Hydroxyapatite-Modified Zeolite for Fluoride Removal from Drinking Water: Adsorption Mechanism Investigation and Column Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Modification of Raw Zeolite
2.3. Characterization
2.4. Batch Study
2.5. Isotherm Study
2.6. Kinetic Study
2.7. Regeneration and Re-Use
2.8. Column Study
3. Results and Discussion
3.1. Characterization of the Modified Zeolite
3.1.1. EDS
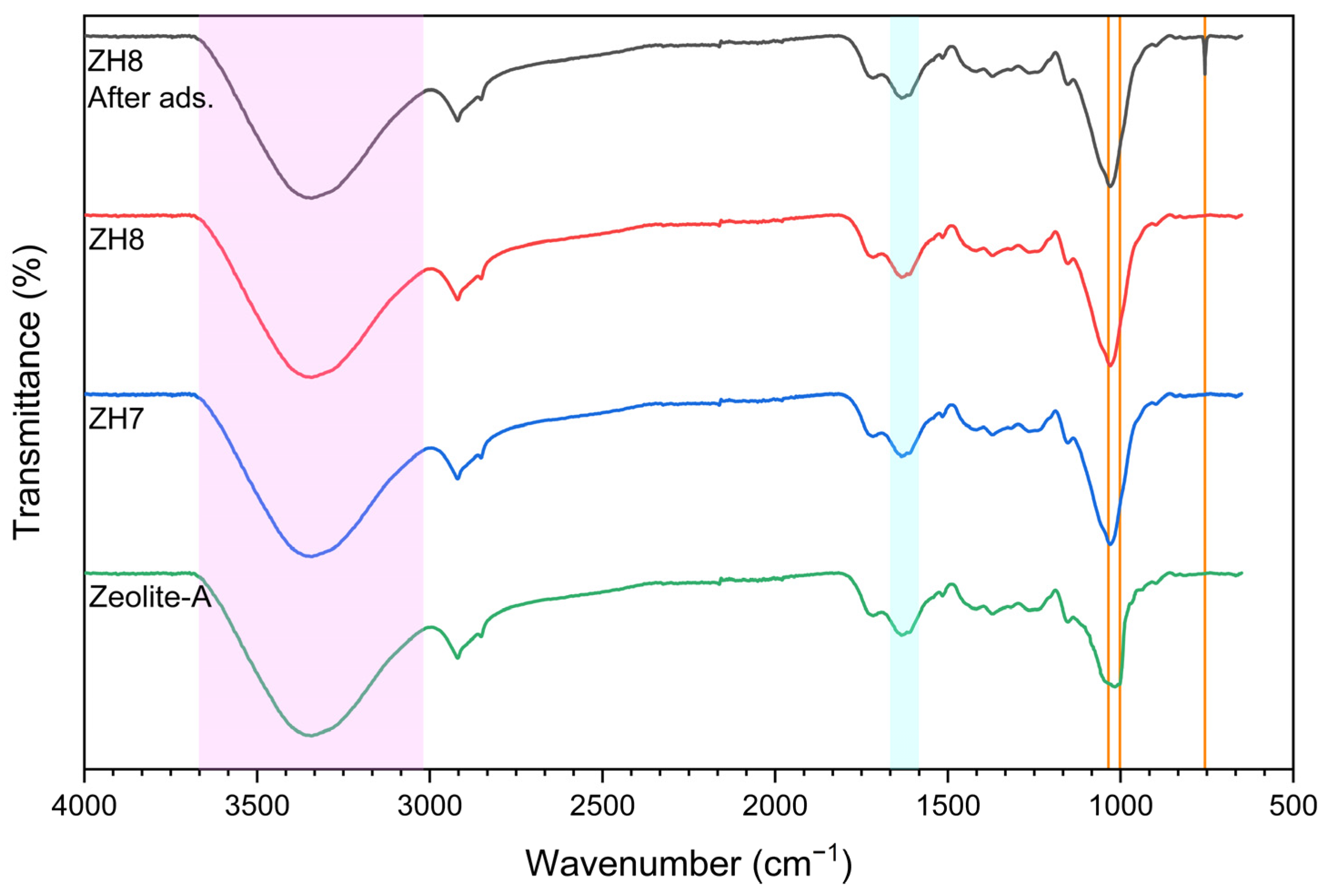
3.1.2. FTIR
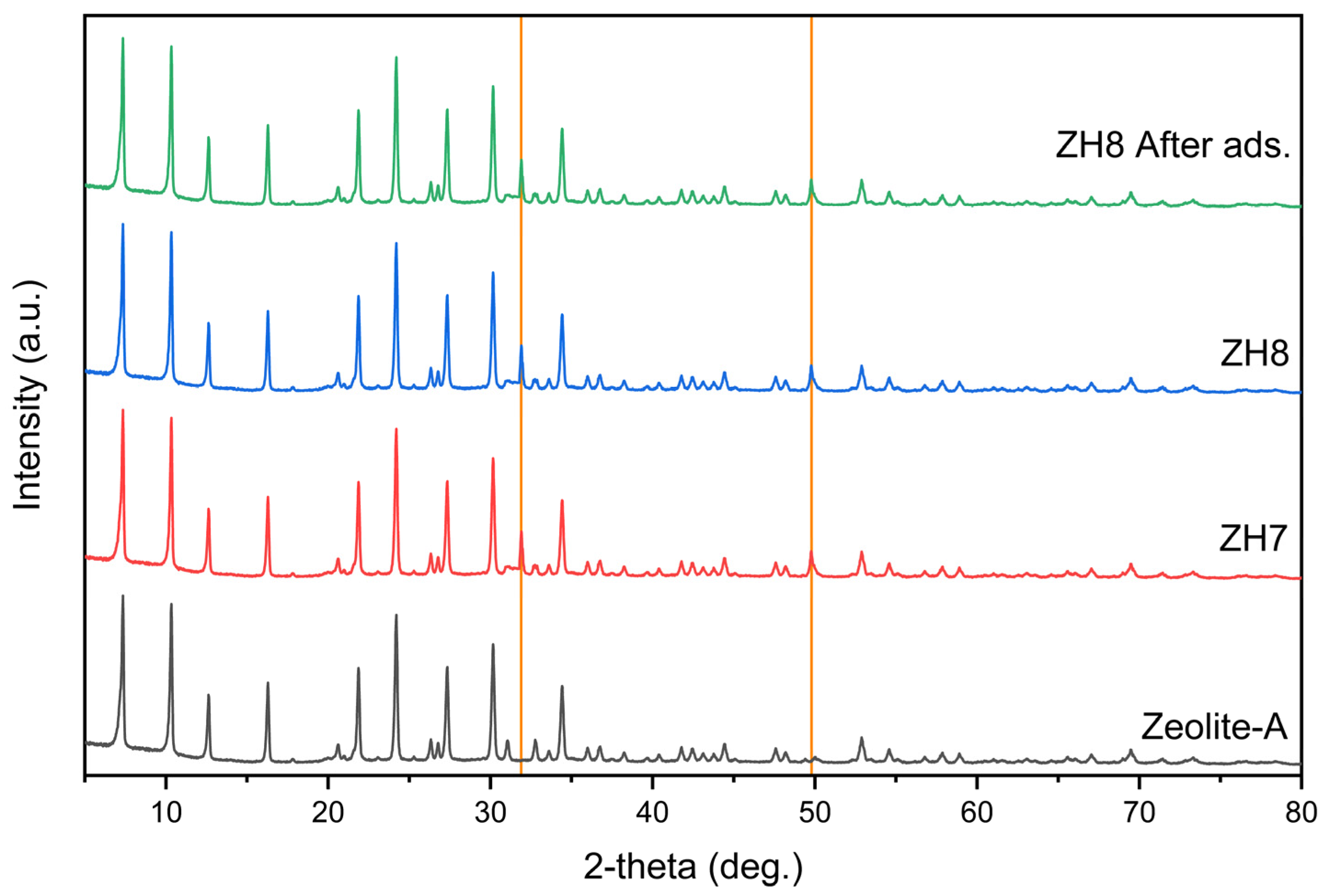
3.1.3. XRD
3.2. Adsorption Performance
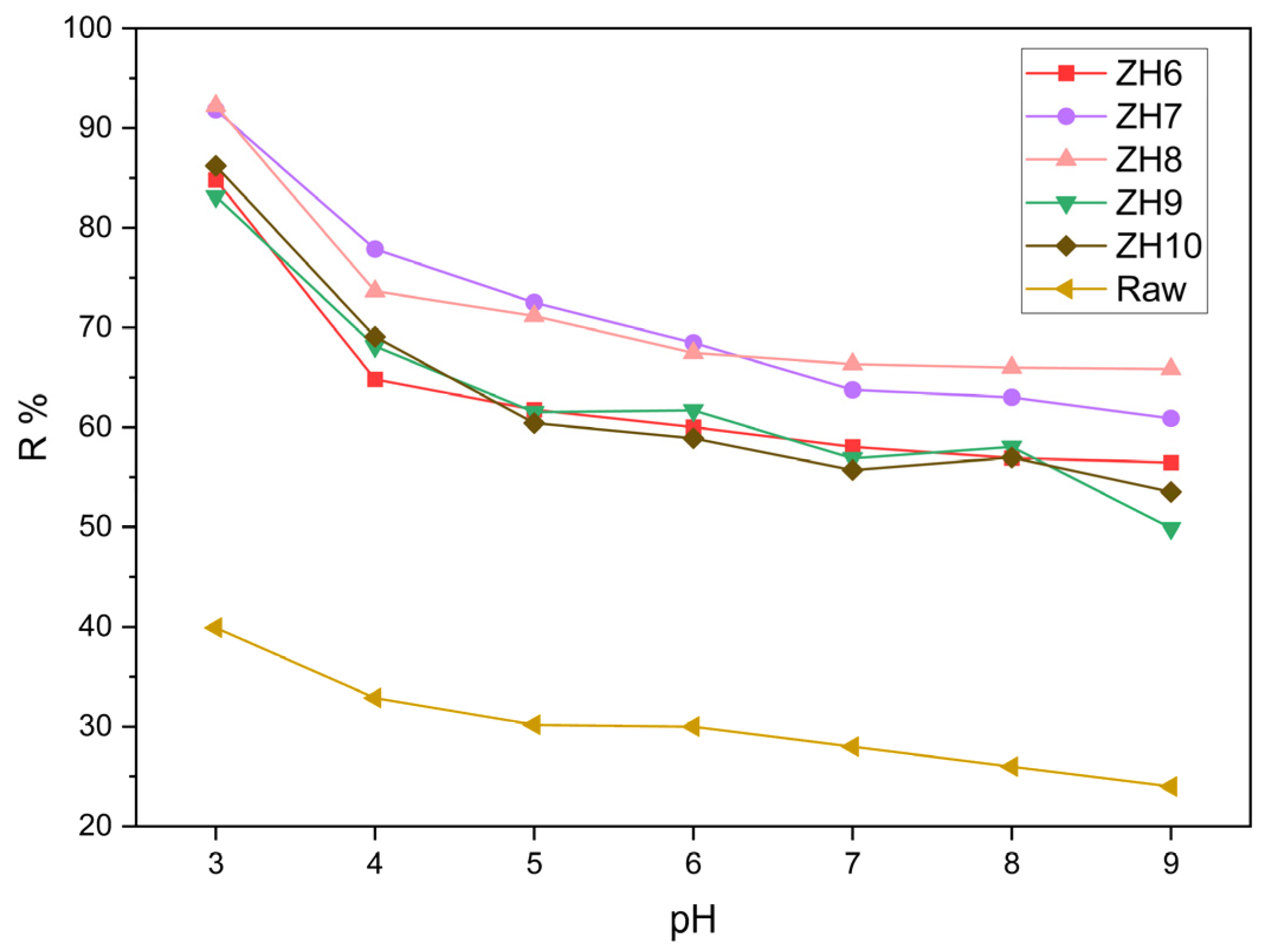
3.2.1. Effect of pH on Removal Efficiency
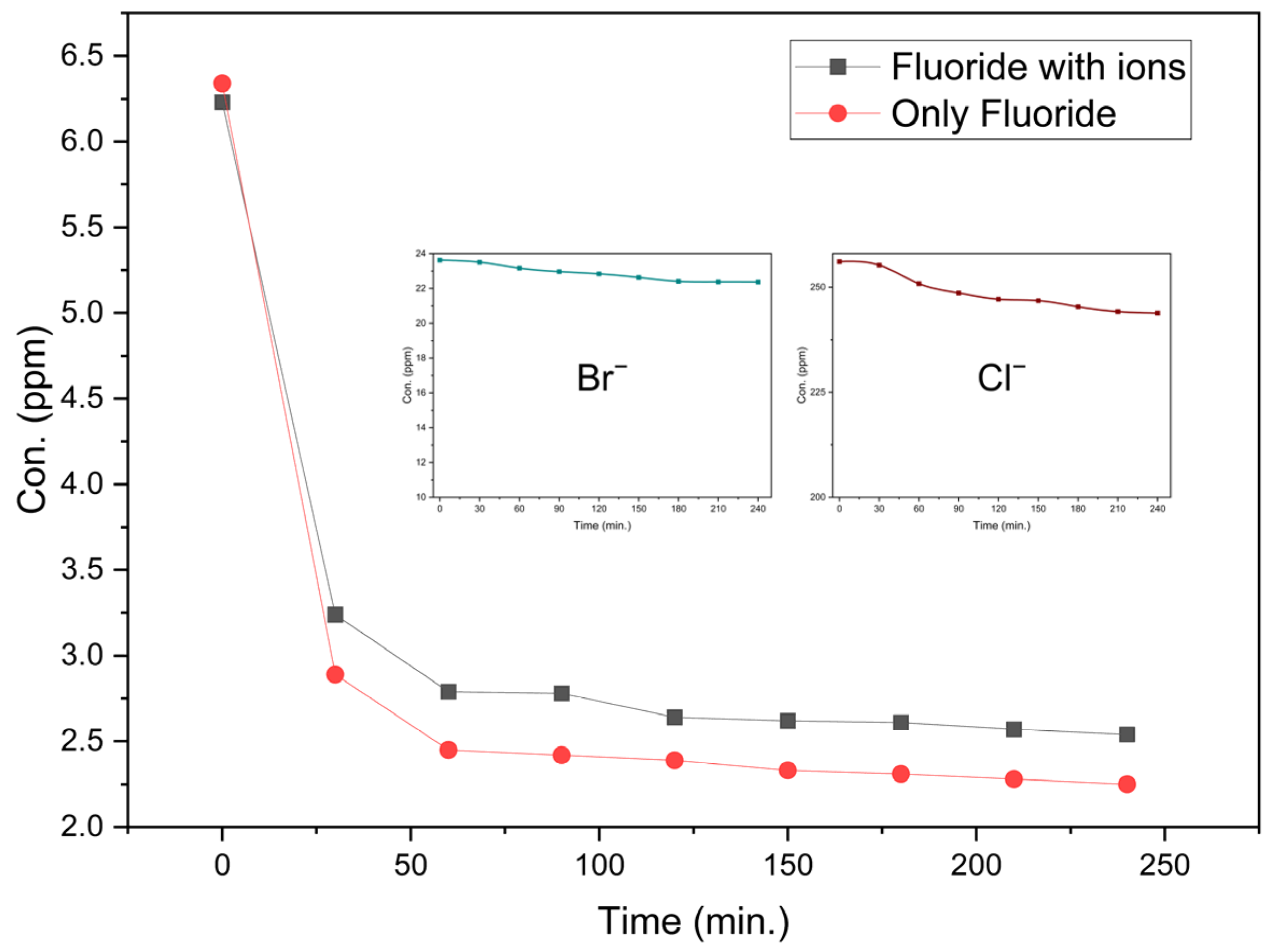
3.2.2. Effect of Competing Ions
3.3. Adsorption Mechanism
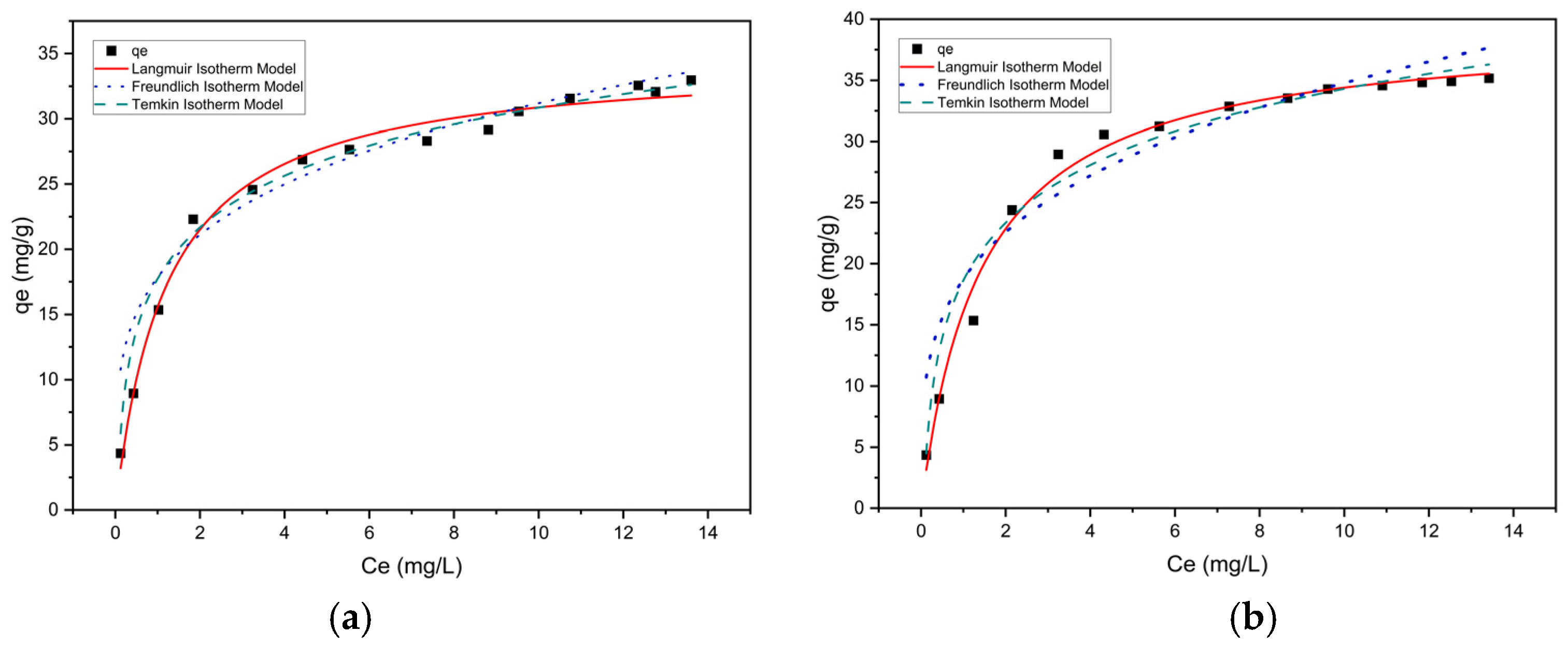
3.3.1. Isotherm Study
3.3.2. Kinetic Study
3.4. Practical Application Study
3.4.1. Regeneration and Re-Use
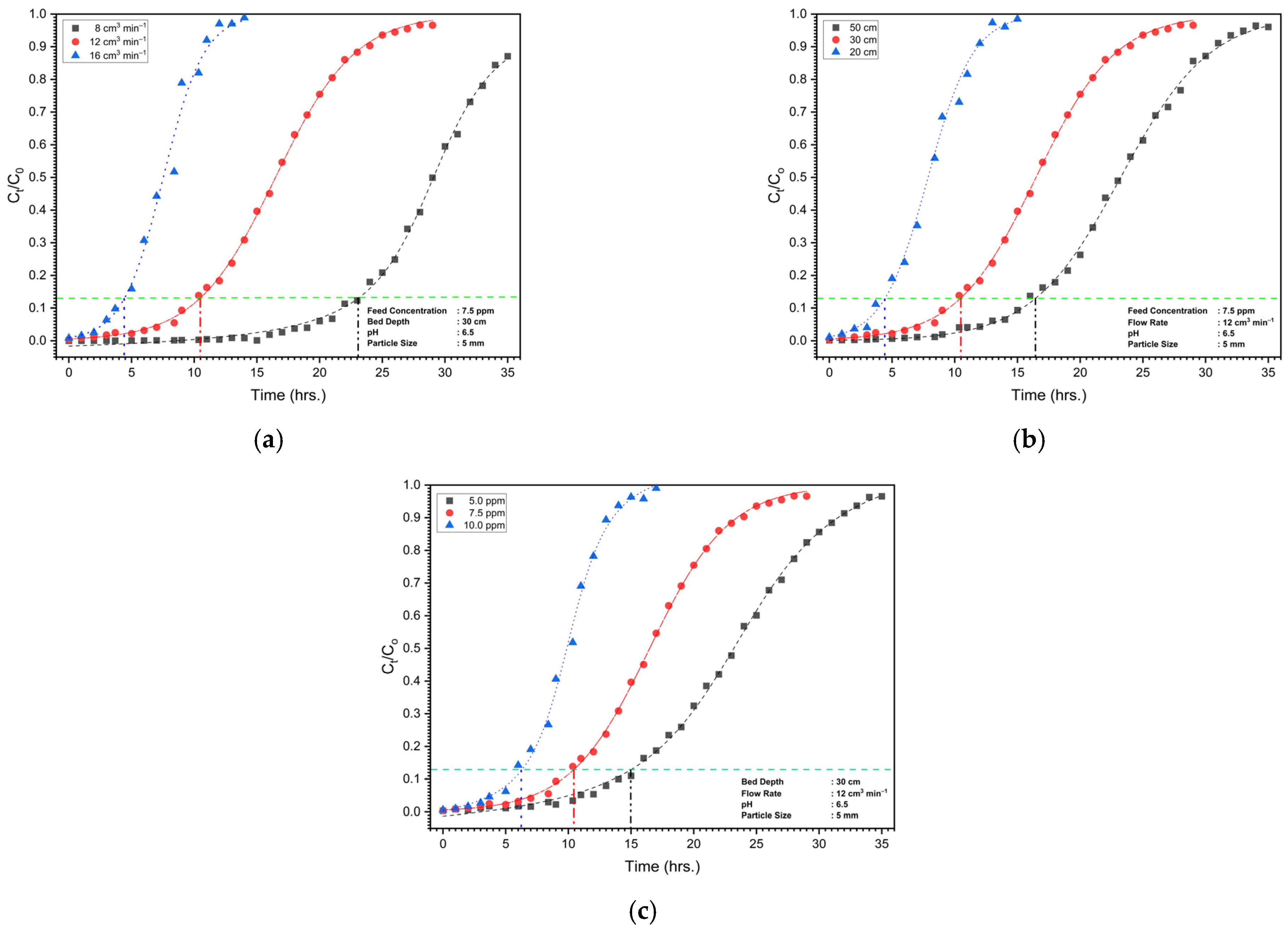
3.4.2. Column Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dar, F.A.; Kurella, S. Fluoride in Drinking Water: An in-Depth Analysis of Its Prevalence, Health Effects, Advances in Detection and Treatment. Mater. Today Proc. 2024, 102, 349–460. [Google Scholar] [CrossRef]
- Ahmad, S.; Singh, R.; Arfin, T.; Neeti, K. Fluoride Contamination, Consequences and Removal Techniques in Water: A Review. Environ. Sci. Adv. 2022, 1, 620–661. [Google Scholar] [CrossRef]
- Din, I.U.; Ali, W.; Muhammad, S.; Shaik, M.R.; Shaik, B.; Rehman, I.u.; Tokatli, C. Spatial Distribution and Potential Health Risk Assessment for Fluoride and Nitrate via Water Consumption in Pakistan. J. Geochem. Explor. 2024, 259, 349–360. [Google Scholar] [CrossRef]
- Huang, S.; Guo, J.; Xie, Y.; Bian, R.; Wang, N.; Qi, W.; Liu, H. Distribution, Sources, and Potential Health Risks of Fluoride, Total Iodine, and Nitrate in Rural Drinking Water Sources of North and East China. Sci. Total Environ. 2023, 898, 165561. [Google Scholar] [CrossRef] [PubMed]
- Tashauoei, H.R.; Mahdavi, M.; Mahvi, A.H.; Fatehizadeh, A. Dataset of Fluoride Concentration and Health Risk Assessment in Drinking Water in the Saveh City of Markazi Province, Iran. Data Brief 2023, 50, 109466. [Google Scholar] [CrossRef]
