Advances in Hydrothermal Carbonization for Biomass Wastewater Valorization: Optimizing Nitrogen and Phosphorus Nutrient Management to Enhance Agricultural and Ecological Outcomes
Abstract
1. Introduction
2. Hydrothermal Carbonization Technology
2.1. The Basic Principle of Hydrothermal Carbonization
2.1.1. Physical Chemistry Process
2.1.2. Influencing Factors
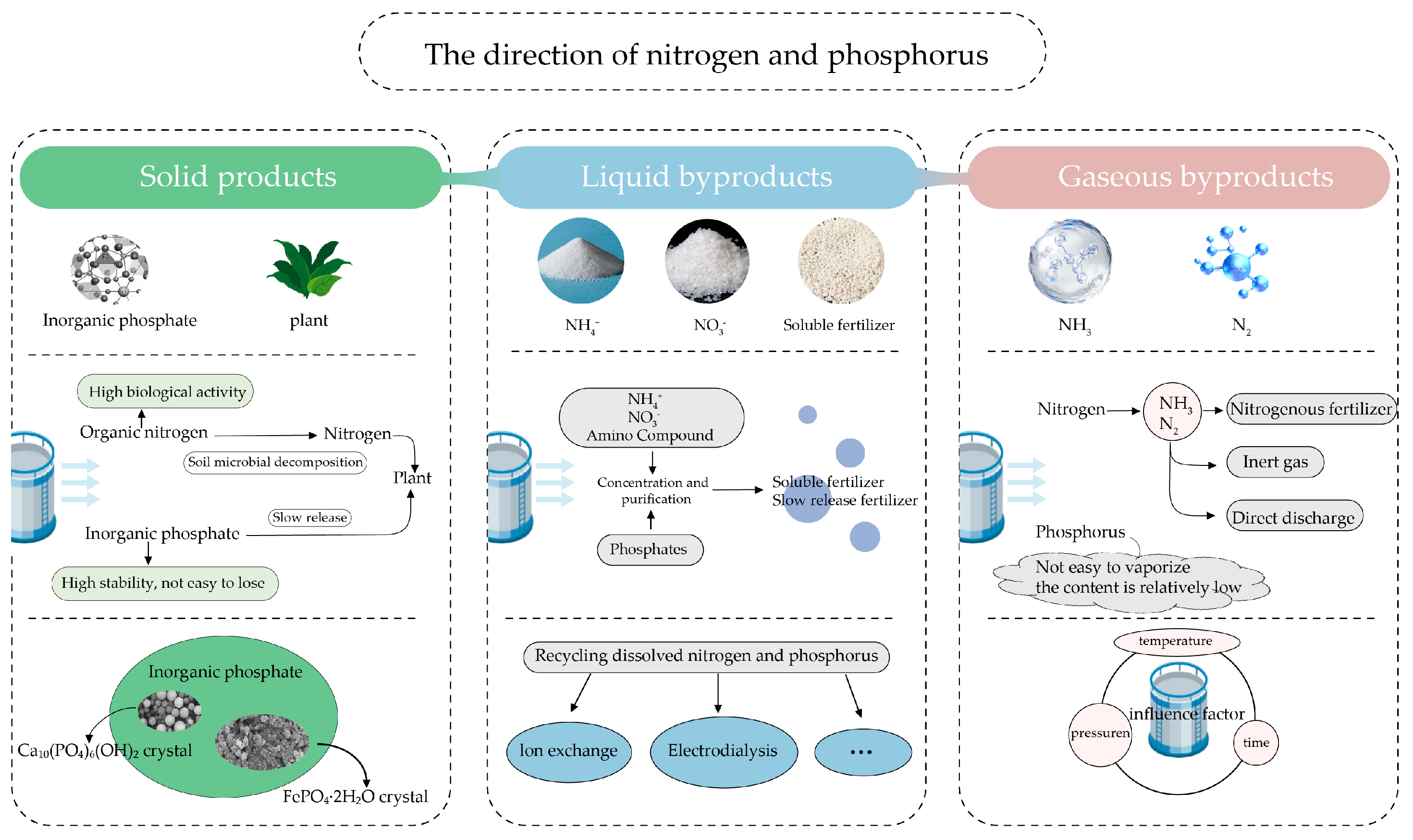
2.1.3. Products
2.2. Application Status of Hydrothermal Carbonization Technology
2.2.1. Application to Different Types of Biomass Wastewater
2.2.2. Comparison of the Hydrothermal Carbonization Effects of Different Types of Biomass Wastewater
3. Nitrogen–Phosphorus Nutrient Management
3.1. The Roles and Challenges Associated with Nitrogen and Phosphorus Nutrients in Agriculture
3.1.1. Environmental Problems Due to the Overuse of Nitrogen–Phosphorus Nutrients
3.1.2. Resource Utilization Potential of Nitrogen and Phosphorus Nutrients in Biomass Wastewater
3.2. Nitrogen and Phosphorus Migration and Transformation During the Hydrothermal Carbonization Process
3.2.1. Mechanism of Hydrothermal Carbonization Regarding Nitrogen and Phosphorus
3.2.2. Fate of Nitrogen and Phosphorus Under Different Conditions
3.3. Management of Nitrogen and Phosphorus Nutrients in Hydrothermal Carbonization Products
3.3.1. Nitrogen and Phosphorus Content and Their Fertilizer Efficiency in Hydrochar
3.3.2. Utilization of Nitrogen and Phosphorus in Liquid Byproducts
3.3.3. Nitrogen and Phosphorus Recovery Technologies
4. Applications Within the Circular Economic Framework
4.1. The Concept of the Circular Economy and Its Application in Agriculture
4.2. The Role of Hydrothermal Carbonization Technology in the Circular Economy
4.3. Nitrogen and Phosphorus Cycling and Their Agro-Environmental Benefits
5. Analysis of Agro-Environmental Benefits
5.1. Agricultural Benefits of Nitrogen and Phosphorus Management
5.2. Contributions of Hydrothermal Carbonization Technology to Carbon Mitigation
6. Conclusions and Agro-Environmental Implications
- The application of HTC changes conventional biomass wastewater management by converting residues such as straw, rice husks, and fruit shells into hydrochar, aqueous products, and gaseous products with high added value. This approach reduces the environmental pollution from waste accumulation and burning, provides organic fertilizers and renewable energy, and promotes cyclical utilization and sustainable development in agriculture, thereby contributing to a more stable and resilient agricultural production system.
- As a soil amendment, hydrochar enhances soil water and nutrient retention, improves the soil structure, and increases the soil organic matter content, creating favorable conditions for crop growth. Moreover, its long-term stability in soil supports carbon sequestration, reduces the atmospheric CO2 concentrations, lowers greenhouse gas emissions, and mitigates climate change.
- By reducing the use of chemical fertilizers and pesticides, HTC technology enhances the ecological adaptability and market competitiveness of agriculture, laying a solid foundation for its long-term healthy development.
- HTC technology offers new economic growth opportunities for rural areas. By developing high-value-added carbonized products, it diversifies rural economies, increases farmers’ income streams, and improves the living standards of rural populations.
- Integrating HTC technology with modern agricultural production techniques facilitates the advancement of agricultural modernization, elevates the overall technological level of agriculture, and establishes a solid foundation for long-term agricultural development.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| co-HTC | co-hydrothermal carbonization |
| DOC | dissolved organic carbon |
| GWP | global warming potential |
| HHV | higher heating value |
| HMEs | heavy metal elements |
| HTC | hydrothermal carbonization |
| MAP | magnesium ammonium phosphate |
| MB | methylene blue |
| MBR | membrane bioreactor |
| N | nitrogen |
| NOR | norfloxacin |
| OP | organic-P |
| P | phosphorus |
| PDS | peroxydisulfate |
| PMS | peroxymonosulfate |
| QC | quinoline acid |
| RO | reverse osmosis |
| RS | Phragmites australis |
| SOC | soil organic carbon |
| SS HTL | sewage sludge hydrothermal liquefaction |
| SY | sunset yellow |
| TKN | total Kjeldahl nitrogen |
| TOC | total organic carbon |
References
- Chu, H.Q.; Yang, C.H.; Zhang, Z.K.; Liu, Z.L.; Rui, Z.C.; Xu, N. Advances in resource utilization of waste in phase change materials. J. Energy Storage 2024, 99, 113342. [Google Scholar] [CrossRef]
- Paul, S.; Mazumder, C.; Mukherjee, S. Challenges faced in commercialization of biofuel from biomass energy resources. Biocatal. Agric. Biotechnol. 2024, 60, 103312. [Google Scholar] [CrossRef]
- Zoppi, M.; Falasco, E.; Schoefs, B.; Bona, F. Turning waste into resources: A comprehensive review on the valorisation of Elodea nuttallii biomass. J. Environ. Manag. 2024, 369, 122258. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Zhang, T.; Tsang, D.C.W.; Li, G.X. Effects of external additives Biochar, bentonite, phosphate, on co-composting for swine manure and corn straw. Chemosphere 2020, 248, 125927. [Google Scholar] [CrossRef]
- Li, H.H.; Zhang, T.; Shaheen, S.M.; Abdelrahman, H.; Ali, E.F.; Bolan, N.S.; Li, G.X.; Rinklebe, J. Microbial inoculants and struvite improved organic matter humification and stabilized phosphorus during swine manure composting: Multivariate and multiscale investigations. Bioresour. Technol. 2022, 351, 126976. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, X.S.; Shaheen, S.M.; Rinklebe, J.; Bolan, N.S.; Ali, E.F.; Li, G.X.; Tsang, D.C.W. Effects of microorganism-mediated inoculants on humification processes and phosphorus dynamics during the aerobic composting of swine manure. J. Hazard. Mater. 2021, 416, 125738. [Google Scholar] [CrossRef]
- Emmanuel, O.; Rozina; Ezeji, T.C. Utilization of biomass-based resources for biofuel production: A mitigating approach towards zero emission. Sustain. Chem. One World 2024, 2, 100007. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, T.; Niu, Y.Q.; Mukherjee, S.; Abou-Elwafa, S.F.; Nguyen, N.S.H.; Al Aboud, N.M.; Wang, Y.K.; Pu, M.J.; Zhang, Y.; et al. A comprehensive review on agricultural waste utilization through sustainable conversion techniques, with a focus on the additives effect on the fate of phosphorus and toxic elements during composting process. Sci. Total Environ. 2024, 942, 173567. [Google Scholar] [CrossRef]
- Rasaq, W.A.; Matyjewicz, B.; Świechowski, K.; Lazar, Z.; Kupaj, P.; Janek, T.; Valentin, M.; Białowiec, A. Food waste recycling to Yarrowia biomass due to combined hydrothermal carbonization and biological treatment. J. Clean. Prod. 2024, 456, 142385. [Google Scholar] [CrossRef]
- Azman, N.F.; Katahira, T.; Nakanishi, Y.; Chisyaki, N.; Uemura, S.; Yamada, M.; Takayama, K.; Oshima, I.; Yamaguchi, T.; Hara, H.; et al. Sustainable oil palm biomass waste utilization in Southeast Asia: Cascade recycling for mushroom growing, animal feedstock production, and composting animal excrement as fertilizer. Clean. Circ. Bioeconomy 2023, 6, 100058. [Google Scholar] [CrossRef]
- Bai, L.L.; Shi, P.; Li, Z.B.; Li, P.; Zhao, Z.; Dong, J.B.; Cui, L.Z. Effects of vegetation patterns on soil nitrogen and phosphorus losses on the slope-gully system of the Loess Plateau. J. Environ. Manag. 2022, 324, 116288. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Tao, F.L.; Chen, Y. Effects of climate change, crop planting structure, and agricultural management on runoff, sediment, nitrogen and phosphorus losses in the Hai-River Basin since the 1980s. J. Clean. Prod. 2022, 359, 132066. [Google Scholar] [CrossRef]
- Kyllmar, K.; Bechmann, M.; Blicher-Mathiesen, G.; Fischer, F.K.; Fölster, J.; Iital, A.; Lagzdiņš, A.; Povilaitis, A.; Rankinen, K. Nitrogen and phosphorus losses in Nordic and Baltic agricultural monitoring catchments—Spatial and temporal variations in relation to natural conditions and mitigation programmes. Catena 2023, 230, 107205. [Google Scholar] [CrossRef]
- Morais, T.G.; Teixeira, R.F.M.; Lauk, C.; Theurl, M.C.; Winiwarter, W.; Mayer, A.; Kaufmann, L.; Haberl, H.; Domingos, T.; Erb, K.H. Agroecological measures and circular economy strategies to ensure sufficient nitrogen for sustainable farming. Glob. Environ. Change 2021, 69, 102313. [Google Scholar] [CrossRef]
- Moursi, H.; Youssef, M.A.; Poole, C.A.; Castro-Bolinaga, C.F.; Chescheir, G.M.; Richardson, R.J. Drainage water recycling reduced nitrogen, phosphorus, and sediment losses from a drained agricultural field in eastern North Carolina, USA. Agric. Water Manag. 2023, 279, 108179. [Google Scholar] [CrossRef]
- Sieczko, A.K.; van de Vlasakker, P.C.H.; Tonderski, K.; Metson, G.S. Seasonal nitrogen and phosphorus leaching in urban agriculture: Dominance of non-growing season losses in a Southern Swedish case study. Urban For. Urban Green. 2023, 79, 127823. [Google Scholar] [CrossRef]
- Song, K.; Qin, Q.; Yang, Y.F.; Sun, L.J.; Sun, Y.F.; Zheng, X.Q.; Lu, W.G.; Xue, Y. Drip fertigation and plant hedgerows significantly reduce nitrogen and phosphorus losses and maintain high fruit yields in intensive orchards. J. Integr. Agric. 2023, 22, 598–610. [Google Scholar] [CrossRef]
- Zou, T.T.; Meng, F.L.; Zhou, J.C.; Ying, H.; Liu, X.J.; Hou, Y.; Zhao, Z.X.; Zhang, F.S.; Xu, W. Quantifying nitrogen and phosphorus losses from crop and livestock production and mitigation potentials in Erhai Lake Basin, China. Agric. Syst. 2023, 211, 103745. [Google Scholar] [CrossRef]
- Crittenden, S.; Clayton, G.; Boyce, M.; Deng, X.M.; Grant, C. Canola variety, nitrogen, phosphorus, and sulfur fertilization affect yield, quality, and fatty acid profile. Can. J. Plant Sci. 2023, 104, 1–12. [Google Scholar] [CrossRef]
- Shi, Z.H.; Liang, G.L.; Liu, W.H.; Li, S.D.; Qin, Y. Optimization of nitrogen and phosphorus fertilization for enhanced forage production and quality of Festuca Krylovianacv. Huanhu artificial grassland in alpine regions. Heliyon 2024, 10, e35116. [Google Scholar] [CrossRef]
