Removal of Nitrogen and Phosphorus by a Novel Salt-Tolerant Strain Pseudomonas sediminis D4
Abstract
1. Introduction
2. Materials and Methods
2.1. Medium Used
2.2. Sample and Identification
2.3. The Antibiotic Sensitivity of Strain D4
2.4. The Biosafety Assessment of Strain D4
2.5. Effect of Environmental Conditions on the Removal of Nitrogen and Phosphorus
2.6. Simultaneous Nitrogen and Phosphorus Removal at Varying Salinity Levels
2.7. SNDPR Enzyme Activity Assay
2.8. Analytical Method and Statisticfval Analysis
3. Results and Discussion
3.1. Identification of Strain D4
3.2. Features of Strain D4: Biological and Ecological Safety
3.3. Effect of Different Environmental Conditions on the SNDPR Ability of Strain D4
3.3.1. Carbon Sources
3.3.2. C/N Ratio
3.3.3. P/N Ratio
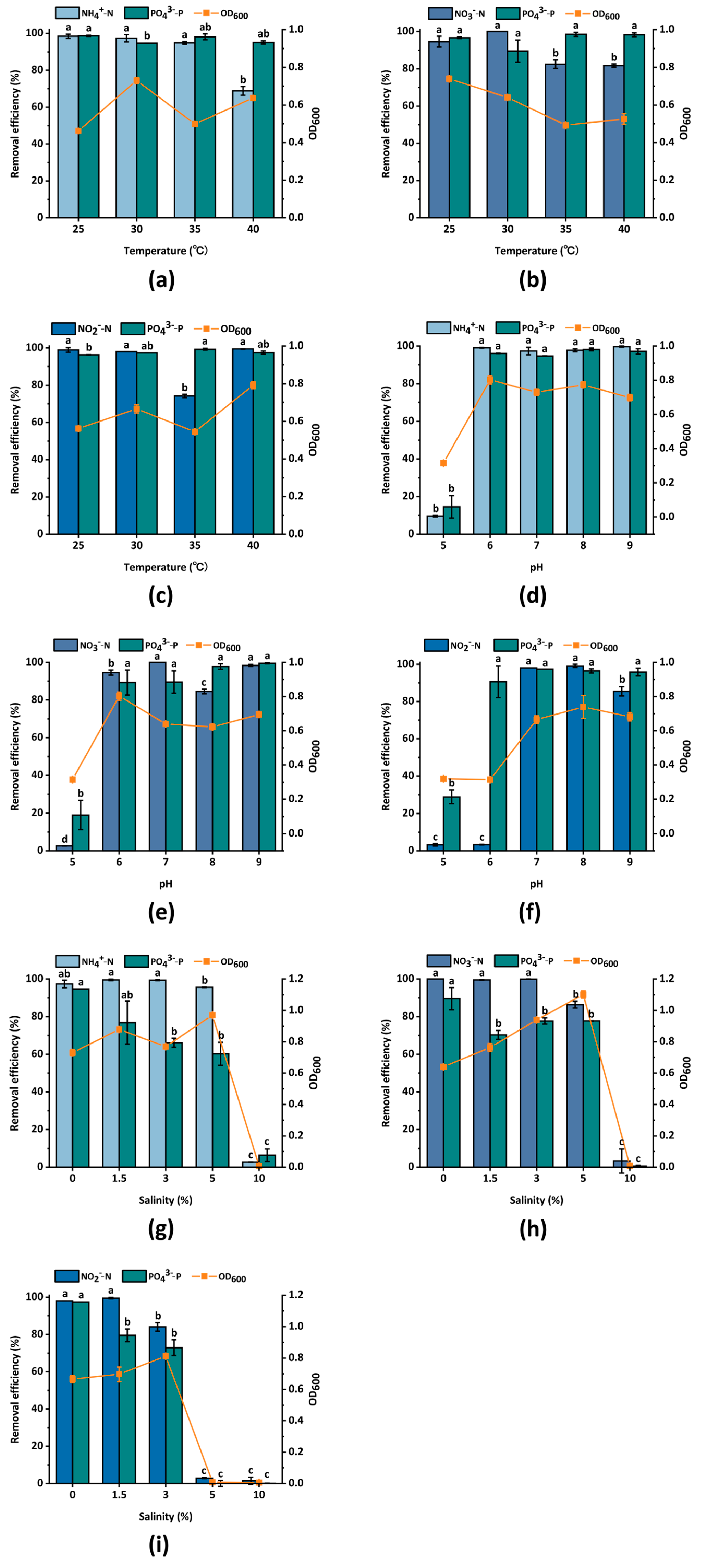
3.3.4. Temperature
3.3.5. pH
3.3.6. Salinity
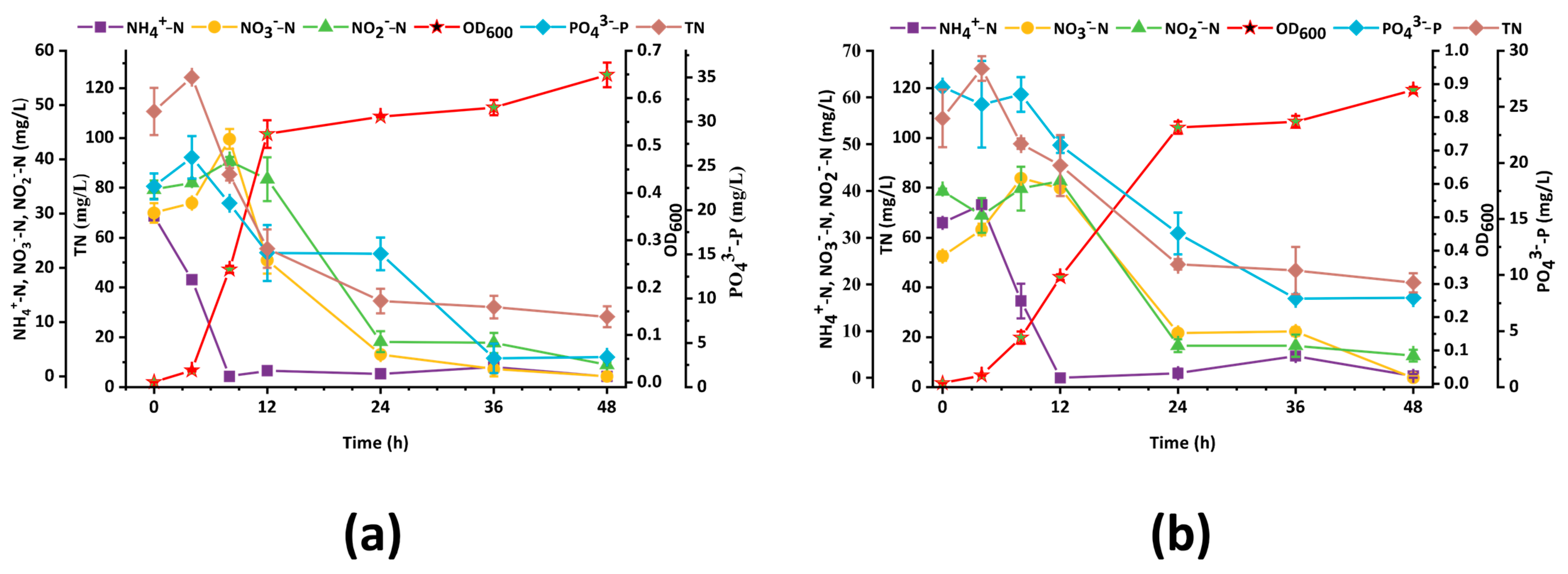
3.4. Nitrogen and Phosphorus Removal Ability of Strain D4 at Different Salinity Levels
3.5. Enzyme Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation, 1st ed.; Food and Agriculture Organization of the United Nation: Rome, Italy, 2022; pp. 1–93. [Google Scholar]
- Shukla, S.; Rajta, A.; Setia, H.; Bhatia, R. Simultaneous nitrification–denitrification by phosphate accumulating microorganisms. World J. Microbiol. Biotechnol. 2020, 36, 151. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhang, F.; Zhang, L.; Liu, H.; Zhang, Q.; Xing, Z.; Zhao, T. Characterization of a novel salt-tolerant strain Sphingopyxis sp. CY-10 capable of heterotrophic nitrification and aerobic denitrification. Bioresour. Technol. 2022, 358, 127353. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liang, X.; Jin, Y.; Wu, C.; Zhou, R. Isolation and Nitrogen Removal Characteristics of an Aerobic Heterotrophic Nitrifying-Denitrifying Bacterium, Klebsiella sp. TN-10. Appl. Biochem. Biotechnol. 2019, 188, 540–554. [Google Scholar] [CrossRef]
- Chen, X.; Li, S.; Zhang, W.; Li, S.; Gu, Y.; Ouyang, L. A Newly Isolated Rhodococcus sp. S2 from Landfill Leachate Capable of Heterotrophic Nitrification and Aerobic Denitrification. Water 2024, 16, 431. [Google Scholar] [CrossRef]
