study of Carbon in Inland Waters—From Isolated Ecosystems to Players in the Global Carbon Cycle. Limnol. Oceanogr. Lett. 2018, 3, 41–48. [Google Scholar] [CrossRef]
- Butman, D.; Striegl, R.; Stackpoole, S.; del Giorgio, P.; Prairie, Y.; Pilcher, D.; Raymond, P.; Pellat, F.P.; Alcocer, J. Chapter 14: Inland waters. In Second State of the Carbon Cycle Report (SOCCR2): A Sustained Assessment Report; Cavallaro, N., Shrestha, G., Birdsey, R., Mayes, M.A., Najjar, R.G., Reed, S.C., Romero-Lankao, P., Zhu, Z., Eds.; U.S. Global Change Research Program: Washington, DC, USA, 2018; pp. 1–470. Available online: https://carbon2018.globalchange.gov/chapter/14 (accessed on 21 November 2024).
- Heathcote, A.J.; Downing, J.A. Impacts of Eutrophication on Carbon Burial in Freshwater Lakes in an Intensively Agricultural Landscape. Ecosystems 2012, 15, 60–70. [Google Scholar] [CrossRef]
- Anderson, N.J.; Bennion, H.; Lotter, A.F. Lake Eutrophication and Its Implications for Organic Carbon Sequestration in Europe. Glob. Chang. Biol. 2014, 20, 2741–2751. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Jiang, Q.; Liu, E.; Chang, J.; Zhang, E. Climate Change Dominates Recent Sedimentation and Organic Carbon Burial in Lake Chenghai, Southwest China. J. Limnol. 2018, 77, 372–384. [Google Scholar] [CrossRef]
- Huang, C.; Chen, Z.; Gao, Y.; Luo, Y.; Huang, T.; Zhu, A.; Yang, H.; Yang, B. Enhanced Mineralization of Sedimentary Organic Carbon Induced by Excess Carbon from Phytoplankton in a Eutrophic Plateau Lake. J. Soils Sediments 2019, 19, 2613–2623. [Google Scholar] [CrossRef]
- Alcocer, J.; Ruiz-Fernández, A.C.; Escobar, E.; Pérez-Bernal, L.H.; Oseguera, L.A.; Ardiles-Gloria, V. Deposition, Burial and Sequestration of Carbon in an Oligotrophic, Tropical Lake. J. Limnol. 2014, 73, 223–235. [Google Scholar] [CrossRef]
- Ngochera, M.J.; Bootsma, H.A. Carbon, Nitrogen and Phosphorus Content of Seston and Zooplankton in Tropical Lake Malawi: Implications for Zooplankton Nutrient Cycling. Aquat. Ecosyst. Health Manag. 2018, 21, 185–192. [Google Scholar] [CrossRef]
- Durán Calderón, I.; Escolero Fuentes, O.A.; Muñoz Salinas, E.; Castillo Rodríguez, M.; Silva Romo, G. Cartografía geomorfológica a escala 1:50000 del Parque Nacional Lagunas de Montebello, Chiapas (México). Boletín Soc. Geológica Mex. 2014, 66, 263–277. [Google Scholar] [CrossRef]
- Alcocer, J.; Vargas-Sánchez, M.; Rivera-Herrera, E.M.; Oseguera, L.A.; Sánchez-Carrillo, S. Limnological Comparison of Pristine and Impacted Lakes from a Tropical, High-Altitude Karst Region in Southern Mexico. Inland. Waters 2024, 1–12. [Google Scholar] [CrossRef]
- Alcocer, J.; Merino-Ibarra, M.; Oseguera, L.A.; Escolero, O. Anthropogenic Impacts on Tropical Karst Lakes: “Lagunas de Montebello,” Chiapas. Ecohydrology 2018, 11, e2029. [Google Scholar] [CrossRef]
- Caballero, M.; Mora, L.; Muñoz, E.; Escolero, O.; Bonifaz, R.; Ruiz, C.; Prado, B. Anthropogenic Influence on the Sediment Chemistry and Diatom Assemblages of Balamtetik Lake, Chiapas, Mexico. Environ. Sci. Pollut. Res. 2020, 27, 15935–15943. [Google Scholar] [CrossRef]