- Wang, N.; Ai, Z.P.; Zhang, Q.Y.; Leng, P.F.; Qiao, Y.F.; Li, Z.; Tian, C.; Cheng, H.F.; Chen, G.; Li, F.D. Impacts of nitrogen (N), phosphorus (P), and potassium (K) fertilizers on maize yields, nutrient use efficiency, and soil nutrient balance: Insights from a long-term diverse NPK omission experiment in the North China Plain. Field Crops Res. 2024, 318, 109616. [Google Scholar] [CrossRef]
- Yang, L.; Wang, R.Z.; Shi, J.W.; Wang, R.; Guo, S.L. Nitrogen fertilization management is required for soil phosphorus mobilization by phoD community assembly and pqqC keystone taxa. Pedosphere 2024. [Google Scholar] [CrossRef]
- Liu, B.H.; Zhang, Y.L.; Yi, X.Y.; Zheng, H.T.; Ni, K.; Ma, Q.X.; Cai, Y.J.; Ma, L.F.; Shi, Y.Z.; Yang, X.D.; et al. Partially replacing chemical fertilizer with manure improves soil quality and ecosystem multifunctionality in a tea plantation. Agric. Ecosyst. Environ. 2025, 378, 109284. [Google Scholar] [CrossRef]
- Pourhosseini, S.H.; Azizi, A.; Sadat Seyedi, F.; Hadian, J. Bio-fertilizer as a pathway to minimize nitrate leaching from chemical fertilizer in high yield peppermint production. J. Clean. Prod. 2024, 468, 143100. [Google Scholar] [CrossRef]
- Raza, A.; Chen, C.Q.; Luo, L.; Asghar, M.A.; Li, L.; Shoaib, N.; Yin, C.Y. Combined application of organic and chemical fertilizers improved the catechins and flavonoids biosynthesis involved in tea quality. Sci. Hortic. 2024, 337, 113518. [Google Scholar] [CrossRef]
- Wang, H.T.; Liang, X.Y.; Qiu, X.F.; Yao, Z.L.; Wang, J.D. Anaerobic digester liquor replacing chemical fertilizer in reducing greenhouse gas emissions under drip irrigation: Factors, pathways, and strategies. Chem. Eng. J. 2024, 494, 153233. [Google Scholar] [CrossRef]
- Raza, S.; Miao, N.; Wang, P.Z.; Ju, X.T.; Chen, Z.J.; Zhou, J.B.; Kuzyakov, Y. Dramatic loss of inorganic carbon by nitrogen-induced soil acidification in Chinese croplands. Glob. Change Biol. 2020, 26, 3738–3751. [Google Scholar] [CrossRef]
- Morugan-Coronado, A.; Perez-Rodriguez, P.; Insolia, E.; Soto-Gomez, D.; Fernandez-Calvino, D.; Zornoza, R. The impact of crop diversification, tillage and fertilization type on soil total microbial, fungal and bacterial abundance: A worldwide meta-analysis of agricultural sites. Agric. Ecosyst. Environ. 2022, 329, 107867. [Google Scholar] [CrossRef]
- Gupta, D.; Garg, A.; Mahajani, S. Investigation on hydrochar and macromolecules recovery opportunities from food waste after hydrothermal carbonization. Sci. Total Environ. 2020, 749, 142294. [Google Scholar] [CrossRef]
- Khanzada, A.K.; Al-Hazmi, H.E.; Kurniawan, T.A.; Majtacz, J.; Piechota, G.; Kumar, G.; Ezzati, P.; Saeb, M.R.; Rabiee, N.; Karimi-Maleh, H.; et al. Hydrochar as a bio-based adsorbent for heavy metals removal: A review of production processes, adsorption mechanisms, kinetic models, regeneration and reusability. Sci. Total Environ. 2024, 945, 173972. [Google Scholar] [CrossRef]
- Wang, T.F.; Zhai, Y.B.; Zhu, Y.; Li, C.T.; Zeng, G.M. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sust. Energ. Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Deng, Y.X.; Zhang, T.; Clark, J.; Aminabhavi, T.; Kruse, A.; Tsang, D.C.W.; Sharma, B.K.; Zhang, F.S.; Ren, H.Q. Mechanisms and modelling of phosphorus solid–liquid transformation during the hydrothermal processing of swine manure. Green Chem. 2020, 22, 5628–5638. [Google Scholar] [CrossRef]
- He, X.Y.; Zhang, T.; Xue, Q.; Zhou, Y.L.; Wang, H.L.; Bolan, N.S.; Jiang, R.F.; Tsang, D.C.W. Enhanced adsorption of Cu (II) and Zn (II) from aqueous solution by polyethyleneimine modified straw hydrochar. Sci. Total Environ. 2021, 778, 146116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Zhang, T. Biowaste valorization to produce advance carbon materials-hydrochar for potential application of Cr (VI) and Cd (II) adsorption in wastewater: A review. Water 2022, 14, 3675. [Google Scholar] [CrossRef]
- Liu, G.Q.; Xu, Q.; Abou-Elwafa, S.F.; Ali Alshehri, M.; Zhang, T. Hydrothermal carbonization technology for wastewater treatment under the “Dual Carbon” goals current status, trends, and challenges. Water 2024, 16, 1749. [Google Scholar] [CrossRef]
- Chen, Y.X.; Zhu, H.S.; Gao, F.; Xiong, H.R.; Yang, H.; Xu, Z.X.; Duan, P.G.; Zheng, L.J.; Osman, S.M.; Luque, R. Green wood bio-adhesives from cellulose-derived bamboo powder hydrochars. Chem. Eng. J. 2024, 498, 155667. [Google Scholar] [CrossRef]
- Karim, A.A.; Martínez-Cartas, M.L.; Cuevas-Aranda, M. Production of hydrochar fuel by microwave-hydrothermal carbonisation of olive pomace slurry from olive oil industry for combustion application. J. Anal. Appl. Pyrolysis 2024, 183, 106801. [Google Scholar] [CrossRef]
- Liu, X.G.; Peng, L.; Deng, P.Y.; Xu, Y.M.; Wang, P.S.; Tan, Q.T.; Zhang, C.Q.; Dai, X.H. Co-hydrothermal carbonization of sewage sludge and rice straw to improve hydrochar quality: Effects of mixing ratio and hydrothermal temperature. Bioresour. Technol. 2024, 415, 131665. [Google Scholar] [CrossRef]
- Ren, S.J.; Tong, Z.Y.; Yong, X.Y.; Xi, Y.L.; Liu, F.W.; Zhou, J. The new strategies of using nitrogen and iron modified hydrochar to enhance methane production during co-anaerobic digestion of cow manure and corn straw. J. Environ. Chem. Eng. 2024, 12, 114127. [Google Scholar] [CrossRef]
- Saha, S.; Pezzenti, S.; Reza, T. Functionalization of pyrolyzed hydrochar with nitrogen containing deep eutectic solvent for carbon capture at low and high pressure. J. Anal. Appl. Pyrolysis 2024, 183, 106765. [Google Scholar] [CrossRef]
- Siddhartha, T.R.; Kooy, E.; Kashif, M.; Che, C.A.; Ghysels, S.; Wu, D.; Ronsse, F.; Heynderickx, P.M. Evaluation of South Korean marine waste resources for hydrochar production: Effect of process variables. Bioresour. Technol. 2024, 410, 131286. [Google Scholar] [CrossRef] [PubMed]
- Tafete, G.A.; Uysal, A.; Habtu, N.G.; Abera, M.K.; Yemata, T.A.; Duba, K.S.; Kinayyigit, S. Hydrothermally synthesized nitrogen-doped hydrochar from sawdust biomass for supercapacitor electrodes. Int. J. Electrochem. Sci. 2024, 19, 100827. [Google Scholar] [CrossRef]
- Tian, X.C.; Sun, A.; Wang, C.Y.; Ding, K.L. Removal of quinoline in aqueous solutions using chemically modified and unmodified hydrochars from lotus seedpods: A comprehensive experimental study. Colloid Surf. A-Physicochem. Eng. Asp. 2024, 702, 135166. [Google Scholar] [CrossRef]
- Wang, K.C.; Xu, J.Z.; Guo, H.; Min, Z.H.; Wei, Q.; Chen, P.; Sleutel, S. Reuse of straw in the form of hydrochar: Balancing the carbon budget and rice production under different irrigation management. Waste Manag. 2024, 189, 77–87. [Google Scholar] [CrossRef]
- Wu, C.Y.; Zhao, Z.H.; Zhong, J.; Lv, Y.; Yan, X.F.; Wu, Y.Y.; Zhang, H.H. Adsorption of dye through hydrochar derived from co-hydrothermal carbonization of garden waste and sewage sludge: The adsorption enhancement mechanism of lignin component. J. Water Process. Eng. 2024, 67, 106233. [Google Scholar] [CrossRef]
- Ahmad, S.; Zhu, X.D.; Luo, J.W.; Zhou, S.J.; Zhang, C.; Fan, J.J.; Clark, J.H.; Zhang, S.C. Phosphorus and nitrogen transformation in antibiotic mycelial residue derived hydrochar and activated pyrolyzed samples: Effect on Pb (II) immobilization. J. Hazard. Mater. 2020, 393, 122446. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Friedl, J.; Vahidi, M.; Rowlings, D.W.; Bai, Z.H.; Dunn, K.; O’Hara, I.M.; Zhang, Z.Y. Effects of hydrochar derived from hydrothermal treatment of sludge and lignocellulose mixtures on soil properties, nitrogen transformation, and greenhouse gases emissions. Chemosphere 2022, 307, 135792. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Wang, N.; Fu, H.B.; Xie, H.F.; Xue, L.H.; Feng, Y.F.; Poinern, G.E.J.; Chen, D.L. Manure-derived hydrochar superior to manure: Reducing non-point pollution risk by altering nitrogen and phosphorus fugacity in the soil–water system. Waste Manag. 2023, 168, 440–451. [Google Scholar] [CrossRef]
- Liu, X.G.; Chen, Y.D.; Wang, H.; Yuan, S.J.; Dai, X.H. Obtain high quality hydrochar from waste activated sludge with low nitrogen content using acids and alkali pretreatment by enhancing hydrolysis and catalyzation. J. Anal. Appl. Pyrolysis 2024, 177, 106315. [Google Scholar] [CrossRef]
- Liu, X.G.; Yuan, S.J.; Dai, X.H. Thermal hydrolysis prior to hydrothermal carbonization resulted in high quality sludge hydrochar with low nitrogen and sulfur content. Waste Manag. 2024, 176, 117–127. [Google Scholar] [CrossRef]
- Xu, M.X.; Wang, Y.; Liu, T.G.; Yang, L.J.; Liu, H.T.; Xu, D.H. Evaluation on phosphorus extraction potential in hydrochar obtained from hydrothermal liquefaction of sewage sludge. Biomass Bioenergy 2024, 182, 107121. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Lu, T.T.; Xu, G.C.; Luo, Y.L.; Zhang, X.L.; Wu, X.P.; Han, X.Z.; Tester, J.W.; Wang, K. Hydrothermal co-carbonization of rice straw and acid whey for enhanced hydrochar properties and nutrient recovery. Green Energy Environ. 2024, 2, 100077. [Google Scholar] [CrossRef]
- Xie, S.Y.; Zhang, T.; Mishra, A.; Tiwari, A.; Bolan, N.S. Assessment of catalytic thermal hydrolysis of swine manure slurry as liquid fertilizer Insights into nutrients and metals. Front. Environ. Sci. 2022, 10, 1005290. [Google Scholar] [CrossRef]
- Bahadırlı, N.P.; Geçgel, C.; Yabalak, E. Agricultural application of ammonium-enriched hydrochars: Cultivation practices to improve quality and yield of Matricaria recutita L. Ind. Crop. Prod. 2025, 225, 120436. [Google Scholar] [CrossRef]
- Ge, X.F.; Chen, X.Y.; Liu, M.X.; Wang, C.S.; Zhang, Y.Y.; Wang, Y.K.; Tran, H.T.; Joseph, S.; Zhang, T. Toward a better understanding of phosphorus nonpoint source pollution from soil to water and the application of amendment materials research trends. Water 2023, 15, 1531. [Google Scholar] [CrossRef]
