Abstract
The water distribution system is a critical infrastructure aiming to deliver safe and clean drinking water, with pipeline materials significantly influencing water quality and efficiency. One critical factor in selecting pipeline materials is the potential for biofilm formation on the inner surfaces of pipes. This study investigates the effects of three iron salts—iron (II) sulfate heptahydrate, iron (III) nitrate nonahydrate, and iron (III) chloride on biofilm formation by Escherichia coli and Enterococcus faecalis in pipeline environments, focusing on water distribution systems. While previous research has examined the effects of iron on various bacterial species, there are limited data on E. coli and E. faecalis biofilm formation in the context of water distribution systems. Results reveal that iron (III) chloride significantly inhibited E. coli biofilm formation by up to 80%, while E. faecalis biofilm growth was promoted by iron (II) sulfate heptahydrate, with an increase of approximately 45%. These findings underscore the critical role of managing iron concentrations to mitigate biofilm-related issues, which influence water quality, infrastructure durability, and microbial resistance. The study highlights the importance of integrating these insights into sustainable water management practices and advancing pipeline material innovations to enhance public health and environmental resilience.
1. Introduction
Ensuring safe and efficient water distribution is a fundamental challenge in modern infrastructure. The material choice of pipelines is a critical factor influencing system performance, water quality, and public health risks. Iron and plastic pipes are common, but their long-term impact on water chemistry, particularly in multicomponent materials systems, is not yet thoroughly understood [1]. This study investigates the impact of different iron salts on bacterial growth, biofilm formation, and antibiotic resistance. Specifically, it examines how various salts influence fecal indicator bacteria, Escherichia coli and Enterococcus faecalis, which are crucial for assessing water quality and public health risks. The findings will improve pipeline material selection and biofilm-associated risk management in water networks and can lead to lead mobilization in wastewater systems.
Iron’s mechanical strength and potential antimicrobial effects make it a popular choice. Still, its susceptibility to corrosion introduces significant challenges, including biofilm formation and the release of iron salts into the water [2,3]. In contrast, plastic pipes, such as polyvinyl chloride (PVC) or high-density polyethylene (HDPE) pipes, are cost-effective and easier to install but lack the strength and antimicrobial benefits of iron [4]. They are lightweight, which reduces transportation and handling costs, and their flexibility can simplify the installation process [5,6]. When older infrastructure contains lead-based materials, such as lead pipes or solder, corrosion of nearby iron pipes can destabilize these lead materials and cause them to be released into the water [7,8,9]. Additionally, the chemical changes caused by iron corrosion, such as increased acidity and the presence of certain oxidizing agents, can enhance the solubility and mobility of lead ions in water. These interactions exacerbate the potential for lead contamination in systems with mixed-material pipelines [10,11]. Furthermore, studies show that iron corrosion products can change water chemistry in ways that favor the dissolution of lead-based materials and pose a serious threat to public health [12].
Iron’s susceptibility to corrosion also introduces significant challenges. Over time, corrosion could leach iron ions or salts like iron sulfate or iron nitrate into the water. In this sense, iron corrosion affects water taste and color but also influences microbial dynamics. Various studies have examined the benefits and drawbacks of using iron in water systems, where it is simultaneously seen as an essential trace element and a pollutant. For example, corrosion in iron pipes can introduce metal ions that interact with microbial communities [3].
Biofilm formation, particularly by fecal indicator bacteria like Escherichia coli and Enterococcus faecalis, poses significant challenges in pipeline systems. These biofilms can serve as reservoirs for antibiotic-resistant bacteria, increasing public health risks. Additionally, biofilm growth contributes to material degradation, reducing pipeline lifespan, and causing operational inefficiencies such as flow restrictions and increased maintenance costs. While iron pipes are known to influence microbial dynamics, the effects of specific iron salts—iron (II) sulfate heptahydrate, iron (III) nitrate nonahydrate, and iron (III) chloride—on E. coli and E. faecalis biofilm formation have not been extensively studied in the context of water distribution systems [13,14]. They form on metal, plastic, and concrete surfaces in pipeline environments, resulting in various operational problems. In this regard, biofilm formation may cause biofouling and decreased flow efficiency, and increased energy consumption may be caused by pipeline blockage [15,16]. Various studies have concluded that iron, where there is fecal contamination, can contribute significantly to the development of biofilms. At the same time, some research also suggested that high iron concentrations might lead to the development of antibiotic-resistant strains [17]. Also, biofilms can raise the corrosion processes, leading to material degradation, which might end in pipeline failures [18,19]. Studies have shown that corroded iron pipes can increase iron concentrations in drinking water, sometimes exceeding the EPA’s secondary maximum contaminant level (SMCL) of 0.3 mg/L set, leading to taste and discoloration issues [3].
