Abstract
Caffeine in aquatic ecosystems is an emerging contaminant causing significant environmental concern. In this work, spent coffee ground (SCG) was pyrolyzed at 300, 450, and 600 °C to produce pristine SCG biochars (CG), which were then ball-milled to produce ball-milled SCG biochars (BMCG). A batch experiment with ball-milled and pristine biochars showed that ball-milled biochars pyrolyzed at 450 °C and 600 °C had the highest capacities to adsorb caffeine. Subsequently, ball-milled CG450 (BMCG450) was selected for further analysis. The results showed that ball milling dramatically augmented the specific surface area and oxygen-containing functional groups of the biochar. The Langmuir maximum caffeine adsorption capacity was 82.65 mg/g. Both solution pH and ionic strength affected caffeine removal by BMCG450. As pH increased, increased electrostatic repulsion limited caffeine adsorption onto the biochar. However, an increase in ion strength slightly enhanced caffeine adsorption because of the electrostatic screening effect of cations. The ball-milled SCG biochar also showed high adsorption efficiency in a completely mixed flow reactor under continuous flow conditions. Our study indicates that ball-milled SCG biochar at 450 °C can serve as a viable sorbent for the removal of caffeine from water.
1. Introduction
Caffeine (1, 3, 7-trimethylxanthine) is an emerging contaminant that has attracted widespread attention because it has been detected in many water bodies, with potential risks to ecosystems and public health. Caffeine, an alkaloid of the methylxanthine family (Figure 1), naturally exists in coffee, tea, and cocoa beans [1]. Nowadays, caffeine, as a food additive, is used to produce different foods and beverages, such as energy drinks, chewing gum, and candies [2]. Caffeine can stimulate the human central nervous system and reduce drowsiness; therefore, it is consumed all over the world.
Figure 1.
Chemical structure of caffeine.
The average daily caffeine consumption varies in different countries, and the worldwide average is about 70 mg per person [3]. In the USA, Drewnowski and Rehm [4] found that adults consume 173 mg of caffeine per person per day on average, while in Europe, the daily caffeine consumption ranges from 137 to 275 mg per person [5]. Meanwhile, spent coffee ground (SCG), as the primary waste produced in the coffee industry, annually reaches approximately 60 million tons globally [6]. Although SCG is a carbon-rich material, it is often labeled as waste and generally disposed of, becoming an environmental burden. The main portion of ingested caffeine is metabolized by the human liver, and about 3% of caffeine enters the sewage system by urine excretion [7]. Moreover, the disposal of caffeine-containing medicine from hospitals and unconsumed caffeine-containing products from restaurants and households also inputs caffeine into the water environment through sewage systems and landfill leaching.
Caffeine is detected in different types of water bodies, including rivers (14.5~127,092 ng/L), lakes (6~250 ng/L), seawater (5.2~1528.2 ng/L), and groundwater (88 ng/L) [8,9,10,11,12,13,14]. The effect of caffeine on species living in different types of aquatic ecosystems has been investigated. Gaudet-Hull et al. [15] found that the development of Xenopus laevis egg was affected by caffeine in the water. Moore et al. [16] pointed out that caffeine could impair the reproduction of Ceriodaphnia dubia and inhibit the growth of Pimephales promelas. Besides affecting freshwater species, caffeine could also negatively impact marine organisms, including algae, Bivalvia, and polychaeta. Aguirre-Martínez et al. [17] showed that caffeine caused growth inhibition of Isochrysis galbana. Capolupo et al. [18] illustrated that caffeine exposure could increase the oxidative stress of Ruditapes philippinarum. Pires et al. [19] found that long-term caffeine exposure could affect the regenerative capacity of Diopatra neapolitana.
Various technologies, such as adsorption [20], advanced oxidative processes [21], biodegradation [22], and membrane separation [23] have been developed to remove caffeine from water. A catalytic MnO2/MXene/chitosan nanocomposite nanofiltration membrane was fabricated and had a remarkable caffeine removal rate of 99.9% [24]. Under optimized environmental conditions, the fungus Trametes versicolor can achieve a caffeine degradation efficiency of up to 98% [25]. In addition, activated carbon derived from artichoke leaves was used as an adsorbent, with a maximum adsorption capacity of 290.86 mg/g [26]. Among the available technologies, adsorption is considered as a practical and feasible approach because it is affordable, highly efficient, and user-friendly, with no sludge formation [27]. Several adsorbents, especially novel carbonaceous material adsorbents with porous structures, have been examined to determine their performance in the sorption of caffeine [28,29,30].
Biochar is a carbonaceous adsorbent produced by the pyrolysis of biomass under an oxygen-limited environment [31]. Biochar has a high specific surface area and porosity with diverse functional groups, serving as a viable sorbent for the removal of contaminants from water [32]. Several engineering modifications have been applied to biochar, such as surface oxidation [33], acid/base treatment [34], and surface coating [35], to further enhance its adsorption capacity. Most of these modifications are chemical-based, requiring additional chemical reagents, which results in extra costs and the formation of by-products. Ball milling, different from most modifications mentioned, is an environment-friendly and effective physical treatment. It mechanically turns biochar particles into microscale- or nanoscale-size powder with low energy consumption, resulting in increases in its specific surface area (SSA) and oxygen-containing functional groups [36,37]. Previous studies have investigated the effects of ball milling on biochar’s performance in the adsorption of organic pollutants. Huang, Zimmerman, Chen, and Gao [31] indicated that ball milling increased the sulfamethoxazole removal capacity of hickory biochar by up to 83.3%. Zhang et al. [38] applied ball milling to enhance the adsorption of synthetic musk by wheat straw biochar. They found that, after ball milling, biochar’s adsorption capacity for synthetic musk increased dramatically from 609 mg/kg to 2098 mg/kg. However, so far, there has been no research on the application of ball-milled biochar, especially biochar derived from SCG, in removing caffeine from water. Furthermore, current research on the removal of caffeine by biochar has only focused on batch studies to evaluate biochar’s adsorption capacities. To obtain a better understanding of biochar’s performance under more practical circumstances, continuously flowing reactors—such as completely mixed flow reactors (CMFRs)—need to be applied as a method for caffeine removal. CMFRs offer several advantages, including a continuous capacity for removing contaminants, a consistent concentration of contaminants in the effluent, and a constant level of removal efficiency [39].
In this study, we investigated the adsorption of ball-milled SCG biochar for caffeine removal, along with the key influencing factors affecting caffeine adsorption. Additionally, we explored the adsorption mechanisms to better understand the interactions between caffeine and the biochar. Finally, we evaluated caffeine removal efficiency under dynamic conditions in a CMFR. The novelty of this study lies in the utilization of SCG, a byproduct of the coffee production process, to synthesize biochar for the adsorption of caffeine, an emerging contaminant in aquatic environments. This approach effectively addresses two environmental challenges within the coffee industry: (1) the management of SCG and (2) the mitigation of caffeine-induced water pollution. By integrating waste reusage with pollutant removal, this study aims to provide a sustainable and circular solution, achieving a ‘two birds with one stone’ effect.
2. Materials and Methods
2.1. Materials
SCG was collected from home kitchens and school break rooms. It was dried at 80 °C to remove moisture prior to biochar production. Caffeine was purchased from Thermo Scientific (Waltham, MA, USA). A caffeine stock solution (500 mg/L) was prepared and then diluted for subsequent experiments using deionized (DI) water (Thermo Scientific Barnstead Nanopure). The caffeine solution was processed by sonication and then stored at 25 °C. All required chemicals were of analytical grade.
