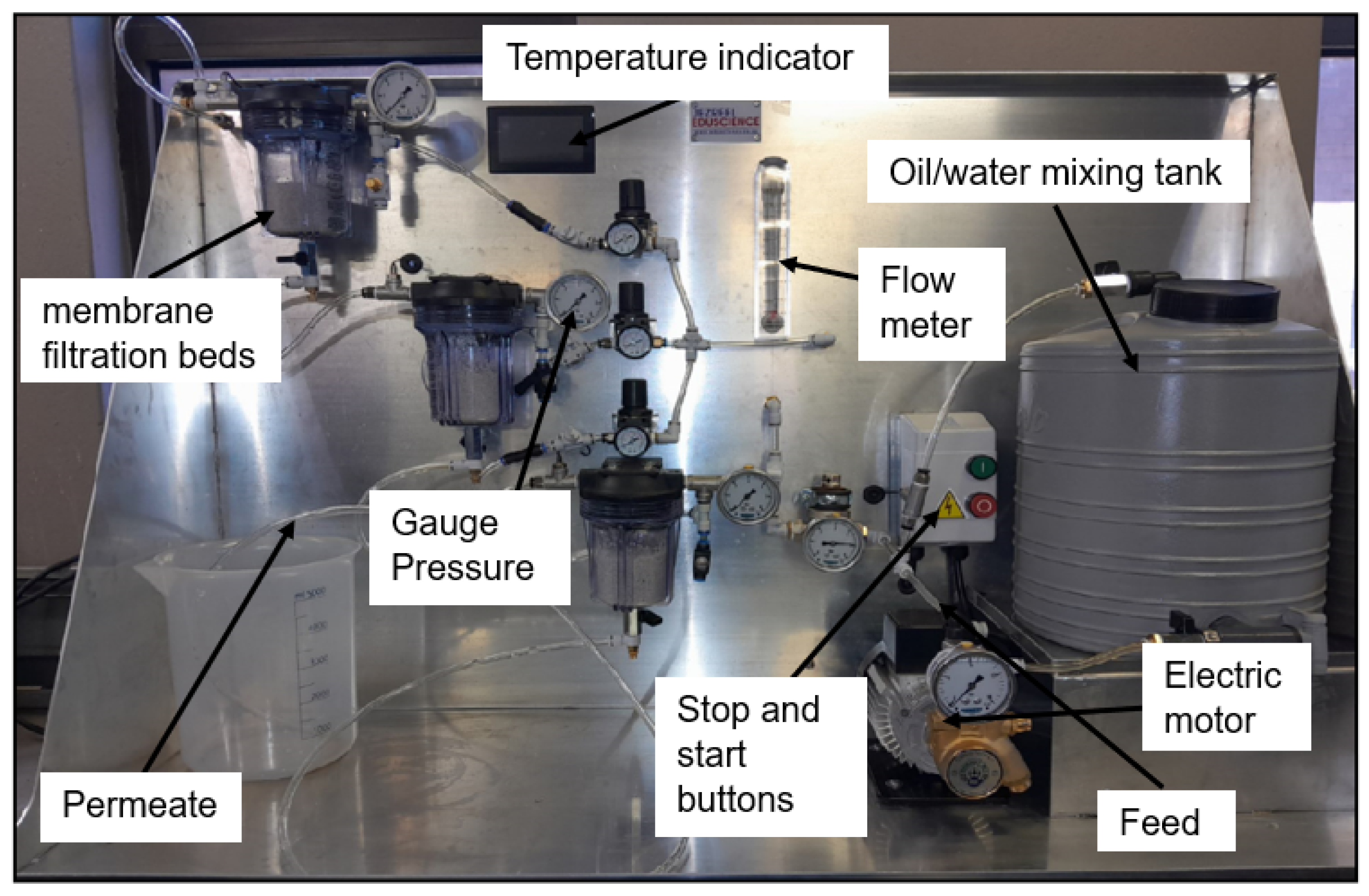
The experimental outcomes represent the culmination of detailed characterization and rigorous performance testing to evaluate the effectiveness of the developed filtration system. A suite of advanced analytical methods and practical assessments was applied to gain a thorough understanding of the material’s properties and separation performance. Scanning Electron Microscopy (SEM) was utilized to analyze the surface morphology of the quartz samples, both in their untreated form and after modification with hydrophilic nanoparticles. Raman spectroscopy was employed to identify chemical bonds, and functional groups present on the quartz surface, while X-Ray Diffraction (XRD) provided critical insights into the crystalline structure of the material before and after nanoparticle integration. Additionally, oil and grease analysis quantified the residual oil content in the permeate, offering a precise measure of the system’s separation efficiency.
