3.1. Taxonomic Synopsis
Family Atyidae De Haan, 1849
Genus Atyoida Chace, 1983
3.1.1. Atyoida pilipes (Newport, 1847)
Atya pilipes Newport, 1847 [
18]: 160 [type locality: “Apia, Upolu, New Zealand” (corrected to “Apia, Upolu, Navigator or Samoan group” by Dana, 1852 [
19]: 533] (see Lorang et al., 2020 [
20]).
Atya pilipes-Holthuis, 1982 [
9]: 609.
Atyoida pilipes-Chace, 1983 [
21]: 13, Fig. 3; Cai et al., 2006 [
22]: 392; Lorang et al., 2020 [
20]: 6.
Caridina acuminata Stimpson, 1860 [
23]: 98 [type locality: Ogasawara (Bonin)] Islands, Japan].
Caridina brevirostris Stimpson, 1860 [
23]: 98 [type locality: Okinawa (Loo Choo) Island, Ryukyu Islands, Japan].
None.
Lorang et al. (2020 [
20]) stated that in Nuku-Hiva (Marquesas islands), females of
A. pilipes occur mainly in water with high velocities and on coarse substrata, while males were indifferent to water velocity. Large males appeared more frequently where the riverbeds have less vegetation, while small males were more frequent in shallow habitats, with the authors concluding that females with their atyoid chelae are adapted to filter-feeding and males, with their caridinoid chelae, to sweeping. Females are thereby localized in the currents and on the rocks and males in vegetation.
The species distribution spans from the Philippines and Indonesia to Pacific high islands, as far north as Kume-Jima in the Ryukyus at about 26° N (Cai & Shokita, 2006a [
24]), as far south as New Caledonia, and as far east as Marquesas (Lorang et al., 2020 [
20]).
Lorang et al. (2020 [
20]) studied numerous specimens of
Atyoida collected from Polynesia morphologically and genetically, finding that a molecular study proved that the specimens were split into two different clades separated by 7% genetic distance (16S) and geographically structured such that they can be considered as two distinct species, and re-validated
A. tahitensis Stimpson, 1860, from Eastern Polynesia, as a distinct species. Their morphological study, however, did not find characteristics that could be used to distinguish the two species easily.
Atyoida pilipes in New Guinea is so far only reported from Papua, Indonesia (Holthuis, 1982 [
9]).
Genus Atyopsis Chace, 1983.
3.1.2. Atyopsis spinipes (Newport, 1847)
Atya spinipes Newport, 1847 [
18]: 159 [type locality: Philippine Islands].
Atya spinipes Holthuis, 1982 [
9]: 609.
Atya moluccensis—Nobili, 1905 [
1]: 480; J. Roux, 1917 [
4]: 595; de Man, 1915 [
3]: 407; Figs. 5a–d.
Atyopsis spinipes—Chace, 1983 [
21]: 35, Figs. 20–22; Shokita, 1990 [
25]: 311; Cai & Ng, 2001 [
26]: 266, Figs. 2a–d; Cai & Anker, 2004 [
27]: 236; Cai & Shokita, 2006b [
28]: 2134; 2006a [
24]: 246; Cai et al., 2009 [
29]: 66; de Mazancourt et al., 2024 [
30]: 7.
Two females, cl 4.3–4.4 mm, RMNH 700, two creeks near Seroei, Yapen Island, Papua, Indonesia, 22 February 1955.
This species inhabits areas with strong water currents, usually in the higher course of rivers or mountain streams.
From the Philippines and Indonesia, northwards to Tokuno-shima in the Ryukyus, and eastwards to Samoa.
Atyopsis spinipes has been reported from three parts of New Guinea (Nobili, 1905 [
1], De Man, 1915 [
3], Holthuis, 1982 [
9]), and the specimens examined here have been reported by Cai et al. (2007 [
31]) as comparative materials to
Atyopsis moluccensis.
Genus Caridina H. Milne Edwards, 1837.
3.1.3. Caridina typus H. Milne Edwards, 1837
Caridina typus H. Milne Edwards, 1837 [
32] (in H. Milne Edwards, 1834–1840): 363, pl. 25bis, Figs. 4, 5 [type locality: unknown, likely Mauritius] (see de Mazancourt et al., 2020 [
15]).
Caridina exilirostris Stimpson, 1860 [
23]: 29 [type locality (neotype): Okuma River, Okinawa Island, Ryukyu Islands, Japan].
Caridina typus-Holthuis, 1982 [
9]: 609; Yeo et al., 1999 [
33]: 225; Cai et al., 2006 [
22]: 412–418, Figs. 13–15; Naiyanetr, 2007 [
34]: 34; Soomro et al., 2011 [
35]: 41; Bernardes et al., 2017 [
11]: 1, Figs. 1–3 (part, ARC clade); de Mazancourt et al., 2017 [
36]: 226, Fig. 4; de Mazancourt et al., 2019b [
37]: 167, 169–170; de Mazancourt et al., 2020 [
15]: 34, Figs. 2D, 10; de Mazancourt et al., 2023 [
38]: 391; de Mazancourt et al., 2024 [
30]: 17; Cai & Naiyanetr, 2024 [
16]: 770.
Syntypes of Caridina typus H. Milne Edwards, 1837: 3 females, cl 7.0–8.4 mm, MNHN-Na 930, locality unknown, no date. Others: 1 male, cl 4.0 mm, 3 females, cl 4.6–5.5 mm, ZRC, small rocky stream on north side of PTFI Etnol Bay camp, 3°58.10′ S 134°57.69′ E, 50 m elev., West Papua, Indonesia, 28 March 1997; 59 males, cl 2.8–4.3 mm, 12 females, cl 3.7–5.0 mm, 7 ovigerous females, cl 4.2–4.9 mm, RMNH, Creek at near Kampong Ambai, Ambai Island, Papua, Indonesia, 22 February 1955; 5 males, cl 4.5–4.9 mm, 1 ovigerous female, cl 6.8 mm, RMNH 615, creek above Marine barracks, Jayapura, Irian Jaya (Papua), Indonesia, in a small pool, among water plants, coll. L. D. Brongersma, M. Boeseman and L. B. Holthuis, 31 October 1954; 22 males, cl 3.2–5.3 mm, RMNH 624, creek above Marine barracks, Jayapura, Papua, Indonesia, coll. L. B. Holthuis, 10 November 1954; 1 female, cl 6.1 mm, RMNH 717, Wasi River, west of Manokwari, West Papua, Indonesia, 9 March 1955; 1 ovigerous female, cl 8.8 mm, RMNH, Manainoemi River and another river near Seroei, Yapen Island, Papua, Indonesia, coll. L. B. Holthuis (specimen caught by students), 22 February 1955; 1 female, cl 7.0 mm, RMNH 408, Pieni River near Walwali, Aitape, Sub-district Sepik, District Territory of New Guinea (=Papua New Guinea), altitude lower than 100 m, depth of water 0.5–2.5 m, R. D. Hoogtand, Jun 1961; 1 female, cl 4.6 mm, RMNH 281–345, Wanigela, Northern District, Territory of Papua, Papua New Guinea, coll. R. D. Hoogland, June–September1954; 3 females, NMB 39t, River Ga, Waigeo Island, West Papua, Indonesia, no date.
This species is found from the lower to higher course of rivers.
Indo-West Pacific region, ranging from South Africa, Madagascar, and the Seychelles to Japan, Malaysia, the Philippines, Australia, Micronesia, Papua New Guinea, Solomon Islands, New Caledonia, Vanuatu, and Fiji (de Mazancourt et al., 2020 [
15]).
Bernardes et al. (2017 [
11]) studied the phylogeography of
Caridina typus, finding that both mitochondrial DNA (mtDNA) and multilocus datasets recovered the same three major clades, viz., ARC, SUL, and TAL. The application of several different species delimitation methods indicates that each lineage/clade represents a distinct (cryptic) species, contradicting the current morphospecies delimitation of a single
C.
typus taxon. Among the three genetic lineages, only the ARC, which is widely distributed in both Indian ocean and West Pacific, represents
Caridina typus sensu stricto. While the lineage TAL was subsequently assigned to
Caridina zhujianensis Chen et al. 2018 [
39] by de Mazancourt et al. (2023 [
38]), the lineage SUL is assigned to
C. jeani Cai, 2010 (see remarks under
Caridina zhujianensis and
Caridina jeani).
de Mazancourt et al. (2020 [
15]) reported
Caridina typus, sensu lato, from Walindi River and Rangihi swamp of New Britain, Papua New Guinea, and redescribed this species based on specimens from most of its distribution range. They commented that the length of setae on the telson (namely plumose terminal setae on the telson subequal to lateral ones or slightly longer) is a better criterion to characterize
Caridina typus species group that agree with their molecular results and consider some species like
C. turipi or
C. sumatrensis that have numerous dorsal rostral teeth to be part of the
C. typus group. However, this grouping practice is different from the original idea on the recognition of species groups/complexes, which is based on morphological taxonomy, instead of a recognition of the phylogenetic group based on molecular data. Chen et al. (2018 [
39]) did not compare their new species
Caridina zhujiangensis with
C. fasciata Hung, Chan & Yu, 1993, which is morphologically a very close species that is so far only known from Taiwan. de Mazancourt et al. (2020 [
15]) described
C. poarae and
C. paratypus as members of the
C. weberi group. However, morphologically, both species should be referred to the
C. typus group, as it is traditionally recognized.
Currently, the species group includes C. typus H. Milne Edwards, 1837, C. siamensis Giebel, 1863, C. fasciata Hung, Chan & Yu, 1993, C. jeani Cai, 2010, C. zhujiangensis Chen, Chen & Guo, 2018. C. poarae de Mazancourt, Boseto, Marquet & Keithy, 2020, C. paratypus de Mazancourt, Boseto, Marquet & Keithy, 2020, and C. ravisankarani Vijayamma, Dhamorikar & Manchi, 2021.
3.1.4. Caridina zhujiangensis Chen, Chen & Guo, 2018
Caridina zhujiangensis Chen, Chen & Guo, 2018 [
39]: 319, Figs. 4–6 [Type locality: Near the Resort Hotel, Dong’ ao Island, Guangdong Province, China (E113_4200300, N 22_0100600, al. 19 m, stn. 4)].
Caridina zhujiangensis-Xu et al., 2020 [
40]: 21, Figs. 6, 7; Chen et al., 2020 [
41]: 18, Figs. 6, 7; Feng et al., 2021 [
42]: 34, Fig. 2.
Caridina typus-Bernardes et al., 2017 [
11]: Figs. 1A, 2 (part, TAL clade).
None.
This species is found from the lower to the higher course of rivers.
China (Guangdong), Vietnam, Thailand, Malaysia, the Philippines (Mindanao, Mindoro, Samar, Luzon, and Palawan), and Indonesia (Sulawesi, Taliabu, and Papua).
Bernardes et al. (2017 [
11]) reported a single specimen from Mindoro under the name
Caridina typus, belonging to their clade TAL. de Mazancourt et al. (2023 [
38]) based their study on Xu et al. (2020 [
40]), who published mitochondrial DNA sequences of
C. zhujiangensis from the type locality to confirm the identity of this specimen. Bernardes et al. (2017 [
11]:
Figure 1A) indicated that their clad TAL also found at Aru Islands.