2.2. Biochar Production and Ball Milling
The pristine SCG biochar was produced following a similar process of previous studies [40,41]. Briefly, the dried SCG was placed in an N2-filled tubular furnace (Kejia Furnace KJ-T1200, Zhengzhou, China) and pyrolyzed at a heating rate of 10 °C/min to three different final temperatures (300, 450, and 600 °C); each temperature was held for 1 h. The SCG biochar samples were labeled based on pyrolysis temperature (e.g., CG300 for SCG biochar at 300 °C). The yield percentage of the final product at each pyrolysis temperature was as follows: CG300 yielded 75.29%, CG450 32.41%, and CG600 26.38%.
To produce ball-milled SCG biochar samples, 1.8 g of each pristine biochar was mixed with 180 g of grinding agate balls (diameter = 6 mm) in a 500 mL agate jar. The jars were subsequently placed within a planetary ball milling machine (Across International PQ-N2, Livingston, NJ, USA) operated at 300 rpm for 12 h, with a change in rotation direction every 3 h. The ball-milled biochar was identified by adding the prefix ‘BM’ to the labels (e.g., BMCG300 for SCG biochar at 300 °C with ball-milling treatment).
2.3. Batch Adsorption Experiment
The biochar screening experiment was conducted at room temperature (25 ± 0.5 °C) by adding 100 mg of pristine or ball-milled biochar into 50 mL of a 100 mg/L caffeine solution in a conical centrifuge tube (Thermo Scientific Nunc, Waltham, MA, USA). The tubes were placed on a mechanical shaker at 200 rpm for 24 h. Samples were collected using sterile syringes (Fisherbrand, Waltham, MA, USA) and then filtered through 0.45 μm nylon membrane filters (Thermo Scientific Choice, Waltham, MA, USA) immediately. Based on the study of Atomssa and Gholap [42], caffeine concentrations in the solution were measured with a UV–Vis spectrophotometer (Thermo Scientific, Evolutio 60S, Waltham, MA, USA) at a wavelength of 273 nm. The caffeine removal efficiency (or rate) was determined based on Equation (1):
where C0 is the initial concentration (mg/L) and Ct is the final concentration after treatment (mg/L). The sample (BMCG450) with the best sorption performance was then selected as the adsorbent in the rest of the adsorption, characterization, and CMFR experiments.
Adsorption kinetics experiments were conducted by adding 50 mg of BMCG450 into 50 mL of caffeine (50 mg/L) solution in conical centrifuge tubes on the mechanical shaker. The centrifuge tubes were withdrawn from the shaker at time intervals of 0.17, 0.5, 1, 2, 4, 8, 12, 16, 24, and 36 h, respectively. Caffeine concentrations in the aqueous phase were measured as previously described. The pseudo-first-order, pseudo-second-order, Elovich, and Ritchie kinetic models, respectively, defined in Equations (2)–(5), were applied to simulate the caffeine adsorption kinetics of BMCG450.
where qt is adsorption capacity at time t (mg/g), which is calculated by the mass of caffeine sorbed in the solid phase divided by the mass of biochar; qe is equilibrium adsorption capacity (mg/g); k1 is the pseudo-first-order rate constant (h−1); k2 is the pseudo-second-order rate constant (h−1); α is the initial adsorption rate constant (mg/g·h); β is the desorption constant related to surface coverage (g/mg); kR is the Ritchie kinetic rate constant (h−1); and n is the reaction order parameter (dimensionless).
Adsorption isotherm experiments were conducted by adding 50 mg of BMCG450 into 50 mL of different dosage solutions of caffeine (5, 10, 20, 25, 50, 75, 100, 150, and 200 mg/L) in centrifuge tubes placed on the shaker. After shaking for 24 h, the samples were withdrawn to measure the aqueous caffeine concentrations. The Langmuir, Freundlich, Redlich–Peterson, and Freundlich–Langmuir isotherm models, given by Equations (6)–(9), respectively, were used to simulate the isotherm.
where qe is the adsorption capacity at equilibrium (mg/g); qL is the maximum monolayer adsorption capacity (mg/g); Ce is the equilibrium concentration of the adsorbate in solution (mg/L); KL is the Langmuir adsorption constant (L/mg), related to the affinity of binding sites; KF is the Freundlich adsorption constant (mg/g)·(L/mg)n, representing adsorption capacity; n is the adsorption intensity parameter (dimensionless), indicating the degree of favorability of adsorption; KR is the Redlich–Peterson isotherm constant (L/g); αR is the Redlich–Peterson isotherm constant (L/mg); β is the exponent parameter (dimensionless), ranging between 0 and 1; qFL is the Freundlich–Langmuir maximum adsorption capacity (mg/g); KFL is the Freundlich–Langmuir adsorption constant (L/mg); and 1/n is a dimensionless exponent related to the heterogeneity of the adsorption surface.
The effect of pH on the caffeine removal efficiency of BMCG450 was determined by adding 25 mg of BMCG450 into 25 mL caffeine solutions (25 mg/L) with five different pH values (3, 5, 7, 9, and 11). The pH was adjusted using 0.1 N HCl and 0.1 N NaOH solutions. The effect of ionic strength on the caffeine removal efficiency of BMCG450 was determined by adding 25 mg of BMCG450 into 25 mL caffeine solutions (50 mg/L) with five different ionic strength values (0, 25, 50, 75, and 100 mmol/L). The ionic strength was adjusted using NaCl (Fisher Chemical, Waltham, MA, USA). All experimental conditions were the same as in the biochar screening experiment.
2.4. Sorbent Characterization
The Bruauer–Emmet–Teller (BET, Micromeritics ASAP2460, Norcross, GA, USA) method was used to analyze the specific surface area (SSA) of all the biochar samples. The surface morphology of the selected biochar samples (CG450 and BMCG450) was analyzed via scanning electron microscopy (SEM, Hitachi SU8020, Tokyo, Japan). The changes in the functional groups of CG450 and BMGG450 were recorded using Fourier transform infrared (FTIR, Nicolet IS 10, Waltham, MA, USA) spectroscopy and X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250Xi, Waltham, MA, USA).
2.5. Caffeine Removal in CMFR
A bench-scale CMFR was constructed, as shown in Figure 2. The whole CMFR system comprised (1) a 1 L beaker (a) with 10 mg/L caffeine solution; (2) a 1 L beaker (b) where 2 g/L of BMCG450 suspension was constantly agitated with an overhead stirrer (Corning, Corning, NY, USA) to ensure homogeneous adsorbent concentrations; (3) a 750 mL reactor (c), where the adsorbent and sorbate were mixed with an overhead stirrer (Corning, USA); and (4) an 800 mL clarifier (d), where the treated solution was collected and the adsorbent settled out of the solution. Hydraulic retention time (HRT—contact time between adsorbents and sorbates in reactors), sorbate concentration, and adsorbent amount are essential factors affecting the design and performance of the reactor. To initiate the adsorption experiment in the CMFR, adsorbent and caffeine were pumped with tube pumps (Masterflex L/S, Vernon Hills, IL, USA) from reactors (a) and (b) at a flow rate of 1 mL/min. Thus, the adsorbent concentration in reactor (c) was 1 g/L, and the caffeine concentration was 5 mg/L. The mixture in reactor (c) then flowed into reactor (d). In reactor (d), the adsorbents settled down in the container, and the effluent was collected to measure the caffeine concentration.
