Stable Water Isotopes Across Marsh, River, and Lake Environments in the Zoige Alpine Wetland on the Tibetan Plateau
Abstract
1. Introduction
2. Materials and Methods
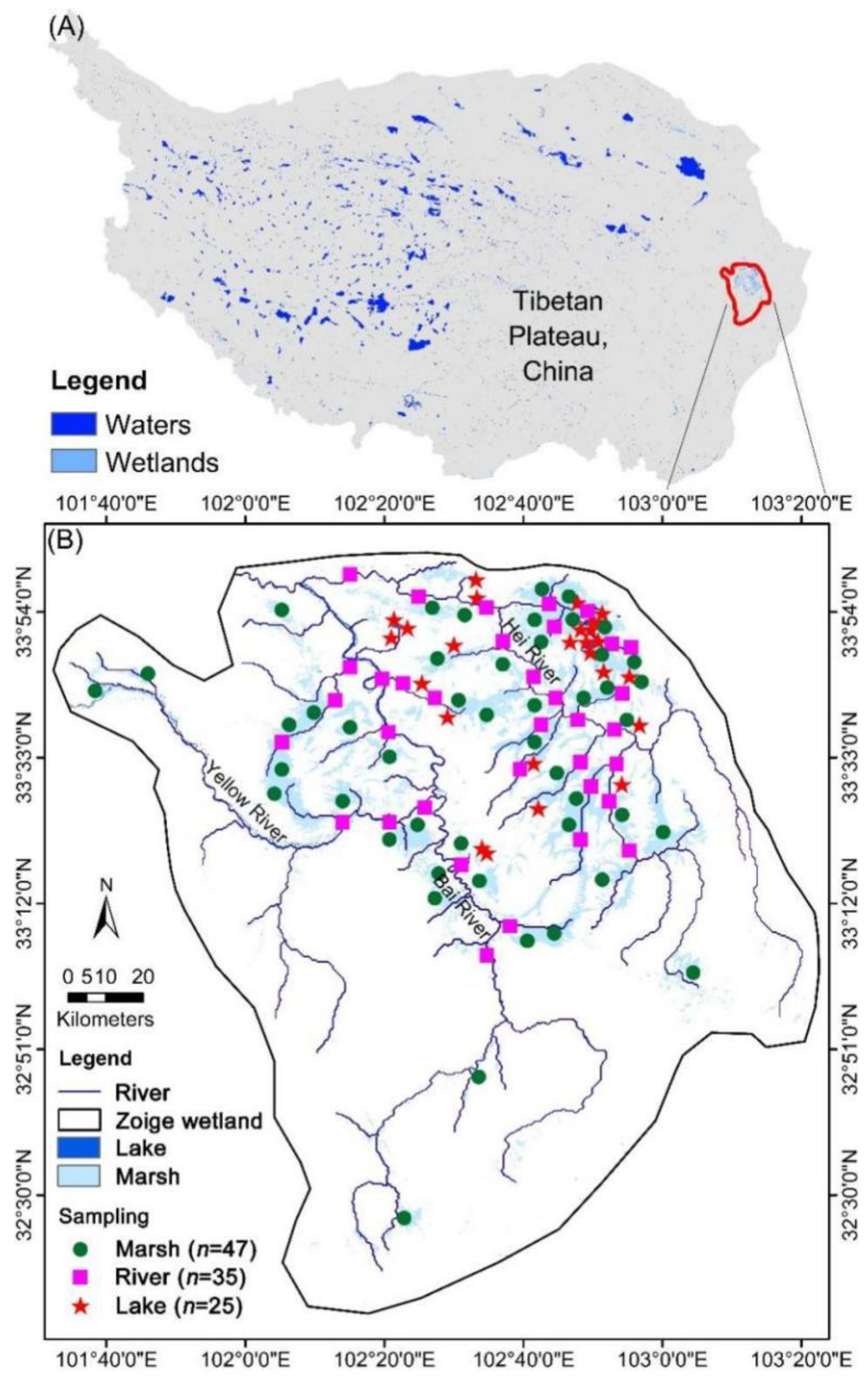
2.1. Study Area
2.2. Field Sampling
2.3. Laboratory Analysis of Water Stable Isotopes
2.4. Statistical Analysis
3. Results
3.1. Isotopic Characteristics of Atmospheric Precipitation in the Study Area
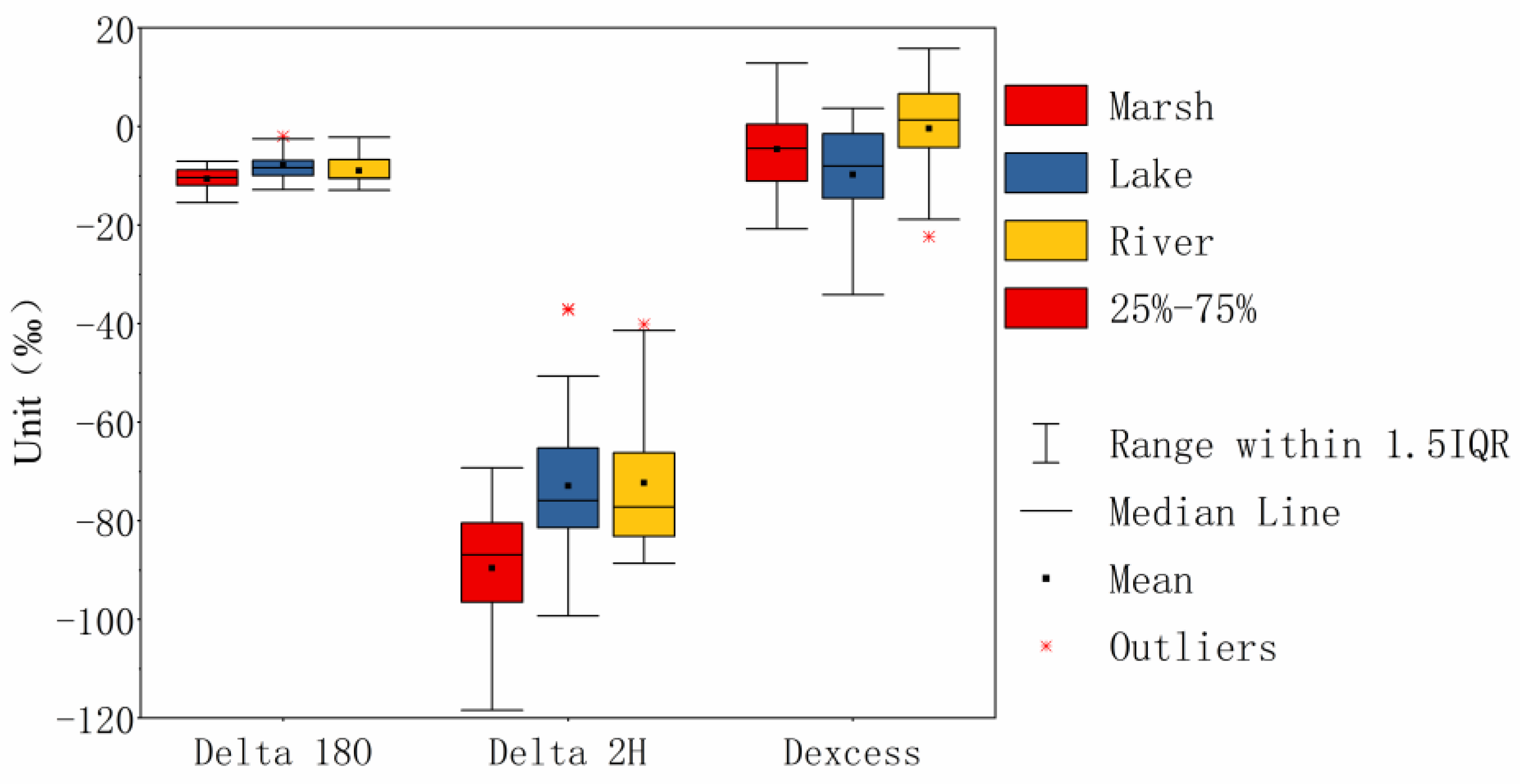
3.2. Statistical Analysis of δD, δ18O, and d-Excess
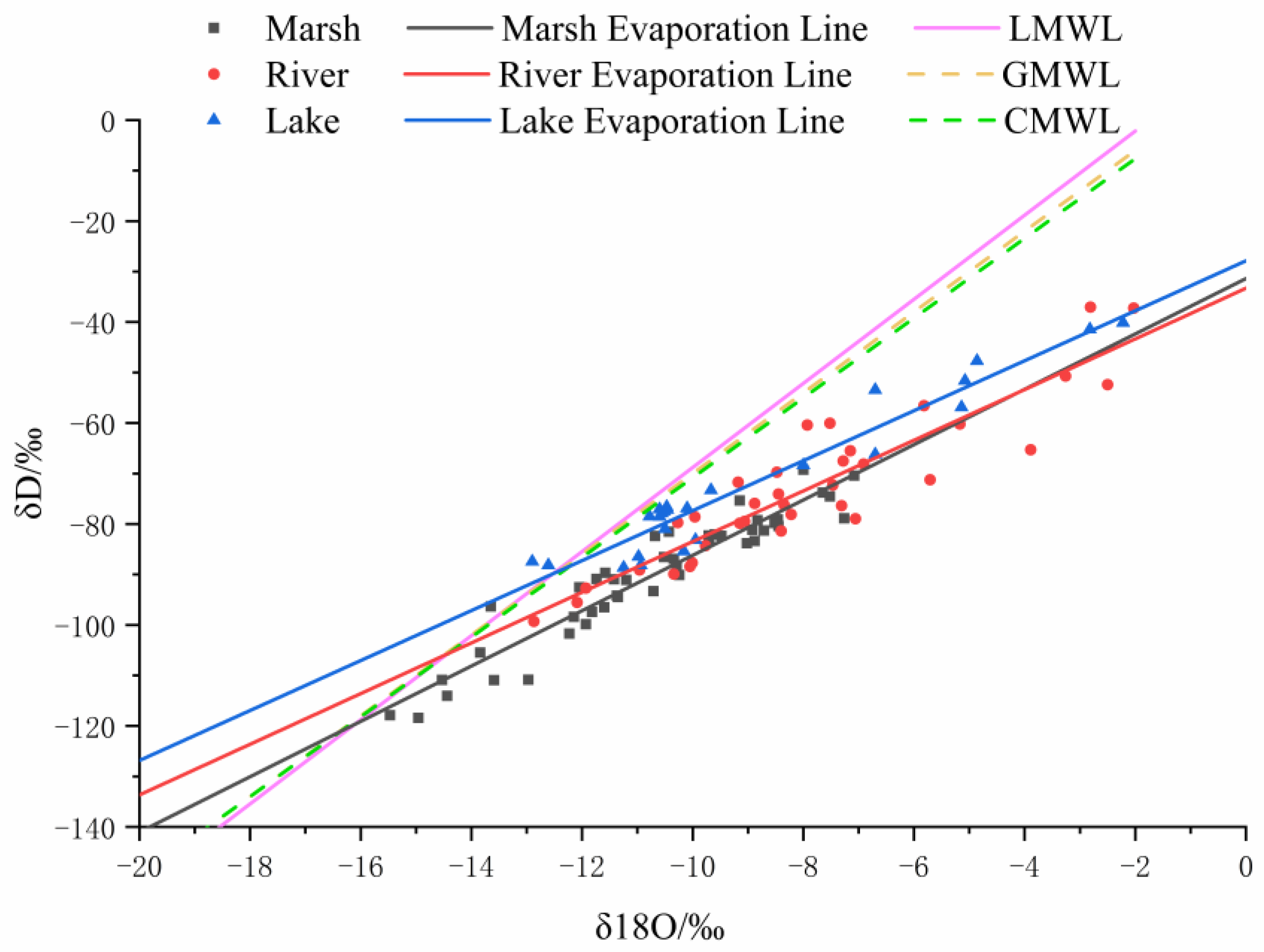
3.3. δD/δ18O Ratios
3.4. Estimation of Evaporation Losses from Different Water Bodies
4. Discussion
4.1. Isotope Analysis of Water Bodies Reveals Evaporation and Water Source Relationships
4.2. Factors Influencing Variations in δD and δ18O Values
4.3. Influence of Hydrological Connectivity on Water Stable Isotopes in Alpine Wetlands
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, Y.; Beamish, R. Isotopic composition of otoliths as a chemical tracer in population identification of sockeye salmon (Oncorhynchus nerka). Can. J. Fish. Aquat. Sci. 1999, 56, 2062–2068. [Google Scholar] [CrossRef]
- Yin, Y.; Xia, R.; Liu, X.; Chen, Y.; Song, J.; Dou, J. Spatial response of water level and quality shows more significant heterogeneity during dry seasons in large river-connected lakes. Sci. Rep. 2024, 14, 8373. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.J.; Holmes, T.; Stadnyk, T.A.; Birks, S.J.; Eby, P.; Pietroniro, A. Isotopic constraints on water balance and evapotranspiration partitioning in gauged watersheds across Canada. J. Hydrol. Reg. Stud. 2021, 37, 100878. [Google Scholar] [CrossRef]
- Li, C.; Lian, E.; Yang, C.; Deng, K.; Qian, P.; Xiao, S.; Liu, Z.; Yang, S. Seasonal variability of stable isotopes in the Changjiang (Yangtze) river water and its implications for natural climate and anthropogenic impacts. Environ. Sci. Eur. 2020, 32, 84. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Gao, Y.; Li, J.; Yang, Y.; Wang, P. Allocation, morphology, physiology: Multiple aspects of above-and below-ground responses to water table stress, duration of drainage in alpine wetland plants (Carex muliensis). Plant Soil 2024, 505, 703–718. [Google Scholar] [CrossRef]
