Hydrochemical Characterization, Source Identification, and Irrigation Water Quality Assessment in the Voghji River Catchment Area, Southern Armenia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sites Description
2.2. Water Sampling
2.3. Sample Analysis
2.4. Data Analysis and Processing
3. Assessment of Irrigation Water Quality and Agroecological Hazards
- Low sodium hazard (S1): SAR < 10. Water in this category is generally safe for irrigation, posing minimal risk to soil structure.
- Medium sodium hazard (S2): SAR = 10–18. This water may lead to moderate sodium accumulation, particularly in soils with low permeability, such as clay-rich soils.
- High sodium hazard (S3): SAR = 18–26. Water in this range can significantly affect the soil structure by causing clay dispersion, reducing permeability, and restricting aeration.
- Very high sodium hazard (S4): SAR > 26. Irrigation with such water is generally unsuitable without prior treatment, as it can lead to severe soil degradation, reduced infiltration rates, and long-term agricultural unsustainability.
- Low salinity risk (C1): EC < 250 µS/cm. Water in this category is suitable for most crops and soil types, posing minimal risk to plant growth.
- Medium salinity risk (C2): EC = 250–750 µS/cm. This water is generally suitable for soils with moderate drainage.
- High salinity risk (C3): EC = 750–2250 µS/cm. Water in this range may be problematic for soils with poor drainage, as excessive salt buildup can reduce crop yields.
- Very high salinity risk (C4): EC > 2250 µS/cm. This water is generally unsuitable for irrigation under normal conditions.
4. Results and Discussion
4.1. Assessment of EC and Major Ions in Surface Waters
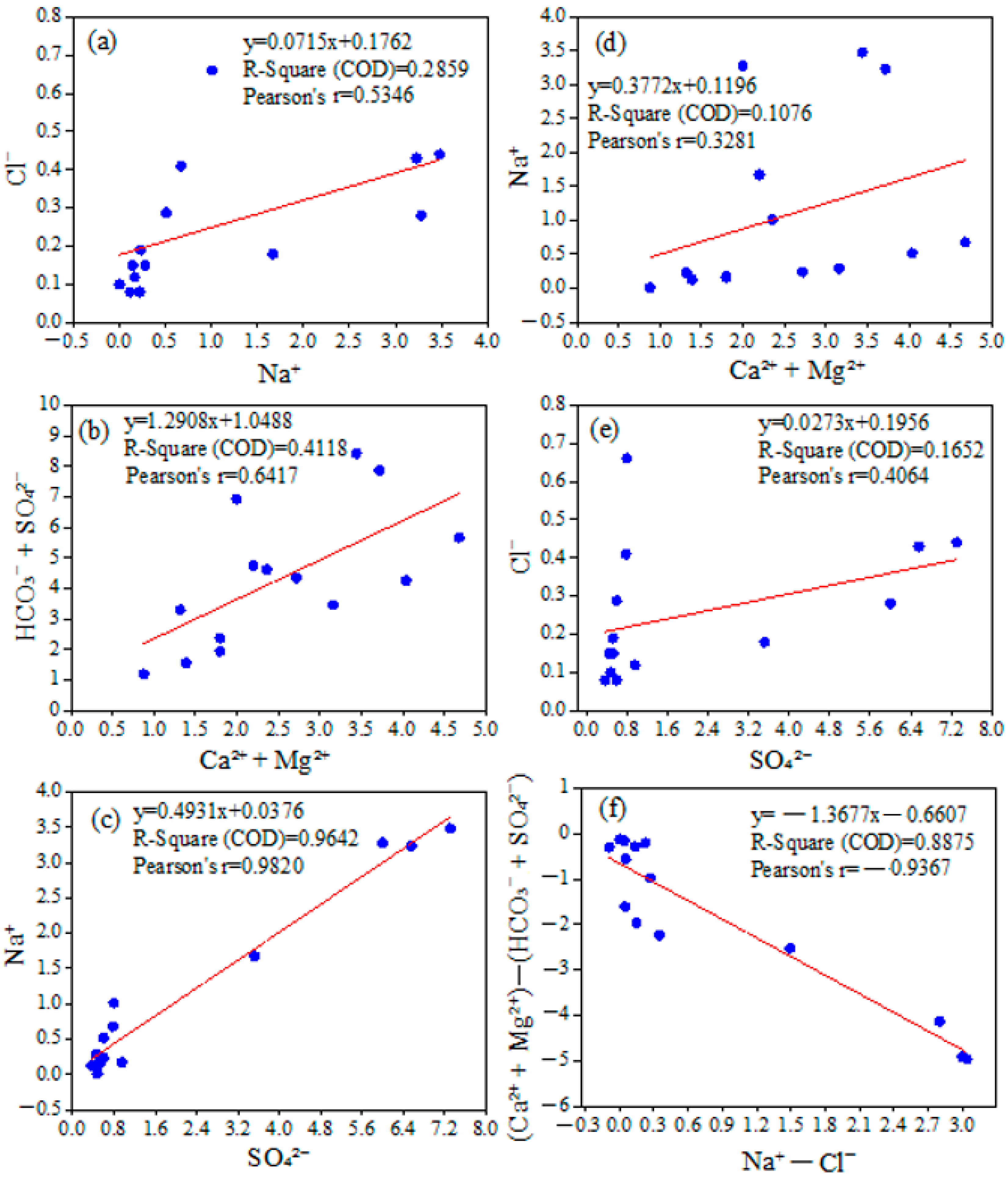
4.2. Hydrochemical Facies and Source Identification in Surface Waters
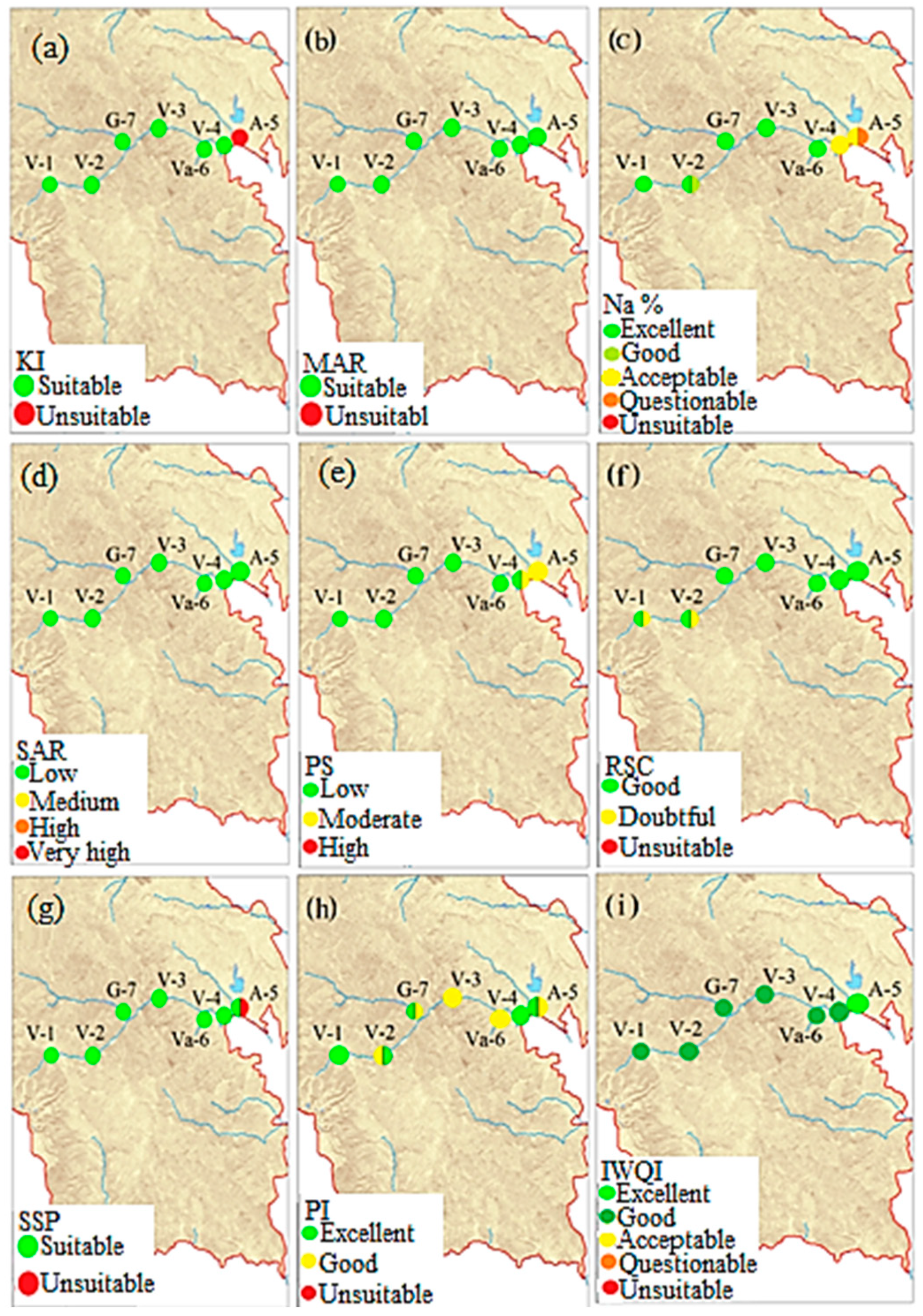
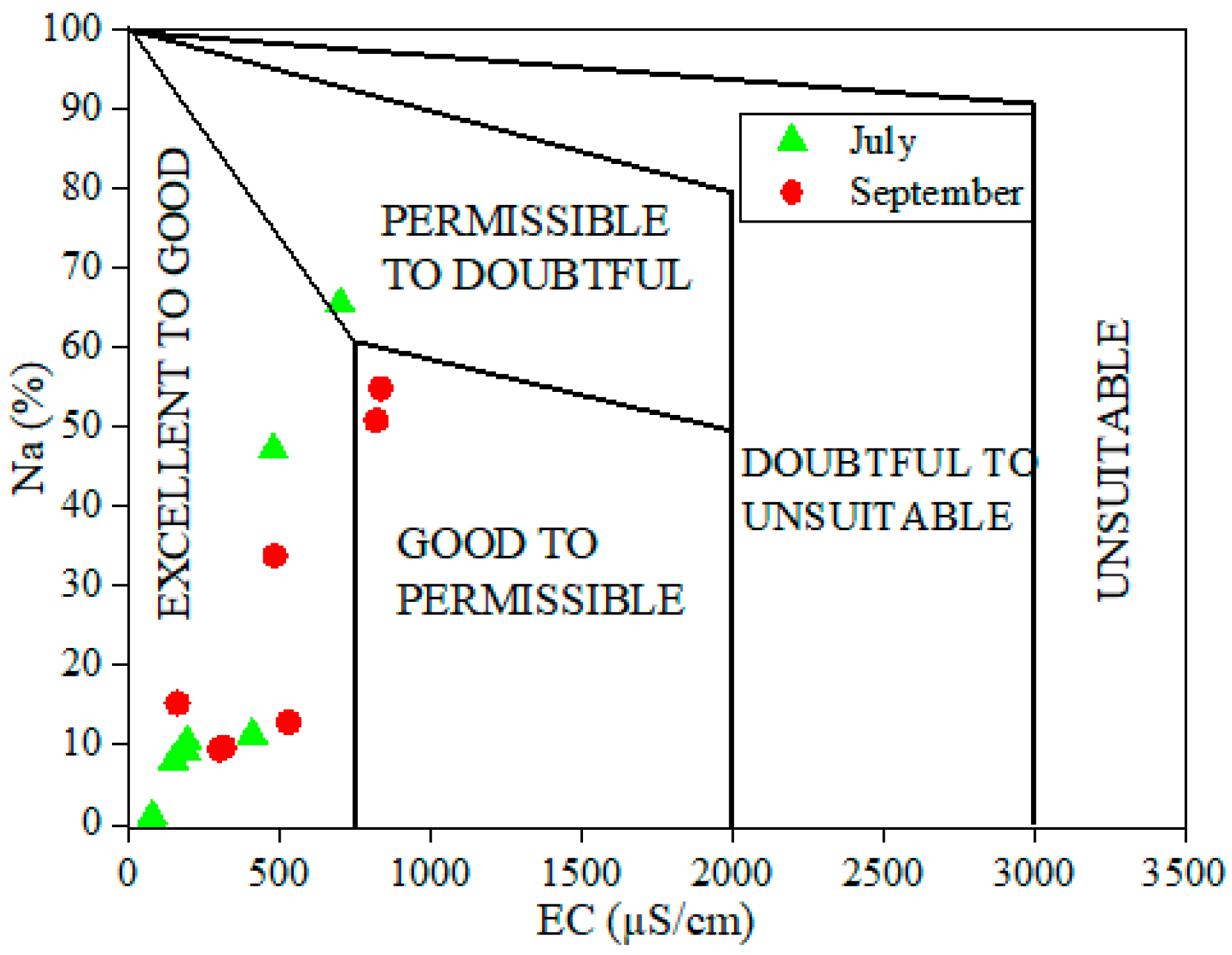
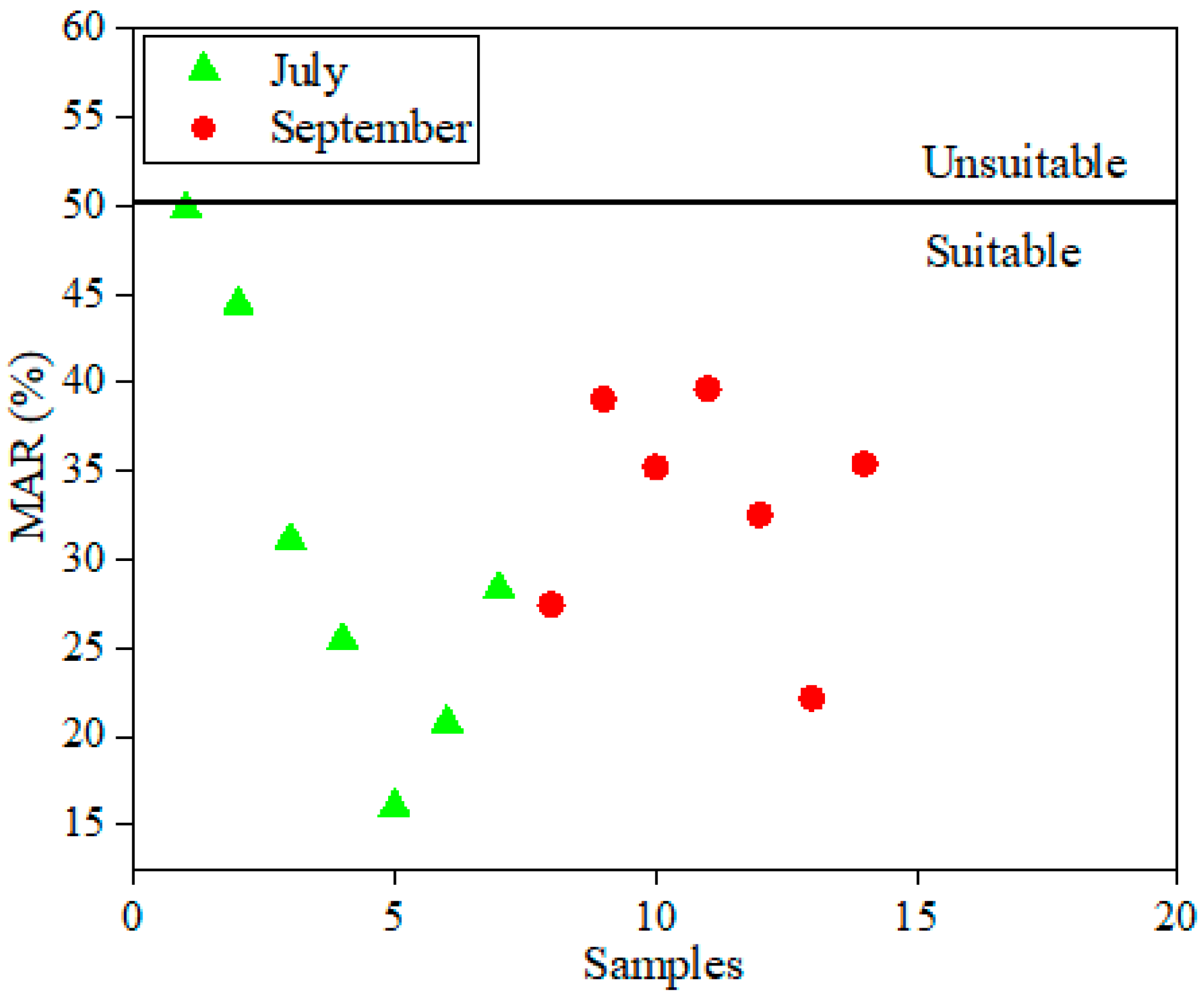
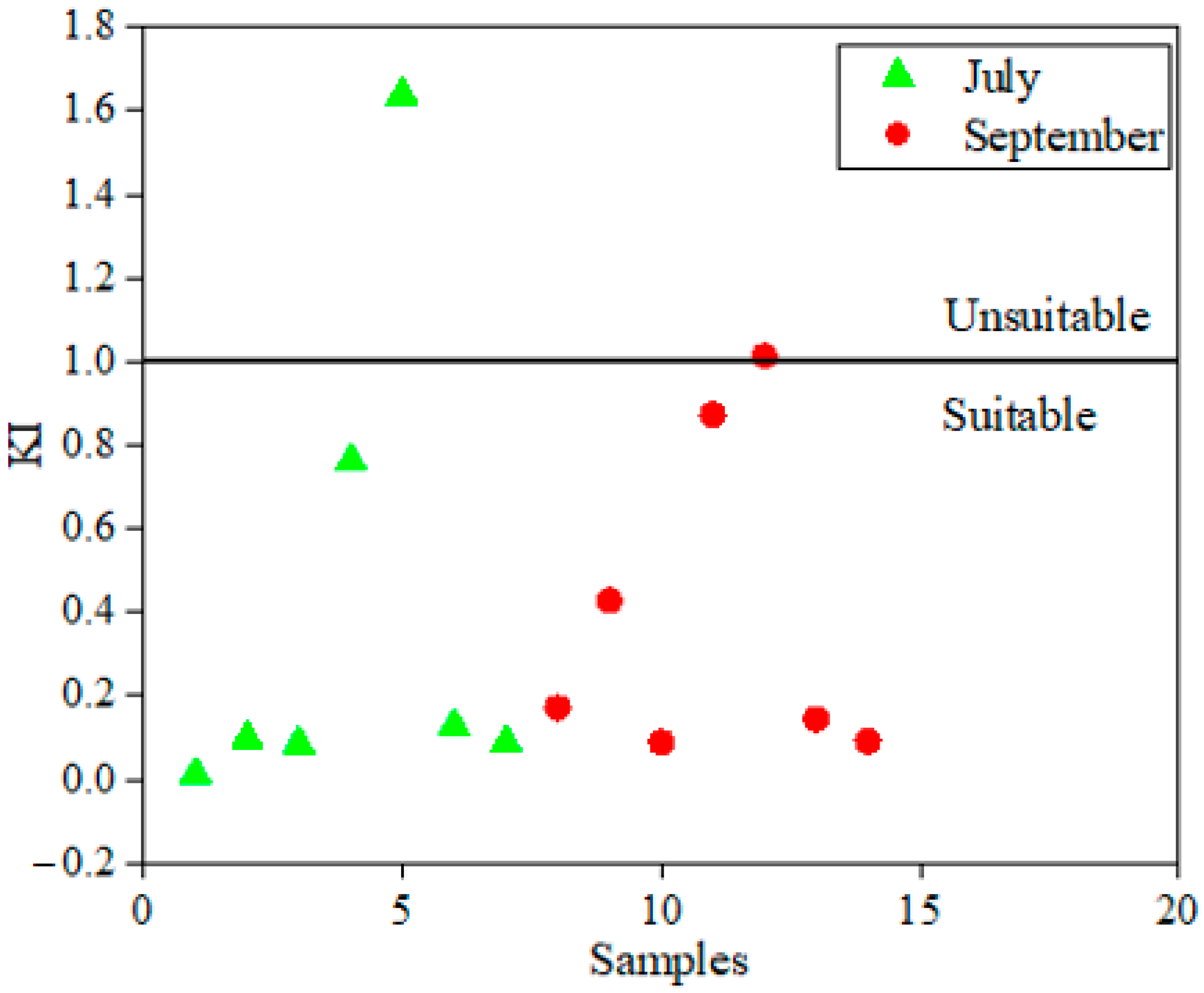
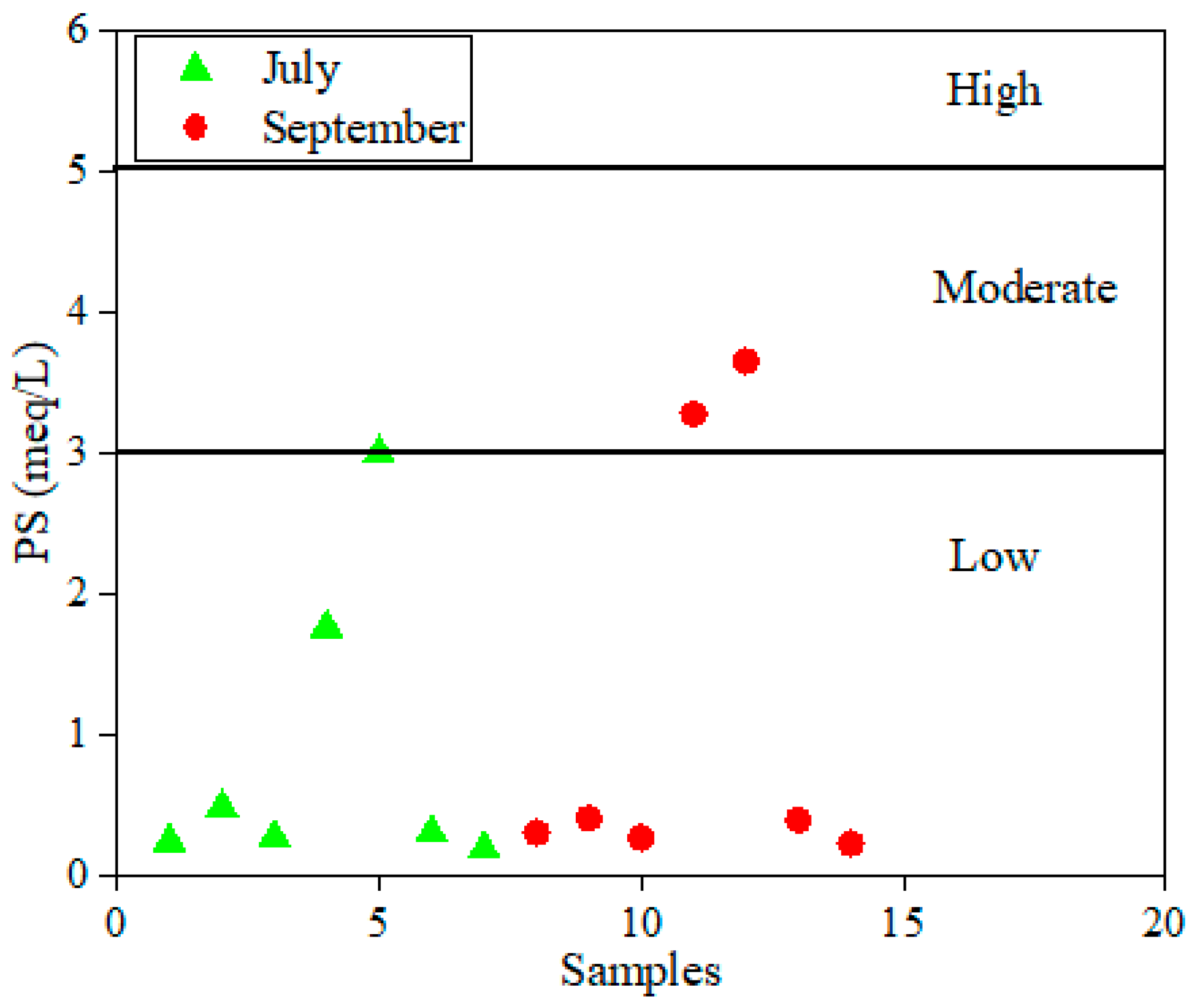
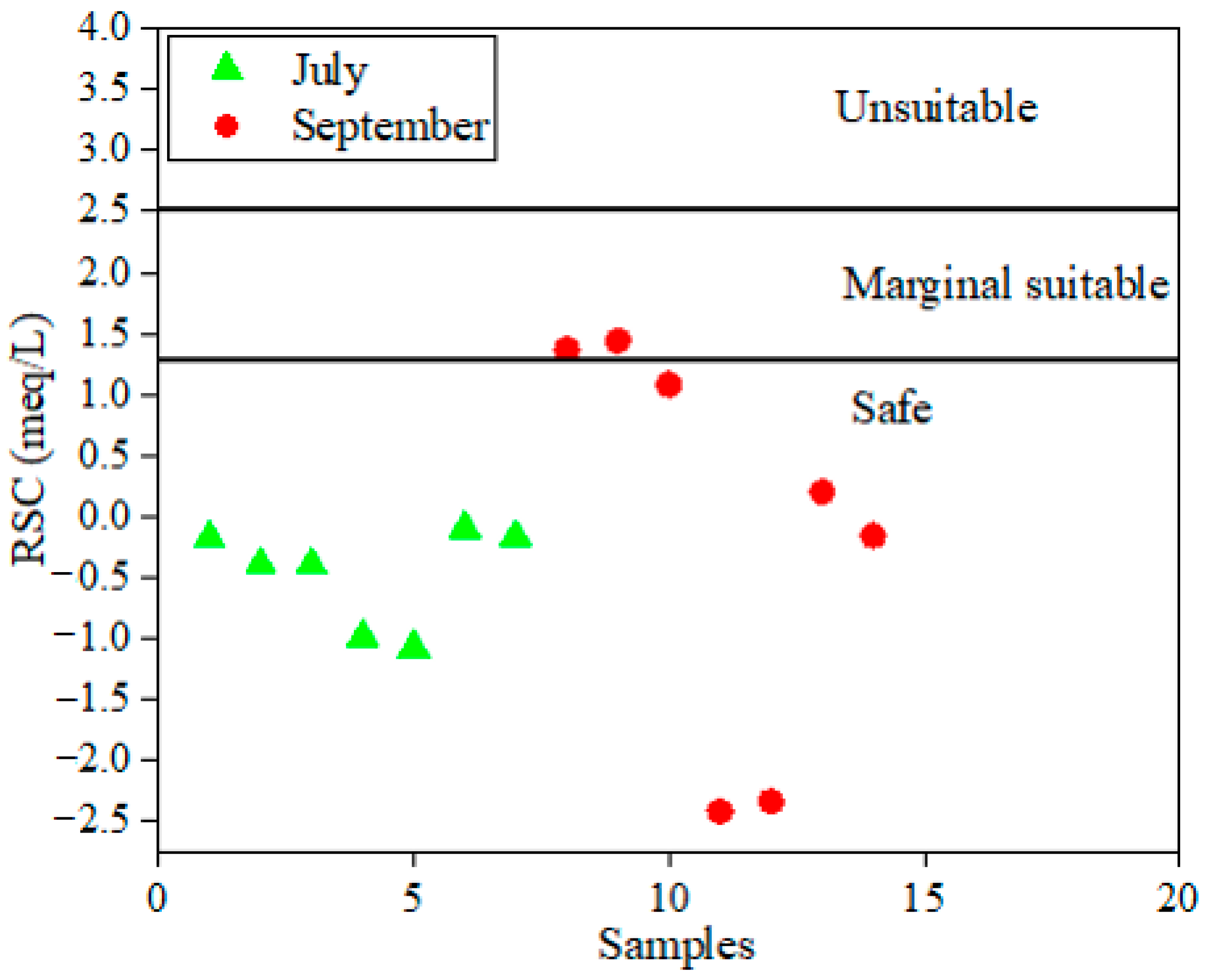
4.3. Assessment of Irrigation Surface Water Quality