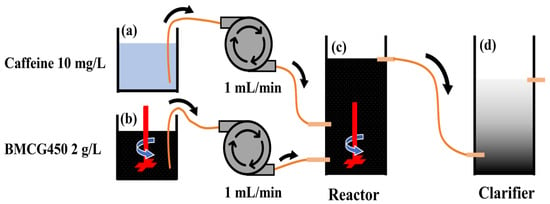
Figure 2.
Schematic flow chart of caffeine removal in CMFR: (a) 10 mg/L of caffeine solution, (b) 2 g/L BMCG450 in DI water, (c) reactor where BMCG450 adsorbs caffeine, (d) clarifier where treated water is collected and spent BMCG450 settles down.
All adsorption experiments were conducted with two replicates, with a blank as the control. Mean values are reported. The least significant difference (LSD) test was conducted to compare the difference between means at α = 0.05. Experimental data were fitted to Equations (2)–(9) to reach the best model performance in terms of goodness-of-fit statistics, including the Akaike Information Criterion (AIC), sum of squared estimate of errors (SSE), and correlation between measured and modeled values (R2). Only R2 values are reported.
3. Results and Discussion
3.1. Initial Assessment
Two ball-milled biochars, BMCG450 and BMCG600, had significantly higher caffeine removal efficiencies, up to 65%, than the un-milled biochar and BMCG300, which showed almost no sorption (Figure 3). There are two significant factors that could affect the caffeine removal efficiency of the biochar: the ball-milling process and pyrolysis temperature. Ball milling can enhance biochar caffeine sorption by increasing biochar SSA and surface functional groups [43]. Moreover, the surface area of biochar generally increases with increasing pyrolysis temperature, causing the enhancement of adsorption [44]. Both CG300 and BMCG300 showed negative caffeine removal, meaning they released caffeine into the solution. This could be attributed to the incomplete carbonization of caffeine residues in SCG at 300 °C. Furthermore, after ball milling, BMCG300 looked like a sticky brown mush, confirming that it was not fully carbonized.
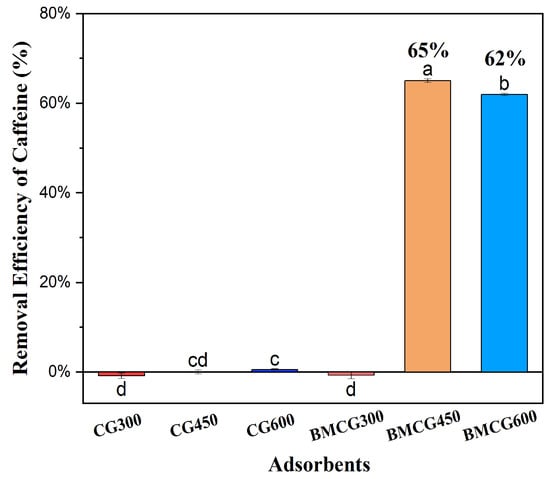
Figure 3.
Removal efficiency of caffeine by different biochar adsorbents. Means with the same letter are not significantly different at the significance level of 0.05.
According to the results of this initial caffeine adsorption experiment, BMCG450 showed the most outstanding caffeine removal efficiency among the six biochar treatments. It was thus selected as the representative ball-milled SCG biochar in the follow-up experiments.
3.2. Properties of Adsorbents
Ball milling, as a modification method, can crush the granular pristine biochar into ultra-fine particles with porous and rough surfaces, increasing SSA. SEM analysis confirmed the surface morphological changes on CG450 and BMCG450 (Figure 4). Moreover, the BET analysis results also confirmed that ball milling enhanced the surface areas of all biochar samples (Table 1). A roughly 167-fold increase in the surface area for CG450 and a roughly 137-fold increase for CG600 were achieved with ball milling. This BET result is, to some extent, corroborated by the initial caffeine removal assessment (Figure 3) that the two ball-milled biochars showed the highest removal efficiency of caffeine from water.
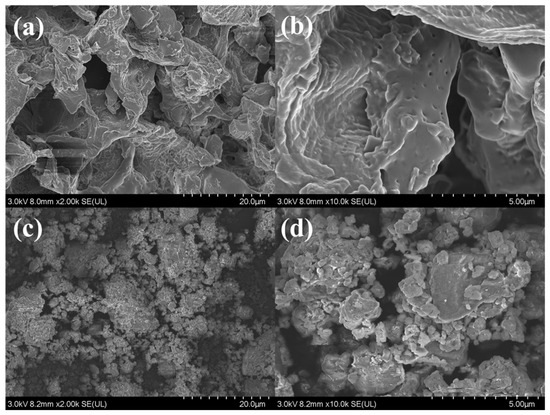
Figure 4.
SEM images of CG450 at (a) 2.00k× and (b) 10.00k× magnifications and BMCG450 at (c) 2.00k× and (d) 10.00k× magnifications.

Table 1.
BET specific surface area of biochar produced at three pyrolysis temperatures before and after ball milling.
The FTIR spectroscopic analysis revealed differences in surface functional groups between CG450 and BMCG450 (Figure 5). There was no new outstanding peak showing up after the ball milling, indicating no dramatic changes in the species of the functional groups. However, there was a considerable enhancement in the amount of each functional group, including C-H (2934, 2854, 1440, and 1375 cm−1), C=O (1693 cm−1), C=C (1595 cm−1), C-O (1156 cm−1), and aromatic C-H (879, 802, and 759 cm−1) [45]. Therefore, compared with pristine biochar (CG450), BMCG450 had many more functional groups, especially oxygen-containing functional groups, consistent with the findings of previous studies [31,46].
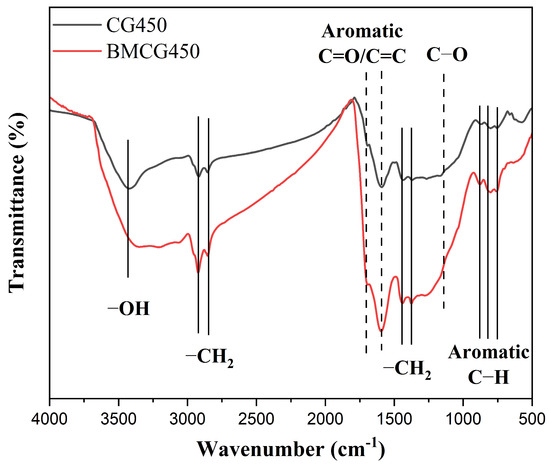
Figure 5.
FTIR spectra of CG450 (black line) and BMCG450 (red line).
XPS spectra analysis was used to reveal the effects of ball milling on the elemental composition and bonding state of the SCG biochars. There was a slight decrease in C1s, from 87.4% (CG450) to 84.1% (BMCG450). Meanwhile, the corresponding O1s values increased from 9.84% to 11.13% after ball milling. According to the C1s spectra in Figure 6a,b, the changes in three key spikes, C-C (284.8 eV), C-O-C (286 eV), and O-C=O (288.5 eV), in CG450 and BMCG450 indicate that ball milling enhanced the formation of oxygen-containing bonds, indicative of more oxygen-containing functional groups on the ball-milled biochar surface. Moreover, the O1s spectra in Figure 6c,d of the two samples suggest that ball milling might increase the proportion of carboxyl functional groups (C=O, 531.71 eV), but reduce the proportion of hydroxyl groups (C-O, 533.12 eV). In comparison to hydroxyl groups, carboxyl groups are more easily dissociated and more prone to chemical reactions with caffeine in water.
