Bioremediation of Endocrine Disruptors (EDs): A Systematic Review of Fungal Application in ED Removal from Wastewater
Abstract
1. Introduction
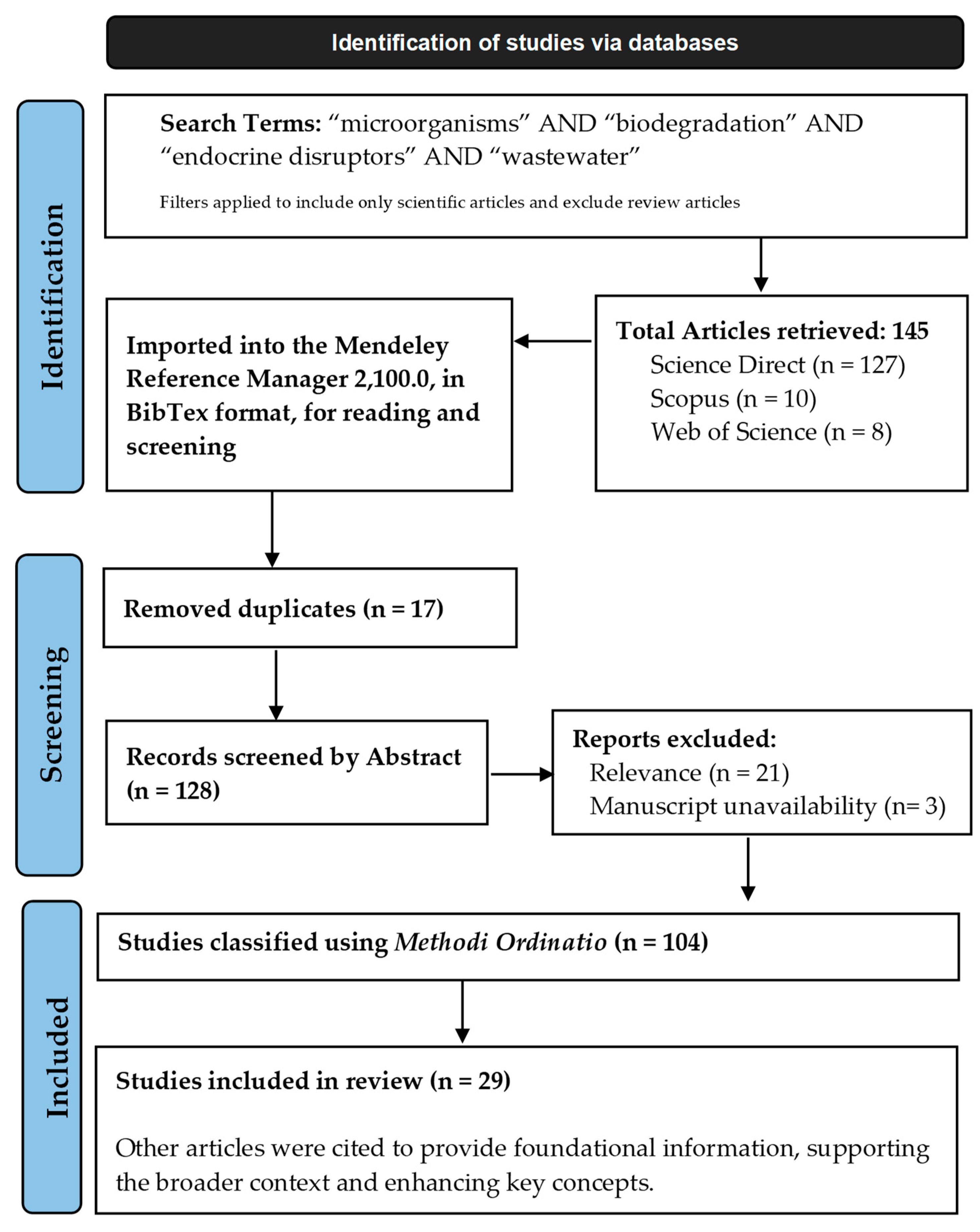
2. Methodology
3. Results and Discussion
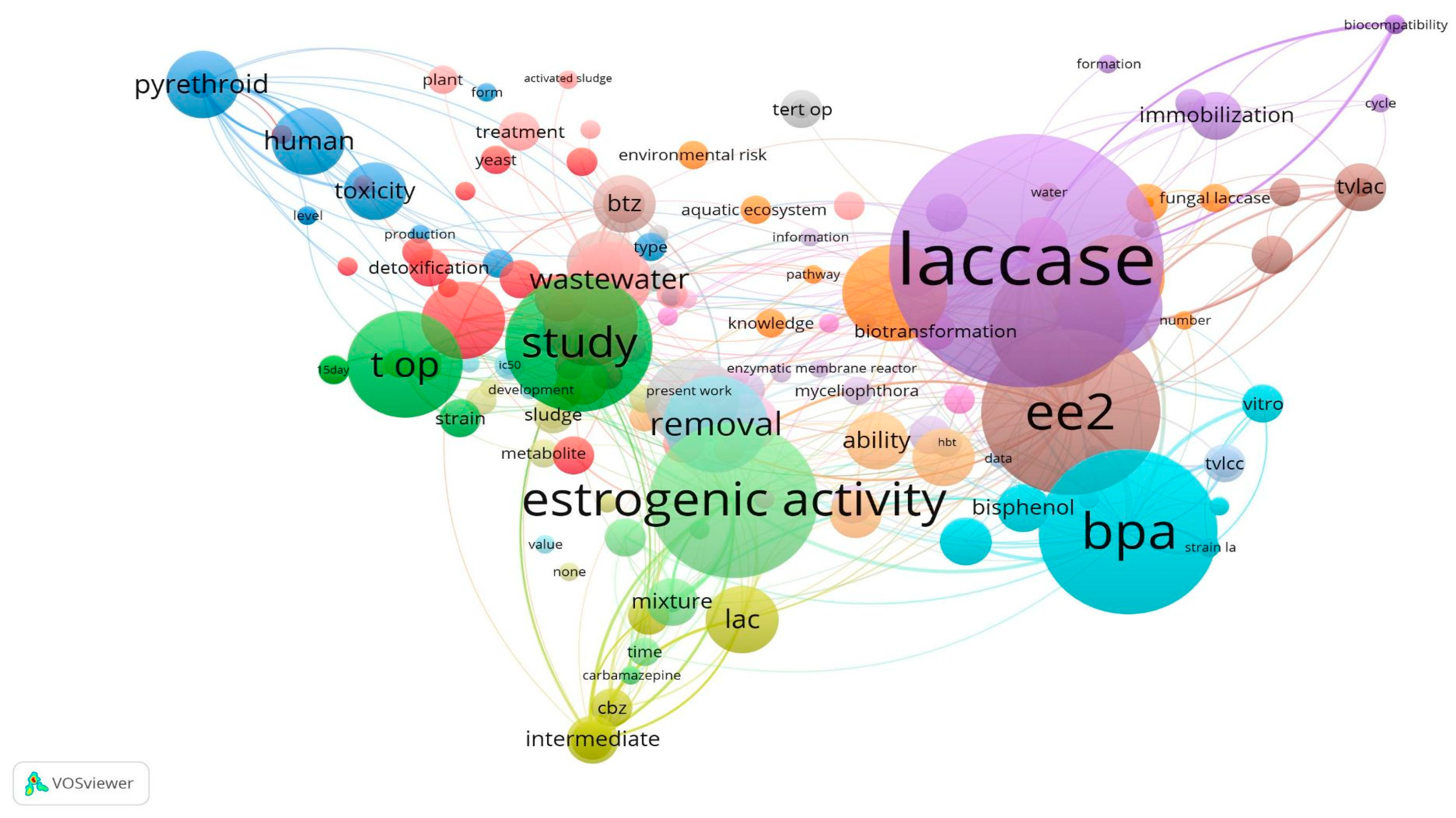
3.1. Overview of Key Findings
3.2. Fungal Applications for the Removal of Endocrine Disruptors
3.3. Laccase-Mediated Degradation of Endocrine Disruptors
3.4. Applications of Fungi and Their Enzymes in Real Wastewater Treatment
| Fungus | EDs | Conditions | Main Results | Ref. |
|---|---|---|---|---|
| Trametes versicolor, Pleurotus ostreatus, Phanerochaete chrysosporium | PhOH, Parabens, Phthalates | Fed-batch and starvation strategies reduced fresh biomass input and external nutrients. The fungus operated in two bioreactors over one week with five consecutive degradation cycles of EDs. | Best results with T. versicolor It efficiently removed all EDs without additional nutrients, showing potential for repeated cycles in bioreactors. Biotransformation was the primary removal mechanism, with minimal biosorption. | [16] |
| Trametes versicolor NRRL 66313 | E2, single A mixture of EDs: E1, E2, EE2, BPA, ATZ, CBZ, DEET, OBZ, TCS | Fungus was grown in glucose-amended, sterile wastewater (5 g/L). Removals performed in aerated Erlenmeyer incubated for 8 days at room temperature (25 ± 2 °C) and spiked with 5 mg/L of E2 or the mixture of EDCs (350 μg/L each). Abiotic and heat-killed fungus controls were also tested. | T. versicolor reduced E2 from 5 mg/L to below detection levels within 5 h, with E1 as a metabolite, which was subsequently removed. For the mixture of EDs, 62–100% removal was achieved within 3.5 h, and estrogenic activity reduced by 77% (compared to 4–8% in controls). After 12 h, estrogenic activity reduction exceeded 98% (vs. 24–42% for controls). | [28] |
| Trametes versicolor | ATZ, BPA, CBZ, TCS | Commercial enzymes, biodegradation of estrogenic pollutants in wastewater. | Near-total reduction in estrogenic activity. >80% of atrazine in contaminated water within 72 h by laccase. High efficiency across 5 degradation cycles without external nutrients. Laccase. The process breaks down complex aromatic pesticide structures into simpler, less toxic byproducts, which were further degraded by microbial consortia. | [30] |
| Pleurotus pulmonarius LBM 105 Trametes sanguinea LBM 023 | PCBs | Single culture vs. consortium in bioremediation of PCB-contaminated transformer oil. | Pleurotus pulmonarius LBM 105 showed the highest PCB degradation 95.4% PCB removal, outperforming Trametes sanguinea LBM 023 and fungal consortium. | [35] |
| Anthracophyllum discolor | PAHs B[a]P | Biodegradation in liquid medium and autoclaved contaminated soil. | 75% PAH removal in soil. Manganese peroxidase production linked to degradation. Lower efficiency in non-autoclaved soils. | [36] |
| Aspergillus niger AN 400 | ATZ | Batch reactors with dispersed fungal biomass, glucose as co-substrate. | 40% ATZ removal without co-substrate, doubled efficiency with glucose addition at 3 g/L. Higher glucose levels reduced degradation due to competition. | [37] |