Iron sulfate, iron nitrate, and iron chloride differ in chemical properties, solubility, and reactivity, leading to varied interactions with bacterial communities. Iron sulfate, for instance, tends to precipitate out of solution more readily than iron nitrate, which remains soluble at higher concentrations. These differences influence how each salt interacts with bacterial cells, potentially affecting the biofilm formation and metabolic activity. Studies focusing on the role of iron salts in microbial ecosystems have provided insights, but the effects of these salts on fecal bacteria in pipeline environments remain underexplored [20,21].
Heavy metals, including iron, have also been linked to the co-selection of antibiotic resistance in bacterial communities exposed to sub-lethal iron concentrations [22]. This co-selection for resistance is a growing concern in water and wastewater systems. Additionally, bacterial metabolic activity is a critical marker of bacterial viability under stress conditions, such as exposure to iron salts. However, research on how iron salts influence metabolic activity in bacteria, particularly those involved in fecal contamination, remains limited [18].
Although much research has examined iron’s role in water systems, few studies have directly compared the impact of different iron salts on bacterial growth, antibiotic resistance, and metabolic activity. Antibiotic resistance has become a growing global concern, particularly in wastewater systems where bacteria are frequently exposed to antibiotics and metal contaminants that can co-select for resistance traits. Specifically, no studies have investigated the influence of a series of iron salts on crucial bacterial characteristics such as growth, biofilm, and antibiotic resistance in wastewater systems. This is particularly so for fecal indicator bacteria such as E. coli and E. faecalis, which are of the utmost significance in the context of water quality and public health risks. The current study fills this gap by scientifically assessing the impact of different iron salts on these bacterial parameters. The findings are expected to yield useful information for effective pipeline material selection and biofilm-associated risk management in water networks.
2. Materials and Methods
2.1. Iron Salts
The iron samples used for the testing were iron (II) sulfate heptahydrate (FeSO4 × 7H2O, F8263, Sigma-Aldrich, Steinheim, Germany), iron (III) nitrate nonahydrate (FeN3O9 × 9H2O, I/1075/50, Fisher Scientific, Waltham, MA, USA), and iron (III) chloride (FeCl3, 157740, Sigma-Aldrich, Steinheim, Germany). The initial concentration was 100 mg/mL total mass concentration.
The choice of iron (II) sulfate heptahydrate and iron (III) nitrate nonahydrate included was based on their distinct chemical properties. Both salts dissociate in water to release iron ions in different oxidation states (Fe2+ and Fe3+), which may have varying effects on bacterial growth, biofilm formation, and antibiotic resistance. Iron (III) chloride was included as a representative form of iron commonly found in water systems in its chloride form.
2.2. Bacterial Strains
The bacterial strains used were E. coli ATCC 14169, E. coli ATCC 25922, E. faecalis ATCC 19433, and E. faecalis ATCC 29212. The bacterial strains were cultured overnight at 37 °C on Mueller Hinton (MH) agar (Liofilchem Ltd., Roseto degli Abruzzi, Italy). The bacterial suspension used in the research was 0.5 McFarland (1.5 × 108 CFU/mL) inoculum made in MH broth (Liofilchem Ltd., Italy). Then, 100 µL of the 0.5 McFarland inoculum was added to 9.9 mL of MH broth.
2.3. Antibacterial Effect
Four different concentrations (25, 50, 75, and 100 mg/mL) of iron (II) sulfate heptahydrate, iron (III) nitrate nonahydrate, and iron (III) chloride were used to examine the antibacterial effect of iron salts. The initial concentration of the iron salts was 100 mg/mL. The bacterial suspension used in the research was 0.5 McFarland (1.5 × 108 CFU/mL) inoculum made in MH broth. The inoculum was plated onto MH agar plates. Three wells (triplicate) were made in each agar plate, and 80 uL of the sample was added to the wells. The plates were incubated at 37 °C for 24 h. After the incubation period, the zones of inhibition were measured in mm.
2.4. Tissue Culture Plate Method
The tissue culture plate method determined the minimal inhibitory concentration (MIC). The negative controls used were MH broth. The positive controls (MH broth with bacteria) differed for each tested strain. Serial dilutions were made in MH broth in 96-well plates. The plates were inoculated with 20 µL of bacteria. The plates were incubated for 24 h at 37 °C. The optical density (OD) was measured in quadruplets at 595 nm using an ELISA plate reader (EPOCH, Agilent Technologies, Inc. Santa Clara, CA, USA).
