Road Salt Collection and Redistribution at an Urban Rain Garden on Sandy Soil, Gary, Indiana
Abstract
1. Introduction
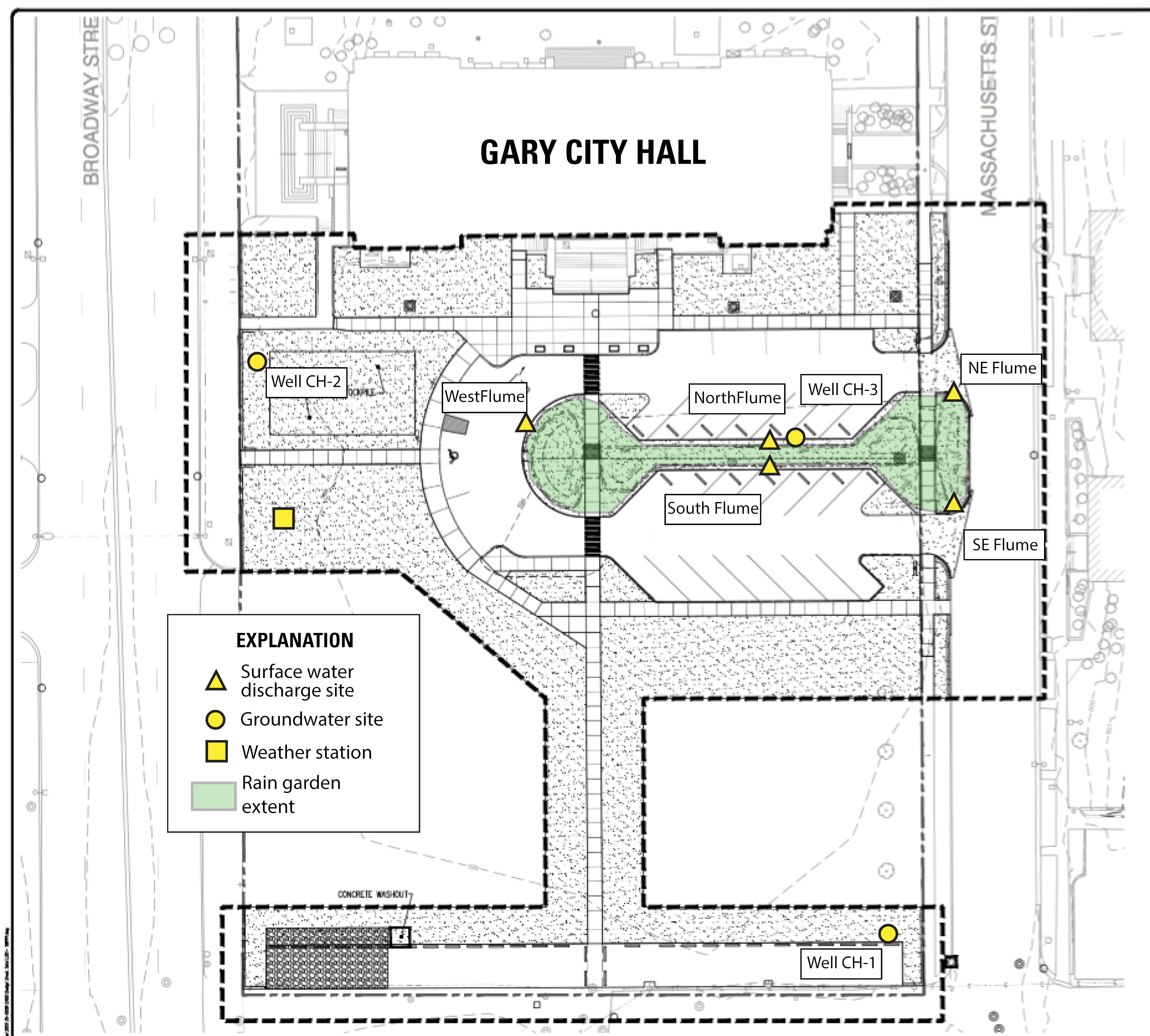
2. Study Area
3. Materials and Methods
3.1. Quality Assurance Methods
3.2. Computing Road Salt Collection from Discrete Chloride Concentrations, Continuous Specific Conductance Data, and Flume Discharges
- a = the slope of the linear regression relation;
- b = the value of chloride (in mg/L) when specific conductance (in μS/cm) equals zero.