| Trametes hirsuta La-7 | BPA, E1, E2 | In vivo and in vitro degradation using extracellular laccase and mycelium. | >80% BPA removal within 6 h. Metabolized EDs through six mechanisms, unaffected by BPA presence in plant test. | [38] |
| Phanerochaete chrysosporium | 6:2 FTOH | Transformation of PFAS in bioreactors with Kirk medium with and without glucose, supplemented with organic nutrients like lignocellulosic powder. | Phanerochaete chrysosporium biotransformed 6:2 FTOH into perfluorocarboxylic acids (PFCAs), polyfluorocarboxylic acids, and intermediates within 28 days. Main product was 5:3 FTCA, making up 32–43% of the initial 6:2 FTOH, with minor amounts of PFCAs (5.9%). Efficient EDs degradation, but with some residual estrogenic activity. | [39] |
| Trametes versicolor,
Irpex lacteus, Bjerkandera adusta, Phanerochaete chrysosporium, Phanerochaete magnoliae, Pleurotus ostreatus, Pycnoporus cinnabarinus, Dichomitus squalens | NP, n-NP, BPA, EE2, TCS | Biodegradation in static conditions at 28 °C, malt extract–glucose medium. | I. lacteus and P. ostreatus were the most efficient degraders, >90% and >80% in 7 days, respectively. Both fungi degraded pollutants below detection limit within the first 3 days. Estrogenic activities decreased with advanced degradation, but residual activity was observed in cultures of I. lacteus, P. ostreatus, and P. chrysosporium (28–85% of initial). B. adusta showed an increase in estrogenic activity during NP degradation, suggesting endocrine-active intermediates. Ligninolytic enzyme activity was affected by the ED, indicating potential stimulation or suppression during biodegradation. | [40] |
| Pleurotus ostreatus HK 35 | BPA, E2 | Trickle-bed reactor, lab and real wastewater treatment. | Degraded >90% of EDs in 12 days, >76% ED removal in pilot reactor. | [60] |
| Trametes versicolor (pellets) | PhACs, EDs | Fluidized bed bioreactor treating hospital wastewater under sterile and non-sterile conditions. | Removed 46 out of 51 detected EDs and PhACs. 83.2% removal in sterile conditions; 53.3% in non-sterile environments. Complete removal of DIF. | [61] |
4. Methodologies for Assessing Estrogenic Activity
5. Critical Assessment of Research Gaps and Limitations
6. Conclusions and Future Perspectives
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. Persistent organic chemicals of emerging environmental concern. In Environmental Deterioration and Human Health: Natural and Anthropogenic Determinants; Abdul, M., Elisabeth, G., Rais, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 163–213. ISBN 978-94-007-7889-4. [Google Scholar]
- Ismail, N.A.H.; Wee, S.Y.; Aris, A.Z. Multiclass of endocrine disrupting compounds in aquaculture ecosystems and health impacts in exposed biota. Chemosphere 2017, 188, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Morin-Crini, N.; Lichtfouse, E.; Liu, G.; Balaram, V.; Ribeiro, A.R.L.; Lu, Z.; Stock, F.; Carmona, E.; Teixeira, M.R.; Picos-Corrales, L.A.; et al. Emerging Contaminants: Analysis, Aquatic Compartments and Water Pollution. In Emerging Contaminants, Vol. 1. Environmental Chemistry for a Sustainable World; Morin-Crini, N., Lichtfouse, E., Crini, G., Eds.; Springer: Cham, Switzerland, 2021; Volume 65. [Google Scholar] [CrossRef]