2.5. Biofilm Formation Assay
Iron salts were used to evaluate their effect on biofilm formation for tested bacteria. MH broth was used as a negative control, while the positive controls (MH broth with bacteria) were specific for each tested strain. The serial dilutions were made in MH broth in 96-well plates. The plates were incubated for 24 h at 37 °C. Afterward, they were washed, stained with 0.1% crystal violet, and treated with 96% ethanol. The OD was measured in quadruplets at 595 nm using the ELISA plate reader. The optical density cut-off value (ODc) was calculated with the following formula [23]:
ODc = average OD of negative control + 3 × standard deviation of negative control.
The classification of biofilm formation is given in Table 1.

Table 1.
The classification of biofilm formation [23].
2.6. Antibiotic Susceptibility Testing
The antibiotic susceptibility testing (AST) of the four reference bacterial strains was conducted in duplicates using the Kirby–Bauer disc diffusion method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines [24]. The testing was performed for the strains treated with the subminimum dose (0.5 McFarland) of iron salts. The antimicrobial discs (Liofilchem Ltd., Italy) used were Doxycycline 30 µg (DXT 30), Amoxicillin 30 µg (AML 30), Mezlocillin (MEZ 75), Cefuroxime 30 µg (CXM 30), Ceftazidime 30 µg (CAZ 30), Ceftriaxone 30 µg (CRO 30), Ampicillin 2 µg (AMP 2), Amoxicillin and clavulanic acid 30 µg (AUG 30), Ceftazidime and clavulanic acid 40 µg (CAL 40), Ciprofloxacin 5 µg (CIP 5), Gentamicin 30 µg (CN 30), Gentamicin 10 µg (CN 10), Kanamycin 30 µg (K 30), Tobramycin 10 µg (TOB 10), and Tetracycline 30 µg (TE 30).
2.7. Growth Curves
To test the effect of the subminimum concentrations of iron salts, the growth of E. coli and E. faecalis strains was measured throughout 48 h at 20 °C by measuring their absorbance in duplicates at 595 nm using the ELISA plate reader. The untreated strains were compared to the strains growing in the presence of iron salts. All tested strains had an initial McFarland equivalent of 105 cells/mL.
2.8. Cell Viability Assay
A cell viability assay was used to determine the viability of tested bacterial strains after adding iron salts. MH broth was used as a negative control, while the positive controls (MH broth with bacteria) were specific for each bacterial strain. The serial dilutions were made in MH broth in the 96-well plates. The plates were incubated for 24 h at 37 °C. Moreover, 0.015 g was diluted in 100 mL of phosphate buffer saline (PBS) for the resazurin dye solution, whilst 60 µL of resazurin dye solution was added to each well and the plates were incubated for 3 h at 37 °C.
The OD was measured in quadruplets at 562 and 595 nm using the ELISA plate reader. The measurements were taken at 0 h and 3 h after adding the resazurin dye solution. Cell innate metabolic activity can be calculated using a formula for reduction percentage [25]:
where are the molar extinction coefficients for resazurin (Table 2), A is the measured absorbance at a given wavelength, t0 is the first measurement, and tx is the measurement at a given time x.

Table 2.
The molar extinction coefficients for resazurin [25].
3. Results and Discussion
3.1. Antibacterial Effect Results
Iron salts exhibited concentration-dependent inhibitory effects against the four pathogens. The antibacterial effect showed that mostly 100 mg/mL and 75 mg/mL of all the iron salts had the greatest zones of inhibition (Table 3 and Table 4).

Table 3.
The antibacterial effect on E. coli strains.

Table 4.
The antibacterial effect on E. faecalis strains.
3.2. Minimum Inhibitory Concentration of Iron Salts
Iron salts demonstrated concentration-dependent inhibitory effects on bacterial growth. Iron (III) chloride showed the lowest MIC values, particularly for E. coli strains, suggesting a higher potency compared to iron (II) sulfate heptahydrate and iron (III) nitrate nonahydrate (Table 5).

Table 5.
The minimum inhibitory concentration of iron salts.
At the lower concentration (3.125 mg/mL), Fe3+ ions likely exert partial inhibitory effects on E. faecalis ATCC 19433. The bacteria may still maintain some growth due to their ability to regulate iron uptake and mitigate oxidative stress. At the higher concentration (6.250 mg/mL), the inhibitory effects become more pronounced, likely overwhelming the bacterial defense mechanisms. The elevated Fe3+ concentration increases reactive oxygen species (ROS) production, disrupts metabolic pathways, and leads to more significant growth inhibition.