- Σ is the sum of NaCl estimated for all sequential periods of continuous specific conductance and chloride measurements, in kg;
- Cl is the mean concentration of chloride estimated from continuous specific conductance over time t, in mg/L;
- 58.443 is the atomic weight of NaCl, in g/mol;
- 35.445 is the atomic weight of Cl, in g/mol;
- 28.317 is a unit-conversion factor, in L/feet3;
- Q is discharge, in feet3/s;
- t is the time increment between successive specific conductance and flume discharge measurements, in s;
- 1/1000 is a unit conversion factor, kg/mg.
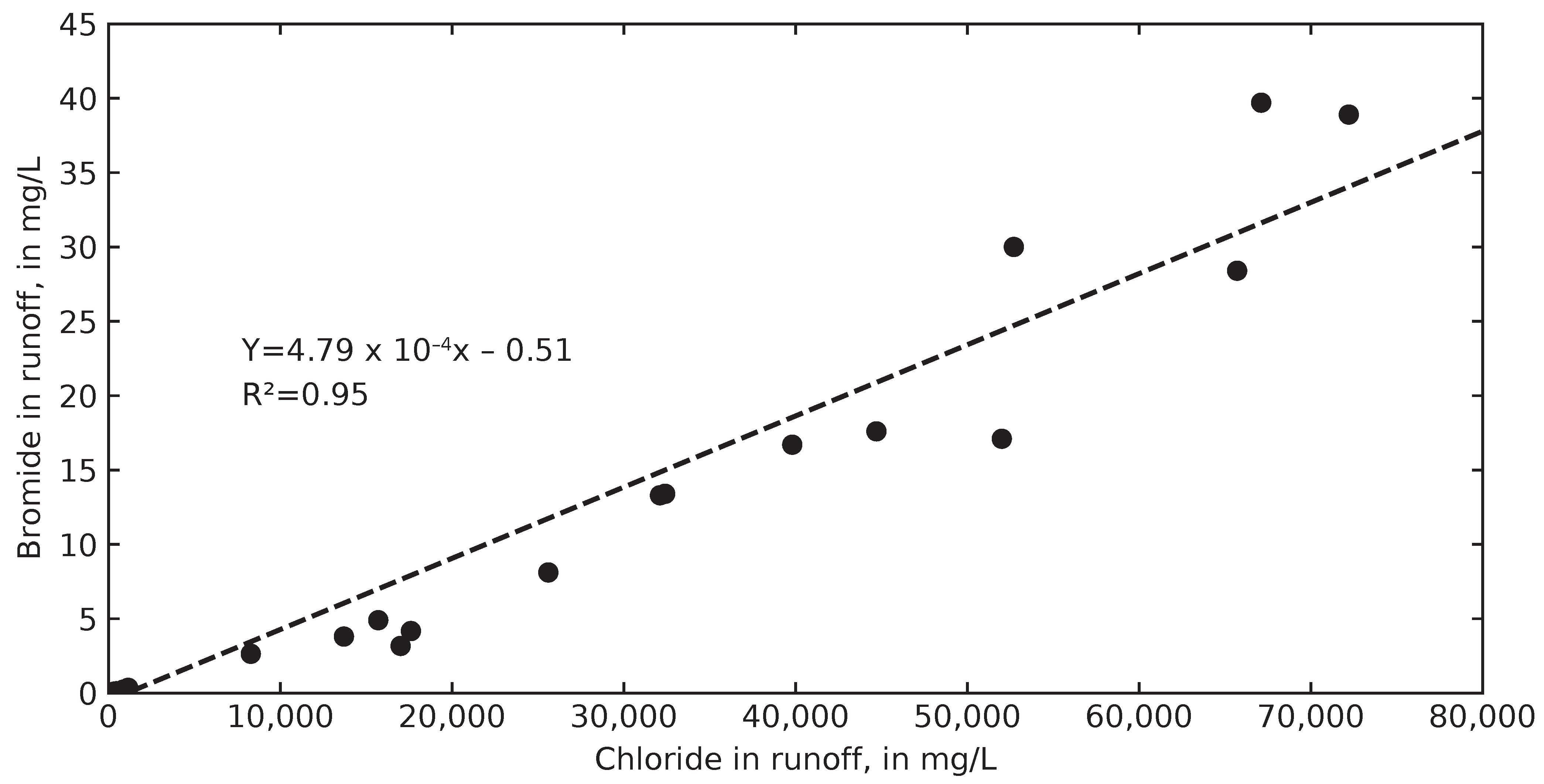
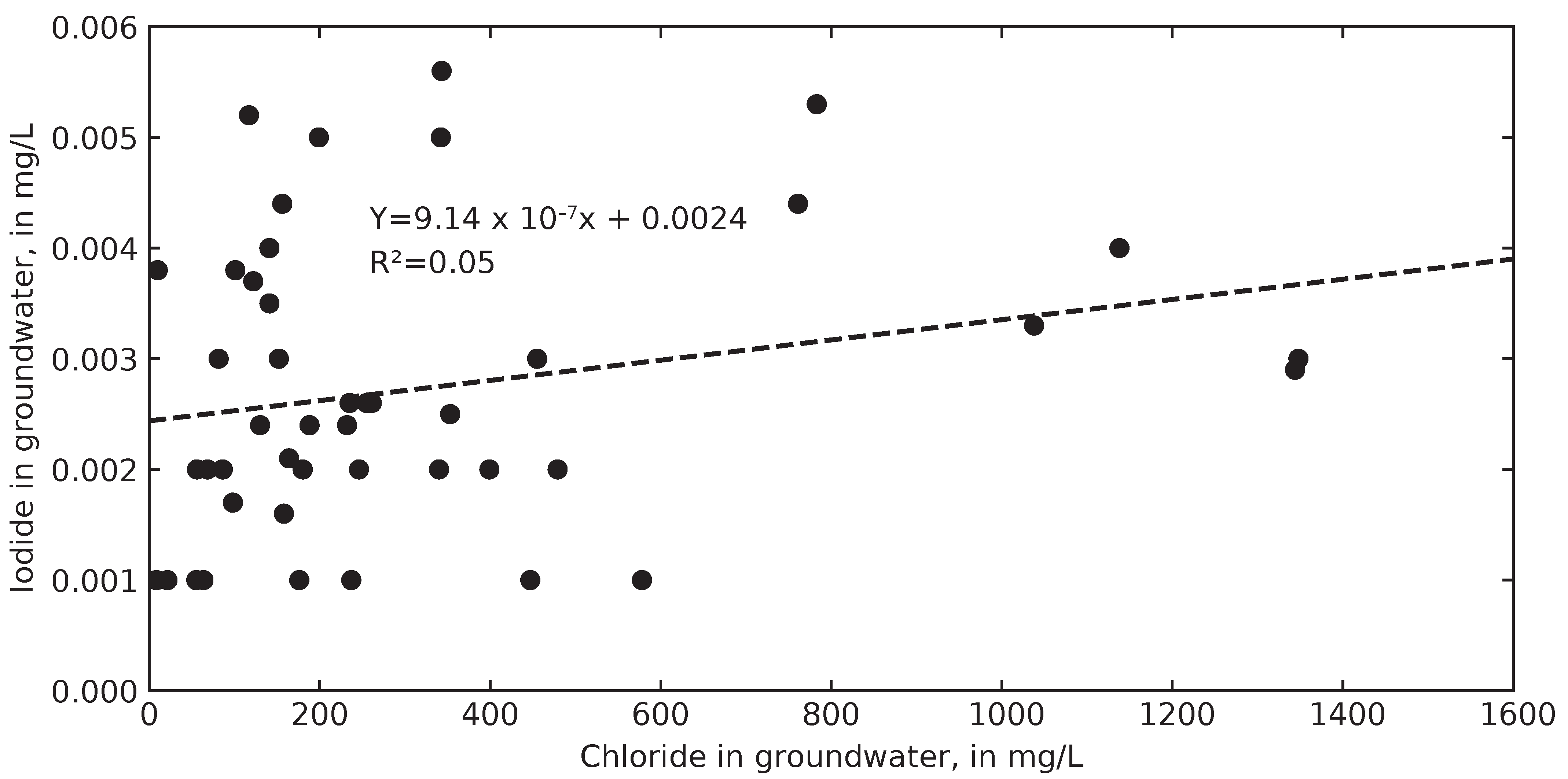
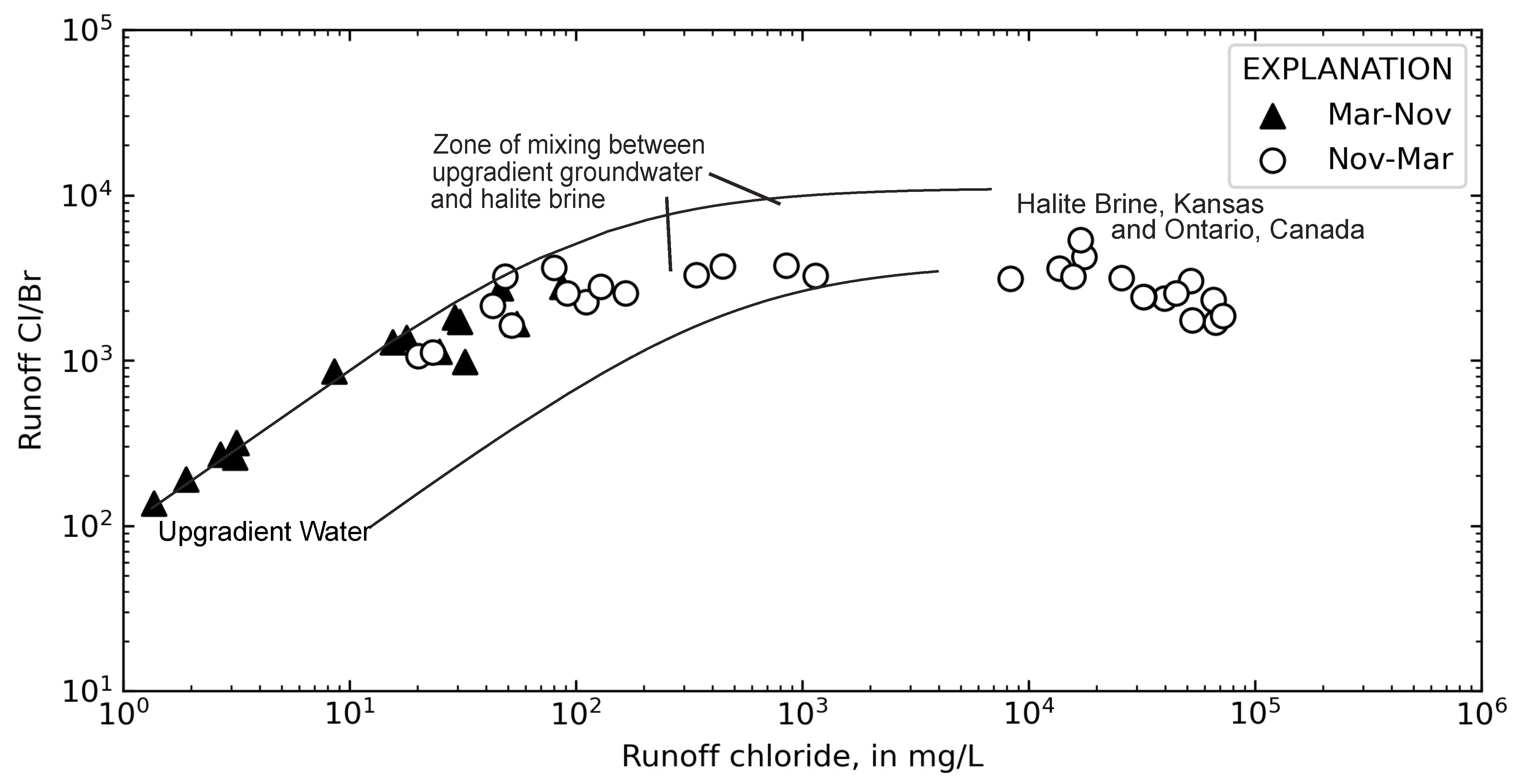
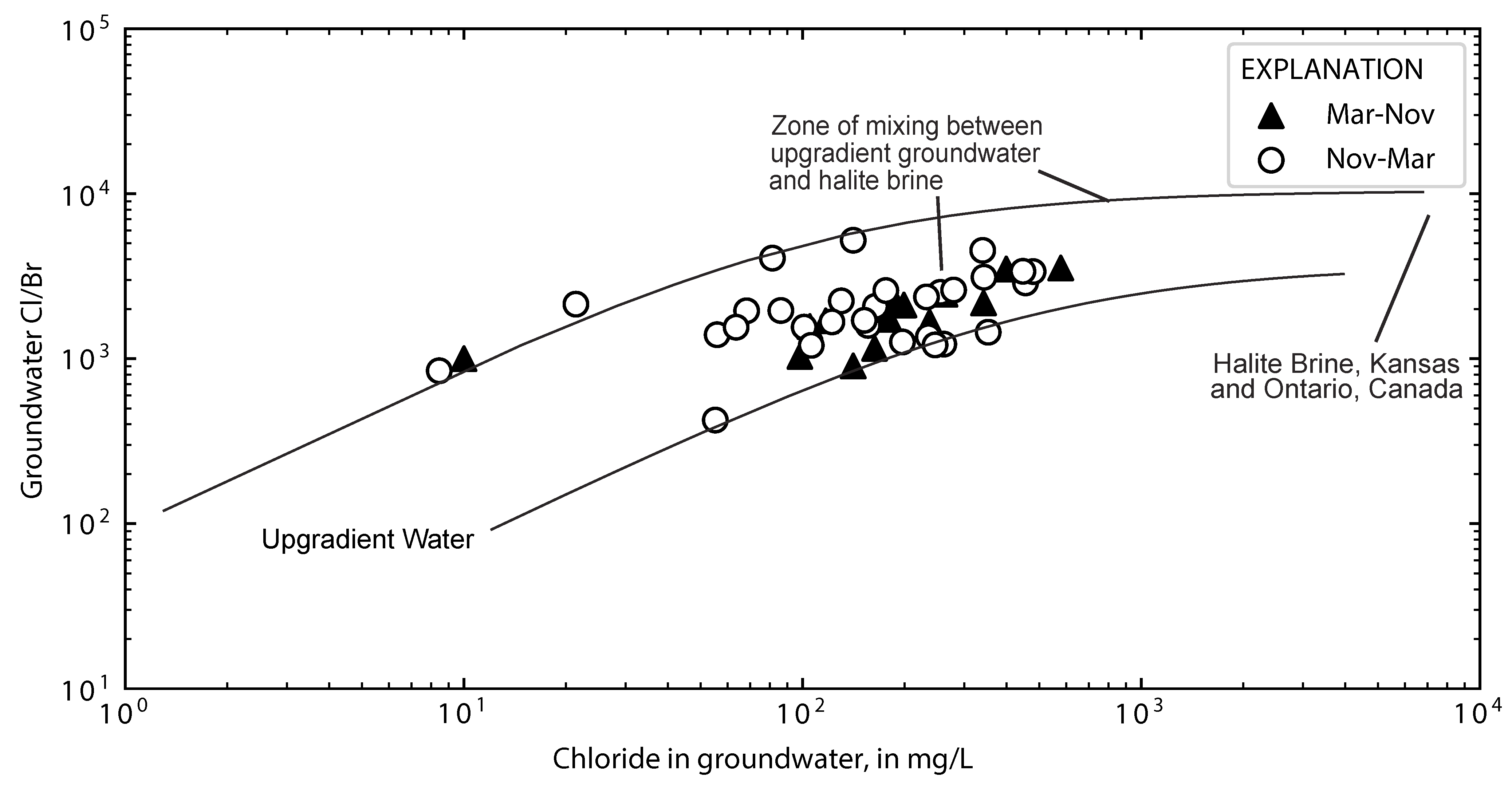
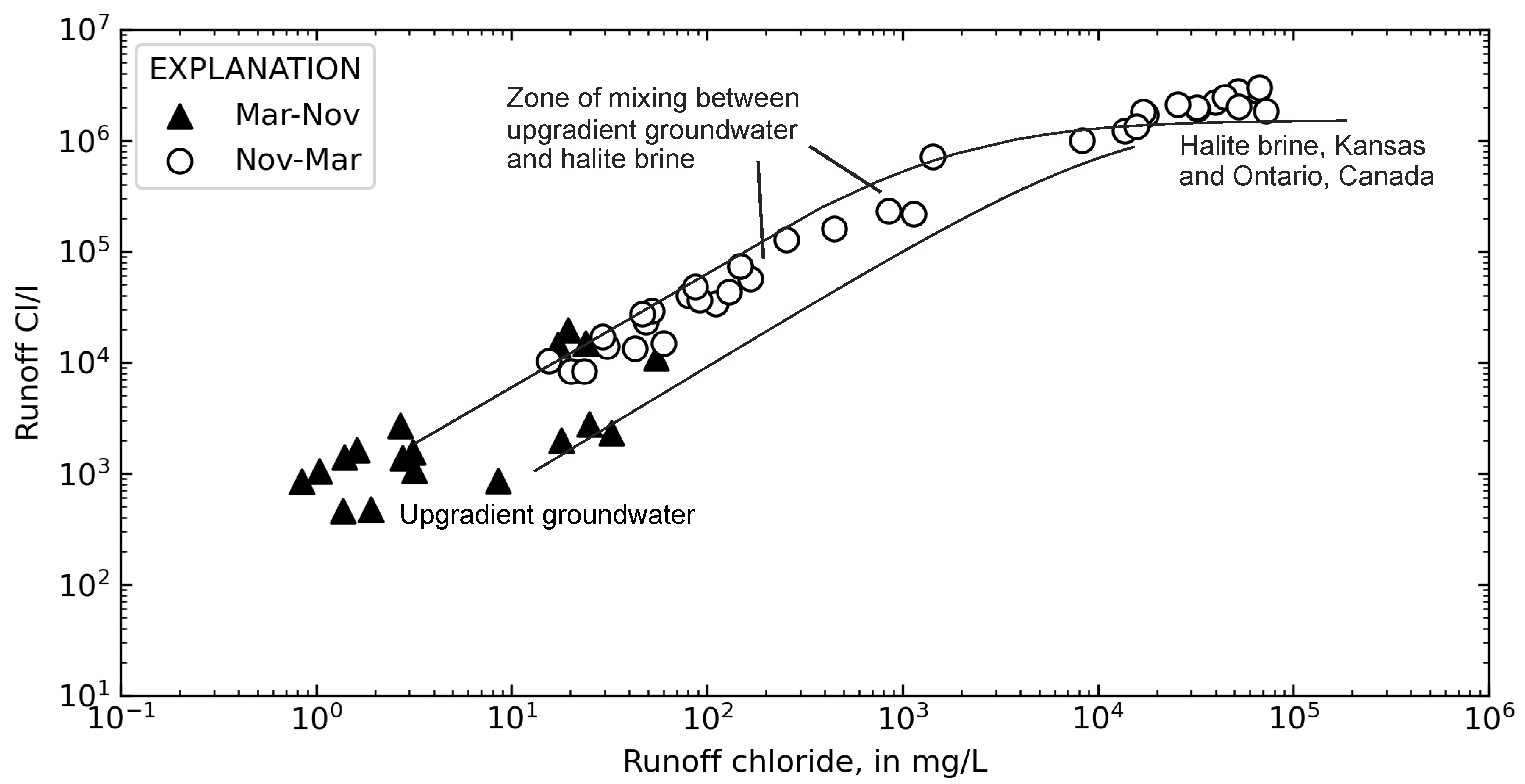
3.3. Inferring Chloride Sources from Ion Ratios
4. Results
4.1. Deicer Composition
- Ca0.23 Mg0.64 Na99.05 K0.08 Cl 99.79 (SO4)0.20;
- Ca0.18 Mg0.64 Na99.09 K0.10 Cl99.77 (SO4)0.23;
- Ca0.12 Mg0.63 Na99.17 K0.10 Cl99.78 (SO4)0.23.