- Werkneh, A.A.; Gebru, S.B.; Redae, G.H.; Tsige, A.G. Removal of endocrine disrupters from the contaminated environment: Public health concerns, treatment strategies and future perspectives—A review. Heliyon 2022, 8, e09206. [Google Scholar] [CrossRef]
- Jeon, H.-K.; Chung, Y.; Ryu, J.-C. Simultaneous determination of benzophenone-type UV filters in water and soil by gas chromatography–mass spectrometry. J. Chromatogr. A 2006, 1131, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Matike, D.M.E.; Ngole-Jeme, V.M. A Review of Phthalates and Phenols in Landfll Environments: Occurrence, Fate and Environmental Implications. Int. J. Environ. Res. 2024, 18, 79. [Google Scholar] [CrossRef]
- Lzaod, S.; Dutta, T. Biotransformation of 4,4′-dihydroxybiphenyl and dienestrol by laccase from Trametes versicolor. J. Hazard. Mater. Adv. 2022, 8, 100169. [Google Scholar] [CrossRef]
- Kasonga, T.K.; Coetzee, M.A.A.; van Zijl, C.; Momba, M.N.B. Removal of pharmaceutical estrogenic activity of sequencing batch reactor effluents assessed in the T47D-KBluc reporter gene assay. J. Environ. Manag. 2019, 240, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Alves, M. Chapter 4: Dyes—Environmental impact and remediation. In Environmental Protection Strategies for Sustainable Development, Strategies for Sustainability; Malik, A., Grohmann, E., Eds.; Springer Science + Business Media B.V.: Dordrecht, The Netherlands, 2012; pp. 111–154. [Google Scholar] [CrossRef]
- Lloret, L.; Eibes, G.; Ló-Chau, T.A.; Moreira, M.T.; Feijoo, G.; Lema, J.M. Laccase-catalyzed degradation of anti-inflammatories and estrogens. Biochem. Engin. J. 2010, 51, 124–131. [Google Scholar] [CrossRef]
- Asif, M.B.; Hai, F.I.; Hou, J.; Price, W.E.; Nghiem, L.D. Impact of Wastewater Derived Dissolved Interfering Compounds on Growth, Enzymatic Activity and Trace Organic Contaminant Removal of White Rot Fungi-A Critical Review. J. Environ. Manag. 2017, 201, 89–109. [Google Scholar] [CrossRef]
- Asif, M.B.; Hai, F.I.; Singh, L.; Price, W.E.; Nghiem, L.D. Degradation of Pharmaceuticals and Personal Care Products by White-Rot Fungi-Critical Review. Water Res. 2017, 123, 503–520. [Google Scholar] [CrossRef]
- Elayaperumal, S.; Sivamani, Y.; Bhattacharya, D.; Lahiri, D.; Nag, M. Eco-Friendly Biosurfactant Solutions for Petroleum Hydrocarbon Cleanup in Aquatic Ecosystems. Sustain. Chem. Environ. 2025, 100207. [Google Scholar] [CrossRef]
- Mishra, S.; Lin, Z.; Pang, S.; Zhang, W.; Bhatt, P.; Chen, S. Recent Advanced Technologies for the Characterization of Xenobiotic-Degrading Microorganisms and Microbial Communities. Front Bioeng Biotechnol. 2021, 9, 632059. [Google Scholar] [CrossRef]
- Pezzella, C.; Macellaro, G.; Sannia, G.; Raganati, F.; Olivieri, G.; Marzocchella, A.; Schlosser, D.; Piscitelli, A. Exploitation of Trametes Versicolor for Bioremediation of Endocrine Disrupting Chemicals in Bioreactors. PLoS ONE 2017, 12, e0178758. [Google Scholar] [CrossRef]