3.1. Scanning Electron Microscopy (SEM) Quartz Surface Analysis
The following SEM images provide a detailed representation of the quartz surface at various stages of preparation and coating. The sequence begins with the untreated raw quartz surface, showcasing its natural morphology. Next, the surface is displayed after being thoroughly washed with a descaling chemical to remove impurities and enhance its cleanliness. The progression continues with the application of the first nanoparticle coating, revealing the initial layer of modification. Subsequent images capture the quartz surface after the addition of the second, third, and fourth coatings, illustrating the incremental buildup of material layers and changes in surface structure as each coating is applied.
The Scanning Electron Microscopy (SEM) image of raw quartz in
Figure 2 reveals a heterogeneous surface morphology, characterized by irregularities, sharp edges, micro-cracks, and layered formations. The presence of these features suggests that the quartz has undergone mechanical stress, likely during the mining and crushing process [
19]. The rough surface lacks significant porosity, which may influence its interaction with water and oil droplets during filtration. Additionally, the layered structure observed in the image is characteristic of silica-rich minerals, which can play a role in nanoparticle adhesion when the quartz is subjected to surface modification [
20]. The high surface roughness could also impact oil–water separation efficiency, affecting how water permeates through the system.
The elemental mapping analysis provides insights into the chemical composition and distribution of key elements present in the raw quartz. The carbon (C-K) mapping indicates a low presence of carbonaceous material, suggesting that the quartz is relatively free from organic contaminants or hydrocarbon deposits. This is beneficial for filtration applications, as it minimizes unwanted fouling. The oxygen (O-K) mapping shows a high and uniform distribution, which aligns with the expected silicon dioxide (SiO2) composition of quartz. The strong oxygen signal confirms the oxidized state of the material, indicating no significant elemental impurities affecting its chemical stability. Additionally, aluminum (Al-K) mapping reveals minor traces of alumina (Al2O3), which are common in natural quartz deposits. The presence of aluminum could slightly influence the hydrophilicity of the quartz, as alumina-based compounds tend to alter surface wettability. Finally, the silicon (Si-K) mapping demonstrates a dominant and homogenous presence of SiO2, verifying that the quartz is structurally stable and chemically pure, with no noticeable non-silica-based inclusions.
From a filtration perspective, the observed morphological and chemical properties have several implications. The rough and uneven surface of the raw quartz provides an advantageous platform for nanoparticle adhesion, which is crucial for hydrophilic modification in oil–water separation systems [
21]. However, excessive surface roughness could lead to non-uniform coating distribution, necessitating precise nanoparticle deposition techniques. The low carbon presence ensures that the quartz is free from organic fouling, reducing the likelihood of oil absorption during filtration. Additionally, the layered microstructure suggests that the material is mechanically durable, although prolonged high-pressure flow conditions could contribute to surface erosion over extended operational cycles [
22]. The minor presence of alumina (Al
2O
3) may introduce surface charge variations, which could influence the hydrophilicity and water permeability of the quartz material.
The scanning electron microscopy (SEM) image of washed quartz in
Figure 3 presents a smoother and more refined surface morphology compared to raw quartz, indicating the removal of surface impurities, residual debris, and fine dust through the washing process. The surface appears to have undergone structural rearrangement, exhibiting reduced microfractures and fewer surface irregularities. However, while the roughness has diminished, some surface undulations and minor imperfections remain, which may influence nanoparticle adhesion and coating uniformity in later modification steps [
23]. The reduction in surface irregularities suggests that the washing procedure effectively eliminated weakly bound particles, thereby improving the structural stability of the quartz material. This enhanced smoothness is expected to facilitate more uniform hydrophilic nanoparticle coating, which is critical for optimizing oil–water separation performance in filtration applications.