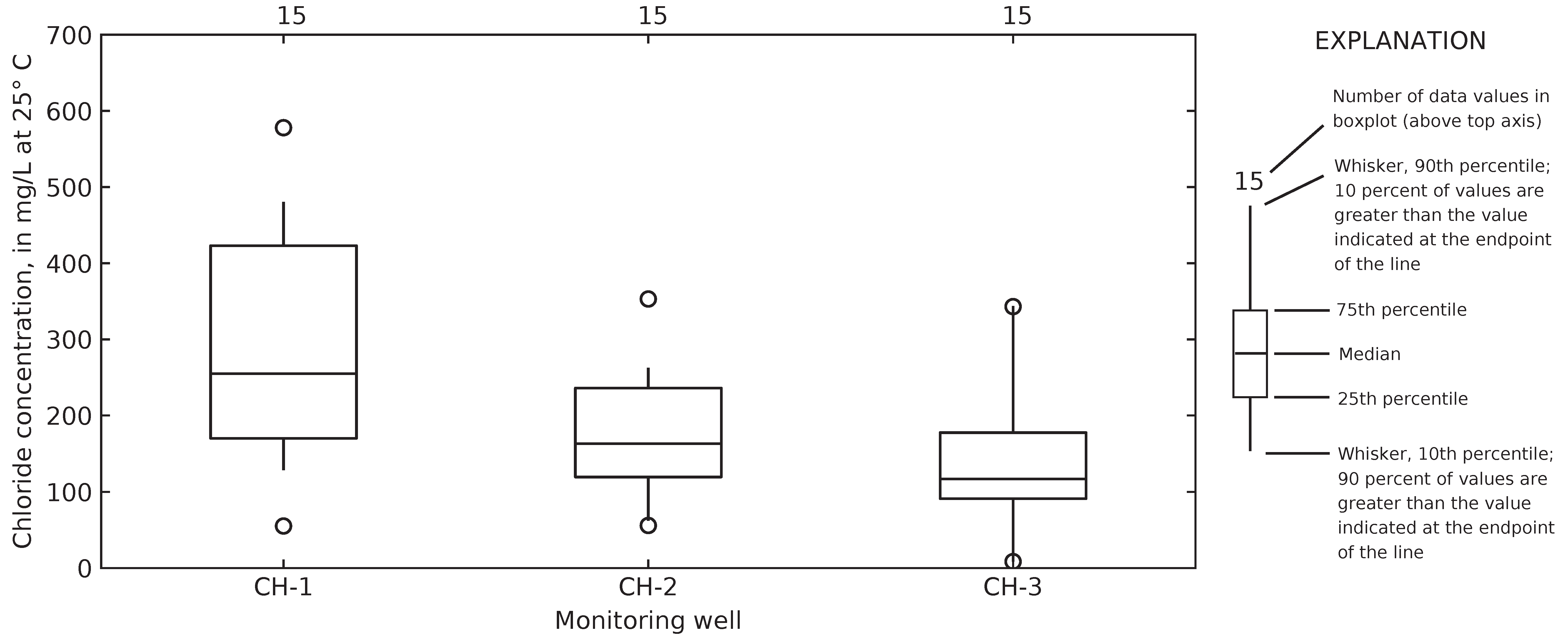
4.2. Chloride Concentrations in Groundwater and Runoff
4.3. Temporal Sources of Chloride
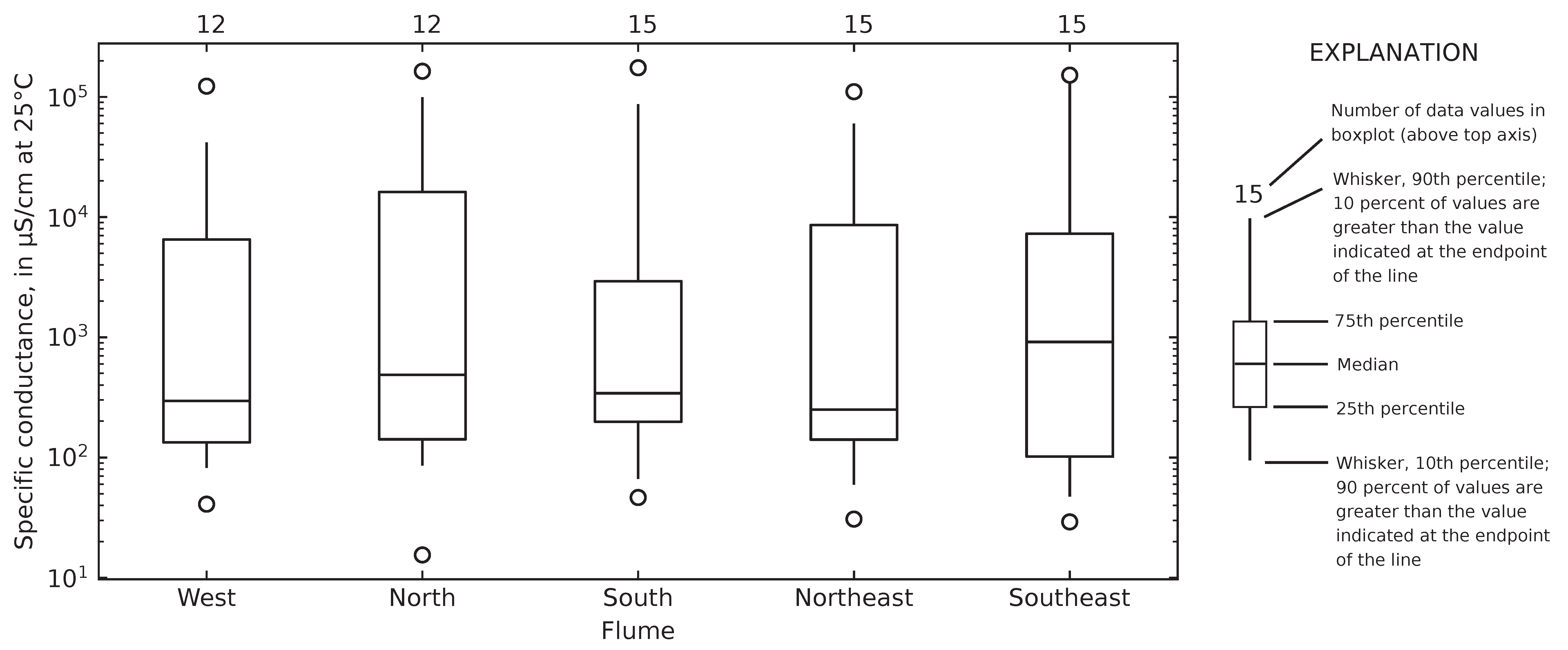
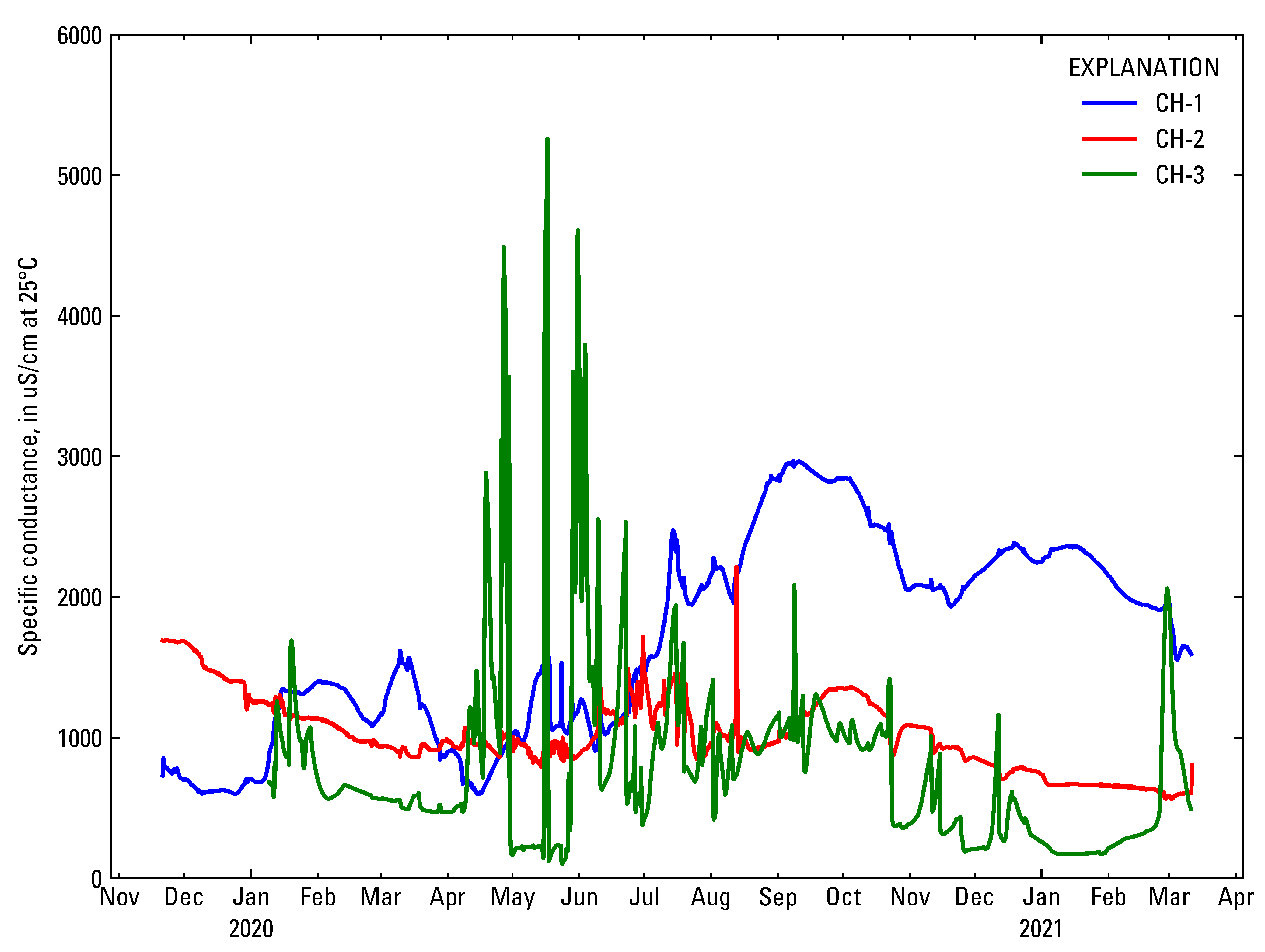
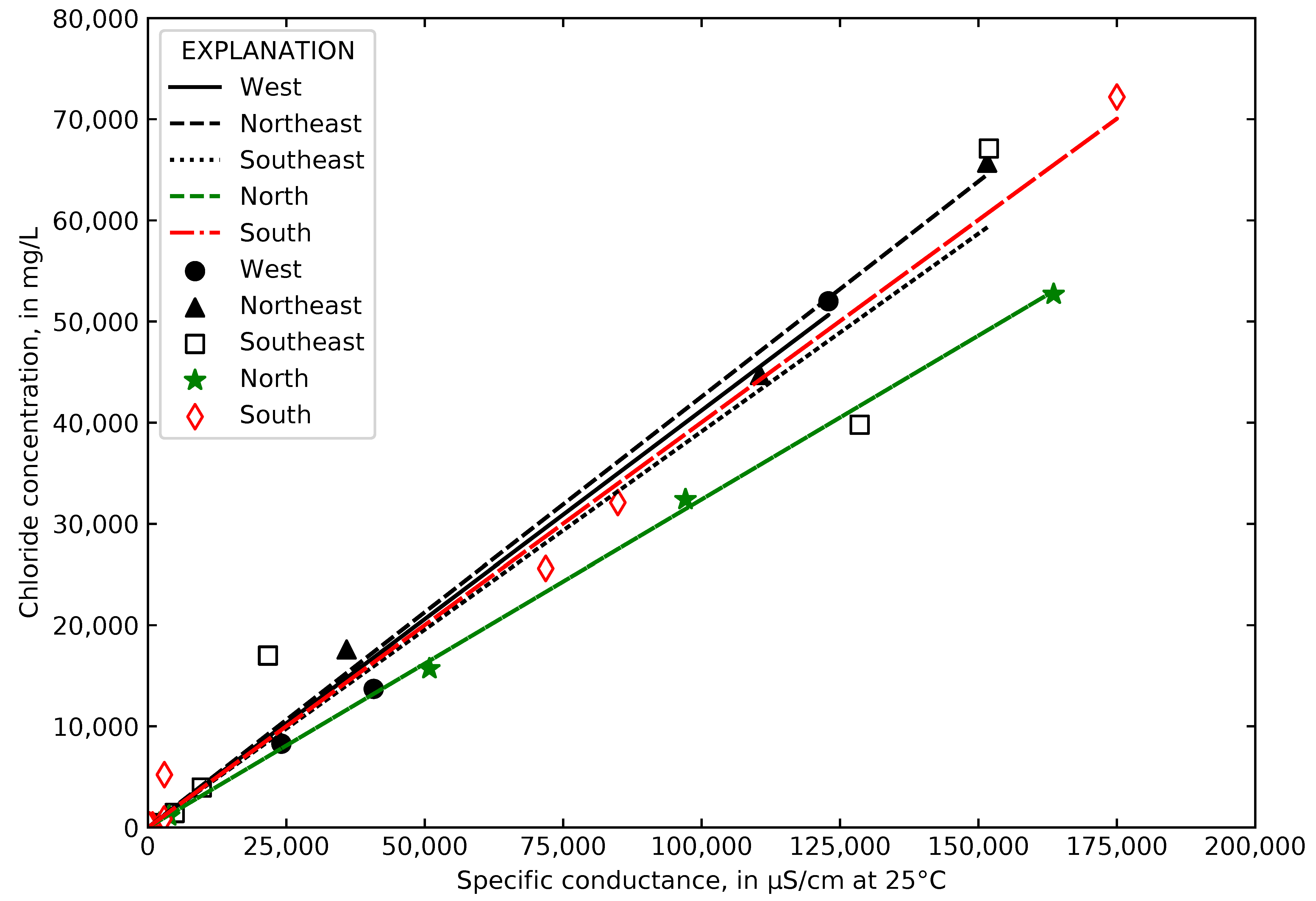
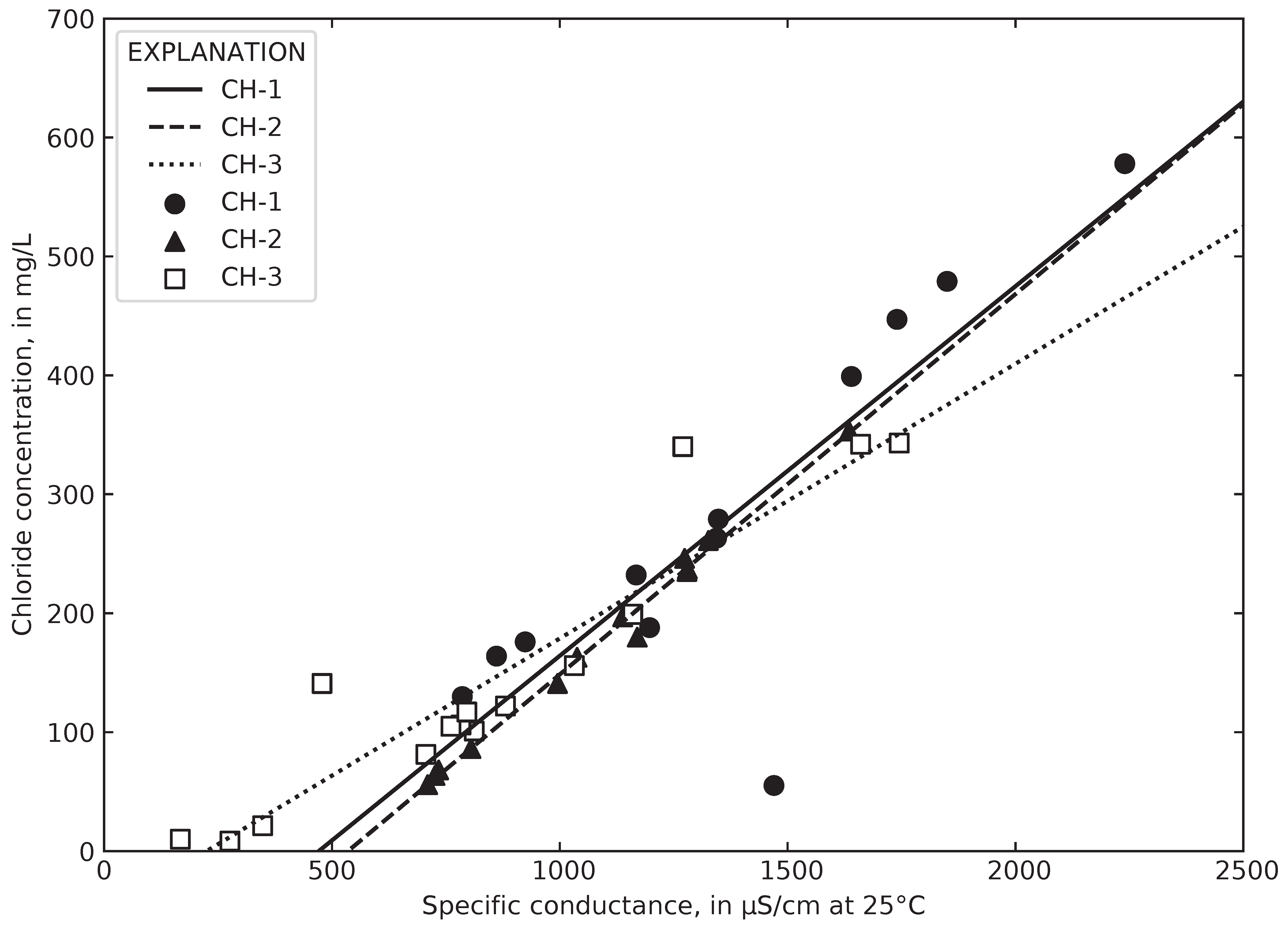
4.4. Relating Chloride to Specific Conductance at Gary City Hall
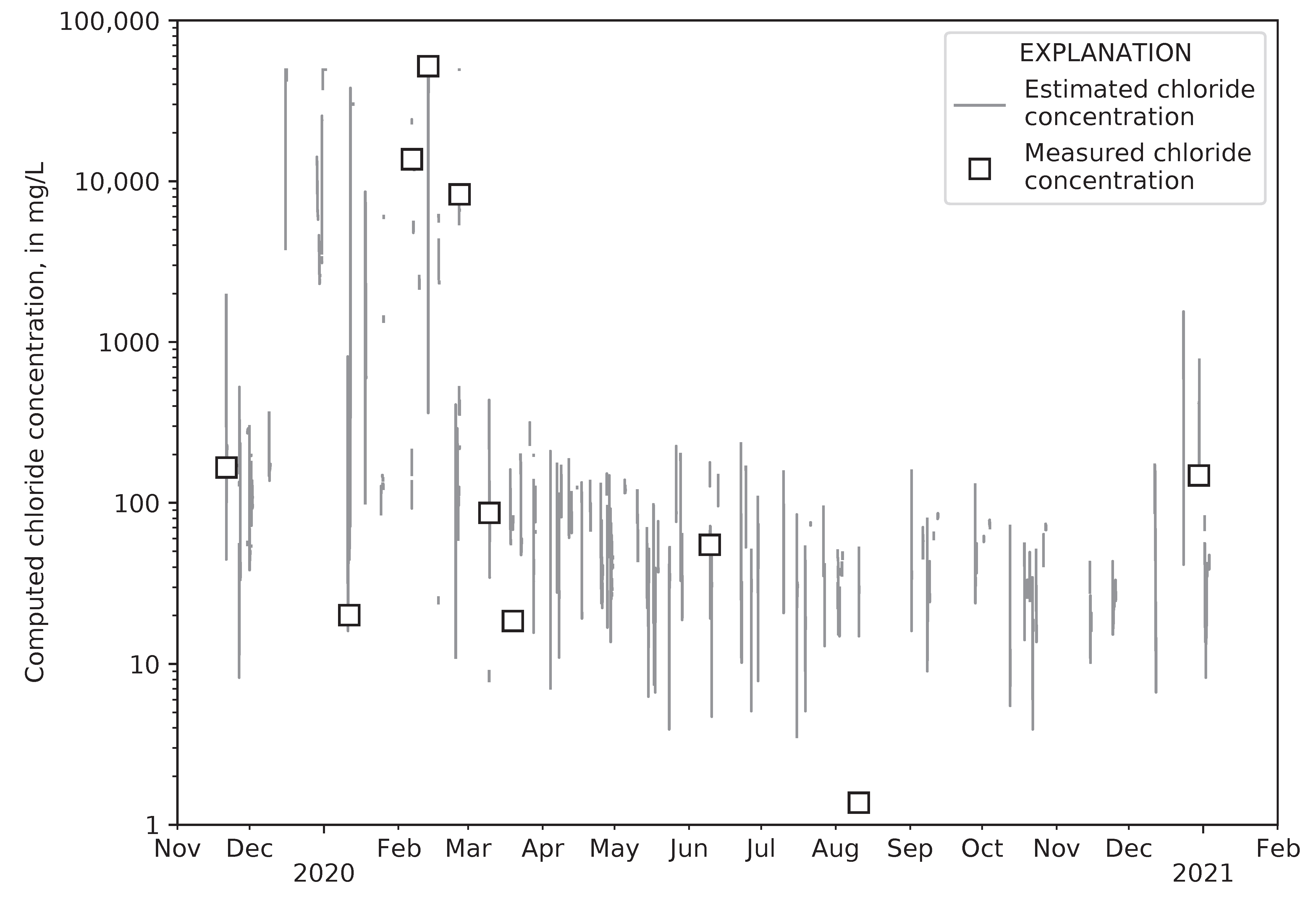
4.5. Computed Chloride Concentrations and Total Road Salt Mass in Collected Runoff
4.6. Computed Chloride Concentrations in Groundwater
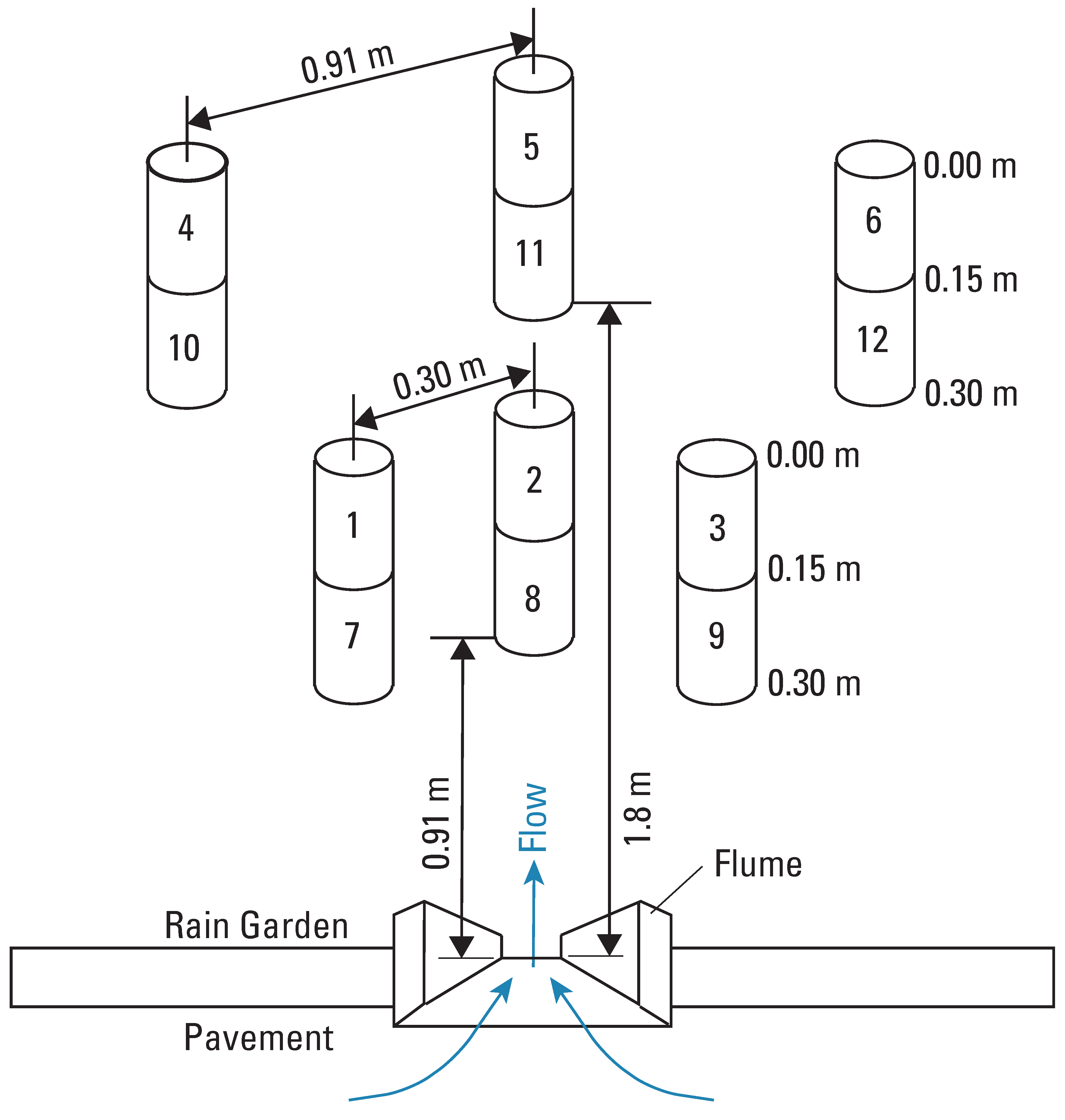
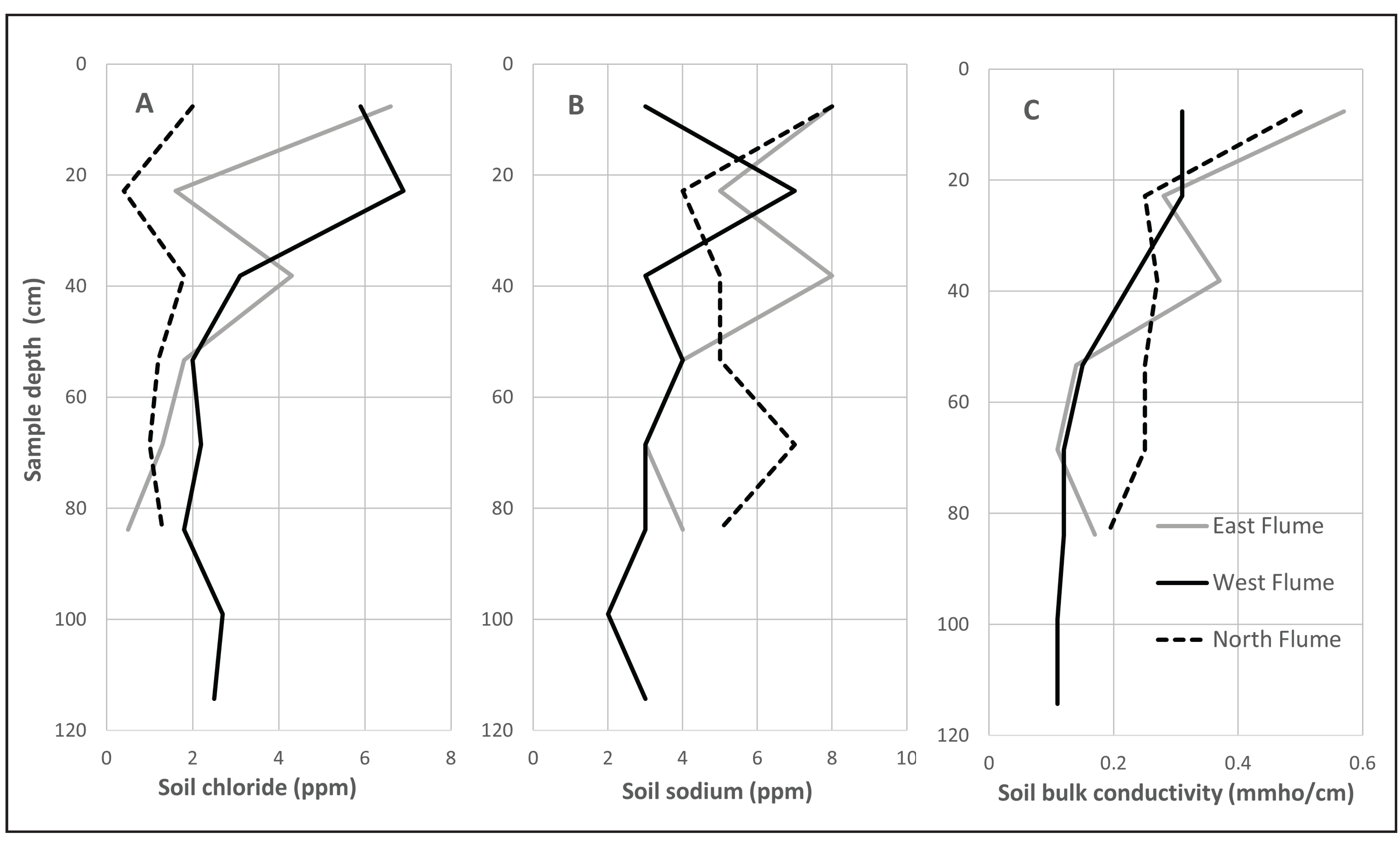
4.7. Soil Chemistry
4.8. Soil Permeability
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, N.T.; Sullivan, D.J.; Selbig, W.R.; Haefner, R.J.; Lampe, D.C.; Bayless, E.R.; McHale, M.R. Green Infrastructure in the Great Lakes—Assessment of Performance, Barriers, and Unintended Consequences; U.S. Geological Survey Circular 1496; U.S. Geological Survey: Reston, VA, USA, 2022; p. 70. [CrossRef]
- Lindsey, B.D.; Cravotta, C.A.; Szabo, Z.; Belitz, K.; Stackelberg, P. Relation between road-salt application and increasing radium concentrations in a low-pH aquifer, southern New Jersey. ACS EST Water 2021, 1, 2541–2547. [Google Scholar] [CrossRef]
- McQuiggan, R.; Andres, S.A.; Roros, A.; Sturchio, N.C. Stormwater drives seasonal geochemical processes beneath an infiltration basin. J. Environ. Qual. 2022, 51, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Haefner, R.J.; Hoard, C.J.; Shuster, W. Hydrologic Study of Green Infrastructure in Poorly Drained Urbanized Soils at RecoveryPark, Detroit, Michigan, 2014–21; U.S. Geological Survey Scientific Investigations Report 2024–5018; U.S. Geological Survey: Reston, VA, USA, 2024; p. 29. [CrossRef]