5. Limitations and Future Outlook
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EC | Electroconductivity |
| FAO | Food and Agriculture Organization |
| GIS | Geographic Information System |
| IWQI | Irrigation Water Quality Index |
| KI | Kelly Index |
| MAR | Magnesium Absorption Ratio |
| MESC | Ministry of Education, Science, Culture, and Sports |
| SAR | Sodium Adsorption Ratio |
| SSP | Soluble Sodium Percentage |
| PI | Permeability Index |
| RSC | Residual sodium carbonate |
| TDS | Total Dissolved Solids |
References
- Ingrao, C.; Strippoli, R.; Lagioia, G.; Huisingh, D. Water Scarcity in Agriculture: An Overview of Causes, Impacts and Approaches for Reducing the Risks. Heliyon 2023, 9, e18507. [Google Scholar] [CrossRef]
- Feng, T.; Xiong, R.; Huan, P. Productive Use of Natural Resources in Agriculture: The Main Policy Lessons. Resour. Policy 2023, 85, 103793. [Google Scholar] [CrossRef]
- Pawlak, K.; Kołodziejczak, M. The Role of Agriculture in Ensuring Food Security in Developing Countries: Considerations in the Context of the Problem of Sustainable Food Production. Sustainability 2020, 12, 5488. [Google Scholar] [CrossRef]
- Vaseashta, A. Introduction to Water Safety, Security and Sustainability. In Water Safety, Security and Sustainability; Advanced Sciences and Technologies for Security Applications; Vaseashta, A., Maftei, C., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 3–22. ISBN 978-3-030-76007-6. [Google Scholar]
- Mishra, B.; Kumar, P.; Saraswat, C.; Chakraborty, S.; Gautam, A. Water Security in a Changing Environment: Concept, Challenges and Solutions. Water 2021, 13, 490. [Google Scholar] [CrossRef]
- Hanjra, M.A.; Qureshi, M.E. Global Water Crisis and Future Food Security in an Era of Climate Change. Food Policy 2010, 35, 365–377. [Google Scholar] [CrossRef]
- Okello, C.; Githiora, Y.W.; Sithole, S.; Owuor, M.A. Nature-Based Solutions for Water Resource Management in Africa’s Arid and Sem-Arid Lands (ASALs): A Systematic Review of Existing Interventions. Nat.-Based Solut. 2024, 6, 100172. [Google Scholar] [CrossRef]
