A Review on Cytotoxic Antibiotics: Occurrence in Water Matrices, Degradation by Advanced Oxidation Processes, and By-Product Formation
Abstract
1. Introduction
| Review | Focus and Novelty | This Review |
|---|---|---|
| Occurrence and fate of pharmaceutical pollutants in wastewater: Insights on ecotoxicity, health risk, and state-of-the-art removal [33]. | Occurrence and impacts of PhACs, along with their treatment by different methods, are extensively analyzed, offering an in-depth and comprehensive overview of their environmental implications. | Occurrence and impacts of CA, along with its treatment using AOPs, which are underrepresented in the literature. This highlights the limitations of AOPs, particularly by demonstrating their inability to achieve complete pollutant mineralization. |
| Challenges and Emerging Trends in Advanced Oxidation Technologies and Integration of Advanced Oxidation Processes with Biological Processes for Wastewater Treatment [32]. | Extensively reports on real wastewater degradation processes and addresses a broad range of pollutants. Establishes the challenges of wastewater treatment and highlights the introduction of contaminants into water bodies from various sources. | Focus on the degradation of specific pollutants, providing detailed insights into their behavior at each stage of various degradation processes. It also offers a comprehensive analysis of CA consumption and its concentrations detected in different aquatic and solid matrices. |
| Anticancer drugs in wastewater and natural environments: A review on their occurrence, environmental persistence, treatment, and ecological risks [34]. | Emphasizes the importance of generating transformation products through physicochemical processes and provides a state-of-the-art review on the application of AOPs for the degradation of cytostatic drugs. | Presents various transformation products of CA and establishes its generation pathways through different AOPs. It also provides a state-of-the-art analysis of the application of AOPs and their combinations, incorporating the use of novel nanomaterials. |
| Critical review of technologies for the on-site treatment of hospital wastewater: From conventional to combined advanced processes [35]. | Includes an extensive characterization of the main chemical parameters in hospital influents and reports the concentrations of a wide range of PhACs in hospital wastewater. | Provides a systematic analysis of the presence of CA in various water matrices and WWTP sludge, offering a broader perspective on the issue and establishes the causes of CA removal through transfer between matrices and traces the route of contaminants throughout their cycle. |
| Trends in Fenton and photo-Fenton processes for degradation of antineoplastic agents in water matrices: current knowledge and future challenges evaluation using a bibliometric and systematic analysis [36]. | A systematic bibliometric analysis to assess trends in the use of Fenton and photo-Fenton processes. Additionally, it focuses on the identification of transformation products and their subsequent toxicological evaluation. | Highlights the persistence, toxicity, and specific transformation routes of CA, providing detailed information on its excretion and environmental impact. It also analyzes a wider variety of AOPs, including photocatalysis, ozonation, electro-oxidation, and combined processes. |
| Wastewater treatment by anodic oxidation in electrochemical advanced oxidation process: Advance in mechanism, direct and indirect oxidation detection methods [37]. | Details anodic oxidation mechanisms, highlighting both direct and indirect oxidation methods. It also explores specific electrode materials and conditions aimed at improving the efficiency of anodic oxidation. | Addresses CA, focusing on global data, environmental persistence, and transformation pathways through a wide range of AOPs, describing the fabrication of materials used and listing the experimental parameters involved. |
2. Cancer and Anticancer Drugs
3. Occurrence of Cytotoxic Antibiotics in Water
3.1. Cytotoxic Antibiotics Degradation by Biological Methods
3.2. Cytotoxic Antibiotics Degradation by Advanced Oxidation Processes
4. Issues and Prospects
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AOPs | Advanced oxidation processes |
| APC | Assisted photocatalysis |
| APEC | Assisted photoelectrocatalysis |
| BCN | Bulk g-C3N4 |
| BDD | Boron-doped diamond |
| BLEO | Bleomycin |
| CA | Cytotoxic antibiotics |
| CC | Carbon cloth |
| CD | Cytostatic drugs |
| CEA | Cellulose acetate |
| CN | Metal-free graphitic nitride |
| CNO | Carbon nano-onions |
| CPCN | C-doped g-C3N4 |
| DAC | Dactinomycin |
| DAU | Daunorubicin |
| DB | Degradation by-products |
| DNA | Deoxyribonucleic Acid |
| DOX | Doxorubicin |
| EC-O | Electrochemical oxidation |
| EP | Emerging pollutants |
| EPI | Epirubicin |
| GO | Grapheme oxide |
| HUSM | Federal University of Santa Maria |
| IBP | Ixabepilone |
| IDAU | Idarubicin |
| HRMS-ESI | High-resolution mass spectrometer with an electrospray ion source |
| MIT | Mitomycin |
| MXT | Mitoxantrone |
| NOCN-1 | 1.6 g CPCN |
| NOCN-2 | 1.9 g CPCN |
| NOCN-3 | 2.2 g CPCN |
| NPs | Nanoparticles |
| PAN | Polyacrylonitrile |
| PCN | Polymeric g-C3N4 |
| POCIS | Polar organic chemical integrative sampler |
| PRB | Pirarubicin |
| rGO | Reduced graphene oxide |
| RNA | Ribonucleic Acid |
| TOC | Total organic carbon |
| WHO | World health organization |
| WRF | White rot fungi |
| WWTP | Wastewater treatment plants |
| PhACs | Pharmaceutical compounds |
| PhCat | Photocatalysis |
| Si | Silicone |
References
- Vargas-Berrones, K.; Bernal-Jácome, L.; Díaz de León-Martínez, L.; Flores-Ramírez, R. Emerging pollutants (EPs) in Latin América: A critical review of under-studied EPs, case of study -Nonylphenol-. Sci. Total Environ. 2020, 726, 138493. [Google Scholar] [CrossRef] [PubMed]
- Finčur, N.; Sfîrloagă, P.; Putnik, P.; Despotović, V.; Lazarević, M.; Uzelac, M.; Abramović, B.; Vlazan, P.; Ianăși, C.; Alapi, T.; et al. Removal of Emerging Pollutants from Water Using Environmentally Friendly Processes: Photocatalysts Preparation, Characterization, Intermediates Identification and Toxicity Assessment. Nanomaterials 2021, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- González-González, R.B.; Flores-Contreras, E.A.; Parra-Saldívar, R.; Iqbal, H.M.N. Bio-removal of emerging pollutants by advanced bioremediation techniques. Environ. Res. 2022, 214, 113936. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Naushad, M.; Govarthanan, M.; Iqbal, J.; Alfadul, S.M. Emerging contaminants of high concern for the environment: Current trends and future research. Environ. Res. 2022, 207, 112609. [Google Scholar] [CrossRef]
- Tang, Y.; Yin, M.; Yang, W.; Li, H.; Zhong, Y.; Mo, L.; Liang, Y.; Ma, X.; Sun, X. Emerging pollutants in water environment: Occurrence, monitoring, fate, and risk assessment. Water Environ. Res. 2019, 91, 984–991. [Google Scholar] [CrossRef]
- Riva, F.; Castiglioni, S.; Fattore, E.; Manenti, A.; Davoli, E.; Zuccato, E. Monitoring emerging contaminants in the drinking water of Milan and assessment of the human risk. Int. J. Hyg. Environ. Health 2018, 221, 451–457. [Google Scholar] [CrossRef]