- Mora Palomino, L.; García, L.A.; Ramos, Y.R.; Bonifaz, R.; Escolero, O. Description of Chemical Changes in a Large Karstic System: Montebello, Mexico. Procedia Earth Planet. Sci. 2017, 17, 829–832. [Google Scholar] [CrossRef]
- Alvarado Velázquez, J.A.; García-Meneses, P.M.; Esse, C.; Saavedra, P.; Trosino, R.M.; Alfonzo, R.B.; Mazari-Hiriart, M. Spatially Explicit Vulnerability Analysis of Contaminant Sources in a Karstic Watershed in Southeastern Mexico. Appl. Geogr. 2022, 138, 102606. [Google Scholar] [CrossRef]
- Olea-Olea, S.; Escolero, O. Nutrients Load Estimation to a Lake System through the Local Groundwater Flow: Los Lagos de Montebello, México. J. S. Am. Earth Sci. 2018, 84, 201–207. [Google Scholar] [CrossRef]
- García Amaro, E. Modificaciones al Sistema de Clasificación Climática de Köppen: Para Adaptarlo a las Condiciones de la República Mexicana, Segunda Edición, Corregida y Aumentada; Universidad Nacional Autónoma de México: Mexico City, México, 1988. [Google Scholar]
- Comisión Nacional de Áreas Naturales Protegidas (CONANP). Programa de Conservación y Manejo Parque Nacional Lagunas de Montebello; Secretaría de Medio Ambiente y Recursos Naturales: Mexico City, Mexico, 2007; pp. 9–10. ISBN 978-968-817-848-5. [Google Scholar]
- Mora P, L.; Bonifaz, R.; López-Martínez, R. Unidades geomorfológicas de la cuenca del Río Grande de Comitán, Lagos de Montebello, Chiapas-México. Boletín Soc. Geológica Mex. 2016, 68, 377–394. [Google Scholar] [CrossRef]
- Ortega-Gutiérrez, F.; Mitre, S.L.; Roldán, Q.J.; Aranda, G.J.J.; Morán, Z.D.; Alaniz, A.S.; Nieto, S.A. Carta Geológica de La República Mexicana, 1:2000000 (map); Universidad Nacional Autónoma de México, Institutoo de Geología: Mexico City, Mexico, 1992; 78p. [Google Scholar]
- Alcocer, J.; Oseguera, L.A.; Sánchez, G.; González, C.G.; Martínez, J.R.; González, R. Bathymetric and Morphometric Surveys of the Montebello Lakes, Chiapas. J. Limnol. 2016, 75, 56–65. [Google Scholar] [CrossRef]
- Bloesch, J. A Review of Methods Used to Measure Sediment Resuspension. Hydrobiologia 1994, 284, 13–18. [Google Scholar] [CrossRef]
- Gardner, W.D.; Hinga, K.R.; Marra, J. Observations on the Degradation of Biogenic Material in the Deep Ocean with Implications on Accuracy of Sediment Trap Fluxes. J. Mar. Res. 1983, 41, 195–214. [Google Scholar] [CrossRef]
- Lee, C.; Hedges, J.I.; Wakeham, S.G.; Zhu, N. Effectiveness of Various Treatments in Retarding Microbial Activity in Sediment Trap Material and Their Effects on the Collection of Swimmers. Limnol. Oceanogr. 1992, 37, 117–130. [Google Scholar] [CrossRef]
- Goto, N.; Hisamatsu, K.; Yoshimizu, C.; Ban, S. Effectiveness of Preservatives and Poisons on Sediment Trap Material in Freshwater Environments. Limnology 2016, 17, 87–94. [Google Scholar] [CrossRef]
- Arar, E.J.; Collins, G.B. In Vitro Determination of Chlorophyll and Pheophytin a in Marine and Freshwater Algae by Fluorescence; U.S. Environmental Protection Agency: Washington, DC, USA, 1997. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 21 November 2024).
- Ogle, D.H.; Doll, J.C.; Wheeler, A.P.; Dinno, A. FSA: Simple Fisheries Stock Assessment Methods. R Package Version 0.9.5. 2023. Available online: https://CRAN.R-project.org/package=FSA (accessed on 21 November 2024).