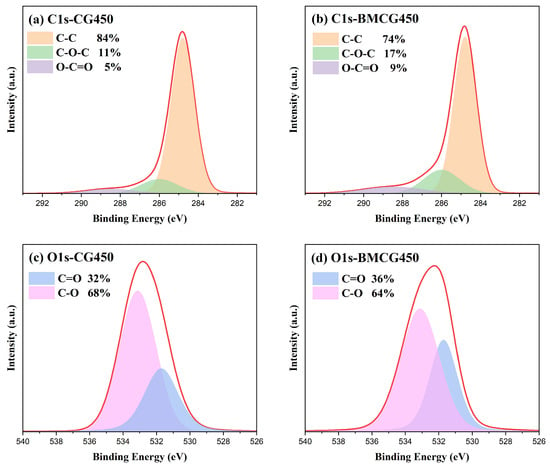
Figure 6.
XPS spectra of (a) C1s-CG450, (b) C1s-BMCG450, (c) O1s-CG450, and (d) O1s-BMCG450.
3.3. Caffeine Adsorption Kinetics and Isotherms
Establishing the adsorption kinetics is necessary for us to understand how quickly BMCG450 reaches equilibrium (equilibration time), providing insights into the mechanisms governing the adsorption process. Figure 7a shows that caffeine adsorption on BMCG450 increased dramatically in the first two hours, then attained equilibrium within eight hours. At the equilibrium, caffeine adsorption reached about 27.26 mg/g. The pseudo-first-order, pseudo-second-order, Elovich, and Ritchie kinetic models were applied to simulate the caffeine adsorption kinetics of BMCG450 (Table 2). Among them, the pseudo-second-order kinetic model aligned the best with the experimental data (R2 = 0.996), indicating that the caffeine adsorption onto BMCG450 could be a heterogenous chemisorption process affected by multiple factors, such as surface area and functional groups [47]. Considering that the Ritchie kinetic model (R2 = 0.995) also provided a strong fit, this suggests that while chemisorption is the dominant mechanism (as indicated by the pseudo-second-order model), additional physical interactions such as surface diffusion or van der Waals forces may also contribute to the overall adsorption process. This implies that the adsorption of caffeine onto BMCG450 is governed by a combination of mechanisms rather than a single dominant process.
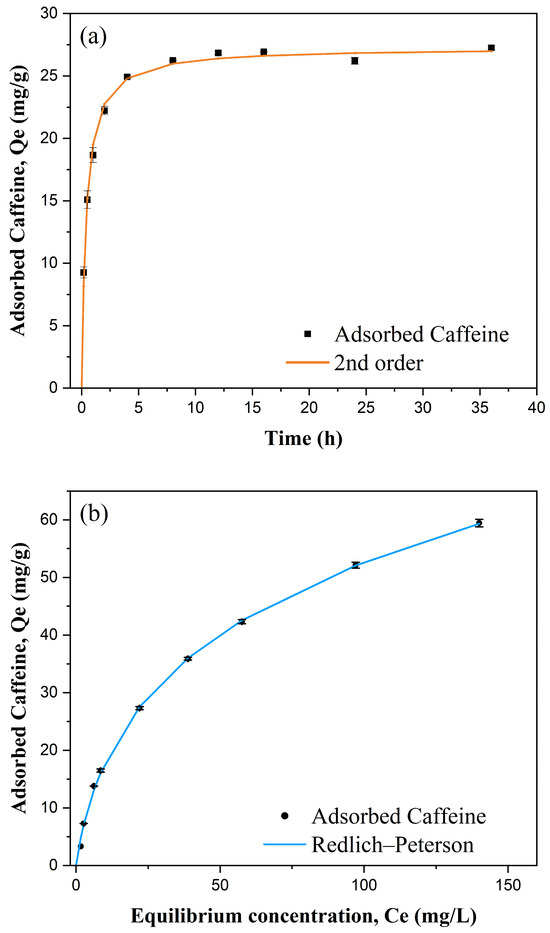
Figure 7.
Kinetics (a) and isotherms (b) of caffeine adsorption onto BMCG450. Symbols represent experimental data and lines represent model results.

Table 2.
Best-fit adsorption models and their parameters, as defined in Equations (2)–(9).
Adsorption isotherms show the relationship between initial sorbate concentration and the adsorbent capacity of the sorbent. The adsorption capacity of BMCG450 for caffeine increased as the caffeine initial concentration increased (Figure 7b). The Langmuir, Freundlich, Redlich–Peterson, and Freundlich–Langmuir isotherm models were used to simulate the isotherm (Table 2). According to the Langmuir model, the maximum caffeine adsorption of BMCG450 was about 82.65 mg/g, higher than that of many other carbonaceous adsorbents in the literature [29,48,49]. The Freundlich–Langmuir isotherm model gives an even higher maximum sorption capacity of 100.44 (mg/g). Among all the models, Redlich–Peterson and Freundlich–Langmuir provided the best fit (R2 = 0.999), indicating that caffeine adsorption onto BMCG450 is governed by a combination of physical and chemical adsorption mechanisms. The high specific surface area and surface functional groups of BMCG450 facilitate multiple interactions, including Van der Waals forces, π-π stacking, hydrogen bonding, electrostatic attraction, and surface complexation. The strong fit of these hybrid models further supports the presence of both monolayer and multilayer adsorption processes, making BMCG450 an effective adsorbent for caffeine removal from water.
3.4. Effect of pH on Caffeine Adsorption
Solution pH plays an essential role in the adsorption of organic pollutants onto biochar because pH can influence the charge of the biochar surface, as well as the degree of ionization of organics in the solution. Here, the pH effect was investigated by changing the initial solution pH from 3 and 11, and the corresponding caffeine removal rates by BMCG450 are shown in Figure 8. Generally, as pH increased, caffeine removal by BMCG450 decreased. The caffeine removal rate by BMCG450 slightly decreased when the pH increased from 3 to 5, followed by a relatively stable phase in the pH range of 5 to 9. However, as pH rose to 11, the caffeine removal rate showed significant decrease.
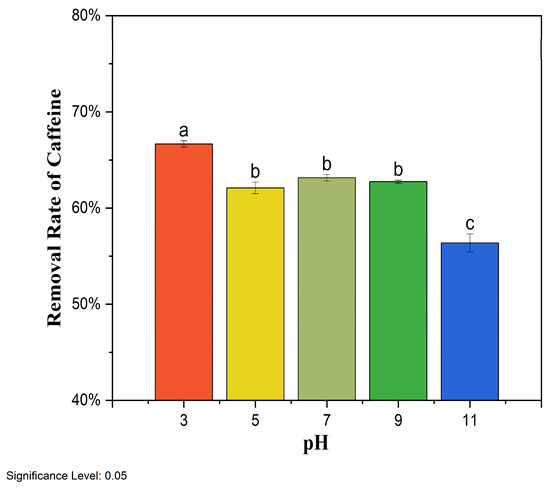
Figure 8.
Effect of pH on the removal of caffeine by BMCG450. Means with the same letter are not significantly different at the significance level of 0.05.