- Zhang, T.; Li, P.; Fang, C.; Jiang, R.F. Phosphate recovery from animal manure wastewater by struvite crystallization and CO2 degasification reactor. Ecol. Chem. Eng. S. 2014, 21, 89–99. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Q.C.; Ding, L.L.; Ren, H.Q.; Xu, K.; Wu, Y.G.; Sheng, D. Modeling assessment for ammonium nitrogen recovery from wastewater by chemical precipitation. J. Environ. Sci. 2011, 23, 881–890. [Google Scholar] [CrossRef]
- Chen, H.; Yang, G.T.; Xiao, Y.; Zhang, G.H.; Yang, G.X.; Wang, X.C.; Hu, Y.G. Effects of nitrogen and phosphorus fertilizer on the eating quality of indica rice with different amylose content. J. Food Compos. Anal. 2023, 118, 105167. [Google Scholar] [CrossRef]
- Du, T.Y.; Hu, Q.F.; Mao, W.J.; Yang, Z.; Chen, H.; Sun, L.N.; Zhai, M.Z. Metagenomics insights into the functional profiles of soil carbon, nitrogen, and phosphorus cycles in a walnut orchard under various regimes of long-term fertilisation. Eur. J. Agron. 2023, 148, 126887. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, H.K.; Chen, W.J.; Xue, H.X.; Liu, H.F.; Wang, J.; Mao, S.J.; Liu, G.B.; Xue, S. The combined nitrogen and phosphorus fertilizer application reduced soil multifunctionality in Qinghai-Tibet plateau grasslands, China. Eur. J. Soil Biol. 2024, 123, 103684. [Google Scholar] [CrossRef]
- Zhao, C.; Peng, D.P.; Huang, Z.J.; Wu, Z.; Huang, T. Magnesium-modified starch cryogels for the recovery of nitrogen and phosphorus from wastewater and its potential as a fertilizer substitute: A novel nutrient recovery approach. J. Environ. Chem. Eng. 2024, 12, 114044. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, X.S.; Shaheen, S.M.; Zhao, Q.; Liu, X.J.; Rinklebe, J.; Ren, H.Q. Ammonium nitrogen recovery from digestate by hydrothermal pretreatment followed by activated hydrochar sorption. Chem. Eng. J. 2020, 379, 122254. [Google Scholar] [CrossRef]
- Bhakta Sharma, H.; Panigrahi, S.; Dubey, B.K. Food waste hydrothermal carbonization: Study on the effects of reaction severities, pelletization and framework development using approaches of the circular economy. Bioresour. Technol. 2021, 333, 125187. [Google Scholar] [CrossRef]
- Cavali, M.; Libardi Junior, N.; Mohedano, R.D.A.; Belli Filho, P.; Da Costa, R.H.R.; de Castilhos Junior, A.B. Biochar and hydrochar in the context of anaerobic digestion for a circular approach: An overview. Sci. Total Environ. 2022, 822, 153614. [Google Scholar] [CrossRef] [PubMed]
- Dhull, S.B.; Rose, P.K.; Rani, J.; Goksen, G.; Bains, A. Food waste to hydrochar: A potential approach towards the sustainable Development Goals, carbon neutrality, and circular economy. Chem. Eng. J. 2024, 490, 151609. [Google Scholar] [CrossRef]
- Emmanuel, S.S.; Adesibikan, A.A. Hydrothermal valorization of biomass waste into hydrochar towards circular economy and sustainable adsorptive dye contaminants clean-up: A review. Desalin. Water Treat. 2024, 320, 100801. [Google Scholar] [CrossRef]
- González-Arias, J.; Torres-Sempere, G.; González-Castaño, M.; Baena-Moreno, F.M.; Reina, T.R. Hydrochar and synthetic natural gas co-production for a full circular economy implementation via hydrothermal carbonization and methanation: An economic approach. J. Environ. Sci. 2024, 140, 69–78. [Google Scholar] [CrossRef]
- Suksaroj, C.; Jearat, K.; Cherypiew, N.; Rattanapan, C.; Suksaroj, T.T. Promoting circular economy in the palm oil industry through biogas codigestion of palm oil mill effluent and empty fruit bunch pressed wastewater. Water 2023, 15, 2153. [Google Scholar] [CrossRef]
- Supraja, K.V.; Doddapaneni, T.R.K.C.; Ramasamy, P.K.; Kaushal, P.; Ahammad, S.Z.; Pollmann, K.; Jain, R. Critical review on production, characterization and applications of microalgal hydrochar: Insights on circular bioeconomy through hydrothermal carbonization. Chem. Eng. J. 2023, 473, 145059. [Google Scholar] [CrossRef]
- Urrea Vivas, M.A.; Seguí-Amórtegui, L.; Tomás Pérez, C.; Guerrero-García Rojas, H. Technical–economic evaluation of water reuse at the WWTP El Salitre (Bogotá, Colombia): Example of circular economy. Water 2023, 15, 3374. [Google Scholar] [CrossRef]
- Zvimba, J.N.; Musvoto, E.V.; Nhamo, L.; Mabhaudhi, T.; Nyambiya, I.; Chapungu, L.; Sawunyama, L. Energy pathway for transitioning to a circular economy within wastewater services. Case Stud. Chem. Environ. Eng. 2021, 4, 100144. [Google Scholar] [CrossRef]
- Deng, Y.X.; Zhang, T.; Sharma, B.K.; Nie, H.Y. Optimization and mechanism studies on cell disruption and phosphorus recovery from microalgae with magnesium modified hydrochar in assisted hydrothermal system. Sci. Total Environ. 2019, 646, 1140–1154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, X.S.; Fan, X.; Tsang, D.C.W.; Li, G.X.; Shen, Y.J. Corn waste valorization to generate activated hydrochar to recover ammonium nitrogen from compost leachate by hydrothermal assisted pretreatment. J. Environ. Manag. 2019, 236, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.Y.; He, X.Y.; Alshehri, M.A.; Abou-Elwafa, S.F.; Zhang, T. Elevated effect of hydrothermal treatment on phosphorus transition between solid-liquid phase in swine manure. Results Eng. 2024, 24, 102887. [Google Scholar] [CrossRef]
- He, X.Y.; Zhang, T.; Niu, Y.Q.; Xue, Q.; Ali, E.F.; Shaheen, S.M.; Tsang, D.C.W.; Rinklebe, J. Impact of catalytic hydrothermal treatment and CaAl-modified hydrochar on lability, sorption, and speciation of phosphorus in swine manure: Microscopic and spectroscopic investigations. Environ. Pollut. 2022, 299, 118877. [Google Scholar] [CrossRef]
- Yang, C.; Xia, P.; Zhao, L.Y.; Wang, K.; Wang, B.; Huang, R.; Yang, H.; Yao, Y.Z. Hydrothermal carbonization of woody waste: Changes in the physicochemical properties and the structural evolution mechanisms of hydrochar during this process. Chemosphere 2024, 366, 143524. [Google Scholar] [CrossRef]
- Feng, Z.T.; Xiong, J.B.; Wang, G.F.; Li, L.; Zhou, C.F.; Zhou, C.H.; Huang, H.J. Treatment of swine manure by hydrothermal carbonization: The influential effect and preliminary mechanism of surfactants. Sci. Total Environ. 2024, 946, 174233. [Google Scholar] [CrossRef]
- Wang, C.Y.; Sun, W.B.; Zhou, H.; Sun, X.H.; Su, Y.; He, C. Adsorption of tetracycline by hydrochar derived from co-hydrothermal carbonization of polyvinyl chloride and garden waste: Adsorption characteristics and enhancement mechanism. J. Clean. Prod. 2024, 467, 143007. [Google Scholar] [CrossRef]
- Wei, X.; Liu, P.B.; Huang, S.; Li, X.Q.; Wu, Y.Q.; Wu, S.Y. Hydrothermal carbonization characteristics and mechanism of penicillin mycelial residues and CO2 gasification performance of hydrochars. Biomass Bioenergy 2024, 183, 107130. [Google Scholar] [CrossRef]
- Dang, H.; Xu, R.S.; Zhang, J.L.; Wang, M.Y.; Xu, K. Hydrothermal carbonization of waste furniture for clean blast furnace fuel production: Physicochemical, gasification characteristics and conversion mechanism investigation. Chem. Eng. J. 2023, 469, 143980. [Google Scholar] [CrossRef]
- Qiu, Y.J.; Wang, F.; Ma, X.J.; Yin, F.; Li, D.N.; Li, J. Carbon quantum dots derived from cassava stems via acid/alkali-assisted hydrothermal carbonization: Formation, mechanism and application in drug release. Ind. Crop. Prod. 2023, 204, 117243. [Google Scholar] [CrossRef]
- Shen, Q.; Zhu, X.Q.; Peng, Y.; Xu, M.; Huang, Y.; Xia, A.; Zhu, X.; Liao, Q. Structure evolution characteristic of hydrochar and nitrogen transformation mechanism during co-hydrothermal carbonization process of microalgae and biomass. Energy 2024, 295, 131028. [Google Scholar] [CrossRef]
- Hong, J.; Bao, J.; Liu, Y. Removal of methyleneblue from simulated wastewater based upon hydrothermal carbon activated by phosphoric acid. Water 2025, 17, 733. [Google Scholar] [CrossRef]
- Shi, Y.J.; Zhang, T.; Ren, H.Q.; Kruse, A.; Cui, R.F. Polyethylene imine modified hydrochar adsorption for chromium (VI) and nickel (II) removal from aqueous solution. Bioresour. Technol. 2018, 247, 370–379. [Google Scholar] [CrossRef]
- Guo, S.; Gan, J.Y.; Yang, L.; Sun, B.Z.; Qu, H.W.; Li, X.C.; Zhao, D. Simulation and mechanistic exploration of the mid to late-stage hydrothermal carbonization process in biomass. Process Saf. Environ. Protect. 2025, 194, 437–452. [Google Scholar] [CrossRef]
- Srivastava, S.; Reddy, P.M.; Rao, P.V. Valorization of chicken offal waste to solid fuel by hydrothermal carbonization: Characterization, effect of process parameters on combustion behavior and reaction pathways. Fuel 2025, 384, 133983. [Google Scholar] [CrossRef]
- Sun, R.X.; Li, C.; Kong, L.H.; Zhang, L.J.; Zhang, S.; Cui, Z.H.; Wang, D.; Leng, C.J.; Hu, X. Torrefaction and hydrothermal carbonization of cellulose make marked difference in subsequent pyrolysis. J. Energy Inst. 2025, 119, 101978. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Nadarajah, K.; Suarez-Toriello, V.A.; Bandala, E.R.; Goonetilleke, A. Engineered hydrochar production methodologies, key factors influencing agriculture wastewater treatment, and life cycle analysis: A critical review. J. Water Process. Eng. 2023, 56, 104483. [Google Scholar] [CrossRef]
- Sudibyo, H.; Budhijanto, B.; Marbelia, L.; Güleç, F.; Budiman, A. Kinetic and thermodynamic evidences of the Diels-Alder cycloaddition and Pechmann condensation as key mechanisms of hydrochar formation during hydrothermal conversion of Lignin-Cellulose. Chem. Eng. J. 2024, 480, 148116. [Google Scholar] [CrossRef]
- Ali, M.A.; Chambers, C.; Reza, M.T.; Aich, N. Application of corn stover derived pyrolyzed hydrochars for efficient phosphorus removal from water: Influence of pyrolysis temperature. Chem. Eng. J. Adv. 2024, 18, 100613. [Google Scholar] [CrossRef]
- Atallah, E.; Kwapinski, W.; Ahmad, M.N.; Leahy, J.J.; Zeaiter, J. Effect of water-sludge ratio and reaction time on the hydrothermal carbonization of olive oil mill wastewater treatment: Hydrochar characterization. J. Water Process. Eng. 2019, 31, 100813. [Google Scholar] [CrossRef]
- Deepak, K.R.; Mohan, S.; Dinesha, P.; Balasubramanian, R. CO2 uptake by activated hydrochar derived from orange peel (Citrus reticulata): Influence of carbonization temperature. J. Environ. Manag. 2023, 342, 118350. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Huang, J.C.; Wang, J.W.; Wang, Z.Q.; Qiao, Y. Hydrothermal carbonization of sewage sludge for hydrochar valorization: Role of feedwater pH on sulfur removal and transformation. Fuel 2024, 377, 132813. [Google Scholar] [CrossRef]