- Indika, S.; Hu, D.; Wei, Y.; Yapabandara, I.; Cooray, T.; Makehelwala, M.; Jinadasa, K.B.S.N.; Weragoda, S.K.; Weerasooriya, R.; Pang, Z. Spatiotemporal Variation of Groundwater Quality in North Central Province, Sri Lanka. ACS EST Water 2023, 3, 1687–1698. [Google Scholar] [CrossRef]
- Wasana, H.M.S.; Aluthpatabendi, D.; Kularatne, W.M.T.D.; Wijekoon, P.; Weerasooriya, R.; Bandara, J. Drinking Water Quality and Chronic Kidney Disease of Unknown Etiology (CKDu): Synergic Effects of Fluoride, Cadmium and Hardness of Water. Environ. Geochem. Health 2016, 38, 157–168. [Google Scholar] [CrossRef]
- Imbulana, S.; Oguma, K.; Takizawa, S. Evaluation of Groundwater Quality and Reverse Osmosis Water Treatment Plants in the Endemic Areas of Chronic Kidney Disease of Unknown Etiology (CKDu) in Sri Lanka. Sci. Total Environ. 2020, 745, 140870. [Google Scholar] [CrossRef]
- Dar, F.A.; Kurella, S. Recent Advances in Adsorption Techniques for Fluoride Removal—An Overview. Groundw. Sustain. Dev. 2023, 23, 101017. [Google Scholar] [CrossRef]
- Jha, P.K.; Tripathi, P. Arsenic and Fluoride Contamination in Groundwater: A Review of Global Scenarios with Special Reference to India. Groundw. Sustain. Dev. 2021, 13, 100576. [Google Scholar] [CrossRef]
- Behbahani, M.; Moghaddam, M.R.A.; Arami, M. Techno-Economical Evaluation of Fluoride Removal by Elec-trocoagulation Process: Optimization through Response Surface Methodology. Desalination 2011, 271, 209–218. [Google Scholar] [CrossRef]
- Haldar, D.; Duarah, P.; Purkait, M.K. MOFs for the Treatment of Arsenic, Fluoride and Iron Contaminated Drinking Water: A Review. Chemosphere 2020, 251, 126388. [Google Scholar] [CrossRef] [PubMed]
- Gangani, N.; Joshi, V.C.; Sharma, S.; Bhattacharya, A. Fluoride Contamination in Water: Remediation Strate-gies through Membranes. Groundw. Sustain. Dev. 2022, 17, 100751. [Google Scholar] [CrossRef]
- Kabay, N.; Arar, Ö.; Samatya, S.; Yüksel, Ü.; Yüksel, M. Separation of Fluoride from Aqueous Solution by Electrodialysis: Effect of Process Parameters and Other Ionic Species. J. Hazard. Mater. 2008, 153, 107–113. [Google Scholar] [CrossRef]