- Zeng, W.; He, Z.; Bai, W.; He, L.; Chen, X.; Chen, J. Identification of ecological security patterns of alpine wetland grasslands based on landscape ecological risks: A study in Zoigê County. Sci. Total Environ. 2024, 928, 172302. [Google Scholar] [CrossRef]
- Yu, D.-W.; Liu, Y.; Xie, D.-J.; Mu, C.-L.; Sun, Z.-Y.; Zhou, M.-J.; Rao, J.-P.; Suolang, D.; Xiong, Y.-Q.; Chen, J.-S. Effects of driving factors on water supply function under different basins and spatial scale in Zoige alpine wetland, China. Ecol. Indic. 2024, 158, 111403. [Google Scholar] [CrossRef]
- Carlson, B.Z.; Hébert, M.; Van Reeth, C.; Bison, M.; Laigle, I.; Delestrade, A. Monitoring the Seasonal hydrology of alpine wetlands in response to snow cover dynamics and summer climate: A novel approach with sentinel-2. Remote Sens. 2020, 12, 1959. [Google Scholar] [CrossRef]
- Balwan, W.K.; Kour, S. Wetland-An Ecological Boon for the Environment. East Afr. Sch. J. Agric. Life Sci. 2021, 4, 38–48. [Google Scholar] [CrossRef]
- Kang, X.; Li, Y.; Wang, J.; Yan, L.; Zhang, X.; Wu, H.; Yan, Z.; Zhang, K.; Hao, Y. Precipitation and temperature regulate the carbon allocation process in alpine wetlands: Quantitative simulation. J. Soils Sediments 2020, 20, 3300–3315. [Google Scholar] [CrossRef]
- Yang, A.; Kang, X.; Li, Y.; Zhang, X.; Zhang, K.; Kang, E.; Yan, Z.; Li, M.; Wang, X.; Niu, Y. Alpine wetland degradation reduces carbon sequestration in the Zoige Plateau, China. Front. Ecol. Evol. 2022, 10, 980441. [Google Scholar] [CrossRef]
- Fan, S.; Qin, J.; Sun, H.; Jia, Z.; Chen, Y. Alpine soil microbial community structure and diversity are largely influenced by moisture content in the Zoige wetland. Int. J. Environ. Sci. Technol. 2022, 19, 4369–4378. [Google Scholar] [CrossRef]