- Shemer, H.; Wald, S.; Semiat, R. Challenges and Solutions for Global Water Scarcity. Membranes 2023, 13, 612. [Google Scholar] [CrossRef]
- Smith, P.; Ashmore, M.R.; Black, H.I.J.; Burgess, P.J.; Evans, C.D.; Quine, T.A.; Thomson, A.M.; Hicks, K.; Orr, H.G. REVIEW: The Role of Ecosystems and Their Management in Regulating Climate, and Soil, Water and Air Quality. J. Appl. Ecol. 2013, 50, 812–829. [Google Scholar] [CrossRef]
- Musie, W.; Gonfa, G. Fresh Water Resource, Scarcity, Water Salinity Challenges and Possible Remedies: A Review. Heliyon 2023, 9, e18685. [Google Scholar] [CrossRef]
- Baggio, G.; Qadir, M.; Smakhtin, V. Freshwater Availability Status across Countries for Human and Ecosystem Needs. Sci. Total Environ. 2021, 792, 148230. [Google Scholar] [CrossRef]
- Cosgrove, W.J.; Loucks, D.P. Water Management: Current and Future Challenges and Research Directions. Water Resour. Res. 2015, 51, 4823–4839. [Google Scholar] [CrossRef]
- Bashir, I.; Lone, F.A.; Bhat, R.A.; Mir, S.A.; Dar, Z.A.; Dar, S.A. Concerns and Threats of Contamination on Aquatic Ecosystems. In Bioremediation and Biotechnology; Hakeem, K.R., Bhat, R.A., Qadri, H., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–26. ISBN 978-3-030-35690-3. [Google Scholar]
- Issaka, S.; Ashraf, M.A. Impact of Soil Erosion and Degradation on Water Quality: A Review. Geol. Ecol. Landsc. 2017, 1, 1–11. [Google Scholar] [CrossRef]
- Anh, N.T.; Can, L.D.; Nhan, N.T.; Schmalz, B.; Luu, T.L. Influences of Key Factors on River Water Quality in Urban and Rural Areas: A Review. Case Stud. Chem. Environ. Eng. 2023, 8, 100424. [Google Scholar] [CrossRef]
- Dinar, A. Challenges to Water Resource Management: The Role of Economic and Modeling Approaches. Water 2024, 16, 610. [Google Scholar] [CrossRef]
- Levidow, L.; Zaccaria, D.; Maia, R.; Vivas, E.; Todorovic, M.; Scardigno, A. Improving Water-Efficient Irrigation: Prospects and Difficulties of Innovative Practices. Agric. Water Manag. 2014, 146, 84–94. [Google Scholar] [CrossRef]