- Almeida, B.T. Heterogeneidad Espacial En El Sistema Lacustre de Montebello: Estructura Funcional y Taxonómica Del Fitoplancton; UNAM, FES Iztacala: Tlalnepantla, México, 2014. [Google Scholar]
- Martínez, M. Caracterización de Los Suelos de La Cuenca Del Río Grande, Como Soporte Para Entender La Dinámica de Contaminantes Que Llegan al Sistema Lagunar de Montebello, Chiapas; UNAM, FES Zaragoza: Tlalnepantla, México, 2015. [Google Scholar]
- Mora, L.; Prado, B. Edafología. In Atlas del Socioecosistema Río Grande de Comitán-Lagos de Montebello, Chiapas; Mazari-Hiriart, M., García-Meneses, P.M., Eds.; Seminario Universitario de Sociedad, Medio Ambiente e Instituciones (SUSMAI), Universidad Nacional Autónoma de México, UNAM: Mexico City, México, 2024. [Google Scholar] [CrossRef]
- INEGI. Guía Para la Interpretación de Cartografía: Uso del Suelo y Vegetación: Scala 1:250, 000: Serie V; Instituto Nacional de Estadística y Geografía (INEGI): México City, Mexico, 2014; 195p. [Google Scholar]
- Castro, R.; Bonifaz, R.; Alvarado, V.J.; Mazari, M. Uso de Suelo y Vegetación. In Atlas del Socioecosistema Río Grande de Comitán-Lagos de Montebello, Chiapas; Mazari-Hiriart, M., García-Meneses, P.M., Eds.; Secretaría de Desarrollo Institucional: Ciudad de México, México, 2024. [Google Scholar] [CrossRef]
- Kruk, C.; Peeters, E.t.h.m.; Van Nes, E.H.; Huszar, V.L.M.; Costa, L.S.; Scheffer, M. Phytoplankton Community Composition Can Be Predicted Best in Terms of Morphological Groups. Limnol. Oceanogr. 2011, 56, 110–118. [Google Scholar] [CrossRef]