- Wu, Y.; He, X.; Wang, X.; Xv, J.; Muddassir, M.; Ansari, I.A.; Zhong, A. Synergistic efficacy unleashed: Co/Ni-based catalysts as a versatile powerhouse for photocatalytic degradation of ornidazole. Inorg. Chim. Acta 2024, 568, 122115. [Google Scholar] [CrossRef]
- Dias, R.; Sousa, D.; Bernardo, M.; Matos, I.; Fonseca, I.; Vale Cardoso, V.; Neves Carneiro, R.; Silva, S.; Fontes, P.; Daam, M.A.; et al. Study of the Potential of Water Treatment Sludges in the Removal of Emerging Pollutants. Molecules 2021, 26, 1010. [Google Scholar] [CrossRef]
- Rempel, A.; Pedó Gutkoski, J.; Torres Nazari, M.; Nadal Biolchi, G.; Biduski, B.; Treichel, H.; Colla, L.M.J. Microalgae growth with a high concentration of emerging pollutants and phytotoxicity evaluation of cultivation wastewater. Water Process Eng. 2022, 46, 102616. [Google Scholar] [CrossRef]
- Arman, N.Z.; Salmiati, S.; Aris, A.; Salim, M.R.; Nazifa, T.H.; Muhamad, M.S.M.; Marpongahtun, M. A Review on Emerging Pollutants in the Water Environment: Existences, Health Effects and Treatment Processes. Water 2021, 13, 3258. [Google Scholar] [CrossRef]
- Ranjan, N.; Singh, P.K.; Maurya, N.S. Pharmaceuticals in water as emerging pollutants for river health: A critical review under Indian conditions. Ecotoxicol. Environ. Saf. 2022, 247, 114220. [Google Scholar] [CrossRef]
- Peña-Guzmán, C.; Ulloa-Sánchez, S.; Mora, K.; Helena-Bustos, R.; Lopez-Barrera, E.; Alvarez, J.; Rodriguez-Pinzón, M.J. Emerging pollutants in the urban water cycle in Latin America: A review of the current literature. Environ. Manag. 2019, 237, 408–423. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mentha, S.S.; Misra, Y.; Dwivedi, N. Emerging pollutants of severe environmental concern in water and wastewater: A comprehensive review on current developments and future research. Water-Energy Nexus 2023, 6, 74–95. [Google Scholar] [CrossRef]
- Egbuna, C.; Amadi, C.N.; Patrick-Iwuanyanwu, K.C.; Ezzat, S.M.; Awuchi, C.G.; Ugonwa, P.O.; Orisakwe, O.E. Emerging pollutants in Nigeria: A systematic review. Environ. Toxicol. Pharmacol. 2021, 85, 103638. [Google Scholar] [CrossRef]
- He, Z.; Cheng, X.; Kyzas, G.Z.; Fu, J. Pharmaceuticals pollution of aquaculture and its management in China. J. Mol. Liq. 2016, 223, 781–789. [Google Scholar] [CrossRef]
- Moreno Ríos, A.L.; Gutierrez-Suarez, K.; Carmona, Z.; Ramos, C.G.; Silva Oliveira, L.F. Pharmaceuticals as emerging pollutants: Case naproxen an overview. Chemosphere 2022, 291, 132822. [Google Scholar] [CrossRef]
- Zhou, S.; Di Paolo, C.; Wu, X.; Shao, Y.; Seiler, T.B.; Hollert, H. Optimization of screening-level risk assessment and priority selection of emerging pollutants—The case of pharmaceuticals in European surface waters. Environ. Int. 2019, 128, 1–10. [Google Scholar] [CrossRef]
- Tiwari, B.; Sellamuthu, B.; Ouarda, Y.; Drogui, P.; Tyagi, R.D.; Buelna, G. Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour. Technol. 2017, 224, 1–12. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, F.S.A.; Mubarak, N.M.; Khalid, M.; Tan, Y.H.; Mazari, S.A.; Karri, R.R.; Abdullah, E.C. Emerging pollutants and their removal using visible-light responsive photocatalysis—A comprehensive review. J. Environ. Chem. Eng. 2021, 9, 106643. [Google Scholar] [CrossRef]
- Kar, P.; Shukla, K.; Jain, P.; Sathiyan, G.; Gupta, R.K. Semiconductor based photocatalysts for detoxification of emerging pharmaceutical pollutants from aquatic systems: A critical review. Nano Mater. Sci. 2021, 3, 25–46. [Google Scholar] [CrossRef]
- Velempini, T.; Prabakaran, E.; Pillay, K. Recent developments in the use of metal oxides for photocatalytic degradation of pharmaceutical pollutants in water—A review. Mater. Today Chem. 2021, 19, 100380. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Anubha, M.; Jayashree, S. Degradation of toxic agrochemicals and pharmaceutical pollutants: Effective and alternative approaches toward photocatalysis. Environ. Pollut. 2022, 298, 118844. [Google Scholar] [CrossRef]
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging pollutants in wastewater: A review of the literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U., Jr.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- Neha, R.; Adithya, S.; Sai Jayaraman, R.; Gopinath, K.P.; Pandimadevi, M.; Praburaman, L.; Arun, J. Nano-adsorbents an effective candidate for removal of toxic pharmaceutical compounds from aqueous environment: A critical review on emerging trends. Chemosphere 2021, 272, 129852. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameed, B.H. Removal of emerging pharmaceutical contaminants by adsorption in a fixed-bed column: A review. Ecotoxicol. Environ. Saf. 2018, 149, 257–266. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, C.; Li, J.; Yu, X.; Feng, M. Treatment-driven removal efficiency, product formation, and toxicity evolution of antineoplastic agents: Current status and implications for water safety assessment. Water Res. 2021, 206, 117729. [Google Scholar] [CrossRef]
- Eniola, J.O.; Kumar, R.; Barakat, M.A.; Rashid, J. A review on conventional and advanced hybrid technologies for pharmaceutical wastewater treatment. J. Clean. Prod. 2022, 356, 131826. [Google Scholar] [CrossRef]
- Adeoye, J.B.; Tan, Y.H.; Lau, S.Y.; Tan, Y.Y.; Chiong, T.; Mubarak, N.M.; Khalid, M. Advanced oxidation and biological integrated processes for pharmaceutical wastewater treatment: A review. J. Environ. Manag. 2024, 353, 120170. [Google Scholar] [CrossRef]
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review. ChemEngineering 2022, 6, 8. [Google Scholar] [CrossRef]
- Gopalakrishnan, G.; Jeyakumar, R.B.; Somanathan, A. Challenges and Emerging Trends in Advanced Oxidation Technologies and Integration of Advanced Oxidation Processes with Biological Processes for Wastewater Treatment. Sustainability 2023, 15, 4235. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Lin, C.; Bui, X.T.; Rakib, M.R.J.; Nguyen, H.L.; Truong, Q.M.; Hoang, H.G.; Tran, H.T.; Malafaia, G.; Idris, A.M. Occurrence and fate of pharmaceutical pollutants in wastewater: Insights on ecotoxicity, health risk, and state–of–the-art removal. Chemosphere 2024, 354, 141678. [Google Scholar] [CrossRef]
- Castellano-Hinojosa, A.; Gallardo-Altamirano, M.J.; González-López, J.; González-Martínez, A. Anticancer drugs in wastewater and natural environments: A review on their occurrence, environmental persistence, treatment, and ecological risks. J. Hazard. Mater. 2023, 447, 130818. [Google Scholar] [CrossRef]
- Pariente, M.I.; Segura, Y.; Álvarez-Torrellas, S.; Casas, J.A.; de Pedro, Z.M.; Diaz, E.; García, J.; López-Muñoz, M.J.; Marugán, J.; Mohedano, A.F.; et al. Critical review of technologies for the on-site treatment of hospital wastewater: From conventional to combined advanced processes. J. Environ. Manag. 2022, 320, 115769. [Google Scholar] [CrossRef]
- Sanabria, P.; Wilde, M.L.; Ruiz-Padillo, A.; Sirtori, C. Trends in Fenton and photo-Fenton processes for degradation of antineoplastic agents in water matrices: Current knowledge and future challenges evaluation using a bibliometric and systematic analysis. Environ. Sci. Pollut. Res. 2022, 29, 42168–42184. [Google Scholar] [CrossRef]
