Fluoride in Drinking Water and Tea in the Arusha Region of Tanzania
Abstract
1. Introduction
2. Materials and Methods
2.1. Water Supply
2.2. Household Surveys
2.3. Tea
3. Results and Discussion
3.1. Water Quality
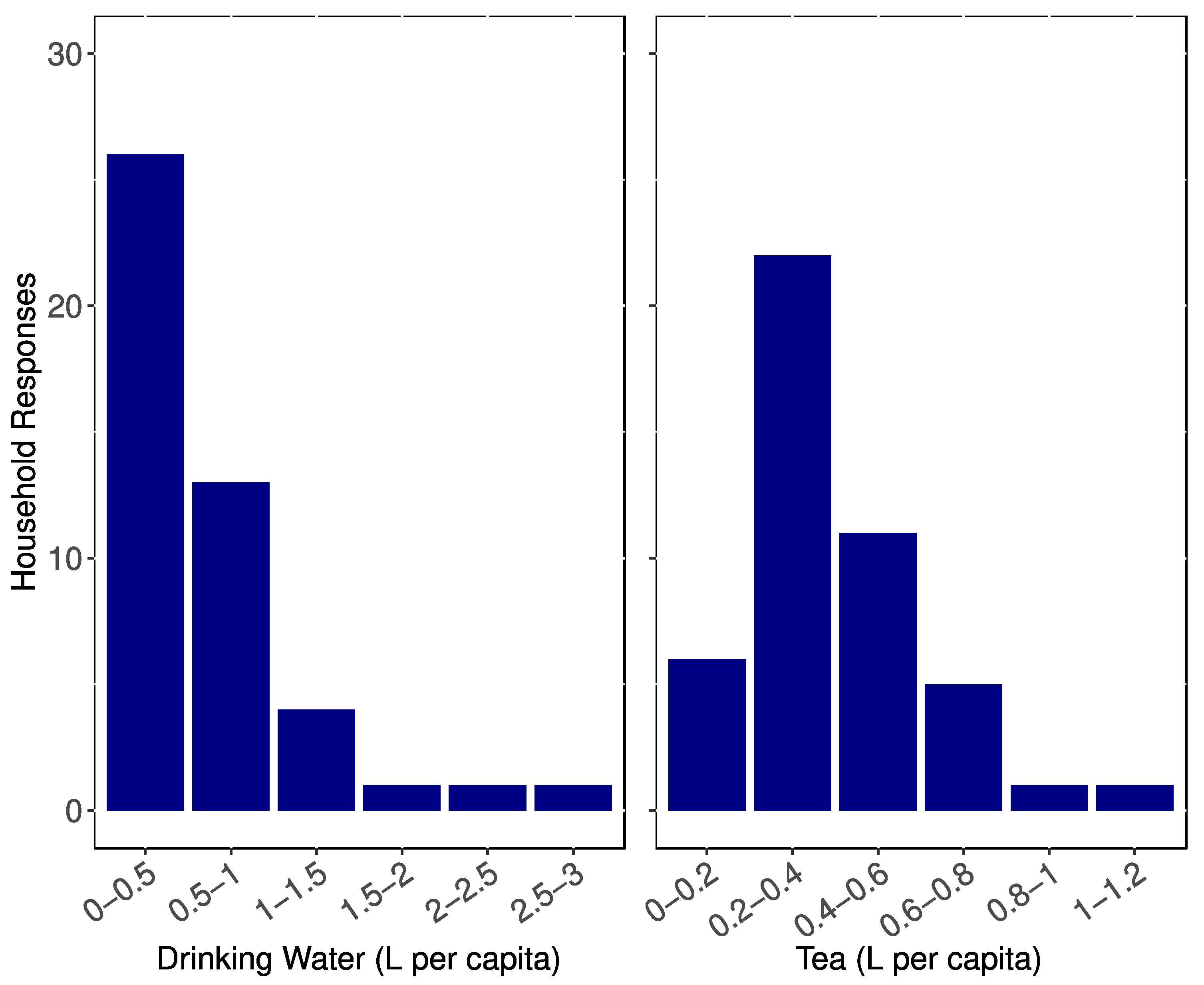
3.2. Water Consumption
3.3. Tea
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDHHS, U.S. Public Health Service Recommendation for Fluoride Concentration in Drinking Water for the Prevention of Dental Caries; Association of Schools of Public Health: Washington, DC, USA, 2015; Volume 130, pp. 318–331. [Google Scholar]
- Jagtap, S.; Yenkie, M.K.; Labhsetwar, N.; Rayalu, S. Fluoride in Drinking Water and Defluoridation of Water. Chem. Rev. 2012, 112, 2454–2466. [Google Scholar] [CrossRef] [PubMed]
- Ayoob, S.; Gupta, A.K. Fluoride in Drinking Water: A Review on the Status and Stress Effects. Crit. Rev. Environ. Sci. Technol. 2006, 36, 433–487. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; World Health Organization: Geneva, Switzerland, 2017; p. 631. [Google Scholar]
- Aulestia, F.J.; Groeling, J.; Bomfim, G.H.S.; Costiniti, V.; Manikandan, V.; Chaloemtoem, A.; Concepcion, A.R.; Li, Y.; Wagner, L.E.; Idaghdour, Y.; et al. Fluoride Exposure Alters Ca2+ Signaling and Mitochondrial Function in Enamel Cells. Sci. Signal. 2020, 13, eaay0086. [Google Scholar] [CrossRef] [PubMed]
- Solanki, Y.S.; Agarwal, M.; Gupta, A.B.; Gupta, S.; Shukla, P. Fluoride Occurrences, Health Problems, Detection, and Remediation Methods for Drinking Water: A Comprehensive Review. Sci. Total Environ. 2022, 807, 150601. [Google Scholar] [CrossRef]
- Pramanik, S.; Saha, D. The Genetic Influence in Fluorosis. Environ. Toxicol. Pharmacol. 2017, 56, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Sellami, M.; Riahi, H.; Maatallah, K.; Ferjani, H.; Bouaziz, M.C.; Ladeb, M.F. Skeletal Fluorosis: Don’t Miss the Diagnosis! Skelet. Radiol. 2020, 49, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Podgorski, J.; Berg, M. Global Analysis and Prediction of Fluoride in Groundwater. Nat. Commun. 2022, 13, 4232. [Google Scholar] [CrossRef]