The assignment of clad TAL to Caridina zhujiangensis is only based on molecular data, follow up morphological confirmation is required for the identity of clad TAL in its distribution range.
3.1.5. Caridina jeani Cai, 2010
Caridina typus var.
brevirostris Roux, 1911 [
2]: 87. [type locality: Kei Islands, Indonesia; name preoccupied by
Caridinabrevirostris Stimpson, 1860].
Caridina jeani Cai, 2010b [
43]: 80, Figs. 1, 2. (new name for
Caridina typus var.
brevirostris Roux, 1911); Klotz et al., 2023 [
44]: Fig. 6, appendix 1 (ZMB 30708-1 (2235)); K. von Rintelen et al., 2024 [
45]: Table 1 (part), Figs. 3, Appendix AI (ZMB 29092 (DNA Code 80), ZMB 30708 (DNA Code 2235), Fig. FA1.
Caridina typus-Bernardes et al., 2017 [
11]: Figs. 1A; 2 (part, SUL clade).
None.
This species is found from the lower to the higher course of rivers.
Indonesia (Sulawesi, Bali, Java, Halmahera, Aru Islands, West Papua) and the Philippines (Samar, Mindoro, Palawan, Luzon).
Bernardes et al. (2017 [
11]: Figure 1A) indicated that their clad SUL is a cryptic species that is genetically different from
C. typus, and has been found at West Papua and Aru Islands.
Klotz et al. (2023 [
44]) recently described a new species
Caridina clandestina from the upper reaches of Lariang River in Central Sulawesi, listed
Caridina jeani (collected from Central Sulawesi: River at road Malino to Watambayoli) as one of the comparative species in their molecular phylogeny of all described endemic species of
Caridina from Sulawesi. Von Rintelen et al. (2024 [
45]) recently conducted a phylogeographic study on atyid shrimps of Maros, southern Sulawesi, which “includes a specimen from Bantimurung (ZMB 29092 in Figure 3, Table A–I in the Appendix), which was assigned to
C. typus in previous publications (von Rintelen et al., 2012 [
46]; Bernardes et al., 2017 [
11]: both with published sequences under ZMB 29092; Table A–I in the Appendix).” Von Rintelen et al. (2024 [
45]) found that their Maros specimens are genetically (with sequences of 16S and COI) and morphologically identical to the specimens reported by Klotz et al. (2023 [
44]) as
Caridina jeani Cai, 2010.
Von Rintelen et al. (2024 [
45]) further commented that “As the Maros karst specimens from Cai & Ng (2009 [
47]) were published with locality data only (from Gua Tanette and Bantimurung), they are also likely to belong to
C. jeani instead of
C. typus”. As highlighted by Cai (2010b [
43]),
C. jeani could be easily distinguished from
C. typus by possessing sexual dimorphism on the third and fourth pereiopods if both genders of specimens are available. However, as the specimens examined by Cai & Ng (2009 [
47]) are all females, with a large body size (cl 7.2–8.1 mm), while those type specimens of
C. jeani have a cl 4.3–6.3 mm (mostly less than 5 mm), the assignment of specimens from Cai & Ng (2009 [
47]) to
C. jeani could be premature, and further investigation with more specimens collected will help to clarify their identity.
The current assignment of clad SUL to Caridina jeani is only based on molecular data, follow up morphological confirmation is required for the identity of clad SUL in its distribution range.
3.1.6. Caridina siamensis Giebel, 1863
Caridina siamensis Giebel, 1863 [
48]: 329 [type locality: Siam (Thailand)].
Caridina typus var.
longirostris De Man, 1892 [
49]: 369, pl. 22, Figs. 22 f-I (junior homonym of
Caridina longirostris H. Milne Edwards, 1837 [
32]) [type locality: River near Reo, Flores, Indonesia; River near Palopo, Sulawesi, Indonesia; Benteng, Saleyer, Indonesia].
Caridina typus-Bouvier, 1925 [
50]: 251.
Caridina villadolidi Blanco, 1939 [
51]: 389, pl. 1, Figs. 1–9 [type locality: Laoag River, Laoag, Ilocos Norte Province, Luzon, the Philippines].
Caridina villadolidi-Hung et al., 1993 [
52]: 485, Figs. 1B, 3; Chace, 1997 [
53]: 21, Fig. 12; Shy & Yu, 1998 [
54]: 62; Cai & Ng, 2001 [
26]: 668, Figs. 4a–e; Liang, 2004 [
55]: 156, Fig. 74; Cai & Shokita, 2006a [
24]: 248; Cai et al., 2009 [
29]: 66; de Mazancourt et al., 2023 [
38]: 392; Klotz et al., 2023 [
44]: 19, Fig. 1.
Caridina siamensis-Cai & Naiyanetr, 2024 [
16]: 772.
Five females, cl 4.5–6.7 mm, 3 ovigerous females, cl 5.0–7.8 mm, RMNH 700, two creeks near Seroei, Yapen Island, Papua, Indonesia, 22 February 1955; 12 males, cl 3.3–4.6 mm, 7 females, cl 2.7–6.0 mm, 5 ovigerous females, cl 5.5–6.5 mm, RMNH 704, Manainoemi River at Seroei, Yapen Island, Papua, Indonesia, 21 February 1956; 7 ovigerous females, cl 6.3–7.2 mm, RMNH 689, Seroei, Yapen Island, Geelvink Bay, Papua, Indonesia, coll. D. L. Leiker, 1954; 1 ovigerous female, cl 6.9 mm, Sorong, RMNH, North West of New Guinea, West Papua, Indonesia, coll. M. A. Lieftiuck, 28 August–6 September 1948; 1 spec., SMF 7955, Wokam, Aru Islands, Indonesia, coll. H. Merton, 17 April 1908.
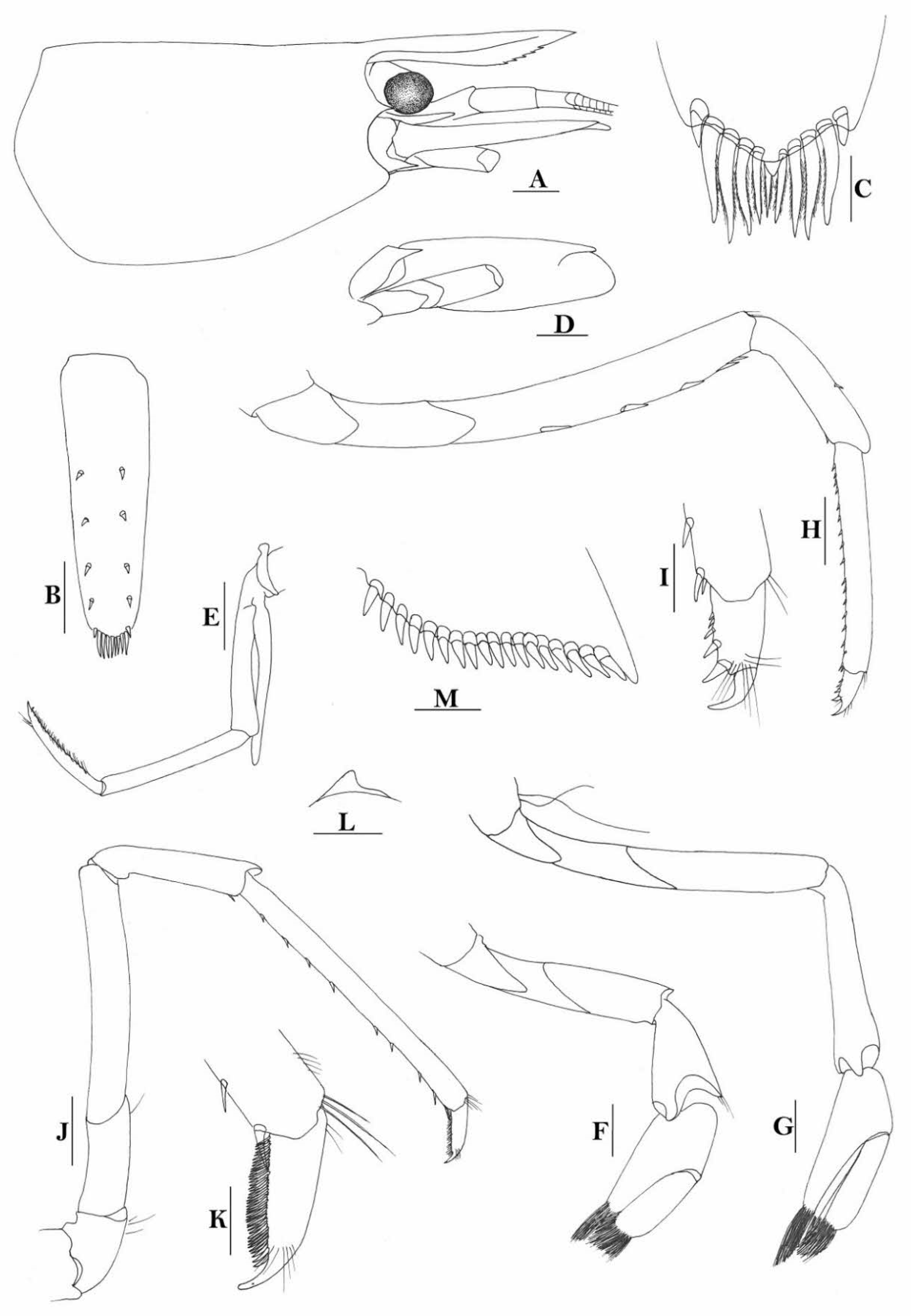
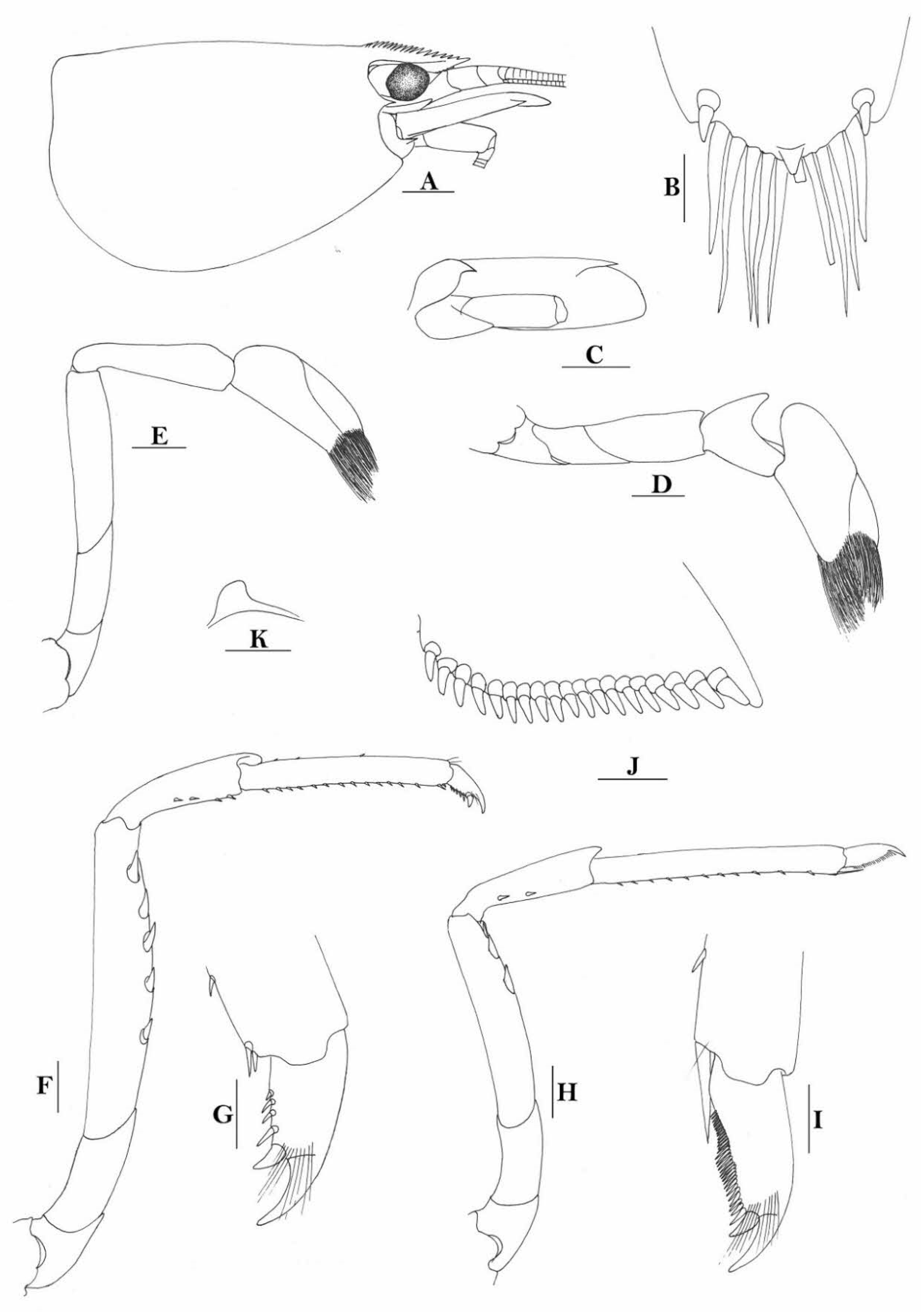
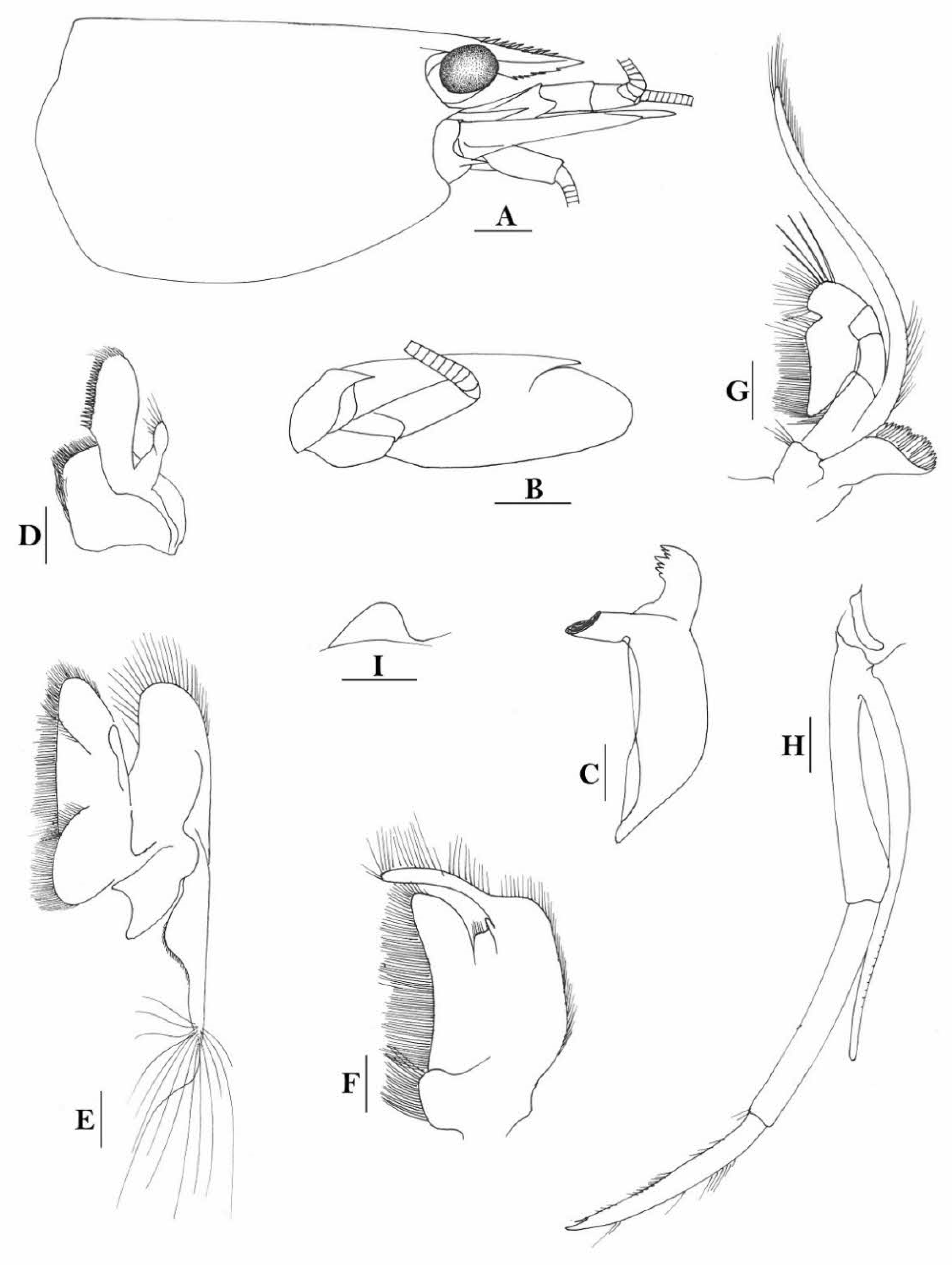
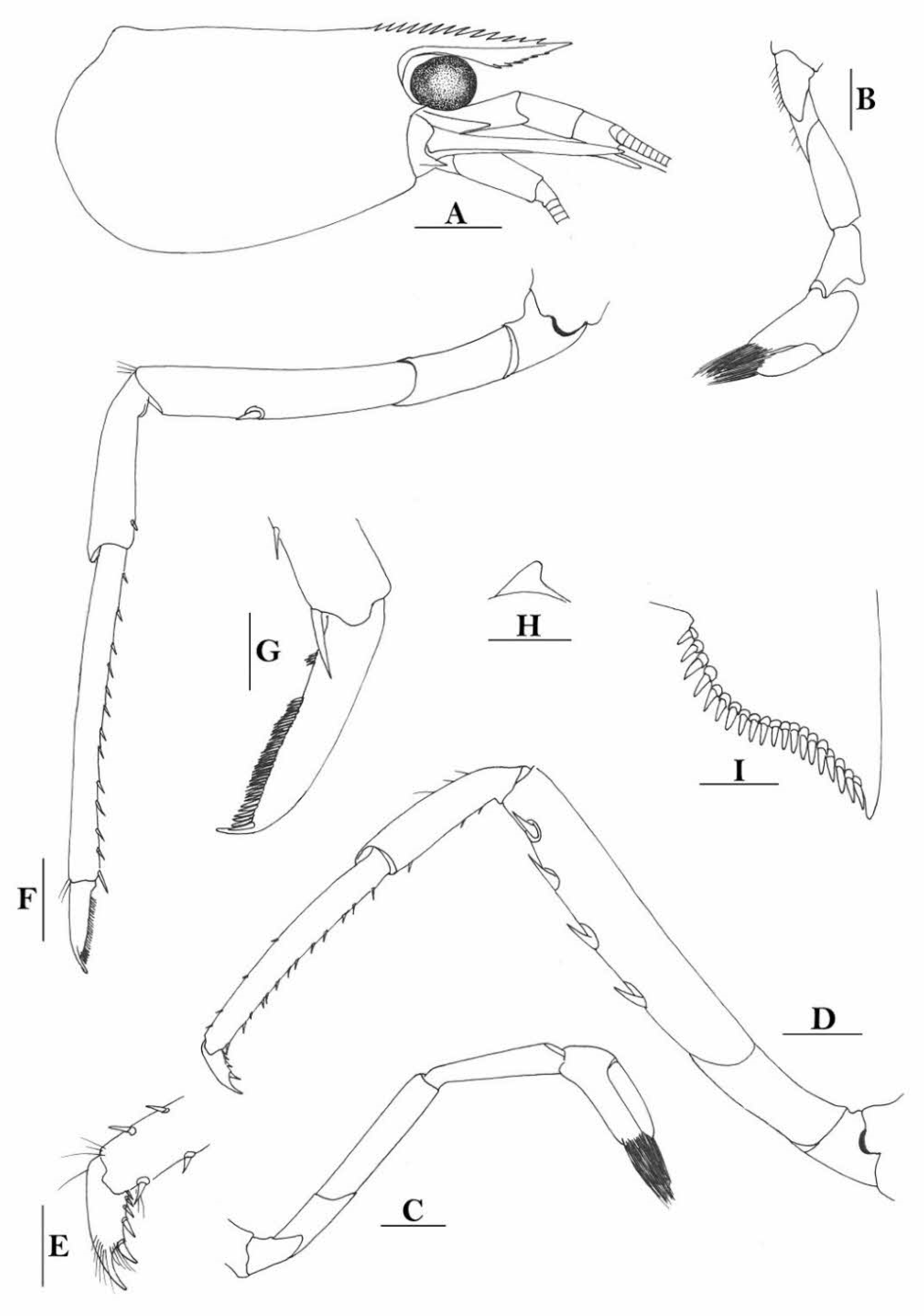
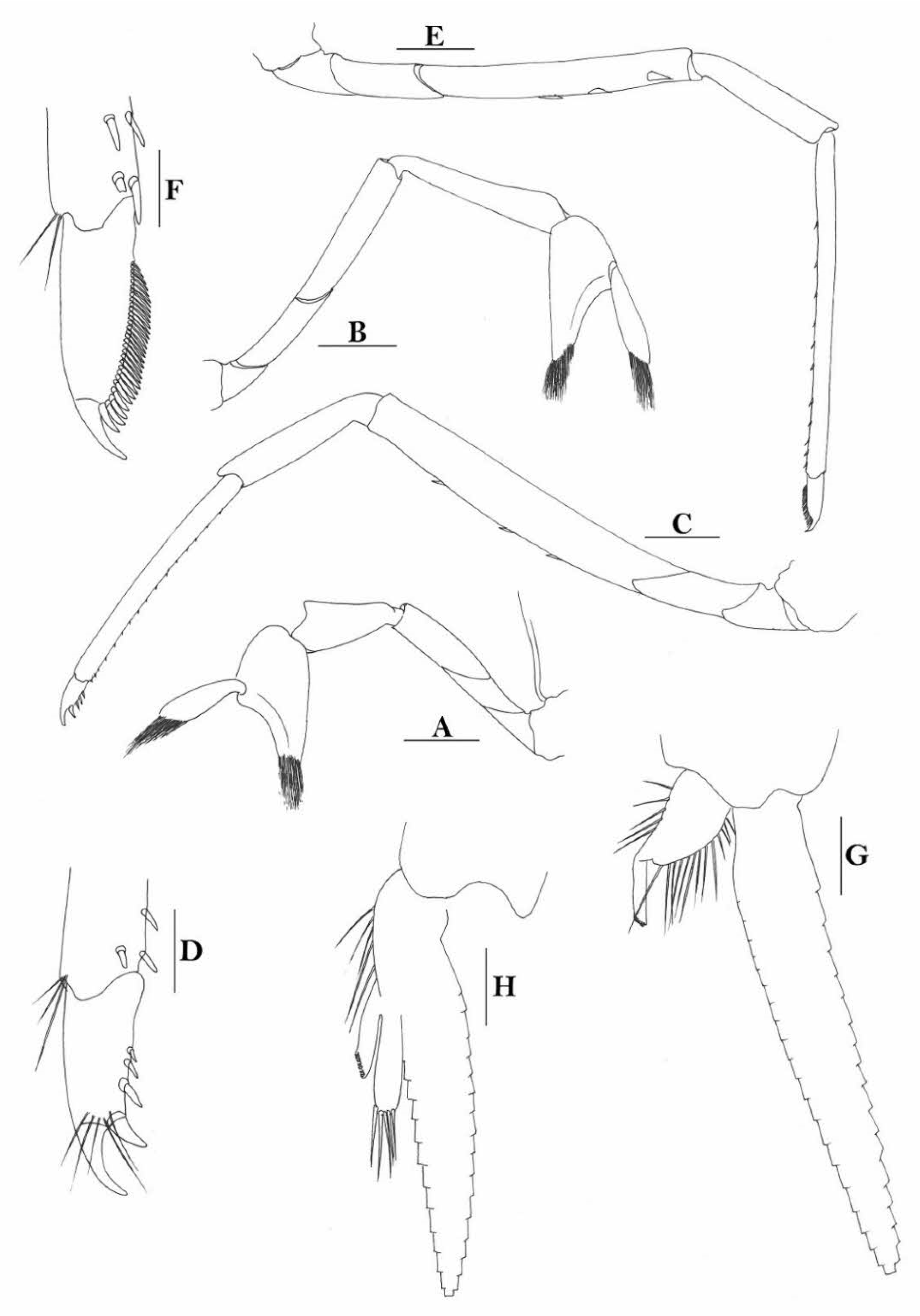
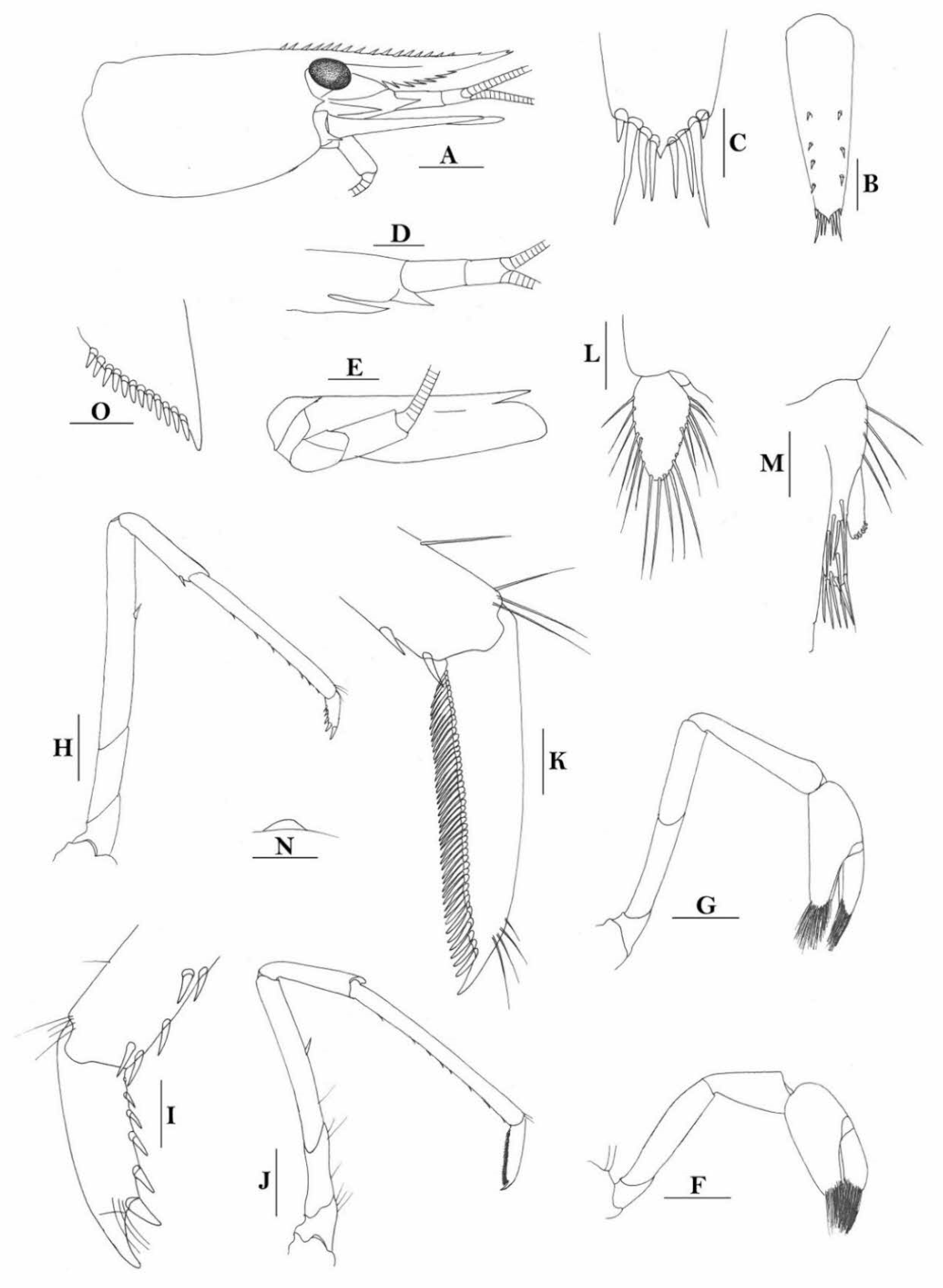
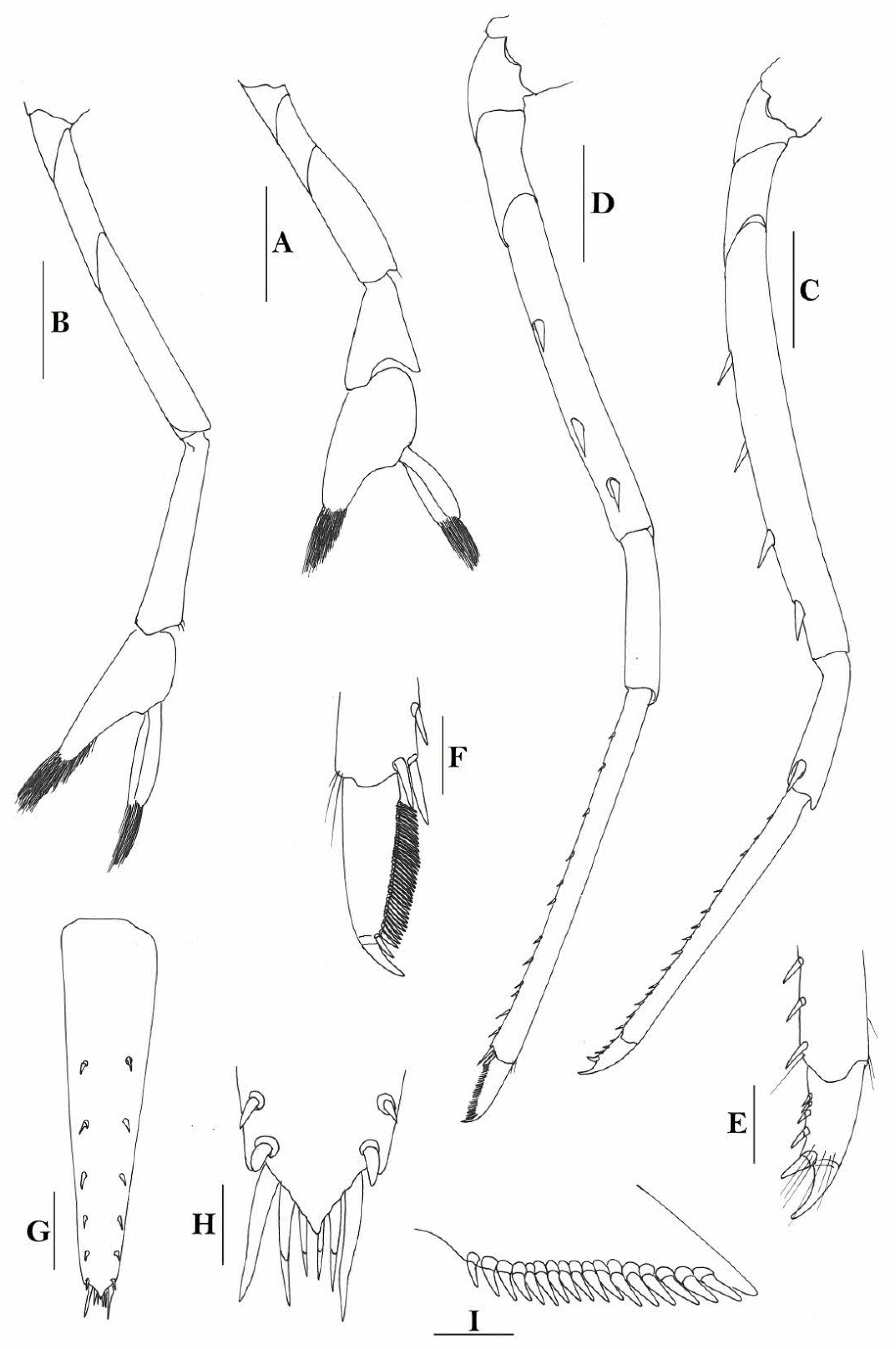
Rostrum straight, reaching near to or slightly beyond end of antennular peduncle, dorsal margin nearly horizontal, unarmed dorsally, armed ventrally with 1–7 inconspicuous teeth. Suborbital angle completely fused with antennal spine; pterygostomian margin narrowly rounded.
Sixth abdominal somite 0.58 times as long as carapace, 1.36 times as long as fifth somite, shorter than telson. Telson 3.1 times as long as wide, terminal margin rounded, ending in a projection, with four pairs of dorsal spinules and one pair of dorsolateral spinules; distal end with about four to five pairs of spines, lateral pair slightly longer or as long as intermediate pairs. Preanal carina moderately high, without spine.
Anterior end of eye reaching 0.7 length of basal segment of antennular peduncle. Antennular peduncle 0.64 times as long as carapace; anterolateral angle of basal segment reaching 0.20 length of the second segment. Stylocerite reaching 0.8 length of basal segment of antennular peduncle. Scaphocerite 3.2 times as long as wide.
Palp of first maxilliped ending in a broad triangle. Third maxilliped reaching to end of antennular peduncle, with ultimate segment distinctly shorter than penultimate segment.
Epipods well developed on first four pereiopods. First pereiopod reaching to distal end of basal segment of antennular peduncle; merus 2.3 times as long as broad, as long as carpus; carpus excavated anteriorly, shorter than chela, 1.5 times as long as high; chela 2.3 times as long as broad; fingers slightly longer than palm. Second pereiopod reaching to end of second segment of antennular peduncle; merus slightly shorter than carpus, 5.0 times as long as broad; carpus slightly longer than chela, 4.3 times as long as high; chela 2.6 times as long as broad; fingers 1.5 times as long as palm. Third pereiopod reaching slightly beyond end of scaphocerite, propodus 8.7 times as long as broad, 4.7 times as long as dactylus; dactylus 2.0 times as long as wide (spines included), terminating in one claw, with five accessory spines on flexor margin. Fifth pereiopod reaching to end of basal segment of antennular peduncle, propodus 12 times as long as broad, 5.2 times as long as dactylus, dactylus 2.6 times as long as wide (spinules included), terminating in one claw, with 42 spinules on flexor margin. Endopod of male first pleopod sub-triangular, appendix interna reaching beyond distal end of endopod by most of its length, 2.4 times as long as wide, 1/3 length of exopod.
Uropodal diaeresis with 18–20 movable spinules.
Eggs 0.40 × 0.25 mm in diameter.
Rivers.
Indonesia (Sulawesi, Halmahera, the Philippines, Papua), Taiwan, to Sri Lanka and Thailand.
Caridina siamensis was recently resurrected and regarded as a senior synonym for
C. villadolidi by Cai & Naiyanetr (2024 [
16]), who included these New Guinea specimens as comparative materials. An ovigerous female specimen, from Yapen island of Papua, Indonesia was used to illustrate the species (
Figure 2).
3.1.7. Caridina weberi De Man, 1892
Caridina Weberi De Man, 1892 [
49]: 371, pl. 22: Figs. 23–23g [type locality: Kotting, Flores, Indonesia].
Caridina Weberi- Bouvier, 1925 [
50]: 242, Figs. 562-571; Holthuis, 1978b [
56]: 30; Chace, 1997 [
53]: 12 (part); Cai & Ng, 2001 [
26]: 666, Fig. 3.
Caridina cf.
weberi sp. 2- de Mazancourt et al., 2019a [
14]: 166, 169–170; de Mazancourt et al., 2020 [
15]: 49, Figs. 2T, 15, 26C.
Not
Caridina weberi- Edmondson, 1935 [
7]: 8, Figs. 3a–f, 4g, h.
One ovigerous female, cl 4.8 mm, RMNH 717, Wosi River, west of Manokwari, 9 March 1955; one ovigerous female, cl 4.1 mm, RMNH 717, Wasi River, west of Manokwari, Irian Jaya (Papua), Indonesia, 9 March 1955.
Lesser Sunda Islands: Lectotype (here designated): 1 ovigerous female, cl 6.8 mm, RMNH D1770, syntypes of Caridina weberi De Man, 1892, Kotting, Flores, Indonesia, coll. M. Weber, 1888. Paralectotypes: 2 ovigerous females, cl 6.7–7.0 mm, RMNH D1770, syntypes of Caridina weberi De Man, 1892, Kotting, Flores, Indonesia, coll. M. Weber, 1888; 4 males, cl 3.7–4.0 mm, 1 female, cl 4.3 mm, 3 ovigerous females, cl 6.0–7.1 mm, North Sumba, Indonesia, coll. Ten Kate, no date. Sulawesi: 7 males, cl 3.3–3.9 mm, 5 females, cl 3.3–4.2 mm, ZMA De 102608, syntypes of Caridina weberi, Bantimurong near Maros, southern Sulawesi, coll. M. Weber, 1888. Moluccas: 1 male, cl 3.0 mm, 1 ovigerous female, cl 5.0 mm, ZRC, Sungai Ifis, Halmahera, September 1994, leg. D. Robb. Bali: 1 ovigerous female, cl 5.9 mm, ZRC MK 92–24, coll. M. Kottelat, November 1992. Java: 1 female, cl 4.6 mm, 1 ovigerous female, cl 4.5 mm, MZB Cr1116, Sungai Taman Jaya, Barten, Java, Indonesia, coll. D. I. Hartoto, January 1984.