Iron (II) sulfate heptahydrate demonstrated a lower MIC against E faecalis strains compared to iron (III) nitrate nonahydrate and iron (III) chloride. This finding suggests that iron (II) sulfate heptahydrate may exert a more potent antimicrobial effect on E. faecalis, potentially due to its unique chemical properties, such as solubility and ion availability, which may influence microbial growth.
3.3. The Effect of Iron Salts on the Planktonic Growth
The results demonstrate that the iron (II) sulfate heptahydrate and iron (III) nitrate nonahydrate concentrations ranging from 3.125 to 100 mg/mL hinder E. coli growth. For the iron (III) chloride, the concentrations range from 1.563 to 100 mg/mL (Figure 1a,b). Regarding E. faecalis, iron (III) nitrate nonahydrate and iron (III) chloride concentrations range from 3.125 to 100 mg/mL. In contrast, for iron (II) sulfate heptahydrate, the concentrations range from 1.563 to 100 mg/mL (Figure 1c,d). These findings agree with Xiao et al., who reported that while low iron concentrations can stimulate microbial growth, high concentrations can have inhibitory effects due to generating ROS [26]. The current study further advances this knowledge by establishing the specific concentration range at which these inhibitive effects occur. This may help determine dosage ranges for limiting bacterial growth in pipeline systems, whether for drinking water or wastewater.
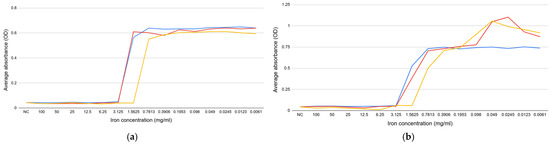
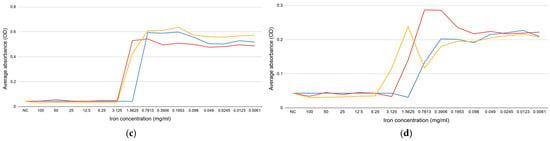
Figure 1.
The effect of iron (II) sulfate heptahydrate (blue), iron (III) nitrate nonahydrate (red), and iron (III) chloride (yellow) on the planktonic growth: (a) E. coli ATCC 14169; (b) E. coli ATCC 25922; (c) E. faecalis ATCC 19433; (d) E. faecalis ATCC 29212. NC—negative control.
3.4. The Effect of Iron Salts on the Biofilm Formation
The results of this study provide important insights into the impact of iron salts on biofilm formation in water distribution systems. The findings demonstrate that iron salts have species-specific effects, with iron (III) chloride showing the strongest inhibition of E. coli biofilm formation, while iron (II) sulfate heptahydrate promoted biofilm growth in E. faecalis. These results align with existing literature indicating that microbial responses to iron vary significantly based on species, environmental conditions, and the chemical form of iron. The effect of the iron salts on the biofilm formation of E. coli can be seen in Figure 2a,b, while the effect on E. faecalis biofilm formation is shown in Figure 2c,d.
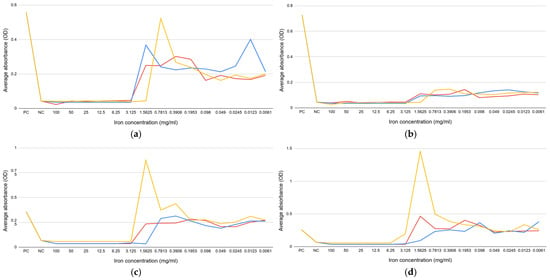
Figure 2.
The effect of iron (II) sulfate heptahydrate (blue), iron (III) nitrate nonahydrate (red), and iron (III) chloride (yellow) on the biofilm formation: (a) E. coli ATCC 14169; (b) E. coli ATCC 25922; (c) E. faecalis ATCC 19433; (d) E. faecalis ATCC 29212. PC—positive control; NC—negative control.