- Chan, S.S.; Khoo, K.S.; Chew, K.W.; Ling, T.C.; Show, P.L. Recent advances biodegradation and biosorption of organic compounds from wastewater: Microalgae-bacteria consortium—A review. Bioresour. Technol. 2022, 344, 126159. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Wang, H.; Ma, X.; Wang, C.; Wang, M.; Liu, Z.; Lu, M.; Cao, J.; Ke, X. Efficient nitrogen removal of a novel Pseudomonas chengduensis strain BF6 mainly through assimilation in the recirculating aquaculture systems. Bioresour. Technol. 2023, 379, 129036. [Google Scholar] [CrossRef]
- Li, B.; Jing, F.; Wu, D.; Xiao, B.; Hu, Z. Simultaneous removal of nitrogen and phosphorus by a novel aerobic denitrifying phosphorus-accumulating bacterium, Pseudomonas stutzeri ADP-19. Bioresour. Technol. 2021, 321, 124445. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, Y.; Qiu, G.; Liang, D.; Li, Y.; Cheng, J.; Chen, Y.; Wang, G.; Xie, J.; Zhu, X. Genomics and metabolic characteristics of simultaneous heterotrophic nitrification aerobic denitrification and aerobic phosphorus removal by Acinetobacter indicus CZH-5. Bioresour. Technol. 2024, 395, 130322. [Google Scholar] [CrossRef]
- Oehmen, A.; Carvalho, G.; Freitas, F.; Reis, M.A.M. Assessing the abundance and activity of denitrifying polyphosphate accumulating organisms through molecular and chemical techniques. Water Sci. Technol. 2010, 61, 2061–2068. [Google Scholar] [CrossRef]
- Zeng, W.; Bai, X.; Guo, Y.; Li, N.; Peng, Y. Interaction of “Candidatus Accumulibacter” and nitrifying bacteria to achieve energy-efficient denitrifying phosphorus removal via nitrite pathway from sewage. Enzym. Microb. Technol. 2017, 105, 1–8. [Google Scholar] [CrossRef]
- Huang, M.-Q.; Cui, Y.-W.; Huang, J.-L.; Sun, F.-L.; Chen, S. A novel Pseudomonas aeruginosa strain performs simultaneous heterotrophic nitrification-aerobic denitrification and aerobic phosphate removal. Water Res. 2022, 221, 118823. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, X.-H.; Cui, S.; Ren, Y.-X.; Yu, J.; Chen, N.; Xiao, Q.; Guo, L.-K.; Wang, R.-H. Simultaneous removal of nitrogen and phosphorous by heterotrophic nitrification-aerobic denitrification of a metal resistant bacterium Pseudomonas putida strain NP5. Bioresour. Technol. 2019, 285, 121360. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Ma, Y.; Lu, H.; Sun, H.; Zhao, J.; Ruan, Z.; Zhou, J.; Liu, Y.; Liu, F.; Xu, J.; et al. Simultaneous aerobic nitrogen and phosphate removal capability of novel salt-tolerant strain, Pseudomonas mendocina A4: Characterization, mechanism and application potential. Bioresour. Technol. 