- Fu, R.; Zhang, P.S.; Jiang, Y.X.; Sun, L.; Sun, X.H. Wastewater treatment by anodic oxidation in electrochemical advanced oxidation process: Advance in mechanism, direct and indirect oxidation detection methods. Chemosphere 2023, 311, 136993. [Google Scholar] [CrossRef]
- WHO-World Health Organization. International Agency for Research on Cancer, Global Cancer Observatory, Cancer Today. Available online: https://gco.iarc.fr/today/en/fact-sheets-populations#regions (accessed on 20 October 2023).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Garcia-Costa, A.L.; Alves, A.; Madeira, L.M.; Santos, M.S.F. Oxidation processes for cytostatic drugs elimination in aqueous phase: A critical review. J. Environ. Chem. Eng. 2021, 9, 104709. [Google Scholar] [CrossRef]
- Jureczko, M.; Kalka, J. Cytostatic pharmaceuticals as water contaminants. Eur. J. Pharmacol. 2020, 866, 172816. [Google Scholar] [CrossRef]
- Roque-Diaz, Y.; Sanadar, M.; Han, D.; López-Mesas, M.; Valiente, M.; Tolazzi, M.; Melchior, A.; Veclani, D. The Dark Side of Platinum Based Cytostatic Drugs: From Detection to Removal. Processes 2021, 9, 1873. [Google Scholar] [CrossRef]
- Novak, M.; Žegura, B.; Modic, B.; Heath, E.; Filipič, M. Cytotoxicity and genotoxicity of anticancer drug residues and their mixtures in experimental model with zebrafish liver cells. Sci. Total Environ. 2017, 601–602, 293–300. [Google Scholar] [CrossRef]
- Villarini, M.; Gianfredi, V.; Levorato, S.; Vannini, S.; Salvatori, T.; Moretti, M. Occupational exposure to cytostatic/antineoplastic drugs and cytogenetic damage measured using the lymphocyte cytokinesis-block micronucleus assay: A systematic review of the literature and meta-analysis. Mutat. Res. Rev. Mutat. Res. 2016, 770, 35–45. [Google Scholar] [CrossRef]
- Yadav, A.; Rene, E.R.; Sharma, M.; Kumar, V.; Mandal, M.K.; Dubey, K.K. Source, Occurrence, and Risk Assessment of Antineoplastic Medicines in Aquatic Environments: A Comprehensive Review. Curr. Pollut. Rep. 2023, 9, 391–409. [Google Scholar] [CrossRef]
- Toolaram, A.P.; Kümmerer, K.; Schneider, M. Environmental risk assessment of anti-cancer drugs and their transformation products: A focus on their genotoxicity characterization-state of knowledge and short comings. Mutat. Res. Rev. Mutat. Res. 2014, 760, 18–35. [Google Scholar] [CrossRef]
- NIPH-Norwegian Institute of Public Health. ATC/DDD Index, Antineoplastic and Immunomodulating Agents, Antineoplastic Agents, Cytotoxic Antibiotics and Related Substances. Available online: https://atcddd.fhi.no/atc_ddd_index/?code=L01D&showdescription=no (accessed on 7 November 2023).
- Jureczko, M.; Przystaś, W. Ecotoxicity risk of presence of two cytostatic drugs: Bleomycin and vincristine and their binary mixture in aquatic environment. Ecotoxicol. Environ. Saf. 2019, 172, 210–215. [Google Scholar] [CrossRef]
- Rowney, N.C.; Johnson, A.C.; Williams, R.J. Cytotoxic drugs in drinking water: A prediction and risk assessment exercise for the thames catchment in the United Kingdom. Environ. Toxicol. Chem. 2009, 28, 2733–2743. [Google Scholar] [CrossRef]
- Ardestani, F.; Sheikhi, M. Evaluation of the Efficiency of Activated Sludge Process in Cefazolin and Doxorubicin Antibiotics Removal from Hospital Wastewater. Iran J. Energy Environ. 2020, 11, 351–359. [Google Scholar] [CrossRef]
- Mello Souza, D.; Reichert, J.F.; Ramos do Nascimento, V.; Figueiredo Martins, A. Ozonation and UV photolysis for removing anticancer drug residues from hospital wastewater. J. Environ. Sci. Health-Toxic/Hazard. 2022, 57, 635–644. [Google Scholar] [CrossRef]
- Besse, J.P.; Latour, J.F.; Garric, J. Anticancer drugs in surface waters: What can we say about the occurrence and environmental significance of cytotoxic, cytostatic and endocrine therapy drugs? Environ. Int. 2012, 39, 73–86. [Google Scholar] [CrossRef]
- El-Kawy, O.A.; Abdelaziz, G. Preparation, characterization and evaluation of [125I]-pirarubicin: A new therapeutic agent for urinary bladder cancer with potential for use as theranostic agent. Appl. Radiat. Isot. 2022, 179, 110007. [Google Scholar] [CrossRef]
- Gómez-Canela, C.; Campos, B.; Barata, C.; Lacorte, S. Degradation and toxicity of mitoxantrone and chlorambucil in water. Int. J. Environ. Sci. Technol. 2015, 12, 633–640. [Google Scholar] [CrossRef]
- Benet, L.Z.; Broccatelli, F.; Oprea, T.I. BDDCS Applied to Over 900 Drugs. AAPS J. 2011, 13, 519–547. [Google Scholar] [CrossRef]
- Mahnik, S.N.; Lenz, K.; Weissenbacher, N.; Mader, R.M.; Fuerhacker, M. Fate of 5-fluorouracil, doxorubicin, epirubicin, and daunorubicin in hospital wastewater and their elimination by activated sludge and treatment in a membrane-bio-reactor system. Chemosphere 2007, 66, 30–37. [Google Scholar] [CrossRef]
- Bull, R.J.; Crook, J.; Whittaker, M.; Cotruvo, J.A. Therapeutic dose as the point of departure in assessing potential health hazards from drugs in drinking water and recycled municipal wastewater. Regul. Toxicol. Pharmacol. 2011, 60, 1–19. [Google Scholar] [CrossRef]
- Kozuch, P.; Hoff, P.M.; Hess, K.; Adams, J.; Newman, R.A.; Lee, F.; Pazdur, R. Phase I bioequivalency study of MitoExtra and mitomycin C in patients with solid tumors. Cancer 2001, 91, 815–821. [Google Scholar] [CrossRef]
- Christinat, A. Miscellaneous. In ESMO Handbook: Clinical Pharmacology of Anti-Cancer Agents; Sessa, C., Gianni, L., Garassino, M., van Halteren, H., Eds.; ESMO Press: Lugano, Switzerland, 2012; pp. 103–106. [Google Scholar]
- Mahnik, S.N.; Rizovski, B.; Fuerhacker, M.; Mader, R.M. Development of an analytical method for the determination of anthracyclines in hospital effluents. Chemosphere 2006, 65, 1419–1425. [Google Scholar] [CrossRef]
- Franquet-Griell, H.; Cornadó, D.; Caixach, J.; Ventura, F.; Lacorte, S. Determination of cytostatic drugs in Besòs River (NE Spain) and comparison with predicted environmental concentrations. Environ. Sci. Pollut. Res. 2017, 24, 6492–6503. [Google Scholar] [CrossRef]
- Da Silva, R.F.; de Lima Moura, L.; Gavião, L.O.; Brito Alves Lima, G.; Dausacker Bidone, E. Local environmental risk assessment of anticancer drugs in a developing country. Hum. Ecol. Risk Assess. 2020, 26, 2142–2161. [Google Scholar] [CrossRef]
- Gómez-Canela, C.; Cortés-Francisco, N.; Oliva, X.; Pujol, C.; Ventura, F.; Lacorte, S.; Caixach, J. Occurrence of cyclophosphamide and epirubicin in wastewaters by direct injection analysis–liquid chromatography–high-resolution mass spectrometry. Environ. Sci. Pollut. Res. 2012, 19, 3210–3218. [Google Scholar] [CrossRef] [PubMed]
- Askarian, M.; Momeni, M.; Danaei, M. The management of cytotoxic drug wastes in Shiraz, Iran: An overview of all government and private chemotherapy settings, and comparison with national and international guidelines. Waste Manag. Res. 2013, 31, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Ortiz de García, S.; Pinto Pinto, G.; García Encina, P.; Irusta Mata, R. Consumption and occurrence of pharmaceutical and personal care products in the aquatic environment in Spain. Sci. Total Environ. 2013, 444, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Sinawe, H.; Casadesus, D. Mitomycin. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562249/ (accessed on 8 August 2024).