These findings are consistent with the literature that indicates that increasing iron availability encourages the growth of biofilms inside metallic pipelines [17] and that metals such as iron tend to support thicker biofilms compared to plastic surfaces [27]. Biofilms in water supply and wastewater systems can alter flow dynamics, harbor pathogens, and impact water quality. For instance, the corrosion of iron pipes and rough surfaces facilitates the attachment and embedding of biofilms—a possible reason behind the stimulation of biofilm-related problems in aging wastewater systems [28]. Our findings show that controlling iron salt concentration may be a viable way to regulate biofilm formation and control microbial populations in supply and drainage systems. The effects of biofilm on water flow are complex. Biofilms may block the pipes, reduce the flow rates, and increase the chances of pathogen proliferation. The pipe material can influence the types of microorganisms that dominate the biofilm and, hence, eventually affect the quality and safety of the water [29].
One of the key implications of this study is the potential to use targeted iron salt concentrations to manage biofilm formation in water pipelines. The observed inhibitory effects of iron (III) chloride on E. coli biofilms highlight its potential as a tool for reducing biofilm formation in water distribution systems. However, the promotion of E. faecalis biofilm formation by certain iron salts suggests that careful consideration must be given to the microbial composition of the system when selecting materials and treatment protocols.
Our findings are in partial agreement with the results obtained by Zhang et al., who noticed that Fe3+ ions inhibited bacterial growth, although microbial strains different from those studied here were used [11]. On the other hand, Hua et al. demonstrated that Fe2+ ions can act as the cofactors of enzymes involved in the process of biofilm formation in Gram-positive bacteria [18]. This corresponds to our observations obtained for E. faecalis.
The differences in our results from previous studies may be due to the varying experimental conditions, such as iron salt concentrations, bacterial strains, and environmental pH. For example, Zhu et al. showed that Fe3+ ions under alkaline conditions increased the production of ROS, which could account for the inhibition of biofilms observed in this study [12].
3.5. Antibiotic Susceptibility Testing Results
Interestingly, after being treated with sub-inhibitory concentrations of iron salts, only E. faecalis ATCC 29212 developed resistance (R) to Ceftazidime after treatment with each iron salt (Table 6 and Table 7). This finding is significant in wastewater systems, where mixed microbial populations and trace metal presence may play a similar role in determining antibiotic resistance.

Table 6.
The antibiotic susceptibility for E. coli.

Table 7.
The antibiotic susceptibility for E. faecalis.
A two-way ANOVA was conducted to assess the impact of treatment or various iron salts on the antibiotic susceptibility of different bacterial strains compared to untreated controls. The analysis revealed several statistically significant differences in susceptibility (p < 0.05). For E. coli ATCC 25922, a significant difference was observed with iron sulfate treatment (p = 0.0106). Similarly, for E. coli ATCC 14169, significant differences were found with nitrate (p = 0.000029) and chloride (p = 0.02998) treatments. For E. faecalis ATCC 19433, significant effects were identified for sulfate (p = 0.0019), nitrate (p = 0.00085), and chloride (p = 0.02039) treatments. Additionally, for E. faecalis ATCC 29212, nitrate treatment resulted in a significant difference (p = 0.007). These findings suggest that the type of iron salt and bacterial strain play a critical role in modulating antibiotic susceptibility, with certain treatments causing significant alterations.
3.6. Growth Curves Results
In order to examine the effect of subminimum doses of iron salts, a growth curve for E. coli ATCC 14169 (Figure 3a) and E. coli ATCC 25922 (Figure 3b) was constructed by measuring the absorbance each hour for 48 h at 595 nm. Iron (II) sulfate heptahydrate and iron (III) nitrate nonahydrate decrease the growth of E. coli ATCC 14169 and E. coli ATCC 25922 compared to the positive control. The same testing was done for E. faecalis ATCC 19433 (Figure 3c) and E. faecalis ATCC 29212 (Figure 3d). Iron (II) sulfate heptahydrate increased the growth of E. faecalis ATCC 19433 and E. faecalis ATCC 29212, while iron (III) chloride decreased their growth.
Figure 3.
Growth curves in the presence of iron (II) sulfate heptahydrate (blue), iron (III) nitrate nonahydrate (red), and iron (III) chloride (yellow): (a) E. coli ATCC 14169 (green); (b) E. coli ATCC 25922 (green); (c) E. faecalis ATCC 19433 (green); (d) E. faecalis ATCC 29212 (green).
For E. faecalis, the increased initial absorbance at time zero for iron (II) sulfate heptahydrate could be due to some bacterial-specific interaction with the iron salt, such as the adsorption of iron ions to its cell wall or the promotion of aggregation, thereby increasing turbidity. Furthermore, the metabolic activity of E. faecalis might alter the local pH or produce iron-chelating compounds that could affect the solubility and oxidation state of iron sulfate and thus its optical properties. These did not occur for E. coli, probably because of differences in the structure of the cell wall, metabolic activity, or interaction with the medium.