2024, 393, 130047. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, J.; Wang, X.; Wang, E.; Li, B.; He, R.; Yuan, H. Removal of nitrogen by heterotrophic nitrification–aerobic denitrification of a phosphate accumulating bacterium Pseudomonas stutzeri YG-24. Bioresour. Technol. 2015, 182, 18–25. [Google Scholar] [CrossRef]
- Behera, P.; Mahapatra, M.; Seuylemezian, A.; Vaishampayan, P.; Ramana, V.V.; Joseph, N.; Joshi, A.; Shouche, Y.; Suar, M.; Pattnaik, A.K.; et al. Taxonomic description and draft genome of Pseudomonas sediminis sp. nov., isolated from the rhizospheric sediment of Phragmites karka. J. Microbiol. 2018, 56, 458–466. [Google Scholar] [CrossRef]
- Song, K.; Gao, Y.; Yang, Y.; Guo, B.-Q.; Wang, Y.-Z. Performance of simultaneous carbon and nitrogen removal of high-salinity wastewater in heterotrophic nitrification-aerobic denitrification mode. J. Environ. Chem. Eng. 2023, 11, 109682. [Google Scholar] [CrossRef]
- Duan, J.; Fang, H.; Su, B.; Chen, J.; Lin, J. Characterization of a halophilic heterotrophic nitrification–aerobic denitrification bacterium and its application on treatment of saline wastewater. Bioresour. Technol. 2015, 179, 421–428. [Google Scholar] [CrossRef]
- Dinçer, A.R.; Kargi, F. Salt Inhibition of Nitrification and Denitrification in Saline Wastewater. Environ. Technol. 1999, 20, 1147–1153. [Google Scholar] [CrossRef]
- Man, Q.; Zhang, P.; Huang, W.; Zhu, Q.; He, X.; Wei, D. A heterotrophic nitrification-aerobic denitrification bacterium Halomonas venusta TJPU05 suitable for nitrogen removal from high-salinity wastewater. Front. Environ. Sci. Eng. 2021, 16, 69. [Google Scholar] [CrossRef]
- Brenner, D.J.; Krieg, N.R.; Staley, J.T. Bergey’s Manual® of Systematic Bacteriology: Volume 2: The Proteobacteria, Part B: The Gammaproteobacteria, 2nd ed.; Springer: New York, NY, USA, 2013; pp. 323–442. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2020; pp. 42–45. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests, 11th ed.; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2012; pp. 25–29. [Google Scholar]