- Shi, F.; Rao, Z.; Cao, J.; Huang, C.; Wu, D.; Yang, W.; Sun, W. Meltwater is the dominant water source controlling α-cellulose δ18O in a vascular-plant-dominated alpine peatland in the Altai Mountains, Central Asia. J. Hydrol. 2019, 572, 192–205. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, W.; Zhang, S.; Sun, Z.; Li, Y.; Huang, Y.; Wang, W.; Qi, P.; Zou, Y.; Jiang, M. The role of snowmelt discharge to runoff of an alpine watershed: Evidence from water stable isotopes. J. Hydrol. 2022, 604, 127209. [Google Scholar] [CrossRef]
- Chazette, P.; Flamant, C.; Sodemann, H.; Totems, J.; Monod, A.; Dieudonné, E.; Baron, A.; Seidl, A.; Steen-Larsen, H.C.; Doira, P. Experimental investigation of the stable water isotope distribution in an Alpine lake environment (L-WAIVE). Atmos. Chem. Phys. 2021, 21, 10911–10937. [Google Scholar] [CrossRef]
- Dogramaci, S.; Skrzypek, G.; Dodson, W.; Grierson, P.F. Stable isotope and hydrochemical evolution of groundwater in the semi-arid Hamersley Basin of subtropical northwest Australia. J. Hydrol. 2012, 475, 281–293. [Google Scholar] [CrossRef]
- Cao, S.; Cao, G.; Wang, Z.; Hou, Y.; Wang, Y.; Kang, L. Isotopic hydrological links among precipitation, river water and groundwater in an alpine mountain basin, NE Qinghai–Tibet Plateau in warm seasons. Environ. Earth Sci. 2022, 81, 366. [Google Scholar] [CrossRef]
- Yang, K.; Han, G. Controls over hydrogen and oxygen isotopes of surface water and groundwater in the Mun River catchment, northeast Thailand: Implications for the water cycle. Hydrogeol. J. 2020, 28, 1021–1036. [Google Scholar] [CrossRef]
- Hou, M.; Ge, J.; Gao, J.; Meng, B.; Li, Y.; Yin, J.; Liu, J.; Feng, Q.; Liang, T. Ecological risk assessment and impact factor analysis of alpine wetland ecosystem based on LUCC and boosted regression tree on the Zoige Plateau, China. Remote Sens. 2020, 12, 368. [Google Scholar] [CrossRef]
- Coplen, T.B. New guidelines for reporting stable hydrogen, carbon, and oxygen isotope-ratio data. Geochim. Cosmochim. Acta 1996, 60, 3359–3360. [Google Scholar] [CrossRef]