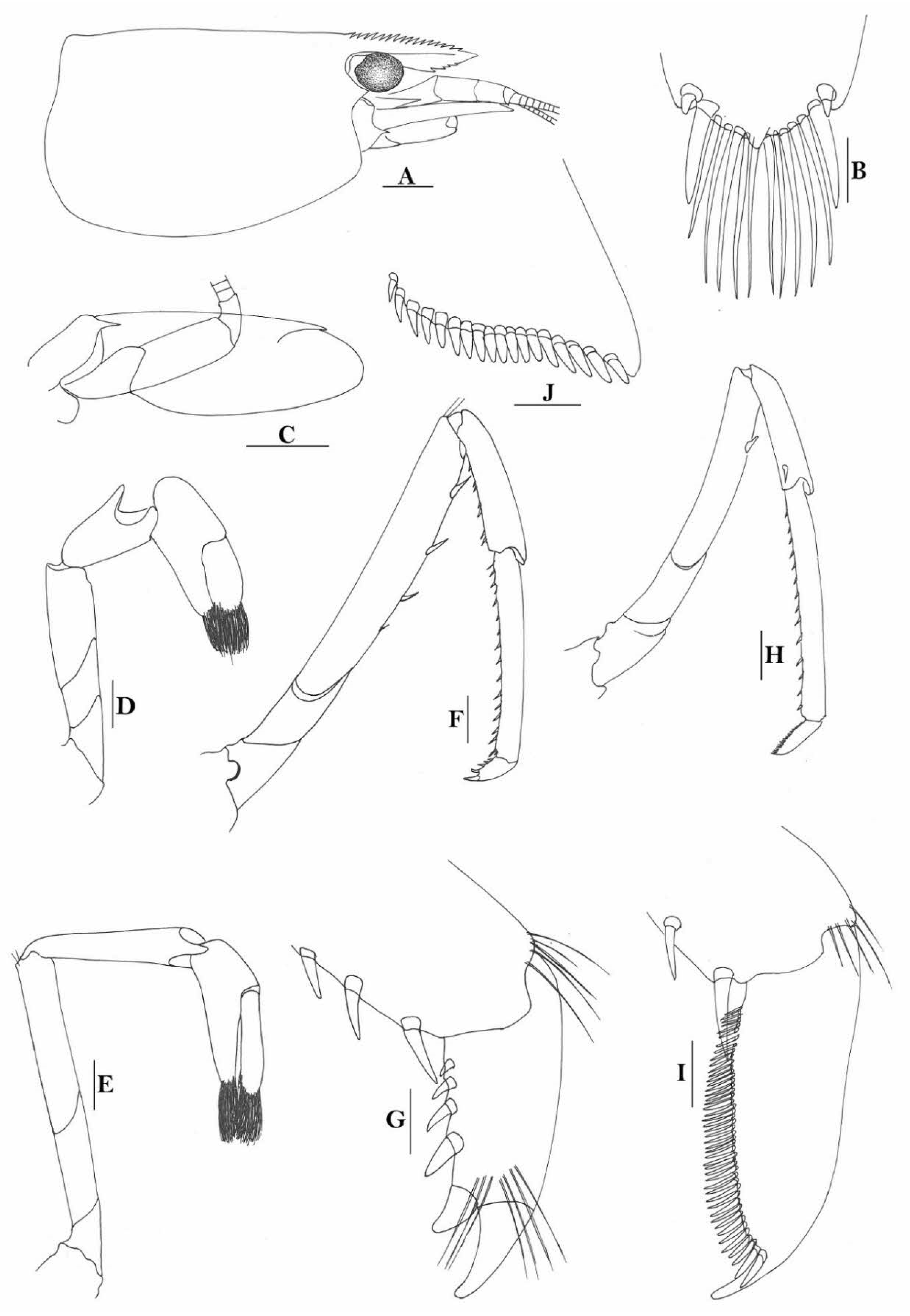
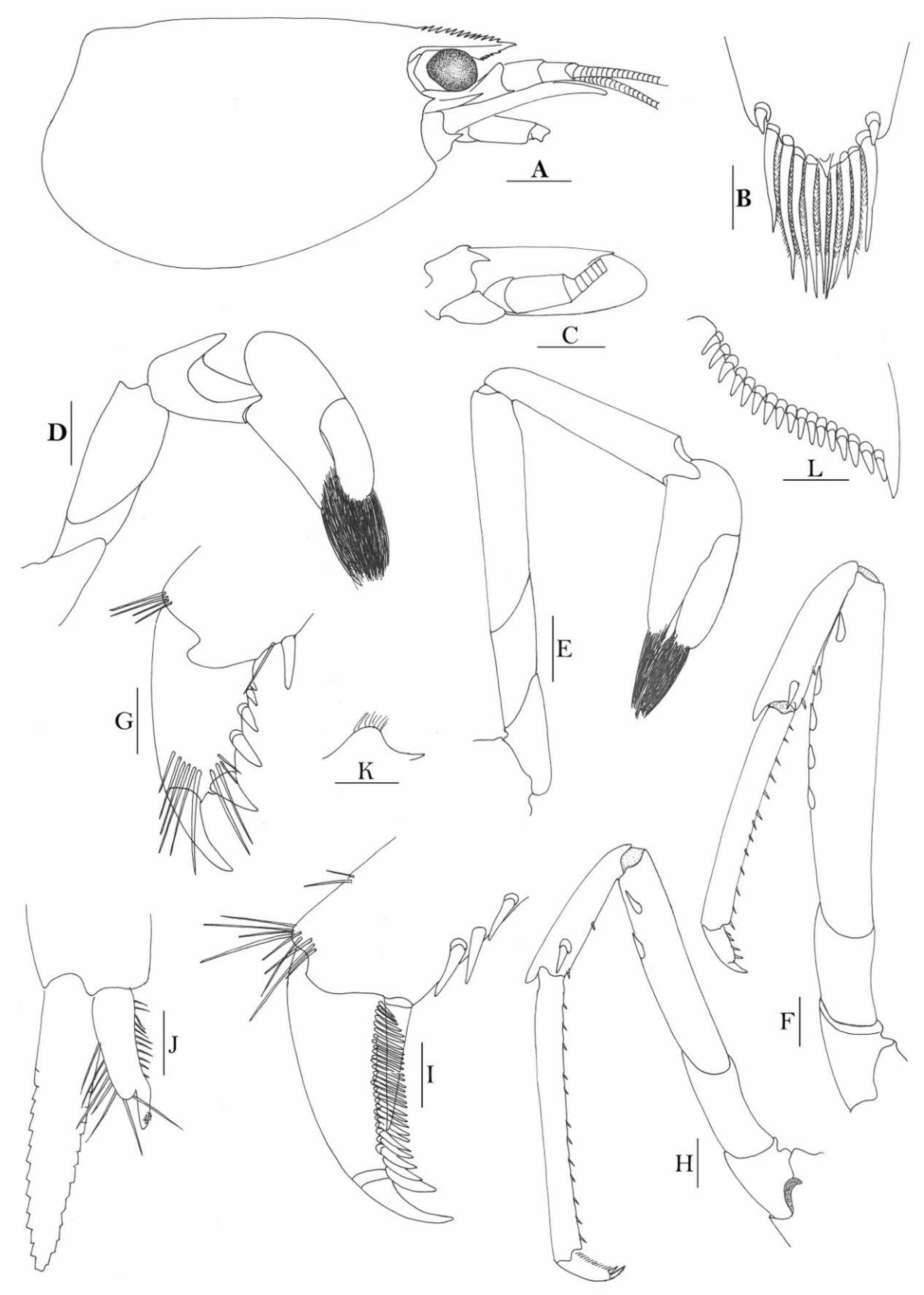
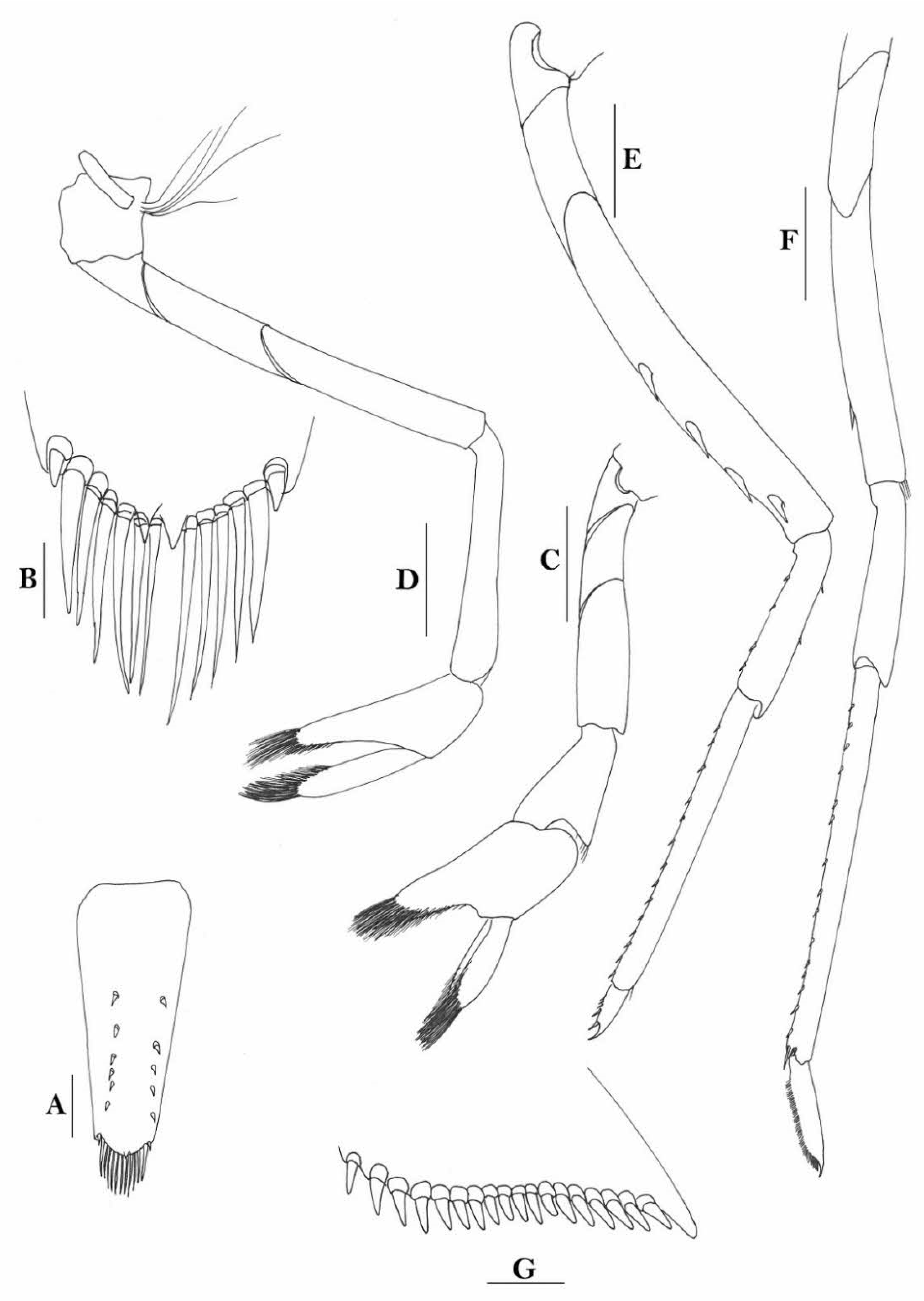
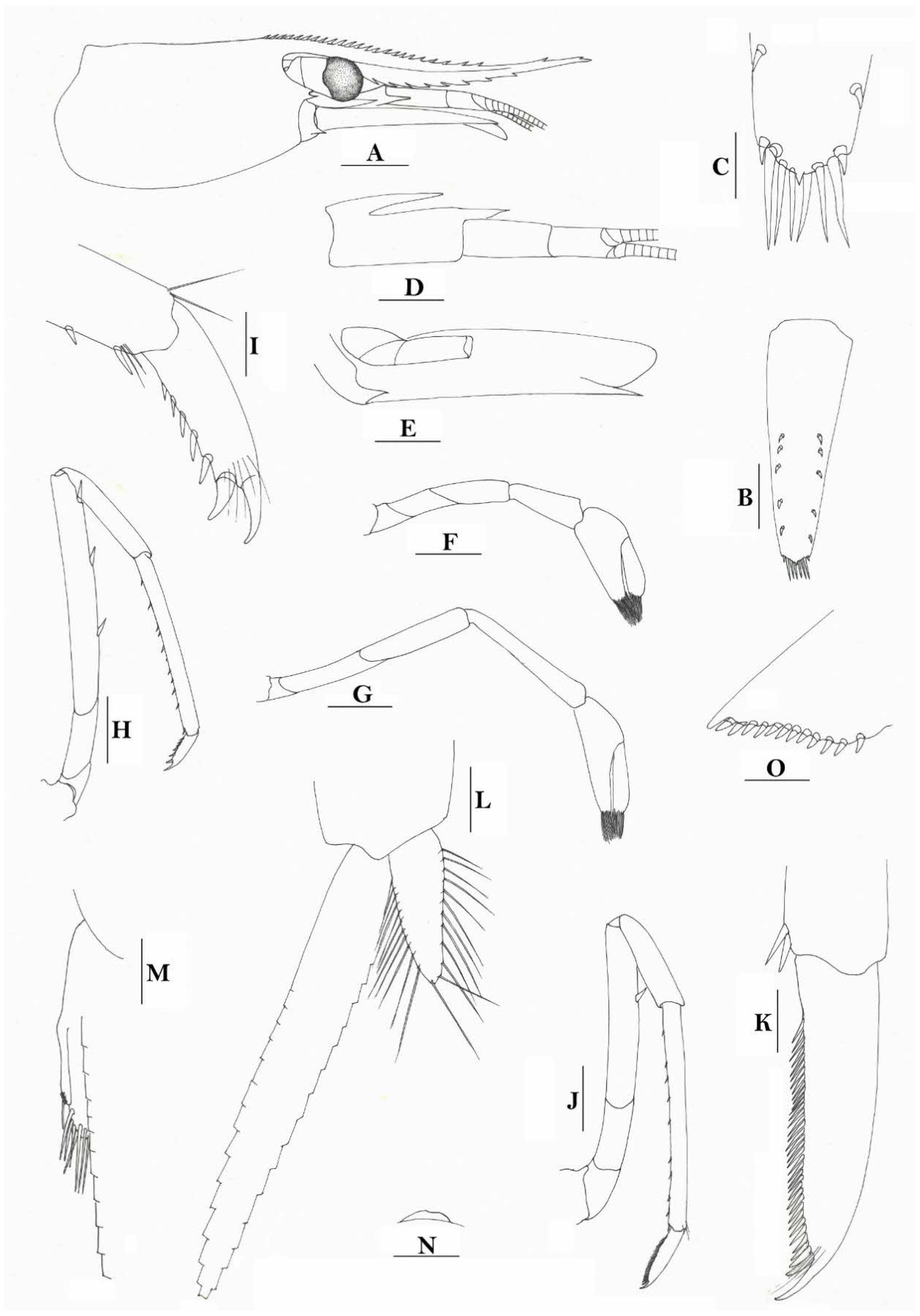
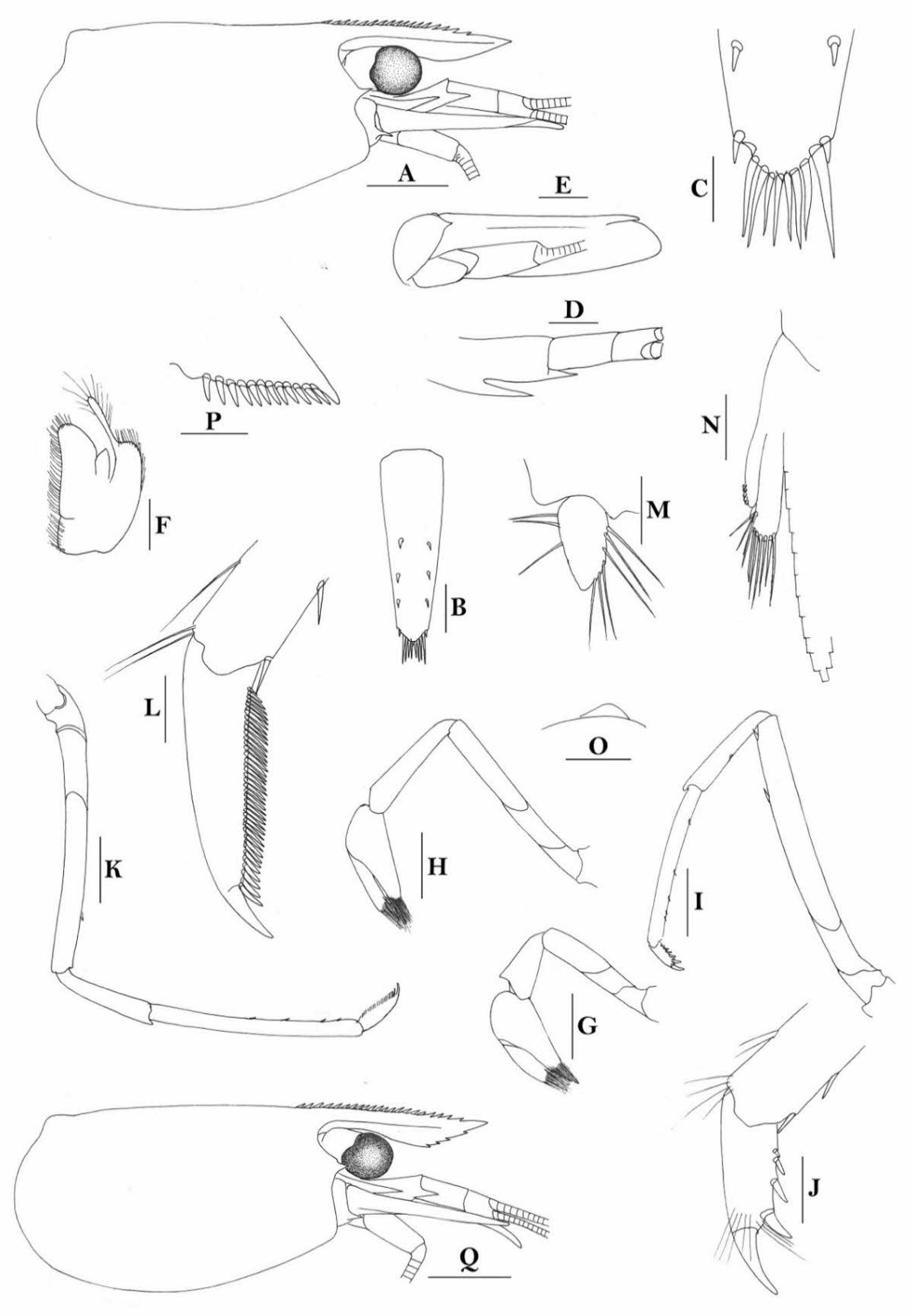
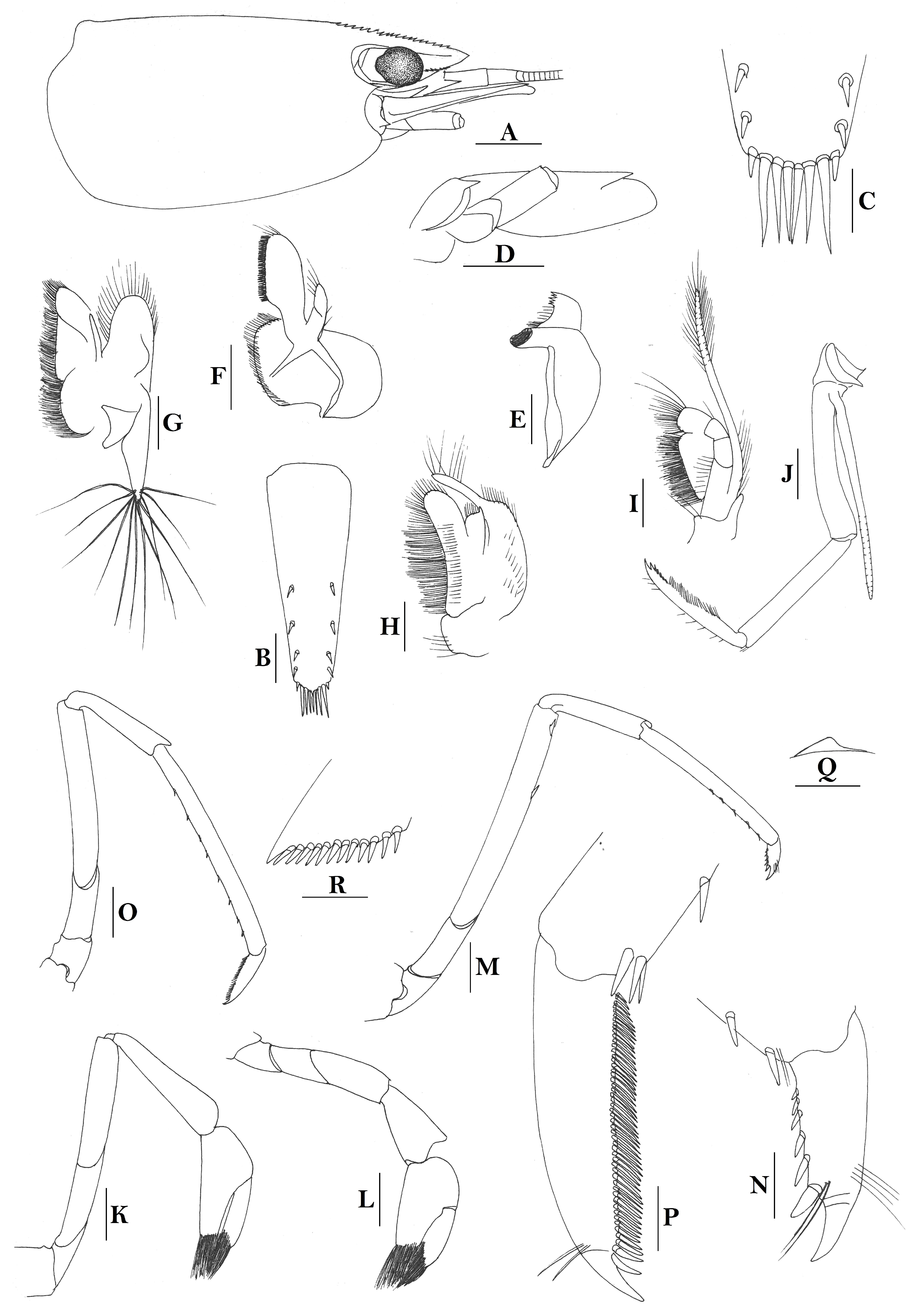
Rostrum straight, reaching to base or near middle of second segment of antennular peduncle, dorsal margin nearly horizontal, rostral formula: 0+14-19/2-4; rarely have 1–2 teeth on carapace posterior to orbital margin. Antennal spine fused with inferior orbital angle; pterygostomian margin rounded.
Sixth abdominal somite 0.45 times as long as carapace, 1.2 times as long as fifth somite, shorter than telson. Telson 2.9 times as long as wide, terminating in a projection, with five pairs of dorsal spinules and one pair of dorsolateral spinules; distal end with about four to five pairs of spines and plumose setae, lateral pair of spines distinctly shorter than intermediate pairs of setae, distal margin broadly rounded. Preanal carina high, without spine.
Anterior end of eye reaching 0.7 length of basal segment of antennular peduncle. Antennular peduncle 0.60 times as long as carapace; anterolateral angle of basal segment reaching 0.20 length of the second segment. Stylocerite reaching 0.8 length of basal segment of antennular peduncle. Scaphocerite 2.8 times as long as wide.
Palp of first maxilliped ending in broad triangle. Third maxilliped reaching to end of antennular peduncle, with ultimate segment distinctly shorter than penultimate segment. Epipods well developed on first four pereiopods. First pereiopod reaching to distal end of basal segment of antennular peduncle; merus 2.0 times as long as broad, as long as carpus; carpus excavated anteriorly, shorter than chela, 1.3–1.8 times as long as high; chela 2.2 times as long as broad; fingers subequal to length of palm. Second pereiopod reaching to end of antennular peduncle; merus as long as carpus, 4.6 times as long as broad; carpus slightly longer than chela, 4.2 times as long as high; chela 2.8 times as long as broad; fingers 1.8 times as long as palm. Third pereiopod reaching beyond end of scaphocerite by 1/3 of its propodus length, propodus 9.4 times as long as broad, 4.7 times as long as dactylus; dactylus 2.4 times as long as wide (spines included), terminating in one stout claw, with five accessory spines on flexor margin. Fifth pereiopod reaching to end of basal segment of antennular peduncle, propodus 10 times as long as broad, 4.3–5.0 times as long as dactylus; dactylus 2.7 times as long as wide (spinules included), terminating in one elongated claw, with 46–55 spinules on flexor margin.
Endopod of male first pleopod subtriangular, reaching 3/7 length of exopod, appendix interna reaching beyond distal end of endopod by most of its length. Appendix masculina of male second pleopod reaching 4/7 length of endopod.
Uropodal diaeresis with 17–19 movable spinules.
Eggs 0.40–0.42 × 0.20–0.25 mm in diameter.
Rivers.
Indonesia (Flores, Bali, Java, Moluccas, Sulawesi, and Papua), Papua New Guinea (New Britain) and the Solomon Islands (Kolombangara and Malaita).
Compared to species that were described earlier, Caridina weberi is morphologically close to C. multidentata Stimpson, 1860. However, it can be easily separated from C. multidentata by the form of rostrum, which is not crested over the post-orbital margin, the shorter sixth abdominal somite (sixth abdominal somite is 1.2 times as long as fifth one vs. 1.5 times in C. multidentata); the form for the spines on the dactylus of the third pereiopod, of which, the subterminal one is distinctly longer than the following accessory spine (vs. distinctly shorter in C. multidentata); the distal end of the propodus does not have an extra-long spine (vs. long spine present in C. multidentata), and the distal part of telson has one pair of spines, the others being setae (vs. all are spines in C. multidentata).
To stabilize the taxonomy of the species, a lectotype is here designated (ovigerous female, cl 6.8 mm, RMNH D1770, syntypes of Caridina weberi De Man, 1892, Kotting, Flores, Indonesia, coll. M. Weber).
de Mazancourt et al. (2020 [
15]) reported the occurrence of
C. weberi in Huvenganga River, Galaku River and Wogan River, New Britain, Papua New Guinea.
3.1.8. Caridina papuana Nobili, 1905
Caridina weberi var.
celebensis Schenkel, 1902 [
57]: 499 [type locality: Kalaena, Luwu, Sulawesi (Celebes), Indonesia].
Caridina weberi var.
papuana Nobili, 1905 [
1]: 481, Figs. 1a, 1b. [Type locality: small forest stream, Stephansort, Madang Province, Papua New Guinea].
Caridina papuana-Holthuis, 1982 [
9]: 609; de Mazancourt et al., 2019a [
14]: 166, Figs. 2–5; de Mazancourt et al., 2020 [
15]: 46, Figs. 2N, 14, 26E; de Mazancourt et al., 2023 [
38]: 390.
Caridina cf.
weberi papuana—de Mazancourt et al., 2017 [
36]: Fig. 4.
One male, cl 3.0 mm, 1 female, cl 3.8 mm, RMNH 704, Manainoemi River at Seroei, Yapen Island, Papua, Indonesia, 21 February 1956; 1 male, cl 3.5 mm, RMNH D619, Stream Ibaroe at Kampong Nangoepkoe near Kampong Benjon, District of Genyem, Papua, Indonesia, 3 November 1954; 39 males, cl 3.9–4.4 mm, 2 ovigerous females, cl 6.1–6.2 mm, RMNH 611, creek above Kloof Kamp, Jayapura, Papua, Indonesia, coll. L. D. Brongersma, M. Boeseman, L. B. Holthuis, 25 October 1954; 30 males, cl 2.5–3.5 mm, 8 females, cl 3.9–4.8 mm, 3 ovigerous females, cl 4.1–4.5 mm, RMNH 705, creek at Ambai, near Kampong Ambai, Papua, Indonesia, 22 February 1955.
Solomon Islands: 1 ovigerous female, with eggs 0.36 × 0.25 mm, USNM 90866, Solomon Islands. Moluccas: 1 male, 2 females, 15 ovigerous females, ZMA D240145, upper stream Rioeapa, west Seram, Indonesia, coll. De Beaufort, 21 January 1910; 1 spec., River Emme, at sidestream, west Seram, Indonesia, coll. De Beaufort, February 1910. Sulawesi: 1 male, cl 3.0 mm, 2 ovigerous females, cl 5.1–5.4 mm, eggs 0.4 × 0.3 mm, ZRC, Sungei Batang, on road from Palopo to Wotu, south Sulawesi, coll. M. Kottelat, 1989. Lesser Sunda: 5 females, 5 ovigerous females, SMF, Sambelia, Lombok Island, Indonesia, coll. J. Elbert, 1909.