Caffeine has a pKa value of 8.3 [50]. Beltrame, Cazetta, de Souza, Spessato, Silva, and Almeida [30] pointed out that neutral-form caffeine dominates when solution pH is below 5.5. The anionic form of caffeine exists in solutions with a pH above 5.5. After pH exceeds 8.3, the anionic form of caffeine with a negative charge becomes the dominant species. Meanwhile, with the pH change, BMCG450’s surface charge was also affected. The pHpzc, the pH at which the net charge of the adsorbent surface is zero [51], may also provide some insight. According to the study of Nguyen et al. [52], the pHpzc of SCG biochar pyrolyzed at 500 °C is about 7.25.
At low pH < 5 in this study, the BMCG450 surface was protonated, and caffeine existed in the neutral form. They may interact with each other through mechanisms such as hydrogen bonding and π-π stacking [53]. This leads to effective adsorption, though a slight decline is observed as pH increases from 3 to 5. In the pH range of 5 to 9, some caffeine molecules become anionic, but the BMCG450 surface remains partially protonated, maintaining the electrostatic attraction between negatively charged caffeine species and positively charged biochar sites. However, some hydrogen bonding interactions may weaken. At a pH of 11, caffeine existed predominantly in the anionic form, and the surface of BMCG450 mainly carried negative charges due to deprotonation. Electrostatic repulsion between the negatively charged caffeine and biochar surface reduces adsorption efficiency.
3.5. Effect of Ionic Strength on Caffeine Adsorption
The adsorption of caffeine by BMCG450 slightly increased as NaCl concentrations increased from 0 to 100 mmol/L (Figure 9). There are conflicting reports on the impact of solution ionic strength on adsorption, depending on specific circumstances. Wang et al. [54] found that ionic strength had no significant impact on ciprofloxacin adsorption onto activated carbon. However, Couto Jr, Matos, da Fonseca, Arroyo, da Silva, and de Barros [53] found that Ca2+ and Mg2+ in solution decreased the caffeine adsorption of activated carbon due to a competition effect. Some reports have suggested that increasing ionic strength could suppress the adsorption of organic chemicals onto carbon nanotubes due to the formation of a highly compacted structure, the so-called squeezing-out effect [55,56,57]. Liu et al. [58] found that increasing NaCl and CaCl2 concentrations enhanced the sorption of ketoprofen and considered that this resulted from the electrostatic screening effect by the cations. Similarly, the increased adsorption of caffeine on BMCG450 with increasing ionic strength could result from the electrostatic screening effect.
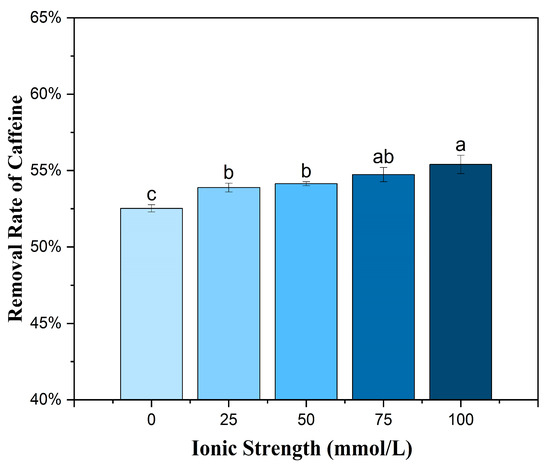
Figure 9.
Effect of ionic strength on the removal of caffeine by BMCG450. Means with the same letter are not significantly different at the significance level of 0.05.
3.6. Caffeine Adsorption in CMFR
Caffeine removal by BMCG450 under continuously flowing conditions was determined in the CMFR. During the operation, the removal rate of caffeine by BMCG450 increased with time until the reaction system reached equilibrium and stability after about 90 min, and then the removal rate remained at about 43% (Figure 10). There was no concentration breakthrough during the experiment, indicating that BMCG450 effectively removed caffeine from the water. This removal efficiency can be further enhanced by adjusting the caffeine concentration, BMCG450 dosage, or flow rate. The results from the CMFR indicated that BMCG450 can serve as an effective adsorbent under continuous flow conditions for the fast and convenient removal of caffeine from water.
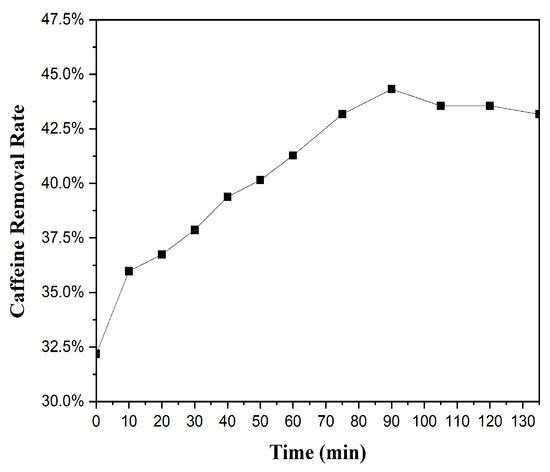
Figure 10.
Caffeine removal rate of BMCG450 in a completely mixed flow reactor (CMFR) under continuous flow conditions.
3.7. Practical Considerations
This study shows that ball-milled SCG biochar is a promising adsorbent for environmental applications. Pyrolysis temperature and engineering modifications are key practical considerations that need to be factored in the production of biochar derived from spent coffee ground. In the literature, a magnetic activated SCG biochar produced at a pyrolysis temperature of 700 °C was reported for the removal of ibuprofen from ground water and lakes [59]. These authors indicated that the simultaneous magnetization and activation of the biochar was an effective approach to improving the adsorption capacity of ibuprofen. In another study, SCG biochar produced at a pyrolysis temperature of 450 °C was modified by phosphoric acid and mercaptoacetic acid [60]. This biochar exhibited an impressive Cd2+ adsorption capacity of 205 mg/g. An excellent sorption of norfloxacin at the maximum absorption capacity of 69.8 mg/g was also noted with SCG biochar produced at a pyrolysis temperature of 500 °C [52]. This biochar was pristine without modification and the optimal pyrolysis temperature was found through testing pyrolysis temperatures of 300 °C, 500 °C, 700 °C, and 900 °C. In addition to its application in water treatment, biochar derived from spent coffee grounds produced at pyrolysis temperatures of 400–600 °C was tested for the capture of carbon dioxide from flue gas [61]. Biochar produced at 600 °C performed the best. Clearly, the selection of modification methods, if needed, for biochar derived from spent coffee grounds is dependent on the specific contaminant of concern. Ball milling, as shown in our study, is an effective physical modification method that significantly increases the SSA and functional groups of biochar. Our study and the existing literature generally support an optimal pyrolysis temperature range of 450–600 °C.