- Dell’ Anno, F.; Rastelli, E.; Sansone, C.; Brunet, C.; Ianora, A.; Dell’ Anno, A. Bacteria, Fungi and Microalgae for the Bioremediation of Marine Sediments Contaminated by Petroleum Hydrocarbons in the Omics Era. Microorganisms 2021, 9, 1695. [Google Scholar] [CrossRef] [PubMed]
- Razia, S.; Hadibarata, T.; Lau, S.Y. A review on biodegradation of Bisphenol A (BPA) with bacteria and fungi under laboratory conditions. Int. Biodet. Biod. 2024, 195, 105893. [Google Scholar] [CrossRef]
- Latif, W.; Ciniglia, C.; Iovinella, M.; Shafiq, M.; Papa, S. Role of white rot fungi in industrial wastewater treatment: A review. Appl. Sci. 2023, 13, 8318. [Google Scholar] [CrossRef]
- Zhuo, R.; Fan, F. A Comprehensive Insight into the Application of White Rot Fungi and Their Lignocellulolytic Enzymes in the Removal of Organic Pollutants. Sci. Total Environ. 2021, 778, 146132. [Google Scholar] [CrossRef]
- Harms, H.; Schlosser, D.; Wick, L. Untapped potential: Exploiting fungi in bioremediation of hazardous chemicals. Nat. Rev. Microbiol. 2011, 9, 177–192. [Google Scholar] [CrossRef]
- Kumar, A.; Chandra, R. Ligninolytic enzymes and their mechanisms for degradation of lignocellulosic waste in the environment. Heliyon 2020, 6, e03170. [Google Scholar] [CrossRef] [PubMed]
- Dinakarkumar, Y.; Ramakrishnan, G.; Gujjula, K.R.; Vasu, V.; Balamurugan, P.; Murali, G. Fungal bioremediation: An overview of the mechanisms, applications and future perspectives. Environ. Chem. Ecotox. 2024, 6, 293–302. [Google Scholar] [CrossRef]
- Bala, S.; Garg, D.; Thirumalesh, B.V.; Sharma, M.; Sridhar, K.; Inbaraj, B.S.; Tripathi, M. Recent Strategies for Bioremediation of Emerging Pollutants: A Review for a Green and Sustainable Environment. Toxics 2022, 10, 484. [Google Scholar] [CrossRef]
- Pagani, R.N.; Kovaleski, J.L.; Resende, L.M. Methodi Ordinatio: A proposed methodology to select and rank relevant scientific papers enc.ompassing the impact factor, number of citation, and year of publication. Scientometrics 2015, 105, 2109–2135. [Google Scholar] [CrossRef]
- Mesacasa, L.; Cabral, F.S.; Fochi, D.A.T.; Oliveira, W.S.; Oliveira, F.; Kersting, M.; Colares, G.S.; Rodriguez, A.L.; Lutterbeck, C.A.; Konrad, O.; et al. Constructed wetlands and the role of the fungal community for wastewater treatment: A review. Ecohydrol. Hydrobiol. 2024; in press. [Google Scholar] [CrossRef]
- Pundir, A.; Thakur, M.S.; Prakash, S.; Kumari, N.; Sharma, N.; Parameswari, E.; He, Z.; Nam, S.; Thakur, M.; Puri, S.; et al. Fungi as versatile biocatalytic tool for treatment of textile wastewater effluents. Environ. Sci. Eur. 2024, 36, 185. [Google Scholar] [CrossRef]
- Shreve, M.J.; Brockman, A.; Hartleb, M.; Prebihalo, S.; Dorman, F.L.; Brennan, R.A. The white-rot fungus Trametes versicolor reduces the estrogenic activity of a mixture of emerging contaminants in wastewater treatment plant effluent. Int. Biodet. Biodeg. 2016, 109, 132–140. [Google Scholar] [CrossRef]
- Lloret, L.; Eibes, G.; Moreira, M.T.; Feijoo, G.; Lema, J.M. Removal of Estrogenic Compounds from Filtered Secondary Wastewater Effluent in a Continuous Enzymatic Membrane Reactor. Identification of Biotransformation Products. Environ. Sci. Technol. 2013, 47, 4536–4543. [Google Scholar] [CrossRef] [PubMed]