- Saleem, T.; Kasi, A. Daunorubicin. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559073/ (accessed on 8 August 2024).
- Ibrahim, N.K. Ixabepilone: Overview of Effectiveness, Safety, and Tolerability in Metastatic Breast Cancer. Front. Oncol. 2021, 11, 617874. [Google Scholar] [CrossRef]
- Li, J.; Ren, J.; Sun, W. Systematic review of ixabepilone for treating metastatic breast cancer. Breast Cancer 2017, 24, 171–179. [Google Scholar] [CrossRef]
- Li, D.; Chen, H.; Liu, H.; Schlenk, D.; Mu, J.; Lacorte, S.; Ying, G.G.; Xie, L. Anticancer drugs in the aquatic ecosystem: Environmental occurrence, ecotoxicological effect and risk assessment. Environ. Int. 2021, 153, 106543. [Google Scholar] [CrossRef]
- Negreira, N.; López de Alda, M.; Barceló, D. Cytostatic drugs and metabolites in municipal and hospital wastewaters in Spain: Filtration, occurrence, and environmental risk. Sci. Total Environ. 2014, 497–498, 68–77. [Google Scholar] [CrossRef]
- Martín, J.; Camacho-Muñoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Simultaneous determination of a selected group of cytostatic drugs in water using high-performance liquid chromatography–triple-quadrupole mass spectrometry. J. Sep. Sci. 2011, 34, 3166–3177. [Google Scholar] [CrossRef]
- Martín, J.; Camacho-Muñoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence and Ecotoxicological Risk Assessment of 14 Cytostatic Drugs in Wastewater. Water Air Soil Pollut. 2014, 225, 1896. [Google Scholar] [CrossRef]
- Gouveia, T.I.A.; Silva, A.M.T.; Freire, M.G.; Sousa, A.C.A.; Alves, A.; Santos, M.S.F. Multi-target analysis of cytostatics in hospital effluents over a 9-month period. J. Hazard. Mater. 2023, 448, 130883. [Google Scholar] [CrossRef]
- Gouveia, T.I.A.; Mota, I.H.; Silva, A.M.T.; Alves, A.; Santos, M.S.F. Are cytostatic drugs in surface waters a potential threat? Sci. Total Environ. 2022, 853, 158559. [Google Scholar] [CrossRef] [PubMed]
- Souza, D.M.; Reichert, J.F.; Martins, A.F. A simultaneous determination of anti-cancer drugs in hospital effluent by DLLME HPLC-FLD, together with a risk assessment. Chemosphere 2018, 201, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Popowicz, J.; Koszelnik, P. The influence of the physicochemical properties on the methods of inactivation and removal of cytostatic drugs from water and wastewater. Acta Sci. Pol. Form. Circumiectus 2015, 14, 107–125. [Google Scholar] [CrossRef]
- Maghsodian, Z.; Sanati, A.M.; Mashifana, T.; Sillanpää, M.; Feng, S.; Nhat, T.; Ramavandi, B. Occurrence and Distribution of Antibiotics in the Water, Sediment, and Biota of Freshwater and Marine Environments: A Review. Antibiotics 2022, 11, 1461. [Google Scholar] [CrossRef]
- Danner, M.C.; Robertson, A.; Behrends, V.; Reiss, J. Antibiotic pollution in surface fresh waters: Occurrence and effects. Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the Soil Environment-Degradation and Their Impact on Microbial Activity and Diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Booker, V.; Halsall, C.; Llewellyn, N.; Johnson, A.; Williams, R. Prioritising anticancer drugs for environmental monitoring and risk assessment purposes. Sci. Total Environ. 2014, 473–474, 159–170. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mehta, D.; Dhangar, K.; Kumar, M. Environmental fate, distribution and state-of-the-art removal of antineoplastic drugs: A comprehensive insight. J. Chem. Eng. 2021, 407, 127184. [Google Scholar] [CrossRef]
- Kosjek, T.; Heath, E. Occurrence, fate and determination of cytostatic pharmaceuticals in the environment. TrAC Trends Anal. Chem. 2011, 30, 1065–1087. [Google Scholar] [CrossRef]
- Kelbert, M.; Daronch, N.A.; Pereira, C.S.; Cesca, K.; Michels, C.; Soares, H.M. Inhibitory impact of the anticancer drug doxorubicin on anaerobic microbial community. Aquat. Toxicol. 2023, 264, 106706. [Google Scholar] [CrossRef]
- Franquet-Griell, H.; Medina, A.; Sans, C.; Lacorte, S. Biological and photochemical degradation of cytostatic drugs under laboratory conditions. J. Hazard. Mater. 2017, 323, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Chang, V.W.C.; She, Q.; Tang, C.Y. Removal of cytostatic drugs from wastewater by an anaerobic osmotic membrane bioreactor. J. Chem. Eng. 2018, 339, 153–161. [Google Scholar] [CrossRef]