Another consideration for the practical application of caffeine removal is the maximum caffeine adsorption capacity of the biochar. For BMCG450 in this study, the maximum caffeine adsorption capacity reached 82.65 mg/g, as obtained by the Langmuir model (Table 2). This maximum adsorption capacity can be compared with several adsorbents that were tested for caffeine removal. A MgAl-LDH/biochar composite using bovine bone biochar as a support for Layered Double Hydroxide (1:2) nanoparticles exhibited a maximum adsorption capacity of 26.22 mg/g at 40 °C [62]. These authors noted that physical adsorption was the dominant mechanism, possibly relating to the layered nature of LDH and the decreased sorption as temperature increased. A palm-activated carbon had a Langmuir maximum adsorption capacity of only 8.50 mg/g at 30 °C [63]. These authors did not report the specific surface area of the sorbent, yet they stated that chemisorption was the dominant adsorption mechanism, and the Langmuir maximum adsorption capacity did not change much with temperature. A thermally modified bentonite (at 400 °C) had a Langmuir maximum adsorption capacity of 80.3 mg/g [64]. Bentonite is a 2:1-type clay, typically featuring a temperature-dependent specific surface area (around 100–200 m2/g). The thermal modification contributed to the large adsorption capacity, likely in association with the increased SSA. The highest value reported in the literature is 367.2 mg/g from a grape stalk biochar, which exhibited an exceptionally high specific surface area of 1100 m2/g [65]. The literature generally supports that the maximum caffeine adsorption capacity is a valid engineering parameter for the design of adsorption systems. Sorbents offering both physical and chemical sorption are preferred, and BMCG450—examined in this study—possesses such properties with reasonably high adsorption capacity for practical applications.
The final practical consideration is the recovery and reusability of the ball-milled SCG biochar. Through not experimentally examined in this study, scaling up the system shown in the CMFR is critically important to assess its cost-effectiveness. Note that ball-milled SCG biochar has an extremely fine particle size and high dispersibility, meaning that it easily flows with water. This can potentially lead to significant loss of the adsorbent if not controlled well. The recovery and reuse of ball-milled biochar in flowing water systems remain critical challenges that need to be addressed, currently limiting its large-scale practical application. Thermal, solvent, and magnetic methods are common regeneration methods [66] and deserve our future examination. Nevertheless, given SCG is a byproduct of the coffee production process and the growing concerns about caffeine in aquatic environments, this study offers a technical basis for developing a sustainable and circular solution by integrating waste reuse with pollutant removal.
4. Conclusions
This study demonstrates that ball-milled SCG biochar is an excellent adsorbent for the removal of caffeine from water. Ball milling dramatically increased the SSA of biochar by up to 168 times. Meanwhile, FTIR and XPS analyses revealed more oxygen-containing functional groups on the surface of the biochar after the ball milling. All these could be factors contributing to the increased adsorption of caffeine onto ball-milled SCG biochar. The Langmuir maximum caffeine adsorption capacity of BMCG450 reached 82.65 mg/g. Both solution pH and ionic strength affected caffeine removal by BMCG450. As pH increased, caffeine adsorption onto the biochar decreased due to the increase in electrostatic repulsion. However, caffeine adsorption was stable in the pH range of 5 to 9. Increases in ionic strength slightly enhanced caffeine adsorption, possibly due to the electrostatic screening effect of cations. BMCG450 also showed good performance in a CMFR under continuous flow conditions. All of these findings indicate that BMCG450 biochar can be used as a low-cost and efficient adsorbent for the removal of caffeine from water under practical conditions. The regeneration and reusability of biochar will be a key focus for further exploration.
Author Contributions
Y.Y.: Data curation, Formal analysis, Methodology, Writing—original draft. Y.W.: Conceptualization, Validation, Writing—review and editing. J.C., H.C., Y.L. and R.M.-C.: Supervision. Y.Z. (Yulin Zheng), J.H. and Y.Z. (Yue Zhang): Methodology, Investigation, Validation. B.G.: Methodology, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly funded by USDA-NIFA 2023-38821-39959.
Data Availability Statement
Data available on request due to restrictions, e.g., privacy or ethical. The data presented in this study are available on request from the corresponding author.
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Conflicts of Interest
The authors assert that they have no financial interests or personal relationships that could have potentially influenced the research presented in this paper.
References
- Ashihara, H.; Suzuki, T. Distribution and biosynthesis of caffeine in plants. Front. Biosci. (Landmark Ed.) 2004, 9, 1864–1876. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, L.S.; Mihalov, J.J.; Carlson, S.J.; Mattia, A. Regulatory status of caffeine in the United States. Nutr. Rev. 2014, 72, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Rigueto, C.V.T.; Nazari, M.T.; De Souza, C.F.; Cadore, J.S.; Brião, V.B.; Piccin, J.S. Alternative techniques for caffeine removal from wastewater: An overview of opportunities and challenges. J. Water Process Eng. 2020, 35, 101231. [Google Scholar] [CrossRef]
- Drewnowski, A.; Rehm, C.D. Sources of caffeine in diets of US children and adults: Trends by beverage type and purchase location. Nutrients 2016, 8, 154. [Google Scholar] [CrossRef]
- Gracia-Lor, E.; Rousis, N.I.; Zuccato, E.; Bade, R.; Baz-Lomba, J.A.; Castrignanò, E.; Causanilles, A.; Hernández, F.; Kasprzyk-Hordern, B.; Kinyua, J. Estimation of caffeine intake from analysis of caffeine metabolites in wastewater. Sci. Total Environ. 2017, 609, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Forcina, A.; Petrillo, A.; Travaglioni, M.; di Chiara, S.; De Felice, F. A comparative life cycle assessment of different spent coffee ground reuse strategies and a sensitivity analysis for verifying the environmental convenience based on the location of sites. J. Clean. Prod. 2023, 385, 135727. [Google Scholar] [CrossRef]
- Sauvé, S.; Aboulfadl, K.; Dorner, S.; Payment, P.; Deschamps, G.; Prévost, M. Fecal coliforms, caffeine and carbamazepine in stormwater collection systems in a large urban area. Chemosphere 2012, 86, 118–123. [Google Scholar] [CrossRef]
- Montagner, C.C.; Jardim, W.F. Spatial and seasonal variations of pharmaceuticals and endocrine disruptors in the Atibaia River, São Paulo State (Brazil). J. Braz. Chem. Soc. 2011, 22, 1452–1462. [Google Scholar] [CrossRef]