- Xu, N.; Liao, M.; Liang, Y.; Guo, J.; Zhang, Y.; Xie, X.; Fan, Q.; Zhu, Y. Biological nitrogen removal capability and pathways analysis of a novel low C/N ratio heterotrophic nitrifying and aerobic denitrifying bacterium (Bacillus thuringiensis strain WXN-23). Environ. Res. 2021, 195, 110797. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, R.; Chen, Y. Effects of ZnO Nanoparticles on Wastewater Biological Nitrogen and Phosphorus Removal. Environ. Sci. Technol. 2011, 45, 2826–2832. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhao, L.; Li, Q.; Huang, L.; Qin, Y.; Wang, P.; Zhu, C.; Yan, Q. The involvement of the T6SS vgrG gene in the pathogenicity of Pseudomonas plecoglossicida. J. Fish Dis. 2023, 46, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- López, J.R.; Lorenzo, L.; Marcelino-Pozuelo, C.; Marin-Arjona, M.C.; Navas, J.I. Pseudomonas baetica: Pathogenicity for marine fish and development of protocols for rapid diagnosis. FEMS Microbiol. Lett. 2016, 364, fnw286. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Zhao, X.; Senbati, Y.; Song, K.; He, X. Nitrogen removal by heterotrophic nitrifying and aerobic denitrifying bacterium Pseudomonas sp. DM02: Removal performance, mechanism and immobilized application for real aquaculture wastewater treatment. Bioresour. Technol. 2021, 322, 124555. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, J.; Li, Y.; Hao, H.-h.; Chen, J.-m. Characteristics of a novel thermophilic heterotrophic bacterium, Anoxybacillus contaminans HA, for nitrification–aerobic denitrification. Appl. Microbiol. Biotechnol. 2015, 99, 10695–10702. [Google Scholar] [CrossRef]
- Rout, P.R.; Bhunia, P.; Dash, R.R. Simultaneous removal of nitrogen and phosphorous from domestic wastewater using Bacillus cereus GS-5 strain exhibiting heterotrophic nitrification, aerobic denitrification and denitrifying phosphorous removal. Bioresour. Technol. 2017, 244, 484–495. [Google Scholar] [CrossRef]
- Gu, X.; Leng, J.; Zhu, J.; Zhang, K.; Zhao, J.; Wu, P.; Xing, Q.; Tang, K.; Li, X.; Hu, B. Influence mechanism of C/N ratio on heterotrophic nitrification-aerobic denitrification process. Bioresour. Technol. 2022, 343, 126116. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, H.; Cai, L.; Sun, P.; Ma, B.; Li, J.; Chen, G.; Ruan, Y. C/N ratios inform sustainable aerobic denitrification for nitrogen pollution control: Insights into the key parameter from a view of metabolic division. J. Clean. Prod. 2023, 414, 137565. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, W.; Zhu, S.; Liu, F.; Qin, L.; Xu, C.; Wang, Z. Biological nitrogen and phosphorus removal by a phosphorus-accumulating bacteria Acinetobacter sp. strain C-13 with the ability of heterotrophic nitrification–aerobic denitrification. Bioresour. Technol. 2021, 322, 124507. [Google Scholar] [CrossRef]
- Song, T.; Zhang, X.; Li, J.; Wu, X.; Feng, H.; Dong, W. A review of research progress of heterotrophic nitrification and aerobic denitrification microorganisms (HNADMs). Sci. Total Environ. 2021, 801, 149319. [Google Scholar] [CrossRef]
- Tian, J.; He, F.; Cheng, Z.; Zhang, X.; Yang, C.; Gao, B.; Xu, Z.; Tian, Y. Aerobic Denitrification of Pseudomonas stutzeri yjy-10 and Genomic Analisis of This Process. Appl. Biochem. Microbiol. 2022, 58, 294–301. [Google Scholar] [CrossRef]
- Huang, F.; Pan, L.; Lv, N.; Tang, X. Characterization of novel Bacillus strain N31 from mariculture water capable of halophilic heterotrophic nitrification–aerobic denitrification. J. Biosci. Bioeng. 2017, 124, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Wang, C.; Jiang, J.; Liu, S.; Zheng, Y.; Yang, M.; Zhang, Y. Nitrate removal by alkali-resistant Pseudomonas sp. XS-18 under aerobic conditions: Performance and mechanism. Bioresour. Technol. 2022, 344, 126175. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, B.; Liu, M.; Jiang, K.; Wang, L. Stutzerimonas frequens strain TF18 with superior heterotrophic nitrification-aerobic denitrification ability for the treatment of aquaculture effluent. Process Biochem. 2023, 130, 156–165. [Google Scholar] [CrossRef]
- Huang, M.-Q.; Cui, Y.-W.; Yang, H.-J.; Xu, M.-J.; Cui, Y.; Chen, Z. A halophilic aerobic-heterotrophic strain Halomonas venusta SND-01: Nitrogen removal by ammonium assimilation and heterotrophic nitrification-aerobic denitrification. Bioresour. Technol. 2023, 374, 128758. [Google Scholar] [CrossRef]
- Huang, X.; Li, W.; Zhang, D.; Qin, W. Ammonium removal by a novel oligotrophic Acinetobacter sp. Y16 capable of heterotrophic nitrification–aerobic denitrification at low temperature. Bioresour. Technol. 2013, 146, 44–50. [Google Scholar] [CrossRef]