- Watson, L.R.; Bayless, E.R.; Buska, P.M.; Wilson, J.T. Effects of Highway-Deicer Application on Ground-Water Quality in a Part of the Calumet Aquifer, Northwestern Indiana; U.S. Geological Survey Water Resources Investigations Report 01-4260; U.S. Geological Survey: Reston, VA, USA, 2001; p. 148. [CrossRef]
- Bäckström, M.; Karlsson, S.; Bäckman, L.; Folkeson, L.; Lind, B. Mobilisation of heavy metals by deicing salts in a roadside environment. Water Res. 2004, 38, 720–732. [Google Scholar] [CrossRef] [PubMed]
- Amrhein, C.; Strong, J.E.; Mosher, P.A. Effect of deicing salts on metal and organic matter mobilization in roadside soils. Environ. Sci. Technol. 1992, 26, 703–709. [Google Scholar] [CrossRef]
- Davis, S.N.; Whettenmore, D.O.; Fabryka-Martin, J. Uses of chloride/bromide ratios in studies of potable water. Groundwater 1998, 36, 338–350. [Google Scholar] [CrossRef]
- Vengosh, A.; Pankratov, I. Chloride/bromide and chloride/fluoride ratios of domestic sewage effluents and associated contaminated ground water. Groundwater 1998, 36, 815–824. [Google Scholar] [CrossRef]
- Risch, M.R.; Robinson, B.A. Use of Borehole and Surface Geophysics to Investigate Ground-Water Quality Near a Road-Deicing Salt-storage Facility, Valparaiso, Indiana; U.S. Geological Survey Water-Resources Investigations Report 00-4070; U.S. Geological Survey: Reston, VA, USA, 2001; p. 65. [CrossRef]
- Richter, B.C.; Kreitler, C.W. Identification of Sources of Ground-Water Salinization Using Geochemical Techniques; U.S. Environmental Protection Agency EPA-600/2-91/064; U.S. Environmental Protection Agency: Ada, OK, USA, 1991; p. 259. Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=30000HXE.TXT (accessed on 28 January 2025).
- Holser, W.T. Trace elements and isotopes in evaporates. In Marine Minerals; Burns, R.G., Ed.; Mineralogical Society of America, Short Course Notes; Mineralogical Society of America: Washington, DC, USA, 1979; Volume 6, Chapter 9; pp. 295–345. [Google Scholar] [CrossRef]