Elemental mapping analysis through energy-dispersive X-ray spectroscopy (EDS) reveals key chemical composition characteristics of the washed quartz. The carbon (C-K) signal appears more prominent than in raw quartz, possibly due to adsorption of trace organic residues from the washing solution. However, the carbon presence remains minimal, indicating that the quartz surface is still largely free from hydrocarbon contamination, which is beneficial for maintaining high water affinity. The oxygen (O-K) mapping displays an intensified and homogeneously distributed signal, consistent with the silicon dioxide (SiO2) composition of quartz. This suggests that surface silica groups are more exposed after washing, enhancing reactivity and potential bonding sites for further nanoparticle deposition.
The presence of aluminium (Al-K) remains detectable, though its distribution is relatively sparse, indicating that alumina (Al2O3) impurities persist despite washing. These residual aluminium traces could subtly influence surface charge properties, potentially affecting water permeability and oil repellence. Meanwhile, the silicon (Si-K) signal exhibits a well-defined, structured distribution, confirming a high-purity quartz framework with no significant phase separations or structural degradation. The phosphorus (P-K) mapping introduces a new element that was absent in raw quartz, likely resulting from descaling agents or washing additives. The phosphorus appears to be concentrated in localized regions, suggesting possible surface interactions with quartz that may influence its hydrophilicity and long-term chemical stability.
The changes observed in surface morphology and elemental composition have significant implications for the filtration performance of the quartz material [
24]. The smoother and cleaner surface provides a more uniform substrate for nanoparticle adhesion, ensuring consistent hydrophilic modifications that are essential for oil–water separation applications. However, the persistence of aluminium impurities may introduce variations in wettability, while the presence of phosphorus suggests potential secondary surface interactions that require further investigation. The washing process appears to have successfully enhanced the surface purity of quartz, but minor structural imperfections and elemental variations may still influence filtration efficiency and long-term stability.
The scanning electron microscopy (SEM) image of the first-coated quartz in
Figure 4 demonstrates a well-distributed nanoparticle layer, making it the optimal coating stage for filtration applications. The surface appears significantly smoother and more structured than uncoated quartz, indicating that the nanoparticles have adhered effectively without excessive agglomeration. This uniform distribution enhances the hydrophilicity of the surface, ensuring efficient water permeability while repelling oil droplets, which is a critical factor in oil–water separation systems. The moderate surface roughness observed in the micrograph promotes enhanced interaction with water molecules, preventing oil accumulation and fouling, a common limitation in traditional filtration systems.
The energy-dispersive X-ray spectroscopy (EDS) elemental mapping further confirms the efficacy of the first coating by highlighting the balanced chemical composition of the modified quartz surface. The oxygen (O-K) signal is strong and well-distributed, reinforcing the presence of hydrophilic functional groups, which play a crucial role in improving water attraction and oil repellence. The silicon (Si-K) mapping remains dominant, ensuring that the structural integrity of the quartz is maintained, preventing excessive alteration of the material’s mechanical stability. Notably, the fluorine (F-K) and phosphorus (P-K) signals are homogeneously dispersed, suggesting enhanced surface modification without localized clustering, which is essential for ensuring consistent performance across the entire filtration medium. Unlike higher coatings where nanoparticle agglomeration leads to pore clogging, the first coating achieves an optimal balance, allowing high flux rates while maintaining excellent separation efficiency.
The minimal presence of aluminum (Al-K) and carbon (C-K) suggests that surface contamination is negligible, ensuring that the filtration system remains stable over extended operational cycles. The combination of structural integrity, optimal nanoparticle dispersion, and well-regulated surface roughness makes the first coating the most efficient stage in enhancing the performance of quartz-based filtration membranes. Its high permeability, improved water affinity, and controlled nanoparticle integration ensure long-term durability, minimal fouling, and sustained oil rejection efficiency, making it the ideal candidate for scalable oil–water separation technologies [
25].