- Lei, X.; Jia, Y.; Chen, Y.; Hu, Y. Simultaneous nitrification and denitrification without nitrite accumulation by a novel isolated Ochrobactrum anthropic LJ81. Bioresour. Technol. 2019, 272, 442–450. [Google Scholar] [CrossRef]
- Yang, J.-R.; Wang, Y.; Chen, H.; Lyu, Y.-K. Ammonium removal characteristics of an acid-resistant bacterium Acinetobacter sp. JR1 from pharmaceutical wastewater capable of heterotrophic nitrification-aerobic denitrification. Bioresour. Technol. 2019, 274, 56–64. [Google Scholar] [CrossRef]
- He, T.; Zhang, M.; Ding, C.; Wu, Q.; Chen, M.; Mou, S.; Cheng, D.; Duan, S.; Wang, Y. New insight into the nitrogen removal capacity and mechanism of Streptomyces mediolani EM-B2. Bioresour. Technol. 2022, 348, 126819. [Google Scholar] [CrossRef]




| Antibiotic Type | Inhibition Circle Diameter/mm | Result |
|---|---|---|
| penicillin | - | R |
| benzoxacillin | - | R |
| amoxicillin | 25.00 ± 0.00 | S |
| cefthiophene | - | R |
| cefazolin | 14.00 ± 0.58 | R |
| cefoxitin | 9.67 ± 0.88 | R |
| imipenem | 31.00 ± 0.00 | S |
| amitrazine | 22.00 ± 0.58 | S |
| streptomycin | 13.67 ± 1.33 | I |
| neomycin | 17.00 ± 0.58 | S |
| polymyxin B | 15.00 ± 0.58 | S |
| norfloxacin | 28.00 ± 1.00 | S |
| ciprofloxacin | 22.00 ± 1.15 | S |
| erythromycin | 10.00 ± 0.58 | R |
| sulfisoxazole | 20.00 ± 1.15 | S |
| tetracycline | 22.00 ± 1.00 | S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yin, P.; Zhou, J.; Ma, Y.; Lai, X.; Lin, J.; Peng, H.; Shu, H.; Huang, W. Removal of Nitrogen and Phosphorus by a Novel Salt-Tolerant Strain Pseudomonas sediminis D4. Water 2025, 17, 502. https://doi.org/10.3390/w17040502
Liu Y, Yin P, Zhou J, Ma Y, Lai X, Lin J, Peng H, Shu H, Huang W. Removal of Nitrogen and Phosphorus by a Novel Salt-Tolerant Strain Pseudomonas sediminis D4. Water. 2025; 17(4):502. https://doi.org/10.3390/w17040502
Chicago/Turabian StyleLiu, Yuting, Peng Yin, Jiayi Zhou, Yonghao Ma, Xunheng Lai, Junduo Lin, Huilin Peng, Hu Shu, and Wen Huang. 2025. "Removal of Nitrogen and Phosphorus by a Novel Salt-Tolerant Strain Pseudomonas sediminis D4" Water 17, no. 4: 502. https://doi.org/10.3390/w17040502
APA StyleLiu, Y., Yin, P., Zhou, J., Ma, Y., Lai, X., Lin, J., Peng, H., Shu, H., & Huang, W. (2025). Removal of Nitrogen and Phosphorus by a Novel Salt-Tolerant Strain Pseudomonas sediminis D4. Water, 17(4), 502. https://doi.org/10.3390/w17040502