- De Vargas, J.P.R.; Bastos, M.C.; Al Badany, M.; Gonzalez, R.; Wolff, D.; Dos Santos, D.R.; Labanowski, J. Pharmaceutical compound removal efficiency by a small constructed wetland located in south Brazil. Environ. Sci. Pollut. Res. 2021, 28, 30955–30974. [Google Scholar] [CrossRef] [PubMed]
- Jureczko, M.; Przystaś, W.; Krawczyk, T.; Gonciarz, W.; Rudnicka, K. White-rot fungi-mediated biodegradation of cytostatic drugs—Bleomycin and vincristine. J. Hazard. Mater. 2021, 407, 124632. [Google Scholar] [CrossRef]
- Rybczyńska-Tkaczyk, K. Enhanced Efficiency of the Removal of Cytostatic Anthracycline Drugs Using Immobilized Mycelium of Bjerkandera adusta CCBAS 930. Molecules 2021, 26, 6842. [Google Scholar] [CrossRef]
- Kelbert, M.; Pereira, C.S.; Daronch, N.A.; Cesca, K.; Michels, C.; de Oliveira, D.; Soares, H.M. Laccase as an efficacious approach to remove anticancer drugs: A study of doxorubicin degradation, kinetic parameters, and toxicity assessment. J. Hazard. Mater. 2021, 409, 124520. [Google Scholar] [CrossRef]
- Saravanan, A.; Deivayanai, V.C.; Senthil Kumar, P.; Rangasamy, G.; Hemavathy, R.V.; Harshana, T.; Gayathri, N.; Alagumalai, K. A detailed review on advanced oxidation process in treatment of wastewater: Mechanism, challenges and future outlook. Chemosphere 2022, 308, 136524. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. J. Chem. Eng. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef]
- Dewil, R.; Mantzavinos, D.; Poulios, I.; Rodrigo, M.A. New perspectives for Advanced Oxidation Processes. J. Environ. Manag. 2017, 195, 93–99. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.K.G.; Ferreira Garcia, L.; Lobón, G.S.; Barroso Brito, L.; Rodrigues Oliveira, G.A.; Luque, R.; de Souza Gil, E. Ecotoxicological assessment and electrochemical remediation of doxorubicin. Ecotoxicol. Environ. Saf. 2019, 179, 143–150. [Google Scholar] [CrossRef]
- Ferreira Garcia, L.; Moreno, E.K.G.; Barroso Brito, L.; Rodrigues de Oliveira, G.A.; Linares, J.J.; de Souza Gil, E. Effective degradation of the antineoplastic doxorubicin by electrochemical oxidation on boron doped diamond. J. Electroanal. Chem. 2020, 870, 114252. [Google Scholar] [CrossRef]
- De Souza Gil, E.; Moreno, E.K.; Ferreira Garcia, L.; Linares León, J.J. Performance Evaluation of Active and Non-active Electrodes for Doxorubicin Electro-oxidation. KnE Eng. 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Jafarizad, A.; Rostamizadeh, M.; Zarei, M.; Gharibian, S. Mitoxantrone removal by electrochemical method: A comparison of homogenous and heterogenous catalytic reactions. Environ. Health Eng. Manag. 2017, 4, 185–193. [Google Scholar] [CrossRef]
- Wu, J.; Lin, M.; Weng, X.; Owens, G.; Chen, Z. Pre-adsorption and Fenton-like oxidation of mitoxantrone using hybrid green synthesized rGO/Fe nanoparticles. J. Chem. Eng. 2021, 408, 127273. [Google Scholar] [CrossRef]
- Wu, J.; Lin, Z.; Weng, X.; Owens, G.; Chen, Z. Removal mechanism of mitoxantrone by a green synthesized hybrid reduced graphene oxide @ iron nanoparticles. Chemosphere 2020, 246, 125700. [Google Scholar] [CrossRef]
- Garcia-Costa, A.L.; Gouveia, T.I.A.; Pereira, M.F.R.; Silva, A.M.T.; Madeira, L.M.; Alves, A.; Santos, M.S.F. Intensification strategies for cytostatics degradation by ozone-based processes in aqueous phase. J. Hazard. Mater. 2022, 440, 129743. [Google Scholar] [CrossRef]
- Cavalcante, R.P.; da Rocha Sandim, L.; Bogo, D.; Jacques Barbosa, A.M.; Osugi, M.E.; Blanco, M.; de Oliveira, S.C.; de Fatima Cepa Matos, M.; Machulek, A., Jr.; Souza Ferreria, V. Application of Fenton, photo-Fenton, solar photo-Fenton, and UV/H2O2 to degradation of the antineoplastic agent mitoxantrone and toxicological evaluation. Environ. Sci. Pollut. Res. 2013, 20, 2352–2361. [Google Scholar] [CrossRef]
- Calza, P.; Medana, C.; Sarro, M.; Rosato, V.; Aigotti, R.; Baiocchi, C.; Minero, C. Photocatalytic degradation of selected anticancer drugs and identification of their transformation products in water by liquid chromatography–high resolution mass spectrometry. J. Chromatogr. A 2014, 1362, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Štenglová Netíková, I.R.; Slušná, M.; Tolasz, J.; Šťastný, M.; Popelka, Š.; Štengl, V. A new possible way of anthracycline cytostatics decontamination. New J. Chem. 2017, 41, 3975–3985. [Google Scholar] [CrossRef]