- Viviano, G.; Valsecchi, S.; Polesello, S.; Capodaglio, A.; Tartari, G.; Salerno, F. Combined use of caffeine and turbidity to evaluate the impact of CSOs on river water quality. Water Air Soil Pollut. 2017, 228, 330. [Google Scholar] [CrossRef]
- Jagoda, A.; Żukowski, W.; Dąbrowska, B. Investigations of the presence of caffeine in the Rudawa River, Kraków, Poland. Environ. Monit. Assess. 2015, 187, 566. [Google Scholar] [CrossRef]
- Buerge, I.J.; Poiger, T.; Müller, M.D.; Buser, H.-R. Caffeine, an anthropogenic marker for wastewater contamination of surface waters. Environ. Sci. Technol. 2003, 37, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Szymczycha, B.; Borecka, M.; Białk-Bielińska, A.; Siedlewicz, G.; Pazdro, K. Submarine groundwater discharge as a source of pharmaceutical and caffeine residues in coastal ecosystem: Bay of Puck, southern Baltic Sea case study. Sci. Total Environ. 2020, 713, 136522. [Google Scholar] [CrossRef]
- Siegener, R.; Chen, R. Caffeine in Boston harbor seawater. Mar. Pollut. Bull. 2002, 44, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Knee, K.L.; Gossett, R.; Boehm, A.B.; Paytan, A. Caffeine and agricultural pesticide concentrations in surface water and groundwater on the north shore of Kauai (Hawaii, USA). Mar. Pollut. Bull. 2010, 60, 1376–1382. [Google Scholar] [CrossRef]
- Gaudet-Hull, A.M.; Rayburn, J.R.; Bantle, J.A.; Burton, D.T.; Turley, S.D.; Dawson, D.A.; Dumont, J.N.; Finch, R.A.; Maurice, M.A.; Fort, D.J. FETAX interlaboratory validation study: Phase II testing. Environ. Toxicol. Chem. Int. J. 1994, 13, 1629–1637. [Google Scholar] [CrossRef]
- Moore, M.; Greenway, S.; Farris, J.; Guerra, B. Assessing caffeine as an emerging environmental concern using conventional approaches. Arch. Environ. Contam. Toxicol. 2008, 54, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Martínez, G.; Owuor, M.; Garrido-Pérez, C.; Salamanca, M.; Del Valls, T.; Martín-Díaz, M. Are standard tests sensitive enough to evaluate effects of human pharmaceuticals in aquatic biota? Facing changes in research approaches when performing risk assessment of drugs. Chemosphere 2015, 120, 75–85. [Google Scholar] [CrossRef]
- Capolupo, M.; Valbonesi, P.; Kiwan, A.; Buratti, S.; Franzellitti, S.; Fabbri, E. Use of an integrated biomarker-based strategy to evaluate physiological stress responses induced by environmental concentrations of caffeine in the Mediterranean mussel Mytilus galloprovincialis. Sci. Total Environ. 2016, 563, 538–548. [Google Scholar] [CrossRef]
- Pires, A.; Almeida, Â.; Calisto, V.; Schneider, R.J.; Esteves, V.I.; Wrona, F.J.; Soares, A.M.; Figueira, E.; Freitas, R. Long-term exposure of polychaetes to caffeine: Biochemical alterations induced in Diopatra neapolitana and Arenicola marina. Environ. Pollut. 2016, 214, 456–463. [Google Scholar] [CrossRef]
- Gil, A.; Taoufik, N.; García, A.; Korili, S.A. Comparative removal of emerging contaminants from aqueous solution by adsorption on an activated carbon. Environ. Technol. 2018, 40, 3017–3030. [Google Scholar] [CrossRef]
- Souza, F.S.; Féris, L.A. Degradation of caffeine by advanced oxidative processes: O3 and O3/UV. Ozone Sci. Eng. 2015, 37, 379–384. [Google Scholar] [CrossRef]
- Matamoros, V.; Uggetti, E.; García, J.; Bayona, J.M. Assessment of the mechanisms involved in the removal of emerging contaminants by microalgae from wastewater: A laboratory scale study. J. Hazard. Mater. 2016, 301, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Lopera, A.E.-C.; Ruiz, S.G.; Alonso, J.M.Q. Removal of emerging contaminants from wastewater using reverse osmosis for its subsequent reuse: Pilot plant. J. Water Process Eng. 2019, 29, 100800. [Google Scholar] [CrossRef]
- Arshad, F.; Ali, M.S.; Ali, L.; Zou, L. Catalytic MnO2/MXene/chitosan nanocomposite membrane in removing caffeine from wastewater. Sep. Purif. Technol. 2025, 361, 131504. [Google Scholar] [CrossRef]
- Dave, B.; Moysa, E.L.; Kuźnik, A. Enhancing fungal adaptation for efficient caffeine degradation in wastewater: Biomimetic approach and environmental optimization. Desalination Water Treat. 2025, 321, 100938. [Google Scholar] [CrossRef]
- Melliti, A.; Touihri, M.; Kofroňová, J.; Hannachi, C.; Sellaoui, L.; Bonilla-Petriciolet, A.; Vurm, R. Sustainable removal of caffeine and acetaminophen from water using biomass waste-derived activated carbon: Synthesis, characterization, and modelling. Chemosphere 2024, 355, 141787. [Google Scholar] [CrossRef]
- Zhuo, N.; Lan, Y.; Yang, W.; Yang, Z.; Li, X.; Zhou, X.; Liu, Y.; Shen, J.; Zhang, X. Adsorption of three selected pharmaceuticals and personal care products (PPCPs) onto MIL-101 (Cr)/natural polymer composite beads. Sep. Purif. Technol. 2017, 177, 272–280. [Google Scholar] [CrossRef]
- Sotelo, J.L.; Ovejero, G.; Rodríguez, A.; Álvarez, S.; Galán, J.; García, J. Competitive adsorption studies of caffeine and diclofenac aqueous solutions by activated carbon. Chem. Eng. J. 2014, 240, 443–453. [Google Scholar] [CrossRef]
- Gil, A.; Santamaría, L.; Korili, S. Removal of caffeine and diclofenac from aqueous solution by adsorption on multiwalled carbon nanotubes. Colloid Interface Sci. Commun. 2018, 22, 25–28. [Google Scholar] [CrossRef]
- Beltrame, K.K.; Cazetta, A.L.; de Souza, P.S.; Spessato, L.; Silva, T.L.; Almeida, V.C. Adsorption of caffeine on mesoporous activated carbon fibers prepared from pineapple plant leaves. Ecotoxicol. Environ. Saf. 2018, 147, 64–71. [Google Scholar] [CrossRef]
- Huang, J.; Zimmerman, A.R.; Chen, H.; Gao, B. Ball milled biochar effectively removes sulfamethoxazole and sulfapyridine antibiotics from water and wastewater. Environ. Pollut. 2020, 258, 113809. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, Y.; Zeng, G.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, B.; Lee, X.; Lehmann, J.; Gao, B. Sorption and desorption of Pb (II) to biochar as affected by oxidation and pH. Sci. Total Environ. 2018, 634, 188–194. [Google Scholar] [CrossRef]
- Zeng, H.; Zeng, H.; Zhang, H.; Shahab, A.; Zhang, K.; Lu, Y.; Nabi, I.; Naseem, F.; Ullah, H. Efficient adsorption of Cr (VI) from aqueous environments by phosphoric acid activated eucalyptus biochar. J. Clean. Prod. 2021, 286, 124964. [Google Scholar] [CrossRef]
- Hudcová, B.; Fein, J.B.; Tsang, D.C.; Komárek, M. Mg-Fe LDH-coated biochars for metal (loid) removal: Surface complexation modeling and structural change investigations. Chem. Eng. J. 2022, 432, 134360. [Google Scholar] [CrossRef]
- Richard, S.; Rajadurai, J.S.; Manikandan, V. Influence of particle size and particle loading on mechanical and dielectric properties of biochar particulate-reinforced polymer nanocomposites. Int. J. Polym. Anal. Charact. 2016, 21, 462–477. [Google Scholar] [CrossRef]