- Lakshmi, M.V.V.C.; Chandana Lakshmip, M.V.V.; Karthikp, K.V.S. Removal of Fluoride from Groundwater Using Various Technologies: A Review. Int. J. Innov. Sci. Eng. Technol. 2019, 6, 20–37. [Google Scholar]
- El Messaoudi, N.; Franco, D.S.P.; Gubernat, S.; Georgin, J.; Şenol, Z.M.; Ciğeroğlu, Z.; Allouss, D.; El Hajam, M. Advances and Future Perspectives of Water Defluoridation by Adsorption Technology: A Review. Environ. Res. 2024, 252, 118857. [Google Scholar] [CrossRef] [PubMed]
- Gebrewold, B.D.; Werkneh, A.A.; Kijjanapanich, P.; Rene, E.R.; Lens, P.N.L.; Annachhatre, A.P. Low Cost Materials for Fluoride Removal from Groundwater. J. Environ. Manag. 2024, 370, 122937. [Google Scholar] [CrossRef]
- He, J.; Yang, Y.; Wu, Z.; Xie, C.; Zhang, K.; Kong, L.; Liu, J. Review of Fluoride Removal from Water Environ-ment by Adsorption. J. Environ. Chem. Eng. 2020, 8, 104516. [Google Scholar] [CrossRef]
- Yadav, K.K.; Gupta, N.; Kumar, V.; Khan, S.A.; Kumar, A. A Review of Emerging Adsorbents and Current Demand for Defluoridation of Water: Bright Future in Water Sustainability. Environ. Int. 2018, 111, 80–108. [Google Scholar] [CrossRef]
- Manna, S.; Das, P.; Basak, P.; Sharma, A.K.; Singh, V.K.; Patel, R.K.; Pandey, J.K.; Ashokkumar, V.; Pugazhendhi, A. Separation of Pollutants from Aqueous Solution Using Nanoclay and Its Nanocomposites: A Review. Chemosphere 2021, 280, 130961. [Google Scholar] [CrossRef]
- Bahmanzadegan, F.; Ghaemi, A. A Comprehensive Review on Novel Zeolite-Based Adsorbents for Environ-mental Pollutant. J. Hazard. Mater. Adv. 2025, 17, 100617. [Google Scholar] [CrossRef]
- Luo, Q.; Wei, J.; Guo, Z.; Song, Y. Adsorption and Immobilization of Phosphorus from Water and Sediments Using a Lanthanum-Modified Natural Zeolite: Performance, Mechanism and Effect. Sep. Purif. Technol. 2024, 329, 125187. [Google Scholar] [CrossRef]
- Sifuna Wanyonyi, F.; Fidelis, T.T.; Louis, H.; Kyalo Mutua, G.; Orata, F.; Rhyman, L.; Ramasami, P.; Pembere, A.M.S. Simulation Guided Prediction of Zeolites for the Sorption of Selected Anions from Water: Machine Learning Predictors for Enhanced Loading. J. Mol. Liq. 2022, 355, 118913. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, X.; Li, J.; Li, F.; Li, X.; Yu, J.; Guo, L.; Song, G.; Xiao, C.; Zhou, F.; et al. Efficient Removal of Phosphate and Fluoride from Phosphogypsum Leachate by Lanthanum-Modified Zeolite: Synchronous Ad-sorption Behavior and Mechanism. J. Environ. Chem. Eng. 2024, 12, 113294. [Google Scholar] [CrossRef]