- Bigtan, M.H.; Mahanpoor, K. Preparation, characterization, and evaluation of the photocatalytic effect of α-Fe2O3/SAPO-34 in the elimination process of the anti-cancer drug doxorubicin from aqueous solution. Stud. Univ. Babes-Bolyai Chem. 2017, 62, 203–212. [Google Scholar] [CrossRef]
- Zhao, C.; Li, C.; Chen, M.; Niu, T.; Zhao, Q.; Ni, T.; Yan, D.; Wu, W.; Liu, D. Effective removal of antineoplastic doxorubicin by 0D Nb2O5 quantum dots embed 3D porous C-doped g-C3N4: Degradation mechanism, pathway and toxicity assessment. Appl. Surf. Sci. 2023, 612, 155861. [Google Scholar] [CrossRef]
- Garg, T.; Renu; Nitansh; Aggarwal, D.; Kumar, V.; Tikoo, K.; Paulik, C.; Kaushik, A.; Singhal, S. Rational construction of g-C3N4/SnO2/CoFe2O4 dual Z-scheme system as a potential scaffold for fluorescence sensing and visible light driven photocatalytic degradation of lethal pollutants. J. Environ. Chem. Eng. 2023, 11, 110744. [Google Scholar] [CrossRef]
- Racles, C.; Zaltariov, M.F.; Silion, M.; Macsim, A.M.; Cozan, V. Photo-oxidative degradation of doxorubicin with siloxane MOFs by exposure to daylight. Environ. Sci. Pollut. Res. 2019, 26, 19684–19696. [Google Scholar] [CrossRef]
- Abbasi, M.A.; Amin, K.M.; Ali, M.; Ali, Z.; Atif, M.; Ensinger, W.; Khalid, W. Synergetic effect of adsorption-photocatalysis by GO-CeO2 nanocomposites for photodegradation of doxorubicin. J. Environ. Chem. Eng. 2022, 10, 107078. [Google Scholar] [CrossRef]
- Teymourinia, H.; Sánchez, L.; Mollaie, F.; Ghalkhani, M.; Ramazani, A.; Hublikar, L.V.; Aminabhavi, T.M. Novel plasmonic Z-scheme-based photocatalysts and electrochemical aptasensor for the degradation and determination of epirubicin. J. Chem. Eng. 2024, 480, 148307. [Google Scholar] [CrossRef]
- Da Rosa, A.P.P.; Cavalcante, R.P.; da Silva, D.A.; da Silva, L.M.; da Silva, T.F.; Gozzi, F.; McGlynn, E.; Brady-Boyd, A.; Casagrande, G.A.; Wender, H.; et al. H2O2-assisted photoelectrocatalytic degradation of Mitoxantrone using CuO nanostructured films: Identification of by-products and toxicity. Sci. Total Environ. 2019, 651, 2845–2856. [Google Scholar] [CrossRef]
- Somensi, C.A.; Simionatto, E.L.; Dalmarco, J.B.; Gaspareto, P.; Radetski, C.M. A comparison between ozonolysis and sonolysis/ozonolysis treatments for the degradation of the cytostatic drugs methotrexate and doxorubicin: Kinetic and efficiency approaches. J. Environ. Sci. Health-Toxic/Hazard. 2012, 47, 1543–1550. [Google Scholar] [CrossRef]
- Dumitru, R.; Ianculescu, A.; Păcurariu, C.; Lupa, L.; Pop, A.; Vasile, B.; Surdu, A.; Manea, F. BiFeO3-synthesis, characterization and its photocatalytic activity towards doxorubicin degradation from water. Ceram. Int. 2019, 45, 2789–2802. [Google Scholar] [CrossRef]
- Bahmani, E.; Seyyed Zonouzi, H.; Koushkbaghi, S.; Keyvani Hafshejani, F.; Fassadi Chimeh, A.; Irani, M. Electrospun polyacrylonitrile/cellulose acetate/MIL-125/TiO2 composite nanofibers as an efficient photocatalyst and anticancer drug delivery system. Cellulose 2020, 27, 10029–10045. [Google Scholar] [CrossRef]
- Yogesh Kumar, K.; Prashanth, M.K.; Alduaij, O.K.; Yousef, T.A.; Abualnaja, K.M.; Raghu, M.S. Mentha arvensis mediated green synthesis of platinum doped TiO2 nanocomposite for enhanced anti-cancer and photocatalytic degradation activity: Insights from molecular docking and DFT studies. Inorg. Chem. Commun. 2021, 134, 108987. [Google Scholar] [CrossRef]
- Štenglová-Netíková, I.R.; Petruželka, L.; Šťastný, M.; Štengl, V. Anthracycline antibiotics derivate mitoxantrone-Destructive sorption and photocatalytic degradation. PLoS ONE 2018, 13, e0193116. [Google Scholar] [CrossRef] [PubMed]
- Medana, C.; Calza, P.; Dal Bello, F.; Baiocchi, C. LC-HRMS Determination of Anticancer Drugs as Occupational Contaminants Applied to Photocatalytic Degradation of Molecules of Different Stability. LCGC N. Am. 2013, 11, 30–37. [Google Scholar]
- Gomes, J.; Lincho, J.; Domingues, E.; Quinta-Ferreira, R.M.; Martins, R.C. N–TiO2 Photocatalysts: A Review of Their Characteristics and Capacity for Emerging Contaminants Removal. Water 2019, 11, 373. [Google Scholar] [CrossRef]
- Silerio-Vázquez, F.d.J.; Núñez-Núñez, C.M.; Proal-Nájera, J.B.; Alarcón-Herrera, M.T. A Systematic Review on Solar Heterogeneous Photocatalytic Water Disinfection: Advances over Time, Operation Trends, and Prospects. Catalysts 2022, 12, 1314. [Google Scholar] [CrossRef]
