Efficient Removal of Cu(II) from Wastewater Using Chitosan Derived from Shrimp Shells: A Kinetic, Thermodynamic, Optimization, and Modelling Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Chitosan
2.3. Characterization of the Prepared Chitosan
2.4. Preparation of Cu(II) Solutions and Quantification
2.5. Adsorption Experiments
2.5.1. Batch Equilibrium and Kinetics Studies
2.5.2. Testing for Optimizing and Modelling Adsorption Processes
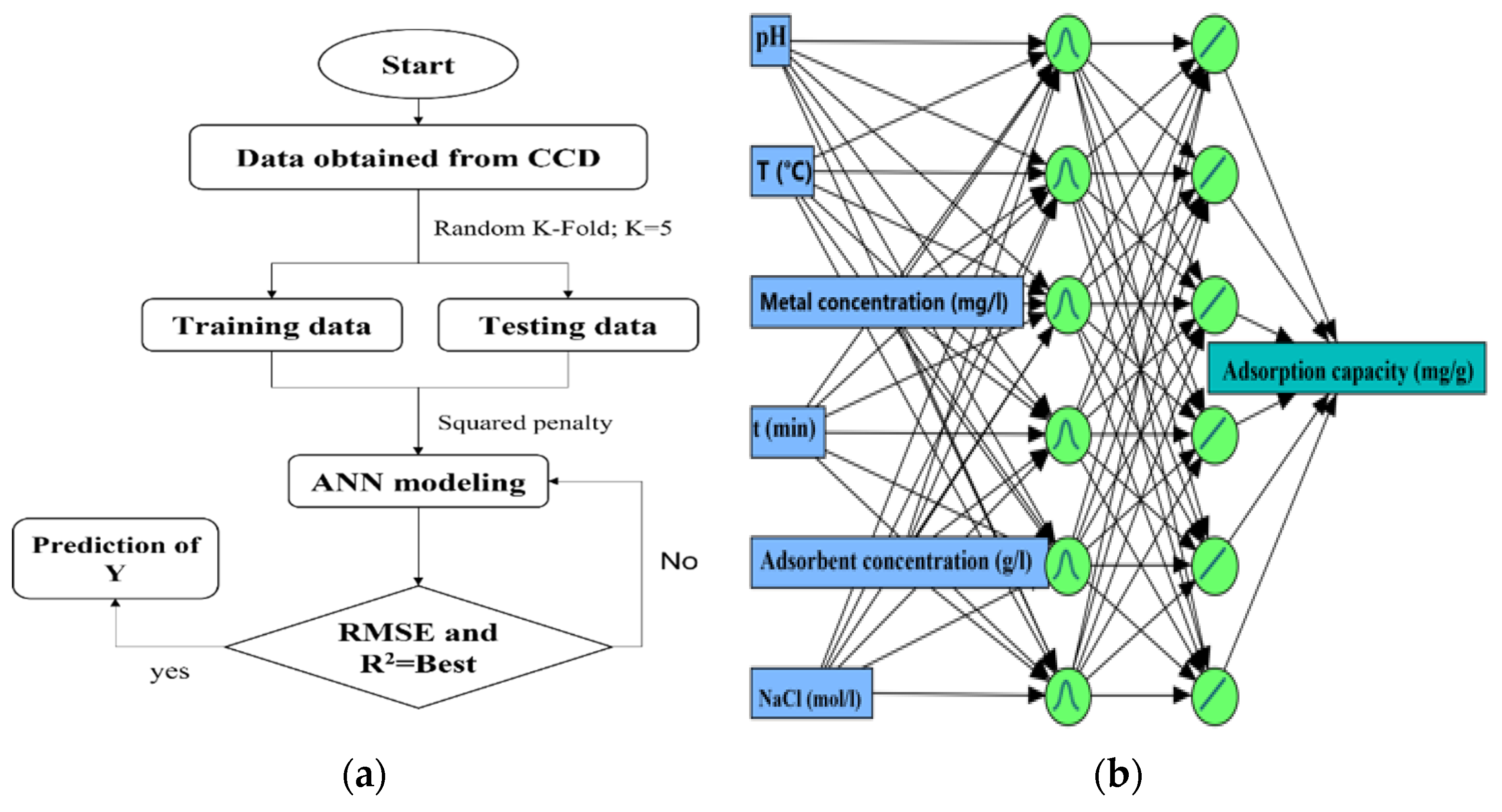
2.6. Design of Experiments, Optimization, Response Surface Methodology (RSM), and Artificial Neural Network (ANN) Modelling
3. Results and Discussion
3.1. Results of the Characterization of Chitosan
3.1.1. Characterization of Chitosan with Degree of Deacetylation (DD) Using Conductometric Titration and Viscosity
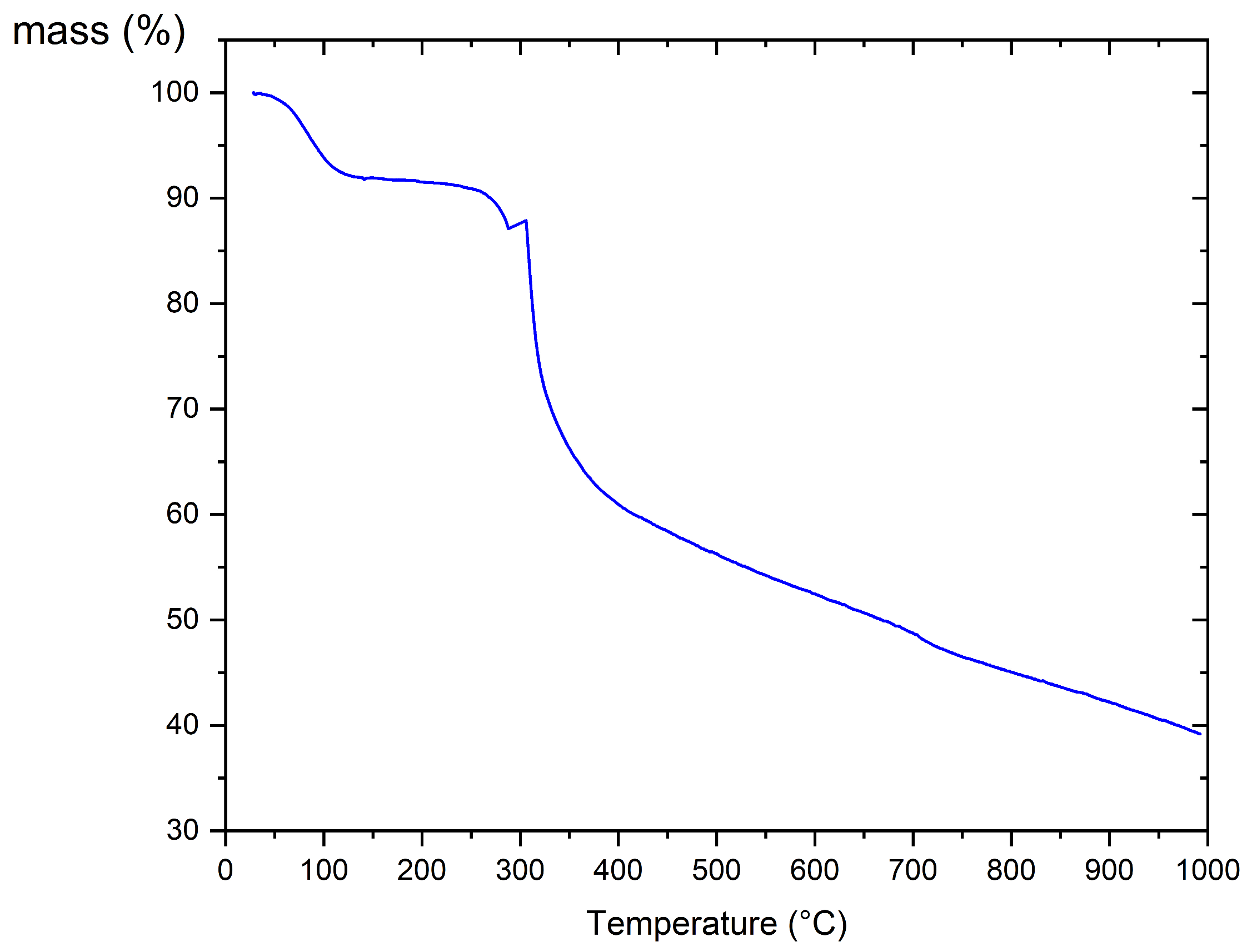
3.1.2. Thermogravimetric Analysis (TGA)
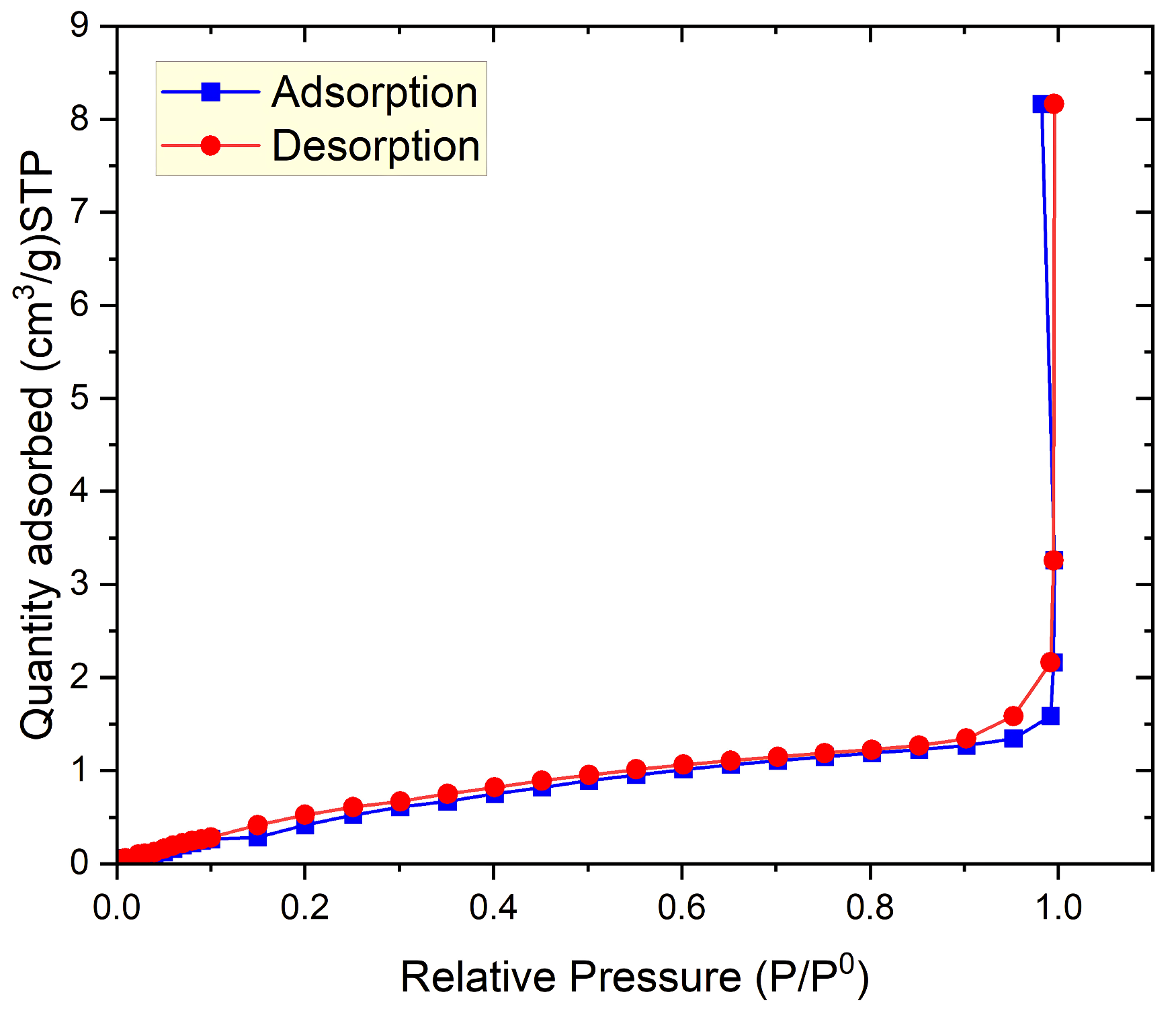
3.1.3. BET Specific Surface Area Analysis
3.1.4. SEM Analysis
3.2. Adsorption Studies
3.2.1. Equilibrium Studies and Adsorption Kinetics Modelling
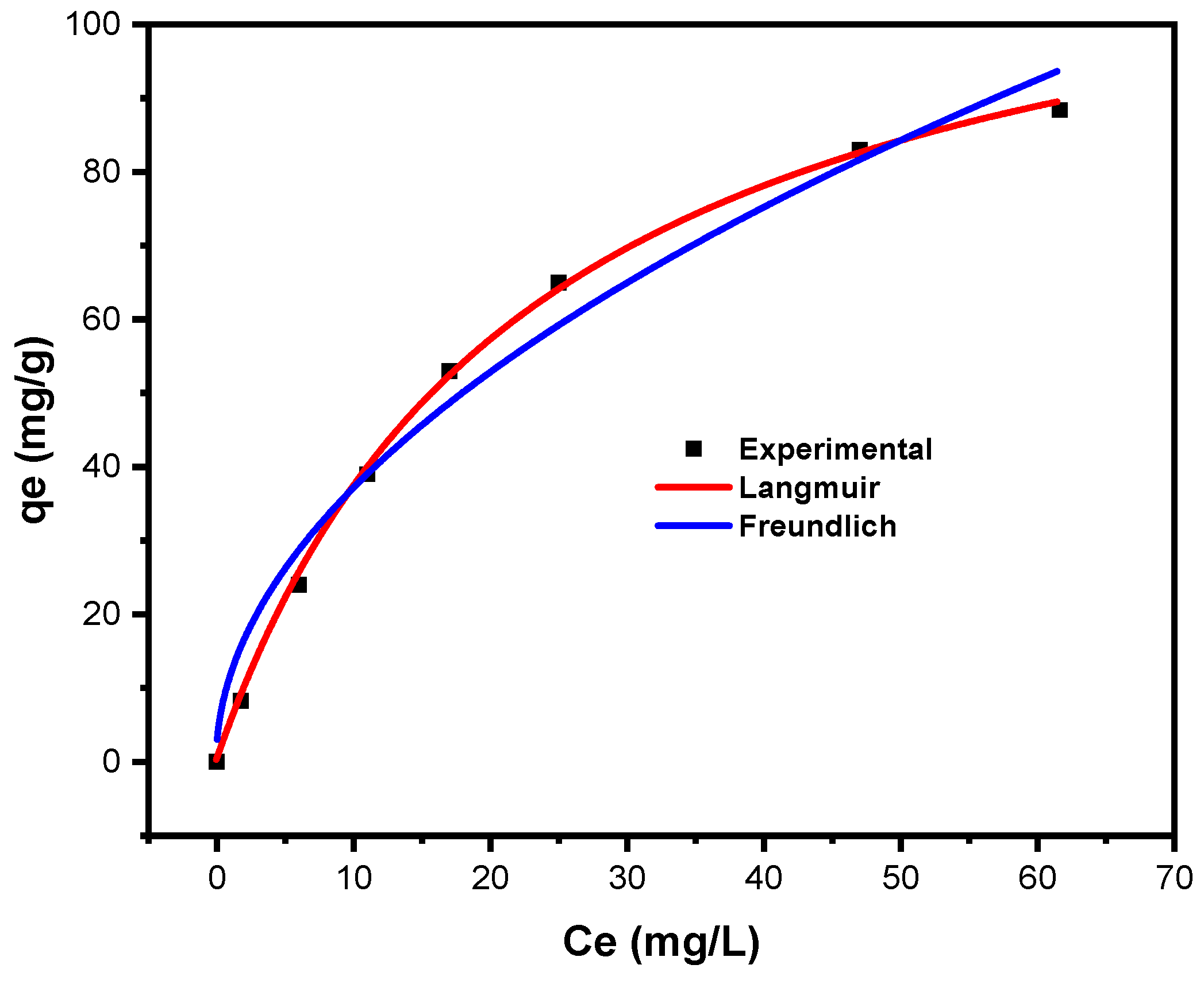