- Meng, X.; Li, H.; Liu, X.; Li, B.; Liu, Y.; Li, M.; Sun, D.; Yang, Y.; Gao, Y.; Pei, J. Drinking Brick Tea Containing High Fluoride Increases the Prevalence of Osteoarthritis in Tibetan, China. Int. J. Environ. Health Res. 2024, 34, 3760–3770. [Google Scholar] [CrossRef] [PubMed]
- Onipe, T.; Edokpayi, J.N.; Odiyo, J.O. A Review on the Potential Sources and Health Implications of Fluoride in Groundwater of Sub-Saharan Africa. J. Environ. Sci. Health Part A 2020, 55, 1078–1093. [Google Scholar] [CrossRef] [PubMed]
- Smedley, P.L.; Nkotagu, H.; Pellig-Ba, K.; MacDonald, A.M.; Tyler-Whittle, R.; Whitehead, E.J.; Kinniburgh, D.G. Fluoride in Groundwater from High-Fluoride Areas of Ghana and Tanzania; Fluoride in Groundwater from High-Fluoride Areas of Ghana and Tanzania: Nottingham, UK, 2002; p. 72. [Google Scholar]
- Kut, K.M.K.; Sarswat, A.; Srivastava, A.; Pittman, C.U.; Mohan, D. A Review of Fluoride in African Groundwater and Local Remediation Methods. Groundw. Sustain. Dev. 2016, 2–3, 190–212. [Google Scholar] [CrossRef]
- Malinowska, E.; Inkielewicz, I.; Czarnowski, W.; Szefer, P. Assessment of Fluoride Concentration and Daily Intake by Human from Tea and Herbal Infusions. Food Chem. Toxicol. 2008, 46, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Kaushik, C.P.; Haritash, A.K.; Singh, B.; Raghuvanshi, S.P.; Kansal, A. Determination of Exposure and Probable Ingestion of Fluoride Through Tea, Toothpaste, Tobacco and Pan Masala. J. Hazard. Mater. 2007, 142, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Karak, T.; Bhagat, R.M. Trace Elements in Tea Leaves, Made Tea and Tea Infusion: A Review. Food Res. Int. 2010, 43, 2234–2252. [Google Scholar] [CrossRef]