- Yang, R.; Chen, J.; Zhang, Z.; Wu, D. Performance and Mechanism of Lanthanum-Modified Zeolite as a Highly Efficient Adsorbent for Fluoride Removal from Water. Chemosphere 2022, 307, 136063. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-q.; Yang, K.; Yang, C.; Tian, Z.-l.; Guo, W.-c.; Li, J. Thermodynamics and Kinetics of Fluoride Removal from Simulated Zinc Sulfate Solution by La(III)-Modified Zeolite. Trans. Nonferrous Met. Soc. China 2018, 28, 783–793. [Google Scholar] [CrossRef]
- Chen, J.; Chen, H.; Li, X.; He, X. Adsorption Characteristics of Chitosan Loaded Zirconium-Zeolite Composite Adsorbents for Removal of F from Water. Lizi Jiaohuan Yu Xifu/Ion Exch. Adsorpt. 2017, 33, 537–546. [Google Scholar]
- Chen, J.; Yang, R.; Zhang, Z.; Wu, D. Removal of Fluoride from Water Using Aluminum Hydroxide-Loaded Zeolite Synthesized from Coal Fly Ash. J. Hazard. Mater. 2022, 421, 126817. [Google Scholar] [CrossRef]
- Ebsa, D.G. Defluoridation of Drinking Water by Modified Natural Zeolite with Cationic Surfactant, in the Case of Ziway Town, Ethiopia. Clean. Eng. Technol. 2023, 12, 100596. [Google Scholar] [CrossRef]
- Gao, Y.; Li, M.; Ru, Y.; Fu, J. Fluoride Removal from Water by Using Micron Zirconia/Zeolite Molecular Sieve: Characterization and Mechanism. Groundw. Sustain. Dev. 2021, 13, 100567. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Q.; Weng, X.; Mang, C.; Si, L.; Guan, Z.; Cheng, L.; Ma, Z.; Weng, X. Fluoride Ion Adsorption from Wastewater Using Magnesium(II), Aluminum(III) and Titanium(IV) Modified Natural Zeolite: Kinetics, Thermodynamics, and Mechanistic Aspects of Adsorption. J. Water Reuse Desalination 2018, 8, 479–489. [Google Scholar] [CrossRef]
- Bera, B.; Saha Chowdhury, S.; Sonawane, V.R.; De, S. High Capacity Aluminium Substituted Hydroxyapatite Incorporated Granular Wood Charcoal (Al-HApC) for Fluoride Removal from Aqueous Medium: Batch and Column Study. Chem. Eng. J. 2023, 466, 143264. [Google Scholar] [CrossRef]