3.2.2. Adsorption Isotherms
Langmuir Isotherm
Freundlich Isotherm
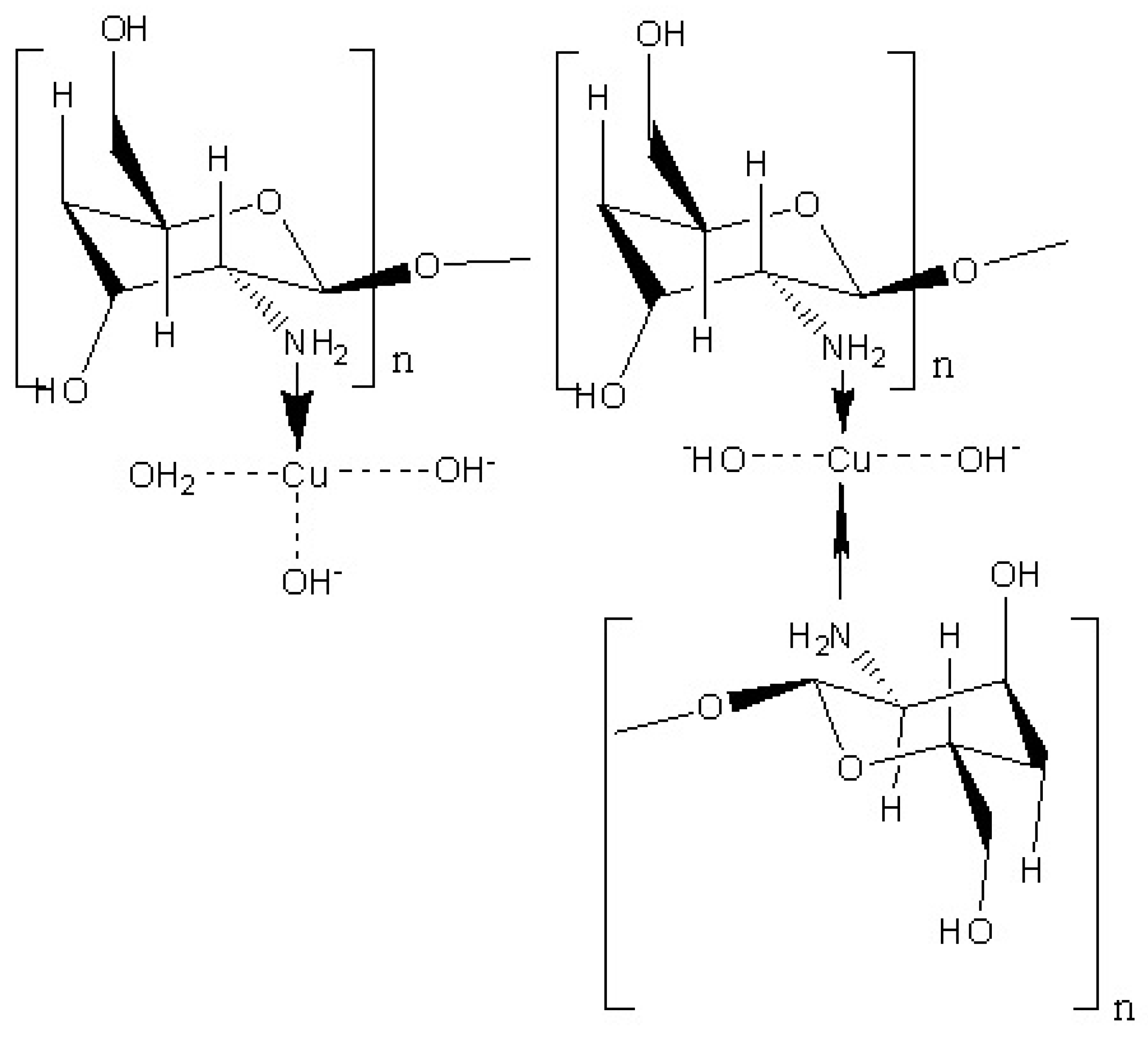
3.2.3. Mechanism of Metal Ion Removal by Chitosan
3.3. Optimization and Modelling via the Response Surface Methodology (RSM) Using the Experimental Design (DOE)
3.3.1. Analysis of the Results Obtained Through Planning by the Experimental Design (DOE)
3.3.2. Analysis of Variance (ANOVA)
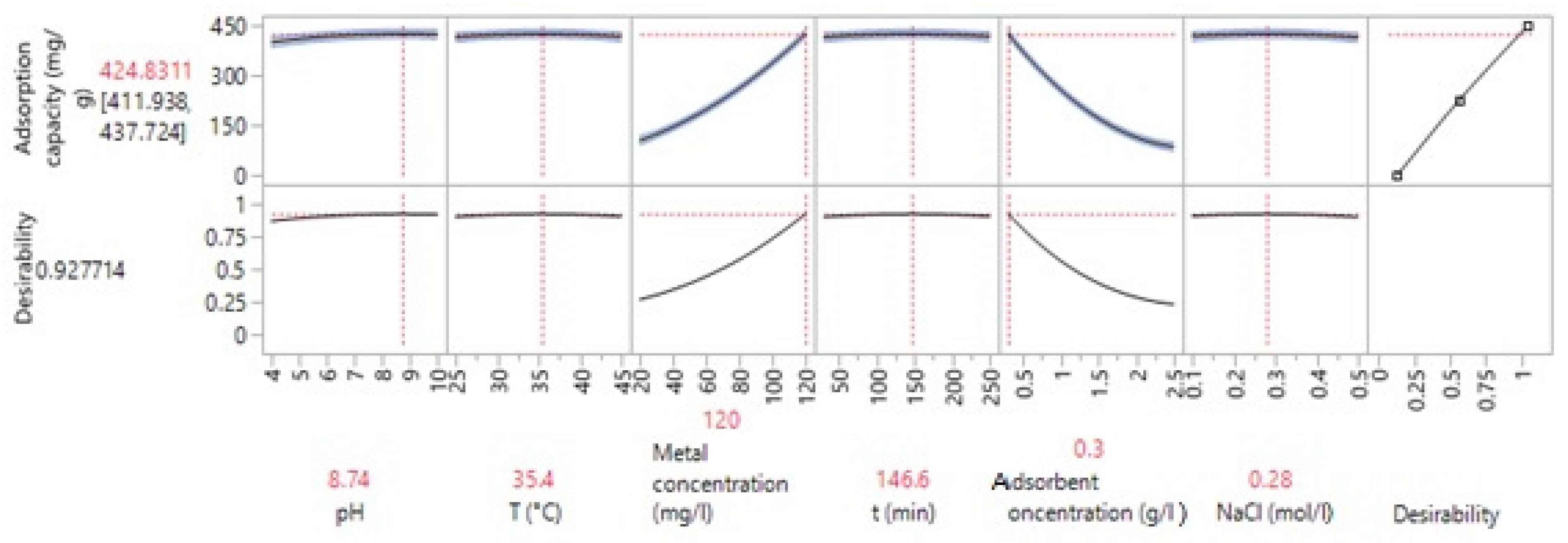
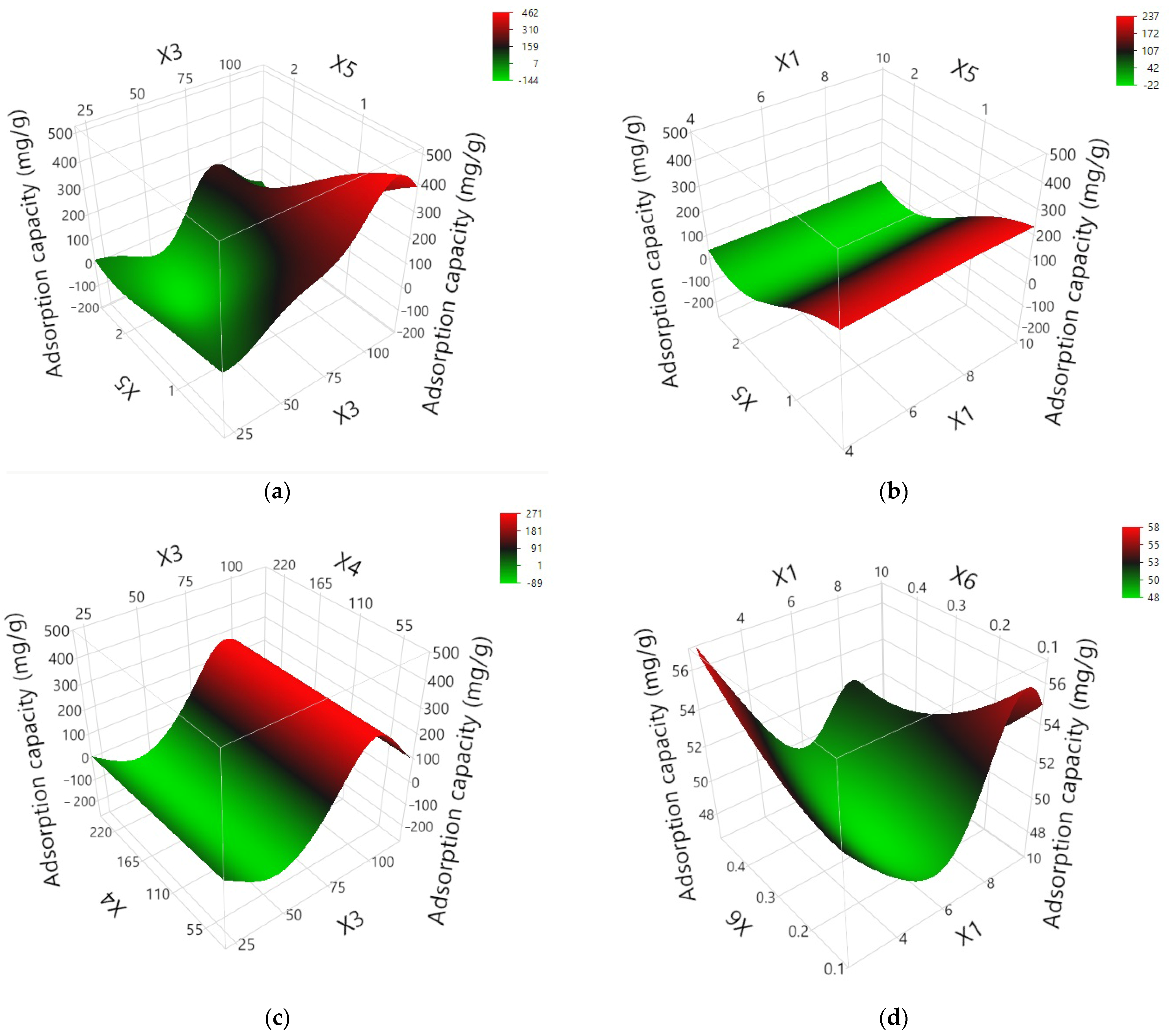
3.3.3. Optimization of the Values of the Variables
3.4. Artificial Neural Networks (ANNs)
3.4.1. Artificial Neural Networking Strategy
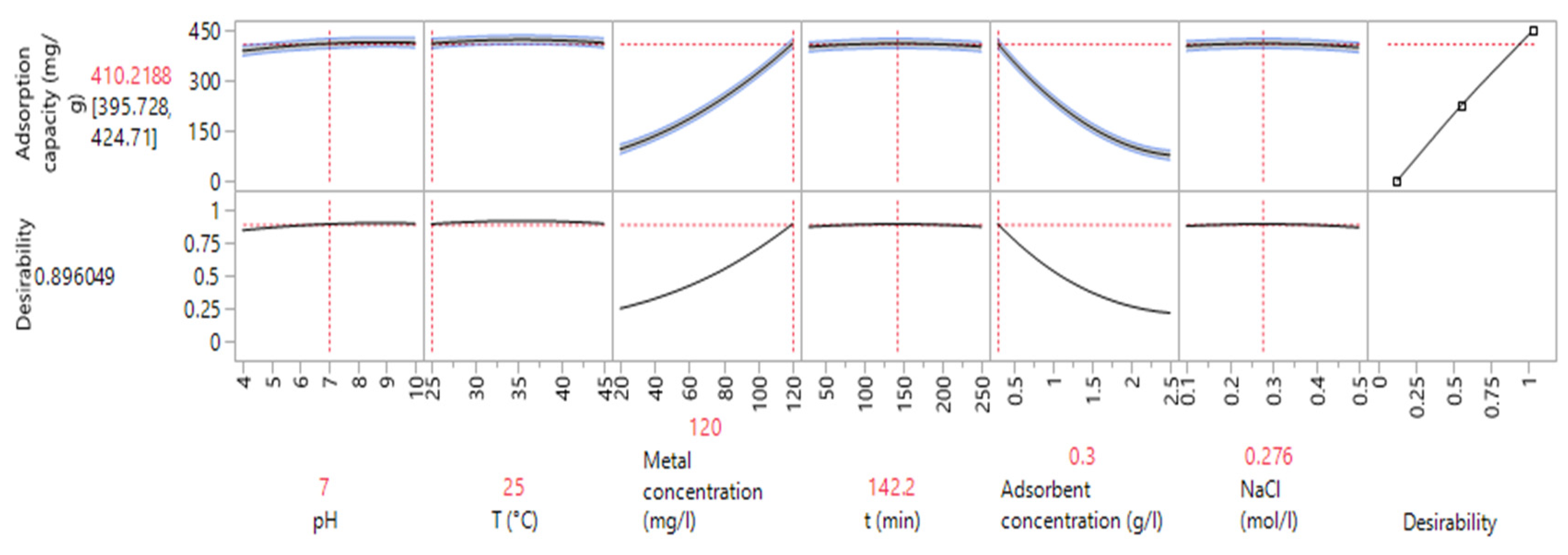
3.4.2. Optimization of Variable Values and Maximization of Adsorption Capacity Using ANNs
3.4.3. Mathematical Modelling by ANNs
3.4.4. RSM Evolution by ANNs and Effects of Parameters
3.5. Thermodynamic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nieder, R.; Benbi, D.K. Potentially toxic elements in the environment–a review of sources, sinks, pathways and mitigation measures. Rev. Environ. Health 2024, 39, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E. What are heavy metals? Long-standing controversy over the scientific use of the term ‘heavy metals’–proposal of a comprehensive definition. Toxicol. Environ. Chem. 2018, 100, 6–19. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Maskawat Marjub, M.; Rahman, N.; Dafader, N.C.; Tuhen, F.S.; Sultana, S.; Ahmed, F.T. Acrylic acid-chitosan blend hydrogel: A novel polymer adsorbent for adsorption of lead (II) and copper (II) ions from wastewater. J. Polym. Eng. 2019, 39, 883–891. [Google Scholar] [CrossRef]
- Bawa, U. Heavy metals concentration in food crops irrigated with pesticides and their associated human health risks in Paki, Kaduna State, Nigeria. Cogent Food Agric. 2023, 9, 2191889. [Google Scholar] [CrossRef]
- Yang, J.; Ma, S.; Zhou, J.; Song, Y.; Li, F. Heavy metal contamination in soils and vegetables and health risk assessment of inhabitants in Daye, China. J. Int. Med. Res. 2018, 46, 3374–3387. [Google Scholar] [CrossRef]
- Vilas–Boas, J.A.; Cardoso, S.J.; Senra, M.V.X.; Rico, A.; Dias, R.J.P. Ciliates as model organisms for the ecotoxicological risk assessment of heavy metals: A meta–analysis. Ecotoxicol. Environ. Saf. 2020, 199, 110669. [Google Scholar] [CrossRef]
- Alam, M.; Rohani, M.F.; Hossain, M.S. Heavy metals accumulation in some important fish species cultured in commercial fish farm of Natore, Bangladesh and possible health risk evaluation. Emerg. Contam. 2023, 9, 100254. [Google Scholar] [CrossRef]
- Singh, A.; Pal, D.B.; Mohammad, A.; Alhazmi, A.; Haque, S.; Yoon, T.; Srivastava, N.; Gupta, V.K. Biological remediation technologies for dyes and heavy metals in wastewater treatment: New insight. Bioresour. Technol. 2022, 343, 126154. [Google Scholar] [CrossRef]
- Browner, C.M. Development Document for Effluent Limitations Guidelines and Standards for the Centralized Waste Treatment Industry: Final; US Environmental Protection Agency, Office of Water: Washington, DC, USA, 2000; Volume 2.
- Organisation Mondiale de la Santé. Directives Sur la Qualité de L’eau de Boisson: Quatrième Edition Intégrant le Premier Additif, 4th ed.; Organisation Mondiale de la Santé: Geneva, Switzerland, 2017.