- Karp, S.G.; Ávila, P.F.; Woiciechowski, A.L.; Soccol, V.T.; Soccol, C.R. White-rot fungi for bioremediation processes in the environment: A review. Bioresour. Technol. 2021, 313, 123707. [Google Scholar]
- Zabel, R.A.; Morrell, J.J. Chapter Three—The characteristics and classification of fungi and bacteria. In Wood Microbiology, 2nd ed.; Zabel, R.A., Morrell, J.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 55–98. [Google Scholar] [CrossRef]
- Dhakar, K.; Pandey, A. Laccase Production from a Temperature and pH Tolerant Fungal Strain of Trametes hirsuta (MTCC 11397). Enzyme Res. 2013, 2013, 869062. [Google Scholar] [CrossRef]
- Sarma, H.; Bhattacharyya, P.N.; Jadhav, D.A.; Pawar, P.; Thakare, M.; Pandit, S.; Mathuriya, A.S.; Prasad, R. Fungal-mediated electrochemical system: Prospects, applications and challenges. Cur. Res. Microbial Sci. 2021, 2, 100041. [Google Scholar] [CrossRef]
- Kurniawati, S.; Nicell, J.A. Characterization of Trametes versicolor laccase for the transformation of aqueous phenol. Bioresour. Technol. 2008, 99, 7825–7834. [Google Scholar] [CrossRef] [PubMed]
- Benitez, S.F.; Sadañoski, M.A.; Velázquez, J.E.; Zapata, P.D.; Fonseca, M.I. Comparative Study of Single Cultures and a Consortium of White Rot Fungi for Polychlorinated Biphenyls Treatment. J. Appl. Microbiol. 2021, 131, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, F.; Pizzul, L.; Castillo, M.D.P.; Cuevas, R.; Diez, M.C. Degradation of Polycyclic Aromatic Hydrocarbons by the Chilean White-Rot Fungus Anthracophyllum Discolor. J. Hazard. Mater. 2011, 185, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Marinho, G.; Barbosa, B.C.A.; Rodrigues, K.; Aquino, M.; Pereira, L. Potential of the filamentous fungus Aspergillus niger AN 400 to degrade atrazine in wastewaters. Biocatal. Agric. Biotechnol. 2017, 9, 162–167. [Google Scholar] [CrossRef]
- Liu, J.; Sun, K.; Zhu, R.; Wang, X.; Waigi, M.G.; Li, S. Biotransformation of bisphenol A in vivo and in vitro by laccase-producing Trametes hirsuta La-7: Kinetics, products, and mechanisms. Environ. Pollut. 2023, 321, 121155. [Google Scholar] [CrossRef] [PubMed]
- Tseng, N.; Wang, N.; Szostek, B.; Mahendra, S. Biotransformation of 6:2 Fluorotelomer Alcohol (6:2 FTOH) by a Wood-Rotting Fungus. Environ. Sci. Technol. 2014, 48, 4012–4020. [Google Scholar] [CrossRef] [PubMed]
- Cajthaml, T.; Křesinová, Z.; Svobodová, K.; Möder, M. Biodegradation of Endocrine-Disrupting Compounds and Suppression of Estrogenic Activity by Ligninolytic Fungi. Chemosphere 2009, 75, 745–750. [Google Scholar] [CrossRef]
- Rajendran, R.K.; Huang, S.-L.; Lin, C.-C.; Kirschner, R. Biodegradation of the endocrine disrupter 4-tert-octylphenol by the yeast strain Candida rugopelliculosa RRKY5 via phenolic ring hydroxylation and alkyl chain oxidation pathways. Biores. Technol. 2017, 226, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Merino, N.; Wang, N.; Gao, Y.; Wang, M.; Mahendra, S. Roles of various enzymes in the biotransformation of 6:2 fluorotelomer alcohol (6:2 FTOH) by a white-rot fungus. J. Hazard. Mater. 2023, 450, 131007. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, K.; Salgado, R.; Galhanas, D.; Bermudez, V.M.S.; Silva, G.M.M.; da Mata, A.M.Á.T.; Pereira, L. Thriving in salty environments: Aspergillus niger’s halotolerance and BTEX biodegradation potential. World J. Microbiol. Biotechnol. 2025, 41, 6. [Google Scholar] [CrossRef]