The scanning electron microscopy (SEM) image of the second-coated quartz surface in
Figure 5 reveals a more complex morphology, with visible changes in surface texture and nanoparticle distribution compared to the first coating. The surface exhibits increased roughness and irregularities, indicating that the additional nanoparticle layer has altered the structural uniformity. Unlike the first coating, where nanoparticle dispersion was relatively uniform, the second coating shows localized agglomeration, suggesting non-homogeneous deposition in certain regions. This could impact filtration performance, as excessive nanoparticle accumulation can lead to higher surface resistance and reduced permeability in oil–water separation applications.
The energy-dispersive X-ray spectroscopy (EDS) elemental mapping provides further insights into the chemical composition of the coated quartz. The oxygen (O-K) signal remains strong and widely distributed, confirming the continued presence of hydrophilic surface modifications, which enhance water attraction and improve oil rejection efficiency. The silicon (Si-K) mapping still dominates, ensuring that the structural integrity of the quartz remains intact despite the additional coating. However, the carbon (C-K) intensity has slightly increased, indicating potential organic residue accumulation, which could result from nanoparticle bonding agents or surface contaminants introduced during the second coating process. The presence of fluorine (F-K) and phosphorus (P-K) suggests that surface interactions between the nanoparticles and the quartz are occurring, potentially modifying the surface energy and wettability properties. While fluorine remains evenly dispersed, phosphorus appears more clustered, reinforcing the observation of localized agglomeration.
The increased surface roughness and non-uniform particle distribution observed in the second coating may have implications for filtration performance. While the enhanced hydrophilic properties promote water permeability, the excessive aggregation of nanoparticles in certain areas could contribute to higher resistance to fluid flow, thereby reducing the overall filtration efficiency compared to the first-coated quartz. Additionally, agglomerated nanoparticles may be more prone to detachment under high-flow conditions, potentially affecting the long-term stability of the coated membrane [
26]. Despite these challenges, the strong elemental signals for oxygen, silicon, and fluorine suggest that the coating remains chemically stable, making it a viable candidate for further optimization in oil–water separation applications.
The Scanning Electron Microscopy (SEM) image of the third-coated quartz in
Figure 6 exhibits a notable increase in surface roughness and complexity, indicating a higher degree of nanoparticle accumulation compared to previous coatings. The surface appears more irregular, with visible clusters of nanoparticle agglomeration, suggesting that the deposition process has reached a saturation point where additional coatings contribute to excessive particle aggregation rather than uniform dispersion. This increased agglomeration could lead to partial pore blockage, potentially reducing filtration efficiency by restricting water permeability and increasing hydraulic resistance during oil–water separation.
The energy-dispersive X-ray spectroscopy (EDS) elemental mapping provides further insight into the chemical composition and distribution of the coated surface. The oxygen (O-K) signal remains consistently strong, reinforcing the presence of hydrophilic surface properties, which are essential for water affinity and oil repellence. The silicon (Si-K) mapping continues to dominate, confirming that the quartz structure remains intact despite multiple coatings. However, carbon (C-K) intensity is noticeably higher, indicating that the third coating may have introduced more organic residues, likely from excessive nanoparticle deposition or bonding agents used during the modification process.
A key observation is the increased presence of fluorine (F-K) and phosphorus (P-K), both of which appear more dispersed yet concentrated in specific regions, further confirming nanoparticle clustering. The fluorine signal is well-distributed, suggesting that it continues to contribute to surface modifications, whereas phosphorus shows dense accumulation, potentially leading to localized regions of varying surface energy. The presence of aluminum (Al-K) remains sparse, indicating that the coating process has not significantly altered the underlying quartz composition, but rather affected surface interactions with nanoparticles.
The structural and chemical characteristics of the third coating highlight both advantages and challenges for filtration applications. While the hydrophilic nature is preserved, the excessive aggregation of nanoparticles may reduce the membrane’s overall efficiency by limiting water permeability and increasing resistance to flow, similar results were observed by Jiaonan et al. [
27]. This suggests that beyond a certain number of coatings, performance gains begin to diminish, emphasizing the need for controlled deposition techniques to maintain an optimal balance between surface coverage and functional efficiency. Despite these challenges, the coated quartz remains chemically stable, making it suitable for oil–water separation, provided that agglomeration control strategies are implemented to sustain consistent and efficient filtration performance.