- Komárek, M.; Čadková, E.; Chrastný, V.; Bordas, F.; Bollinger, J.-C. Contamination of vineyard soils with fungicides: A review of environmental and toxicological aspects. Environ. Int. 2010, 36, 138–151. [Google Scholar] [CrossRef]
- Leng, S.; Guo, X.; Ma, N.; Li, B.; Zhang, X.; Ding, Y.; Zhao, L. Bottom-Up Synthesis of an Oligo-dithiocarbamate Chelating Precipitant and Its Direct Removal of Complexed Heavy Metals from Acidic Wastewater. ACS ES&T Water 2023, 3, 2581–2589. [Google Scholar]
- Doyo, A.N.; Kumar, R.; Barakat, M.A. Recent advances in cellulose, chitosan, and alginate based biopolymeric composites for adsorption of heavy metals from wastewater. J. Taiwan Inst. Chem. Eng. 2023, 151, 105095. [Google Scholar] [CrossRef]
- Ozcan, A.S.; Gök, O.; Ozcan, A. Adsorption of lead(II) ions onto 8-hydroxy quinoline-immobilized bentonite. J. Hazard. Mater. 2009, 161, 499–509. [Google Scholar] [CrossRef]
- Cheikh, S.; Imessaoudene, A.; Bollinger, J.-C.; Manseri, A.; Bouzaza, A.; Hadadi, A.; Hamri, N.; Amrane, A.; Mouni, L. Adsorption behavior and mechanisms of the emerging antibiotic pollutant norfloxacin on eco-friendly and low-cost hydroxyapatite: Integrated experimental and response surface methodology optimized adsorption process. J. Mol. Liq. 2023, 392, 123424. [Google Scholar] [CrossRef]
- Bouchelkia, N.; Mouni, L.; Belkhiri, L.; Bouzaza, A.; Bollinger, J.C.; Madani, K.; Dahmoune, F. Removal of lead (II) from water using activated carbon developed from jujube stones, a low-cost sorbent. Sep. Sci. Technol. 2016, 51, 1645–1653. [Google Scholar] [CrossRef]
- Ye, X.; Shang, S.; Zhao, Y.; Cui, S.; Zhong, Y.; Huang, L. Ultra-efficient adsorption of copper ions in chitosan–montmorillonite composite aerogel at wastewater treatment. Cellulose 2021, 28, 7201–7212. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Ahmed, M.A.; Mohamed, A.A. Synthesis, characterization and application of chitosan/graphene oxide/copper ferrite nanocomposite for the adsorptive removal of anionic and cationic dyes from wastewater. RSC Adv. 2023, 13, 5337–5352. [Google Scholar] [CrossRef]
- Javad Sajjadi Shourije, S.M.; Dehghan, P.; Bahrololoom, M.E.; Cobley, A.J.; Vitry, V.; Azar, G.T.P.; Kamyab, H.; Mesbah, M. Using fish scales as a new biosorbent for adsorption of nickel and copper ions from wastewater and investigating the effects of electric and magnetic fields on the adsorption process. Chemosphere 2023, 317, 137829. [Google Scholar] [CrossRef]
- Fundji, W.N.; Igberase, E.; Osifo, P.; Kabuba, J. Modification of chitosan with cellulose and gelatin for the removal of Cu2+, Fe2+, and Pb2+ from oily wastewater. Can. J. Chem. Eng. 2023, 101, 4711–4730. [Google Scholar] [CrossRef]
- Roosen, J.; Van Roosendael, S.; Borra, C.R.; Van Gerven, T.; Mullens, S.; Binnemans, K. Recovery of scandium from leachates of Greek bauxite residue by adsorption on functionalized chitosan-silica hybrid materials. Green Chem. Int. J. Green Chem. Resour. GC 2016, 18, 2005–2013. [Google Scholar] [CrossRef]
- Teng, D.; Jin, P.; Guo, W.; Liu, J.; Wang, W.; Li, P.; Cao, Y.; Zhang, L.; Zhang, Y. Recyclable Magnetic Iron Immobilized onto Chitosan with Bridging Cu Ion for the Enhanced Adsorption of Methyl Orange. Molecules 2023, 28, 2307. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Hao, H.; Wang, X.; Shao, Z. Chitosan-based composite film adsorbents reinforced with nanocellulose for removal of Cu(II) ion from wastewater: Preparation, characterization, and adsorption mechanism. Int. J. Biol. Macromol. 2022, 213, 369–380. [Google Scholar] [CrossRef]
- Imessaoudene, A.; Cheikh, S.; Hadadi, A.; Hamri, N.; Bollinger, J.C.; Amrane, A.; Tahraoui, H.; Manseri, A.; Mouni, L. Mouni, Adsorption performance of zeolite for the removal of congo red dye: Factorial design experiments, kinetic, and equilibrium studies. Separations 2023, 10, 57. [Google Scholar] [CrossRef]
- Antony, J. Design of Experiments for Engineers and Scientists, 3rd ed.; Elsevier: London, UK, 2023. [Google Scholar]
- Greenhill, S.; Rana, S.; Gupta, S.; Vellanki, P.; Venkatesh, S. Bayesian Optimization for Adaptive Experimental Design: A Review. IEEE Access 2020, 8, 13937–13948. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Beresford, R. Basic concepts of artificial neural network (ANN) modeling and its application in pharmaceutical research. J. Pharm. Biomed. Anal. 2000, 22, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Fiyadh, S.S.; Alardhi, S.M.; Al Omar, M.; Aljumaily, M.M.; Al Saadi, M.A.; Fayaed, S.S.; Ahmed, S.N.; Salman, A.D.; Abdalsalm, A.H.; Jabbar, N.M.; et al. A comprehensive review on modelling the adsorption process for heavy metal removal from waste water using artificial neural network technique. Heliyon 2023, 9, e15455. [Google Scholar] [CrossRef]
- Benzouz, K.; Bouchelkia, N.; Imessaoudene, A.; Bollinger, J.; Amrane, A.; Assadi, A.; Zeghioud, H.; Mouni, L. Efficient and Low-Cost Water Remediation for Chitosan Derived from Shrimp Waste, an Ecofriendly Material: Kinetics Modeling, Response Surface Methodology Optimization, and Mechanism. Water 2023, 15, 3728. [Google Scholar] [CrossRef]
- Truong, T.O.; Hausler, R.; Monette, F.; Niquette, P. Valorisation des résidus industriels de pêches pour la transformation de chitosane par technique hydrothermo-chimique. Rev. Des Sci. L’eau 2007, 20, 253–262. [Google Scholar] [CrossRef]