- Liu, X.M.; Fan, Y.W.; Zhai, Y.B.; Liu, X.P.; Wang, Z.X.; Zhu, Y.; Shi, H.R.; Li, C.T.; Zhu, Y. Co-hydrothermal carbonization of rape straw and microalgae: pH-enhanced carbonization process to obtain clean hydrochar. Energy 2022, 257, 124733. [Google Scholar] [CrossRef]
- Tamires Da Silva Carvalho, N.; Silveira, E.A.; de Paula Protásio, T.; Trugilho, P.F.; Bianchi, M.L. Hydrotreatment of eucalyptus sawdust: The influence of process temperature and H2SO4 catalyst on hydrochar quality, combustion behavior and related emissions. Fuel 2024, 360, 130643. [Google Scholar] [CrossRef]
- Phang, F.J.F.; Tiong, S.I.X.; Wang, Y.S.; Soh, M.; Chew, J.J.; Khaerudini, D.S.; Thangalazhy-Gopakumar, S.; How, B.S.; Loh, S.K.; Yusup, S.; et al. Hydrochars derived via wet torrefaction of empty fruit bunches: Effect of temperature and time, comparison to oil palm trunks counterpart, and their pyrolysis behavior. J. Anal. Appl. Pyrolysis 2024, 179, 106441. [Google Scholar] [CrossRef]
- Xia, R.; Wang, J.; Yang, X.X.; Li, Q.H.; Zhou, H.; Sun, H.; Zhang, Y.G. Comprehensive compositional analysis of liquid organic product prepared by industrialized hydrothermal cracking of biomass waste and its potential application as fertilizer. Sci. Total Environ. 2024, 951, 175264. [Google Scholar] [CrossRef]
- Zhang, H.W.; Li, G.Q.; Li, W.Y.; Li, Y.Z.; Zhang, S.H.; Nie, Y. Biochemical properties of sludge derived hydrothermal liquid products and microbial response of wastewater treatment. Process Biochem. 2024, 144, 294–305. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, T.; Zhang, T.; Zhang, Z.R.; Wang, W.Z.; Peng, H.; Li, D.; Zhu, Z.P. Volatile fatty acid release and metal ion concentration in hydrothermal carbonization liquid. J. Anal. Appl. Pyrolysis 2024, 183, 106815. [Google Scholar] [CrossRef]
- Guo, S.; Mu, J.; Zhao, D.; Qu, H.; Sun, B.; Li, X.; Yang, L. Gasification performance of wet hydrochar from co-hydrothermal carbonization of high-moisture sludge and fungus bran. J. Environ. Chem. Eng. 2024, 12, 113901. [Google Scholar] [CrossRef]
- Qi, J.W.; Wang, Y.J.; Xu, P.C.; Hu, M.; Huhe, T.L.; Ling, X.; Yuan, H.R.; Li, J.D.; Chen, Y. Biomass hydrothermal gasification characteristics study: Based on deep learning for data generation and screening strategies. Energy 2024, 312, 133492. [Google Scholar] [CrossRef]
- Zhang, X.S.; Shi, S.L.; Men, X.Y.; Hu, D.B.; Yang, Q.L.; Zhang, L.M. Elevating clean energy through sludge: A comprehensive study of hydrothermal carbonization and co-gasification technologies. J. Environ. Manag. 2024, 369, 122388. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, M.; Moser, B.R.; Alharthi, F.A.; Rashid, U. Efficient production of biodiesel from palm fatty acid distillate using a novel hydrochar-based solid acid catalyst derived from palm leaf waste. Process Saf. Environ. Protect. 2024, 187, 1126–1139. [Google Scholar] [CrossRef]
- Baytar, O.; Şahin, Ö.; Ekinci, A. Effect of environmentally friendly and efficient metal-free hydrochars as catalysts on sodium borohydride hydrolysis. Fuel 2023, 346, 128308. [Google Scholar] [CrossRef]
- Comak, G.; Bayram, G.; Görmez, Ö.; Çağlayan, U.; Gözmen, B. Synthesis of biomass-based BiOI@ hydrochar heterogeneous catalyst and investigation of its activity in sonocatalytic process. Desalin. Water Treat. 2024, 320, 100625. [Google Scholar] [CrossRef]
- Guo, H.X.; Isoda, Y.; Honma, T.; Shen, F.; Smith, R.L., Jr. Sustainably-derived sulfonated pinecone-based hydrochar catalyst for carbohydrate dehydration. Renew. Energy 2024, 232, 121145. [Google Scholar] [CrossRef]
- Xiao, Y.; Ding, L.; Leghari, A.; Hungwe, D.; Gao, M.; Gao, Y.F.; Zhang, Y.Y.; Chen, X.L.; Wang, F.C. Ferric sludge derived pyrolyzed-hydrochar supported iron catalysts for catalytic cracking of toluene. Chem. Eng. J. 2024, 491, 152001. [Google Scholar] [CrossRef]
- Picone, A.; Volpe, M.; Codignole Lùz, F.; Malik, W.; Volpe, R.; Messineo, A. Co-hydrothermal carbonization with process water recirculation as a valuable strategy to enhance hydrochar recovery with high energy efficiency. Waste Manag. 2024, 175, 101–109. [Google Scholar] [CrossRef]
- Zhang, X.F.; Li, Y.L.; Zhang, X.W.; Ma, P.Y.; Xing, X.J. Co-combustion of municipal solid waste and hydrochars under non-isothermal conditions: Thermal behaviors, gaseous emissions and kinetic analyses by TGA–FTIR. Energy 2023, 265, 126373. [Google Scholar] [CrossRef]
- Chen, S.S.; Tang, X.Y.; Chen, J.Q.; Xue, Y.Y.; Wang, Y.H.; Xu, D.H. Regulation of slow-release performance of high-sugar biomass waste filter mud and sugarcane bagasse by co-hydrothermal carbonization and potential evaluation of hydrochar-based slow-release fertilizers. Biomass Bioenergy 2025, 193, 107557. [Google Scholar] [CrossRef]
- Yao, H.; Cheng, Y.D.; Kong, Q.X.; Wang, X.; Rong, Z.G.; Quan, Y.; You, X.W.; Zheng, H.; Li, Y.Q. Variation in microbial communities and network ecological clusters driven by soil organic carbon in an inshore saline soil amended with hydrochar in yellow river delta, China. Environ. Res. 2025, 264, 120369. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Zhong, F.; Liang, X.R.; Xu, W.X.; Yuan, Q.X.; Niu, W.J.; Meng, H.B. Microwave–assisted hydrothermal conversion of crop straw: Enhancing the properties of liquid product and hydrochar by varying temperature and medium. Energy Conv. Manag. 2023, 290, 117192. [Google Scholar] [CrossRef]
- Ding, C.X.; Ye, C.; Zhu, W.; Zeng, G.Y.; Yao, X.M.; Ouyang, Y.; Rong, J.; Tao, Y.P.; Liu, X.Y.; Deng, Y.C. Engineered hydrochar from waste reed straw for peroxymonosulfate activation to degrade quinclorac and improve solanaceae plants growth. J. Environ. Manag. 2023, 347, 119090. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, J.L.; Hubble, A.H.; Ma, Q.L.; Volpe, M.; Severini, G.; Andreottola, G.; Fiori, L. Valorization of cow manure via hydrothermal carbonization for phosphorus recovery and adsorbents for water treatment. J. Environ. Manag. 2022, 308, 114561. [Google Scholar] [CrossRef]
- Xie, S.Y.; Zhang, T.; You, S.M.; Mukherjee, S.; Pu, M.J.; Chen, Q.; Wang, Y.S.; Ali, E.F.; Abdelrahman, H.; Rinklebe, J.; et al. Applied machine learning for predicting the properties and carbon and phosphorus fate of pristine and engineered hydrochar. Biochar 2025, 7, 19. [Google Scholar] [CrossRef]
- Ding, S.D.; Li, J.; Wang, Y.; He, S.Y.; Xie, H.F.; Fu, H.B.; Feng, Y.F.; Shaheen, S.M.; Rinklebe, J.; Xue, L.H. Manure derived hydrochar reduced phosphorus loss risk via an alteration of phosphorus fractions and diversified microbial community in rice paddy soil. Sci. Total Environ. 2024, 918, 170582. [Google Scholar] [CrossRef]
- McIntosh, S.; Padilla, R.V.; Rose, T.; Rose, A.L.; Boukaka, E.; Erler, D. Crop fertilisation potential of phosphorus in hydrochars produced from sewage sludge. Sci. Total Environ. 2022, 817, 153023. [Google Scholar] [CrossRef]
- Zhou, Y.; Shi, W.K.; Engler, N.; Nelles, M. High-value utilization of kitchen waste derived hydrochar in energy storage regulated by circulating process water. Energy Conv. Manag. 2021, 229, 113737. [Google Scholar] [CrossRef]
- Ali Khan, M.; Hameed, B.H.; Raza Siddiqui, M.; Alothman, Z.A.; Alsohaimi, I.H. Physicochemical properties and combustion kinetics of food waste derived hydrochars. J. King Saud Univ. Sci. 2022, 34, 101941. [Google Scholar] [CrossRef]
- Zhou, Y.D.; Xiao, H.T.; Liu, Q.; Wang, L.; Gong, Y.; Remón, J. Synergistic production of nitrogen-rich hydrochar and solid biofuels via co-hydrothermal carbonization of microalgae and macroalgae: When nitrogen circularity matters. Environ. Res. 2025, 268, 120749. [Google Scholar] [CrossRef]
- Ansah, E.; Wang, L.; Zhang, B.; Shahbazi, A. Catalytic pyrolysis of raw and hydrothermally carbonized Chlamydomonas debaryana microalgae for denitrogenation and production of aromatic hydrocarbons. Fuel 2018, 228, 234–242. [Google Scholar] [CrossRef]
- Fu, J.Q.; Bai, L.; Chi, M.S.; Xu, X.L.; Yu, K.C.; Wang, M. Synergistic effect of Fenton pretreatment and hydrothermal carbonization of lignin on the physicochemical properties of the resulting hydrochar. Green Chem. 2023, 25, 9857–9872. [Google Scholar] [CrossRef]
- Inkoua, S.; Li, C.; Rashid, M.; Naeem, M.M.; Zhang, S.; Gao, W.; Gholizadeh, M.; Hu, X. Unveiling drastic influence of cross-interactions in hydrothermal carbonization of spirulina with cellulose, lignin or poplar on nature of hydrochar and activated carbon. J. Environ. Manag. 2024, 366, 121713. [Google Scholar] [CrossRef]
- Li, X.Z.; Yu, Y.C.; He, R.M.; Zhen, Q.; She, D. Mechanistic insights into cadmium removal from environmental waters using silicon-enhanced lignin-derived hydrochar and pyrochar. Sep. Purif. Technol. 2025, 355, 129727. [Google Scholar] [CrossRef]
- Habchi, S.; Lahboubi, N.; Asbik, M.; Bari, H.E. Enhancing biomethane production from food waste using olive pomace hydrochar: An optimization study. Environ. Adv. 2024, 15, 100477. [Google Scholar] [CrossRef]
- Alves, D.M.S.; Ferreira, W.M.; Da Fonseca, M.P.S.; de Carvalho, J.L.V.; Pimentel, C. Top dressing of nitrogen and phosphorus fertilizer increases yield and leads to biofortification of a local cowpea genotype. Sci. Hortic. 2024, 334, 113204. [Google Scholar] [CrossRef]
- Brookfield, A.E.; Hansen, A.T.; Sullivan, P.L.; Czuba, J.A.; Kirk, M.F.; Li, L.; Newcomer, M.E.; Wilkinson, G. Predicting algal blooms: Are we overlooking groundwater? Sci. Total Environ. 2021, 769, 144442. [Google Scholar] [CrossRef]
- Ding, S.M.; Chen, M.S.; Gong, M.D.; Fan, X.F.; Qin, B.Q.; Xu, H.; Gao, S.S.; Jin, Z.F.; Tsang, D.C.W.; Zhang, C.S. Internal phosphorus loading from sediments causes seasonal nitrogen limitation for harmful algal blooms. Sci. Total Environ. 2018, 625, 872–884. [Google Scholar] [CrossRef]
- Mustafa, S.; Zaman, M.I.; Khan, S. Temperature effect on the mechanism of phosphate anions sorption by β-MnO2. Chem. Eng. J. 2008, 141, 51–57. [Google Scholar] [CrossRef]