- Dansgaard, W. Stable isotopes in precipitation. Tellus 1964, 16, 436–468. [Google Scholar] [CrossRef]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Majoube, M. Fractionnement en oxygène 18 et en deutérium entre l’eau et sa vapeur. J. Chim. Phys. 1971, 68, 1423–1436. [Google Scholar] [CrossRef]
- Qiao, Y.; Meng, Z.; Zhang, C.; Cheng, Y.; Shi, L.; Xu, A.; Zhang, Q.; Li, Y. Characteristics of mine aquifer with complex structure and identification of its hydraulic connection. J. China Coal Soc. 2021, 46, 4010–4020. [Google Scholar]
- Wu, Z.S.R.; Zhang, W. Hydrochemistry and Isotopic Characteristics of Various Types of Water Bodies in Zoige Wetland and their Water Recharge Relationship. Wetl. Sci. 2024, 22, 418–427. [Google Scholar]
- Zheng, S.H.; Hou, F.G.; Ni, B.L. The studies of hydrogen and oxygen stable isotopes in atmospheric precipitation in China. Chin. Sci. Bull. 1983, 13, 801–806. [Google Scholar]
- George Darling, W.; Bath, A.H.; Gibson, J.J.; Rozanski, K. Isotopes in Water; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Xia, Z.; Winnick, M.J. The competing effects of terrestrial evapotranspiration and raindrop re-evaporation on the deuterium excess of continental precipitation. Earth Planet. Sci. Lett. 2021, 572, 117120. [Google Scholar] [CrossRef]
- Mao, H.; Wang, G.; Shi, Z.; Liao, F.; Xue, Y. Spatiotemporal variation of groundwater recharge in the lower reaches of the Poyang Lake Basin, China: Insights from stable hydrogen and oxygen isotopes. J. Geophys. Res. Atmos. 2021, 126, e2020JD033760. [Google Scholar] [CrossRef]
- Wang, R.; Gentine, P.; Yin, J.; Chen, L.; Chen, J.; Li, L. Long-term relative decline in evapotranspiration with increasing runoff on fractional land surfaces. Hydrol. Earth Syst. Sci. 2021, 25, 3805–3818. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, W.; Li, Y.; Zhang, J.; Xu, Z. Multiple Isotopes Reveal a Hydrology Dominated Control on the Nitrogen Cycling in the Nujiang River Basin, the Last Undammed Large River Basin on the Tibetan Plateau. Environ. Sci. Technol. 2022, 56, 4610–4619. [Google Scholar] [CrossRef]