- Długaszek, M.; Kaszczuk, M. Assessment of the Nutritional Value of Various Teas Infusions in Terms of the Macro- and Trace Elements Content. J. Trace Elem. Med. Biol. 2020, 59, 126428. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Mehra, A.; Saikat, S.; Lynch, P. Human Exposure Assessment of Fluoride from Tea (Camellia sinensis L.): A UK Based Issue? Food Res. Int. 2013, 51, 564–570. [Google Scholar] [CrossRef]
- Wong, M.H.; Fung, K.F.; Carr, H.P. Aluminium and Fluoride Contents of Tea, with Emphasis on Brick Tea and Their Health Implications. Toxicol. Lett. 2003, 137, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.; Shaw, L.; Smith, A.J. The Bio-Availability of Fluoride from Black Tea. J. Dent. 2001, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Chandrajith, R.; Abeypala, U.; Dissanayake, C.B.; Tobschall, H.J. Fluoride in Ceylon Tea and Its Implications to Dental Health. Environ. Geochem. Health 2007, 29, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Gardner, E.J.; Ruxton, C.H.S.; Leeds, A.R. Black Tea—Helpful or Harmful? A Review of the Evidence. Eur. J. Clin. Nutr. 2007, 61, 3–18. [Google Scholar] [CrossRef]
- Shen, C.-L.; Yeh, J.K.; Cao, J.J.; Wang, J.-S. Green Tea and Bone Metabolism. Nutr. Res. 2009, 29, 437–456. [Google Scholar] [CrossRef]
- Fung, K.F.; Zhang, Z.Q.; Wong, J.W.C.; Wong, M.H. Fluoride Contents in Tea and Soil from Tea Plantations and the Release of Fluoride into Tea Liquor During Infusion. Environ. Pollut. 1999, 104, 197–205. [Google Scholar] [CrossRef]
- Yi, J.; Cao, J. Tea and Fluorosis. J. Fluor. Chem. 2008, 129, 76–81. [Google Scholar] [CrossRef]
- Pattaravisitsate, N.; Phetrak, A.; Denpetkul, T.; Kittipongvises, S.; Kuroda, K. Effects of Brewing Conditions on Infusible Fluoride Levels in Tea and Herbal Products and Probabilistic Health Risk Assessment. Sci. Rep. 2021, 11, 14115. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms; EPA: Washington, DC, USA, 2002; p. 275.
- LaPierre, R. Bigelow Tea Response—Ref#000217704A 2019.