- Whittemore, D.O.; Pollock, L.M. Determination of Salinity Sources in Water Resources of Kansas by Minor Alkali Metal and Halide Chemistry; Kansas State University, Kansas Water Resources Research Institute Contribution 208: Manhattan, NY, USA, 1979; p. 28. [Google Scholar]
- Knuth, M.; Jackson, J.L.; Whittemore, D.O. An integrated approach to identifying the salinity source contaminating a ground-water supply. Groundwater 1990, 28, 207–214. [Google Scholar] [CrossRef]
- Howard, K.W.F.; Beck, P.J. Hydrochemical interpretation of groundwater flow systems in Quaternary sediments of southern Ontario. Can. J. Earth Sci. 1986, 23, 938–947. [Google Scholar] [CrossRef]
- Keller, S.J. Analyses of Subsurface Brines in Indiana; Indiana Geological Survey Occasional Paper 41; Indiana Department of Natural Resources: Bloomington, IN, USA, 1983; p. 30. Available online: https://hdl.handle.net/2022/28165 (accessed on 28 January 2025).
- Masoner, J.R.; Kolpin, D.W.; Cozzarelli, I.M.; Barber, L.B.; Burden, D.S.; Foreman, W.T.; Forshay, K.J.; Furlong, E.T.; Groves, J.F.; Hladik, M.L.; et al. Urban stormwater: An overlooked pathway of extensive mixed contaminants to surface and groundwaters in the United States. Environ. Sci. Technol. 2019, 53, 10070–10081. [Google Scholar] [CrossRef] [PubMed]
- Granato, G.E.; DeSimone, L.A.; Barbaro, J.R.; Jeznach, L.C. Methods for Evaluating Potential Sources of Chloride in Surface Water and Groundwaters of the Conterminous United States; U.S. Geological Survey Open-File Report 2015-1080; U.S. Geological Survey: Reston, VA, USA, 2015; p. 89. [CrossRef]
- Snodgrass, J.W.; Moore, J.; Lev, S.M.; Casey, R.E.; Ownby, D.R.; Flora, R.F.; Izzo, G. Influence of modern stormwater management practices on transport of road salt to surface waters. Environ. Sci. Technol. 2017, 51, 4165–4172. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.S.; Relyea, R.A. A Review of the Combined Threats of Road Salts and Heavy Metals to Freshwater Systems. BioScience 2018, 68, 327–335. [Google Scholar] [CrossRef]