- Rott, E.; Steinmetz, H.; Metzger, J.W. Organophosphonates: A review on environmental relevance, biodegradability and removal in wastewater treatment plants. Sci. Total Environ. 2018, 615, 1176–1191. [Google Scholar] [CrossRef]
- Sajjad, M.; Huang, Q.; Khan, S.; Nawab, J.; Khan, M.A.; Ali, A.; Ullah, R.; Kubar, A.A.; Guo, G.; Yaseen, M.; et al. Methods for the removal and recovery of nitrogen and phosphorus nutrients from animal waste: A critical review. Ecol. Front. 2024, 44, 2–14. [Google Scholar] [CrossRef]
- He, K.; Xu, Y.; He, G.; Zhao, X.H.; Wang, C.P.; Li, S.J.; Zhou, G.K.; Hu, R.B. Combined application of acidic biochar and fertilizer synergistically enhances miscanthus productivity in coastal saline-alkaline soil. Sci. Total Environ. 2023, 893, 164811. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.J.; Yi, G.W.; Hao, Y.F.; Li, L.T.; Shen, L.C.; Zhang, Q.Z. The effect of combined application of biochar and phosphate fertilizers on phosphorus transformation in saline-alkali soil and its microbiological mechanism. Sci. Total Environ. 2024, 951, 175610. [Google Scholar] [CrossRef] [PubMed]
- Rivett, M.O.; Buss, S.R.; Morgan, P.; Smith, J.W.N.; Bemment, C.D. Nitrate attenuation in groundwater: A review of biogeochemical controlling processes. Water Res. 2008, 42, 4215–4232. [Google Scholar] [CrossRef]
- Ai, H.S.; Fan, B.; Zhou, Z.Q.; Liu, J.H. The impact of nitrogen Fertilizer application on air Pollution: Evidence from China. J. Environ. Manag. 2024, 370, 122880. [Google Scholar] [CrossRef]
- Hu, M.C.; Xue, H.W.; Wade, A.J.; Gao, N.; Qiu, Z.J.; Long, Y.U.; Shen, W.S. Biofertilizer supplements allow nitrogen fertilizer reduction, maintain yields, and reduce nitrogen losses to air and water in China paddy fields. Agric. Ecosyst. Environ. 2024, 362, 108850. [Google Scholar] [CrossRef]
- Mirbagheri, S.A.; Nejati, S.; Moshirvazir, S. Numerical simulation of dissolved oxygen, algal biomass, nitrate, organic nitrogen, ammonia, and dissolved phosphorus in waste stabilization ponds. Desalin. Water Treat. 2018, 135, 188–197. [Google Scholar] [CrossRef]
- Tong, Y.; Zhang, W.J.; Zhou, J.H.; Liu, S.Q.; Kang, B.Y.; Wang, J.H.; Jiang, S.J.; Leng, L.J.; Li, H.L. Machine learning prediction and exploration of phosphorus migration and transformation during hydrothermal treatment of biomass waste. Sci. Total Environ. 2024, 955, 176780. [Google Scholar] [CrossRef]
- Yu, J.Y.; Yu, W.Q.; Chang, B.; Li, X.; Jia, J.; Wang, D.F.; Xu, Z.N.; Zhang, X.L.; Liu, H.; Zhou, W.J. Waste-yeast biomass as nitrogen/phosphorus sources and carbon template: Environment-friendly synthesis of N,P-Mo2C nanoparticles on porous carbon matrix for efficient hydrogen evolution. Chin. Chem. Lett. 2022, 33, 3231–3235. [Google Scholar] [CrossRef]
- Aragón-Briceño, C.I.; Pozarlik, A.K.; Bramer, E.A.; Niedzwiecki, L.; Pawlak-Kruczek, H.; Brem, G. Hydrothermal carbonization of wet biomass from nitrogen and phosphorus approach: A review. Renew. Energy 2021, 171, 401–415. [Google Scholar] [CrossRef]
- He, C.; Wang, K.; Yang, Y.H.; Amaniampong, P.N.; Wang, J.Y. Effective nitrogen removal and recovery from dewatered sewage sludge using a novel integrated system of accelerated hydrothermal deamination and air stripping. Environ. Sci. Technol. 2015, 49, 6872–6880. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.X.; Fang, C.; Lu, X.W.; Jiang, R.F.; Tang, Y.Z. Transformation of phosphorus during (hydro) thermal treatments of solid biowastes: Reaction mechanisms and implications for P reclamation and recycling. Environ. Sci. Technol. 2017, 51, 10284–10298. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.C.; Tan, F.R.; Wu, B.; He, M.X.; Wang, W.G.; Tang, X.Y.; Hu, Q.C.; Zhang, M. Immobilization of phosphorus in cow manure during hydrothermal carbonization. J. Environ. Manag. 2015, 157, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.C.; Yang, B.; Li, H.; Tan, F.R.; Zhu, N.M.; Zhu, Q.L.; He, M.X.; Ran, Y.; Hu, G.Q. A synergistic combination of nutrient reclamation from manure and resultant hydrochar upgradation by acid-supported hydrothermal carbonization. Bioresour. Technol. 2017, 243, 860–866. [Google Scholar] [CrossRef]
- Becker, G.C.; Wuest, D.; Koehler, H.; Lautenbach, A.; Kruse, A. Novel approach of phosphate-reclamation as struvite from sewage sludge by utilising hydrothermal carbonization. J. Environ. Manag. 2019, 238, 119–125. [Google Scholar] [CrossRef]
- Idowu, I.M. Hydrothermal Carbonization of Food Waste for Nutrient Recovery and Reuse. Master’s Thesis, Civil Engineering, University of South Carolina, Columbia, SC, USA, 2018. [Google Scholar]
- Kalderis, D.; Stavroulakis, G.; Tsubota, T.; Çalhan, S.D. Valorization of aloe vera waste for the production of Ca and P-rich hydrochars. Sustain. Chem. Environ. 2024, 5, 100057. [Google Scholar] [CrossRef]
- Chen, H.H.; Rao, Y.; Cao, L.C.; Shi, Y.; Hao, S.L.; Luo, G.; Zhang, S.C. Hydrothermal conversion of sewage sludge: Focusing on the characterization of liquid products and their methane yields. Chem. Eng. J. 2019, 357, 367–375. [Google Scholar] [CrossRef]
- Gou, L.; Dai, L.Y.; Wang, Y.Y. Coupling of struvite crystallization and aqueous phase recirculation for hydrochar upgrading and nitrogen recovery during hydrothermal carbonization of sewage sludge. Sci. Total Environ. 2024, 929, 172682. [Google Scholar] [CrossRef]
- Köchermann, J.; Görsch, K.; Wirth, B.; Mühlenberg, J.; Klemm, M. Hydrothermal carbonization: Temperature influence on hydrochar and aqueous phase composition during process water recirculation. J. Environ. Chem. Eng. 2018, 6, 5481–5487. [Google Scholar] [CrossRef]
- Picone, A.; Volpe, M.; Malik, W.; Volpe, R.; Messineo, A. Role of reaction parameters in hydrothermal carbonization with process water recirculation: Hydrochar recovery enhancement and energy balance. Biomass Bioenerg. 2024, 181, 107061. [Google Scholar] [CrossRef]
- Su, X.H.; Zhang, T.; Zhao, J.Y.; Mukherjee, S.; Alotaibi, N.M.; Abou-Elwafa, S.F.; Tran, H.T.; Bolan, N.S. Phosphorus fraction in hydrochar from co-hydrothermal carbonization of swine manure and rice straw an optimization analysis based on response surface methodology. Water 2024, 16, 2208. [Google Scholar] [CrossRef]
- Geng, M.X.; Zeng, T.; Deng, X.Y.; Zhang, Z.Y.; Xiao, C.Q.; Chi, R.A. Innovative strategies for ammonia nitrogen removal in rare earth tailings: An advanced element recycling process using leaching, chemical precipitation and adsorption. J. Water Process. Eng. 2024, 67, 106205. [Google Scholar] [CrossRef]
- Li, J.T.; Chen, C.Y.; Luo, Z.F.; Qiu, J.R.; Zhao, L.; Zhang, J.; Xiao, X.; Lin, X.J.; Wang, X.J.; Cai, Q.Y.; et al. Nitrogen and phosphorus recovery from livestock wastewater via magnesium ammonium phosphate precipitation: A critical review of methods, progress, and insights. J. Water Process. Eng. 2024, 67, 106139. [Google Scholar] [CrossRef]
- Tong, Y.F.; Liu, W.; Wang, Z.P.; Liu, J.; Zhou, J.B. Method for preparing high-purity struvite by extracting nitrogen and phosphorus from sewage sludge using a coupled process of hydrothermal carbonization and oxalic acid leaching. Sustain. Chem. Pharm. 2025, 43, 101885. [Google Scholar] [CrossRef]
- Trotta, S.; Adani, F.; Fedele, M.; Salvatori, M. Nitrogen and phosphorus recovery from cow digestate by struvite precipitation: Process optimization to maximize phosphorus recovery. Results Eng. 2023, 20, 101478. [Google Scholar] [CrossRef]
- Zhang, L.F.; Huang, X.D.; Fu, G.K.; Zhang, Z. Aerobic electrotrophic denitrification coupled with biologically induced phosphate precipitation for nitrogen and phosphorus removal from high-salinity wastewater: Performance, mechanism, and microbial community. Bioresour. Technol. 2023, 372, 128696. [Google Scholar] [CrossRef]
- Zhang, L.F.; Su, J.F.; Liu, S.Y.; Huang, T.L.; Wang, Z.; Liu, Y.; Hou, C.X.; Wang, X.J. Calcium self-release bioremediation system combined with microbially induced calcium precipitation for the removal of ammonium nitrogen, phosphorus and heavy metals. J. Environ. Chem. Eng. 2024, 12, 114190. [Google Scholar] [CrossRef]
- Abayie, S.O.; Leiviskä, T. Removal of nitrate from underground mine waters using selective ion exchange resins. J. Environ. Chem. Eng. 2022, 10, 108642. [Google Scholar] [CrossRef]
- Cyganowski, P.; Gruss, Ł.; Skorulski, W.; Kabat, T.; Piszko, P.; Jermakowicz-Bartkowiak, D.; Pulikowski, K.; Wiatkowski, M. Field installation of ion exchange technology for purification of retention reservoirs from nitrogen-based nutrient contamination. J. Water Process. Eng. 2024, 59, 104959. [Google Scholar] [CrossRef]
- Ruiz-Cosgaya, L.; Izquierdo, W.A.; Martínez-Guijarro, R.; Serralta, J.; Barat, R. Ion exchange columns. A promising technology for nitrogen and phosphorus recovery in the main line of a wastewater treatment plant. J. Environ. Manag. 2024, 370, 122719. [Google Scholar] [CrossRef]
- Tang, Y.C.; Wen, Q.X.; Chen, Z.Q. Simultaneous removal of nitrogen and phosphorus nutrients from secondary effluent by magnetic resin containing two types of quaternary ammonium adsorption sites: Preparation, characterization, and application. Chem. Eng. J. 2023, 477, 147137. [Google Scholar] [CrossRef]
- Zeng, B.Z.; Tao, B.C.; Pan, Z.X.; Shen, L.G.; Zhang, J.Z.; Lin, H.J. A low-cost and sustainable solution for nitrate removal from secondary effluent: Macroporous ion exchange resin treatment. J. Environ. Manag. 2023, 347, 119142. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, R.R.; Nakagawa, K.; Kumagai, K.; Hasegawa, S.; Matsuoka, A.; Li, Z.; Mai, Z.H.; Yoshioka, T.; Hori, T.; Matsuyama, H. Hybrid osmotically assisted reverse osmosis and reverse osmosis (OARO-RO) process for minimal liquid discharge of high strength nitrogenous wastewater and enrichment of ammoniacal nitrogen. Water Res. 2023, 246, 120716. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Wang, Z.; Su, J.F.; Wang, X.J.; Ali, A.; Li, X. Microbial induced calcium precipitation by Zobellella denitrificans sp. LX16 to simultaneously remove ammonia nitrogen, calcium, and chemical oxygen demand in reverse osmosis concentrates. Environ. Res. 2024, 240, 117484. [Google Scholar] [CrossRef]