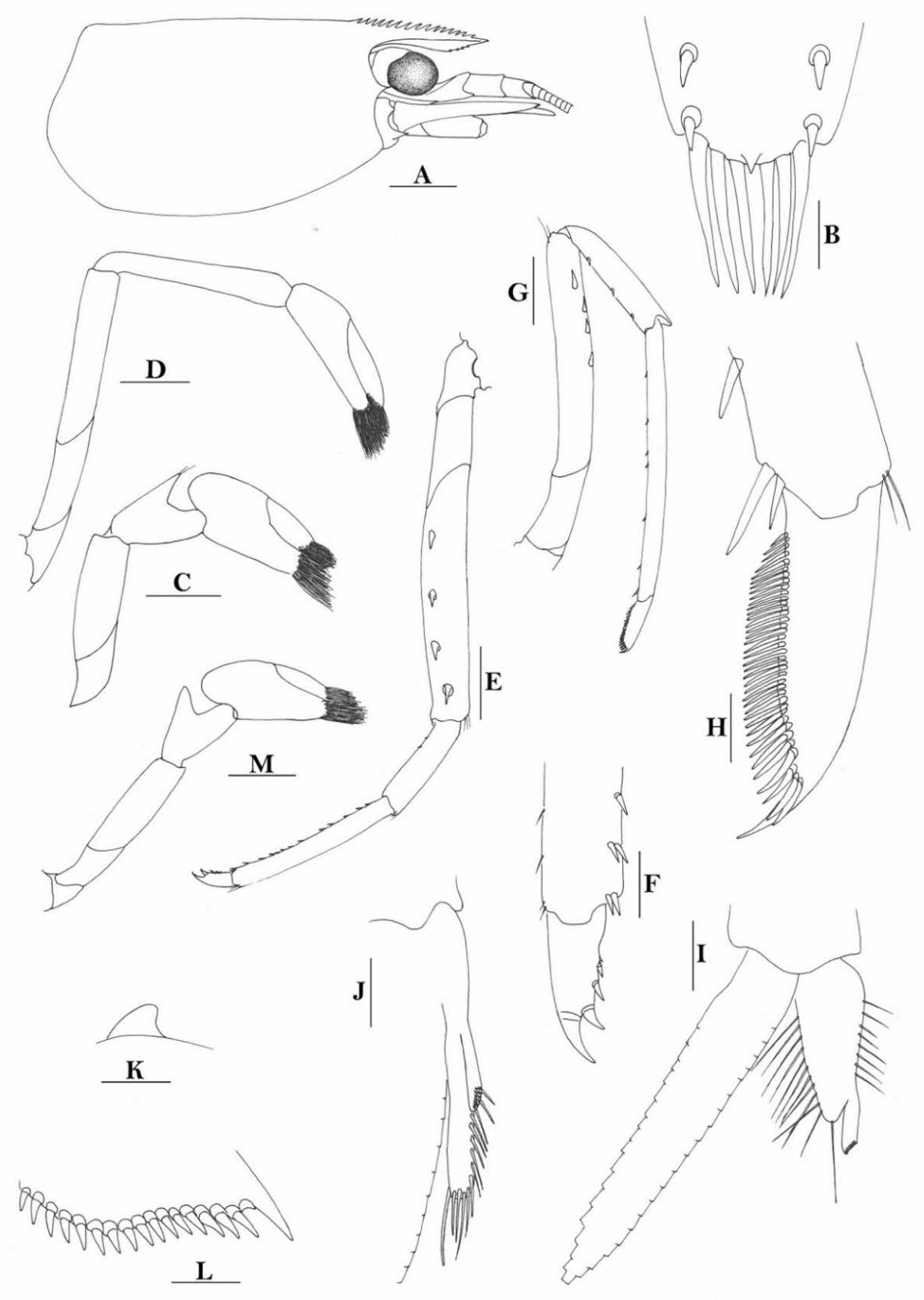
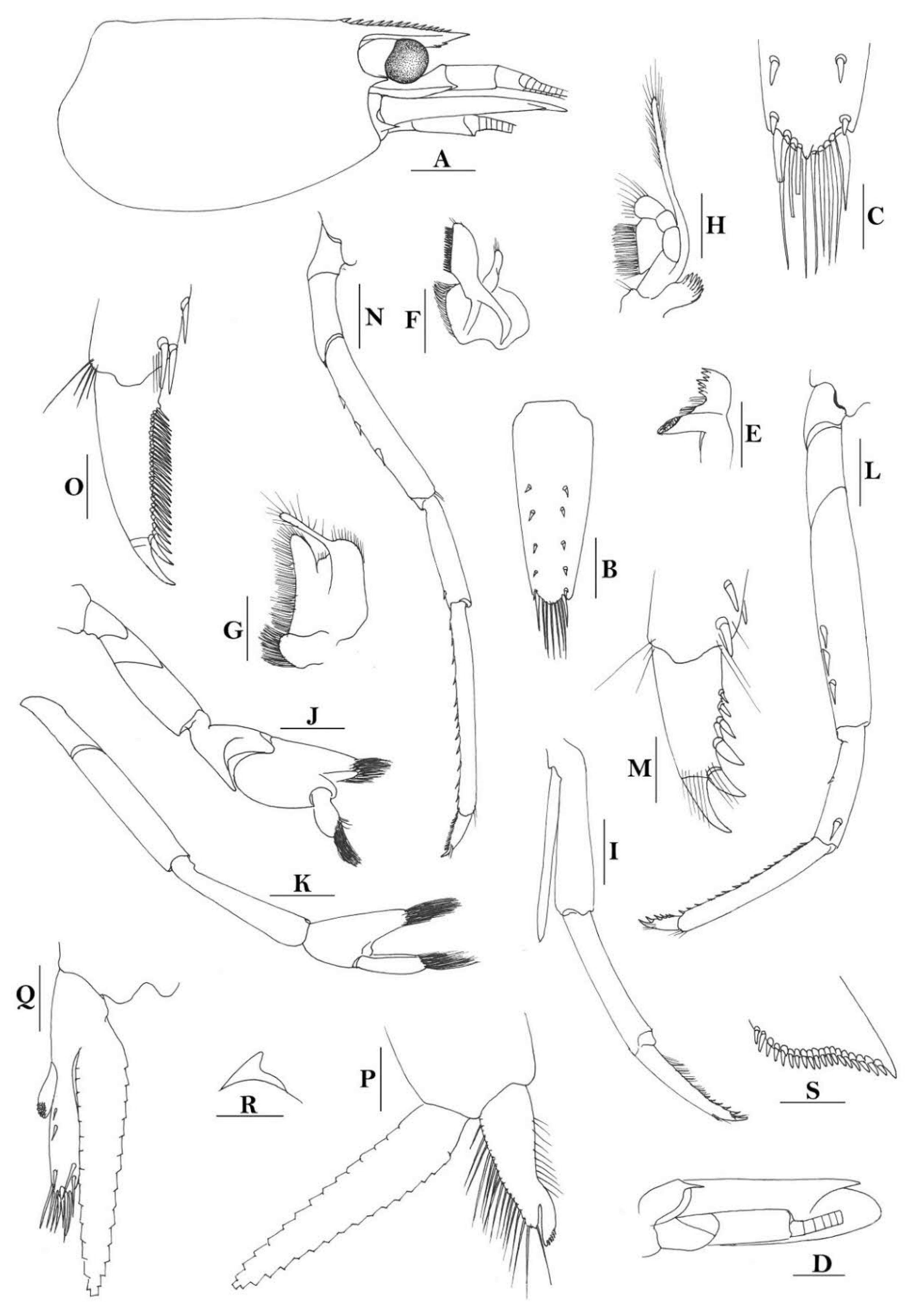
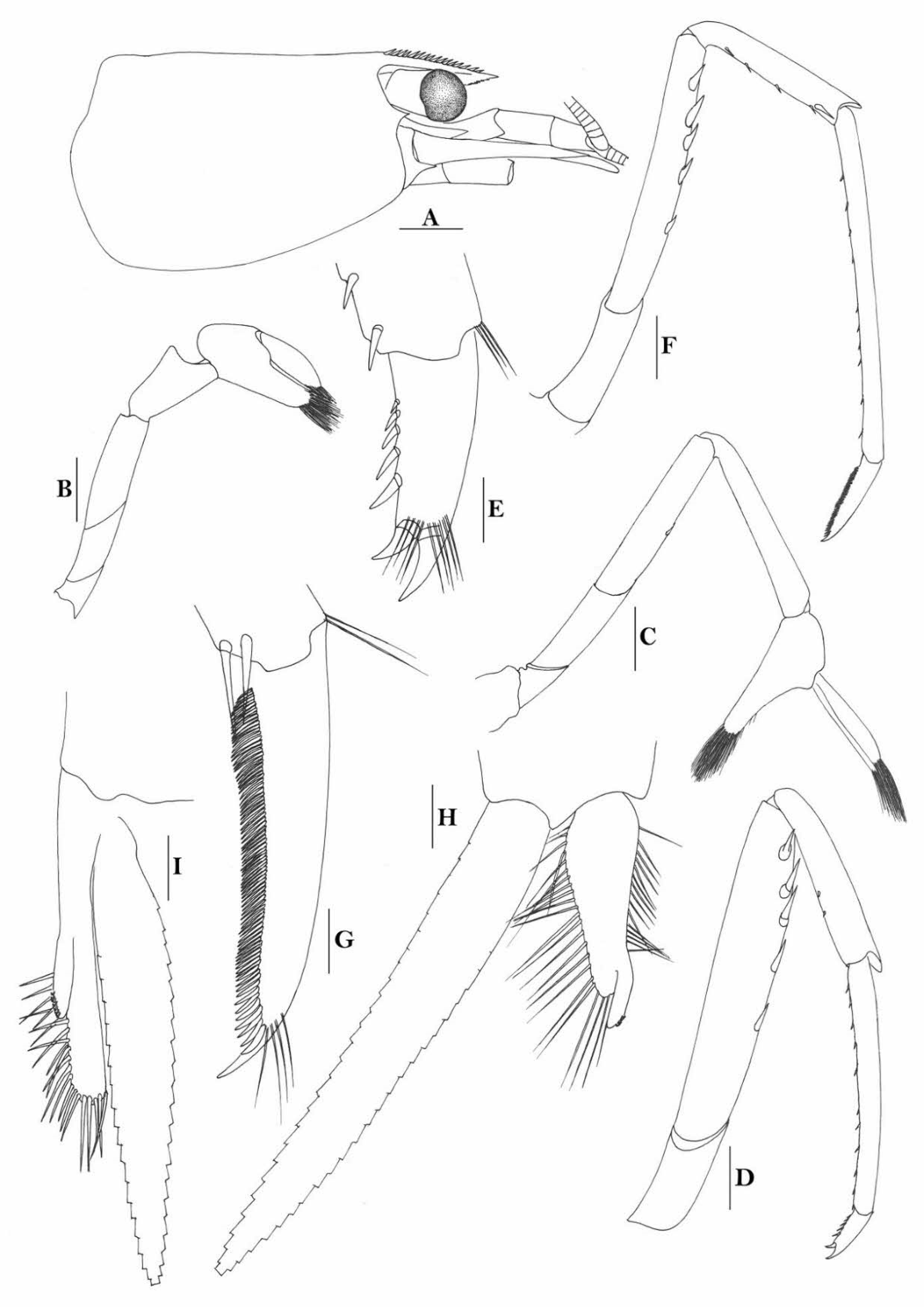
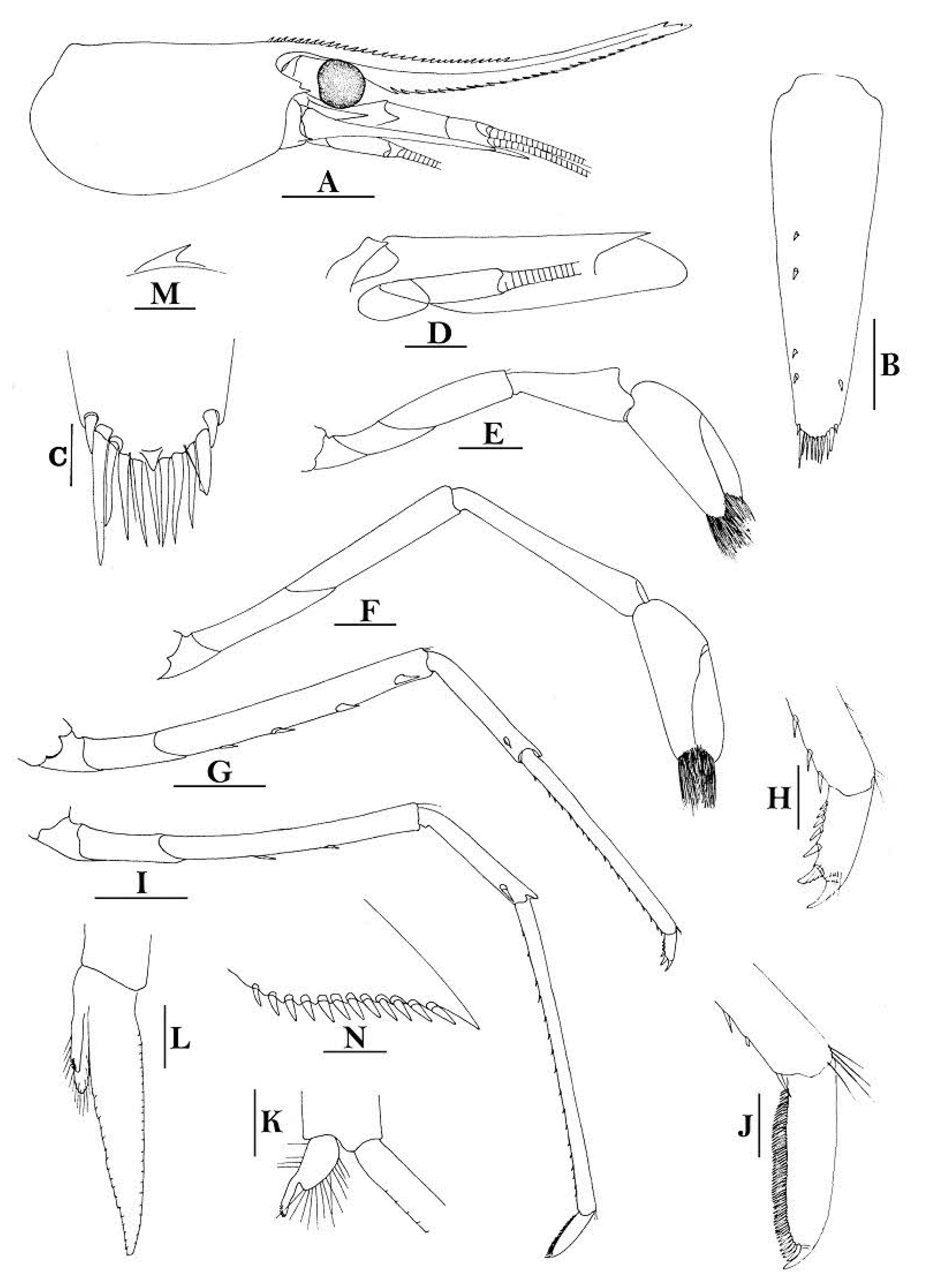
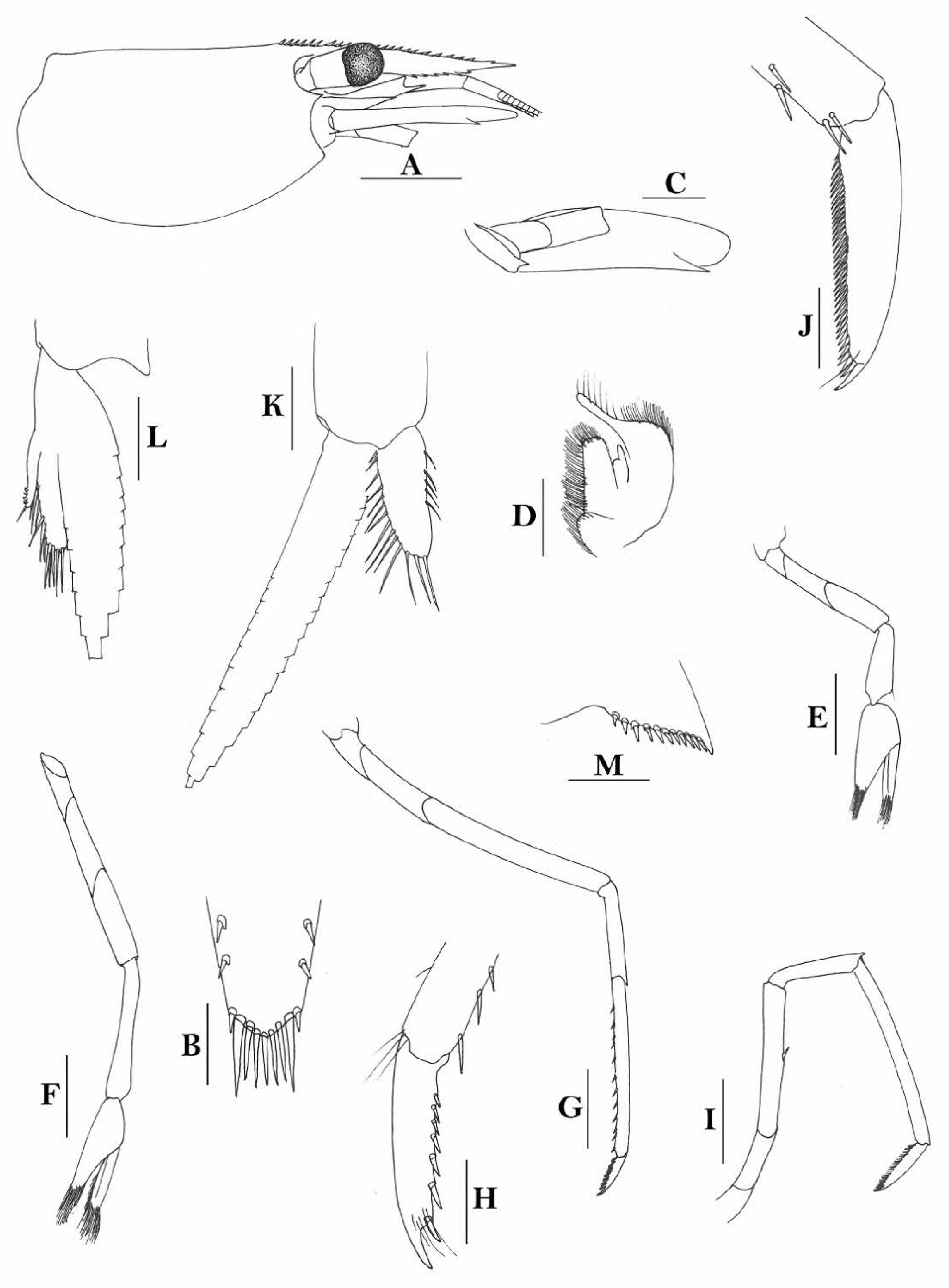
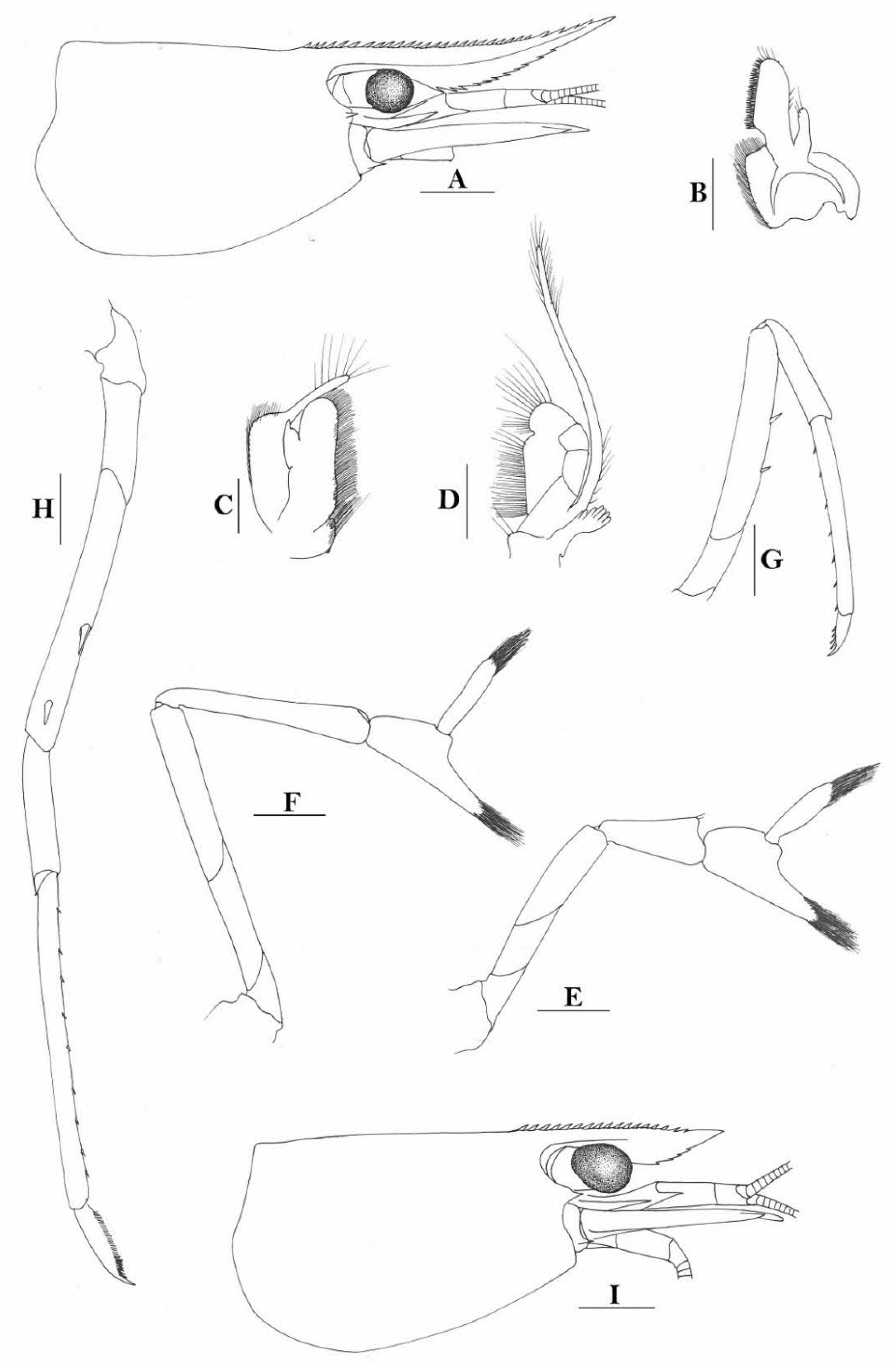
Rostrum straight, reaching to or slightly beyond end of basal segment of antennular peduncle, rostral formula: 0+9-16/2-5. Antennal spine fused with inferior orbital angle; pterygostomian margin rounded.
Sixth abdominal somite 0.44 times as long as carapace, 1.2 times as long as fifth somite, shorter than telson. Telson 2.7 times as long as wide, terminating in a projection, with four pairs of dorsal spinules and one pair of dorsolateral spinules; distal end with about four to five pairs of spines and plumose setae, lateral pair of spines shorter than intermediate pairs of setae, distal margin broadly rounded. Preanal carina high, without spine.
Anterior end of eye reaching 0.8 length of basal segment of antennular peduncle. Antennular peduncle 0.45–0.52 times as long as carapace; anterolateral angle of basal segment reaching 0.20 length of the second segment. Stylocerite reaching 0.8 length of basal segment of antennular peduncle. Scaphocerite 2.9 times as long as wide.
Palp of first maxilliped ending in broad triangle. Third maxilliped reaching to end of antennular peduncle, with ultimate segment distinctly shorter than penultimate segment.
Epipods well developed on first four pereiopods. First pereiopod reaching to distal end of basal segment of antennular peduncle; merus 2.0 times as long as broad, as long as carpus; carpus excavated anteriorly, shorter than chela, as long as high or slightly longer than high; chela 2.2 times as long as broad; fingers subequal to length of palm. Second pereiopod reaching to end of antennular peduncle; merus as long as carpus, 4.0 times as long as broad; carpus as long as chela, 3.2 times as long as high; chela 2.5 times as long as broad; fingers 1.5 times as long as palm. Third pereiopod reaching beyond end of scaphocerite by its dactylus length, propodus 7.1 times as long as broad, 4.3 times as long as dactylus, armed with a prominent long spine at its distal end; dactylus 2.6 times as long as wide (spines included), terminating in two stout claws, with four accessory spines on flexor margin. Fifth pereiopod reaching to end of second segment of antennular peduncle, propodus 8.5 times as long as broad, 4.3 times as long as dactylus, extra long distal spine on distal end reaching to half length of the dactylus or longer; dactylus 2.6 times as long as wide (spinules included), terminating in one elongated claw, with 28–32 spinules on flexor margin.
Endopod of male first pleopod sub-triangular, reaching 0.4 length of exopod, appendix interna reaching beyond distal end of endopod by most of its length. Appendix masculina of male second pleopod reaching 0.8 length of endopod.
Uropodal diaeresis with 17–19 movable spinules.
Eggs 0.37–0.45 × 0.25–0.28 mm in diameter.
Rivers.
Papua New Guinea, Indonesia (Papua, Sulawesi, Lombok, and Ceram), the Solomon Islands (Choiseul), the Philippines (Mindoro), and Taiwan.
de Mazancourt et al. (2020 [
15]) recently redescribed this species in detail and designated a lectotype among its syntypes. The authors also commented that “All former mentions of this taxa were subspecific; given the results of our study, we decided to erect it to specific level.” However, the authors missed out Holthuis (1982 [
9]: 609), who did use a full species name and indicated “
Caridina papuana Nobili, 1905(= C.
weberi papuana)”.
Caridina papuana closely resembles
C. weberi De Man, 1892. When De Man (1892 [
49]) described
C. weberi, he included one lot of specimens from Sulawesi, with relatively shorter rostrums as co-types (=Syntypes). Schenkel (1902 [
57]) subsequently assigned De Man’s (1892 [
49]) Sulawesi specimens to a new variety,
celebensis, together with one female specimen from Luwu, Sulawesi. Nobili (1905 [
1]) described
C. weberi var.
papuana from New Guinea. Types of
C. papuana are not available to the author, but one lot of specimens collected from the Solomon Islands has all the characteristics of
C. papuana, as described in detail by Nobili (1905 [
1]), and were used together with the redescription by de Mazancourt et al. (2020 [
15]) for comparison. The comparison shows that
C. weberi var.
papuana is identical to
C. weberi var.
celebensis. The differences between the two forms mentioned by Bouvier (1925 [
50]: 246) fall within the variation of the species. The name of
C. weberi celebensis is the senior synonym, but this name cannot be used as it is preoccupied by
Caridina serratirostris var.
celebensis De Man, 1892 [
49]. So, the next available name
C. weberi papuana must be used. Regarding the prominent characters of the rostrum, the distinct form of the third and the fifth dactylus,
C. papuana is easily distinguished from
C. weberi.
3.1.9. Caridina buehleri J. Roux, 1934
Caridina buehleri J. Roux, 1934 [
58]: 219, Figs. 1–5 [type locality: Bimun, western New Ireland, Papua New Guinea].
Caridina buehleri–de Mazancourt et al., 2017 [
36]: 222, Figs. 2, 3; de Mazancourt et al., 2019a [
14]: 166, 169–170; de Mazancourt et al., 2020 [
15]: 40, Figs. 2W, 12, 26A.
Not
Caridina buehleri–Klotz, Karge & von Rintelen, 2007 [
59] (=
Caridina gueryi Marquet et al., 2009)
Holotype: Female, cl 6.7 mm, NMB 822, Bimun, western New Ireland, Papua New Guinea, coll. Buehler, 1932. Others: 9 males, cl 2.7–2.9 mm, 4 females, cl 3.0–4.4 mm, RMNH 705, creek at near Kampong Ambai, Ambai Island, Papua, Indonesia, 22 February 1955; 1 ovigerous female, cl 4.6 mm, eggs 0.4 × 0.2 mm, RMNH 700, two creeks near Seroei, Yapen Island, Papua, Indonesia, 22 February 1955; 1 female, cl 3.8 mm, RMNH, Sorong, West Papua, Indonesia, coll. M. A. Lieftiuck, 8 August–6 September 1948.
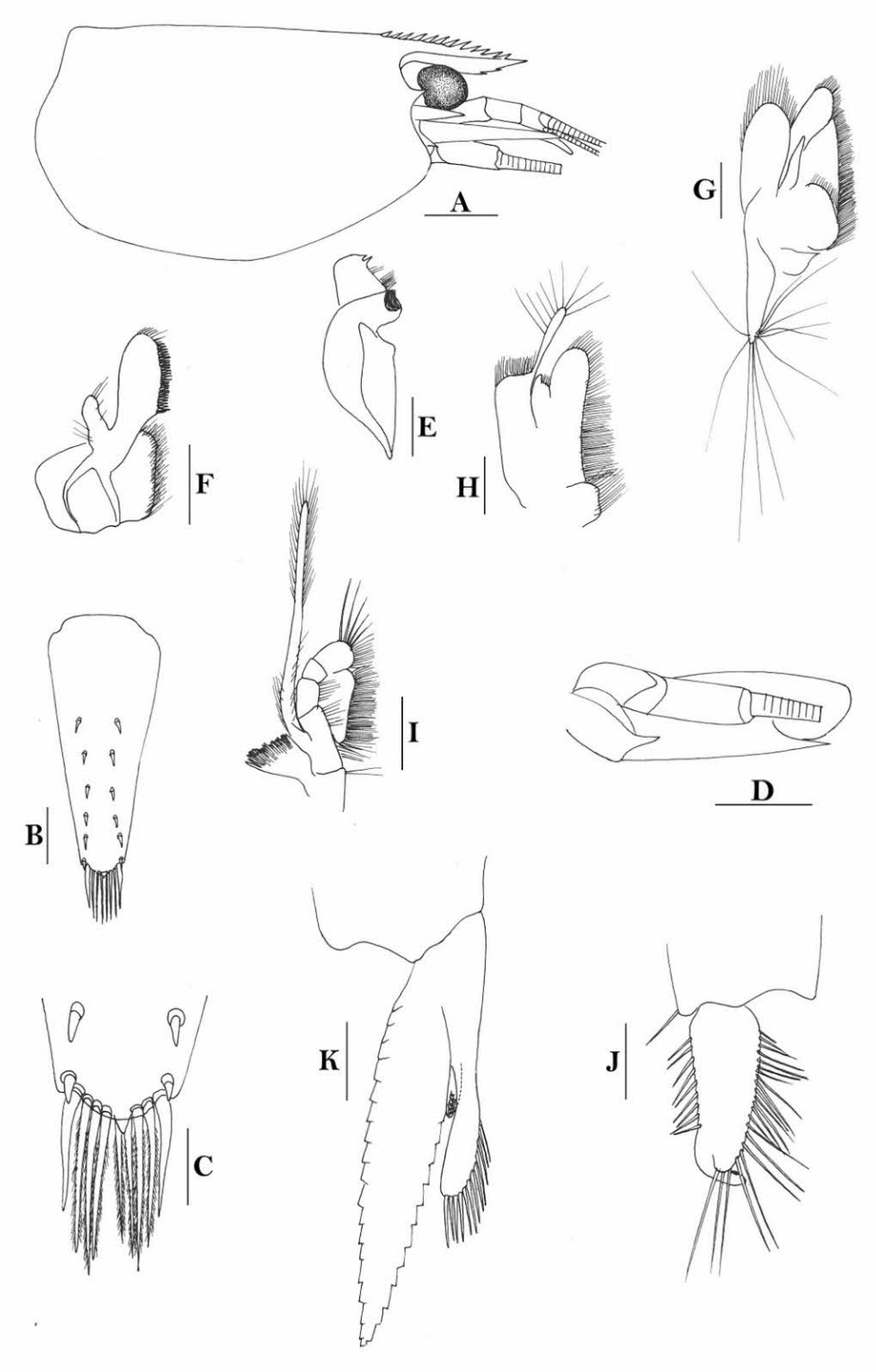
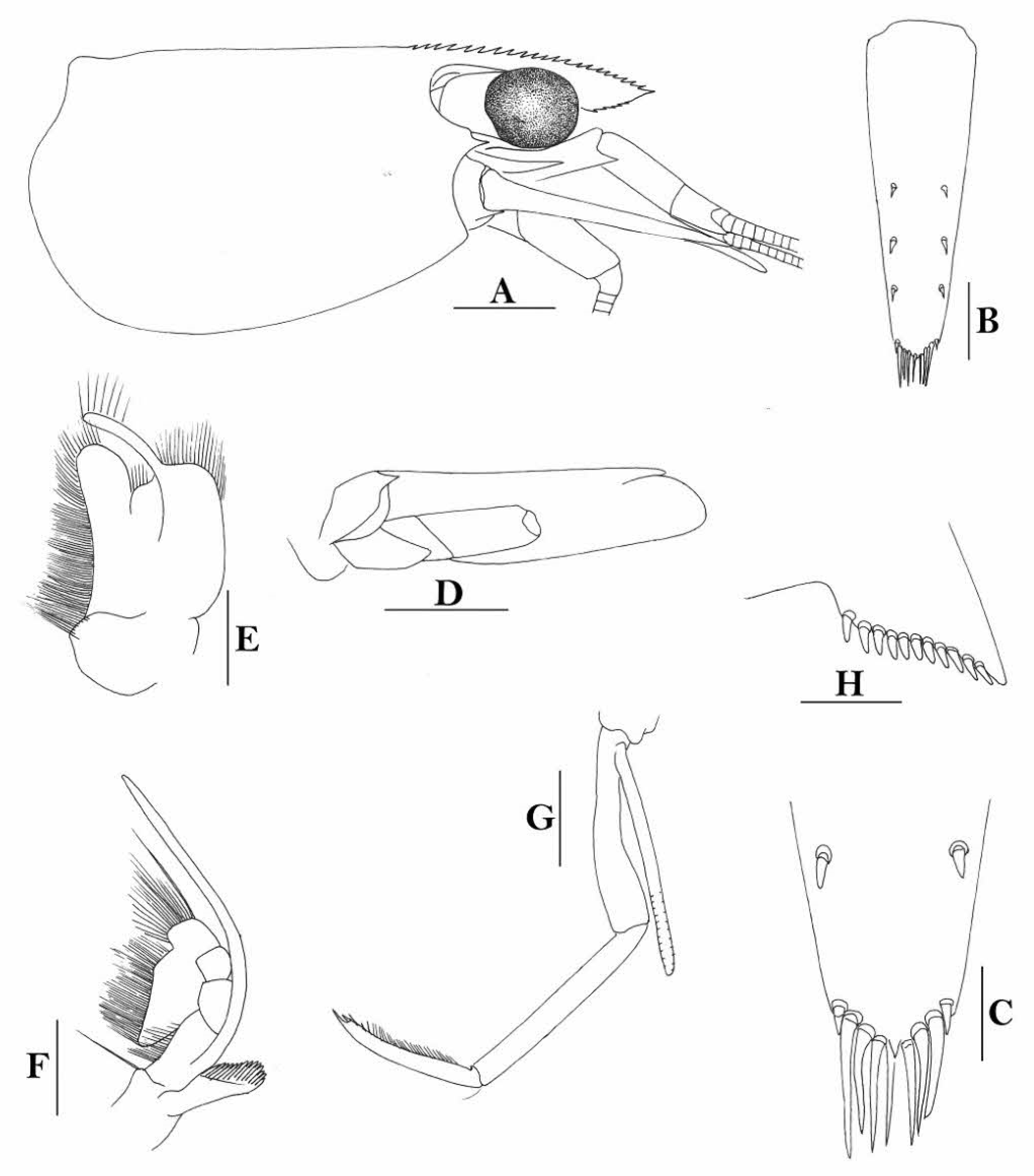
Rostrum straight, reaching to middle of second segment of antennular peduncle or slightly beyond end of antennular peduncle, rostral formula: 2-4+8-13/1-6. Antennal spine fused with inferior orbital angle; pterygostomian margin rectangular.