- Mishra, S.; Kumar, R.; Kumar, M. Use of Treated Sewage or Wastewater as an Irrigation Water for Agricultural Purposes- Environmental, Health, and Economic Impacts. Total Environ. Res. Themes 2023, 6, 100051. [Google Scholar] [CrossRef]
- Stagnari, F.; Jan, S.; Angelica, G.; Michele, P. Sustainable Agricultural Practices for Water Quality Protection. In Water Stress and Crop Plants; Ahmad, P., Ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 75–85. ISBN 978-1-119-05436-8. [Google Scholar]
- World Bank. Armenia—General Water Security Assessment; Europe and Central Asia Region: Yerevan, Armenia, 2024; p. 48. [Google Scholar]
- Varol, M.; Tokatli, C. Evaluation of the Water Quality of a Highly Polluted Stream with Water Quality Indices and Health Risk Assessment Methods. Chemosphere 2023, 311, 137096. [Google Scholar] [CrossRef]
- Mohammed, M.A.A.; Szabó, N.P.; Szűcs, P. Multivariate Statistical and Hydrochemical Approaches for Evaluation of Groundwater Quality in North Bahri City-Sudan. Heliyon 2022, 8, e11308. [Google Scholar] [CrossRef]
- Gevorgyan, G.; Mamyan, A.; Boshyan, T.; Vardanyan, T.; Vaseashta, A. Heavy Metal Contamination in an Industrially Affected River Catchment Basin: Assessment, Effects, and Mitigation. Int. J. Environ. Res. Public Health 2021, 18, 2881. [Google Scholar] [CrossRef]
- Mammeri, A.; Tiri, A.; Belkhiri, L.; Salhi, H.; Brella, D.; Lakouas, E.; Tahraoui, H.; Amrane, A.; Mouni, L. Assessment of Surface Water Quality Using Water Quality Index and Discriminant Analysis Method. Water 2023, 15, 680. [Google Scholar] [CrossRef]
- Chan Kujiek, D.; Sahile, Z.A. Water Quality Assessment of Elgo River in Ethiopia Using CCME, WQI and IWQI for Domestic and Agricultural Usage. Heliyon 2024, 10, e23234. [Google Scholar] [CrossRef]
- Ushurhe, O.; Ozabor, F.; Onyeayana, W.V.; Adekunle, O.; Christabel, I.C.; Chike, D.F. Seasonal Sodium Percentage (%NA), Absorption Ratio (SAR) and Irrigation Water Quality Index (IWQI) Determination for Irrigation Purposes Along River Ethiope, Southern Nigeria. J. Water Resour. Prot. 2024, 16, 523–537. [Google Scholar] [CrossRef]