- Philibert, M.; Villacorte, L.O.; Ekowati, Y.; Abushaban, A.; Salinas-Rodriguez, S.G. Fouling and scaling in reverse osmosis desalination plants: A critical review of membrane autopsies, feedwater quality guidelines and assessment methods. Desalination 2024, 592, 118188. [Google Scholar] [CrossRef]
- Rho, H.; Cho, J.; Chon, K. Rejection behaviors of N-nitrosamines by initially fouled ultrafiltration and reverse osmosis membranes for municipal wastewater reclamation: A pilot study. Desalination 2024, 586, 117877. [Google Scholar] [CrossRef]
- Yu, H.F.; Zhuang, L.L.; Zhang, M.; Zhang, J. The mechanism study of attached microalgae cultivation based on reverse osmosis concentrated water (WROC). Resour. Conserv. Recycl. 2022, 179, 106066. [Google Scholar] [CrossRef]
- Zhao, H.R.; Zhou, Y.; Zou, L.P.; Lin, C.H.; Liu, J.Y.; Li, Y.Y. Pure water and resource recovery from municipal wastewater using high-rate activated sludge, reverse osmosis, and mainstream anammox: A pilot scale study. Water Res. 2024, 266, 122443. [Google Scholar] [CrossRef]
- Cao, Y.H.; Li, X.L.; Zhang, L. Construction of bipolar membrane electrodialysis reactor for removal and recovery of nitrogen and phosphorus from wastewater. Int. J. Electrochem. Sci. 2023, 18, 100051. [Google Scholar] [CrossRef]
- Huang, M.L.; Zhai, Y.B.; Liu, X.M.; Liu, X.P.; Wang, Z.X.; Zhou, Y.; Xu, M. Efficient extraction of phosphorus from food waste biogas digestate ash through two-compartment electrodialysis cell. J. Environ. Chem. Eng. 2022, 10, 108701. [Google Scholar] [CrossRef]
- Liu, Y.X.; Wu, X.Y.; Wu, X.Y.; Dai, L.P.; Ding, J.G.; Ye, X.; Chen, R.Y.; Ding, R.; Liu, J.X.; Jin, Y.C.; et al. Recovery of nickel, phosphorus and nitrogen from electroless nickel-plating wastewater using bipolar membrane electrodialysis. J. Clean. Prod. 2023, 382, 135326. [Google Scholar] [CrossRef]
- Weisz, L.; Reif, D.; Weilguni, S.; Parravicini, V.; Saracevic, E.; Krampe, J.; Kreuzinger, N. Feasibility study of electrodialysis as an ammonium reuse process for covering the nitrogen demand of an industrial wastewater treatment plant. Sci. Total Environ. 2024, 954, 176699. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, R.; Xia, M.T.; Heng, S.L.; Wang, J.D.; Liu, Z.B.; Tian, J.H.; Lu, X.Q.; Zhen, G.Y. Microalgae biofilm barrier to assist the simultaneous nitrogen and phosphorus removal in anammox: Molecular mechanism on sludge granulation and microbial metabolism. Chem. Eng. J. 2024, 502, 157887. [Google Scholar] [CrossRef]
- Ma, Y.G.; Jiang, W.Q.; Nie, Z.B.; Qi, P.; Jiao, Y.; Peng, J.X.; Bian, D.J. Study on the mechanisms of enhanced biological nitrogen and phosphorus removal by denitrifying phosphorus removal in a Micro-pressure swirl reactor. Bioresour. Technol. 2022, 364, 128093. [Google Scholar] [CrossRef]
- Wang, D.Q.; Han, I.; McCullough, K.; Klaus, S.; Lee, J.; Srinivasan, V.; Li, G.Y.; Wang, Z.L.; Bott, C.B.; McQuarrie, J.; et al. Side-Stream Enhanced Biological Phosphorus Removal (S2EBPR) enables effective phosphorus removal in a pilot-scale a-b stage shortcut nitrogen removal system for mainstream municipal wastewater treatment. Water Res. 2024, 251, 121050. [Google Scholar] [CrossRef]
- Zhou, Q.; Sun, H.M.; Jia, L.X.; Wu, W.Z.; Wang, J.L. Simultaneous biological removal of nitrogen and phosphorus from secondary effluent of wastewater treatment plants by advanced treatment: A review. Chemosphere 2022, 296, 134054. [Google Scholar] [CrossRef]
- Guadie, A.; Xia, S.Q.; Zhang, Z.Q.; Guo, W.S.; Ngo, H.H.; Hermanowicz, S.W. Simultaneous removal of phosphorus and nitrogen from sewage using a novel combo system of fluidized bed reactor–membrane bioreactor (FBR–MBR). Bioresour. Technol. 2013, 149, 276–285. [Google Scholar] [CrossRef]
- Iorhemen, O.T.; Hamza, R.A.; Sheng, Z.Y.; Tay, J.H. Submerged aerobic granular sludge membrane bioreactor (AGMBR): Organics and nutrients (nitrogen and phosphorus) removal. Bioresour. Technol. Rep. 2019, 6, 260–267. [Google Scholar] [CrossRef]
- Lee, H.; Yun, G.H.; Kim, S.; Yun, Z. The 4-stage anoxic membrane bioreactor for simultaneous nitrogen and phosphorus removal, and its strengths and weaknesses. Desalin. Water Treat. 2015, 54, 3616–3624. [Google Scholar] [CrossRef]
- Liang, W.F.; Yang, B.; Bin, L.Y.; Hu, Y.D.; Fan, D.P.; Chen, W.R.; Li, P.; Tang, B. Intensifying the simultaneous removal of nitrogen and phosphorus of an integrated aerobic granular sludge-membrane bioreactor by Acinetobacter junii. Bioresour. Technol. 2024, 397, 130474. [Google Scholar] [CrossRef]
- Nie, J.X.; Huang, H.G.; Rao, P.; Chen, H.; Du, X.; Wang, Z.H.; Zhang, W.X.; Liang, H. Composite functional particle enhanced gravity driven ceramic membrane bioreactor for simultaneous removal of nitrogen and phosphorus from groundwater. Chem. Eng. J. 2023, 452, 139134. [Google Scholar] [CrossRef]
- Qin, W.; Dong, J.H.; Huang, H.G.; Nie, J.X.; Du, X.; Tian, J.Y.; Zhang, W.X. Advanced nitrogen and phosphorus removal from groundwater by a composite functional particle-ceramic membrane bioreactor. Sep. Purif. Technol. 2024, 339, 126549. [Google Scholar] [CrossRef]
- Xia, Y.G.; Bai, J.H.; Yang, T.; Yang, X.L.; Song, H.L. Enhanced removal of nitrogen and phosphorus from expressway service area wastewater using step-feed multi-stage Anoxic/Oxic membrane bioreactor optimization process. J. Water Process. Eng. 2025, 69, 106769. [Google Scholar] [CrossRef]
- Zorpas, A.A. The hidden concept and the beauty of multiple “R” in the framework of waste strategies development reflecting to circular economy principles. Sci. Total Environ. 2024, 952, 175508. [Google Scholar] [CrossRef]
- Cong, H.B.; Meng, H.B.; Chen, M.S.; Song, W.; Xing, H.H. Co-processing paths of agricultural and rural solid wastes for a circular economy based on the construction concept of “zero-waste city” in China. Circ. Econ. 2023, 2, 100065. [Google Scholar] [CrossRef]
- Eady, S.; Carre, A.; Grant, T. Life cycle assessment modelling of complex agricultural systems with multiple food and fibre co-products. J. Clean. Prod. 2012, 28, 143–149. [Google Scholar] [CrossRef]
- Li, J.; Sun, W.H.; Lichtfouse, E.; Maurer, C.; Liu, H.B. Life cycle assessment of biochar for sustainable agricultural application: A review. Sci. Total Environ. 2024, 951, 175448. [Google Scholar] [CrossRef]
- Meier, M.S.; Stoessel, F.; Jungbluth, N.; Juraske, R.; Schader, C.; Stolze, M. Environmental impacts of organic and conventional agricultural products—Are the differences captured by life cycle assessment? J. Environ. Manag. 2015, 149, 193–208. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Akaeme, F.C.; Georgin, J.; Schumacher de Oliveira, J.; Franco, D.S.P. Biomass hydrochar: A critical review of process chemistry, synthesis methodology, and applications. Sustainability 2025, 17, 1660. [Google Scholar] [CrossRef]
- Bermúdez, L.A.; Mendoza, V.D.; Díaz, J.C.L.; Pascual, J.M.; Del Mar Muñio Martínez, M.; Capilla, J.M.P. Investigation of the agricultural reuse potential of urban wastewater and other resources derived by using membrane bioreactor technology within the circular economy framework. Sci. Total Environ. 2024, 955, 177011. [Google Scholar] [CrossRef]
- Xu, J.Q.; Fan, Z.P.; Yang, Q.L.; Lu, G.Y.; Liu, P.F.; Wang, D.W. Hydrothermal carbonization of waste wood: Sustainable recycling of biomass by-products and novel performance enhancer for bitumen. Constr. Build. Mater. 2023, 404, 133307. [Google Scholar] [CrossRef]
- Silvestri, S.; Duarte, Á.E.; Bueno, G.G.; Carissimi, E.; Fajardo, A.R. Enhanced photo-oxidation of hormones in water using biomass waste-derived hydrochar composites as photocatalysts. Bioresour. Technol. Rep. 2024, 28, 101977. [Google Scholar] [CrossRef]
- Pan, T.Y.; Guo, Z.C.; Zhang, X.H.; Feng, L. Hydrothermal carbonization of biomass waste and application of produced hydrochar in organic pollutants removal. J. Clean. Prod. 2024, 457, 142386. [Google Scholar] [CrossRef]
- Lawa, Y.; Benu, F.L.; Boimau, K.; Riwu, D.B.N.; Kune, P.; Faria Da Silva, A.; Widyaningrum, B.A.; Darmokoesoemoe, H.; Kusuma, H.S.; Neolaka, Y.A.B. Hydrochar preparation from wild weeds (Amaranthus sp.) And its application as artificial soil for hydroponic system. Kuwait J. Sci. 2024, 51, 100277. [Google Scholar]
- Ding, S.D.; Wang, B.Y.; Feng, Y.Y.; Fu, H.B.; Feng, Y.F.; Xie, H.F.; Xue, L.H. Livestock manure-derived hydrochar improved rice paddy soil nutrients as a cleaner soil conditioner in contrast to raw material. J. Clean. Prod. 2022, 372, 133798. [Google Scholar] [CrossRef]
- Kravchenko, E.; Dela Cruz, T.L.; Sushkova, S.; Rajput, V.D. Effect of wood and peanut shell hydrochars on the desiccation cracking characteristics of clayey soils. Chemosphere 2024, 358, 142134. [Google Scholar] [CrossRef]
- Zhang, R.C.; Zhang, Y.Z.; Goei, R.; Oh, W.D.; Zhang, Z.; He, C. Sustainable utilization of Sedum plumbizincicola as superior hydrochar for efficient nutrients recovery. J. Environ. Manag. 2023, 344, 118441. [Google Scholar] [CrossRef]
- Mahmood Al-Nuaimy, M.N.; Azizi, N.; Nural, Y.; Yabalak, E. Recent advances in environmental and agricultural applications of hydrochars: A review. Environ. Res. 2024, 250, 117923. [Google Scholar] [CrossRef]
- Shan, G.C.; Li, W.G.; Bao, S.S.; Li, Y.Y.; Tan, W.B. Co-hydrothermal carbonization of agricultural waste and sewage sludge for product quality improvement: Fuel properties of hydrochar and fertilizer quality of aqueous phase. J. Environ. Manag. 2023, 326, 116781. [Google Scholar] [CrossRef]
- Xue, G.; Zhang, L.L.; Fan, X.Y.; Luo, K.J.; Guo, S.P.; Chen, H.; Li, X.; Jian, Q.W. Responses of soil fertility and microbiomes of atrazine contaminated soil to remediation by hydrochar and persulfate. J. Hazard. Mater. 2022, 435, 128944. [Google Scholar] [CrossRef]
- Suarez, E.; Tobajas, M.; Mohedano, A.F.; Reguera, M.I.A.; Esteban, E.; de la Rubia, M.A. Evaluation of Green Waste Biochar and Hydrochar Application as Soil Amendment. 2022. Available online: http://generalchemistry.chemeng.ntua.gr/uest/corfu2022/proceedings/III/1430_Suarez_Paper.pdf (accessed on 13 February 2025).