- Vystavna, Y.; Harjung, A.; Monteiro, L.R.; Matiatos, I.; Wassenaar, L.I. Stable isotopes in global lakes integrate catchment and climatic controls on evaporation. Nat. Commun. 2021, 12, 7224. [Google Scholar] [CrossRef] [PubMed]
- Xin, P.; Wilson, A.; Shen, C.; Ge, Z.; Moffett, K.B.; Santos, I.R.; Chen, X.; Xu, X.; Yau, Y.Y.Y.; Moore, W.; et al. Surface Water and Groundwater Interactions in Salt Marshes and Their Impact on Plant Ecology and Coastal Biogeochemistry. Rev. Geophys. 2022, 60, e2021RG000740. [Google Scholar] [CrossRef]
- Zhang, L.; Lan, S.; Zhao, L.; Yi, H.; Han, G. Temporal variation of water-use efficiency and water sources of Phragmites australis in the coastal wetland: Based on stable isotopic composition (δ13C and δ18O). Ecol. Indic. 2022, 139, 108957. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, T.; Mallants, D.; Tian, H.; Owen, D.D. Numerical modelling of stable isotope (2H and 18O) transport in a hydro-geothermal system: Model development and implementation to the Guide Basin, China. J. Hydrol. 2019, 569, 93–105. [Google Scholar] [CrossRef]
- Sun, Z.; Zhu, G.; Zhang, Z.; Xu, Y.; Yong, L.; Wan, Q.; Ma, H.; Sang, L.; Liu, Y. Identifying surface water evaporation loss of inland river basin based on evaporation enrichment model. Hydrol. Process. 2021, 35, e14093. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, C.; Li, X.-Y.; Fu, C.; Wu, H.; Wang, P.; Liu, J. Hydrometeorological Processes and Moisture Sources in the Northeastern Tibetan Plateau: Insights from a 7-Yr Study on Precipitation Isotopes. J. Clim. 2022, 35, 2919–2931. [Google Scholar] [CrossRef]
- Wei, H.; Wang, J.; Li, M.; Wen, M.; Lu, Y. Assessing the Applicability of Mainstream Global Isoscapes for Predicting δ18O, δ2H, and d-excess in Precipitation across China. Water 2023, 15, 3181. [Google Scholar] [CrossRef]