- Joe-Wong, C.; Alemán-Reyes, A.; Le, N.Q.; Salerno, K.M.; Johnson, J.K.; Xia, Z.; Nachman, D.R. Fluoride Re-moval by Calcite and Hydroxyapatite. Environ. Sci. 2023, 9, 1683–1689. [Google Scholar] [CrossRef]
- Rathnayake, A.; Hettithanthri, O.; Sandanayake, S.; Mahatantila, K.; Rajapaksha, A.U.; Vithanage, M. Essence of Hydroxyapatite in Defluoridation of Drinking Water: A Review. Environ. Pollut. 2022, 311, 119882. [Google Scholar] [CrossRef]
- Russo, V.; D’Angelo, A.; Salvi, C.; Paparo, R.; Fortunato, M.E.; Cepollaro, E.M.; Tarallo, O.; Trifuoggi, M.; Di Serio, M.; Tesser, R. Fluoride Adsorption on Hydroxyapatite: From Batch to Continuous Operation. J. Environ. Chem. Eng. 2024, 12, 111973. [Google Scholar] [CrossRef]
- Bakhta, S.; Sadaoui, Z.; Bouazizi, N.; Samir, B.; Cosme, J.; Allalou, O.; Le Derf, F.; Vieillard, J. Successful Re-moval of Fluoride from Aqueous Environment Using Al(OH)3@AC: Column Studies and Breakthrough Curve Modeling. RSC Adv. 2024, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Yang, X.; Huang, L.; Li, Y.; Hao, L.; Pei, Q.; Pei, X. A Critical Review of Breakthrough Models with Analytical Solutions in a Fixed-Bed Column. J. Water Process Eng. 2024, 59, 105065. [Google Scholar] [CrossRef]
- Asrori, M.R.; Santoso, A.; Sumari, S. Proofing the Presence of Metal Oxide Impregnated into Zeolite A without Calcination: XRD and FTIR Studies. Case Stud. Chem. Environ. Eng. 2024, 9, 100676. [Google Scholar] [CrossRef]
- Chen, W.; Tang, H.; Li, H.; Zhao, Y.; Wang, X.; Chen, J.; Chen, Z.; Zhu, Y.; Yang, W. Efficient Defluoridation of Water by Utilizing Nanosized Ce-Fe Bimetal Oxyhydroxides Encapsulated inside Porous Polystyrene Anion Exchanger. Chem. Eng. J. 2023, 461, 141820. [Google Scholar] [CrossRef]
- Adamu, D.B.; Tufa, L.T.; Lee, J.; Zereffa, E.; Segne, T.A.; Razali, M.H. Facile Synthesis of Bismuth and Iron Co-Doped Hydroxyapatite Nanomaterials for High-Performance Fluoride Ions Adsorption. J. Environ. Chem. Eng. 2023, 11, 111196. [Google Scholar] [CrossRef]