- Rodrigues, K.; de Sousa, A.M.X.; dos Santos, A.D.O.; Barbosa, B.C.A.; Silva, A.R.; Pereira, L.; Silva, G.M.M. Decolorization and Detoxification of Industrial Wastewater Containing Indigo Carmine by Aspergillus niger AN400 in Sequential Reactors. Colorants 2024, 3, 73–85. [Google Scholar] [CrossRef]
- Arregui, L.; Ayala, M.; Gómez-Gil, X.; Gutiérrez-Soto, G.; Hernández-Luna, C.E.; Herrera de Los Santos, M.; Levin, L.; Rojo-Domínguez, A.; Romero-Martínez, D.; Saparrat, M.C.N.; et al. Laccases: Structure, Function, and Potential Application in Water Bioremediation. Microb. Cell Fact. 2019, 18, 200. [Google Scholar] [CrossRef]
- Sun, K.; Hong, D.; Liu, J.; Latif, A.; Li, S.; Chu, G.; Qin, W.; Si, Y. Trametes versicolor Laccase-Assisted Oxidative Coupling of Estrogens: Conversion Kinetics, Linking Mechanisms, and Practical Applications in Water Purification. Sci. Total Environ. 2021, 782, 146917. [Google Scholar] [CrossRef]
- Cabana, H.; Alexandre, C.; Agathos, S.N.; Jones, J.P. Immobilization of Laccase from the White Rot Fungus Coriolopsis polyzona and Use of the Immobilized Biocatalyst for the Continuous Elimination of Endocrine Disrupting Chemicals. Biores. Technol. 2009, 100, 3447–3458. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.-D.; Tiwari, A.; Anisha, G.S.; Chen, C.-W.; Singh, A.; Haldar, D.; Patel, A.K.; Singhania, R.R. Laccase: A potential biocatalyst for pollutant degradation. Environm. Poll. 2023, 319, 120999. [Google Scholar] [CrossRef]
- Loi, M.; Glazunova, O.; Fedorova, T.; Logrieco, A.F.; Mulè, G. Fungal Laccases: The Forefront of Enzymes for Sustainability. J. Fungi 2021, 7, 1048. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.; Verma, P. Multicopper oxidase laccases with distinguished spectral properties: A new outlook. Heliyon 2020, 6, e03972. [Google Scholar] [CrossRef]
- Lloret, L.; Eibes, G.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Degradation of estrogens by laccase from Myceliophthora thermophila in fed-batch and enzymatic membrane reactors. J. Hazard. Mater. 2012, 213, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Chappell, H.A.; Milliken, A.; Farmer, C.; Hampton, A.; Wendland, N.; Coward, L.; Gregory, D.J.; Johnson, C.M. Efficient Remediation of 17α-Ethinylestradiol by Lentinula edodes (Shiitake) Laccase. Biocat. Agric. Biotechnol. 2017, 10, 64–68. [Google Scholar] [CrossRef]
- Zofair, S.F.; Hashmi, M.A.; Faridi, I.H.; Rasool, F.; Magani, S.K.J.; Khan, M.A.; Younus, H. Immobilization of Laccase on Poly-L-lysine Modified Silver Nanoparticles Formed by Green Synthesis for Enhanced Stability, Suppressed Estrogenic Activity of 17β-Estradiol, Biocompatibility and Anti-Cancer Action: An In Vitro and In Silico Study. J. Mol. Liq. 2023, 392, 123502. [Google Scholar] [CrossRef]