3.7. Cell-Viability Assay Results
Cell-viability assay results in Table 8, Table 9 and Table 10 confirm the MIC of iron salts for the tested bacterial strains. The reduction percentage of the negative control ranged from 7% to 11%. After MIC, the reduction percentage increased for each iron salt. Chen et al. concluded that the viability of bacteria was affected by exposure to iron-based compounds. Gram-negative bacteria like E. coli were more sensitive to Fe2+, while Gram-positive bacteria showed higher sensitivity to Fe3+ [30].

Table 8.
Reduction percentages for iron (II) sulfate heptahydrate.

Table 9.
Reduction percentages for iron (III) nitrate nonahydrate.

Table 10.
Reduction percentages for iron (III) chloride.
The cell viability assays revealed inhibitory effects of iron (III) chloride on bacterial cells, consistent with its strong biofilm-disrupting properties. This aligns with Berlec et al. and Benov who demonstrated that Fe3+ ions significantly reduced cell viability, likely due to oxidative stress induction [31,32].
From a practical perspective, these results have significant implications for pipeline material selection and water treatment strategies. For instance, selecting pipeline materials that minimize iron leaching, combined with the regular monitoring of iron salt concentrations, could reduce biofilm formation and extend the lifespan of infrastructure. Additionally, incorporating these findings into water management policies could help address biofilm-related issues such as microbial contamination, antibiotic resistance, and increased maintenance costs.
Moreover, such evidence can inform water management policy by identifying the need for selective interventions to avoid biofilm formation. For instance, employing optimized disinfection protocols or altering water chemistry to limit the availability of reactive iron salts would serve to reduce the risk of microbial contamination. Since biofilms themselves are reservoirs of antibiotic-resistant bacteria, avoiding their formation could complement overall public health efforts to contain antimicrobial resistance in water supply systems. Follow-up investigations should determine the long-term effect of exposure to iron salt under simulated natural conditions such as varying pH levels, flow regimes, and pipe material interaction.
4. Conclusions
This study demonstrates that iron salts impact the growth and biofilm formation of E. coli and E. faecalis in pipeline environments. Quantitatively, iron (III) chloride at concentrations as low as 3.125 mg/mL demonstrated a mild inhibitory effect on E. faecalis growth, with stronger inhibition observed at 6.250 mg/mL. Similarly, iron (III) chloride concentrations above 6.250 mg/mL markedly reduced E. coli growth and biofilm formation, indicating a threshold-dependent inhibitory effect. On the other hand, iron (II) sulfate heptahydrate exhibited a contrasting effect, promoting biofilm formation in E. faecalis within the tested concentration range, potentially due to its contribution to metabolic processes or biofilm-supportive conditions. These findings have important implications for pipeline material selection and water treatment strategies, emphasizing the need to carefully manage iron concentrations in water systems.
One limitation of the present study is that it focuses on specific laboratory strains that do not fully represent microbial diversity in real pipeline systems. While laboratory findings provide valuable insights, real-world pipeline systems involve complex variables such as temperature fluctuations, flow dynamics, and diverse microbial communities. The study’s controlled conditions may not fully replicate these complexities. Nevertheless, the results underscore the importance of managing iron concentrations in water systems to minimize biofilm formation and its associated risks.
Future research should focus on testing these interactions under dynamic, real-world conditions and exploring advanced pipeline materials and coatings that mitigate biofilm formation while maintaining durability and cost-effectiveness.
Author Contributions
Conceptualization, M.A. and A.E.S.; methodology, S.D., A.G. and N.C.; validation, A.E.S. and M.H.; formal analysis, S.D.; investigation, S.D. and N.C.; data curation, N.C.; writing—original draft preparation, S.D. and A.G.; writing—review and editing, M.H. and M.A.; supervision, M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article.