- Kunz, M.J.; Anselmetti, F.S.; Wüest, A.; Wehrli, B.; Vollenweider, A.; Thüring, S.; Senn, D.B. Sediment Accumulation and Carbon, Nitrogen, and Phosphorus Deposition in the Large Tropical Reservoir Lake Kariba (Zambia/Zimbabwe). J. Geophys. Res. 2011, 116, G03003. [Google Scholar] [CrossRef]
- Ohlendorf, C.; Bigler, C.; Goudsmit, G.-H.; Lemcke, G.; Livingstone, D.M.; Lotter, A.F.; Müller, B.; Sturm, M. Causes and Effects of Long Periods of Ice Cover on a Remote High Alpine Lake. J. Limnol. 2000, 59, 65–80. [Google Scholar] [CrossRef]
- Oseguera, L.A.; Alcocer, J.; Vilaclara, G. Relative Importance of Dust Inputs and Aquatic Biological Production as Sources of Lake Sediments in an Oligotrophic Lake in a Semi-Arid Area. Earth Surf. Process. Landf. 2011, 36, 419–426. [Google Scholar] [CrossRef]
- Oseguera, L.A.; Alcocer, J.; Hernández-Hernández, B. Variación Del Flujo de Carbono Orgánico Particulado En Un Lago Oligotrófico Con Dominancia de Fitoplancton de Talla Grande [Variation of Particulate Organic Carbon Flux in a Large Size Phytoplankton-Dominant Oligotrophic Lake]. In Estado Actual del Conocimiento del Ciclo del Carbono y sus Interacciones en México: Síntesis a 2013; Programa Mexicano del Carbono, Colegio de Posgraduados, Universidad Autónoma de Chapingo e Instituto Tecnológico y de Estudios Superiores de Monterrey: Texcoco, México, 2013. [Google Scholar]