Sixth abdominal somite 0.48 times as long as carapace, 1.2 times as long as fifth somite, shorter than telson. Telson 3.0 times as long as wide, terminating in a projection, with four pairs of dorsal spinules and one pair of dorsolateral spinules; distal end with one pair of spines and about 3–4 pairs of plumose setae, lateral pair of spines shorter than intermediate pairs of setae, distal margin broadly rounded. Preanal carina high, without spine.
Anterior end of eye reaching 0.8 length of basal segment of antennular peduncle. Antennular peduncle 0.66 times as long as carapace; anterolateral angle of basal segment reaching 0.20 length of the second segment. Stylocerite very long, reaching to or slightly beyond end of basal segment of antennular peduncle. Scaphocerite 3.4 times as long as wide.
Palp of first maxilliped ending in a triangular projection. Third maxilliped reaching to end of antennular peduncle, with ultimate segment distinctly shorter than penultimate segment.
Epipods well developed on first four pereiopods. First pereiopod reaching to distal end of basal segment of antennular peduncle; merus 1.7 times as long as broad, slightly longer than carpus; carpus excavated anteriorly, shorter than chela, 1.2 times as long as high; chela 1.9 times as long as broad; fingers short, 0.5 times as long as palm. Second pereiopod reaching end of antennular peduncle; merus as long as carpus, 4.2 times as long as broad; carpus 1.3 times as long as chela, 4.5 times as long as high; chela 2.0 times as long as broad; fingers 1.4 times as long as palm. Third pereiopod reaching to end of scaphocerite, propodus 7.8 times as long as broad, 3.9 times as long as dactylus; dactylus 2.8 times as long as wide (spines included), terminating in one claw, with five accessory spines on flexor margin. Fifth pereiopod reaching to end of second segment of antennular peduncle, propodus 12 times as long as broad, 3.8 times as long as dactylus, dactylus 4.1 times as long as wide (spinules included), terminating in one elongated claw, with 28 spinules on flexor margin.
Endopod of male first pleopod sub-triangular, reaching 0.4 length of exopod, appendix interna reaching beyond distal end of endopod by half of its length. Appendix masculina of male second pleopod stout, reaching 0.6 length of endopod.
Uropodal diaeresis with 19 movable spinules.
Eggs 0.40 × 0.25 mm in diameter.
Creeks.
Indonesia (Papua), Papua New Guinea (New Guinea. New Ireland, New Britain), Solomon Islands (Kolombangara, Vella Lavella) and western Samoa (Upolu).
de Mazancourt et al. (2020 [
15]) reported it from Vaavu River, Walindi River, New Britain, Papua New Guinea.
3.1.10. Caridina cf. sikipozo
Caridina sikipozo de Mazancourt et al., 2020 [
15]: 62, Fig. 20 [Type locality: Choiseul Island, Solomon Islands, Lokataveva Creek; 06°59.085′ S, 156°47.454′ E]
Fourteen males, cl 3.4–4.4 m, 2 ovigerous females, cl 6.3–6.9 mm, RMNH 615, creek above Marine barracks, Jayapura, Papua, Indonesia, in a small pool, among water plants, coll. L. D. Brongersma, M. Boeseman and L. B. Holthuis, 31 October 1954; 33 males, cl 3.6–4.1 mm, 5 ovigerous females, cl 6.4–6.7 mm, eggs 4.8 × 2.8 mm, RMNH 624, creek above Marine barracks, Jayapura, Papua, Indonesia, coll. L. B. Holthuis, 10 November 1954; 2 ovigerous females, cl 4.1–4.8 mm, RMNH 717, Wasi River, west of Manokwari, West Papua, Indonesia, 9 March 1955.
Rostrum straight or sloping down, short, reaching near to end of basal segment or near end of second segment of antennular peduncle, rostral formula: 0–2(mode 0)+12-19/0-5. Antennal spine fused with inferior orbital angle; pterygostomian margin rounded. Sixth abdominal somite 0.46 times as long as carapace, 1.3 times as long as fifth somite, as long as telson. Telson 2.4 times as long as wide, terminating in a projection, with six pairs of dorsal spinules and one pair of dorsolateral spinules; distal end with about one pair of spines and 4–5 pairs of plumose setae, lateral pair of spines distinctly shorter than intermediate pairs of setae, distal margin broadly rounded. Preanal carina high, without spine.
Anterior end of eye reaching 0.8 length of basal segment of antennular peduncle. Antennular peduncle 0.59–0.75 times as long as carapace; anterolateral angle of basal segment reaching 0.20 length of the second segment. Stylocerite reaching 0.8–0.9 length of basal segment of antennular peduncle. Scaphocerite 2.7 times as long as wide.
Palp of first maxilliped ending in a finger-like projection. Third maxilliped reaching to end of antennular peduncle, with the ultimate segment slightly shorter than the penultimate segment.
Epipods well developed on first four pereiopods. First pereiopod reaching to distal end of basal segment of antennular peduncle; merus 2.6–2.9 times as long as broad, longer than carpus; carpus excavated anteriorly, shorter than chela, 1.5 times as long as high; chela 2.2–2.3 times as long as broad; fingers as long as palm or slightly longer than palm. Second pereiopod reaching to end of antennular peduncle; merus slightly shorter than carpus, 5.4 times as long as broad; carpus 1.2–1.3 times as long as chela, 5.6–5.8 times as long as high; chela 3.0–3.6 times as long as broad; fingers 1.7 times as long as palm. Third pereiopod reaching beyond end of scaphocerite by its dactylus length, propodus 10–11 times as long as broad, 5.0–5.1 times as long as dactylus; dactylus 2.9–3.0 times as long as wide (spines included), terminating in two stout claws, with five accessory spines on flexor margin. Fifth pereiopod reaching to end of antennular peduncle, propodus 15–16 times as long as broad, 3.2–3.8 times as long as dactylus; dactylus 3.8–4.3 times as long as wide (spinules included), terminating in one elongated claw, with 81–88 spinules on flexor margin.
Endopod of male first pleopod sub-triangular, reaching 1/3 length of exopod, appendix interna reaching beyond distal end of endopod by half of its length. Appendix masculina of male second pleopod reaching half length of endopod.
Uropodal diaeresis with 18–21 movable spinules.
Eggs 0.40–0.48 × 0.25–0.27 mm in diameter.
Found in a small pool, among water plants.
Known only from Papua (Irian Jaya), Indonesia.
This species morphologically resembles Caridina sikipozo de Mazancourt, Boseto, Marquet & Keith, 2020, but differs by the number of post-orbital teeth (0–2 (mode 0) vs. 2–3 in C. sikipozo), stouter carpus of first pereiopods (1.5 times as long as high vs. 1.7–1.8 times in C sikipozo, and a slenderer chela of the second pereiopods (3.0–3.6 times as long as wide vs. 2.3–2.4 times in C. sikipozo). However, since C. sikipozo was only known from two small male specimens, the differences described here may not represent the real case. Our specimens have a large number of spinules on flexor margin of dactylus of the fifth pereiopods, which is unusually high (81–88) as compared to other species of the Caridina weberi species group. Unfortunately, the fifth pereiopods are missing in both type specimens. The current specimens are thus tentatively assigned to C. sikipozo with doubt, waiting for further investigation. Caridina cf sikipozo is morphologically similar to C. tupaia de Mazancourt, Marquet & Keith, 2019, in the form of the rostrum, the form of the carpus of the first two pereiopods, and the form of the male sexual appendages, but can be distinguished by the larger number of rostral teeth (12–19 vs. 8–14 in C. tupaia), chela of first pereiopods 2.2–2.3 times as long as broad (vs. 1.9–2.1 times in C. tupaia), fingers as long as palm or slightly longer than palm vs. 0.7–1.0 times as long as palm in C. tupaia, and much higher number of spinules on the flexor margin of dactylus of the fifth pereiopod (81–88 vs. 29–54 in C. tupaia).
3.1.11. Caridina iriana New Species
Holotype: Ovigerous female, cl 4.4 mm, QM W 24129, Mawati River estuary near Timika, southern Papua, Indonesia, 4°48 S 137°08 E, electrofished, coll. Timika Environmental Laboratory, station S760m, freshwater riverine tidal zone, 11 November 1995.
Paratypes: 1 female, cl 4.2 mm, QM 24129, same data as holotype; 1 male, cl 3.6 mm, 1 female, cl 4.8 mm, 1 ovigerous female, cl 4.4 mm, ZRC 2025.0073, same data as holotype.
Others: 1 female, cl 5.0 mm, RMNH, Misool Island, West Papua, Indonesia, coll. A. Z. Beehje, 21 September 1948; 1 male, cl 3.5 mm, RMNH, Waitama River, Misool Island, West Papua, M. A. Lieftiuck, 1–6 October 1948; 1 male, cl 3.6 mm, RMNH 705, creek near Kampong Ambai, Ambai Island, Papua, Indonesia, 22 February 1955.
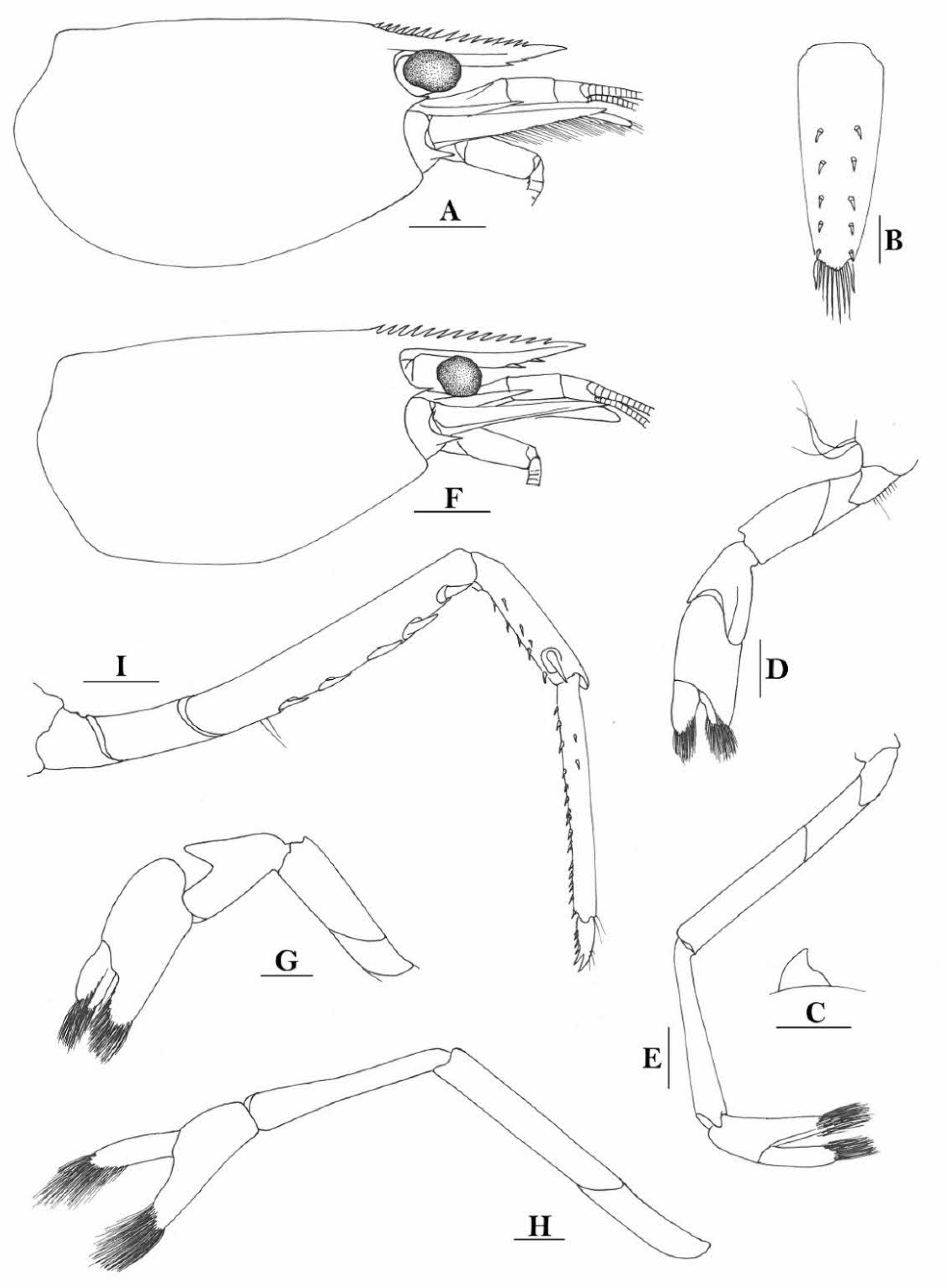
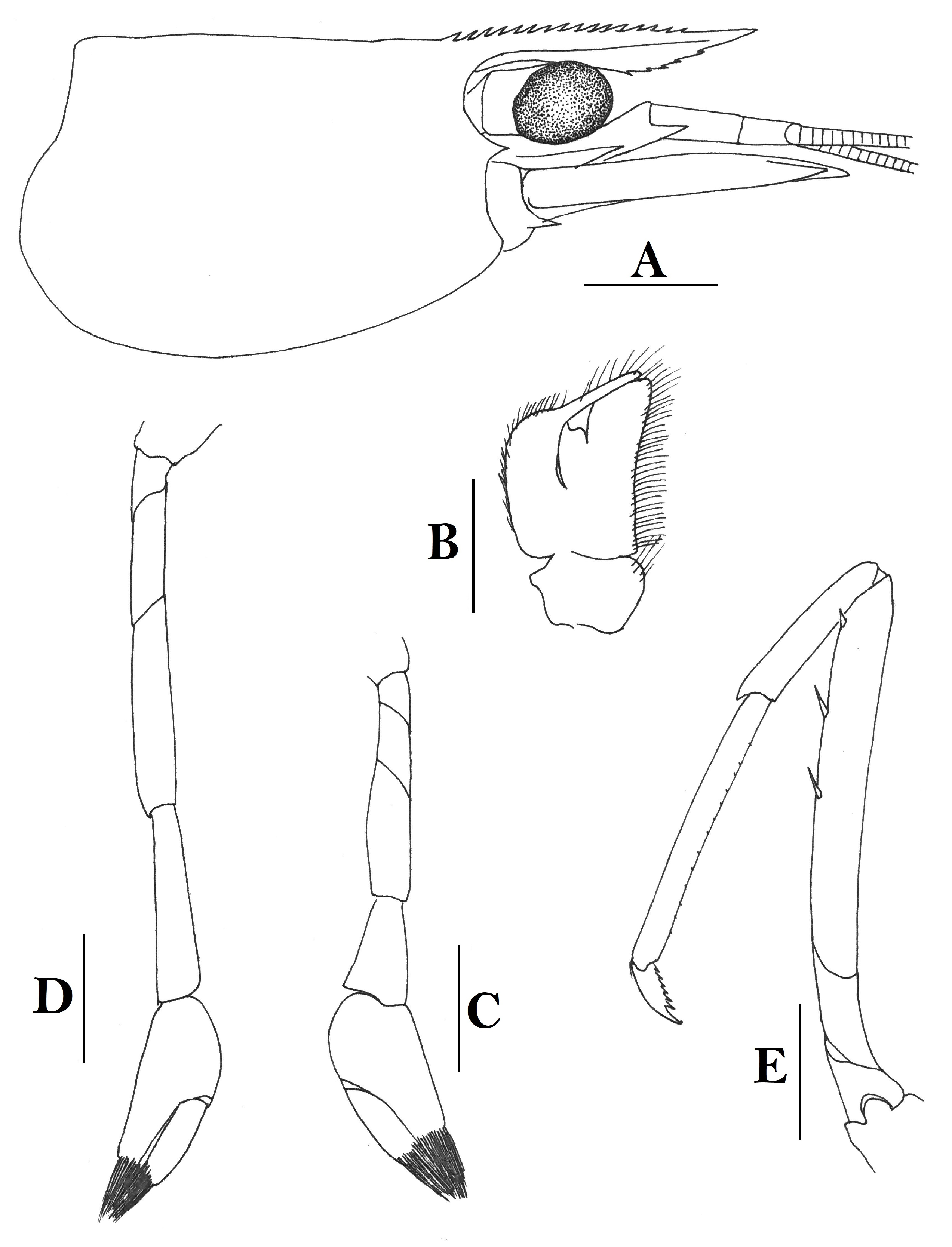
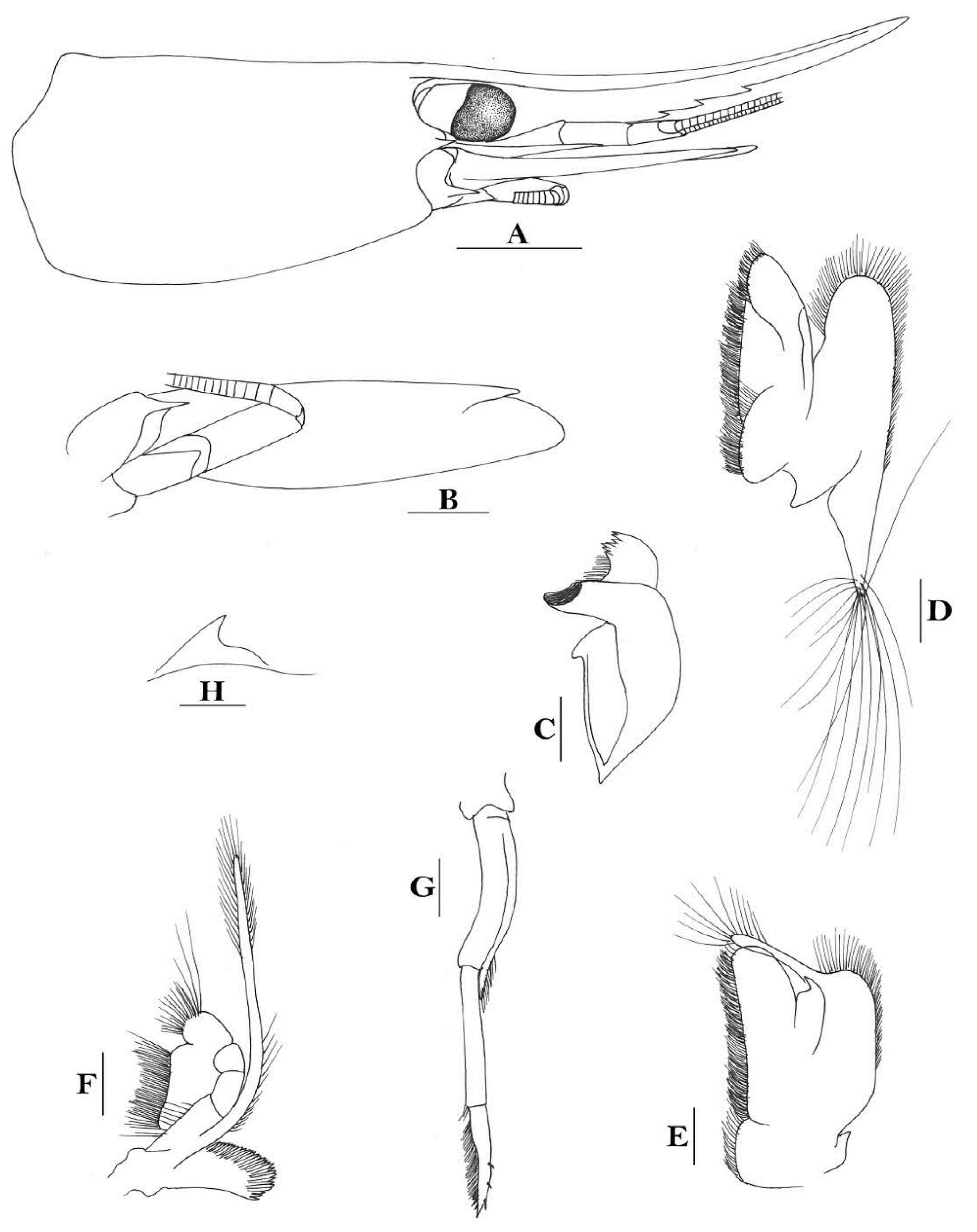
Rostrum straight, reaching middle of second segment of antennular peduncle or to end of this segment, rostral formula: 1-5(2-4) +9-11/0-6(2-4). Antennal spine fused with inferior orbital angle; pterygostomian margin rounded.
Sixth abdominal somite 0.43 times as long as carapace, 1.3 times as long as fifth somite, shorter than telson. Telson 2.3 times as long as wide, terminating in a projection, with five pairs of dorsal spinules and one pair of dorsolateral spinules; distal end with one pair of spines and about 3–4 pairs of plumose setae, lateral pair of spines distinctly shorter than intermediate pairs of setae, distal margin broadly rounded. Preanal carina high, without spine.
Anterior end of eye reaching 0.7–0.8 length of basal segment of antennular peduncle. Antennular peduncle 0.47 times as long as carapace; anterolateral angle of basal segment reaching 0.20 length of the second segment. Stylocerite reaching 0.7 length of basal segment of antennular peduncle. Scaphocerite 2.8 times as long as wide.
Palp of first maxilliped ending in a finger-like projection. Third maxilliped reaching to end of antennular peduncle, with ultimate segment slightly shorter than penultimate segment.
Epipods well developed on first four pereiopods. First pereiopod reaching to distal end of basal segment of antennular peduncle; merus 2.7 times as long as broad, longer than carpus; carpus excavated anteriorly, shorter than chela, 1.1 times as long as high; chela 2.3 times as long as broad; fingers slightly longer than palm. Second pereiopod reaching end of antennular peduncle; merus as long as carpus, 4.1 times as long as broad; carpus 1.3 times as long as chela, 4.5 times as long as high; chela 2.8 times as long as broad; fingers 1.8 times as long as palm. Third pereiopod reaching beyond end of scaphocerite by 1/4 of its propodus length, propodus 8.1 times as long as broad, 4.1 times as long as dactylus; dactylus 2.6 times as long as wide (spines included), terminating in one claw, with five accessory spines on flexor margin. Fifth pereiopod reaching to end of second segment of antennular peduncle, propodus 11 times as long as broad, 3.8 times as long as dactylus, dactylus 4.2 times as long as wide (spinules included), terminating in one elongated claw, with 68 spinules on its flexor margin.
Endopod of male first pleopod sub-triangular, reaching 0.3 length of exopod, appendix interna reaching beyond distal end of endopod by most of its length. Appendix masculina of male second pleopod reaching 0.6 length of endopod.
Uropodal diaeresis with 21 movable spinules.
Eggs 0.40 × 0.25 mm in diameter.
Rivers.
The species is named after the type locality and the currently known distribution range of Irian Jaya, (now Papua), Indonesia, used as a noun in apposition.
Papua (old name Irian Jaya), Indonesia.
With respect to the form of rostrum, the various pereiopods, and the sexual appendages, Caridina iriana, new species, could be easily recognized as members of the C. weberi species group. Within the group, it is close to C. sumatrensis De Man 1892, but can be separated by its number of dorsal teeth on the rostrum situated on the carapace behind orbital margin 1–5 (mode 2–4) vs. 5–6 in C. sumatrensis), relatively shorter carpus of the first pereiopod (1.1 times as long as high vs. 1.6–2.0 in C. sumatrensis), shorter carpus of the second pereiopod (4.5 times as long as high vs. 5.2–6.4 times in C. sumatrensis), and more spinules on the flexor margin of dactylus of the fifth pereiopod (68 vs. 42–49 in C. sumatrensis). The new species looks like Caridina turipi de Mazancourt et al. 2020 in the form of rostrum, and number of post-orbital teeth, but can be differentiated by the stouter chela of the first pereiopod (2.3 times as long as wide vs. 1.9–2.1 times in C. turipi), stouter carpus of the first pereiopod (2.3 times as long as high vs. 1.5–1.8 times in C. turipi), stouter carpus of the second pereiopod (4.5 times as long as high vs. 5.5–6.6 times in C. turipi), stouter chela of the third pereiopod (2.6 times as long as wide vs. 3.0–3.3 times as long as wide), propodus of the third pereiopod (8.1 times as long as wide 8.8–10.5 times in C. turipi), and the larger number of spinules on flexor margin of the propodus of third pereiopod (68 vs. 37–46).
3.1.12. Caridina vitiensis Borradaile, 1898
Caradina vitiensis Borradaile, 1898 [
60]: 1003, pl. 63: Figs. 3, 3a [Type locality: Tamavua River, Viti Levu, Fiji].
Caridina vitiensis-Bouvier, 1905 [
61]: 74; 1912 [
62]: 918; 1913 [
63]: 462; 1925 [
50]: 160, Figs. 336–340; Edmondson, 1935 [
7]: 7; Choy, 1991 [
64]: 356.
?
Caradina vitiensis-Pearson, 1905 [
65]: 76, pl. 1, Fig. 4.
None.
Diagnosis (based on Choy 1991 [
64]):
Rostrum 0.86–1.04 times as long as carapace, rostral formular:1-3+16-23/0-9, sixth abdominal somite 0.67–0.71 times as long as carapace. First pereiopods with carpus 2.0–2.53 times as long as high, second pereiopods with carpus 4.64–4.71 times as long as high, dactylus of fifth pereiopods with 26–35 spinules on its flexor margin. Appendix masculina of male second pleopods 2.0 times as long as appendix interna. Uropodal diaeresis with 9–12 spinules. Ovigerous females with eggs sized 0.25–0.29 × 0.41–0.50 mm.
The type specimens in Fiji were collected from two stations along streams with elevation of 30 m and 100 m, respectively (Choy 1991 [
64]).
Fiji (Viti Levu and Vanua Levu), Solomon Islands and Papua New Guinea.
Edmondson (1935 [
7]) reported additional records of
Caridina vitiensis Borradaile, 1898 from Papua New Guinea, the Bismarck Archipelago and the Solomon Islands; the species having been described from Fiji (Borradaile 1898 [
60]). According to the original description based on a single specimen, the species is characterized by a straight rostrum with 24 teeth on the upper margin, none on the carapace, and 9 on the lower margin. Edmondson (1935 [
7]) commented that “Borradaile considered it close to
Caridina weberi but there are more rostral teeth, the second chelipeds are stouter and the last two legs longer than in that species”. Choy (1991 [
64]) reported the species from two locations from Vanua Levu, provided morphological characteristics for comparison with
C. japonica (=
C. multidentata),
C.weberi and
C. devaneyi.Pearson (1905 [
65]) reported it from Ceylon (Sri Lanka), which Bouvier (1925 [
50]: 260) treated as a doubtful record. Based on the description and figure given by Pearson (1905 [
65]), the species has a rostral formula of 18-20/6, of which three are on the carapace. “The distal joint of the last thoracic legs has a large number of closely packed spines on its posterior border. The sixth abdominal segment is almost twice as long as the fifth, and the telson is equal in length to the sixth segment. The telson bears five pairs of small spines on its dorsal side. Each corner of the posterior border bears a small spine, and there are four pairs of longer spines arranged along the posterior border”. Pearson’s specimens do not fit well with
C. vitiensis.
3.1.13. Caridina brachydactyla De Man, 1908
Caridina nilotica var.
brachydactyla De Man, 1908 [
66]: 269, pl. 20, Figs. 8a-c [type locality: Sulawesi (Celebes), Saleyer and Flores, Indonesia].
Caridina nilotica var.
brachydactyla-J. Roux, 1919 [
5]: 319.