- Obayomi, O.V.; Olawoyin, D.C.; Oguntimehin, O.; Mustapha, L.S.; Kolade, S.O.; Oladoye, P.O.; Oh, S.; Obayomi, K.S. Exploring emerging water treatment technologies for the removal of microbial pathogens. Curr. Res. Biotechnol. 2024, 8, 100252. [Google Scholar] [CrossRef]
- Pandey, A.K.; Reji Kumar, R.; Kalidasan, B.; Laghari, I.A.; Samykano, M.; Kothari, R.; Abusorrah, A.M.; Sharma, K.; Tyagi, V.V. Utilization of solar energy for wastewater treatment: Challenges and progressive research trends. J. Environ. Manag. 2021, 297, 113300. [Google Scholar] [CrossRef]
- Kamati, S.N.; Yan, J.; Fan, J. A review on progresses in reactive dye-containing wastewater treatment. Water Pract. Technol. 2024, 19, 2712–2733. [Google Scholar] [CrossRef]
- Dirani, L.; Ayoub, G.M.; Malaeb, L.; Zayyat, R.M. A review on the occurrence of per- and polyfluoroalkyl substances in the aquatic environment and treatment trends for their removal. J. Environ. Chem. Eng. 2024, 12, 113325. [Google Scholar] [CrossRef]
- Poblete, R.; Bakit, J. Technical and economical assessment of the treatment of vinasse from Pisco production using the advanced oxidation process. Environ. Sci. Pollut. Res. 2023, 30, 70213–70228. [Google Scholar] [CrossRef] [PubMed]



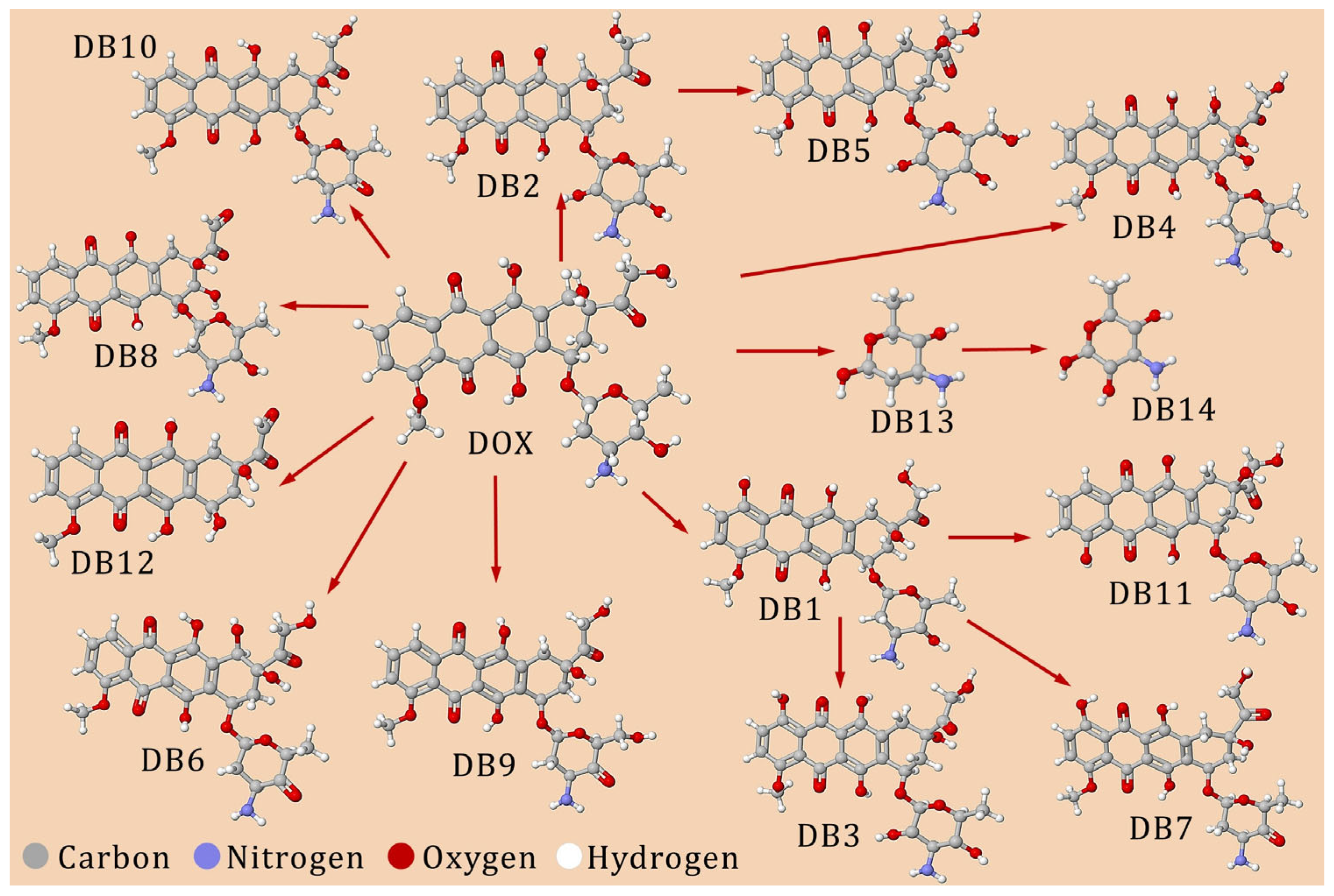
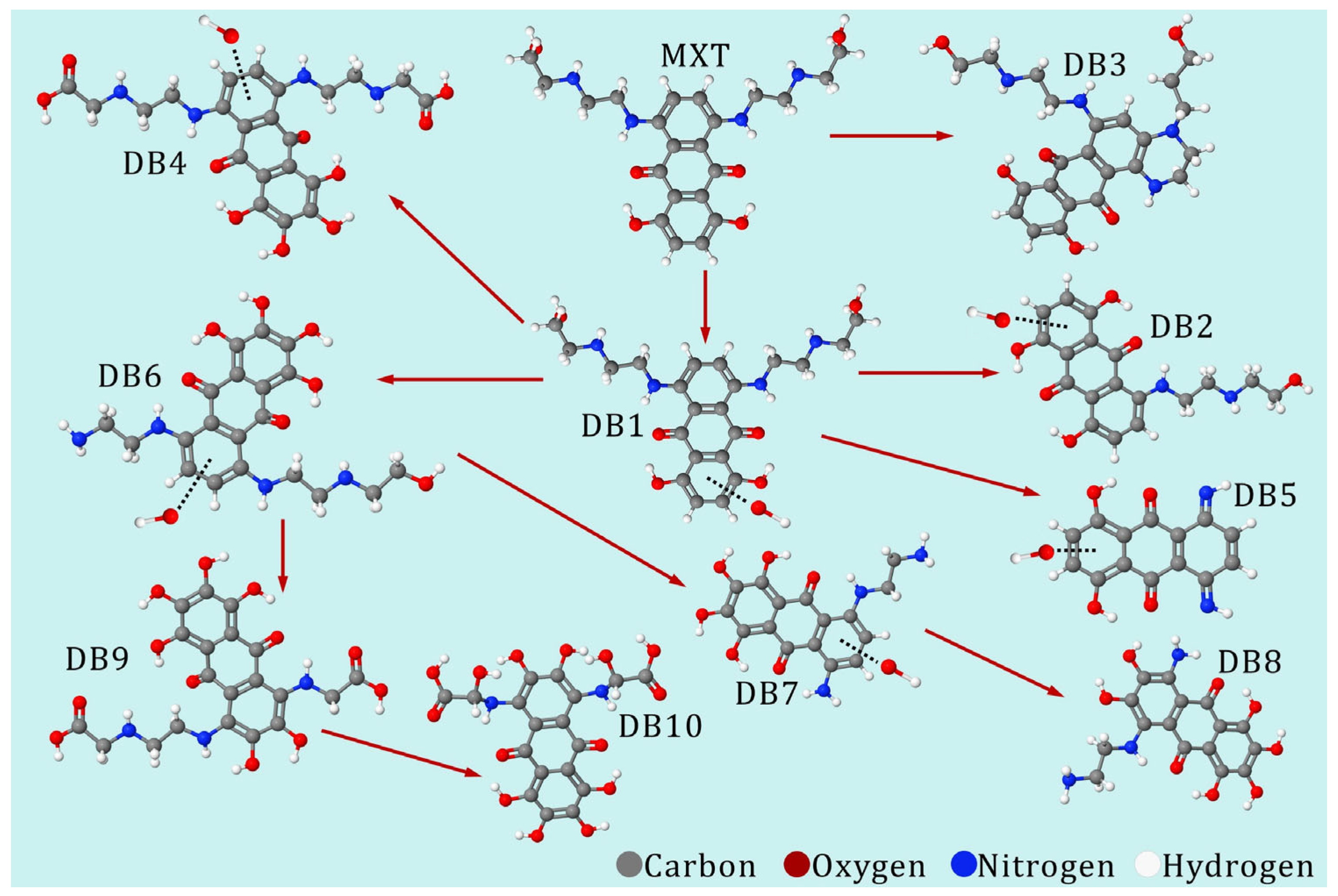
| Group | Sub-Group | Cytotoxic Antibiotics | |||
|---|---|---|---|---|---|
| Name | Code | Name | Code | Name | Code |
| Cytotoxic antibiotics and related substances | L01D | Actinomycines | L01DA | Dactinomycin | L01DA01 |
| Anthracyclines and related substances | L01DB | Doxorubicin | L01DB01 | ||
| Daunorubicin | L01DB02 | ||||
| Epirubicin | L01DB03 | ||||
| Aclarubicin | L01DB04 | ||||
| Zorubicin | L01DB05 | ||||
| Idarubicin | L01DB06 | ||||
| Mitoxantrone | L01DB07 | ||||
| Pirarubicin | L01DB08 | ||||
| Valrubicin | L01DB09 | ||||
| Amrubicin | L01DB10 | ||||
| Pixantrone | L01DB11 | ||||
| Other cytotoxic antibiotics | L01DC | Bleomycin | L01DC01 | ||
| Plicamycin | L01DC02 | ||||
| Mitomycin | L01DC03 | ||||
| Ixabepilone | L01DC04 | ||||
| Cytotoxic Antibiotics | Chemical Formula | Molar Mass | Excretion (%) | Reference | |
|---|---|---|---|---|---|
| Urine | Feces | ||||
| DOX | C27H29NO11 | 543.52 | 3.5–45 | 40–50 | [50,51,57] |
| EPI | C27H29NO11 | 543.52 | 6–20 | 40 | [50,52] |
| DAU | C27H29NO10 | 527.52 | 3–25 | 40–50 | [52,53] |
| BLEO | C55H84N17O21S3+ | 1415.55 | 60–70 | - | [49] |
| MXT | C22H28N4O6 | 444.48 | 6–11 | 25 | [55] |
| IDAU | C26H27NO9 | 497.49 | 2–7 | - | [53] |
| IBP | C27H42N2O5S | 506.69 | 5–6 | - | [56] |
| PRB | C32H37NO12 | 627.64 | 5–9 | - | [54] |
| MIT | C15H18N4O5 | 334.33 | 10 | - | [58,59] |
| DAC | C62H86N12O16 | 1255.42 | 10 | - | [56] |
| Cytotoxic Antibiotics | Location | Concentration (ng/L) | Source | Reference |
|---|---|---|---|---|
| DOX | Vienna, Austria | 260–1350 | Hospital effluent | [57] |
| Spain | 2.5–2.7 | WWTP influent and effluent | [72] | |
| Seville, Spain | 4.5 | WWTP influent | [73] | |
| 20.3–42.4 | WWTP effluent | [74] | ||
| Mazandaran, Iran | 290,000 | WWTP effluent 1 | [51] | |
| 2,690,000 | WWTP effluent 2 | |||
| 950,000 | After activated sludge | |||
| 960,000 | Sludge collection depot | |||
| 810,000 | After chlorination | |||
| Aveiro, Portugal | 37–46 | Hospital effluent | [75] | |
| Portugal | 4.0 | River | [76] | |
| Rio Grande do Sul, Brazil | 2080–4640 | WWTP effluent | [77] | |
| <4640 | [52] | |||
| EPI | Rio Grande do Sul, Brazil | 2270–6220 | WWTP effluent | [77] |
| <6220 | [52] | |||
| Catalonia, Spain | 24,800 | Urban effluent | [64] | |
| DAU | Rio Grande do Sul, Brazil | 1800–3690 | WWTP effluent | [77] |
| <3690 | [52] | |||
| BLEO | - | 30–124,000 | Hospital effluent | [49] |
| 11–19 | WWTP influent | |||
| 11–19 | WWTP effluent | |||
| 5–17 | River water | |||
| 5–13 | Drinking water |
| Process | Cytotoxic Antibiotics | Specifications | Degradation (%) | Parameters | Reference |
|---|---|---|---|---|---|
| EC-O 1 | DOX | AuO-TiO2@G | 97 | [DOX]0 = 5 mg/L | [98] |
| TiO2@G | 90 | 5 volts | |||
| G 3 | 82 | NaCl 10 mmol/L | |||
| BDD-Na2SO4 | 99 | Na2SO4@0.637 Ah/L | [99] | ||
| BDD-NaCl | 99.9 | NaCl@0.318 Ah/L | |||
| AuO-TiO2@G | 100 | [DOX]0 = 1.25 mg/L | [100] | ||
| BDD 4 | 100 | 5 V & 1 mA | |||
| MXT | Fe3O4@GO/CC 5 | 90 | [MXT]0 = 5 mg/L | [101] | |
| TOC 96.9 | pH = 3; 0.2 mM Fe | ||||