- Pascoe-Orrala, T.; Alcocer, J.; Oseguera, L.A. Evaluación de las diferencias de los flujos de carbono particulado en la zona pelágica de un lago tropical profundo. In Tendencias de Investigación en Limnología Tropical: Perspectivas Universitarias en Latinoamérica; Alcocer, J., Merino-Ibarra, M., Escobar-Briones, E., Eds.; Asociación Mexicana de Limnología, A.C., Instituto de Ciencias del Mar y Limnología, UNAM, y Consejo Nacional de Ciencias y Tecnología: Texcoco, México, 2015; pp. 165–170. ISBN 978-607-02-7199-1. [Google Scholar]
- Callieri, C.; Bertoni, R.; De Marco, C.; Contesini, M. Settling Flux and Sinking Velocity of Seston in Lago Di Mergozzo (Northern Italy) and Influence of Microbial Activity on the Decomposition of Entrapped Organic Material. Hydrobiologia 1991, 213, 155–165. [Google Scholar] [CrossRef]
- Callieri, C. Sedimentation and Aggregate Dynamics in Lake Maggiore, a Large, Deep Lake in Northern Italy. Mem. Ist. Ital. Di Idrobiol. 1997, 56, 37–50. [Google Scholar]
- Wei, Y.; Yan, H.; Liu, Z.; Han, C.; Ma, S.; Sun, H.; Bao, Q. The Ballast Effect Controls the Settling of Autochthonous Organic Carbon in Three Subtropical Karst Reservoirs. Sci. Total Environ. 2022, 818, 151736. [Google Scholar] [CrossRef]
- Stallard, R.F. Weathering and Erosion in the Humid Tropics. In Physical and Chemical Weathering in Geochemical Cycles; Lerman, A., Meybeck, M., Eds.; Springer: Dordrecht, The Netherlands, 1988; pp. 225–246. [Google Scholar] [CrossRef]