- Mishima, K.; Du, X.; Miyamoto, N.; Kano, N.; Imaizumi, H. Experimental and Theoretical Studies on the Adsorption Mechanisms of Uranium (VI) Ions on Chitosan. J. Funct. Biomater. 2018, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Ayub, S.; Tripathi, C.N. Isotherms Describing Physical Adsorption of Cr(VI) from Aqueous Solution Using Various Agricultural Wastes as Adsorbents. Cogent Eng. 2016, 3, 1186857. [Google Scholar] [CrossRef]
- Nguyen, D.A.; Nguyen, V.B.; Jang, A. Integrated Adsorption Using Ultrahigh-Porosity Magnesium Oxide with Multi-Output Predictive Deep Belief Networks: A Robust Approach for Fluoride Treatment. Chem. Eng. J. 2024, 484, 149586. [Google Scholar] [CrossRef]
- Cao, L.; Cao, P.; Wang, Z.; Tan, Z.; Zhou, C.; Zhang, G.; Zhang, S.; Wang, J. Performance and environmental impact assessment of a novel zeolite modified cement stabilized calcareous sand material for island eco-construction. Const. Build. Mat. 2025, 458, 139628. [Google Scholar] [CrossRef]
- García Calvo, J.; Carballosa, P.; Padilla, I.; Jarabo, R.; Romero, M.; López-Delgado, A. Use of industrial waste-based zeolites in the fabrication of cementitious materials. Mater. Constr. 2024, 74, e358. [Google Scholar] [CrossRef]
- Graziano, S.F.; Mercurio, M.; Izzo, F.; Langella, A.; Rispoli, C.; Santaniello, N.D.; Benedetto, C.D.; Monetti, V.; Biondi, M.; De Rosa, G.; et al. A recycled natural resource as secondary raw material for versatile technological applications: The quarry waste from zeolite-rich tuffs. Appl. Clay Sci. 2024, 258, 107451. [Google Scholar] [CrossRef]
- Doni, S.; Masciandaro, G.; Macci, C.; Manzi, D.; Mattii, G.B.; Cataldo, E.; Gispert, M.; Vannucchi, F.; Peruzzi, E. Zeolite and Winery Waste as Innovative By-Product for Vineyard Soil Management. Environments 2024, 11, 29. [Google Scholar] [CrossRef]
- Doni, S.; Peruzzi, E.; Manzi, D.; Masini, C.; Mattii, G.B.; Masciandaro, G. Co-composting winery waste and zeolite: A sustainable valorisation example. J. Mater. Cycles Waste Manag. 2024, 26, 1086–1101. [Google Scholar] [CrossRef]
- Teutli-Sequeira, A.; Solache-Ríos, M.; Martínez-Miranda, V.; Linares-Hernández, I. Behavior of Fluoride Removal by Aluminum Modified Zeolitic Tuff and Hematite in Column Systems and the Thermodynamic Parameters of the Process. Water Air Soil Pollut. 2015, 226, 239. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, Y.; Zhong, M. Defluorination of wastewater by calcium chloride modified natural zeolite. Desalination 2011, 276, 246–252. [Google Scholar] [CrossRef]