| Ozonation | DOX | Ozone | 100 | pH = 9; O3 = 1.5 g/h | [52] |
| [DOX]0 = 10 mg/L | |||||
| O3/H2O2 | 8 | O3 = 85 mg/L | [104] | ||
| O3/H2O2/UV | 52 | [H2O2]0 = 7.45 mg/L | |||
| Ozone | 9 | O3 = 0.5 mg/L | [115] | ||
| 41 | [DOX]0 = 30 µg/L | ||||
| O3/Sonolysis | 17 | Sonolysis = 70 W/L | |||
| 47.5 | pH 3 & 9 | ||||
| DAU | Ozone | 100 | pH = 7; O3 = 1.5 g/h | [52] | |
| [DOX]0 = 10 mg/L | |||||
| EPI | Ozone | 100 | pH = 9; O3 = 1.5 g/h | ||
| [DOX]0 = 10 mg/L | |||||
| Fenton | MXT | H2O2 | 11.3 | [H2O2] = 30 mM | [102] |
| rGO/FeNPs/H2O2 | 99.8 | pH = 3 | |||
| FeNPs 6 | 53.1 | [MXT]0 = 10 mg/L | [103] | ||
| rGO 7 | 77.5 | rGO/FeNPs = 0.8 g/L | |||
| rGO/FeNPs | 98.5 | pH = 9 | |||
| UV/H2O2/Fe3+ | 77 | All degradation percentages correspond to TOC | [105] | ||
| UV/H2O2/FeOx | 82 | ||||
| UV/Fe3+ | 13.25 | ||||
| Photolysis | DOX | UV-C | 97.3 | [AC]0 = 10 mg/L | [52] |
| DAU | 88.3 | pH = 9 | |||
| EPI | 99 | UV-C Lamp 254 nm | |||
| MXT | UV-C | 65 | [H2O2] = 18 mmol/L | [105] | |
| UV-C/H2O2 | 100 | Hg lamp 125 W | |||
| PhCat 2 | DOX | TiO2 P25 | 100 | [DOX]0 = 15 mg/L [Cat] = 200 mg/L | [106] |
| Philips TLK/05 lamp 40 W | |||||
| α-Fe2O3/SAPO-34 | 100 | [DOX]0 = 20 mg/L [Cat] = 150 mg/L | [108] | ||
| [H2O2]0 = 4 mol/L; pH = 8 | |||||
| TiO2 | 100 | [DOX]0 = 2 mg/L | [107] | ||
| UV lamp 365 nm, 13 W | |||||
| BiFeO3 | 79 | [DOX]0 = 2 mg/L; UV radiation | [116] | ||
| Cu-A | 100 | [DOX]0 = 30 mg/L; Sunlight | [111] | ||
| H2O2~1% volume | |||||
| 2 mg Cat added | |||||
| PAN 8/CEA 9/MIL-125/TiO2 | 100 | [DOX]0 = 50 mg/L [Cat] = 500 mg/L | [117] | ||
| UV lamp 365 nm, 30 W pH = 3 | |||||
| TiO2 | 62 | Xe visible lamp 300 W pH = 8 | [118] | ||
| Pt/TiO2 | 88 | 30 mg Cat added | |||
| Sonication added | |||||
| GO | 44 | [DOX]0 = 0.5 mM | [112] | ||
| CeO2 | 67 | pH = 7 | |||
| GO-CeO2 | 97 | LED light 9 W–800 lumens | |||
| BCN 10 | 9.2 | [DOX]0 = 10 mg/L | [109] | ||
| PCN 11 | 59.8 | 0.04 g Cat added | |||
| CPCN 12 | 65.3 | pH = 8 | |||
| NOCN-1 13 | 96.2 | Reactor LabSolar 6a | |||
| NOCN-2 14 | 98.6 | Metal halide lamp 500 W | |||
| NOCN-3 15 | 96.6 | Radiation 30 mW/cm2 | |||
| CN 16 | 40.86 | [Cat] = 25 mg/L pH = 2.5 Mercury lamp (λ > 420 nm) H2O2 100 µL added | [110] | ||
| SnO2 | 20.22 | ||||
| CoFe2O4 | 38.62 | ||||
| CSn-20 | 64.05 | ||||
| CSnCo-0.5 | 94.95 | ||||
| CSnCo-1 | 90.29 | ||||
| CSnCo-2 | 90.24 | ||||
| EPI | TiO2 | 98 | [EPI]0 = 2 mg/L | [107] | |
| UV lamp 365 nm, 13 W | |||||
| CNO | 36.4 | [EPI]0 = 10 mg/L; [Cat] = 150 mg/L | [113] | ||
| CNO/MoS2 | 72.4 | Visible light | |||
| CNO/MoS2/Ag | 99.5 | pH = 7 | |||
| MXT | APEC Si/H2O2 | 14 | [MXT]0 = 20 mg/L | [114] | |
| APEC CuO/Si | 14 | Ag/AgCl reference electrode | |||
| APC CuO/Si/H2O2 | 50 | UV-lamp 18 W, 350–400 nm | |||
| APEC CuO/Si/H2O2 | 75 | Potential 1.5 V | |||
| TiO2 | 93.6 | [MXT]0 = 2 mg/L; [Cat] = 500 mg/L | [119] | ||
| Lamp Narva 365 nm, 13 W | |||||
| MIT | TiO2 P25 | 100 | [MIT]0 = 20 mg/L; [Cat] = 200 mg/L | [120] | |
| Lamp TLK/05, 40 W/m2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Burciaga, L.A.; Silerio-Vázquez, F.d.J.; Antileo, C.; Rosales-Castro, M.; Núñez-Núñez, C.M.; Proal-Nájera, J.B. A Review on Cytotoxic Antibiotics: Occurrence in Water Matrices, Degradation by Advanced Oxidation Processes, and By-Product Formation. Water 2025, 17, 628. https://doi.org/10.3390/w17050628
González-Burciaga LA, Silerio-Vázquez FdJ, Antileo C, Rosales-Castro M, Núñez-Núñez CM, Proal-Nájera JB. A Review on Cytotoxic Antibiotics: Occurrence in Water Matrices, Degradation by Advanced Oxidation Processes, and By-Product Formation. Water. 2025; 17(5):628. https://doi.org/10.3390/w17050628
Chicago/Turabian StyleGonzález-Burciaga, Luis A., Felipe de J. Silerio-Vázquez, Christian Antileo, Martha Rosales-Castro, Cynthia M. Núñez-Núñez, and José B. Proal-Nájera. 2025. "A Review on Cytotoxic Antibiotics: Occurrence in Water Matrices, Degradation by Advanced Oxidation Processes, and By-Product Formation" Water 17, no. 5: 628. https://doi.org/10.3390/w17050628
APA StyleGonzález-Burciaga, L. A., Silerio-Vázquez, F. d. J., Antileo, C., Rosales-Castro, M., Núñez-Núñez, C. M., & Proal-Nájera, J. B. (2025). A Review on Cytotoxic Antibiotics: Occurrence in Water Matrices, Degradation by Advanced Oxidation Processes, and By-Product Formation. Water, 17(5), 628. https://doi.org/10.3390/w17050628