- Satish Kumar, V.; Amarender, B.; Dhakate, R.; Sankaran, S.; Raj Kumar, K. Assessment of Groundwater Quality for Drinking and Irrigation Use in Shallow Hard Rock Aquifer of Pudunagaram, Palakkad District Kerala. Appl. Water Sci. 2016, 6, 149–167. [Google Scholar] [CrossRef]
- Mokhtar, A.; Elbeltagi, A.; Gyasi-Agyei, Y.; Al-Ansari, N.; Abdel-Fattah, M.K. Prediction of Irrigation Water Quality Indices Based on Machine Learning and Regression Models. Appl. Water Sci. 2022, 12, 76. [Google Scholar] [CrossRef]
- Eid, M.H.; Elbagory, M.; Tamma, A.A.; Gad, M.; Elsayed, S.; Hussein, H.; Moghanm, F.S.; Omara, A.E.-D.; Kovács, A.; Péter, S. Evaluation of Groundwater Quality for Irrigation in Deep Aquifers Using Multiple Graphical and Indexing Approaches Supported with Machine Learning Models and GIS Techniques, Souf Valley, Algeria. Water 2023, 15, 182. [Google Scholar] [CrossRef]
- Gevorgyan, G.; Varagyan, V.; Varagyan, A. Agricultural Risk Assessment of the Heavy Metal and Mineral Salt Pollution of River Ecosystems in the Voghji River Catchment Area, Armenia. Biol. J. Armen. 2016, 4, 92–97. [Google Scholar]
- Gad, M.; Saleh, A.H.; Hussein, H.; Elsayed, S.; Farouk, M. Water Quality Evaluation and Prediction Using Irrigation Indices, Artificial Neural Networks, and Partial Least Square Regression Models for the Nile River, Egypt. Water 2023, 15, 2244. [Google Scholar] [CrossRef]
- Kramer, I.; Mau, Y. Review: Modeling the Effects of Salinity and Sodicity in Agricultural Systems. Water Resour. Res. 2023, 59, e2023WR034750. [Google Scholar] [CrossRef]
- Varagyan, A.L.; Gevorgyan, G.A.; Varagyan, V.M. Mineral Salt Content in the Water of the Voghji River Catchment Basin (Armenia): Assessment of Potential Effects on Irrigated Soils and Crops. Proc. YSU B Chem. Biol. Sci. 2018, 52, 204–210. [Google Scholar] [CrossRef]
- Viktorovich Larionov, M.; Haykaram Galstyan, M.; Garnik Ghukasyan, A.; Gagik Matevosyan, L.; Lendrush Hakobjanyan, I.; Arayik Gharibyan, P.; Yakhsibek Sayadyan, H.; Shahen Sargsyan, K. The Ecological and Sanitary-Hygienic Assessment of the River Systems Located in the Technogenic Polluted Zone of the Caucasus. Egypt. J. Aquat. Res. 2024, 50, 189–199. [Google Scholar] [CrossRef]