Caridina brachydactyla—Bouvier, 1913 [
63]: 463; Cai & Ng, 2001 [
26]: 671; Fig. 6; Richard & Clark, 2014 [
67]: 308, Fig. 3 (part); de Mazancourt et al., 2019a [
14]: Fig. 1.
Caridina brachydactyla brachydactyla—Tiwari & Pillai, 1971 [
68]: 80, Fig. 1.
Not
Caridina brachydactyla-Leberer & Cai 2003 [
69]: 354. (=
Caridina variabilis de Mazancourt, Marquet, Rogers & Keith, 2018)
Seven males, cl 4.6–4.8 mm, 1 ovigerous female, cl 6.7 mm, RMNH 624, creek above Marine barracks, Jayapura, Papua, Indonesia, coll. L. B. Holthuis, 10 November 1954.
One female, cl 6.7 mm, 2 ovigerous females, cl 5.1–5.3 mm, RMNH D 2552, syntype of Caridina nilotica var. brachydactyla, Luwu, River near Palopo, Celebes (Sulawesi), coll. Max Weber, February 1889. Others: Philippines: 8 males, cl 2.8–3.5 mm, 5 females, cl 3.2–4.9 mm, 1 ovigerous female, cl 3.8 mm, USNM 285324, Calawagan River, River mouth, Mindoro, the Philippines, Albatross Philippines Expedition, 11 December 1908; 6 males, cl 4.0–4.1 mm, 13 ovigerous females, cl 5.3–5.5 mm, ZRC, Philippines: Leyte: Lagu Lagu creek, southern margin of ViSCA, about 7 km north of Baybay, ca. 2 km from the sea, riffles, coll. M. Kottelat, 25 June 1993; 2 males, cl 3.3–3.4 mm, 3 females, cl 3.8–4.8 mm, 7 ovigerous females, cl 5.9–6.2 mm, USNM 285316 Zamboanga Canal, Zaboanga del Sur, Mindanao, the Philippines, Albatross Philippines Expedition, 08 October 1909; 1 male, cl 3.5 mm, 2 ovigerous females, cl 4.7–4.8 mm, USNM 285323, Nato River, Camarines Sur, Luzon, the Philippines, Albatross Philippines Expedition, 18 June 1909. Sulawesi: 3 males, cl 3.1–3.5 mm, 3 females, cl 2.9–3.2 mm, ZRC, Sungai Batang, KMB on road from Palopo to Wotu, coll. M. Kottelat, 22 February 1999; 2 ovigerous females, cl 5.5–7.0 mm, RMNH, Bantimurung, southern Sulawesi, Indonesia, 9 July 1947; 7 males, cl 5.1–5.8 mm, 2 females, cl 6.8–7.0 mm, 16 ovigerous females, cl 6.0–7.1 mm, ZRC, Kabupaten Maros, above Bantimurung water fall, M. Kottelat, 7 June 1988. Lesser Sunda: 3 males, cl 3.8–4.6 mm, 3 females, cl 4.2–5.3mm, 1 ovigerous female, cl 6.4 mm, RMNH, Medewi Atas, river Medewi, Bali, Indonesia, coll. A T. Whitten, 15 June 1992; Java: 2 males, cl 3.0–4.1 mm, 3 females, cl 3.5–4.5 mm, 2 ovigerous females, cl 3.9–5.2 mm, MZB, Bantan, Java, Indonesia, coll. D. I. Hartoto, Aug 1983; 1 male, cl 3.8 mm, 5 juveniles, RMNH N42a, Labuhan, West Java, Indonesia, no date; 1 female, cl 4.2 mm, MZB, S. Cihanggasa, Banten, Java, coll. D. Wowor, 16 August 1982. Timor: 1 male, cl 3.5 mm, 8 ovigerous females, cl 3.6–5.3 mm, RMNH, Kupang, Timor, Indonesia, 17 November 1929; 1 ovigerous female, RMNH D1460, Timor, Indonesia, no date; 1 male, cl 3.5 mm, 8 ovigerous females, cl 4.3–5.0 mm, RMNH, Kupang, Timor, Indonesia, 17 November 1929; 1 female, 2 ovigerous females, 5.3–5.5 mm, North Sumba, Indonesia, coll. Ten Kate, no date.
Rostrum horizontal, reaching end of scaphocerite, slightly shorter or as long as carapace. Rostral formula: 2-4+19-22+1-2/11-12. Antennal spine placed below suborbital angle. Pterygostomian margin rounded.
Sixth abdominal somite 0.66 times as long as carapace, 1.9 times as long as fifth somite, slightly shorter than telson. Telson 3.5 times as long as wide, terminating in a projection, with four pairs of dorsal spinules and one pair of dorsolateral spinules; telson with 4–5 pairs of distal spines, sublateral pair distinctly longer than intermediate pairs. Preanal carina with a spine.
Anterior end of eye reaching 0.7 length of basal segment of antennular peduncle. Antennular peduncle 0.80 times as long as carapace; anterolateral angle of basal segment reaching 0.3 length of second segment. Stylocerite reaching 0.85 length of basal segment of antennular peduncle. Scaphocerite 3.8 times as long as wide.
Third maxilliped reaching to end of second segment of antennular peduncle, with ultimate segment distinctly shorter than penultimate segment. Epipods on first four pereiopods. First pereiopod reaching distal end of eyes, merus of first pereiopod 3.1 times as long as wide, slightly shorter than carpus; carpus shorter than chela, 2.5–3.2 times as long as high; chela 2.3–2.5 times as long as broad, with fingers 2.0 times as long as palm. Second pereiopod reaching to end of second segment of antennular peduncle, merus 5.3 times as long as broad; carpus of second pereiopod 5.0 times as long as high, 1.2 times as long as chela, chela 2.2 times as long as wide, fingers 1.8–2.3 times as long as palm. Third pereiopod reaching beyond end of scaphocerite by its dactylus, propodus 11 times as long as broad, 5.8 times as long as dactylus; dactylus 2.3 times as long as wide (spines included), with 4–7 accessory spines on flexor margin. Fifth pereiopod reaching near end of antennular peduncle, propodus 18 times as long as broad, 6.0 times as long as dactylus, with a large spine at its end in female specimens; dactylus 2.9 times as long as wide (spinules included), with 34–42 spinules on flexor margin.
Endopod of male first pleopod subtriangular, 0.25 length of exopod, appendix interna elongated, with most of its length reaching beyond end of endopod. Appendix masculina of male second pleopod half length of endopod, appendix interna stout.
Uropodal diaeresis with 9–14 movable spinules.
Eggs 0.40–0.42 × 0.25–0.30 mm in diameter.
Lowland freshwater, with sea water influence.
Indonesia (Sulawesi, Papua, Halmahera, Timor, Bali), the Philippines (Luzon, Mindoro, Leyte, Mindanao), and India (Andaman Islands).
Richard & Clark (2010 [
70]) reported
Caridina brachydactyla from South Africa, examined the type series of the species from Indonesia, redescribed and illustrated the species in detail based on specimens from the distributed range of Indonesia and Indian Ocean, and synonymized
Caridina nilotica var.
natalensis De Man 1908,
Caridina nilotica var.
brevidactyla J. Roux, 1919, with
C. brachydactyla. Richard & Clark (2014 [
67]) further synonymized
Caridina meridionalis J. Roux, 1926, with
C. brachydactyla, and extended its range to further east. de Mazancourt et al. (2018 [
13]) conducted morphological and molecular studies based on both old and newly collected specimens, recognized four species that are allied to
C. longirostris H. Milne Edwards, 1837, namely
C. appendiculata Jalihal & Shenoy, 1998,
C. brevidactyla Roux, 1919,
C. gracilipes De Man, 1892, and
C. meridionalis Roux, 1926. de Mazancourt et al. (2019c [
71]), revalidated
Caridina natalensis,
Caridina brevidactyla, and
Caridina meridionalis based on both morphology and molecular data, and concluded that all material previously reported from the West Indian Ocean should be referred to as
C. natalensis, and that
C. brachydactyla is restricted to Indonesia.
J. Roux (1919 [
5]) reported
C. nilotica var.
brachydactyla in Aru Islands.
3.1.14. Caridina gracilipes De Man, 1892
Caridina Wyckii var.
gracilipes De Man, 1892 [
49]: 387, pl. 24: Figs. 29–29e [type localities: Sulawesi (Celebes), and Selajar, Indonesia].
Caridina wyckii- Henderson, 1893 [
72]: 434, Nobili, 1905 [
1]: 480.
Caridina nilotica var.
gracilipes -De Man, 1908 [
66]: 270, Figs. 7a, b; Ueno, 1935 [
73]: 272, Fig. 2; Yu, 1974 [
74]; 52, Figs. 2, 3.
Caridina wyckii var.
gracilipes -Schenkel, 1902 [
57]: 498, fig. 5.
Caridina nilotica bengalensis De Man, 1908 [
66]: 265, Figs. 6a, b [type locality: Calcutta, India].
Caridina acuticaudata Dang, 1975 [
75]: 70, Fig. 4 [type locality: Hoa Binh, North Vietnam].
Caridina gracilipes Richard & Chandran, 1994 [
76]: 246, Fig. 3; Wowor et al., 2004 [
77]: 341 (key), Figs. 6C, D; Cai & Shokita, 2006a [
24]: 250; Cai, 2014 [
78]: 208, Figs. 1–3; Richard & Clark, 2014 [
67]: 310, Figs. 4 and 5; Cai et al. 2007 [
31]: de Mazancourt et al., 2018 [
13]: 1438, Fig. 6; de Mazancourt et al., 2019a [
14]: Fig. 1; Cai, 2020 [
79]: 1407, Figs. 1–3; de Mazancourt et al., 2023 [
38]: 382.
Not
Caridina nilotica gracilipes -Kemp, 1918 [
80]: 275; Liu, 1955 [
81]: 28, Figs. 11–17.
Two males, cl 2.0–2.1 mm, 4 females, cl 2.2–2.6 mm, 3 ovigerous females, cl 2.4–2.8 mm, RMNH 612, Kampond Simboro, near Sentani lake, Papua, Indonesia, coll. L. D. Brongersma, M. Boesenian, L. B. Holthuis, 27 October 1954; 1 female, cl 3.8 mm, RMNH 281–345, Wanigela, Northern District, Territory of Papua, Papua New Guinea, R. D. Hoogland, June–September 1954; 2 females, cl 2.0–3.0 mm, RMNH, east shore of Lake Sentani, at Joka, Papua, Indonesia, coll. L. D. Brongersma & L. B. Holthuis, 20 October 1954; 1 female, cl 2.7 mm, 7 ovigerous females, RMNH, cl 2.3–2.8 mm, Sentani Lake, Koka near Jayapura, Papua, coll. L. V. D. Hamman, 27 December 1953; 1 male, cl 2.4 mm, 5 ovigerous females, cl 2.6–3.0 mm, RMNH 614, Kampong Borowai, west of Sentani Lake, 0–0.5 m in depth, Papua, Indonesia, 19 January 1955; 3 males, cl 2.6–3.2 mm, 31 females, cl 2.5–4.0 mm, QM W24105, Minajerwi River, Main Ajkwa River anastomosis near Timika, southern Papua, Indonesia, freshwater, riverine tidal zone, 9 August 1995; 13 ovigerous females, cl 2.5–3.1 mm, 269 non-ovigerous specimens, cl 1.7–2.6 mm, RMNH 613, Kampong Sisiri, North West of Sentani Lake, Papua, coll. L. D. Brongersma & L. B. Holthuis, 27 October 1954.
Sulawesi: Lectotype: Ovigerous female, cl 5.3 mm, syntypes of Caridina var. gracilipes De Man, 1892, RMNH D 1317, Maros River, Sulawesi, Indonesia, coll. Max Weber, September–October, 1888. Paralectotype: 2 females, cl 4.2–4.4 mm, RMNH D 1317, data same as lectotype. Others: 5 males, cl 3.2–4.2 mm, 2 females, cl 3.7–3.8 mm, Sungai Bula at Desa Poura, Kec. Pampuana Kab. Bone, on road from Sinjai to Singkang, Sulawesi, Indonesia, coll. M. Kottelat, 10 June 1988; 2 males, cl 3.7–3.8 mm, 3 females, cl 2.8–3.7 mm, Sungai Batang, ZRC, on road from Palopo to Wotu, coll. M. Kottelat, 22 February 1999; 14 males, cl 2.6–3.7 mm, 9 females, cl 2.7–4.3 mm, 4 ovigerous females, cl 4.2–4.4 mm, Lake Tondano, North Sulawesi, Indonesia, coll. D. Wowor, 22 September 1983; 9 males, cl 2.3–3.8 mm, 26 females, cl 3.1–4.0 mm, 13 ovigerous females, cl 3.9–4.7 mm, Batoe Batoe (Rocks), Tampe-ancct, Sulawesi, Indonesia, 19 July 1947; 3 males, cl 2.9–4.0 mm, 19 females, cl 2.3–3.1 mm, Sungei Tjimanae, Kamp. Sallima, Singkang, southern Sulawesi, 10 August 1947; 30 specimens, NMB 4IVa, Makassar, South Sulawesi, coll. Sarasin, 1901; 1 male, cl 3.2 mm, 1 female, cl 3.8 mm, 1 ovigerous female, cl 5.3 mm, USNM 39490, Makassar, southern Sulawesi, Indonesia, coll. J. Barbour, 1906–7. Lesser Sunda Islands: 2 males, cl 3.1–3.3 mm, 1 female, cl 3.4 mm, 1 ovigerous female, cl 6.0 mm, from a stagnant pool in a dry river bed, freshwater, Desa Bolo, 21 km south of Bima, Sumbawa Island, Indonesia; 1 female, ZMA De 240106, coll. Boeleleng, 25 January 1907; 1 specimen, ZMA De 240119, vicinity of Ampenan, Lombok, Indonesia, coll. V. Kampen, 26 January 1907; 3 females, 2 ovigerous females, ZMA, river of Konga, Flores, Indonesia, coll. Sande, 10 October 1909; 1 male, 2 females, ZMA D102632, Ba River, near Enden, Flores, Indonesia, coll. M. Weber, 1888; 1 male, 2 ovigerous females, NMB 4IVf, Kmga Flores, Indonesia, exchanged from Museum Amsterdam, 1918; 1 male, 1 female, 2 ovigerous females, NMB 4IVd, Ampenan, Lombok, Indonesia, exchanged from Museum Amsterdam, 1916; 2 males, 1 female, 5 ovigerous females, NMB 4IVe, Makassa, South Sulawesi, exchanged from Amsterdam, 1916. Java: 1 male, cl 3.6 mm, 8 females, cl 3.5–4.5 mm, 1 ovigerous female, cl 4.0 mm, MZB Cru 1060, Bantan, Java, Indonesia, coll. D. I. Hartoto, August 1983; 7 males, cl 2.6–3.8 mm, 2 females, cl 3.3–3.8 mm, RMNH N42a, Labuhan, West Java, Indonesia, no date; 14 males, cl 2.5–3.7 mm, 8 females, cl 2.4–3.4 mm, 11 ovigerous females, cl 3.8–4.8 mm, RMNH, Selamat Datang Bay, river at Kg. Cemara and Kg. Ciujung, West Java, Indonesia, brackish water, 11 November 1941; 1 male, cl 2.6 mm, 9 females, cl 2.0–4.5 mm, RMNH Selamat Datang Bay, West Java, River at Kg. Cemara and Kg. Ciujring, freshwater, 11 November 1941. Borneo: Thirteen males, cl 2.7–4.0 mm, 3 females, cl 3.3–4.5 mm, MZB DW98-43, Sungei Kakap, Kabupaten Kapuas, Kalimantan Barat, coll. D. Wowor, 5 June 1998; 29 males, cl 2.4–5.2 mm, 5 females, cl 3.9–5.6 mm, ZRC THH9692, Borneo, Sarawak: Stream 3.0 km before turnoff to Cape Pelandok and Kg. Pandan, after Lundu town, drains from Gg. Gading, coll. H. H. Tan, 2 September 1996; 2 males, cl 4.0 mm, 1 female, cl 2.7 mm, ZRC 1995.513, East Kalimantan: “tidal creek” near Semunad, coll. M. Kottelat, 10 February 1993. Sumatra: 8 males, cl 3.2–4.0 mm, 5 females, cl 2.5–4.3 mm, 7 ovigerous females, cl 3.6–5.3 mm, RMNH, Pedada Bay, River Pedada, South Sumatra, Indonesia, fresh water, 10 November 1941; 7 males, cl 3.7–4.5 mm, RMNH, Ketang, east coast of Sumatra, Indonesia, 6 November 1941.
Lowland freshwater, with sea water influence, and Lakes.
Southern China (Fujian, Guangdong, Guangxi, Hainan and Taiwan), Vietnam, Singapore, Philippine, Australia, New Guinea, Sulawesi, Lesser Sunda Islands, Borneo, Java, Sumatra, Sri Lanka, and India.
de Mazancourt et al. (2018 [
13]) conducted morphological and genetic studies based on both old and newly collected specimens, recognized four species that are allied to
C. longirostris, namely
C. appendiculata Jalihal & Shenoy, 1998,
C. brevidactyla Roux, 1919,
C. gracilipes De Man, 1892 and
C. meridionalis Roux, 1926.
The records of
C. nilotica gracilipes from Shanghai (Kemp, 1918 [
80]), and Hebei (Liu, 1955 [
81]) China have recently been clarified, with both records being reassigned to
Caridina sheni (Cai, 2020 [
79]).
J. Roux (1919 [
5]) reported
C. nilotica var.
gracilipes in Aru Islands.
3.1.15. Caridina aruensis J. Roux, 1911
Caridina aruensis J. Roux, 1911 [
2]: 82 [type locality: Kepulauan Aru, Indonesia].
Caridina nilotica var.
aruensis J. Roux, 1919 [
5]: 321; 1926 [
82]: 248; Bouvier, 1913 [
63]: 198; 1925 [
50]: 156, Figs. 323-325. Reik, 1953 [
83]: 118, Fig. 7.
Caridina simoni-Johnson, 1963 [
84]: 21 (part); Richard & Clark, 2014 [
67]: 303 (part)
Six males, cl 2.3–3.2 mm, 2 females, cl 2.4–3.5 mm, 2 ovigerous females, cl 3.2–3.5 mm, SMF, syntypes of Caridina aruensis J. Roux, 1911, Ruisseau Panoua Bori, Soungi Manoumbai, Aru, 14 March 1908; 2 ovigerous females, cl 3.5–3.6 mm, ZMA De 102889, syntypes of Caridina aruensis J. Roux, 1911, Matora River, Aru Islands, Indonesia, coll. H. Merton, March 1908; 5 males, cl 2.4–2.8 mm, 2 females, cl 3.0–3.2 mm, 1 ovigerous female, cl 3.2 mm, eggs 0.72 × 0.45 mm, 7 juveniles, SMF 8101, Kobroor, Aru Islands, Indonesia, 30 April 1908; 1 female, cl 3.1 mm, 1 ovigerous female, eggs 0.73 × 4.2 mm, Kobroor, Aru Islands, Indonesia, coll. Merton, 30 April 1908; 10 specimens, SMF, Teiaugan Merapenpen, coll. H. Merton, 6 November 1908; 1 ovigerous female, SMF, Sungei Mannmbai, Pauua Bori, Bach, Aru Islands, Indonesia, coll. H. Merton, April 1908; 60 specimens, SMF, Kobroor, Maremar, Aru Islands, Indonesia, coll. H. Merton, 29 April 1908; 3 males, 6 females, 2 ovigerous females, Trangan, NMB4Va, Aru Islands, Indonesia, coll. H. Merton, 1909; 9 males, 7 females, 1 ovigerous female, NMB4Vb, Kobroor, Aru Islands, Indonesia, coll. H Merton, August 1908.
Rostrum long, sigmoid, reaching distinctly beyond end of antennular peduncle; armed dorsally with 20–27 teeth, including 3 on the carapace posterior to orbital margin and 1–3 subapical teeth, armed ventrally with 6–9 teeth; antennal spine pointed, placed lower than inferior orbital angle; pterygostomian margin broadly rounded. Preanal carina low, no spine.
Sixth abdominal somite 0.65–0.75 times as long as carapace, 2.0 times as long as fifth somite, as long as telson. Telson 3.0 times as long as wide, terminating in a projection, with five pairs of dorsal spinules and one pair of dorsolateral spinules; distal margin with four pairs distal spines, lateral pair of distal spines longer than intermediates. Antennular peduncle 0.82–1.0 as long as carapace, stylocerite 0.9 length of basal segment of antennular peduncle, lateroanterior angle reaching 0.4 length of second segment of antennular peduncle; scaphocerite 4.5 times as long as wide.
Third maxilliped reaching to end of second segment of antennular peduncle, with ultimate segment shorter than penultimate segment. Epipods on first four pereiopods only. Carpus of first pereiopod 2.6 times as long as high, chela 2.1 times as long as broad, fingers slightly longer than palm. Carpus of second pereiopod 8.0 times as long as high, chela 3.0 times as long as broad, fingers 1.3 times as long as palm. Propodus of third pereiopod 3.8–4.3 times as long as dactylus, dactylus 3.3 times as long as wide, with 5–7 spines on flexor margin. Propodus of fifth pereiopods 13 times as long as broad, 3.0–3.2 times as long as dactylus; dactylus terminating in one spine, 4.1 times as long as wide (spinules included), with 35–45 spinules on flexor margin.
Endopod of male first pleopod reaching 0.33 length of exopod, subtriangular, no appendix interna. Appendix masculina of male second pleopod short and slender, appendix interna 0.4 length of appendix masculina.
Uropodal diaeresis with 12 movable spinules.
Eggs 0.70–0.86 × 0.45–0.50 mm in diameter.
Johnson (1963 [
84]) reviewed the ‘
Caridina nilotica complex’ established by Bouvier (1925 [
50]), considered several distinct freshwater shrimp species from the Indo-West Pacific region as junior synonyms of
Caridina simoni Bouvier, 1904, including
C. aruensis. Richard & Clark (2014 [
67]) revised
C. simoni based on examination of type and non-type specimens from Sri Lanka and India. Four syntype specimens of
C. aruensis were also examined and they confirmed the following characters to be shared with
C. simoni: “adult size 20–25 mm; rostrum equal to or slightly longer than the antennal scale, tip pointed, formula (3–4) 20–25/7–9 with 0.25–0.4 of the dorsal margin unarmed distally or interrupted by 1–3 teeth, ventral margin with a short unarmed end distally, posterior margin of the telson rounded with a pair of long lateral spines and two pairs or three intermediate spines of equal length; 6–14 uropod diaeresis spinules; endopod of the male first pleopod with appendix interna and preanal carina unarmed.” They reached the conclusion to consider
C. aruensis a junior synonym of
C. simoni.However, I found that
C. aruensis could be distinguished from
C. simoni, and should remain as a valid species, by comparing a few important morphological characters among the specimens examined by the current study for
C. aruensis, by Bouvier (1925 [
50]), and by Richard & Clark (2014 [
67]), for
C. simoni, viz., the male first pleopod (without appendix interna on its endopod in current examination of syntypes from Aru Islands, vs. with a distinct and prominent appendix interna in Bouvier (1925 [
50]); and all except one with a distinct appendix interna in Richard & Clark (2014 [
67]), number of teeth at the diaeresis (12 vs. 10–11 in Bouvier (1925 [
50]), and 8–14 in Richard & Clark (2014 [
67]), egg size (0.70–0.86 × 0.45–0.50 mm from the current study vs. 0.65–1.0 × 0.45–0.6 mm in Richard & Clark (2014 [
67]), and the shape of the distal margin of the telson (terminating in a median projection in
C. aruensis vs. terminating in a median projection in Bouvier and mostly without a median process in Richard & Clark (2014 [
67]); number of spinules on the flexor margin of the dactylus of the fifth pereiopod (35–45 in
C. aruensis vs. 40–60 in
C. simoni by Bouvier (1925 [
50]), and 35–60 by Richard & Clark (2014 [
67]).
Regarding the form of the rostrum, the large eggs and form of the telson, Caridina aruensis is similar to C. nilotica P. Roux (1833). It can, however, be distinguished from C. nilotica by the presence of distal spines on the telson and the slenderer scaphocerite (4.5 times as long as wide vs. 3.5 times in C. nilotica).
3.1.16. Caridina brevidactyla J. Roux, 1919
Caridina nilotica var.
brevidactyla, Roux, 1919 [
5]: 320 [type locality: 9 localities in Kepulauan Aru, Indonesia].
Caridina nilotica var.
brevidactyla—Roux, 1928 [
85]: 198; 1929 [
86]: 303, Table 1, no. 15.
?
Caridina Modiglianii Nobili, 1900 [
87]: 477 [type locality: “Kifa-juc”, Pulau Enggano, Indonesia].
?
Caridina Modiglianai- Bouvier, 1925 [
50]: 159, Figs. 332–335.
?
Caridina modiglianii-Chace, 1997 [
53]: 16.
Caridina brevidactyla—Cai & Ng, 2001 [
26]: 671, Fig. 5; de Mazancourt et al., 2018 [
13]:1435, Fig. 5.
Caridina brachydactyla- Richard & Clark, 2010 [
70]: 317 (part).
Fifteen males, cl 3.0–4.6 mm, 2 females, cl 4.0–5.7 mm, 2 ovigerous females, cl 6.2–6.3 mm, NMB 4VIb, Sungei Manumbai, Aru Islands, Indonesia, coll. Merton, 15 March 1908; 6 males, cl 3.4–4.8 mm, 2 females, cl 4.1–5.3 mm, SMF 8091, Sungei Manumbai, Aru Islands, Indonesia, coll. Merton, 15 March 1908; 1 male, 25 females (12 ovigerous), SMF 8101, Syntypes, Kobroor, Bei Papakoela, Aru Islands, Indonesia, 30 April 1908; 5 specimens, SMF 7964, syntypes, Aru Islands, Indonesia, coll. Merton, 1909; 5 males, 2 females, 3 ovigerous females, NMB 4VIa, syntypes, Aru Islands, Indonesia, 15 March 1908; 2 males, 2 females, 4 ovigerous females, NMB 4VId, syntypes, Jeltutte, Kobroor, Aru Islands, Indonesia, 1908; 1 male, 1 female, 1 ovigerous female, NMB 4VIc, syntypes, Wokamar Wokam, Aru Islands, Indonesia, 17 April 1908; 12 males, cl 2.6–4.1 mm, 6 females, cl 2.5–4.1 mm, 5 ovigerous females, cl 3.5–4.7 mm, RMNH 700, two creeks near Seroei, Yapen Island, Irian Jaya, Indonesia, 22 February 1955; 8 males, cl 3.0–4.2 mm, 5 females, cl 3.2–4.1 mm, 1 ovigerous female, cl 6.0 mm, RMNH 704, Manainoemi River at Seroei, Yapen Island, Irian Jaya, Indonesia, 21 February 1956; 1 ovigerous female, cl 5.5 mm, RMNH 689, Seroei, Yapen Island, Geelvink Bay, Irian Jaya, Indonesia, coll. D. L. Leiker, 1954; 1 female, cl 4.6 mm, RMNH 615, creek above Marine barracks, Irian Jaya, Indonesia, in a small pool, among water plants, coll. L. D. Brongersma, M. Boeseman and L. B. Holthuis, 31 October 1954; 9 males, cl 3.5–4.5 mm, 14 females, cl 3.4–6.8 mm, 10 ovigerous females, cl 4.7–6.7 mm, RMNH 607, Sago swamp, Irian Jaya, Indonesia, coll. L. D. Brongersma, M. Boeseman & L. B. Holthuis, 10 October 1954; 24 specimens, RMNH 717, Wasi River, west of Manokwari, Irian Jaya, Indonesia, 9 March 1955; 11 specimens ZMA D202884, Rive Waiko, Waigeoe, Irian Jaya, Indonesia, coll. De Beaufort, 20 December 1909; 35 spec. (19 ovig.) RMNH 29111, Fakal Central Misool, A. Z. Beekje, Papua, Indonesia, coll. M. A. Lieftinck, 2 September 1948; 1 female, cl 3.4 mm, Sorong, RMNH 29116, N. W. New Guinea, Bosbeekje, tussen wortelbundels, coll. M. A. Lieftinck, 2 September 1948; 1 ovigerous female, cl 3.5 mm, N. Misool, Kp. Waima doom, Blyza pool, coll. M. A. Lieftinck, 13 September 1948; 1 female, cl 3.6 mm, RMNH 29113, Waitama River, Kafal, Central Misool, New Guinea, coll. M. A. Lieftinck, 1–6 October 1948; 1 female, cl 3.0 mm, RMNH 29115, Sorong, N. W. New Guinea, coll. M. A. Lieftinck, 29 August 1948; 1 male, RMNH 29112, N. Klamono, freshwater, 50 m upstream near a well, 28 February 1949.