- Fei, Y.H.; Zhao, D.; Liu, Y.; Zhang, W.H.; Tang, Y.Y.; Huang, X.X.; Wu, Q.H.; Wang, Y.X.; Xiao, T.F.; Liu, C.S. Feasibility of sewage sludge derived hydrochars for agricultural application: Nutrients (N, P, K) and potentially toxic elements (Zn, Cu, Pb, Ni, Cd). Chemosphere 2019, 236, 124841. [Google Scholar] [CrossRef] [PubMed]
- Baronti, S.; Alberti, G.; Camin, F.; Criscuoli, I.; Genesio, L.; Mass, R.; Vaccari, F.P.; Ziller, L.; Miglietta, F. Hydrochar enhances growth of poplar for bioenergy while marginally contributing to direct soil carbon sequestration. Glob. Change Biol. Bioenergy 2017, 9, 1618–1626. [Google Scholar] [CrossRef]
- Luo, X.; Du, H.Y.; Du, J.; Zhang, X.C.; Xiao, W.Y.; Qin, L. The influence of biomass type on hydrothermal carbonization: Role of calcium oxalate in enhancing carbon sequestration of hydrochar. J. Environ. Manag. 2024, 349, 119586. [Google Scholar] [CrossRef] [PubMed]
- Marris, E. Putting the carbon back: Black is the new green. Nature 2006, 442, 624–626. [Google Scholar] [CrossRef] [PubMed]
- Dinjus, E.; Kruse, A.; Troeger, N. Hydrothermal carbonization: 1. Influence of lignin in lignocelluloses. Chem. Ing. Tech. 2011, 83, 1734–1741. [Google Scholar] [CrossRef]
- Rehman, A.; Ma, H.Y.; Irfan, M.; Ahmad, M. Does carbon dioxide, methane, nitrous oxide, and GHG emissions influence the agriculture? Evidence from China. Environ. Sci. Pollut. Res. 2020, 27, 28768–28779. [Google Scholar] [CrossRef]
- Chen, D.Y.; Zhou, Y.B.; Xu, C.; Lu, X.Y.; Liu, Y.; Yu, S.; Feng, Y.F. Water-washed hydrochar in rice paddy soil reduces N2O and CH4 emissions: A whole growth period investigation. Environ. Pollut. 2021, 274, 116573. [Google Scholar] [CrossRef]
- Hodge, I.; Quille, P.; O’Connell, S. A review of potential feed additives intended for carbon footprint reduction through methane abatement in dairy cattle. Animals 2024, 14, 568. [Google Scholar] [CrossRef]
- Nakazawa, T. Current understanding of the global cycling of carbon dioxide, methane, and nitrous oxide. Proc. Jpn. Acad. Ser. B-Phys. Biol. Sci. 2020, 96, 394–419. [Google Scholar] [CrossRef]
- Al-Naqeb, G.; Sidarovich, V.; Scrinzi, D.; Mazzeo, I.; Robbiati, S.; Pancher, M.; Fiori, L.; Adami, V. Hydrochar and hydrochar co-compost from OFMSW digestate for soil application: 3. Toxicological evaluation. J. Environ. Manag. 2022, 320, 115910. [Google Scholar] [CrossRef]
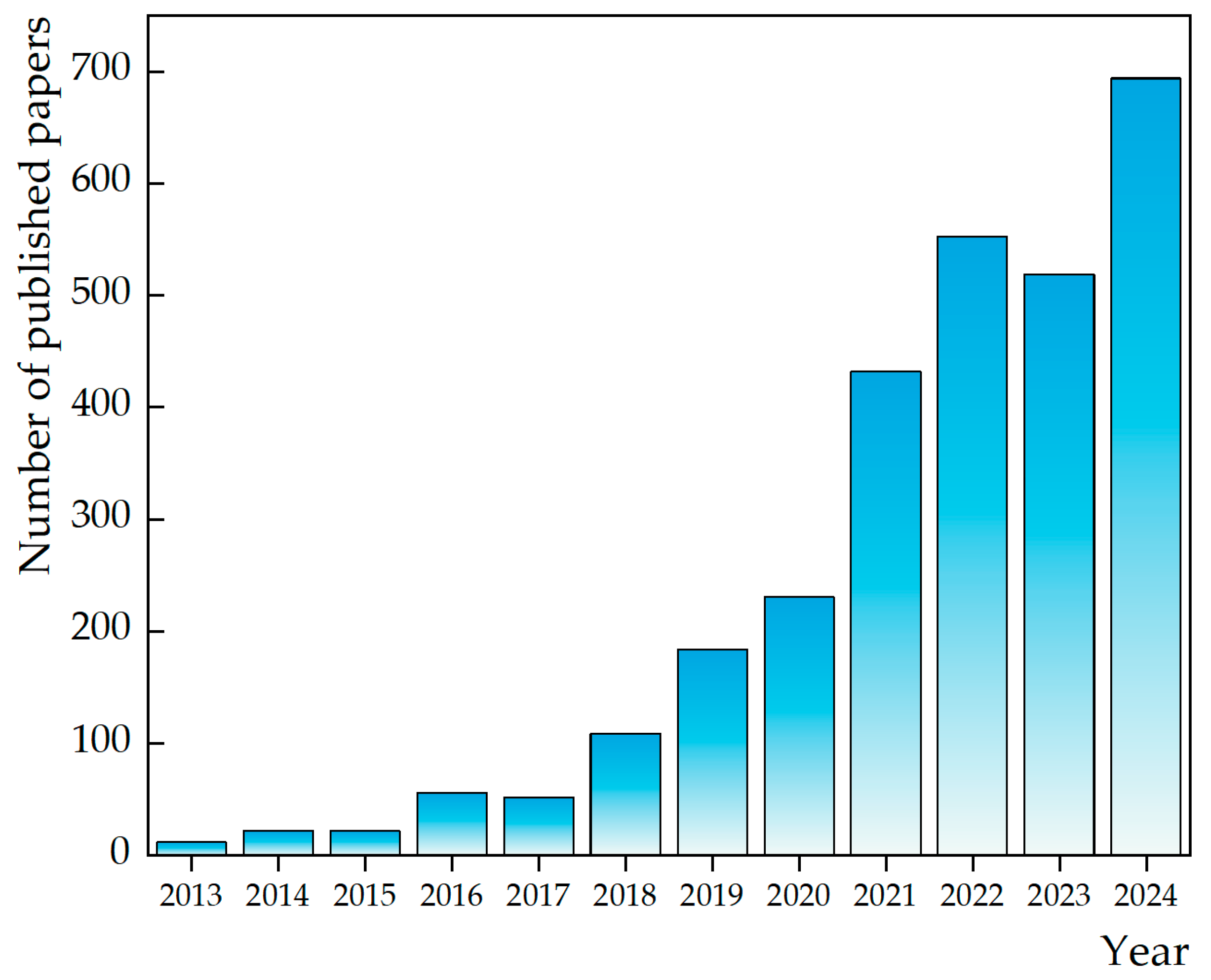
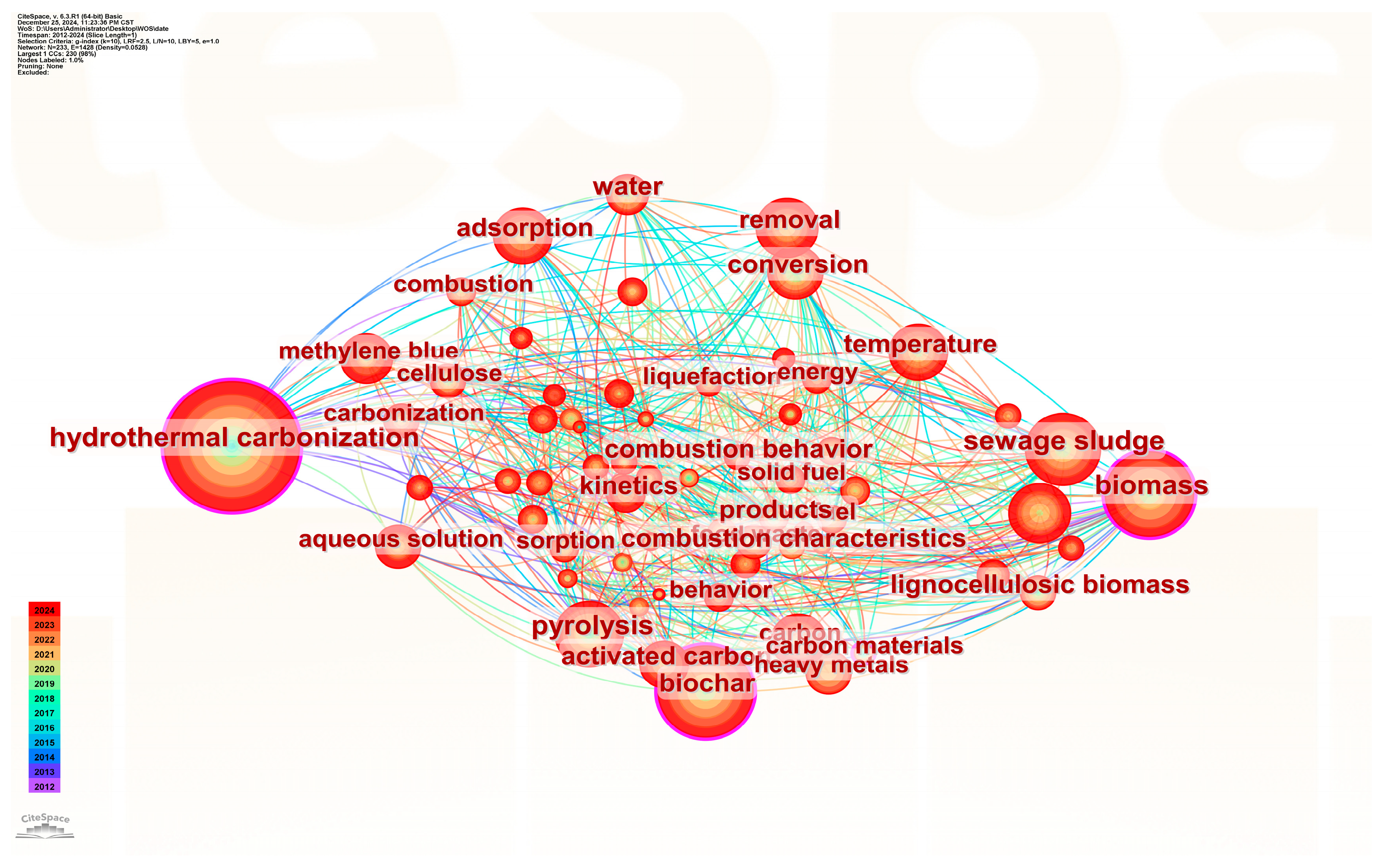
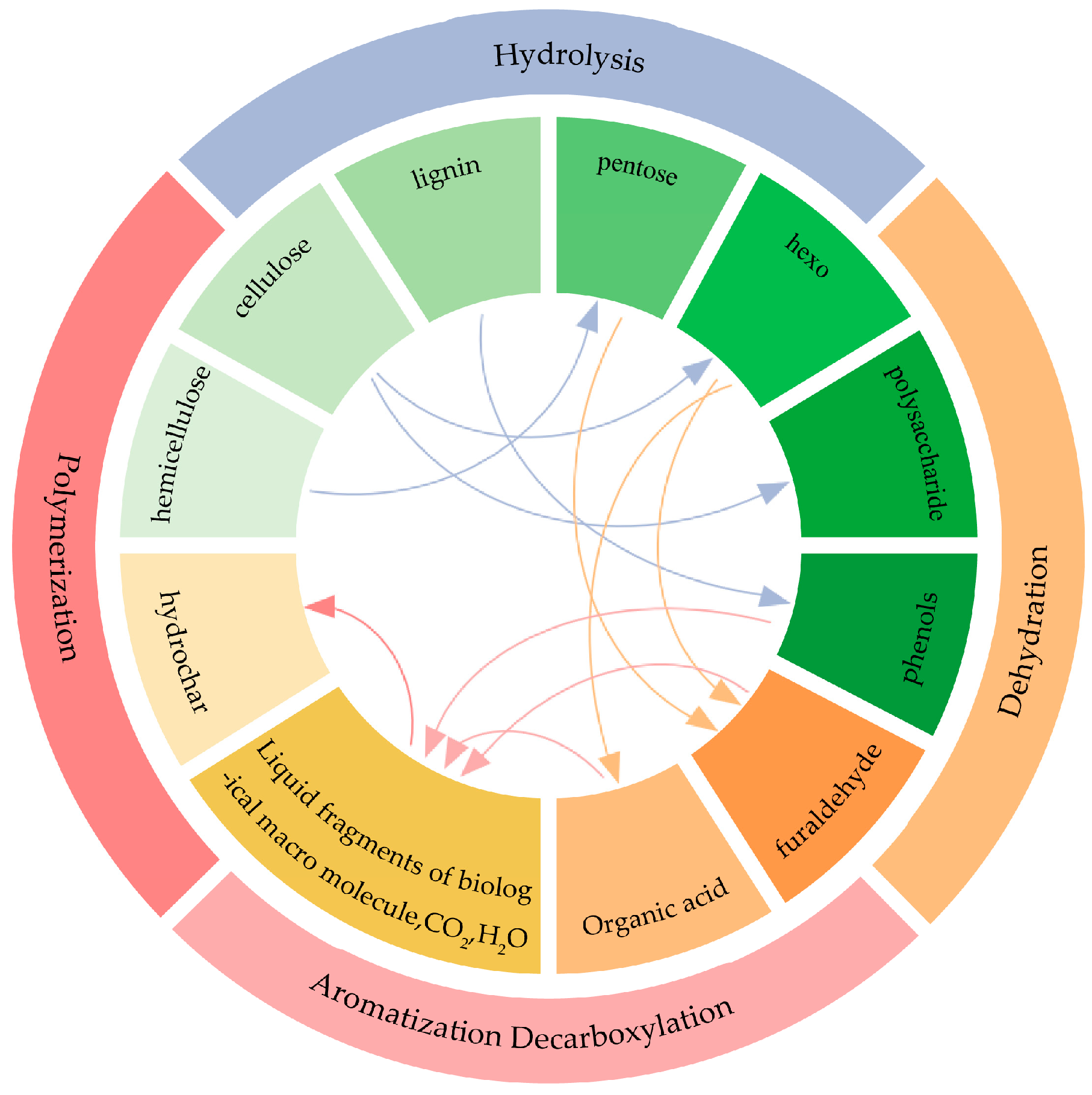
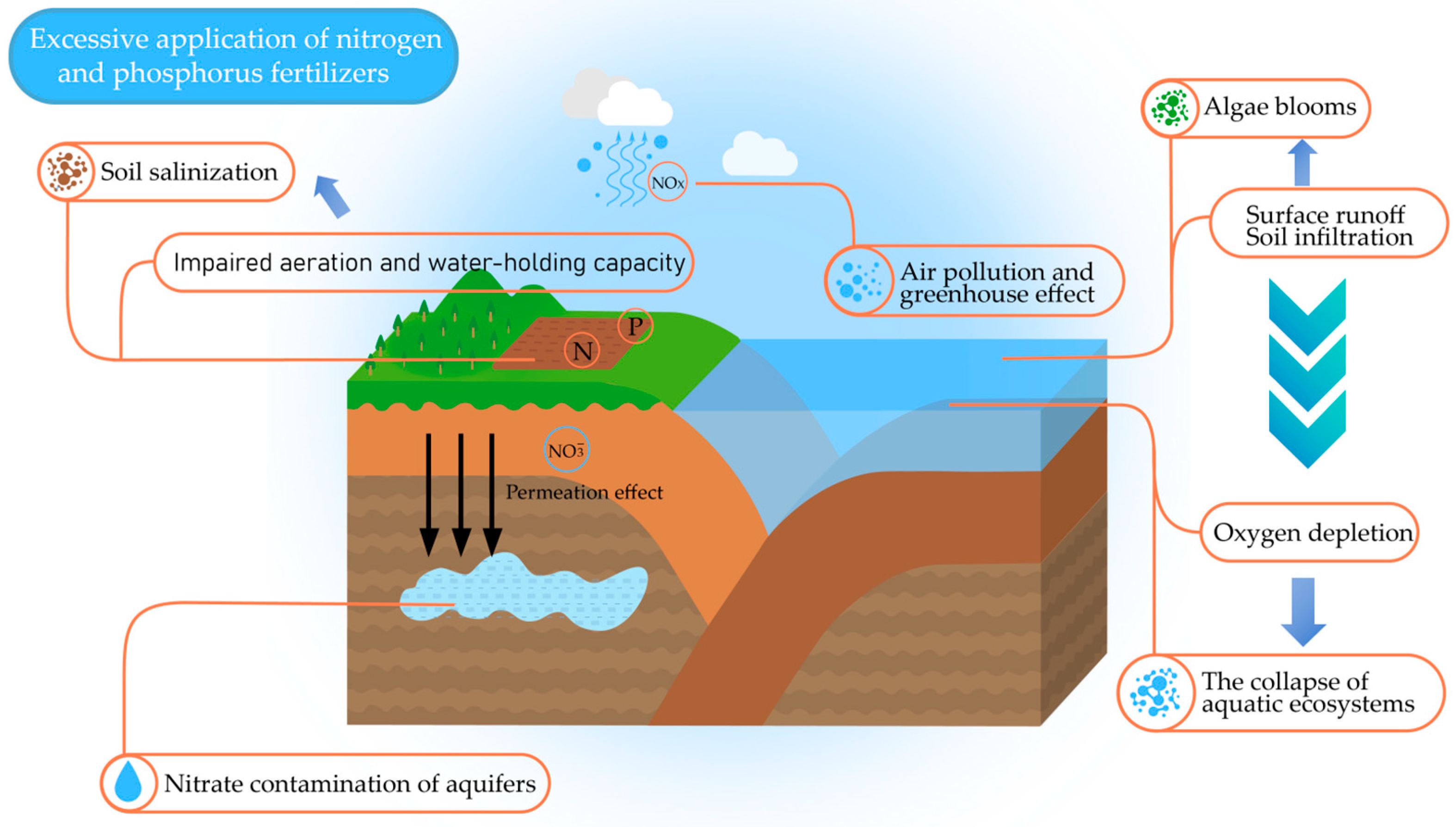
| Influencing Factor | Influence Mechanism | Large Degree of Influence | Small Degree of Influence | References |
|---|---|---|---|---|
| Temperature | The key factor affecting the degree of carbonization, pore structure, and surface functional group distribution of products | Increases the reaction rate and promotes the thermal decomposition of macromolecular organic matter | Hydrolysis and degradation reactions are incomplete, the carbon content of the product is low, and the pore structure is underdeveloped | [92] |
| Time | Affects the complete degree of hydrolysis, polymerization, and the carbonization of biomass | Excessive carbonization, pore structure changes, surface area, and decreased adsorption capacity | Results in the incomplete thermal decomposition of biomass, affecting the carbon yield and carbon quality | [91,96] |
| Pressure | Affects the degree of decomposition of biomass and the structural characteristics of carbonized products | Enhances the absorption of CO2 and increases the surface functional groups and porosity | Low pressure is not conducive to the destruction of the molecular structure of biomass, decreasing the degree of carbonization | [40] |
| pH | Affects the surface properties and pore structures of carbon materials | Under alkaline conditions, lignin degradation and carbonization are promoted, which is conducive to the formation of hydrochar | Under acidic conditions, it is beneficial to the hydrolysis of cellulose and hemicellulose and produces hydrochar with high calorific value | [88,93,94] |
| Type of Raw Material | Raw Material | Temperature (°C) | Time (h) | Modified Condition | Yield of Hydrochar | Results | References |
|---|---|---|---|---|---|---|---|
| Agricultural residues | Straw and rape stalks | 150, 180, and 210 | 1 | Microwave assisted | 43.25 wt%–72.77 wt% | Increased concentrations of organic matter and nutrient ions in liquid products | [112] |
| Agricultural residues | Dried rice straw | 200 | 2 | Microwave assisted | - | Hydrochar has the potential to increase SOC stocks in rice without adverse effects on rice production or carbon emissions | [44] |
| Agricultural residues | Phragmites australis | 200 | 24 | PMS activation | - | Excellent PMS catalytic activity: under the best conditions, it can achieve almost total QC degradation efficiency | [113] |
| Livestock and poultry manure | Cattle manure | 190, 230 | 1, 3 | - | 76 and 57 wt% | The adsorption capacity of hydrochar and recovery purity of hydroxyapatite were improved | [114] |
| Livestock and poultry manure | Swine manure | 180, 220, 260 | 1, 2, 3 | FeCl3 impregnation | 54.7 wt%–89.8 wt% | Improved C stability and P availability in HC-Fe, especially at low pH (4), 220 °C, and 2 h | [115] |
| Livestock and poultry manure | Pig manure | 180 | 1 | - | 53.3 wt% | The proportion of residual phosphorus in soil decreased by 23.8–26.3% | [116] |
| Urban organic waste | Sludge material | 180 | 0.5 | Acid treatment | 75.7 wt% | The P recovery rate is the highest under mild conditions, and the holding time is short | [117] |
| Urban organic waste | Kitchen waste | 225 | 1.5–9.0 | Liquid phase cycle | 67.42 wt%, 66.86 wt% | The hydrochar prepared at 1.5 h showed better electrochemical properties than that at 9.0 h | [118] |
| Urban organic waste | Food waste | 180, 200, and 220 | 2 | - | 69.46 wt%, 68.5 wt%, 65.35 wt% | The hydrochar prepared at 220 °C had the highest calorific value (HHV: 23.61 MJ/kg) | [119] |
| Algae | Chlorella pyrenoidosa, Undaria pinnatifida | 180–260 | 1–4 | - | 12 wt%–35 wt% | Microalgae–macroalgae synergies impact product aspects and nitrogen transformations, being temperature- and time-dependent | [120] |
| Algae | Chlamydomonas debaryana | 200 | 6 | - | 28.3 wt% | Increased carbon content, decreased nitrogen content, and improved HHV in hydrochar | [121] |
| Raw Material | Temperature (°C) | Time (h) | Modified Condition | Utilization Type | Results | References |
|---|---|---|---|---|---|---|
| Sludge | 180 | 0.5 | Acid treatment | Solid-phase product | Maximized recovery of P (99%), as well as carbon (62%) and nitrogen (43%) | [117] |