- Samatya, S.; Yüksel, Ü.; Yüksel, M.; Kabay, N. Removal of Fluoride from Water by Metal Ions (Al3+, La3+ and ZrO2+) Loaded Natural Zeolite. Sep. Sci. Technol. 2007, 42, 2033–2047. [Google Scholar] [CrossRef]
- Waghmare, S.; Lataye, D.H.; Arfin, T.; Rayalu, S. Defluoridation by Nano-Materials, Building Materials and Other Miscellaneous Materials: A Systematic Review. Int. J. Innov. Res. Sci. Eng. Technol. 2015, 4, 11998–12010. [Google Scholar] [CrossRef]
- Onyango, M.S.; Kojima, Y.; Aoyi, O.; Bernardo, E.C.; Matsuda, H. Adsorption equilibrium modeling and solution chemistry dependence of fluoride removal from water by trivalent-cation-exchanged zeolite F-9. J. Colloid Interface Sci. 2004, 279, 341–350. [Google Scholar] [CrossRef]
- Onyango, M.S.; Kojima, Y.; Kumar, A.; Kuchař, D.; Kubota, M.; Matsuda, H. Uptake of Fluoride by Al3+ Pretreated Low-Silica Synthetic Zeolites: Adsorption Equilibrium and Rate Studies. Sep. Sci. Technol. 2006, 41, 683–704. [Google Scholar] [CrossRef]
- Tian, Z.H.; Gan, Y.Q. In situ synthesis of structural hierarchy flowerlike zeolite and its application for fluoride removal in aqueous solution. J. Nanomater. 2019, 2019, 2932973. [Google Scholar] [CrossRef]
- Chakraborty, A.; Naskar, M.K. Sol-gel synthesis of alumina gel@zeolite X nanocomposites for high performance water defluoridation: Batch and column adsorption study. Mater. Adv. 2022, 3, 8544–8556. [Google Scholar] [CrossRef]



| Parameter | Thomas Model | Yoon–Nelson Model | Bohart–Adams Model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | R2 | R2 | |||||||
| Bed Depth (cm) | |||||||||
| 20 | 0.074 | 0.94 | 0.998 | 0.539 | 7.98 | 0.995 | 0.029 | 198.7 | 0.985 |
| 30 | 0.042 | 3.29 | 0.999 | 0.316 | 16.48 | 0.995 | 0.042 | 185.4 | 0.989 |
| 50 | 0.037 | 6.96 | 0.996 | 0.280 | 23.22 | 0.995 | 0.061 | 138.6 | 0.985 |
| Flow Rate (cm3 min−1) | |||||||||
| 8 | 0.020 | 5.84 | 0.998 | 0.230 | 24.81 | 0.988 | 0.055 | 124.5 | 0.985 |