- Weißpflog, J.; Vehlow, D.; Müller, M.; Kohn, B.; Scheler, U.; Boye, S.; Schwarz, S. Characterization of chitosan with different degree of deacetylation and equal viscosity in dissolved and solid state—Insights by various complimentary methods. Int. J. Biol. Macromol. 2021, 171, 242–261. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, F.; Jin, L.; Du, X.; Cai, H. Evaluation of the Deacetylation Degree of Chitosan with Two-Abrupt-Change Coulometric Titration. Electroanalysis 2016, 28, 401–406. [Google Scholar] [CrossRef]
- Abbas, K.; Abdulkarim, S.; Saleh, A.; Ebrahimian, M. Suitability of viscosity measurement methods for liquid food variety and applicability in food industry-A review. J. Food Agric. Environ. 2010, 8, 100–107. [Google Scholar]
- Payet, L. Viscoelasticite et Structure de Gels à Base de Chitosane-Relations Avec Les Propriétés Diffusionnelles de Macromolécules Dans ces Biogels; Université Paris-Diderot-Paris VII: Paris, France, 2005. [Google Scholar]
- Ombaka, O. New metallochromic indicators for Complexometric titration of copper (II) in presence of interfering species. Res. J. Life Sci. Bioinform. Pharm. Chem. Sci. 2020, 6. [Google Scholar] [CrossRef]
- Matias, M.; Sousa, J.F.; Bernardino, E.F.; Vareda, J.P.; Durães, L.; Abreu, P.E.; Marques, J.M.; Murtinho, D.; Valente, A.J. Reduced chitosan as a strategy for removing copper ions from water. Molecules 2023, 28, 4110. [Google Scholar] [CrossRef]
- Sumaila, A.; Ndamitso, M.M.; Iyaka, Y.A.; Abdulkareem, A.S.; Tijani, J.O.; Idris, M.O. Extraction and characterization of chitosan from crab shells: Kinetic and thermodynamic studies of arsenic and copper adsorption from electroplating wastewater. Iraqi J. Sci. 2020, 61, 2156–2171. [Google Scholar]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.; Pernicone, N.; Ramsay, J.D.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of porous solids (Technical Report). Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Mende, M.; Schwarz, D.; Steinbach, C.; Boldt, R.; Schwarz, S. Simultaneous adsorption of heavy metal ions and anions from aqueous solutions on chitosan—Investigated by spectrophotometry and SEM-EDX analysis. Colloids Surf. A Physicochem. Eng. Asp. 2016, 510, 275–282. [Google Scholar] [CrossRef]
- Imessaoudene, A.; Cheikh, S.; Bollinger, J.C.; Belkhiri, L.; Tiri, A.; Bouzaza, A.; El Jery, A.; Assadi, A.; Amrane, A.; Mouni, L. Zeolite Waste Characterization and Use as Low-Cost, Ecofriendly, and Sustainable Material for Malachite Green and Methylene Blue Dyes Removal: Box–Behnken Design, Kinetics, and Thermodynamics. Appl. Sci. 2022, 12, 7587. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Li, D.; Liu, Z.-Q.; Tao, Q.; Zhu, Y.; Yang, J.; Zhang, Y.-M. Kinetics, isotherm, thermodynamic, and adsorption mechanism studies of La (OH)3-modified exfoliated vermiculites as highly efficient phosphate adsorbents. Chem. Eng. J. 2014, 236, 191–201. [Google Scholar] [CrossRef]
- Vitek, R.; Masini, J.C. Nonlinear regression for treating adsorption isotherm data to characterize new sorbents: Advantages over linearization demonstrated with simulated and experimental data. Heliyon 2023, 9, e15128. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Fast and efficient adsorption of methylene green 5 on activated carbon prepared from new chemical activation method. J. Environ. Manag. 2017, 188, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Elfeghe, S.; Anwar, S.; James, L.; Zhang, Y. Adsorption of Cu(II) ions from aqueous solutions using ion exchange resins with different functional groups. Can. J. Chem. Eng. 2023, 101, 2128–2138. [Google Scholar] [CrossRef]
- Khan, J.; Lin, S.; Nizeyimana, J.C.; Wu, Y.; Wang, Q.; Liu, X. Removal of copper ions from wastewater via adsorption on modified hematite (α-Fe2O3) iron oxide coated sand. J. Clean. Prod. 2021, 319, 128687. [Google Scholar] [CrossRef]
- Salman, M.S.; Hasan, M.N.; Hasan, M.M.; Kubra, K.T.; Sheikh, M.C.; Rehan, A.I.; Waliullah, R.M.; Rasee, A.I.; Awual, M.E.; Hossain, M.S.; et al. Improving copper(II) ion detection and adsorption from wastewater by the ligand-functionalized composite adsorbent. J. Mol. Struct. 2023, 1282, 135259. [Google Scholar] [CrossRef]
- Kourim, A.; Malouki, M.A.; Ziouche, A. Thermodynamic and kinetic behaviors of copper (II) and methyl orange (MO) adsorption on unmodified and modified kaolinite clay. Clay Clay Miner. 2021, 3, 45. [Google Scholar]
- Langama, L.M.; Anguile, J.J.; Ndong, A.N.M.M.; Bouraima, A.; Bissielou, C. Adsorption of Copper (II) Ions in Aqueous Solution by a Natural Mouka Smectite and an Activated Carbon Prepared from Kola Nut Shells by Chemical Activation with Zinc Chloride (ZnCl2). Open J. Inorg. Chem. 2021, 11, 131–144. [Google Scholar] [CrossRef]
- Zafar, S.; Khan, M.I.; Lashari, M.H.; Khraisheh, M.; Almomani, F.; Mirza, M.L.; Khalid, N. Removal of copper ions from aqueous solution using NaOH-treated rice husk. Emergent Mater. 2020, 3, 857–870. [Google Scholar] [CrossRef]
- Cardenas, G.; Orlando, P.; Edelio, T. Synthesis and applications of chitosan mercaptanes as heavy metal retention agent. Int. J. Biol. Macromol. 2001, 28, 167–174. [Google Scholar] [CrossRef]
- Chu, K. Removal of copper from aqueous solution by chitosan in prawn shell: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2002, 90, 77–95. [Google Scholar] [CrossRef]