- Tornes, L.H. Effects of Rain Gardens on the Quality of Water in the Minneapolis-St. Paul Metropolitan Area of Minnesota; U.S. Geological Survey Scientific Investigations Report 2002-04; U.S. Geological Survey: Reston, VA, USA, 2005; p. 29. [CrossRef]
- Basu, A.; Sinha, S.; Carpenter, D. A Survey of Barriers and Opportunities to Adopting Green Stormwater Infrastructure in Michigan; Michigan Sea Grant Report MICHU-20-502; Lawrence Technological University: Southfield, MI, USA, 2020; p. 6. Available online: https://www.michiganseagrant.org/wp-content/uploads/2020/01/Executive-Summary-GI-Barriers-Survey-January-2020.pdf (accessed on 28 January 2025).
- Corsi, S.R.; De Cicco, L.A.; Lutz, M.A.; Hirsch, R.M. River chloride trends in snow-affected urban watersheds: Increasing concentrations outpace urban growth rate and are common among all seasons. Sci. Environ. 2015, 508, 488–497. [Google Scholar] [CrossRef]
- Darner, R.A.; Shuster, W.D.; Dumouchelle, D.H. Hydrologic Characteristics of Low-Impact Stormwater Control Measures at Two Sites in Northeastern Ohio, 2008–2013; U.S. Geological Survey Scientific Investigations Report 2015-5030; U.S. Geological Survey: Reston, VA, USA, 2015; p. 38. [CrossRef][Green Version]
- Great Lakes Commission. Great Lakes Regional Green Infrastructure Policy Analysis: Addressing Barriers to Implementation; Great Lakes Commission: Ann Arbor, MI, USA, 2018; p. 51. [Google Scholar]
- Schneider, A.F. Physiography. In Naural Features of Indiana—A Symposium, 1966 (Indiana Sesqucentennial Volume, 1816–1966); Lindsey, A.A., Ed.; Indiana Academy of Science: Indianapolis, IN, USA, 1966; pp. 40–56. [Google Scholar]
- Leverett, F.; Taylor, F.B. The Pleistocene of Indiana and Michigan and the History of the Great Lakes; U.S. Geological Survey Monograph 53; U.S. Geological Survey: Reston, VA, USA, 1915; p. 529.