The scanning electron microscopy (SEM) image of the fourth-coated quartz in
Figure 7 reveals a highly irregular and rough surface morphology, indicating a substantial increase in nanoparticle accumulation and agglomeration. Compared to previous coatings, the surface texture appears more fragmented, with significant nanoparticle clustering, suggesting that the deposition process has exceeded optimal dispersion levels. The excessive accumulation of nanoparticles may lead to pore blockage, thereby reducing the water permeability and efficiency of the filtration system. Additionally, the formation of uneven clusters introduces potential structural instability, which could impact the long-term durability of the coated quartz when subjected to high-flow filtration conditions.
The energy-dispersive X-ray spectroscopy (EDS) elemental mapping highlights further chemical changes associated with the fourth coating. The oxygen (O-K) signal remains prominent, confirming the continued presence of hydrophilic characteristics. However, silicon (Si-K) intensity appears less defined, likely due to the thicker nanoparticle coverage obscuring the underlying quartz structure. The carbon (C-K) mapping shows an increase in scattered intensity, suggesting the possible adsorption of organic compounds or remnants of binding agents from the coating process. The presence of fluorine (F-K) remains consistent with previous coatings, reinforcing its role in modifying surface energy to enhance oil–water separation properties.
A notable concern is the increased presence of phosphorus (P-K), which exhibits a dense and non-uniform distribution, further supporting the observation of nanoparticle agglomeration. The phosphorus clusters could influence surface energy variations, potentially creating localized regions of higher surface resistance, thereby negatively affecting fluid dynamics during filtration. Additionally, aluminum (Al-K) intensity remains low, indicating that the coating primarily affects the surface layer without significant interaction with the underlying quartz structure.
The findings from the fourth coating suggest a decline in filtration efficiency due to excessive nanoparticle deposition and surface congestion. While the hydrophilic nature of the coated quartz is maintained, the agglomeration and reduced surface uniformity introduce higher resistance to fluid flow, which may hinder the effectiveness of the membrane in oil–water separation applications [
28]. Moreover, the structural irregularities indicate that further coatings beyond this stage may not yield additional performance benefits, highlighting the need for optimized nanoparticle deposition techniques to prevent excessive accumulation. Despite these challenges, the fourth-coated quartz still retains hydrophilic properties, making it suitable for selective filtration applications, provided that coating stability and uniformity are controlled to enhance long-term operational efficiency.
Element Composition of Quartz Samples
The data presented in
Table 3 and
Figure 8, in conjunction with the Scanning Electron Microscopy (SEM) results, highlight that the first-coated quartz (C. COAT 1) demonstrates the best filtration efficiency. The elemental composition analysis in
Table 3 shows that the oxygen percentage significantly increases to 82.54% after the first coating, indicating a high degree of hydrophilicity compared to the raw (61.65%) and washed quartz (69.82%). This suggests that the first coating successfully enhances water affinity, a critical property for oil–water separation. Additionally, the fluorine content remains relatively low at 1.14%, ensuring that the surface modification does not introduce excessive fluorine-based alterations, which could otherwise impact the chemical stability of the filtration membrane.
Comparing this to the SEM analysis, the first coating exhibits a uniform nanoparticle distribution with minimal agglomeration, ensuring optimal surface coverage without clogging the filtration pores [
29]. Unlike the second, third, and fourth coatings, which show increasing nanoparticle clustering and structural irregularities, the first coating maintains an even dispersion of nanoparticles, allowing for maximum permeability while ensuring efficient oil rejection. This balance between surface modification and structural integrity prevents excessive resistance to fluid flow, a common issue observed in higher coatings, where excessive agglomeration creates localized areas of reduced permeability.
Furthermore, phosphorus content (5.89%) in the first coating remains moderate, reinforcing the hydrophilic nature of the quartz while preventing over-modification. As observed in subsequent coatings, an increase in phosphorus content leads to higher surface energy variations and greater particle aggregation, which negatively affects filtration efficiency by increasing resistance to fluid passage. The silicon percentage decreases significantly in the first coating (9.97%) compared to raw quartz (37.41%), indicating that the nanoparticle layer has effectively adhered to the quartz surface, modifying its structure for enhanced oil–water separation without compromising its stability.