- Rhazi, M.; Desbrieres, J.; Tolaimate, A.; Rinaudo, M.; Vottero, P.; Alagui, A. Contribution to the study of the complexation of copper by chitosan and oligomers. Polymer 2002, 43, 1267–1276. [Google Scholar] [CrossRef]
- Zia, Q.; Tabassum, M.; Gong, H.; Li, J. A review on chitosan for the removal of heavy metals ions. J. Fiber Bioeng. Inform. 2019, 12, 103–128. [Google Scholar] [CrossRef]
- Rogina, A.; Lončarević, A.; Antunović, M.; Marijanović, I.; Ivanković, M.; Ivanković, H. Tuning physicochemical and biological properties of chitosan through complexation with transition metal ions. Int. J. Biol. Macromol. 2019, 129, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Goupy, J.; Creighton, L. Introduction Aux Plans D’expériences: Avec Applications; Dunod: Paris, France, 2009. [Google Scholar]
- Tran, H.N.; Lima, E.C.; Juang, R.-S.; Bollinger, J.-C.; Chao, H.-P. Thermodynamic parameters of liquid–phase adsorption process calculated from different equilibrium constants related to adsorption isotherms: A comparison study. J. Environ. Chem. Eng. 2021, 9, 106674. [Google Scholar] [CrossRef]
- Salvestrini, S.; Leone, V.; Iovino, P.; Canzano, S.; Capasso, S. Considerations about the correct evaluation of sorption thermodynamic parameters from equilibrium isotherms. J. Chem. Thermodyn. 2014, 68, 310–316. [Google Scholar] [CrossRef]
- Sabnis, S.; Block, L.H. Improved infrared spectroscopic method for the analysis of the degree of N-deacetylation. Polym. Bull. 1997, 39, 67–71. [Google Scholar] [CrossRef]


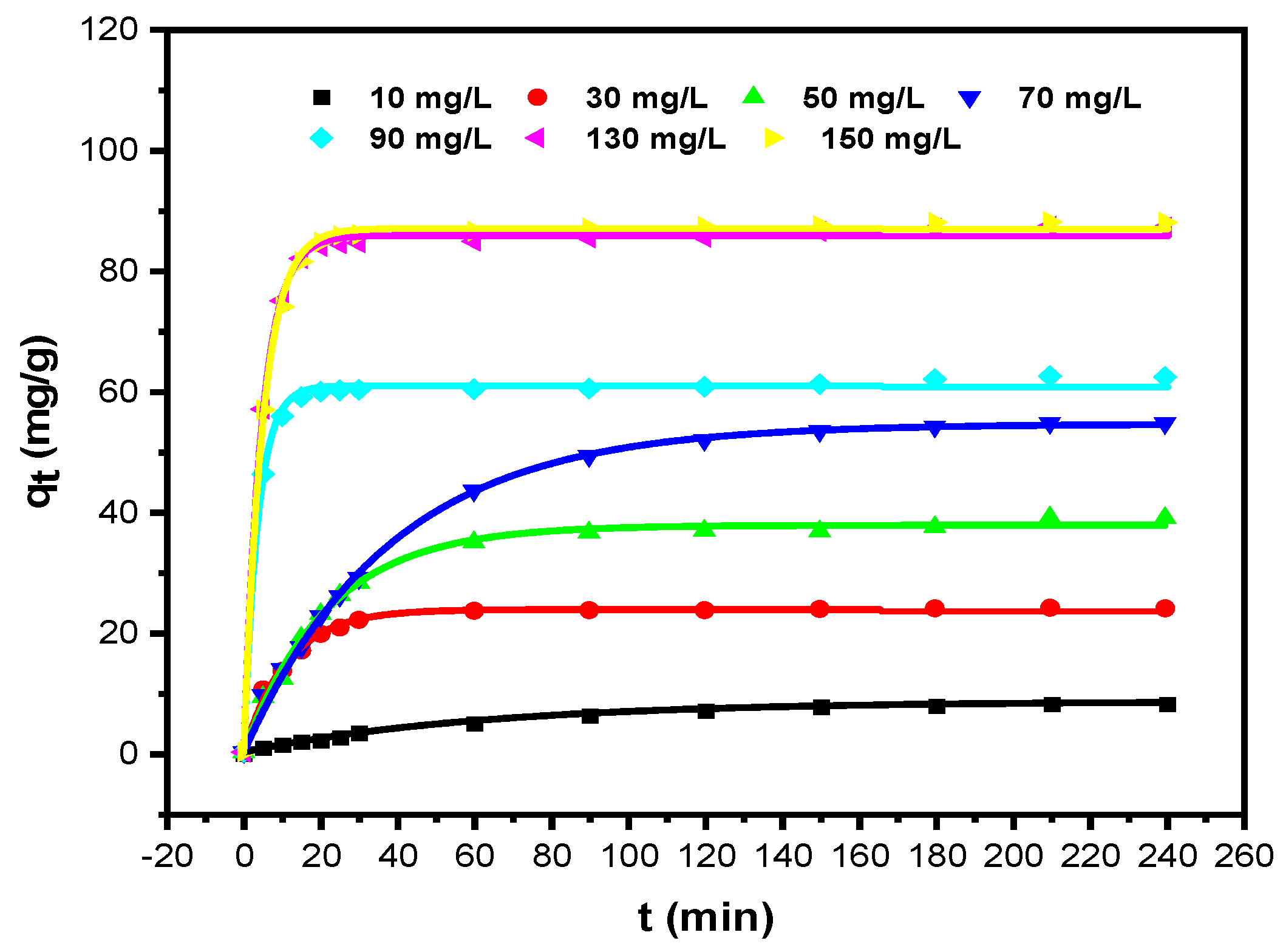



| Pseudo-First Order | Pseudo-Second Order | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C0 (mg/L) | qe (exp) | qe (calc) | K1 (min−1) | R2 | χ2 | qe (calc) | K2 (min−1) | R2 | χ2 |
| 10 | 8.25 | 8.42 ± 0.12 | 0.016 ± 6.8 × 10−4 | 0.99 | 0.032 | 10.93 ± 0.25 | 0.0013 ± 1.11 × 10−4 | 0.99 | 0.03 |
| 30 | 24.00 | 23.69 ± 0.23 | 0.089 ± 0.004 | 0.99 | 0.381 | 25.23 ± 0.38 | 0.0058 ± 5.732 × 10−4 | 0.98 | 0.65 |
| 50 | 39.00 | 37.68 ± 0.37 | 0.046 ± 0.002 | 0.99 | 0.854 | 42.29 ± 0.83 | 0.00136 ± 1.32 × 10−4 | 0.99 | 1.93 |
| 70 | 54.70 | 54.52 ± 0.44 | 0.026 ± 7.1 × 10−4 | 0.99 | 0.842 | 65.39 ± 1.32 | 4.27 × 10−4 ± 3.6 × 10−5 | 0.99 | 2.43 |
| 90 | 62.50 | 60.86 ± 0.29 | 0.273 ± 0.011 | 0.99 | 0.889 | 62.63 ± 0.47 | 0.011 ± 0.0012 | 0.99 | 1.53 |
| 130 | 87.40 | 85.85 ± 0.30 | 0.213 ± 0.005 | 0.99 | 0.893 | 88.98 ± 1.02 | 0.0053 ± 6.9 × 10−4 | 0.99 | 6.72 |
| 150 | 88.10 | 87.01 ± 0.31 | 0.201 ± 0.005 | 0.99 | 0.967 | 90.34 ± 0.98 | 0.0048 ± 5.7 × 10−4 | 0.99 | 6.11 |
| Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|
| qmax (mg/g) | KL (L/mg) | R2 | χ2 | KF [(mg/g) (mg/L)1/n] | 1/n | R2 | χ2 |
| 123.05 ± 2.27 | 0.043 ± 0.001 | 0.999 | 0.958 | 11.34 ± 2.12 | 0.51 ± 0.05 | 0.971 | 30.61 |
| Materials | pH | Maximum Adsorption Capacity (mg/g) | References |
|---|---|---|---|
| Commercial resins Dowex G-26 and Puromet™ MTS9570 | 3.5 | 41.67 and 37.70 mg/g respectively | [46] |
| synthetic hematite (α-Fe2O3) iron oxide-coated sand (HIOCS) | 6 | 3.93 mg/g | [47] |
| Ligand of 4-tert-Octyl-4-((phenyl)diazenyl) phenol-silica | 4 | 184.73 mg/g | [48] |
| Chitosan montmorillonite composite | 6 | 86.95 mg/g | [18] |
| Kaolinite clay | 6 | 34.12 mg/g | [49] |
| Natural smectite (NS) and activated carbon (AC) | 3.5 | 26.6 mg/g and 36.6 mg/g respectively | [50] |
| NaOH-treated rice husk | 6 | 3.75 mg/g | [51] |
| Chitosan using crab shells from nylon shrimps | 2.5 4.5 | 135 mg/g 238 mg/g | [52] |
| Chitosan from partially deacetylated prawn shell | 6 | 16.9 mg/g | [53] |
| Shrimp carapace-derived chitosan | 6.5 | 123 mg/g | This study |
| No. | Variable | Name | Variable Level | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| 01 | X1 | pH | 4 | 7 | 10 |
| 02 | X2 | T (°C) | 25 | 35 | 45 |
| 03 | X3 | Metal concentration (mg/L) | 20 | 70 | 120 |
| 04 | X4 | t (min) | 30 | 140 | 250 |
| 05 | X5 | Adsorbent concentration S/L (g/L) | 0.3 | 1.4 | 2.5 |
| 06 | X6 | NaCl (mol/L) | 0.1 | 0.3 | 0.5 |
| Run | pH | T (°C) | Metal Concentration (mg/L) | t (min) | Adsorbent Concentration (g/L) | NaCl (mol/L) | Observed Adsorption Capacity (mg/g) | Predicted Adsorption Capacity (mg/g) | Residual Adsorption Capacity (mg/g) | Observed Removal (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7 | 35 | 70 | 140 | 2.5 | 0.3 | 27.74 | 30.046 | −2.306 | 99.07 |
| 2 | 4 | 25 | 120 | 250 | 0.3 | 0.5 | 359.08 | 365.584 | −6.504 | 89.77 |
| 3 | 10 | 45 | 20 | 30 | 0.3 | 0.5 | 64.08 | 67.686 | −3.606 | 69.12 |
| 4 | 7 | 35 | 70 | 140 | 1.4 | 0.5 | 49.89 | 47.494 | 2.395 | 99.79 |
| 5 | 7 | 35 | 20 | 140 | 1.4 | 0.3 | 14.17 | 6.548 | 7.621 | 99.2 |
| 6 | 7 | 35 | 70 | 140 | 0.3 | 0.3 | 231.19 | 225.592 | 5.597 | 99.08 |
| 7 | 10 | 45 | 20 | 30 | 2.5 | 0.1 | 6.85 | 0.551 | 6.298 | 85.62 |
| 8 | 10 | 45 | 20 | 250 | 2.5 | 0.5 | 7.71 | 4.512 | 3.197 | 96.37 |
| 9 | 7 | 25 | 70 | 140 | 1.4 | 0.3 | 49.37 | 47.603 | 1.766 | 98.75 |
| 10 | 4 | 35 | 70 | 140 | 1.4 | 0.3 | 49.72 | 43.935 | 5.784 | 99.44 |
| 11 | 10 | 25 | 120 | 250 | 0.3 | 0.1 | 396.22 | 393.957 | 2.262 | 99.05 |
| 12 | 4 | 25 | 120 | 250 | 2.5 | 0.1 | 47.99 | 44.589 | 3.400 | 99.98 |
| 13 | 10 | 45 | 120 | 250 | 2.5 | 0.1 | 47.87 | 51.894 | −4.024 | 99.73 |
| 14 | 4 | 45 | 120 | 30 | 2.5 | 0.1 | 47.69 | 43.100 | 4.589 | 99.36 |