Acknowledgments
The research that has produced research paper has been financially supported by the European Union through the project Introducing Student Research Mobilities to BH Unis -INSTREAM, implemented by a consortium led by International Burch University, but do not necessarily represent the official position of the European Union or International Burch University and are the sole responsibility of the author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abdulah Shrrat Omar, O. Evaluation of Pipe Materials in Water System Networks Using the Theory of Advanced Multi-Criteria Analysis. Sustainability 2023, 15, 4491. [Google Scholar] [CrossRef]
- Lytle, D.A.; Tang, M.; Francis, A.T.; O’Donnell, A.J.; Newton, J.L. The effect of chloride, sulfate, and dissolved inorganic carbon on iron release from cast iron. Water Res. 2020, 183, 116037. [Google Scholar] [CrossRef]
- Sun, H.; Shi, B.; Yang, F.; Wang, D. Effects of sulfate on heavy metal release from iron corrosion scales in drinking water distribution systems. Water Res. 2020, 183, 116037. [Google Scholar] [CrossRef] [PubMed]
- Draper, W.; Li, N.; Solomon, G.; Heaney, Y.; Crenshaw, R.B.; Hinrichs, R.L.; Chandrasena, R. Organic Chemical Contaminants in Water System Infrastructure Following Wildfire. ACS ES&T Water 2022, 2, 357–366. [Google Scholar] [CrossRef]
- Xiong, J.; Zhu, J.; He, Y.; Ren, S.; Huang, W.; Lu, F. The Application of Life Cycle Assessment for the Optimization of Pipe Materials of Building Water Supply and Drainage System. Sustain. Cities Soc. 2020, 60, 102267. [Google Scholar] [CrossRef]
- Goraj, W.; Pytlak, A.; Kowalska, B.; Kowalski, D.; Grządziel, J.; Szafranek-Nakonieczna, A.; Galazka, A.; Stępniewska, Z.; Stepniewski, W. Influence of Pipe Material on Biofilm Microbial Communities Found in Drinking Water Supply System. Environ. Res. 2020, 190, 110433. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Huo, Y.; Leung, A.Y.F.; Zayed, T.; Tsang, D. Corrosion of Pipes for Conveying Drinking Water in Hong Kong: Mechanisms and Controlling Strategies. HKIE Trans. 2024, 31, 20220045. [Google Scholar] [CrossRef]
- Llatas, C.; Soust-Verdaguer, B.; Passer, A. Implementing Life Cycle Sustainability Assessment during design stages in Building Information Modelling: From systematic literature review to a methodological approach. Build. Environ. 2020, 182, 107164. [Google Scholar] [CrossRef]
- Qureshi, M.; Oasmaa, A.; Pihkola, H.; Deviatkin, I.; Tenhunen, A.; Mannila, J.; Minkkinen, H.; Pohjakallio, M.; Laine-Ylijoki, J. Pyrolysis of plastic waste: Opportunities and challenges. J. Anal. Appl. Pyrolysis 2020, 152, 104804. [Google Scholar] [CrossRef]
- Velázquez, J.C.; Hernández-Sánchez, E.; Terán, G.; Capula-Colindres, S.; Díaz-Cruz, M.; Cervantes-Tobón, A. Probabilistic and statistical techniques to study the impact of localized corrosion defects in oil and gas pipelines: A review. Metals 2022, 12, 576. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, L.; Liu, D.; Wang, J.; Zhang, X.; Chen, C. Early period corrosion and scaling characteristics of ductile iron pipe for groundwater supply with sodium hypochlorite disinfection. Water Res. 2020, 176, 115742. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Tang, Y.; Jiang, J.; Zhang, Y.; Wu, M.; Tang, C.; Wu, T.; Zhao, K. Microbial corrosion behavior of pipeline steels in simulation environment of natural gas transportation pipeline. RSC Adv. 2023, 13, 36168–36180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, B.; Guo, F.; Liu, J.; Luan, M.; Liu, Y.; Guan, Y. Taxonomic relatedness and environmental pressure synergistically drive the primary succession of biofilm microbial communities in reclaimed wastewater distribution systems. Environ. Int. 2020, 124, 25–37. [Google Scholar] [CrossRef]
- Best, M.; Cunha-Reis, C.; Ganin, A.; Sousa, A.; Johnston, J.; Oliveira, A.; Smith, D.; Yiu, H.; Cooper, I. Antimicrobial properties of Gallium(III)- and Iron(III)-loaded polysaccharides affecting the growth of Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa, In Vitro. ACS Appl. Bio Mater. 2020, 3, 7589–7597. [Google Scholar] [CrossRef]