- Guo, X.; Gong, X.; Shi, J.; Guo, J.; Dominguez-Villar, D.; Lin, Y.; Wang, H.; Yuan, D. Temporal variations and evaporation control effect of the stable isotope composition of precipitation in the subtropical monsoon climate region, Southwest China. J. Hydrol. 2021, 599, 126278. [Google Scholar] [CrossRef]
- Hathaway, J.M.; Westbrook, C.J.; Rooney, R.C.; Petrone, R.M.; Langs, L.E. Quantifying relative contributions of source waters from a subalpine wetland to downstream water bodies. Hydrol. Process. 2022, 36, e14679. [Google Scholar] [CrossRef]
- Kohfahl, C.; Rodriguez, R.F.; Bermudo, F.R.; Vadillo, I. Vapour source and spatiotemporal variation of precipitation isotopes in Southwest Spain. Hydrol. Process. 2021, 35, e14445. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, M.; Xie, X.; Lv, J.; Zhou, X.; Shen, N. Effect of wetland hydrological connectivity on runoff based on a basin comparison. J. Hydrol. 2023, 619, 129348. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, T.; Zhao, L.; Yang, C.; Zhang, H.; Xie, C.; Li, R.; Wang, W.; Hu, G.; Ni, J.; et al. Exploring the contribution of precipitation to water within the active layer during the thawing period in the permafrost regions of central Qinghai-Tibet Plateau by stable isotopic tracing. Sci. Total Environ. 2019, 661, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Çolak, M.A.; Öztaş, B.; Özgencil, İ.K.; Soyluer, M.; Korkmaz, M.; Ramírez-García, A.; Metin, M.; Yılmaz, G.; Ertuğrul, S.; Tavşanoğlu, Ü.N. Increased water abstraction and climate change have substantial effect on morphometry, salinity, and biotic communities in lakes: Examples from the semi-arid burdur Basin (Turkey). Water 2022, 14, 1241. [Google Scholar] [CrossRef]
- Herrera, C.; Urrutia, J.; Gamboa, C.; Salgado, X.; Godfrey, L.; Rivas, A.; Jódar, J.; Custodio, E.; León, C.; Sigl, V. Evaluation of the impact of the intensive exploitation of groundwater and the mega-drought based on the hydrochemical and isotopic composition of the waters of the Chacabuco-Polpaico basin in central Chile. Sci. Total Environ. 2023, 895, 165055. [Google Scholar] [CrossRef]