- Bretz, J.H. The stages of Lake Chicago; their causes and correlations. Am. J. Sci. 1951, 24, 401–419. [Google Scholar] [CrossRef]
- Hansel, A.K.; Mickelson, D.M.; Schneider, A.F.; Larsen, C.E. Late Wisconsinan and Holocene history of the Lake Michigan basin. In Quarternary Evolution of the Great Lakes; Karrow, P.F., Clakin, P.E., Eds.; Special Paper 30; Geological Association of Canada, The Canadian Copyright Licensing Agency: Toronto, ON, Canada, 1985; pp. 39–53. [Google Scholar]
- Hartke, E.J.; Hill, J.R.; Reshkin, M. Environmental Geology of Lake and Porter Counties, Indiana—An Aid to Planning; Indiana Geological Survey Special Report 11; Indiana Geological and Water Survey: Bloomington, IN, USA, 1975; p. 57. [Google Scholar]
- Brown, S.E.; Thompson, T.A. Geologic Terrains of Northwestern Lake County, Indiana; Indiana Geological Survey Open-File Report 5-05; Indiana Geological and Water Survey: Bloomington, IN, USA, 1995; Available online: https://hdl.handle.net/2022/28847 (accessed on 4 February 2025).
- Fenelon, J.M.; Watson, L.R. Geohydrology and Water Quality of the Calumet Aquifer, in the Vicinity of the Grand Calumet River/Indiana Harbor Canal, Northwestern Indiana; U.S. Geological Survey Water-Resources Investigations Report 92-4115; U.S. Geological Survey: Reston, VA, USA, 1993; p. 151. [CrossRef]
- Lampe, D.C.; Bayless, E.R.; Follette, D.D. Stormwater Reduction and Water Budget for a Rain Garden on Sandy Soil, Gary, Indiana, 2016–2018; U.S. Geological Survey Scientific Investigations Report 2022–5101; U.S. Geological Survey: Reston, VA, USA, 2022; p. 39. [CrossRef]
- Arguez, A.; Durre, I.; Applequist, S.; Vose, R.S.; Squires, M.F.; Yin, X.; Heim, R.R., Jr.; Owen, T.W. NOAA’s U.S. Climate Normals (1981–2010); NOAA National Centers for Environmental Information: Asheville, NC, USA, 2012. [CrossRef]
- Decagon Devices, Inc. ES-2, ES-2F Electrical Conductivity & Temperature Sensor, Operator’s Manual Version 1; Decagon Devices, Inc.: Pullman, WA, USA, 2012; Available online: https://manualzz.com/doc/8424000/es-2-manual (accessed on 28 January 2025).
- U.S. Geological Survey. National Field Manual (NFM) for the Collection of Water-Quality Data; U.S. Geological Survey Techniques and Methods; U.S. Geological Survey: Reston, VA, USA, 2019; Chapter A1–A10. Available online: https://pubs.water.usgs.gov/twri9A (accessed on 14 July 2024).
- Johnson, A.I. Measurement of Infiltration; U.S. Geological Survey Water-Supply Paper 1544-F; U.S. Geological Survey: Reston, VA, USA, 1963; p. 31. [CrossRef]
- ASTM D3385-03; Standard Test Method for Infiltration Rate of Soils in Field Using Double-Ring Infiltrometer. ASTM International: West Conshohocken, PA, USA, 2003. [CrossRef]
- Whittemore, D.O. Bromide as tracer in ground-water studies: Geochemistry and analytical determination. In Proceedings of the Ground Water Geochemistry Conference, Denver, CO, USA, 16–18 February 1988; National Water Well Association: Dublin, OH, USA, 1988; pp. 339–360. [Google Scholar]
- Howard, K.W.F.; Beck, P.J. Hydrogeochemical implications of groundwater contamination by road deicing chemicals. J. Contam. Hydrol. 1993, 12, 245–268. [Google Scholar] [CrossRef]
- Jones, A.L.; Sroka, B.N. Effects of Highway Deicing Chemicals on Shallow Unconsolidated Aquifers in Ohio, Interim Report, 1988–1993; U.S. Geological Survey Water-Resources Investigations Report 97-4027; U.S. Geological Survey: Reston, VA, USA, 1997; p. 139. [CrossRef]
- Schnitzer, M. Soil organic matter—The next 75 years. Soil Sci. 1991, 151, 41–58. [Google Scholar] [CrossRef]
- Henning, S.; Bornemann, L.; Welp, G. Seasonal variability of soil organic carbon fractions under arable land. Pedosphere 2017, 27, 380–386. [Google Scholar] [CrossRef]
- Selbig, W.R.; Balster, N. Evaluation of Turf-Grass and Prairie-Vegetated Rain Gardens in a Clay and Sand Soil, Madison, Wisconsin, Water Years 2004–08; U.S. Geological Survey Scientific Investigations Report 2010-5077; U.S. Geological Survey: Reston, VA, USA, 2010; p. 82. [CrossRef]
- Granato, G.E.; Smith, K.P. Estimating Concentrations of Road-Salt Constituents in Highway-Runoff from Measurements of Specific Conductance; U.S. Geological Survey Water Resources Investigation Report 99-4077; U.S. Geological Survey: Reston, VA, USA, 1999; p. 22. Available online: https://pubs.usgs.gov/wri/wri99-4077/ (accessed on 28 January 2025).
- Lazur, A.; VanDerwerker, T.; Koepenick, K. Review of implications of road salt use on groundwater quality—Corrosivity and mobilization of heavy metals and radionuclides. Water Air Soil Pollut. 2020, 231, 474. [Google Scholar] [CrossRef]
- Baraza, T.; Hasenmueller, E.A. Road salt retention and transport through vadose zone soils to shallow groundwater. Sci. Total Environ. 2021, 755, 142240. [Google Scholar] [CrossRef] [PubMed]
- Burszta-Adamiak, E.; Biniak-Pieróg, M.; Dąbek, P.B.; Sternik, A. Rain garden hydrological performance–Responses to real rainfall events. Sci. Total Environ. 2023, 877, 164153. [Google Scholar] [CrossRef]







| Season | Maximum Temperature, in °C | Minimum Temperature, in °C | Average Temperature, in °C | Precipitation, in m | Snow, in m |
|---|---|---|---|---|---|
| Annual | 15.25 | 6.22 | 10.72 | 1.02 | 1.00 |
| Winter | 1.89 | −5.67 | −1.89 | 0.17 | 0.80 |
| Spring | 14.69 | 4.89 | 9.78 | 0.28 | 0.17 |
| Summer | 27.47 | 17.83 | 22.64 | 0.32 | 0.00 |
| Autumn | 16.97 | 7.83 | 12.39 | 0.25 | 0.03 |
| Well or Flume | USGS Site ID | Equation | r2 |
|---|---|---|---|
| CH-1 | 413610087201001 | y = 0.3106x − 146.28 | 0.72 |
| CH-2 | 413612087201301 | y = 0.319x − 171.02 | 0.99 |
| CH-3 | 413611087201004 | y = 0.2308x − 51.856 | 0.88 |
| West Flume | 413611087201101 | y = 0.4121x | 0.99 |
| Northeast Flume | 413612087200901 | y = 0.4257x | 0.99 |
| Southeast Flume | 413611087200901 | y = 0.3913x | 0.96 |
| North Flume | 413611087201001 | y = 0.3241x | 0.99 |
| South Flume | 413611087201002 | y = 0.4003x | 0.99 |
| Collected Mass of NaCl 2019–20 21 November 2019–10 March 2020 (kg NaCl) | Collected Mass of NaCl 2020 11 March 2020–20 November 2020 (kg NaCl) | Collected Mass of NaCl 2020–2021 21 November 2020–11 March 2021 (kg NaCl) | Total Collected Mass of NaCl by Flume (kg NaCl) | |
|---|---|---|---|---|
| West flume | 325 | 2.43 | 2.06 | 329 |
| North flume | 28.5 | 0.06 | 0.6 | 29.1 |
| Northeast flume | 240 | 15.1 | 5.04 | 260 |
| Southeast flume | 8.06 | 5.59 | 126 | 139 |
| South flume | 696 | 9.49 | 26.1 | 731 |
| Total NaCl collected by season | 1297 | 32.7 | 159 | 1490 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayless, E.R.; Naylor, S.; Lampe, D.C.; Story, A.A.; Artz, C. Road Salt Collection and Redistribution at an Urban Rain Garden on Sandy Soil, Gary, Indiana. Water 2025, 17, 510. https://doi.org/10.3390/w17040510
Bayless ER, Naylor S, Lampe DC, Story AA, Artz C. Road Salt Collection and Redistribution at an Urban Rain Garden on Sandy Soil, Gary, Indiana. Water. 2025; 17(4):510. https://doi.org/10.3390/w17040510
Chicago/Turabian StyleBayless, E. Randall, Shawn Naylor, David C. Lampe, Amy A. Story, and Caleb Artz. 2025. "Road Salt Collection and Redistribution at an Urban Rain Garden on Sandy Soil, Gary, Indiana" Water 17, no. 4: 510. https://doi.org/10.3390/w17040510
APA StyleBayless, E. R., Naylor, S., Lampe, D. C., Story, A. A., & Artz, C. (2025). Road Salt Collection and Redistribution at an Urban Rain Garden on Sandy Soil, Gary, Indiana. Water, 17(4), 510. https://doi.org/10.3390/w17040510