- Lopez-Tenllado, F.J.; Motta, I.L.; Hill, J.M. Modification of biochar with high-energy ball milling: Development of porosity and surface acid functional groups. Bioresour. Technol. Rep. 2021, 15, 100704. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Lyu, H.; Zhao, Q.; Jiang, L.; Liu, L. Ball-milled biochar for galaxolide removal: Sorption performance and governing mechanisms. Sci. Total Environ. 2019, 659, 1537–1545. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Li, Y.; Creamer, A.E.; He, F. Adsorptive removal of arsenate from aqueous solutions by biochar supported zero-valent iron nanocomposite: Batch and continuous flow tests. J. Hazard. Mater. 2017, 322, 172–181. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, B.; Yao, Y.; Fang, J.; Zhang, M.; Zhou, Y.; Chen, H.; Yang, L. Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem. Eng. J. 2014, 240, 574–578. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Y.; Yang, Y.; Huang, J.; Zimmerman, A.R.; Chen, H.; Hu, X.; Gao, B. Mechanisms and adsorption capacities of hydrogen peroxide modified ball milled biochar for the removal of methylene blue from aqueous solutions. Bioresour. Technol. 2021, 337, 125432. [Google Scholar] [CrossRef] [PubMed]
- Atomssa, T.; Gholap, A. Characterization of caffeine and determination of caffeine in tea leaves using uv-visible spectrometer. Afr. J. Pure Appl. Chem. 2011, 5, 1–8. [Google Scholar]
- Kumar, M.; Xiong, X.; Wan, Z.; Sun, Y.; Tsang, D.C.; Gupta, J.; Gao, B.; Cao, X.; Tang, J.; Ok, Y.S. Ball milling as a mechanochemical technology for fabrication of novel biochar nanomaterials. Bioresour. Technol. 2020, 312, 123613. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, D.; Zhu, L. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef]
- Lyu, H.; Gao, B.; He, F.; Zimmerman, A.R.; Ding, C.; Tang, J.; Crittenden, J.C. Experimental and modeling investigations of ball-milled biochar for the removal of aqueous methylene blue. Chem. Eng. J. 2018, 335, 110–119. [Google Scholar] [CrossRef]
- Lee, D.-J.; Cheng, Y.-L.; Wong, R.-J.; Wang, X.-D. Adsorption removal of natural organic matters in waters using biochar. Bioresour. Technol. 2018, 260, 413–416. [Google Scholar] [CrossRef]
- Al-Khateeb, L.A.; Almotiry, S.; Salam, M.A. Adsorption of pharmaceutical pollutants onto graphene nanoplatelets. Chem. Eng. J. 2014, 248, 191–199. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Katsouromalli, A.; Pashalidis, I. Oxidized biochar obtained from pine needles as a novel adsorbent to remove caffeine from aqueous solutions. J. Mol. Liq. 2020, 304, 112661. [Google Scholar] [CrossRef]
- Barbas, C.; Garcıa, A.; Saavedra, L.; Castro, M. Optimization and validation of a method for the determination of caffeine, 8-chlorotheophylline and diphenhydramine by isocratic high-performance liquid chromatography: Stress test for stability evaluation. J. Chromatogr. A 2000, 870, 97–103. [Google Scholar] [CrossRef]
- Kubilay, Ş.; Gürkan, R.; Savran, A.; Şahan, T. Removal of Cu (II), Zn (II) and Co (II) ions from aqueous solutions by adsorption onto natural bentonite. Adsorption 2007, 13, 41–51. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Nguyen, T.-B.; Dat, N.D.; Huu, B.T.; Nguyen, X.-C.; Tran, T.; Bui, M.-H.; Dong, C.-D.; Bui, X.-T. Adsorption of norfloxacin from aqueous solution on biochar derived from spent coffee ground: Master variables and response surface method optimized adsorption process. Chemosphere 2022, 288, 132577. [Google Scholar] [CrossRef] [PubMed]
- Couto, O.M., Jr.; Matos, I.; da Fonseca, I.M.; Arroyo, P.A.; da Silva, E.A.; de Barros, M.A.S.D. Effect of solution pH and influence of water hardness on caffeine adsorption onto activated carbons. Can. J. Chem. Eng. 2015, 93, 68–77. [Google Scholar] [CrossRef]
- Wang, M.; Li, G.; Huang, L.; Xue, J.; Liu, Q.; Bao, N.; Huang, J. Study of ciprofloxacin adsorption and regeneration of activated carbon prepared from Enteromorpha prolifera impregnated with H3PO4 and sodium benzenesulfonate. Ecotoxicol. Environ. Saf. 2017, 139, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shen, J.; Zhong, Y.; Ding, T.; Dissanayake, P.D.; Yang, Y.; Tsang, Y.F.; Ok, Y.S. Sorption of pharmaceuticals and personal care products (PPCPs) from water and wastewater by carbonaceous materials: A review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 727–766. [Google Scholar] [CrossRef]
- Zhang, S.; Shao, T.; Bekaroglu, S.S.K.; Karanfil, T. Adsorption of synthetic organic chemicals by carbon nanotubes: Effects of background solution chemistry. Water Res. 2010, 44, 2067–2074. [Google Scholar] [CrossRef]
- Cho, H.-H.; Huang, H.; Schwab, K. Effects of solution chemistry on the adsorption of ibuprofen and triclosan onto carbon nanotubes. Langmuir 2011, 27, 12960–12967. [Google Scholar] [CrossRef]
- Liu, F.-f.; Zhao, J.; Wang, S.; Du, P.; Xing, B. Effects of solution chemistry on adsorption of selected pharmaceuticals and personal care products (PPCPs) by graphenes and carbon nanotubes. Environ. Sci. Technol. 2014, 48, 13197–13206. [Google Scholar] [CrossRef]
- Shin, J.; Kwak, J.; Kim, S.; Son, C.; Lee, Y.-G.; Baek, S.; Park, Y.; Chae, K.-J.; Yang, E.; Chon, K. Facilitated physisorption of ibuprofen on waste coffee residue biochars through simultaneous magnetization and activation in groundwater and lake water: Adsorption mechanisms and reusability. J. Environ. Chem. Eng. 2022, 10, 107914. [Google Scholar] [CrossRef]
- Jin, Z.; Xue, Z.; Li, B.; Ou, L.; Yan, L.; Yang, L.; Yin, K.; Jouha, J.; Shao, P.; Zeng, Z. High-performance spent coffee grounds-based 3D microporous biochar for the efficient capture of Cd2+ via a multi-pathway mechanism. Chem. Eng. J. 2024, 485, 149537. [Google Scholar] [CrossRef]
- Mukherjee, A.; Borugadda, V.B.; Dynes, J.J.; Niu, C.; Dalai, A.K. Carbon dioxide capture from flue gas in biochar produced from spent coffee grounds: Effect of surface chemistry and porous structure. J. Environ. Chem. Eng. 2021, 9, 106049. [Google Scholar] [CrossRef]
- dos Santos Lins, P.V.; Henrique, D.C.; Ide, A.H.; de Paiva e Silva Zanta, C.L.; Meili, L. Evaluation of caffeine adsorption by MgAl-LDH/biochar composite. Environ. Sci. Pollut. Res. 2019, 26, 31804–31811. [Google Scholar] [CrossRef] [PubMed]
- Melo, L.L.; Ide, A.H.; Duarte, J.L.S.; Zanta, C.L.P.; Oliveira, L.M.; Pimentel, W.R.; Meili, L. Caffeine removal using Elaeis guineensis activated carbon: Adsorption and RSM studies. Environ. Sci. Pollut. Res. 2020, 27, 27048–27060. [Google Scholar] [CrossRef]
- Quintero-Jaramillo, J.A.; Carrero, J.I.; Sanabria-González, N.R. Caffeine Adsorption on a Thermally Modified Bentonite: Adsorbent Characterization, Experimental Design, Equilibrium and Kinetics. Colloids Interfaces 2024, 8, 26. [Google Scholar] [CrossRef]
- Portinho, R.; Zanella, O.; Féris, L.A. Grape stalk application for caffeine removal through adsorption. J. Environ. Manag. 2017, 202, 178–187. [Google Scholar] [CrossRef]
- Gao, P.; Fan, X.; Sun, D.; Zeng, G.; Wang, Q.; Wang, Q. Recent Advances in Ball-Milled Materials and Their Applications for Adsorptive Removal of Aqueous Pollutants. Water 2024, 16, 1639. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).