- Warter, M.M.; Tetzlaff, D.; Ring, A.-M.; Christopher, J.; Kissener, H.L.; Funke, E.; Sparmann, S.; Mbedi, S.; Soulsby, C.; Monaghan, M.T. Environmental DNA, hydrochemistry and stable water isotopes as integrative tracers of urban ecohydrology. Water Res. 2024, 250, 121065. [Google Scholar] [CrossRef]
- Ala-aho, P.; Soulsby, C.; Pokrovsky, O.S.; Kirpotin, S.N.; Karlsson, J.; Serikova, S.; Vorobyev, S.N.; Manasypov, R.M.; Loiko, S.; Tetzlaff, D. Using stable isotopes to assess surface water source dynamics and hydrological connectivity in a high-latitude wetland and permafrost influenced landscape. J. Hydrol. 2018, 556, 279–293. [Google Scholar] [CrossRef]
- Lu, C.; Wu, C.; Sun, Q.; Wu, X.; Yan, L.; Qin, T. Seasonal river–lake-groundwater coupling simulation and groundwater overexploitation and ecological environment assessment in the Aiding Lake Basin, NW China. J. Hydrol. 2024, 632, 130896. [Google Scholar] [CrossRef]
- Wu, H.; Huang, Q.; Fu, C.; Song, F.; Liu, J.; Li, J. Stable isotope signatures of river and lake water from Poyang Lake, China: Implications for river–lake interactions. J. Hydrol. 2021, 592, 125619. [Google Scholar] [CrossRef]
- Arnold, T.E.; Hillman, A.L.; Abbott, M.B.; Werne, J.P.; McGrath, S.J.; Arkush, E.N. Drought and the collapse of the Tiwanaku Civilization: New evidence from Lake Orurillo, Peru. Quat. Sci. Rev. 2021, 251, 106693. [Google Scholar] [CrossRef]
- Halder, J.; Terzer, S.; Wassenaar, L.; Araguás-Araguás, L.; Aggarwal, P. The Global Network of Isotopes in Rivers (GNIR): Integration of water isotopes in watershed observation and riverine research. Hydrol. Earth Syst. Sci. 2015, 19, 3419–3431. [Google Scholar] [CrossRef]
- Li, X.; Weng, B.; Yan, D.; Qin, T.; Wang, K.; Bi, W.; Yu, Z.; Dorjsuren, B. Anthropogenic effects on hydrogen and oxygen isotopes of river water in cities. Int. J. Environ. Res. Public Health 2019, 16, 4429. [Google Scholar] [CrossRef]