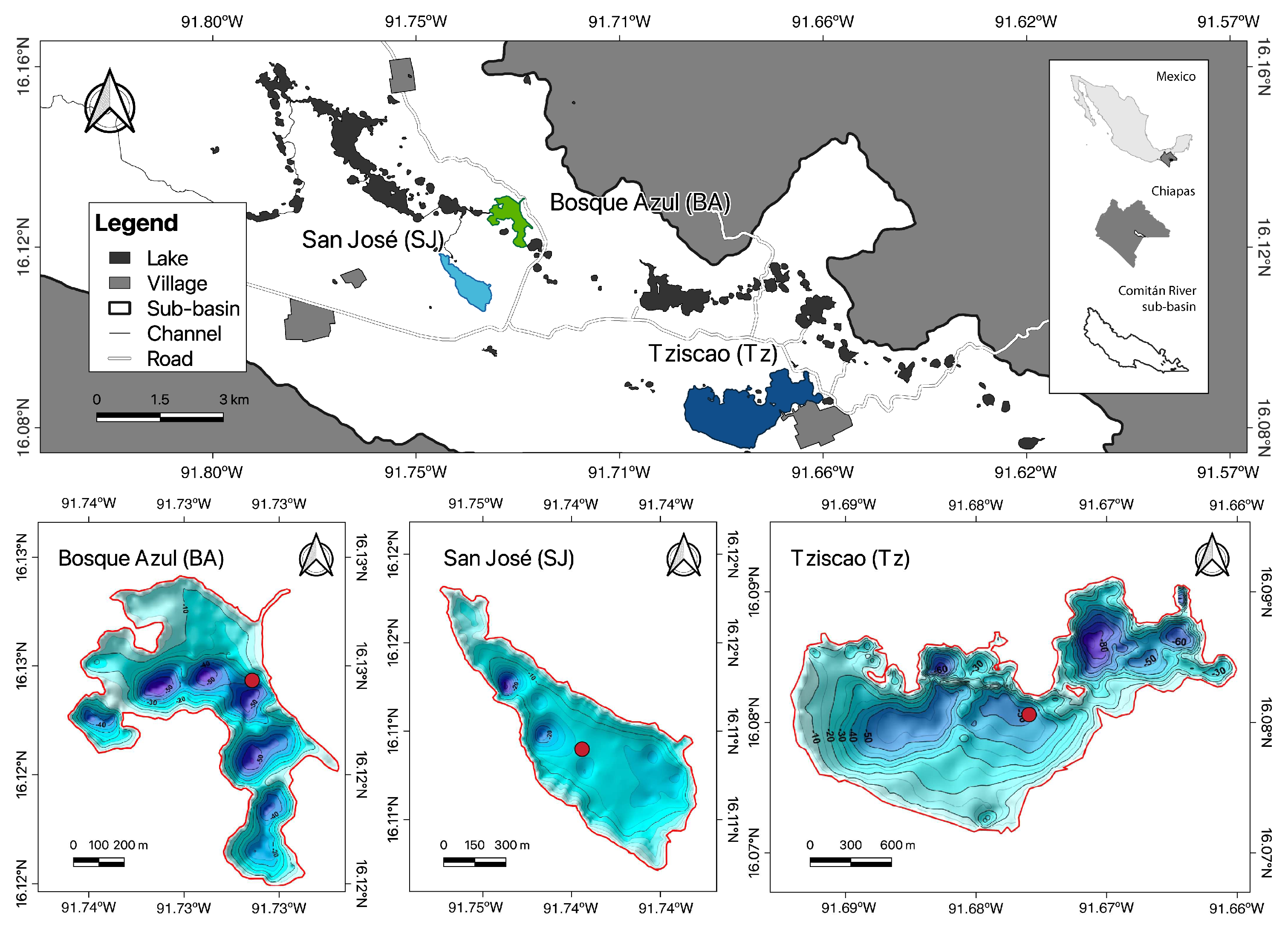
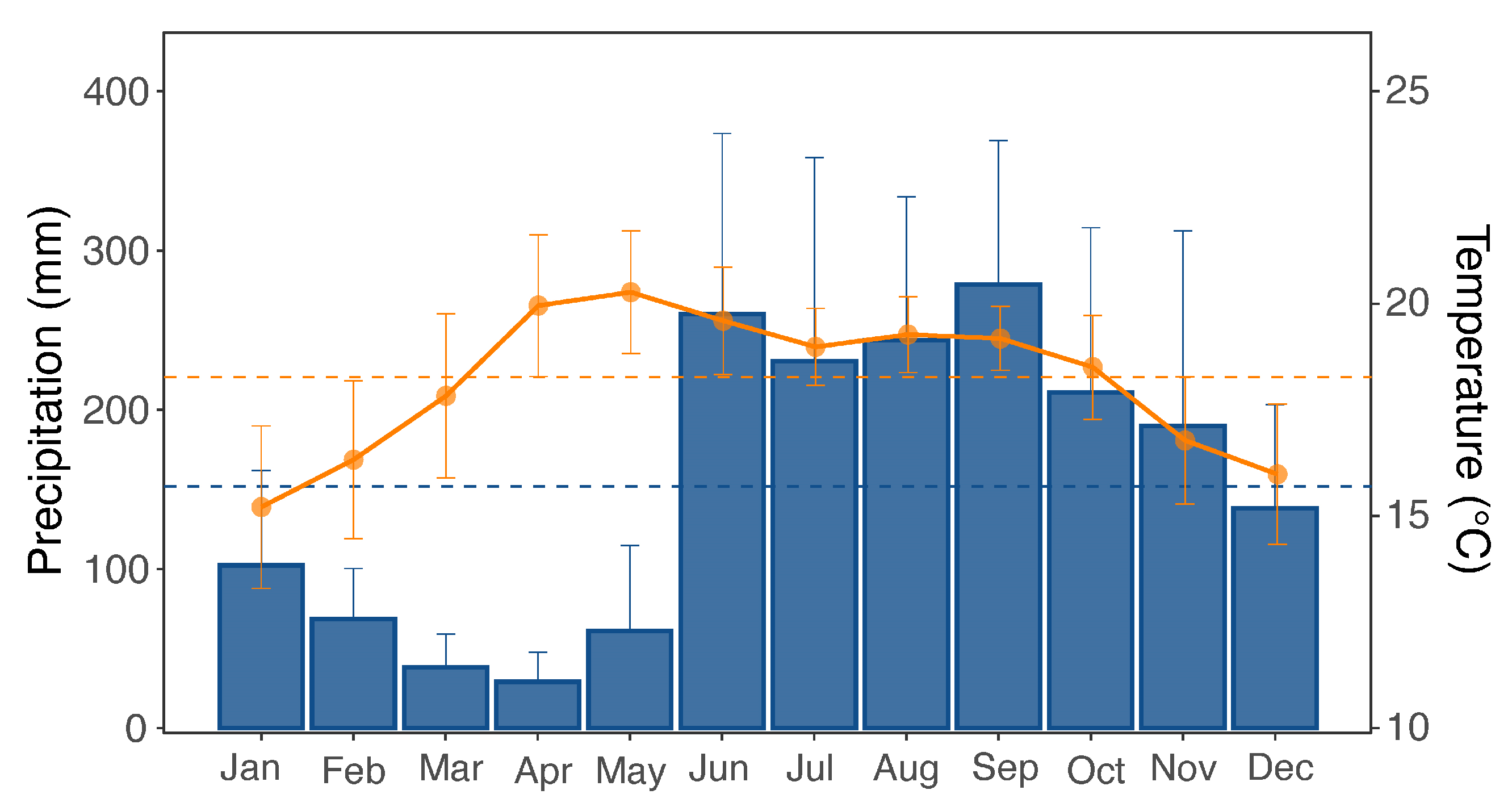
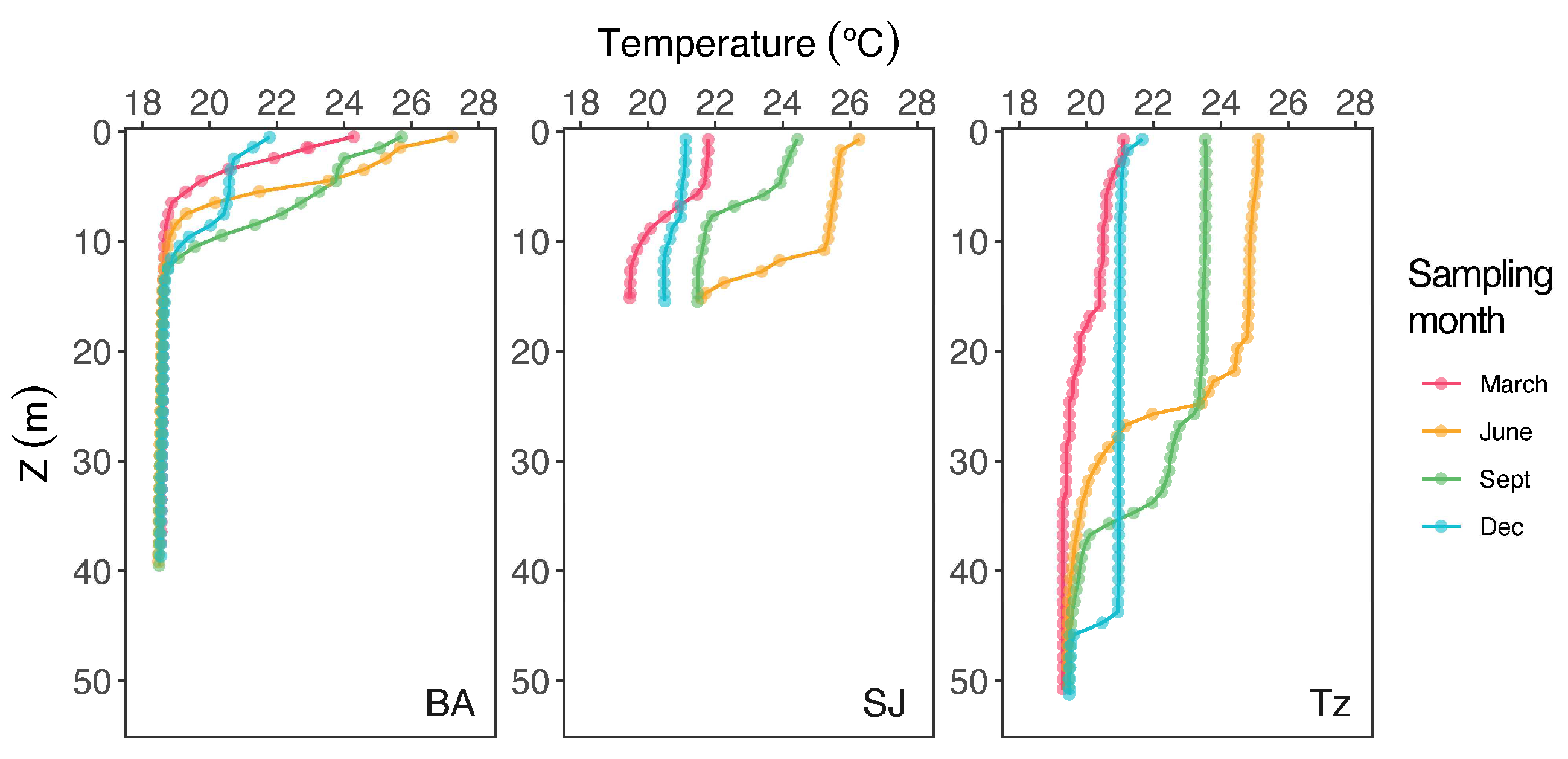
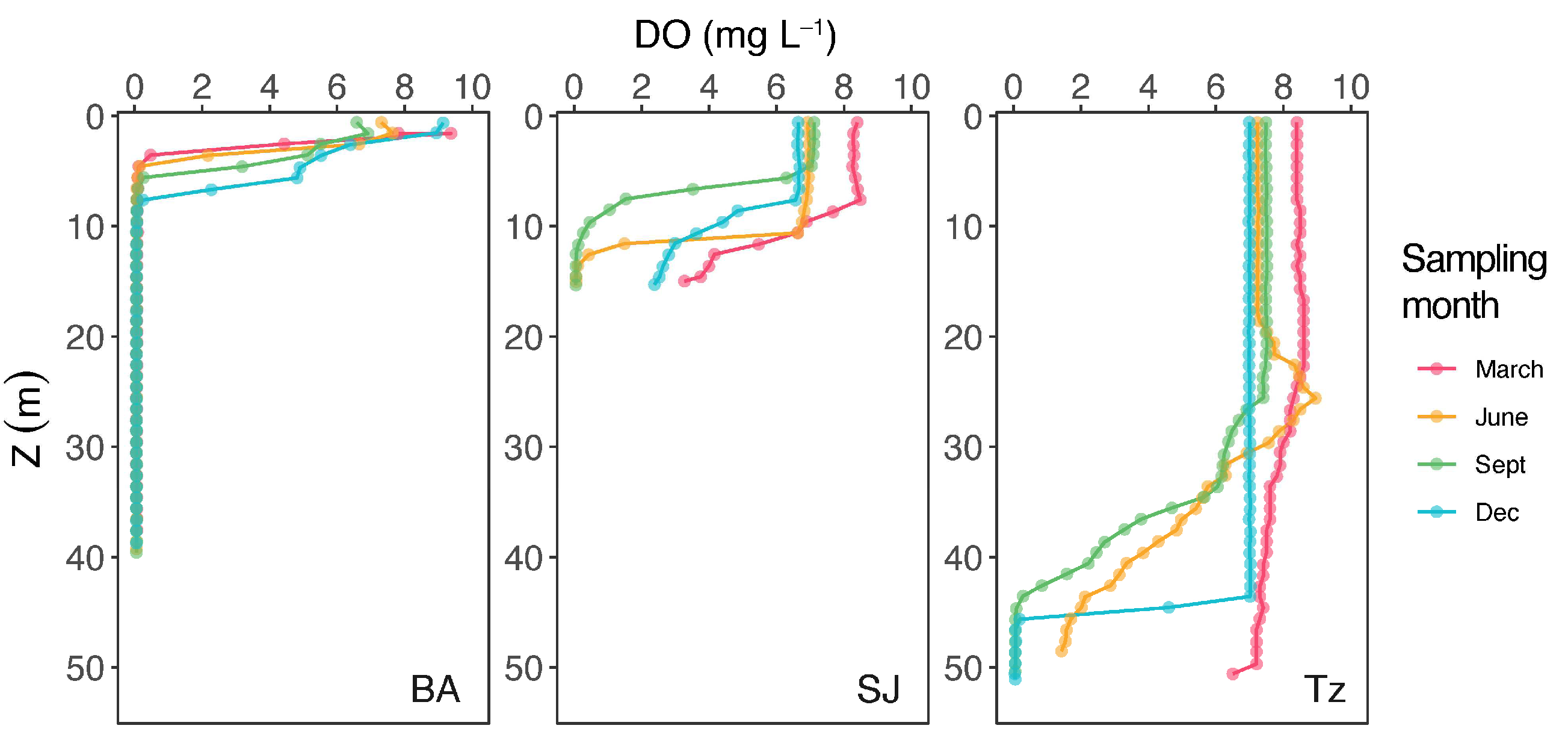
| Lake | Latitude | Longitude | Altitude | Area | Volume | Zmax | Zmean |
|---|---|---|---|---|---|---|---|
| (°N) | (°W) | (m a.s.l) | (ha) | (km3) | (m) | (m) | |
| BA | 16.120–16.131 | 91.729–91.739 | 1458 | 52.5 | 0.01050 | 58 | 20.0 |
| SJ | 16.106–16.119 | 91.738–91.750 | 1454 | 60.6 | 0.00623 | 30 | 10.3 |
| Tz | 16.075–16.093 | 91.665–91.696 | 1490 | 306.6 | 0.08852 | 86 | 28.9 |
| Variable | BA | SJ | Tz | |||
|---|---|---|---|---|---|---|
| MIN–MAX | X ± SD | MIN–MAX | X ± SD | MIN–MAX | X ± SD | |
| ZSD (m) | 0.3–1.1 | 0.8 ± 0.3 | 1.8–4.1 | 3.2 ± 0.9 | 5.0–10.0 | 7.3 ± 1.3 |
| ZEU (m) | 1.6–4.8 | 2.7 ± 1.0 | 6.7–25.2 | 13.7 ± 7.2 | 21.1–28.5 | 25.3 ± 2.7 |
| T (°C) | 17.3–26.4 | 18.9 ± 1.8 | 17.2–24.9 | 20.9 ± 2.2 | 17.5–23.8 | 20.0 ± 1.9 |
| DO (mg L−1) | 0–14.7 | 1.3 ± 2.5 | 0–8.5 | 5.1 ± 2.8 | 0–8.6 | 5.7 ± 2.8 |
| pH | 6.8–9.3 | 7.7 ± 0.6 | 7.0–9.1 | 8.1 ± 0.5 | 7.0–9.3 | 8.2 ± 0.5 |
| K25 (µS cm−1) | 356.1–649.9 | 489.4 ± 59.3 | 286.6–511.3 | 337.7 ± 37.5 | 220.8–412.2 | 248.5 ± 19.9 |
| TP (µmol L−1) | 0.8–11.3 | 4.8 ± 2.6 | 0.5–2.3 | 1.3 ± 0.5 | 0.1–15.7 | 2.1 ± 3.0 |
| TN (µmol L−1) | 39.8–221.4 | 124.1 ± 49.9 | 23.2–118.3 | 46.1 ± 21.9 | 11.8–285.7 | 63.5 ± 52.8 |