- Mkrtchyan, S.S.; Gabrielyan, A.H.; Maghakyan, H.G.; Pafengolts, K.N.; Vardanyants, L.A. (Eds.) Geology of the Armenian SSR, Volume I: Geomorphology; Academy of Sciences Press: Yerevan, Armenia, 1962. (In Russian) [Google Scholar]
- Fichtner Water & Transportation GmbH; Jrtuc LLC. Armenian Small Municipalities Water Project: Environmental Impact Assessment—Kapan; Project No. 610-1182; AWS CJSC: Yerevan, Armenia, 2013. [Google Scholar]
- Gabrielyan, A.V.; Shahnazaryan, G.A.; Minasyan, S.H. Distribution and Identification of Sources of Heavy Metals in the Voghji River Basin Impacted by Mining Activities (Armenia). J. Chem. 2018, 2018, 7172426. [Google Scholar] [CrossRef]
- Gevorgyan, G. Ecological Assessment of the Voghji and Meghriget Rivers and Their Catchment Basins. Ph.D Thesis, Yerevan State University, Yerevan, Armenia, 2011. [Google Scholar]
- Rusydi, A.F. Correlation between Conductivity and Total Dissolved Solid in Various Type of Water: A Review. IOP Conf. Ser. Earth Environ. Sci. 2018, 118, 012019. [Google Scholar] [CrossRef]
- ISO 9964-3:1986; Water Quality—Determination of Calcium and Magnesium—Atomic Absorption Spectrometric Method. International Organization for Standardization ISO: London, UK, 1986.
- ISO 9964-3:1993; Water Quality—Determination of Sodium and Potassium—Part 3: Determination of Sodium and Potassium by Flame Emission Spectrometry. International Organization for Standardization ISO: London, UK, 1993.
- GOST 31957-2012; Water—Methods for Determination of Alkalinity and Mass Concentration of Carbonates and Hydrocarbonates. Interstate Council for Standardization, Metrology, and Certification (ISC): Moscow, Russia, 2012.
- ISO 9297:2011; Water Quality—Determination of Chloride—Silver Nitrate Titration with Chromate Indicator (Mohr’s Method). International Organization for Standardization: London, UK, 2011.
- GOST 4389-72; Drinking Water—Methods for Determination of Sulfate Content. Interstate Council for Standardization, Metrology, and Certification (ISC) Standard Publishing House: Moscow, Russia, 1972.