- Becker, B.D.; Rodriguez-Mozaz, S.; Insa, S.; Schoevaart, R.; Barceló, D.; de Cazes, M.; Belleville, M.-P.; Sanchez-Marcano, J.; Misovic, A.; Oehlmann, J.; et al. Removal of Endocrine Disrupting Chemicals in Wastewater by Enzymatic Treatment with Fungal Laccases. Org. Process Res. Dev. 2017, 21, 480–491. [Google Scholar] [CrossRef]
- Mohidem, N.A.; Mohamad, M.; Rashid, M.U.; Norizan, M.N.; Hamzah, F.; Mat, H.B. Recent Advances in Enzyme Immobilisation Strategies: An Overview of Techniques and Composite Carriers. J. Composites Sci. 2023, 7, 488. [Google Scholar] [CrossRef]
- Akpasi, S.O.; Anekwe, I.M.S.; Tetteh, E.K.; Amune, U.O.; Shoyiga, H.O.; Mahlangu, T.P.; Kiambi, S.L. Mycoremediation as a Potentially Promising Technology: Current Status and Prospects-A Review. Appl. Sci. 2023, 13, 4978. [Google Scholar] [CrossRef]
- Beck, S.; Berry, E.; Duke, S.; Milliken, A.; Patterson, H.; Prewett, D.L.; Rae, T.C.; Sridhar, V.; Wendland, N.; Gregory, B.W. Characterization of Trametes versicolor Laccase-Catalyzed Degradation of Estrogenic Pollutants: Substrate Limitation and Product Identification. Intern. Biodeterior. Biodegrad. 2018, 127, 146–159. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, J.; Hong, D.; Sun, K.; Li, S.; Latif, A.; Si, X.; Si, Y. Fungal laccase-triggered 17β-estradiol humification kinetics and mechanisms in the presence of humic precursors. J. Hazard. Mater. 2021, 412, 125197. [Google Scholar] [CrossRef]
- Antón-Herrero, R.; Chicca, I.; García-Delgado, C.; Crognale, S.; Lelli, D.; Gargarello, R.M.; Herrero, J.; Fischer, A.; Thannberger, L.; Eymar, E.; et al. Main Factors Determining the Scale-Up Effectiveness of Mycoremediation for the Decontamination of Aliphatic Hydrocarbons in Soil. J. Fungi 2023, 9, 1205. [Google Scholar] [CrossRef] [PubMed]
- Křesinová, Z.; Linhartová, L.; Filipová, A.; Ezechiáš, M.; Mašín, P.; Cajthaml, T. Biodegradation of Endocrine Disruptors in Urban Wastewater Using Pleurotus Ostreatus Bioreactor. N Biotechnol. 2018, 43, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Morató, C.; Lucas, D.; Llorca, M.; Rodriguez-Mozaz, S.; Gorga, M.; Petrovic, M.; Barceló, D.; Vicent, T.; Sarrà, M.; MarcoUrrea, E. Hospital Wastewater Treatment by Fungal Bioreactor: Removal Efficiency for Pharmaceuticals and Endocrine Disruptor Compounds. Sci. Total Environ. 2014, 493, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Spina, F.; Romagnolo, A.; Prigione, V.; Tigini, V.; Varese, G. A Scaling-up Issue: The Optimal Bioreactor Configuration for Effective Fungal Treatment of Textile Wastewaters. Chem. Eng. Trans. 2014, 38, 37–42. [Google Scholar] [CrossRef]
- Pilafidis, S.; Diamantopoulou, P.; Gkatzionis, K.; Sarris, D. Valorization of agro-industrial wastes and residues through the production of bioactive compounds by macrofungi in liquid state cultures: Growing circular economy. Appl. Sci. 2022, 12, 11426. [Google Scholar] [CrossRef]
- Salazar-Cerezo, S.; de Vries, R.P.; Garrigues, S. Strategies for the development of industrial fungal producing strains. J. Fungi 2023, 8, 834. [Google Scholar] [CrossRef]