| 15 | 10 | 45 | 20 | 250 | 0.3 | 0.1 | 66.61 | 71.827 | −5.217 | 99.91 |
| 16 | 4 | 45 | 20 | 250 | 2.5 | 0.1 | 7.25 | 14.079 | −6.829 | 90.62 |
| 17 | 10 | 25 | 120 | 30 | 2.5 | 0.1 | 47.77 | 51.995 | −4.225 | 99.53 |
| 18 | 10 | 25 | 20 | 250 | 2.5 | 0.1 | 7.78 | 0.935 | 6.844 | 97.33 |
| 19 | 10 | 25 | 20 | 30 | 2.5 | 0.5 | 7.99 | 8.983 | −0.993 | 99.91 |
| 20 | 4 | 45 | 120 | 250 | 2.5 | 0.5 | 47.99 | 44.726 | 3.263 | 99.98 |
| 21 | 7 | 35 | 70 | 250 | 1.4 | 0.3 | 49.75 | 48.662 | 1.0871 | 99.5 |
| 22 | 10 | 35 | 70 | 140 | 1.4 | 0.3 | 49.33 | 51.823 | −2.493 | 98.67 |
| 23 | 4 | 25 | 20 | 250 | 0.3 | 0.1 | 65.69 | 63.082 | 2.607 | 98.54 |
| 24 | 4 | 25 | 20 | 250 | 2.5 | 0.5 | 7.95 | 11.307 | −3.357 | 99.41 |
| 25 | 7 | 35 | 70 | 140 | 1.4 | 0.3 | 49.5 | 57.947 | −8.447 | 99 |
| 26 | 4 | 45 | 20 | 30 | 0.3 | 0.1 | 60 | 59.143 | 0.856 | 90 |
| 27 | 4 | 25 | 120 | 30 | 2.5 | 0.5 | 47.93 | 42.917 | 5.012 | 99.85 |
| 28 | 10 | 25 | 20 | 30 | 0.3 | 0.1 | 66.61 | 70.079 | −3.469 | 99.91 |
| 29 | 10 | 25 | 120 | 250 | 2.5 | 0.5 | 47.8 | 48.861 | −1.061 | 99.59 |
| 30 | 10 | 45 | 120 | 30 | 2.5 | 0.5 | 47.84 | 50.652 | −2.812 | 99.68 |
| 31 | 7 | 35 | 70 | 30 | 1.4 | 0.3 | 49.44 | 47.236 | 2.203 | 98.89 |
| 32 | 10 | 45 | 120 | 30 | 0.3 | 0.1 | 396.08 | 392.928 | 3.151 | 99.02 |
| 33 | 7 | 45 | 70 | 140 | 1.4 | 0.3 | 49.48 | 47.955 | 1.524 | 98.97 |
| 34 | 4 | 45 | 120 | 30 | 0.3 | 0.5 | 356.77 | 363.820 | −7.050 | 89.19 |
| 35 | 7 | 35 | 70 | 140 | 1.4 | 0.3 | 49.94 | 57.947 | −8.007 | 99.88 |
| 36 | 7 | 35 | 120 | 140 | 1.4 | 0.3 | 180 | 184.330 | −4.330 | 99.79 |
| 37 | 10 | 45 | 120 | 250 | 0.3 | 0.5 | 398.05 | 393.729 | 4.320 | 99.51 |
| 38 | 4 | 25 | 120 | 30 | 0.3 | 0.1 | 366.25 | 369.653 | −3.403 | 91.56 |
| 39 | 10 | 25 | 120 | 30 | 0.3 | 0.5 | 398.66 | 392.035 | 6.6242 | 99.66 |
| 40 | 4 | 25 | 20 | 30 | 2.5 | 0.1 | 7.69 | 12.216 | −4.5260 | 96.12 |
| 41 | 4 | 45 | 120 | 250 | 0.3 | 0.1 | 378.47 | 377.681 | 0.788 | 94.61 |
| 42 | 4 | 45 | 20 | 30 | 2.5 | 0.5 | 7.72 | 10.188 | −2.468 | 96.58 |
| 43 | 7 | 35 | 70 | 140 | 1.4 | 0.1 | 49.87 | 48.974 | 0.895 | 99.75 |
| 44 | 4 | 45 | 20 | 250 | 0.3 | 0.5 | 66.19 | 62.170 | 4.019 | 99.29 |
| 45 | 10 | 25 | 20 | 250 | 0.3 | 0.5 | 63.55 | 68.345 | −4.795 | 95.33 |
| 46 | 4 | 25 | 20 | 30 | 0.3 | 0.5 | 62.33 | 58.511 | 3.818 | 93.5 |
| Factor | DF | Sum of Squares | F-Value | p-Value |
|---|---|---|---|---|
| Adsorbent concentration (X5) | 1 | 325,022.68 | 6531.354 | <0.0001 |
| Metal concentration (X3) | 1 | 268,654.02 | 5398.622 | <0.0001 |
| Metal concentration × Adsorbent concentration (X3X5) | 1 | 152,984.70 | 3074.238 | <0.0001 |
| Adsorbent concentration × Adsorbent concentration (X5X5) | 1 | 11,609.75 | 233.2988 | <0.0001 |
| Metal concentration × Metal concentration (X3X3) | 1 | 3342.72 | 67.1721 | <0.0001 |
| pH × Adsorbent concentration (X1X5) | 1 | 575.28 | 11.5604 | 0.0032 |
| pH (X1) | 1 | 528.83 | 10.6268 | 0.0043 |
| pH× Metal concentration (X1X3) | 1 | 463.30 | 9.3100 | 0.0069 |
| T × T (X2X2) | 1 | 245.83 | 4.9400 | 0.0393 |
| pH × pH (X1X1) | 1 | 241.02 | 4.8433 | 0.0410 |
| t × t (X4X4) | 1 | 237.68 | 4.7762 | 0.0423 |
| NaCl × NaCl (X6X6) | 1 | 224.32 | 4.5077 | 0.0479 |
| Measures | Training | Validation |
|---|---|---|
| R2 | 0.999 | 0.998 |
| RASE | 0.76 | 5.37 |
| MAD | 0.53 | 3.85 |
| Log likelihood | 42.44 | 27.89 |
| SSE | 21.48 | 259.58 |
| Sum of frequency | 37 | 9 |
| T (°K) | KL (L/mg) | KL0 | Ln KL0 | ∆G0 (kJ/mol) | ∆H0 (kJ/mol) | ∆S0 (J/mol·K) |
|---|---|---|---|---|---|---|
| 298.15 | 0.00044 | 27.98 | 3.33 | −8.25 | ||
| 308.15 | 0.00034 | 22.01 | 3.09 | −7.92 | ||
| 318.15 | 0.00026 | 16.69 | 2.81 | −7.44 | −21.78 | −45.14 |
| 328.15 | 0.00021 | 13.25 | 2.58 | −7.05 | ||
| 338.15 | 0.00015 | 09.86 | 2.28 | −6.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benazouz, K.; Bouchelkia, N.; Moussa, H.; Boutheldja, R.; Zamouche, M.; Amrane, A.; Parvathiraja, C.; Al-Lohedan, H.A.; Bollinger, J.-C.; Mouni, L. Efficient Removal of Cu(II) from Wastewater Using Chitosan Derived from Shrimp Shells: A Kinetic, Thermodynamic, Optimization, and Modelling Study. Water 2025, 17, 851. https://doi.org/10.3390/w17060851
Benazouz K, Bouchelkia N, Moussa H, Boutheldja R, Zamouche M, Amrane A, Parvathiraja C, Al-Lohedan HA, Bollinger J-C, Mouni L. Efficient Removal of Cu(II) from Wastewater Using Chitosan Derived from Shrimp Shells: A Kinetic, Thermodynamic, Optimization, and Modelling Study. Water. 2025; 17(6):851. https://doi.org/10.3390/w17060851
Chicago/Turabian StyleBenazouz, Kheira, Nasma Bouchelkia, Hamza Moussa, Razika Boutheldja, Meriem Zamouche, Abdeltif Amrane, Chelliah Parvathiraja, Hamad A. Al-Lohedan, Jean-Claude Bollinger, and Lotfi Mouni. 2025. "Efficient Removal of Cu(II) from Wastewater Using Chitosan Derived from Shrimp Shells: A Kinetic, Thermodynamic, Optimization, and Modelling Study" Water 17, no. 6: 851. https://doi.org/10.3390/w17060851
APA StyleBenazouz, K., Bouchelkia, N., Moussa, H., Boutheldja, R., Zamouche, M., Amrane, A., Parvathiraja, C., Al-Lohedan, H. A., Bollinger, J.-C., & Mouni, L. (2025). Efficient Removal of Cu(II) from Wastewater Using Chitosan Derived from Shrimp Shells: A Kinetic, Thermodynamic, Optimization, and Modelling Study. Water, 17(6), 851. https://doi.org/10.3390/w17060851