| Chl (µg L−1) | 0.9–86.6 | 23.1 ± 20.7 | 0.2–5.7 | 1.2 ± 1.2 | 0.1–2.3 | 0.5 ± 0.4 |
| TSS (mg L−1) | 0.9–27.5 | 5.9 ± 5.0 | 1.0–11.4 | 5.2 ± 3.2 | 0.1–5.2 | 1.2 ± 0.9 |
| Flux | BA | SJ | Tz | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | MIN–MAX | MED ± SD | n | MIN–MAX | MED ± SD | n | MIN–MAX | MED ± SD | |
| FTPC (g m−2 d−1) | 11 | 0.28–0.91 | 0.61 ± 0.2 | 9 | 0.28–4.31 | 1.50 ± 1.2 | 11 | 0.11–0.48 | 0.30 ± 0.1 |
| FPOC (g m−2 d−1) | 10 | 0.20–0.71 | 0.47 ± 0.2 | 11 | 0.12–1.82 | 0.23 ± 0.6 | 11 | 0.11–0.43 | 0.22 ± 0.1 |
| FPIC (g m−2 d−1) | 10 | 0.01–0.24 | 0.08 ± 0.1 | 8 | 0.07–2.63 | 1.11 ± 0.8 | 11 | 0.02–0.14 | 0.07 ± 0.1 |
| Flux | BA | SJ | Tz | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | MIN–MAX | MED ± SD | n | MIN–MAX | MED ± SD | n | MIN–MAX | MED ± SD | |
| FS (g m−2 d−1) | 12 | 3.10–8.79 | 5.61 ± 1.5 | 12 | 4.25–40.8 | 9.10 ± 16.5 | 12 | 1.17–3.31 | 1.81 ± 0.7 |
| FBS (g m−2 d−1) | 12 | 1.27–2.87 | 1.82 ± 0.4 | 12 | 1.21–8.08 | 1.37 ± 2.8 | 12 | 0.35–0.90 | 0.53 ± 0.2 |
| FAB (g m−2 d−1) | 12 | 2.56–6.56 | 3.79 ± 1.4 | 12 | 2.96–35.0 | 7.82 ± 13.8 | 12 | 0.69–2.41 | 1.31 ± 0.5 |
| Flux | Period | BA | SJ | Tz | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | MIN–MAX | MED ± SD | n | MIN–MAX | MED ± SD | n | MIN–MAX | MED ± SD | ||
| 1 | 6 | 0.35–0.96 | 0.65 ± 0.3 | 6 | 0.21–0.30 | 0.26 ± 0.0 | 6 | 0.07–0.11 | 0.09 ± 0.0 | |