- Singh, H.; Janiyani, K.; Gangawane, A.; Pandya, S.; Jasani, S. Engineering cellulolytic fungi for efficient lignocellulosic biomass hydrolysis: Advances in mutagenesis, gene editing, and nanotechnology with CRISPR-Cas innovations. Discov. Appl. Sci. 2024, 6, 665. [Google Scholar] [CrossRef]
- Khan, N.A.; Singh, S.; López-Maldonado, E.A.; Pavithra, N.; Méndez-Herrera, P.M.; López-López, F.J.R.; Baig, U.; Ramamurthy, P.C.; Mubarak, N.M.; Karri, R.R.; et al. Emerging membrane technology and hybrid treatment systems for the removal of micropollutants from wastewater. Desalination 2023, 565, 116873. [Google Scholar] [CrossRef]
- Slaby, S.; Duflot, A.; Zapater, C.; Gómez, A.; Couteau, J.; Maillet, G.; Knigge, T.; Pinto, P.I.S.; Monsinjon, T. The Dicentrarchus labrax estrogen screen test: A relevant tool to screen estrogen-like endocrine disrupting chemicals in the aquatic environment. Chemosphere 2024, 362, 142601. [Google Scholar] [CrossRef]
- Rajendran, R.K.; Lee, Y.-W.; Chou, P.-H.; Huang, S.-L.; Kirschner, R.; Lin, C.-C. Biodegradation of the endocrine disrupter 4-t-octylphenol by the non-ligninolytic fungus Fusarium falciforme RRK20: Process optimization, estrogenicity assessment, metabolite identification and proposed pathways. Chemosphere 2020, 240, 124876. [Google Scholar] [CrossRef] [PubMed]
- Badia-Fabregat, M.; Rodríguez-Rodríguez, C.E.; Gago-Ferrero, P.; Olivares, A.; Piña, B.; Díaz-Cruz, M.S.; Vicent, T.; Barceló, D.; Caminal, G. Degradation of UV Filters in Sewage Sludge and 4-MBC in Liquid Medium by the Ligninolytic Fungus Trametes versicolor. J. Environ. Manag. 2012, 104, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Llorca, M.; Badia-Fabregat, M.; Rodríguez-Mozaz, S.; Caminal, G.; Vicent, T.; Barceló, D. Fungal treatment for the removal of endocrine disrupting compounds from reverse osmosis concentrate: Identification and monitoring of transformation products of benzotriazoles. Chemosphere 2017, 184, 1054–1070. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viana, C.E.M.; Lima, V.d.S.; Rodrigues, K.; Pereira, L.; Silva, G.M.M. Bioremediation of Endocrine Disruptors (EDs): A Systematic Review of Fungal Application in ED Removal from Wastewater. Water 2025, 17, 640. https://doi.org/10.3390/w17050640
Viana CEM, Lima VdS, Rodrigues K, Pereira L, Silva GMM. Bioremediation of Endocrine Disruptors (EDs): A Systematic Review of Fungal Application in ED Removal from Wastewater. Water. 2025; 17(5):640. https://doi.org/10.3390/w17050640
Chicago/Turabian StyleViana, Camila Emanuelle Mendonça, Valquíria dos Santos Lima, Kelly Rodrigues, Luciana Pereira, and Glória Maria Marinho Silva. 2025. "Bioremediation of Endocrine Disruptors (EDs): A Systematic Review of Fungal Application in ED Removal from Wastewater" Water 17, no. 5: 640. https://doi.org/10.3390/w17050640
APA StyleViana, C. E. M., Lima, V. d. S., Rodrigues, K., Pereira, L., & Silva, G. M. M. (2025). Bioremediation of Endocrine Disruptors (EDs): A Systematic Review of Fungal Application in ED Removal from Wastewater. Water, 17(5), 640. https://doi.org/10.3390/w17050640