| Straw and acid whey | 250 | 1 | Co-hydrothermal carbonization | Solid-phase product | HHV increased by 53.6%, yield increased by 20.0%, and carbon content increased by 42.7% | [52] |
| Aloe leaf | 180, 220 | 1–8 | KOH treatment | Solid-phase product | After 8 h of treatment under alkaline conditions, Ca and P concentrations increased to 10.4% and 7382 mg·kg−1, respectively | [147] |
| Sewage sludge | 200, 230, and 260 | 1 | Struvite crystallization and aqueous-phase recycling | Liquid-phase product | Recovery and reuse of nitrogenous nutrients in the water phase | [149] |
| Urban green waste | 180, 220 | 5 | Water-phase recirculation | Liquid-phase product | With water recirculation, the product’s mass yield increases | [150] |
| Orange peel waste | 180–260 | 0.33–4 | Water-phase recirculation | Liquid-phase product | The mass yield of hydrochar was increased by 0.5 to 11 wt% on the dry basis | [151] |
| Technology Type | Method | Application | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Chemical Precipitation | A precipitating agent was added to the liquid byproduct to transform the dissolved nitrogen and phosphorus compounds into less soluble precipitates | Process into solid fertilizer or apply directly to soil | Simple operations and low costs | A large amount of sediment is produced, requiring subsequent treatment | [153,154,155,156,157,158] |
| Ion Exchange | Ion-exchange resin and other materials were used to selectively adsorb nitrogen and phosphorus ions from a hydrothermal solution | Made into fertilizer | High recovery efficiency | The need for the regular regeneration of resin and the relatively high cost | [159,160,161,162,163] |
| Reverse Osmosis | The selective permeability of a semi-permeable membrane is used to separate nitrogen and phosphorus from water in a hydrothermal solution | Made into fertilizer | High-efficiency separation | High equipment investment and operating costs | [164,165,166,167,168,169] |
| Electrodialysis | A DC voltage is applied in the electrolyzer, using the difference in ion migration in the selectively permeable film | Separation of liquid-phase product ions | Automatic control, high separation efficiency | Requires a stable power supply and high equipment maintenance | [170,171,172,173] |
| Biological Methods | Nitrogen and phosphorus nutrients are converted into microbial biomass by microbial metabolism | Nitrogen and phosphorus are absorbed and converted into biomass | Environmental protection, low costs | The treatment cycle is long, and the culture and management of microorganisms are required | [174,175,176,177] |
| Membrane Bioreactor | The metabolic function of microorganisms is combined with the physical screening function of membranes | Achieves biotransformation and solid–liquid separation | High treatment efficiency, less pollution | Membrane material and operation and maintenance costs are relatively high | [178,179,180,181,182,183,184] |
| Raw Material | Temperature (°C) | Time (h) | Modified Condition | Results | Application Aspect | References |
|---|---|---|---|---|---|---|
| Waste wood | 180 | 8 | Asphalt modification | HTC significantly improves the high-temperature and fatigue properties of asphalt | Resource recycling | [192] |
| Agricultural organic residues | 250 | 4 | ZnO or ZnFe2O4 composite | Hydrochar-based composites have higher photocatalytic potential in conjugated estrogens | Resource recycling | [193] |
| Wild almond shell | 160–240 | 4–12 | Chemothermal activation | The maximum adsorption capacities of NOR, MB, and SY were 85.37, 153.46, and 93.35 mg/g, respectively | Resource recycling | [194] |
| Weeds | 200 | 8 | - | The swelling capacity of hydrochar-synthesized soil is 32%, and it has good fertilizer storage and slow-release characteristics | Soil amendment | [195] |
| Cattle manure | 180, 260 | 1 | - | The content of SOC and DOC in soil extracts was significantly increased by hydrochar returning to the field | Soil amendment | [196] |
| Wood and peanut shells | 250 | 1 | - | The addition of hydrochar reduced the fracture strength factor by 43% and 51% | Soil amendment | [197] |
| S. plumbizincicola | 260 | 2 | - | At 45 °C, the maximum phosphate and ammonium adsorption capacities reached 52.46 and 27.56 mg/g, respectively | Soil amendment | [198] |
| Raw Material | Temperature (°C) | Time (h) | Modified Condition | Results | Application Effect | References |
|---|---|---|---|---|---|---|
| Agricultural waste and sewage sludge | 220 | 1 | Co-hydrothermal carbonization | The liquid phase replaces 60% of the chemical fertilizer with liquid fertilizer to promote the growth of cabbage | Reduced fertilizer use | [200] |
| Sewage sludge | 250 | 2 | - | Hydrochar restores the abundance, pH, and urease activity of soil bacteria induced by PDS | Improved soil fertility | [201] |
| Swine and cattle manure | 180, 220, and 260 | 1 | - | Hydrochar changes the composition of N and P in soil–water systems by inhibiting the activity of soil urease and acid phosphatase | Improved soil fertility | [48] |
| Plant fibers | 160 | 4 | Silicon modification | The Cd2+ removal rate of hydrochar in actual water is 93.8% | Reduced environmental pollution | [124] |
| Swine and cattle manure | 180, 220, and 260 | 1 | - | Hydrochar treatment reduces the concentrations of ammonia nitrogen and total phosphorus by 12.9–36.9% and 11.7–20.7% | Reduced environmental pollution | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Zhang, T. Advances in Hydrothermal Carbonization for Biomass Wastewater Valorization: Optimizing Nitrogen and Phosphorus Nutrient Management to Enhance Agricultural and Ecological Outcomes. Water 2025, 17, 800. https://doi.org/10.3390/w17060800
Liu G, Zhang T. Advances in Hydrothermal Carbonization for Biomass Wastewater Valorization: Optimizing Nitrogen and Phosphorus Nutrient Management to Enhance Agricultural and Ecological Outcomes. Water. 2025; 17(6):800. https://doi.org/10.3390/w17060800
Chicago/Turabian StyleLiu, Guoqing, and Tao Zhang. 2025. "Advances in Hydrothermal Carbonization for Biomass Wastewater Valorization: Optimizing Nitrogen and Phosphorus Nutrient Management to Enhance Agricultural and Ecological Outcomes" Water 17, no. 6: 800. https://doi.org/10.3390/w17060800
APA StyleLiu, G., & Zhang, T. (2025). Advances in Hydrothermal Carbonization for Biomass Wastewater Valorization: Optimizing Nitrogen and Phosphorus Nutrient Management to Enhance Agricultural and Ecological Outcomes. Water, 17(6), 800. https://doi.org/10.3390/w17060800