| FChl | 2 | 6 | 2.43–3.18 | 2.77 ± 0.3 | 6 | 0.43–0.75 | 0.57 ± 0.1 | 6 | 0.06–0.10 | 0.08 ± 0.0 |
| (mg m−2 d−1) | 3 | 6 | 1.40–1.89 | 1.71 ± 0.2 | 6 | 0.17–0.35 | 0.24 ± 0.1 | 6 | 0.17–0.31 | 0.23 ± 0.1 |
| 1–3 | 18 | 0.35–3.18 | 1.71 ± 0.9 | 18 | 0.17–0.75 | 0.30 ± 0.2 | 18 | 0.06–0.31 | 0.10 ± 0.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Herrera, M.; Alcocer, J.; Oseguera, L.A.; Vargas-Sánchez, M.; García-Oliva, F.; Sánchez-Carrillo, S. Comparing Particulate Carbon Fluxes in Tropical Karst Lakes with Different Trophic Statuses. Water 2025, 17, 1030. https://doi.org/10.3390/w17071030
Rivera-Herrera M, Alcocer J, Oseguera LA, Vargas-Sánchez M, García-Oliva F, Sánchez-Carrillo S. Comparing Particulate Carbon Fluxes in Tropical Karst Lakes with Different Trophic Statuses. Water. 2025; 17(7):1030. https://doi.org/10.3390/w17071030
Chicago/Turabian StyleRivera-Herrera, Montserrat, Javier Alcocer, Luis A. Oseguera, Mariana Vargas-Sánchez, Felipe García-Oliva, and Salvador Sánchez-Carrillo. 2025. "Comparing Particulate Carbon Fluxes in Tropical Karst Lakes with Different Trophic Statuses" Water 17, no. 7: 1030. https://doi.org/10.3390/w17071030
APA StyleRivera-Herrera, M., Alcocer, J., Oseguera, L. A., Vargas-Sánchez, M., García-Oliva, F., & Sánchez-Carrillo, S. (2025). Comparing Particulate Carbon Fluxes in Tropical Karst Lakes with Different Trophic Statuses. Water, 17(7), 1030. https://doi.org/10.3390/w17071030