| Water Bodies | δD (‰) | δ18O (‰) | δD/δ18O | d-Excess | |
|---|---|---|---|---|---|
| Marshes | Min | −118.4 | −15.47 | 7.05 | −20.78 |
| Max | −69.23 | −7.07 | 10.86 | 12.88 | |
| Mean | −89.58 a | −10.61 a | 8.56 b | −4.63 | |
| STD | 12.45 | 2.16 | 0.76 | 6.64 | |
| Rivers | Min | −99.27 | −12.86 | 7.61 | −34.16 |
| Max | −37.03 | −2.02 | 20.91 | 3.68 | |
| Mean | −72.89 b | −7.89 b | 10.14 a | −9.75 | |
| STD | 14.83 | 2.71 | 3.19 | 10 | |
| Lakes | Min | −88.66 | −12.9 | 6.77 | −22.34 |
| Max | −40.14 | −2.22 | 18.04 | 15.78 | |
| Mean | −72.31 b | −8.98 b | 8.75 b | −0.44 | |
| STD | 15.07 | 2.94 | 2.57 | 9.79 | |
| Waters | Marshes | Rivers | Lakes |
|---|---|---|---|
| Average proportion of water remaining (%) | 64 | 60 | 59 |
| Maximum evaporated proportion (%) | 59 | 91 | 91 |
| Minimum evaporated proportion (%) | 4 | −3 | 12 |
| Average evaporated proportion (%) | 36 | 40 | 41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, Y.; Li, C.; Ning, Y.; Rong, G.; Zhou, Y.; Liu, K.; Li, J.; Wang, H. Stable Water Isotopes Across Marsh, River, and Lake Environments in the Zoige Alpine Wetland on the Tibetan Plateau. Water 2025, 17, 820. https://doi.org/10.3390/w17060820
Zhan Y, Li C, Ning Y, Rong G, Zhou Y, Liu K, Li J, Wang H. Stable Water Isotopes Across Marsh, River, and Lake Environments in the Zoige Alpine Wetland on the Tibetan Plateau. Water. 2025; 17(6):820. https://doi.org/10.3390/w17060820
Chicago/Turabian StyleZhan, Yangying, Chunyi Li, Yu Ning, Guichun Rong, You Zhou, Kexin Liu, Junxuan Li, and Haoyang Wang. 2025. "Stable Water Isotopes Across Marsh, River, and Lake Environments in the Zoige Alpine Wetland on the Tibetan Plateau" Water 17, no. 6: 820. https://doi.org/10.3390/w17060820
APA StyleZhan, Y., Li, C., Ning, Y., Rong, G., Zhou, Y., Liu, K., Li, J., & Wang, H. (2025). Stable Water Isotopes Across Marsh, River, and Lake Environments in the Zoige Alpine Wetland on the Tibetan Plateau. Water, 17(6), 820. https://doi.org/10.3390/w17060820