The second, third, and fourth coatings show a decline in performance, as observed in
Figure 8, where the oxygen percentage decreases after the first coating, stabilizing around 62–65% in higher coatings. This suggests that additional coatings do not further enhance hydrophilicity but rather introduce excessive nanoparticle accumulation, leading to structural irregularities that reduce filtration efficiency. The fluorine and phosphorus contents continue to increase in later coatings, confirming the progressive clustering of nanoparticles, which negatively impacts the membrane’s permeability.
The combined analysis from
Table 3,
Figure 8, and SEM results clearly indicates that the first coating provides the optimal balance between nanoparticle adhesion, hydrophilicity, and surface stability. The controlled nanoparticle distribution ensures high water permeability and effective oil rejection, making it the most efficient coating for quartz-based filtration systems. Beyond this stage, the increased nanoparticle agglomeration and chemical surface variations reduce membrane efficiency, confirming that one coating is optimal for sustainable and scalable oil–water separation applications.
3.3. Raman Spectroscopy for Quartz Surface Analysis
Figure 9,
Figure 10,
Figure 11,
Figure 12,
Figure 13 and
Figure 14 present the Raman spectroscopy analysis of the quartz samples at different stages of treatment and modification. The analysis begins with the raw quartz sample, providing a baseline spectrum to identify its characteristic vibrational modes. The subsequent spectrum for the washed quartz sample highlights changes in the surface composition and potential removal of impurities following the cleaning process.
The coated samples, represented by the first, second, third, and fourth coatings, display progressive modifications to the quartz surface as each layer of coating is applied. These spectra reveal the introduction of new functional groups or bonds, as well as variations in the intensity and position of key Raman peaks, which correspond to changes in crystalline structure. This progression demonstrates how the coating process alters the material’s chemical and physical properties, making Raman spectroscopy a valuable tool for monitoring these transformations.
The Raman spectroscopy spectrum of the raw quartz sample in
Figure 9 reveals distinct intensity peaks in the lower wave number region (0–500 cm
−1), which correspond to the characteristic vibrational modes of SiO
2. These peaks are indicative of the crystalline structure of quartz, confirming its high purity and well-ordered atomic arrangement. The sharp intensity at approximately 465 cm
−1 is attributed to the symmetric stretching of the Si-O-Si bond, a fundamental vibrational mode of quartz. Additionally, smaller peaks within the 100–400 cm
−1 range represent rotational and bending modes, further validating the structural integrity of the raw quartz material. Beyond 500 cm
−1, the spectrum exhibits minimal intensity variations, suggesting a low presence of additional crystalline phases or impurities, which is consistent with the XRD results from
Table 4, where raw quartz was found to contain 94.8% SiO
2 with minor Al
2O
3 content.
When comparing this spectrum to the first-coated quartz sample, significant modifications in surface chemistry and vibrational characteristics are evident. The SEM results confirmed a uniform nanoparticle distribution in the first coating, which is further supported by the Raman spectrum of the coated quartz, where peak broadening and intensity changes indicate enhanced surface interactions due to nanoparticle integration. The increase in Al
2O
3 (17.6%) and P
2O
5 (7.7%) in the first coating contributes to surface modifications that enhance hydrophilicity, as also observed in
Table 3 and
Figure 8, where oxygen content peaked at 82.54%. These compositional changes optimize water permeability and oil rejection, reinforcing the efficacy of the first coating as the best filtration stage.
In contrast, additional coatings (Coat 2, Coat 3, and Coat 4) introduce increasing nanoparticle agglomeration, leading to less uniform spectral features, as observed in their respective Raman spectra and SEM images. This excessive deposition reduces surface homogeneity, increases hydraulic resistance, and negatively impacts filtration efficiency [
31]. Furthermore, the Raman spectra for these higher coatings exhibit increased spectral noise beyond 500 cm
−1, indicating structural inconsistencies caused by non-uniform nanoparticle layering. This aligns with the SEM findings, where excessive nanoparticle accumulation compromised surface smoothness, creating irregularities that hinder water permeability.