Rostrum long, upturned distally, reaching distinctly beyond end of antennular peduncle; armed dorsally with 21–31 teeth, including 2 or 3 on the carapace posterior to orbital margin and 1–3 subapical teeth, armed ventrally with 15–30 teeth; antennal spine pointed, placed lower than inferior orbital angle; pterygostomian margin broadly rounded. Preanal carina with or without a distinct spine.
Sixth abdominal somite 0.76 times as long as carapace, 1.8 times as long as fifth somite, slightly shorter than telson. Telson terminating in a posteromedian projection, with about 7–9 distal spines, lateral pair of distal spines distinctly longer than intermediates. Antennular peduncle slightly longer than carapace, stylocerite 0.9 length of basal segment of antennular peduncle, lateroanterior angle reaching 0.25 length of second segment of antennular peduncle; scaphocerite 4.0 times as long as wide.
Third maxilliped reaching to end of antennular peduncle, ultimate segment distinctly shorter than penultimate segment. Epipods on first four pereiopods. First pereiopod reaching end of basal segment of antennular peduncle, merus 3.0 times as long as wide, slightly shorter than carpus; carpus of first pereiopod 1.7 times as long as high, chela 2.8 times as long as broad, fingers 1.6 times as long as palm. Second pereiopod reaching end of second segment of antennular peduncle, merus 5.2 times as long as wide; carpus of second pereiopod 6.5 times as long as high, chela 2.8 times as long as broad, fingers 1.7 times as long as palm. Third pereiopod reaching end of antennular peduncle, propodus of third pereiopod 15 times as long as wide, 6.0 times as long as dactylus, dactylus 2.8 times as long as wide, with 6 spines on flexor margin. Fifth pereiopod reaching to end of second segment of antennular peduncle, propodus of fifth pereiopods 17 times as long as wide, 5.4 times as long as dactylus, dactylus 3.2 times as long as broad, with 57 spinules on flexor margin.
Endopod of male first pleopod reaching 0.2 length of exopod, subtriangular, appendix interna reaching beyond end of endopod by 0.8 of its length. Appendix masculina of male second pleopod short and slender, reaching 0.4 length of endopod, appendix interna half the length of the appendix masculina.
Uropodal diaeresis with 12 movable spinules.
Eggs 0.40 × 0.22 mm in diameter.
Lowland freshwater.
Indonesia (Aru Islands, Halmahera, Papua), Papua New Guinea, Solomon Islands, Vanuatu, New Caledonia, and Fiji.
With regard to the short dactylus of the last three pereiopods, C. brevidactyla most closely resembles C. brachydactyla De Man, 1908, but can be distinguished by the rostrum having more ventral teeth, proportion of the various joints of pereiopods, and the absence of an enlarged spine at the posterior margin of the propodus of the last three pereiopods. Caridina brevidactyla also differs from another close congener, C. peninsularis Kemp, 1918, by the rostral form. The latter has a rostrum wholly armed with closely placed dorsal teeth.
J. Roux (1919 [
5]) described
C. nilotica var
brevidactyla from Aru. He separated his new subspecies from
C. nilotica brachydactyla by the proportion of the various joints of the pereiopods. However, the specimens have not been compared with
C. grandirostris, to which they are more similar. It can be separated from
C. grandirostris by the longer rostrum (distinctly longer than carapace with one third or slightly more than half untoothed proportion in the dorsal margin vs. subequal to in
C. grandirostris, with about one third untoothed proportion), larger number of ventral rostral teeth (15–30, mode 20–25 vs. 8–21, mode 13–19) and the shorter dactylus of the last three pereiopods (propodus of third pereiopod 6.0 times as long as dactylus and propodus of fifth pereiopod 5.4 times as long as dactylus vs. 5.0–5.5 times and 4.5 times in
C. grandirostris, respectively).
Chace (1997 [
53]: 16) commented that
Caridina modigliani Nobili, 1900, “which is based on a single incomplete female, seems to be identical with
C. longirostris, except for the number of teeth of the dorsal rostral series that are situated on the carapace posterior to the orbital margin”. According to Bouvier (1925 [
50]: 160), “
C. modigliani has five postorbital teeth, whereas I have seen no more than three in any material of
C. longirostris that I have examined.” According to the drawings provided by Bouvier (1925 [
50]) based on the types, it is most probably identical with
C. brevidactyla; both have a long and slender rostrum, with the untoothed rostral proportion wide. The five postorbital rostral teeth of the single type specimen could be an abnormality if Bouvier had correctly counted it. The types are not available to the author, although efforts to re-examine the type of Nobili (1900 [
86]) have been made. Unless the type could be re-examined or fresh material from the type locality becomes available, it is better to treat it as an uncertain species for the time being. If it is shown to be identical to
C. brevidactyla, then the correct name for the species should be
C. modigliani.Richard & Clark (2010 [
70]), based on an examination of type specimens, regarded
C. nilotica var.
brevidactyla a junior synonym of
C. brachydactyla. de Mazancourt et al. (2018 [
13]) recognized
C. brevidactyla Roux, 1919 [
5], as a valid species based on both morphology and molecular data, designed the lectotype, and illustrated and redescribed the species. They found that “the co-types have an equal number of specimens showing a pre-anal carina with a spine and without a spine and are likely a mix of species”. The lectotype designed is a male specimen without a spine on the preanal carina. One of the syntypes that I have examined in the current study is from the same lot of specimens as the lectotype, showing a prominent spine at the preanal carina. The specimen is illustrated here for further investigation.
de Mazancourt et al. (2018 [
13]: Table 1) listed two DNA vouchers (GUC 358, 569) of
Caridina nilotica brevidactyla from Papua New Guinea, specimens originally from Page et al. (2007 [
88]).
3.1.17. Caridina mertoni Roux, 1911
Caridina mertoni Roux, 1911 [
2]: 84. [type locality: Kei Besar Island, Elat, Indonesia].
Caridina mertoni—J. Roux, 1919 [
5]: 328; Bouvier 1925 [
50]: 191, Figs. 398–408; de Mazancourt et al. 2019a [
14]: 166, 169–170; de Mazancourt et al., 2020 [
15]: 27, Figs. 2L, 8, 25G.
Not
Caridina mertoni-Leberer & Cai 2003 [
69]: 354. (=
Caridina variabilis de Mazancourt, Marquet, Rogers & Keith, 2018)
Twenty-nine males, cl 2.5–3.9 mm, 6 females, cl 3.8–4.9 mm, 4 ovigerous females, cl 4.0–5.0 mm, eggs 0.4 × 0.25 mm, RMNH 705, creek near Kampong Ambai, Ambai Island, Papua, Indonesia, 22 February 1955.
One male, cl 4.8 mm, 6 males, 6 females, SMF 8356, Elat and Chinangan, Kei Islands, Indonesia, 3 June 1908, coll. Merton; 3 males, 3 ovigerous females, SMF 8355, Elat and Chinangan, Kei Islands, Indonesia, 3 June 1908, coll. Merton; 1 female, SMF 7956, Kei Islands, 16 March, 1908, coll. Merton.
Rostrum short, sloping down anteriorly, reaching middle of second segment of antennular peduncle; rostral formula: 2-5+19/6. Antennal spine slightly below inferior orbital angle. Pterygostomian margin subrectangular. Telson terminating in a posteromedian projection, with three pairs of dorsal spinules on distal half of telson; with about four pairs of distal spines, lateral pair of distal spines slightly longer or subequal to intermediates. Antennular peduncle 0.9 times as long as carapace, stylocerite reaching 0.7 length of basal segment of antennular peduncle. Epipods on first four pereiopods. Carpus of first pereiopod excavated, 2.1 times as long as high, chela 2.0–2.2 times as long as broad, fingers as long as palm. Carpus of second pereiopod 4.5 times as long as high, chela 2.5 times as long as broad, fingers 1.5 times as long as palm. Propodus of third pereiopod 5.0 times as long as dactylus, dactylus 2.6 times as long as wide, with four spines on flexor margin. Propodus of fifth pereiopods 5.6 times as long as dactylus, dactylus 2.7 times as long as broad, with 30–35 spinules on flexor margin. Endopod of male first pleopod triangular, appendix interna placed near end of inner margin, reaching distinctly beyond distal end of endopod. Appendix masculina of male second pleopod slender, reaching 1/4 length of endopod, appendix interna 1/3 length of appendix masculina. Uropodal diaeresis with 11 movable spinules. Eggs 0.4–0.5 × 0.3 mm in diameter.
Found from the middle to the higher course of rivers, with fresh and well-oxygenated waters, more abundant in areas situated above waterfalls.
Indonesia (Kei Islands, Waigeo, Papua), Solomon Islands and Papua New Guinea.
de Mazancourt et al. (2020 [
15]) assigned
Caridina mertoni to the
C. nilotica species complex based on molecular data (16S) and morphology. The rostrum is more variable in Solomon compared to the type series from Kei Islands; e.g., some of the specimens exhibit the
C. nilotica form of rostrum, having a longer rostrum, with up to ¼ unarmed distally or with one or two subapical teeth.
The specimens reported here represent the first record of Caridina mertoni in New Guinea.
3.1.18. Caridina appendiculata Jalihal & Shenoy, 1998
Caridina gracilirostris De Man, 1892 [
49]:399 part); Holthuis, 1978b [
56]: 35(part).
Caridina appendiculata Jalihal & Shenoy, 1998 [
89]: 128 [type locality: river near Bari, Flores, Indonesia; present designated].
Caridina appendiculata –Klotz et al., 2007 [
59]: 7–9, Figs. 3 (part), 4; de Mazancourt et al., 2018 [
13]: 1433, Figs. 1, 2, 4; de Mazancourt et al., 2020 [
15]: 18, Figs. 2H, 25C; de Mazancourt et al., 2024 [
30]: 22, Figs. 3I, 10A.
Caridina sp. E– Page et al., 2007 [
88]: 648, Table 1; Cook et al., 2011 [
90]: 278, Fig. 3.
One ovigerous female, cl 5.7 mm, RMNH, Blue Cave, Biak Island, collected from the cave, color of the prawn in total white, Papua, Indonesia, coll. L. V. D. Hammen, 19 November 1953.
Lectotype: Male, cl 3.1 mm, Caridina gracilirostris De Man, 1892, syntypes, ZMA De102646, river near Bari, Flores, Indonesia, coll. M. Weber, 1888, designated by Cai & Ng, 2007. Paralectotypes: 1 male, cl 2.9 mm, three females, cl 2.2–4.3 mm, same data as lectotype.
Rostrum end in bifid, reaching far beyond distal end of scaphocerite, 1.5 times as long as carapace, strongly upturned, armed with 12 to 15 dorsal teeth at posterior 2/3, of which, one or two are situated behind the postorbital margin; armed with 13–22 throughout ventral margin; antennal spine long, situated below inferior orbital angle; pterygostomian margin subrectangular.
Sixth abdominal somite 0.76 times as long as carapace, 1.8 times as long as fifth somite, as long as telson. Telson 3.8 times as long as wide, with four pairs of dorsal spinules and one pair of dorsolateral spinules; distal spines stout, lateral pair slightly longer than intermediate pairs; ending in a posteromedian projection. Preanal carina with a spine.
Anterior end of eye reaching 0.8 length of basal segment of antennular peduncle. Antennular peduncle 0.9 times as long as carapace; anterolateral angle of basal segment reaching 0.25 length of the second segment. Stylocerite reaching 0.8 length of basal segment of antennular peduncle. Scaphocerite 4.8 times as long as wide.
Palp of first maxilliped ending in broad triangular form. Third maxilliped reaching to end of second segment of antennular peduncle, with ultimate segment distinctly shorter than penultimate segment.
First pereiopod not reaching beyond end of eye stalk; merus 2.7 times as long as broad; as long as carpus; carpus 2.0 to 2.5 times as long as high; chela 2.4 times as long as broad, fingers slightly longer than palm. Second pereiopod reaching beyond end of basal segment of antennular peduncle, merus shorter than carpus, 5.7 times as long as broad; carpus 1.2 times as long as chela, 5.2 times as long as high; chela 2.8 times as long as broad; fingers 1.3 times as long as palm. Third pereiopod reaching to end of antennular peduncle, propodus 14 times as long as wide, 5.8 times as long as dactylus; dactylus 3.1 times as long as wide (spines included), with 6 spines on flexor margin. Fifth pereiopod reaching to end of basal segment of antennular peduncle, propodus 18 times as long as wide, 4.8 times as long as dactylus; dactylus 3.7 times as long as wide, with 47 spinules on flexor margin.
Endopod of male first pleopod with an elongated appendix interna.
Uropodal diaeresis with 12 movable spinules.
Eggs 0.42 × 0.25 mm.
Lower course of streams or rivers with seawater influence, often with brackish water.
Australia, Indonesia (Flores, Obira, Sumba, Sulawesi, Papua), Solomon Islands (Kolombangara, Isabel), Micronesia (Pohnpei), Palau, Vanuatu (Aneityum, Efate, Epi and Santo), the Philippines (Mindanao), and Madagascar (de Mazancourt et al., 2024 [
30]).
de Mazancourt et al. (2018 [
13]) conducted morphological and genetic studies based on both old and newly collected specimens, recognized four species that are allied to
C. longirostris, namely
C. appendiculata Jalihal & Shenoy, 1998,
C. brevidactyla Roux, 1920,
C. gracilipes De Man, 1892, and
C. meridionalis Roux, 1926.
The specimens reported here represent the first record of Caridina appendiculata in New Guinea.
3.1.19. Caridina intermedia de Mazancourt, Boseto, Marquet & Keith 2020
Caridina sp. Sol.2—Page et al. 2007 [
88]: 649 (GenBank: DQ478545).
Caridina sp. 1 Solomon—de Mazancourt et al., 2019a [
14]: 166, 169–170.
Caridina intermedia de Mazancourt et al., 2020 [
15]: 23, Figs. 2I, 7, 25f. [Type locality: Gu’ma River, Choiseul Island, Solomon Islands].
Caridina nilotica forma
typica-J. Roux, 1917 [
36]: 590.
None.
Brackish water pool or lower part of rivers near the estuary, with tidal influence.
Solomon Islands (Choiseul, Kolombangara, Vella Lavella and Isabel) and Papua New Guinea (New Britain).
The record was based on de Mazancourt et al. (2020 [
15]) who included specimens from several localities of New Britain (Ore River; Garu road, Galuku River, Kokori River and Rangihi swamp) in their original description of
C. intermedia.J. Roux (1917 [
4]) reported
C. nilotica forma
typica from New Guinea based on a single specimen. Based on his brief description on the rostrum, the proportion and various joints of 1–3 pereiopods, as compared with species of the
C. nilotica group that have been found in the island, this specimen is most likely to be
C. intermedia.
3.1.20. Caridina pisuku de Mazancourt, Boseto, Marquet & Keith 2020
C. longirostris—Page et al. 2007 [
88]: 647 (GenBank: DQ478506–DQ478507).
Caridina sp. 3 Solomon—de Mazancourt et al., 2019a [
14]: 166, 169–170.
Caridina pisuku de Mazancourt et al., 2020 [
15]: 30, Figs. 2M, 9. [Type locality: river Pisuku, Choiseul Island, Solomon Islands].
None.
Brackish water pool or lower part of rivers near the estuary, with tidal influence.
Solomon Islands (Choiseul Island), Indonesia (West Papua) and Australia (Queensland).
The record was based on de Mazancourt et al. (2020 [
15]) who included specimen tissue collected from Kayumera, West Papua, in their original description of
C. pisuku. 3.1.21. Caridina gracilirostris De Man, 1892
Caridina gracilirostris De Man, 1892 [
49]: 399 (part), Pl. 25, Figs. 31a-c [type locality: river near Maros, Sulawesi (Celebes), Indonesia].
Caridina gracilirostris-Kemp, 1918 [
80]: 282; Blanco, 1935 [
91]: 32, Figs. 11–17; Riek, 1953 [
82]: 121; Holthuis, 1965 [
92]: 23 (part); 1978b [
56]: 35 (part); Johnson, 1961a [
93]: 124, Figs. 1, 2; 1961b [
94]: 45; Richard & Chandran, 1994 [
76]: 242; Chace, 1997 [
53]:10 (part); Jalihal & Shenoy, 1998 [
89]: 128; Cai & Ng, 2001 [
26]: 674, Fig. 7; Cai & Ng, 2007 [
95]: 1586, Figs. 1–2; de Mazancourt et al. 2019a [
14]: 166, 169–170; de Mazancourt et al., 2020 [
15]: 12, Figs. 2E, 4, 25A; de Mazancourt et al., 2024 [
30]: 25.
Caridina nilotica var.
gracilirostris-J. Roux, 1919 [
5]: 322.
Caridina pseudogracilirostris Thomas et al., 1976 [
96]: 871, Fig. 1 [type locality: Cochin backwater, India].
One male, cl 4.1 mm, 1 female, cl 4.8 mm, MZB, brackish water at river mouth of Ajkwa River, Papua, Indonesia, coll. D. L. Rahayu, 30 May 2000; 3 females, SMF 8354, Wokam, Aru Islands, Indonesia, coll. S. Mertoni, 16 March 1908; 1 female, 1 ovigerous female, NMH 704a, New Guinea, Indonesia, coll. H. Merton, 1919; 1 male, BMNH 704b, Wakamar, Wokam, Aru Islands, Indonesia, coll. H. Merton, 1919.
Sulawesi: Lectotype (designated by Cai & Ng 2007 [
95]): Male, cl 3.5 mm, ZMA DE102645, river near Maros, Sulawesi (Celebes), Indonesia, leg. M. Weber, 1888. Paralectotypes: two ovigerous females, cl 5.6–5.7 mm, data same as lectotype.
Lower parts of streams or rivers with seawater influence, often with brackish water.
Widely distributed in tropical and subtropical Indo-Pacific region, including Indonesia, Singapore, Malaysia, Thailand, Cambodia, Taiwan, Japan, Palau, the Philippines, Australia, Fiji, the Solomon Islands, New Guinea, India, and Madagascar.
J. Roux (1919 [
5]) reported
Caridina gracilirostris from Aru Islands, and Holthuis (1982 [
9]: 609) reported
C. gracilirostris from New Guinea.
Two species were recently described and added in the Caridina gracilirostris species group, namely Caridina leptopoda de Mazancourt, Freitag, K. von Rintelen, Manuel-Santos & T. von Rintelen, 2023, and Caridina wilsoni Klotz, K. von Rintelen & T. von Rintelen, 2024.
3.1.22. Caridina neglecta Cai & Ng, 2007
Caridina neglecta Cai & Ng, 2007 [
95]: 1595, Figs. 4–5 [type locality: Sungai Batang, 13 km on road from Palopo to Wotu, Sulawesi, Indonesia].
Caridina neglecta- de Mazancourt et al., 2020 [
15]: 14, Figs. 2f, 5, 25b.
Nine ovigerous females, cl 5.5–6.6 mm, RMNH 689, Seroei, Yapen Island, Geelvink Bay, Papua, Indonesia, coll. D. L. Leiker, 1954.
Sulawesi: Holotype: male, cl 4.2 mm, MZB, Sungai Batang, 13 Km on road from Palopo to Wotu, Sulawesi, Indonesia, coll. M. Kottela & A. Werner, March 1989. Paratype: one female, cl 4.3 mm, data same as holotype.
Lower course of streams or rivers with seawater influence.
Indonesia (Sulawesi, Papua); the Philippines (Mindanao); Solomon Islands (Choiseul Island, Kolombangara Island, Vella Lavella Island); Vanuatu (Santo Island, Isabel Island)
The current results represent the first record of the species in New Guinea.
3.1.23. Caridina celebensis De Man, 1892
Caridina serratirostris var.
celebensis De Man, 1892 [
49]: 385, pl. 23: Figs. 28f-h [type locality: river at Palopo, Luwu, Sulawesi (Celebes), Indonesia].
Caridina serratirostris koterai Kamita, 1957 [
97]: 75, Figs. A-G [type locality: Shimoko, Iwami Province in southeastern Honshu, Japan].
Caridina serratirostris celebensis-Kamita, 1961 [
98]: 74.
Caridina celebensis-Holthuis, 1978b [
56]: 39, Figs. 14 a–i; Hayashi, 1989 [
99]: 377, Figs. e, g; Yeo et al., 1999 [
33]: 214, Figs. 8, 9; Cai & Shokita, 2006a [
24]: 248; de Mazancourt et al., 2020 [
15]: 71, Figs. 2b, 24; de Mazancourt et al., 2023 [
38]: 390.
Caridina leptocarpa Liang & Zheng, 1988 [
100]: 15, Figs. 1–9 [Minjiang River, Fuzhou, Fujian, southern China]—Liang & Zhou, 1993 [
101]: 231.
Caridina serratirostris-J. Roux, 1919 [
5]: 325; Shy & Yu, 1987 [
102]: 5, pl. 3d; Hung et al., 1993 [
50]: 500, pl. 9e; Chace, 1997 [
51]: 19, Figs. 11a-r; Kubo, 1938 [
103]: 92; Holthuis, 1982 [
9]: 609.
Caridina cf.
serratirostris- Shy & Yu, 1998 [
54]: 60.
Two females, cl 2.8–3.9 mm, RMNH 700, two creeks near Seroei, Yapen Island, Papua, Indonesia, 22 February 1955; 1 male, cl 2.7 mm, RMNH 704, Manainoemi River at Seroei, Yapen Island, Papua, Indonesia, 21 February 1956; 1 female, cl 3.6 mm, 1 ovigerous female, cl 3.6 mm, 1 juvenile, RMNH 717, Wasi River, west of Manokwari, West Papua, Indonesia, 9 March 1955; 1 female, RMNH 6338, Hollandia, N. New Guinea, in stream, coll. W. Stueber, 20 May 1933; 7 specimens (5 ovigerous), SMF 8513, Aru Island, Indonesia, coll. H. Merton, 15 April 1908; 2 ovigerous females, NMB 694a, Aru Islands, Indonesia, coll. H. Merton, 1919.
Lectotype (designed by Cai and Shokita, 2006a [
24]): One male, cl 2.0 mm, ZMA DE 102630, syntypes of
Caridina serratirostris var.
celebensis De Man, 1892, river near Palopo, Luwo, Celebes (=Sulawesi), Indonesia, coll. M. Weber, 1888. Paralectotypes: 3 females, cl 1.9–3.6 mm, 50 ovigerous females, cl 2.7–4.2 mm, data same as lectotype.
Lower course of streams or rivers with seawater influence.
Indonesia, Peninsular Malaysia, the Philippines, Mainland China, Taiwan, Japan, and Papua New Guinea.
J. Roux (1919 [
5]) reported
Caridina serratirostris from Aru Islands and Holthuis (19 82 [
8]) reported it from New Guinea. Their records most probably could be referred to
C. celebensis. de Mazancourt et al. (2020 [
15]) recently redescribed this species in detail and reported it from Papua New Guinea (New Britain). They found that the number of dorsal rostral teeth (17–22 vs. 22–26 in
C. serratirostris) and the form of carpus of the second pereiopods (11.9–12.0 times as long as high vs. 8.2–10.9 in
C. serratirostris) are two morphological characters that could better assist the separation of the two species. de Mazancourt et al. (2024 [
30]) recently described
Caridina rintelenorum from Babeldaob Island, Palau, and Isabel Island, Solomon Islands, as a new member of the
C. serratirostris species group, which looks superficially like
C. celebensis. According to de Mazancourt et al. (2024 [
30]), morphologically, it can be separated from
C. celebensis “by its shorter rostrum, being 0.5–0.6 length of cl (vs. 0.6–0.9), and by its P5 propodus 4.1 times as long as the dactylus (vs. 3.4–3.9)”.
3.1.24. Caridina troglodytes Holthuis, 1978
Caridina troglodytes Holthuis, 1978a [
8]: 214, Figs. 3–4 [type locality: subterranean stagnant pool in Cave near Konogusgus, New Ireland].
None.
Rostrum short, high, reaching to or slightly beyond end of second segment of antennular peduncle; 9-10+14-17/2-10. Antennal spine placed below inferior orbital angle. Pterygostomian margin broadly rounded. Scaphocerite 2.7 times as long as wide. Telson terminating in a small posteromedian projection, with four pairs of dorsal spinules on distal half of telson; with about seven pairs of distal spines, lateral pair of distal spines subequal to intermediates. Eye reduced, with cornea visible as a small black spot at the top. Stylocerite reaching beyond end of basal segment of antennular peduncle. Epipods on first four pereiopods. Carpus of first pereiopod excavated, 4.0 times as long as high, chela 2.0 times as long as broad, fingers 1.5 times as long as palm. Carpus of second pereiopod 6.5 times as long as high, chela 2.1 times as long as broad, fingers 1.8 times as long as palm. Propodus of third pereiopod 4.0 times as long as dactylus, dactylus with two or three spines on flexor margin. Propodus of fifth pereiopods 5.0 times as long as dactylus, dactylus with six small spinules on flexor margin. Endopod of male first pleopod elongated triangular, with no appendix interna. Appendix masculina of male second pleopod stout, appendix interna reaching near end of appendix masculina. Uropodal diaeresis with 10–12 movable spinules. Eggs 0.5 × 0.3 mm in diameter.
Found in subterranean stagnant pools, 0.3 to 0.6 m deep clear water.
Only known from the type locality, New Ireland Island, Papua New Guinea.
Holthuis (1978a [
8]) described
Caridina troglodytes, based on four incomplete specimens from Danmin Cave near Konogusgus, New Ireland. He noted
C. troglodytes shows a remarkable resemblance to
C. rubella which “also has the
C. serratirostris type of rostrum and the long stylocerite. However, it differs from
C. troglodytes in the greater number (11 to 23) of ventral rostral teeth, in the armament of the posterior margin of the telson, which has short spines between the longer outer spines, in the slenderer chelipeds, and in the greater number of spines on the dactylus of the fifth pereiopods”. Apart from abovementioned, it can also be distinguished by its slender scaphocerite (3.4 times as long as broad vs. 2.7 times in
C. troglodytes); a stouter second pereiopod (with chela 3.0 times as long as broad vs. 4.0 times in
C. troglodytes; fingers 1.2 times as long as palm vs. 3–4 times in
C. troglodytes).
3.1.25. Caridina brevicarpalis De Man, 1892
Caridina brevicarpalis De Man, 1892 [
49]: 397, pl. 24: Figs. 30–30d [type locality: near Palopo, Sulawesi (Celebes), Indonesia].
Caridina brevicarpalis -Bouvier, 1925 [
50]: 178, Figs. 372–374; J. Roux, 1928 [
85]: 200; Blanco, 1935 [
91]: 34, pl. 2: Fig. 25; Edmondson, 1935 [
7]: 7, Figs. 2a–f; Holthuis, 1978b [
56]: 38; Hung et al., 1993 [
52]: 492, Figs. 7a–f; Cai & Shokita, 2006a [
24]: 248; Cai et al., 2009 [
29]: 67; Page et al., 2007a [
88]: 649, 653, Fig. 2 (part, Borneo specimen); de Mazancourt et al., 2023 [
38]: 386. 226, Fig. 4 (part, Indonesian specimen).
Caridina brevicarpalis brevicarpalis -Chace, 1997 [
53]: 8.