- Yan, D.; Bai, Z.; Mike, R.; Gu, L.; Ren, S.; Yang, P. Biofilm structure and its influence on clogging in drip irrigation emitters distributing reclaimed wastewater. J. Environ. Sci. 2020, 21, 834–841. [Google Scholar] [CrossRef]
- LaMartina, E.; Mohaimani, A.; Newton, R. Urban wastewater bacterial communities assemble into seasonal steady states. Microbiome 2021, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-y.; Wei, Z.; Sun, G.; Su, H.-L.; Liu, J.; Hu, B.; Zhou, X.; Lou, L. Formation of biofilms from new pipelines at both ends of the drinking water distribution system and comparison of disinfection by-products formation potential. Environ. Res. 2020, 182, 109150. [Google Scholar] [CrossRef]
- Hua, X.; Qi, P.; Zeng, J.; Liu, X.; Li, Y.; Cheng, X. The Role of Iron in Drinking Water Biofilm Formation and Microbial Community Composition. Water Res. 2021, 188, 116523. [Google Scholar] [CrossRef]
- Roguet, A.; Newton, R.; Eren, A.; McLellan, S. Guts of the Urban Ecosystem: Microbial Ecology of Sewer Infrastructure. mSystems 2022, 7, e00118-22. [Google Scholar] [CrossRef]
- Oliveira, F.; Rohde, H.; Vilanova, M.; Cerca, N. The Emerging Role of Iron Acquisition in Biofilm-Associated Infections. Trends Microbiol. 2021, 29, 772–775. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, L.; Huang, T.; Yan, M.; Liu, K.; Miao, Y.; He, H.; Li, S.; Sekar, R. Combined effects of seasonality and stagnation on tap water quality: Changes in chemical parameters, metabolic activity and co-existence in bacterial community. J. Hazard. Mater. 2021, 403, 124018. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, S.; You, G.; Hou, J. Antibiotic resistance genes attenuation in anaerobic microorganisms during iron uptake from zero valent iron: An iron-dependent form of homeostasis and roles as regulators. Water Res. 2021, 195, 116979. [Google Scholar] [CrossRef]
- Panda, P.S.; Chaudhary, U.; Dube, S.K. Comparison of four different methods for detection of biofilm formation by uropathogens. Indian J. Pathol. Microbiol. 2016, 59, 177–179. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard—14th Edition (CLSI Document M02); CLSI: Malvern, PA, USA, 2024; Available online: https://clsi.org/standards/products/microbiology/documents/m02/ (accessed on 23 November 2024).
- Lee, A.; Jain, A. Measuring cell-viability by resazurin (Alamarblue®) assay using photopette® cell. Application Note, Tip Biosystems. 2017. Available online: https://www.tipbiosystems.com/wp-content/uploads/2023/12/AN016-Cell-Viability-using-Resazurin-Dye_2017_05_29.pdf (accessed on 15 November 2024).
- Xiao, R.; Bai, L.; Liu, K.-y.; Shi, Y.-w.; Minakata, D.; Huang, C.-H.; Spinney, R.; Seth, R.; Dionysiou, D.; Wei, Z.; et al. Elucidating sulfate radical-mediated disinfection profiles and mechanisms of Escherichia coli and Enterococcus faecalis in municipal wastewater. Water Res. 2020, 173, 115552. [Google Scholar] [CrossRef] [PubMed]
- Nejati, S.; Mirbagheri, S.; Waimin, J.; Grubb, M.E.; Peana, S.; Warsinger, D.; Rahimi, R. Laser Functionalization of Carbon Membranes for Effective Immobilization of Antimicrobial Silver Nanoparticles. J. Environ. Chem. Eng. 2020, 8, 104109. [Google Scholar] [CrossRef]
- Mulamattathil, S.G.; Bezuidenhout, C.; Mbewe, M. Biofilm formation in surface and drinking water distribution systems in Mafikeng, South Africa. S. Afr. J. Sci. 2020, 110, 1–9. [Google Scholar] [CrossRef]
- Zhu, Z.; Shan, L.; Hu, F.; Li, Z.; Zhong, D.; Yuan, Y.; Zhang, J. Biofilm Formation Potential and Chlorine Resistance of Typical Bacteria Isolated from Drinking Water Distribution Systems. RSC Adv. 2020, 10, 31295–31304. [Google Scholar] [CrossRef]
- Chen, Q.; Li, J.; Wu, Y.; Shen, F.; Yao, M. Biological Responses of Gram-Positive and Gram-Negative Bacteria to nZVI (Fe0), Fe2+, and Fe3+. RSC Adv. 2013, 3, 13835–13842. [Google Scholar] [CrossRef]
- Berlec, A.; Janež, N.; Sterniša, M.; Klančnik, A.; Sabotič, J. Expression of NanoLuc Luciferase in Listeria innocua for development of biofilm assay. Front. Microbiol. 2021, 12, 636421. [Google Scholar] [CrossRef]
- Benov, L. Improved formazan dissolution for bacterial MTT assay. Microbiol. Spectr. 2021, 9, e01637-21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).