- Ayers, R.; Westcott, D. Water Quality for Agriculture—Food and Agricultural Organization of the United Nations; Food and Agricultural Organization of the United Nations: Rome, Italy, 1994. [Google Scholar]
- Kelly, W.P. Permissible Composition and Concentration of Irrigation Water. Trans. Am. Soc. Civ. Eng. 1941, 106, 849–855. [Google Scholar] [CrossRef]
- Szabolcs, I.; Darab, C. The Influences of Irrigation Water of High Sodium Carbonate Contents on Soils. In Proceedings of the 8th International Congress Soil Science Sodic Soils, Bucharest, Romania, 31 August–9 September 1964; pp. 802–812. [Google Scholar]
- Wilcox, L.V. Classification and Use of Irrigation Waters; Department of Agriculture: Washington, DC, USA, 1955. [Google Scholar]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils. Soil Sci. 1954, 78, 154. [Google Scholar] [CrossRef]
- Doneen, L.D. Notes on Water Quality in Agriculture; University of California: Davis, CA, USA, 1964. [Google Scholar]
- Doneen, L.D. The Influence of Crop and Soil on Percolating Waters. In Proceedings of the 1961 Biennial Conference Groundwater Recharge, Bekley, CA, USA, 26–27 June 1962; pp. 156–163. [Google Scholar]
- Eaton, F.M. Significance of Carbonates in Irrigation Waters. Soil Sci. 1950, 69, 123–134. [Google Scholar] [CrossRef]
- Todd, D.K. Groundwater Hydrology; Wiley: Hoboken, NJ, USA, 1980; ISBN 13: 9788126508365. [Google Scholar]
- Gibbs, R.J. Mechanisms Controlling World Water Chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Meireles, A.C.M.; Andrade, E.M.D.; Chaves, L.C.G.; Frischkorn, H.; Crisostomo, L.A. A New Proposal of the Classification of Irrigation Water. Rev. Ciênc. Agron. 2010, 41, 349–357. [Google Scholar] [CrossRef]
- McGeorge, W.T. Diagnosis and Improvement of Saline and Alkaline Soils: By Staff of U. S. Salinity Laboratory, Agriculture Handbook No. 60 U. S. Dept. Agric., Supt. Documents, U.S. Government Printing Office Washington 25, D.C., 1954, 160 Pages, $2.00. Soil Sci. Soc Amer. J. 1954, 18, 348. [Google Scholar] [CrossRef]
- Wilcox, L.V. The Quality of Water for Irrigation Use; United States Department of Agriculture (USDA): Washington, DC, USA, 1948. [Google Scholar] [CrossRef]
- Vaseashta, A.; Gevorgyan, G.; Kavaz, D.; Ivanov, O.; Jawaid, M.; Vasović, D. Exposome, Biomonitoring, Assessment and Data Analytics to Quantify Universal Water Quality. In Water Safety, Security and Sustainability; Advanced Sciences and Technologies for Security Applications; Vaseashta, A., Maftei, C., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 67–114. ISBN 978-3-030-76007-6. [Google Scholar]
- Vaseashta, A.; Duca, G.; Travin, S.O. Handbook of Research on Water Sciences and Society; Engineering Science Reference: Hershey, PA, Canada, 2022; ISBN 978-1-79987-356-3. [Google Scholar]
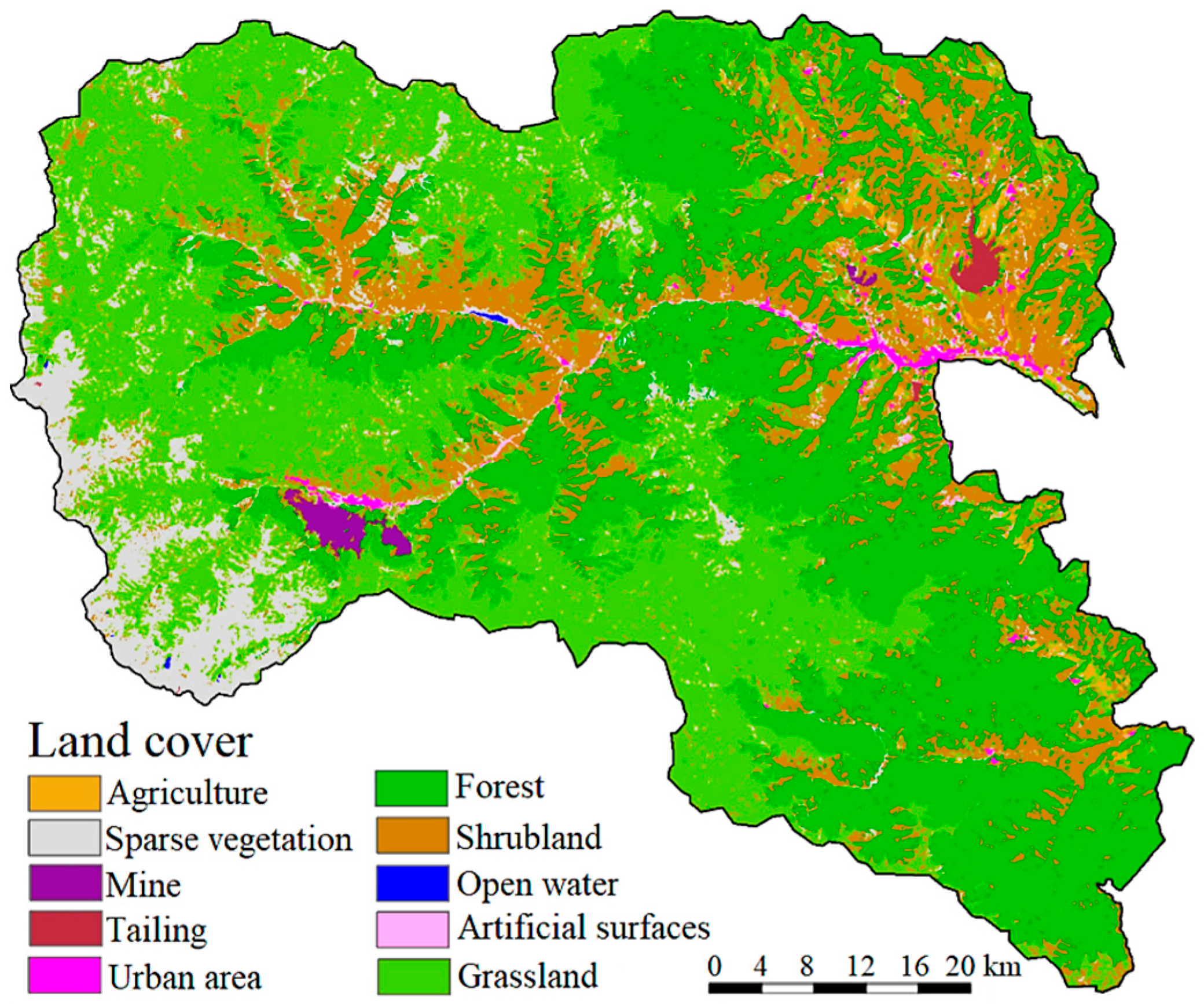
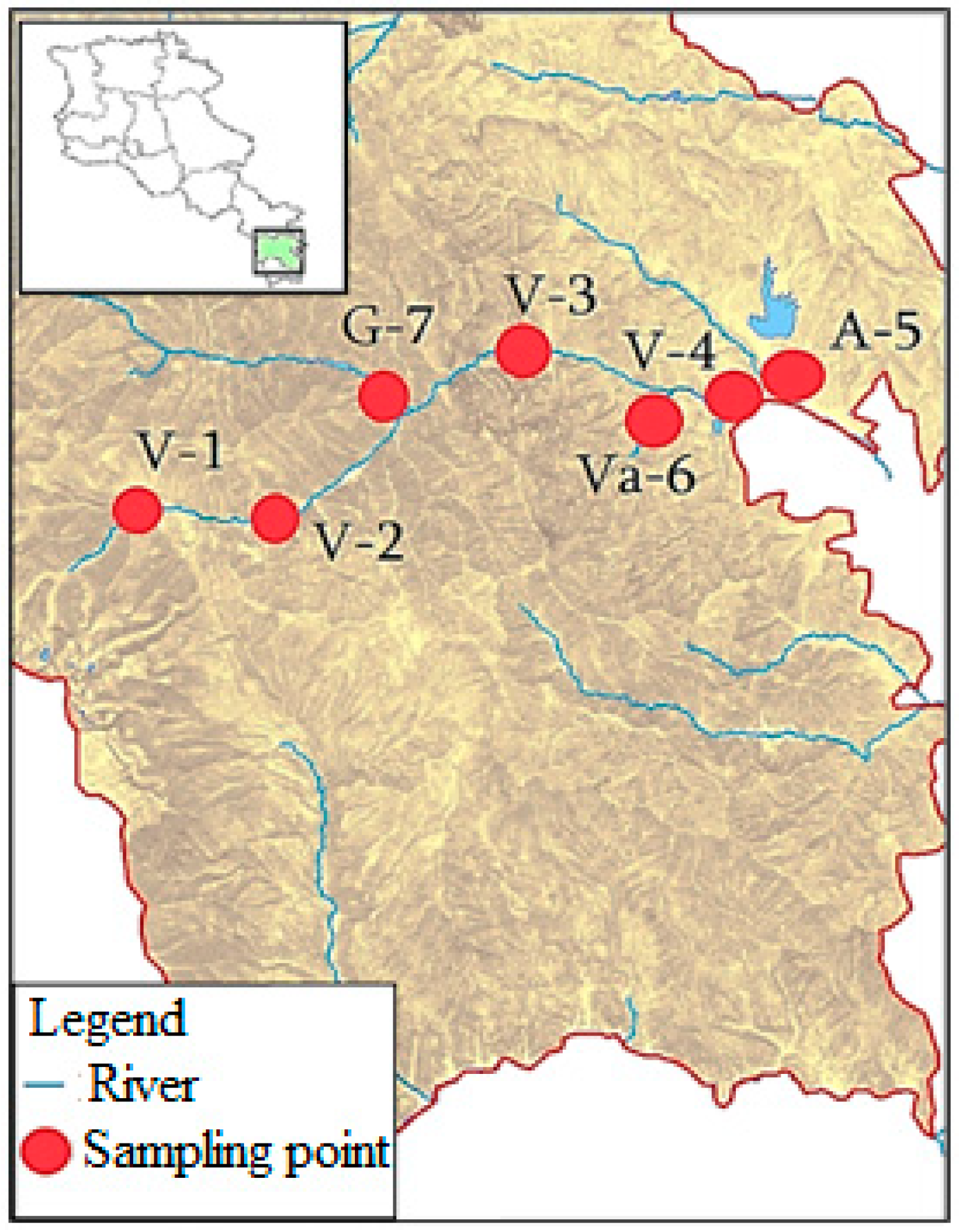





| River | Sampling Point | The Location of the Sampling Point | Geographic Coordinates |
|---|---|---|---|
| Voghji | V-1 | Upstream of Kajaran Town | 39°09′26.9″ N 46°06′47.4″ E |