| 12 | 0.042 | 3.29 | 0.999 | 0.316 | 16.48 | 0.999 | 0.042 | 185.4 | 0.988 |
| 16 | 0.081 | 1.00 | 0.988 | 0.613 | 7.53 | 0.988 | 0.032 | 218.2 | 0.985 |
| Feed Concentration (mg dm−3) | |||||||||
| 5 | 0.031 | 6.43 | 0.998 | 0.250 | 23.0 | 0.998 | 0.025 | 218.6 | 0.985 |
| 7.5 | 0.042 | 3.29 | 0.999 | 0.316 | 16.48 | 0.998 | 0.042 | 185.4 | 0.989 |
| 10 | 0.051 | 1.32 | 0.995 | 0.572 | 9.799 | 0.995 | 0.057 | 146.9 | 0.995 |
| Variation with Bed Depth (cm) | Variation with Flow Rate (cm3 min−1) | Variation with Feed Concentration (ppm) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 20 | 30 | 50 | 8 | 12 | 16 | 5 | 7.5 | 10 | |
| 4.5 | 10.5 | 16.5 | 23 | 10.5 | 4.5 | 15 | 10.5 | 6.5 | |
| 24.3 | 56.7 | 89.1 | 82.8 | 56.7 | 32.4 | 54 | 56.7 | 35.1 | |
| 23.02 | 54.37 | 86.4 | 64.99 | 54.37 | 30.81 | 53.87 | 54.37 | 23.65 | |
| 3.24 | 7.56 | 11.88 | 11.04 | 7.56 | 4.32 | 10.80 | 7.56 | 4.68 | |
| 94.73 | 95.88 | 96.94 | 78.48 | 95.88 | 95.1 | 99.77 | 95.88 | 67.37 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boteju, R.; Zheng, L.; Wasana, H.M.S.; Wu, Q.; Wei, Y.; Zhong, H.; Wang, Y.; de Alwis, A. Hydroxyapatite-Modified Zeolite for Fluoride Removal from Drinking Water: Adsorption Mechanism Investigation and Column Study. Water 2025, 17, 908. https://doi.org/10.3390/w17060908
Boteju R, Zheng L, Wasana HMS, Wu Q, Wei Y, Zhong H, Wang Y, de Alwis A. Hydroxyapatite-Modified Zeolite for Fluoride Removal from Drinking Water: Adsorption Mechanism Investigation and Column Study. Water. 2025; 17(6):908. https://doi.org/10.3390/w17060908
Chicago/Turabian StyleBoteju, Rajinda, Libing Zheng, Hewa M. S. Wasana, Qiyang Wu, Yuansong Wei, Hui Zhong, Yawei Wang, and Ajith de Alwis. 2025. "Hydroxyapatite-Modified Zeolite for Fluoride Removal from Drinking Water: Adsorption Mechanism Investigation and Column Study" Water 17, no. 6: 908. https://doi.org/10.3390/w17060908
APA StyleBoteju, R., Zheng, L., Wasana, H. M. S., Wu, Q., Wei, Y., Zhong, H., Wang, Y., & de Alwis, A. (2025). Hydroxyapatite-Modified Zeolite for Fluoride Removal from Drinking Water: Adsorption Mechanism Investigation and Column Study. Water, 17(6), 908. https://doi.org/10.3390/w17060908