- Kitalika, A.J.; Machunda, R.L.; Komakech, H.C.; Njau, K.N. Fluoride Variations in Rivers on the Slopes of Mount Meru in Tanzania. J. Chem. 2018, 2018, 7140902. [Google Scholar] [CrossRef]
- Bakar, S.; Kahler, D.M.; Bachtlin, A.; Okular, K.; Glancey, K.; Whitewood, A. Assessment of the Fluoride Concentration in Drinking Water and Tea in The Arusha Region of Tanzania. In Duquesne Scholarship Collection; Duquesne University: Pittsburgh, PA, USA, 2021. [Google Scholar]
- Koblar, A.; Tavčar, G.; Ponikvar-Svet, M. Fluoride in Teas of Different Types and Forms and the Exposure of Humans to Fluoride with Tea and Diet. Food Chem. 2012, 130, 286–290. [Google Scholar] [CrossRef]
- Kahler, D.M. Olkokola Meteorological Station 2019. Mendeley Data, V2. [CrossRef]


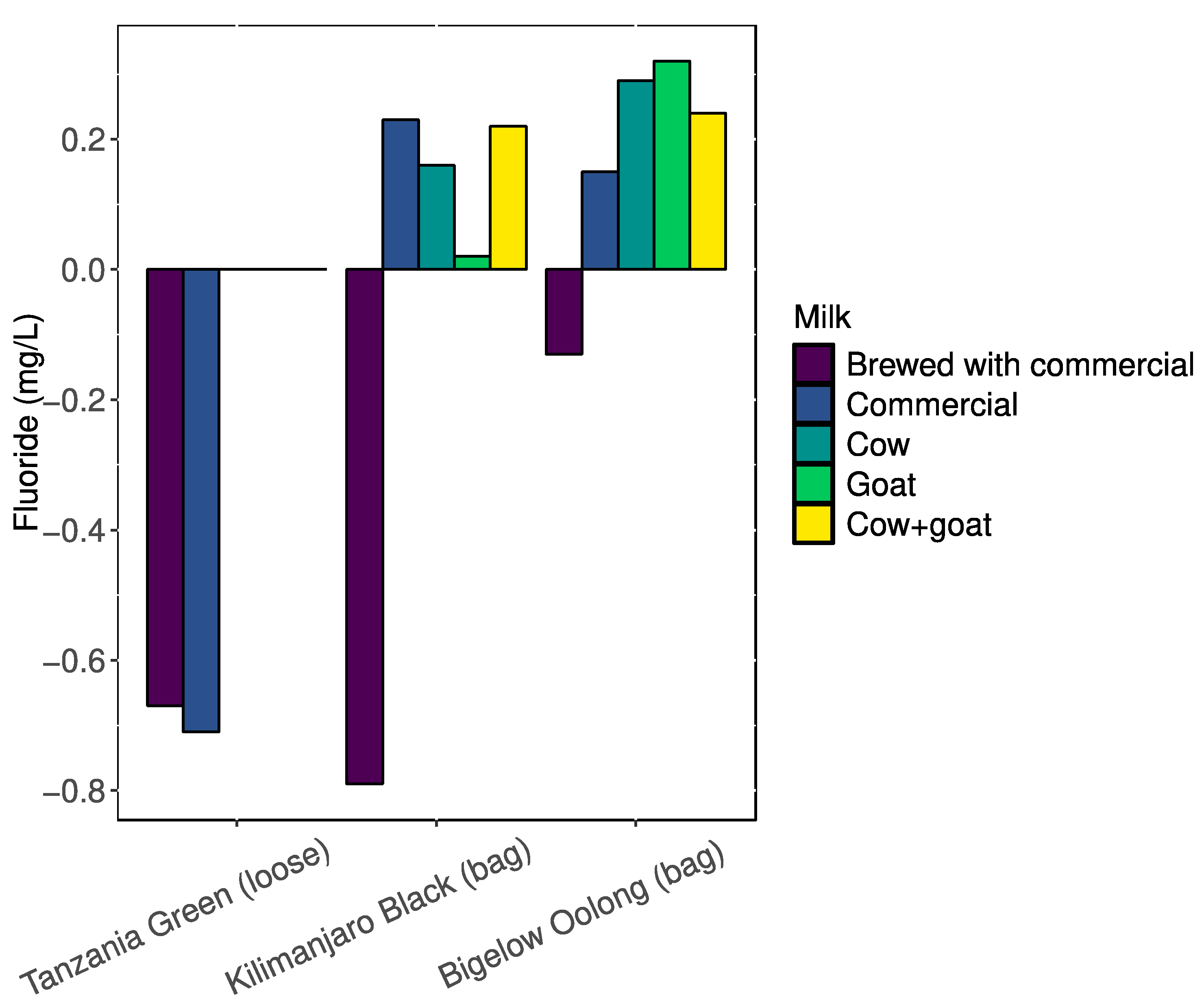
| Milk Sample | Deionized Water | Synthetic Groundwater | ||
|---|---|---|---|---|
| Fluoride (mg/L) | Standard Error | Fluoride (mg/L) | Standard Error | |
| Cow | 0.59 | 0.01 | 0.69 | 0.00 |
| Goat | 0.68 | 0.00 | 0.69 | 0.00 |
| Cow + Goat | 0.72 | 0.00 | 0.68 | 0.00 |
| Commercial | 0.69 | 0.00 | 0.66 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakar, S.A.; Whitewood, A.; Glancey, K.; Okular, K.; Bachtlin, A.; Kahler, D.M. Fluoride in Drinking Water and Tea in the Arusha Region of Tanzania. Water 2025, 17, 546. https://doi.org/10.3390/w17040546
Bakar SA, Whitewood A, Glancey K, Okular K, Bachtlin A, Kahler DM. Fluoride in Drinking Water and Tea in the Arusha Region of Tanzania. Water. 2025; 17(4):546. https://doi.org/10.3390/w17040546
Chicago/Turabian StyleBakar, Sophia A., Abigail Whitewood, Kathleen Glancey, Kara Okular, Alanna Bachtlin, and David M. Kahler. 2025. "Fluoride in Drinking Water and Tea in the Arusha Region of Tanzania" Water 17, no. 4: 546. https://doi.org/10.3390/w17040546
APA StyleBakar, S. A., Whitewood, A., Glancey, K., Okular, K., Bachtlin, A., & Kahler, D. M. (2025). Fluoride in Drinking Water and Tea in the Arusha Region of Tanzania. Water, 17(4), 546. https://doi.org/10.3390/w17040546