One ovigerous female, cl 7.3 mm, 9 ovigerous females, cl 5.5–6.6 mm, RMNH 689, Seroei, Yapen Island, Geelvink Bay, Papua, Indonesia, coll. D. L. Leiker, 1954; 3 males, cl 2.8–4.3 mm, 3 females, cl 3.2–4.0 mm, 10 ovigerous females, cl 4.7–6.8 mm, RMNH 700, two creeks near Seroei, Yapen Island, Papua, Indonesia, 22 February 1955; 15 males, cl 3.5–4.8 mm, 6 females, cl 3.7–4.6 mm, 4 ovigerous females, cl 5.5–6.1 mm, RMNH 704, Manainoemi River at Seroei, Yapen Island, Papua, Indonesia, 21 February 1956; 1 ovigerous female, cl 4.5 mm, RMNH 717, Wasi River, west of Manokwari, West Papua, Indonesia, 9 March 1955.
Lower course of rivers, sometimes in brackish conditions.
Indonesia (Sulawesi, Borneo, Ambon, Waigeo, and Papua) and the Philippines (Palawan, Mindanao, and Mindoro).
de Mazancourt et al. (2020 [
15]) described a new species
Caridina barakoma from Solomon Islands, which looks like the type specimens of
C. brevicarpalis De Man 1892, with their very long rostrum, passing the end of the scaphocerite, without apical tooth and its first pereiopod carpus deeply excavated.
The material examined here represents the first record of the species for New Guinea.
3.1.26. Caridina endehensis De Man, 1892
Caridina brevicarpalis var.
endehensis De Man, 1892 [
49]: 399, pl.24: Fig. 30e [type locality: Nuawari, near Ende, Flores, Indonesia].
Caridina endehensis- Wowor et al., 2004 [
77]: 341, Fig. 5m. Cai & Shokita, 2006a [
24]: 248; Cai et al., 2009 [
29]: 67; de Mazancourt et al., 2023 [
38]: 386.
Caridina brevicarpalis endehensis-Bouvier, 1925 [
50]: 34, 180, pl. 2: Fig. 25; Roux, 1928 [
85]: 218; Blanco, 1935 [
91]: 34, pl. 2-Fig. 25; Chace, 1997 [
53]: 8.
One female, cl 4.6 mm, 5 ovigerous females, cl 5.4–5.8 mm, RMNH 700, two creeks near Seroei, Yapen Island, Papua, Indonesia, 22 February 1955; 8 males, cl 3.0–4.2 mm, 15 females, cl 2.6–4.4 mm, 5 ovigerous females, cl 4.7–6.0 mm, RMNH 717, Wasi River, west of Manokwari, West Papua, Indonesia, 9 March 1955.
Lower course of rivers, sometimes in brackish conditions.
Indonesia (Flores, Sulawesi, Sumba, and Papua) and the Philippines (Luzon, Mindoro, Mindanao).
The specimens examined in this study represent the first record of the species for New Guinea.
3.1.27. Caridina aff. opaensis J Roux, 1904
Caridina opaensis-J. Roux, 1919 [
5]: 327; Holthuis, 1982 [
9]: 609.
None.
Caridina opaensis J Roux, 1904: two females, 2.9–3.3 mm NMB 9a, Opa, Sulawesi, Indonesia, coll. Sarasin, 1904.
J Roux (1919 [
5]) reported
Caridina opaensis from Sungei Waskai, Aru Islands.
Caridina opaensis Roux, 1904, is a riverine species, originally described from Southeast Sulawesi. According to von Rintelen & Cai (2009 [
104]), the species was “reported as the only non-endemic shrimp from the Malili lakes (Woltereck, 1937a [
105], b [
106]). Then, for almost 70 years the state-of-the-art remained unchanged. Brooks (1950 [
107]) and Chace (1997 [
53]) mentioned the species from the lakes, but basically referred to Schenkel (1902 [
57]) and Woltereck (1937a [
105], b [
106])”. Klozt & von Rintelen (2013 [
108]) listed
Caridna opaensis as a species endemic to South-Eastern Sulawesi. The author was unable to locate and reexamine the specimens reported by J. Roux (1919 [
5]); thus, it is tentatively treated as an unknown species morphologically close to
C. opaensis.
3.1.28. Caridina demani Roux, 1911
Caridina demani J. Roux, 1911 [
2]: 94 [type locality: Tawarin River, north coast of West New Guinea, Indonesia].
Caridina demani-De Man, 1915 [
3]: 392; J. Roux, 1927 [
6]: 320; Chace, 1997 [
53]: 9; Karge et al., 2010 [
10]: 134, Figs. 2a–d.
Caridina De Mani-Bouvier, 1925 [
50]: 172, Figs. 361, 362.
One ovigerous female, cl 3.1 mm, eggs 0.7 × 0.4 mm, 3 females, 2.2–2.6 mm, ZMA De 102890, syntypes of Caridina demani J. Roux, 1911, Tawarin River (Papua, Indonesia), New Guinea, coll. New Guinea Exp., 20 June 1903; 1 ovigerous female, cl 4.1 mm, syntype, MNHN 687, Tawarin, New Guinea (Papua, Indonesia), Max Weber, donated by Museum Amsterdam, 1913; 1 male, cl 3.3 mm, syntype, MNHN 688, Tawarin, New Guinea (Papua, Indonesia), Max Weber, donated by Museum Amsterdam, 1913. 1 male, cl 2.7 mm, 2 females, cl 3.3–3.4 mm, 2 ovigerous females, cl 3.4 mm, RMNH, Tawarin, New Guinea (Papua Indonesia), donated by Amsterdam Museum, no date; 4 females, cl 2.8–3.4 mm, RMNH, Berap River at Kg. Berap, northwest of Genyem, 12°46.5′ 140°12′ E, Papua, Indonesia, no date; 2 females, cl 2.9–3.6 mm, 1 ovigerous female, cl 3.7 mm, RMNH 642, creek Ultmondend in northeast of Sentani Lake, Papua, Indonesia, coll. L. D. Brongersma & L. B. Holthuis; 9 males, cl 2.8–3.2 mm, 15 females, cl 2.7–4.3 mm, 1 ovigerous female, cl 4.3 mm, RMNH 640, Klein Beekje Te Ifar near Landmacni Kamp, Papua, Indonesia, coll. L. D. Brongersma M. Boeseman & L. B. Holthuis, 30 November 1954; 1 female, RMNH 2952, Loatbron, New Guinea, New Guinea Expedition, June to July 1910.
Rostrum straight, reaching near to end of antennular peduncle, rostral formula: 2-3+8-17/0-5. Antennal spine fused with inferior orbital angle; pterygostomian margin rounded.
Sixth abdominal somite 0.6 times as long as carapace, 1.7 times as long as fifth somite, as long as telson. Telson 3.0 times as long as wide, terminating in a projection, with three pairs of dorsal spinules and one pair of dorsolateral spinules; distal end with 3–4 pairs of spines, lateral pair longer than intermediate pairs. Preanal carina low, without spine.
Eyes well developed, anterior end reaching 0.7 length of basal segment of antennular peduncle. Antennular peduncle 0.68 times as long as carapace; anterolateral angle of basal segment reaching 0.4 length of the second segment. Stylocerite reaching 0.8 length of basal segment of antennular peduncle. Scaphocerite 3.7 times as long as wide.
Palp of first maxilliped ending in broad triangular form. Third maxilliped reaching to end of antennular peduncle, with ultimate segment distinctly shorter than penultimate segment.
Epipods well developed on first four pereiopods. First pereiopod reaching to distal end of basal segment of antennular peduncle; merus 2.3 times as long as broad, as long as carpus; carpus excavated anteriorly, shorter than chela, 1.8 times as long as high; chela 2.2 times as long as broad; fingers slightly longer than palm. Second pereiopod reaching to beyond end of second segment of antennular peduncle; merus slightly shorter carpus, 4.8 times as long as broad; carpus slightly longer than chela, 4.5 times as long as high; chela 2.6 times as long as broad; fingers 1.5 times as long as palm. Third pereiopod reaching to end of scaphocerite, propodus 10 times as long as broad, 4.4 times as long as dactylus; dactylus 2.9 times as long as wide (spines included), terminating in one claw, with four accessory spines on flexor margin. Fifth pereiopod reaching beyond end of basal segment of antennular peduncle, propodus 13 times as long as broad, 3.9 times as long as dactylus, dactylus 3.5 times as long as wide (spinules included), terminating in one elongated claw, with 40 spinules on flexor margin.
Endopod of male first pleopod subtriangular, 2.1 times as long as wide, reaching 0.33 length of exopod, appendix interna reaching beyond distal end of endopod by half of its length. Appendix masculina of male second pleopod stout, reaching half length of endopod.
Uropod diaeresis with 12–15 movable spinules.
Eggs 0.70–0.82 × 0.40–0.52 mm in diameter.
River and Creeks.
Caridina demani is only known from some rivers in the northern part of New Guinea.
With regard to the rostrum and form of the sexual appendages, Caridina demani is similar to Caridina opaensis J. Roux, 1904, from Sulawesi. It can be separated from C. opaensis by the well-developed epipods (on first four pereiopods vs. only first two in C. opaensis); the stouter scaphocerite (3.7 times as long as wide vs. 4.6 times in C. opaensis); the stouter carpus of the first pereiopod (1.8 times as long as high vs. 2.3–3.2 times in C. opaensis) and the stouter carpus of the second pereiopod (4.5 times as long as high vs. 6.1–6.5 times in C. opaensis).
3.1.29. Caridina fecunda Roux, 1911
Caridina fecunda J. Roux, 1911 [
2]: 95 [type locality: Danau Jamur, Papua, Indonesia].
Caridina fecunda- Bouvier, 1925 [
50]:176, Figs. 368–371; Chace, 1997 [
53]: 10.
Material: 2 ovigerous females, cl 2.5–2.6 mm, eggs 0.8 × 0.5 mm, 1 female, cl 2.6 mm, NMB 659a, Jamovr Lake (Danau Jamur), Papua, Indonesia, August 1903; 4 females, cl 2.3–3.0 mm, 3 ovigerous female, cl 2.5–3.5 mm, ZMA De102891, Jamovr Lake (Danau Jamur), Papua, Indonesia, 10 August 1903; 1 female, cl 3.7 mm, RMNH, Rawah Wan, north of Tanahmerah, Papua, Indonesia, coll. L. D. Browsersma, 13 April 1955; 1 male, cl 3.0 mm, 1 female, cl 3.2 mm, RMNH, Papua, Indonesia, 1910–1911 Exp. 26 June 1910; 1 male, cl 2.8 mm, MNHN Na 713, coll. Max Weber, 1913; 2 males, cl 2.0–2.4 mm, 5 females, cl 1.9–2.9 mm, 5 ovigerous females, cl 2.7–2.9 mm, RMNH 742, freshwater at Plasje, near Kelapa-Lima, Merauke, Southern Papua, Indonesia, coll. L. B. Holthuis, 8 April 1955.
Rostrum straight, reaching to or slightly beyond end of antennular peduncle, rostral formula: 3+15-20/5-7. Antennal spine fused with inferior orbital angle; pterygostomian margin rounded.
Sixth abdominal somite 0.6–0.7 times as long as carapace, 1.6 times as long as fifth somite, as long as or slightly shorter than telson. Telson 3.6 times as long as wide, not terminating in a projection, with three pairs of dorsal spinules and one pair of dorsolateral spinules; distal end with 3–4 pairs of spines, lateral pair longer than intermediate pairs. Preanal carina low, without spine.
Anterior end of eye reaching 0.8 length of basal segment of antennular peduncle. Antennular peduncle 0.82–0.86 times as long as carapace; anterolateral angle of basal segment reaching 0.4 length of the second segment. Stylocerite reaching 0.8 length of basal segment of antennular peduncle. Scaphocerite 3.7 times as long as wide.
Palp of first maxilliped ending in a triangular projection. Third maxilliped reaching to end of antennular peduncle, with ultimate segment distinctly shorter than penultimate segment.
Epipods well developed on first three pereopods, absent from fourth and fifth. First pereiopod reaching to distal end of basal segment of antennular peduncle; merus 2.5 times as long as broad, shorter than carpus; carpus excavated anteriorly, shorter than chela, 2.9 times as long as high; chela 2.3 times as long as broad; fingers 1.4 times as long as palm. Second pereiopod reaching to end of second segment of antennular peduncle; merus slightly shorter carpus, 3.5 times as long as broad; carpus 1.2 times as long as chela, 5.6 times as long as high; chela 3.0 times as long as broad; fingers 1.4 times as long as palm. Third pereiopod reaching to end of scaphocerite, propodus 13 times as long as broad, 3.7 times as long as dactylus; dactylus 4.6 times as long as wide (spines included), terminating in one claw, with 7–8 accessory spines on flexor margin. Fifth pereiopod reaching to end of second segment of antennular peduncle, propodus 13 times as long as broad, 2.7 times as long as dactylus, dactylus 3.8 times as long as wide (spinules included), terminating in one elongated claw, with 35–40 spinules on flexor margin.
Endopod of male first pleopod subtriangular, 2.5 times as long as wide, reaching 0.33 length of exopod, appendix interna reaching beyond distal end of endopod by half of its length. Appendix masculina of male second pleopod stout, reaching 0.6 length of endopod.
Uropodal diaeresis with 9–11 movable spinules.
Eggs 0.80 × 0.50 mm in diameter.
Lakes.
Several sites in Papua, Indonesia.
Caridina fecunda is most similar to C. demani, but can be differentiated from C. demani by the longer antennular peduncle (0.86 times as long as carapace vs. 0.68 times in C. demani); the slenderer carpus on the first pereiopod (2.9 times as long as high vs. 1.8 times) and second pereiopod (5.6 times as long as high vs. 4.5), and the longer dactylus on the fifth pereiopod (propodus 2.7 times as long as dactylus vs. 3.9 times in C. demani).
3.1.30. Caridina cognata De Man, 1915
Caridina cognata De Man, 1915 [
3]: 397, pl.28: Figs. 3–3g, 4–4b [type locality: several localities in northern New Guinea].
Caridina cognata-Chace, 1997 [
53]: 8; Karge et al., 2010 [
10]: 141, Figs. 2e-h.
Material: 1 female, cl 2.9 mm, RMNH D2947, Tjahai River, Netherlands New Guinea (=Papua, Indonesia), 2°50′ S 141°05′ E, North New Guinea Expedition 1910–1911, 12 June 1910; 2 specimens (dried), cl 2.7–4.0 mm, RMNH D2945, River at Niag, North New Guinea (Papua, Indonesia), 14–15 June 1910; 6 specimens, RMNH D2950, River Eussen Tjahai, N. NE Guinea (Papua Indonesia), 13 June 1910; 17 males, cl 2.0–2.4 mm, 16 females, cl 2.0–3.6 mm, 9 ovigerous females, cl 3.1–3.8 mm, eggs 0.8 × 0.5 mm, RMNH D619, Stream Ibaroe at Kampong Nangoepkoe near Kampong Benjon, district of Genyem, Papua, Indonesia, 3 November 1954; 1 ovigerous female, cl 3.4 mm, Ajamaroe, West Papua, Indonesia, coll. L. D. Brongersma & W. J. Roosdrop, June 1952; 1 ovigerous female, cl 2.9 mm, from bank of river, freshwater, North Klamono, 1°08′ S 131°30′ E, West Papua, coll. M. A. Lioffinek, no date; 5 males, cl 2.2–2.7 mm, 9 females, cl 2.0–3.2 mm, 1 ovigerous female, cl 3.0 mm, eggs 0.82 × 0.5 mm, SFM 8101, Kobroor, Bei Papakoela, Aru Islands, Indonesia, 30 April 1908.
Rostrum straight and slightly upturned anterior, reaching to or slightly beyond end of scaphocerite, rostral formula: 2-4+11-27/0-11. Antennal spine fused with inferior orbital angle; pterygostomian margin rounded.
Sixth abdominal somite 0.63 times as long as carapace, 2.0 times as long as fifth somite, as long as telson. Telson 3.0 times as long as wide, terminating in a projection, with three pairs of dorsal spinules and one pair of dorsolateral spinules; distal end with three pairs of spines, lateral pair longer than intermediate pairs. Preanal carina low, without spine.
Anterior end of eye reaching 0.7 length of basal segment of antennular peduncle. Antennular peduncle 0.80 times as long as carapace; anterolateral angle of basal segment reaching 0.4 length of the second segment. Stylocerite reaching 0.8 length of basal segment of antennular peduncle. Scaphocerite 3.6 times as long as wide.
Palp of first maxilliped ending in a finger-like projection. Third maxilliped reaching to end of antennular peduncle, with ultimate segment distinctly shorter than penultimate segment.
Epipods well developed on first four pereiopods. First pereiopod reaching to distal end of basal segment of antennular peduncle; merus 2.2 times as long as broad, shorter than carpus; carpus excavated anteriorly, shorter than chela, 2.0 times as long as high; chela 2.0 times as long as broad; fingers 1.4 times as long as palm. Second pereiopod reaching to end of second segment of antennular peduncle; merus slightly shorter carpus, 3.5 times as long as broad; carpus 1.2 times as long as chela, 5.6 times as long as high; chela 3.0 times as long as broad; fingers 1.4 times as long as palm. Third pereiopod reaching slightly beyond end of antennular peduncle, propodus 11 times as long as broad, 4.1 times as long as dactylus; dactylus 2.9 times as long as wide (spines included), terminating in one claw, with four accessory spines on flexor margin. Fifth pereiopod reaching to end of antennular peduncle, propodus 14 times as long as broad, 3.2 times as long as dactylus, dactylus 3.7 times as long as wide (spinules included), terminating in one elongated claw, with 44 spinules on flexor margin.
Endopod of male first pleopod subtriangular, 1.9 times as long as wide, reaching 0.25 length of exopod, without appendix interna. Appendix masculina of male second pleopod stout, reaching 0.6 length of endopod.
Uropodal diaeresis with 19 movable spinules.
Eggs 0.82–1.00 × 0.40–0.50 mm in diameter.
Lakes, rivers.
Northern part of New Guinea and Aru Islands, Indonesia.
Caridina cognata is morphologically similar to Caridina fecunda in the form of the rostrum. However, it can be separated by the stouter carpus of the first two pereiopods and a telson which terminates in a projection. It differs from C. demani by the longer and slightly upturned rostrum (vs. shorter and straight), relatively longer antennular peduncle (0.80 times as long as carapace vs. 0.68 times in C. demani); and the longer sixth abdominal somite (2.0 times as long as fifth somite vs. 1.7 times in C. demani).
3.1.31. Caridina rouxi De Man, 1915
Caridina rouxi De Man, 1915 [
3]: 387, pl. 27: Figs. 1–11 [type locality: Bouganville Mountains on the north coast of New Guinea, Papua New Guinea].
Caridina rouxi- Chace, 1997 [
53]: 19.
One ovigerous female, cl 4.3 mm, eggs 1.05 × 0.52 mm, 1 female, cl 5.2 mm, NMB 546a, syntypes, Bougainville, Gbe, 500 m, North New Guinea Exp., 1 June 1910; 1 male, cl 4.0 mm, 3 ovigerous females, cl 4.6–5.5 mm, RMNH D 2948, syntypes, Bougainville, Papua New Guinea, North New Guinea Epx., 1 June 1910; 1 male, 1 female, ZMA De 102656, syntypes, Bougainville Mountain, North New Guinea Exp., 1 June 1910.
Rostrum straight, reaching slightly beyond end of basal segment of antennular peduncle, or near end of second segment, rostral formula: 2-4+11-15/3-8. Antennal spine fused with inferior orbital angle; pterygostomian margin rounded.
Sixth abdominal somite 0.55 times as long as carapace, 1.5 times as long as fifth somite, as long as telson. Telson 2.8 times as long as wide, not terminating in a projection, with four pairs of dorsal spinules and one pair of dorsolateral spinules; distal end with three pairs of spines, lateral pair longer than intermediate pairs. Preanal carina low, without spine.
Anterior end of eye reaching 0.8 length of basal segment of antennular peduncle. Antennular peduncle 0.62 times as long as carapace; anterolateral angle of basal segment reaching 0.4 length of the second segment. Stylocerite reaching 0.8 length of basal segment of antennular peduncle. Scaphocerite 3.5 times as long as wide.
Palp of first maxilliped ending in a triangular projection. Second maxilliped with podobranch vestigial. Third maxilliped reaching to end of antennular peduncle, with ultimate segment shorter than penultimate segment.
Epipods well developed on first four pereiopods. First pereiopod reaching to distal end of basal segment of antennular peduncle; merus 2.5 times as long as broad, as long as carpus; carpus excavated anteriorly, shorter than chela, 1.6 times as long as high; chela 2.0 times as long as broad; fingers 1.3 times as long as palm. Second pereiopod reaching to end of antennular peduncle; merus slightly shorter carpus, 4.3 times as long as broad; carpus slightly longer than chela, 3.7 times as long as high; chela 2.3 times as long as broad; fingers 1.2 times as long as palm. Third pereiopod reaching to end of scaphocerite, propodus 11 times as long as broad, 4.5 times as long as dactylus; dactylus 2.9 times as long as wide (spines included), terminating in one claw, with six accessory spines on flexor margin. Fifth pereiopod reaching to end of second segment of antennular peduncle, propodus 14 times as long as broad, 3.6 times as long as dactylus, dactylus 3.4 times as long as wide (spinules included), terminating in one elongated claw, with 55 spinules on flexor margin.
Endopod of male first pleopod subtriangular, reaching 0.4 length exopod, appendix interna reaching beyond distal end of endopod by half of its length. Appendix masculina of male second pleopod stout, reaching 0.6 length of endopod.
Uropodal diaeresis with 19 movable spinules.
Eggs 0.95–1.15 × 0.60–65 mm in diameter.
Mountain streams.
In the Island of New Guinea, Caridina rouxi can only be confused occasionally with C. fecunda. It can easily be separated by its shorter rostrum and the larger eggs (0.95–1.15 × 0.60–65 mm vs. 0.80 × 0.50 mm).
3.1.32. Caridina elisabethae Karge, von Rintelen & Klotz, 2010
Caridina elisabethae Karge et al., 2010 [
10]: 142, Figs. 3–4 [type locality: Papua New Guinea, Morobe, Herzog Mts., Bundun, 700–800 m, 06°51.598′ S, 146°37.07′ E].
None.
Mountain stream at elevation of 700–800 m.
Known only from Papua New Guinea.
Caridina elisabethae shares some characters with
C. demani, but can be distinguished from the latter species by the presence of a small tooth near the distal end of the rostrum and the slenderer first and second chelipeds (Karge et al., 2010 [
10]).
3.1.33. Caridina buergersi Karge, von Rintelen & Klotz, 2010
Caridina buergersi Karge et al., 2010 [
10]: 146, Figs. 5–6 [type locality: Papua New Guinea, former “Deutsch-Neuguinea”, near Mäanderberg].
Five females, cl 2.2–4.1 mm, 1 ovigerous female, cl 4.3 mm, RMNH D 641, Marinierspoel in Beekjeten, north of Westelyijkdeel of Lake Sentani, Papua, Indonesia, coll. L. D. Brongersma, M. Boeseman & L. B. Holthuis, 30 November 1954.
Unknown.
Known only from north of the New Guinea Island at Sepik River (Papua New Guinea) and Lake Sentani (Papua, Indonesia).
Caridina buergersi resembles
C. cognata, but differs by having a distinct tooth on the pterygostomial margin of the carapace, by the antennal spine being situated below the inferior orbitalk angle, and by its slenderer carpi of the first and second pereiopod. The tooth present on the pterygostomial margin in most specimens distinguishes
C. buergersi also from all other
Caridina species known from Papua New Guinea and the surrounding islands (Karge et al., 2010 [
10]).
3.1.34. Caridina yapenensis, New Species
Holotype: Female, cl 5.8 mm, RMNH 700, creek near Seroei, Yapen Island, Papua, Indonesia, 22 February 1955.
Rostrum reaching far beyond distal end of scaphocerite, as long as carapace, strongly upturned, unarmed dorsally, armed ventrally with four large teeth; antennal spine short, situated below inferior orbital angle; pterygostomian margin subrectangular.
Sixth abdominal somite 0.62 times as long as carapace, 1.6 times as long as fifth somite, as long as telson. Telson very slender, ending in a distinct triangular, 3.9 times as long as wide, with four pairs of dorsal spinules and one pair of dorsolateral spinules: distal spines very stout, lateral pair distinctly longer than intermediate spines. Preanal carina with a distinct spine.
Anterior end of eye reaching 0.7 length of basal segment of antennular peduncle. Antennular peduncle 0.76 times as long as carapace; anterolateral angle of basal segment reaching 0.20 length of the second segment. Stylocerite long, reaching to slightly beyond middle of second segment of antennular peduncle. Scaphocerite 3.4 times as long as wide.
Palp of first maxilliped ending in a finger-like projection. Third maxilliped reaching to middle of second segment of antennular peduncle, with ultimate segment distinctly shorter than penultimate segment.
First pereiopod reaching beyond end of eye stalk; merus longer than carpus, 2.9 times as long as wide, carpus 1.4 times as long as high; chela twice as long as broad, fingers slightly longer than palm. Second pereiopod reaching to end of second segment of antennular peduncle, merus as long as carpus, 5.7 times as long as broad; carpus 1.2 times as long as chela, 4.4 times as long as high; chela 2.6 times as long as broad; fingers 1.6 times as long as palm. Third pereiopod reaching to end of scaphocerite, with propodus 12 times as long as wide, 5.3 times as long as dactylus; dactylus 3.0 times as long as wide (spines included), with five spines on flexor margin. Fifth pereiopod reaching to middle of second segment of antennular peduncle, with propodus 16 times as long as wide, 5.5 times as long as dactylus; dactylus 2.7 times as long as wide, with 39 spinules on flexor margin.
Uropodal diaeresis with 17 movable spinules.
Lower course of stream.
The species is named after the type locality, Yen Island, Irian Jaya (now Papua), Indonesia, used as a noun in apposition.
Known only from the type locality, Yapen Island, Papua, Indonesia.
Among all the congeners, Caridina yapenensis, new species, is morphologically most similar to C. siamensis in its general appearance, with an unarmed dorsal margin of the rostrum, and stout pereiopods. It could, however, be easily distinguished from C. siamensis by the rostrum (distinctly up-turned, longer than the carapace, reaching distinctly beyond end of scaphocerite, and armed ventrally with four large teeth vs. straight, distinctly shorter than the carapace, reaching near to scaphocerite, and armed ventrally with 1–7 inconspicuous teeth in C. siamensis), antennal spine situated below the inferior orbital angle (vs. completely fused with inferior orbital angle in C. siamensis), the telson, which terminates in a sharp triangular point (vs. end of distal telson rounded in C. siamensis), and the preanal carina, which is armed with a distinct hook-like spine (vs. no spine in C. siamensis). With its distinct long, upturned, dorsally unarmed rostrum, the pointed triangular form of the distal margin of the telson, and the prominent hock-like spine on the preanal carina, C. yapenensis is very easily distinguished from its other congeners. The new species could not be assigned to any known species group.
3.1.35. Parisia holthuisi Cai, 2010
Parisia holthuisi Cai, 2010a [
12]: 173; Figs. 1–2 [type locality: Tigibi, freshwater at 1600 m altitude, Tari, Papua New Guinea)].
Holotype: Male, cl 4.5 mm, RMNH D 53184, freshwater at elevation of 1600m, Tigibi, Tari, Papua New Guinea, coll. W. Vink, 1 September 1966.
Paratype: one female, cl 2.5 mm, data same as holotype.
Freshwater.
Holthuis (1956 [
109]) established the genus
Parisia based on specimens from Madagascar. The genus currently includes nine species (subspecies) from Madagascar, Australia, the Philippines, and Sulawesi, Indonesia. All are subterranean species except for
P. holthuisi.Von Rintelen et al. (2012 [
46]) commented that “…non-monophyly can be found in the genus
Parisia. As already shown by Page et al. (2008 [
110]), two species of that genus from the same cave in northern Australia (Cutta Cutta Caves) are not closely related despite their morphological similarity. The authors interpret this result as independent cave colonization in Australia along with morphological convergence. Unfortunately, we could not sequence species of
Parisia from Madagascar, Sulawesi, Papua New Guinea (Cai & Ng, 2009; Cai, 2010b) and the Philippines. Nevertheless, the genus
Parisia seems to be in an urgent need of taxonomic revision”.
Parisia holthuisi and P. macrophthalma are the two species with well-developed eyes. P. holthuisi is different from P. macrophthalma in its much shorter and unarmed rostrum.