| Voghji | V-2 | Downstream of Kajaran Town | 39°09′01.8″ N 46°11′34.3″ E |
| Voghji | V-3 | Upstream of Kapan Town | 39°13′27.7″ N 46°20′19.4″ E |
| Voghji | V-4 | Downstream of Kapan Town | 39°11′53.0″ N 46°28′05.9″ E |
| Achanan | A-5 | Near the river mouth | 39°11′54.9″ N 46°28′02.8″ E |
| Vachagan | Va-6 | In Kapan Town | 39°11′53.5″ N 46°23′43.8″ E |
| Geghi | G-7 | Near the river mouth | 39°11′58.3″ N 46°15′31.8″ E |
| qi | EC (µS/cm) | SAR (meq/L) | Na+ (meq/L) | Cl− (meq/L) | HCO3− (meq/L) | |
|---|---|---|---|---|---|---|
| High | 85–100 | 200–750 | <3 | 2–3 | <4 | 1.0–1.5 |
| Medium | 60–85 | 750–1500 | 3–6 | 3–6 | 4–7 | 1.5–4.5 |
| Low | 35–60 | 1500–3000 | 6–12 | 6–9 | 7–10 | 4.5–8.5 |
| Very low | 0–35 | <200 or >3000 | >12 | <2 or >9 | >10 | <1.0 or >8.5 |
| Parameters | wi |
|---|---|
| EC | 0.211 |
| Na+ | 0.204 |
| HCO3− | 0.202 |
| Cl− | 0.194 |
| SAR | 0.189 |
| Total | 1.000 |
| IWQI Values and Type of Restriction | Recommendations for Crops and Soil | |
|---|---|---|
| Type of Plants | Soil | |
| 85–100 (no restriction) | No toxicity | Water can be used for all types of soils as a low risk of soil salinity and sodicity prevails. |
| 70–85 (low restriction) | Avoid the use of salt-sensitive plants | Water can be used for light soil texture with high sand content and moderate to high permeability. |
| 55–70 (moderate restriction) | Moderate salt-tolerance plants | Water can be used for moderate to high permeable soil taking into consideration moderate soil leaching processes. |
| 40–55 (high restriction) | Moderate to high salt-tolerance plants | Water can be used for permeable soil without compact layers and taking into consideration the high frequency of the irrigation schedule for irrigation water with EC > 2000 µS/cm and SAR > 7. |
| 0–40 (severe restriction) | High salt-tolerance plants only | Water cannot be used to irrigate soil under normal conditions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gevorgyan, G.; Khachatryan, G.; Varagyan, A.; Varagyan, V.; Vaseashta, A. Hydrochemical Characterization, Source Identification, and Irrigation Water Quality Assessment in the Voghji River Catchment Area, Southern Armenia. Water 2025, 17, 854. https://doi.org/10.3390/w17060854
Gevorgyan G, Khachatryan G, Varagyan A, Varagyan V, Vaseashta A. Hydrochemical Characterization, Source Identification, and Irrigation Water Quality Assessment in the Voghji River Catchment Area, Southern Armenia. Water. 2025; 17(6):854. https://doi.org/10.3390/w17060854
Chicago/Turabian StyleGevorgyan, Gor, Gor Khachatryan, Anita Varagyan, Vahagn Varagyan, and Ashok Vaseashta. 2025. "Hydrochemical Characterization, Source Identification, and Irrigation Water Quality Assessment in the Voghji River Catchment Area, Southern Armenia" Water 17, no. 6: 854. https://doi.org/10.3390/w17060854
APA StyleGevorgyan, G., Khachatryan, G., Varagyan, A., Varagyan, V., & Vaseashta, A. (2025). Hydrochemical Characterization, Source Identification, and Irrigation Water Quality Assessment in the Voghji River Catchment Area, Southern Armenia. Water, 17(6), 854. https://doi.org/10.3390/w17060854