The Raman spectroscopy results of raw quartz, in combination with SEM, XRD (
Table 4), and elemental composition analysis (
Table 3,
Figure 8), strongly support that the first-coated quartz is the optimal filtration membrane. The controlled surface modification achieved in the first coating enhances hydrophilicity while maintaining structural stability, ensuring efficient oil–water separation. Unlike subsequent coatings that suffer from surface congestion and reduced permeability, the first coating preserves the natural crystalline integrity of quartz while introducing sufficient surface reactivity for enhanced separation efficiency. This comprehensive analysis confirms that one coating is the most effective approach for optimizing quartz-based filtration membranes, balancing nanoparticle distribution, chemical stability, and functional performance in sustainable water treatment applications.
The Raman spectroscopy spectrum of the washed quartz sample in
Figure 10 reveals several key vibrational modes characteristic of silicon dioxide (SiO
2), indicating a high-purity material with minimal contamination. The strong peak at approximately 465 cm
−1 corresponds to the symmetric stretching of the Si-O-Si bonds, which is a signature of crystalline quartz. Additionally, smaller peaks observed in the 100–400 cm
−1 range represent the rotational and bending modes of silica, confirming the structural stability of the washed quartz after the cleaning process. Compared to the raw quartz spectrum, the washed quartz shows a slight sharpening of peaks and a reduction in background noise, indicating the successful removal of surface impurities and improved sample homogeneity.
When compared to the Raman spectrum of the first-coated quartz sample, significant differences in surface chemistry and vibrational properties become evident. The first coating introduces broadening and slight shifts in the characteristic Si-O-Si peak, suggesting enhanced surface interactions due to nanoparticle deposition. The XRD analysis (
Table 4) complements this observation, showing a reduction in SiO
2 content from 94.8% in raw quartz to 74.7% in the first coating, indicating the successful integration of hydrophilic nanoparticles. These modifications enhance the surface energy and hydrophilicity of the quartz, making it ideal for oil–water separation applications.
The elemental composition (
Table 3 and
Figure 8) further supports the effectiveness of the first coating. The oxygen content peaks at 82.54%, and the fluorine and phosphorus percentages remain low and controlled, minimizing surface agglomeration. The SEM analysis confirms a uniform nanoparticle distribution in the first coating, whereas washed quartz lacks any additional surface modification, resulting in a lower hydrophilicity and reduced filtration efficiency. Subsequent coatings (second, third, and fourth) introduce surface agglomeration, as evident in their SEM results and Raman spectra, which compromises permeability and efficiency.
The washed quartz demonstrates improved surface cleanliness and structural integrity compared to raw quartz, but it lacks the functional enhancements provided by nanoparticle coating [
32]. The first coating optimizes surface chemistry, balancing nanoparticle coverage and hydrophilicity while avoiding the agglomeration seen in higher coatings. These properties make the first-coated quartz the most effective material for oil–water separation filtration, offering superior performance through controlled nanoparticle distribution and enhanced water affinity.
The Raman spectroscopy spectrum of the first-coated quartz sample in
Figure 11 reveals significant modifications in surface chemistry and structural properties compared to raw and washed quartz. The dominant peak at approximately 465 cm
−1, attributed to the symmetric stretching of the Si-O-Si bonds, remains prominent, confirming that the quartz crystalline framework is preserved despite surface modifications. However, compared to the spectra of raw and washed quartz, the first coating introduces peak broadening and intensity variations, particularly in the low-wavenumber region (100–500 cm
−1), indicating the successful integration of nanoparticles onto the quartz surface. This spectral modification is associated with enhanced surface interactions between the hydrophilic nanoparticles and the quartz substrate, which is essential for improving water affinity in oil–water separation applications.
The higher intensity of the Raman signal compared to the washed quartz spectrum in
Figure 12 suggests an increase in surface-active sites, confirming that the first coating has effectively altered the quartz surface energy [
33]. This aligns with the elemental composition analysis (
Table 3,
Figure 8), where oxygen content peaked at 82.54%, reinforcing the hydrophilic nature of the coated quartz. Additionally, the moderate fluorine and phosphorus presence ensures that the coating remains stable without excessive chemical alterations, preventing issues such as membrane fouling or loss of permeability. The XRD results (
Table 4) further support this observation, as the SiO
2 content decreases to 74.7% in the first coating, indicating successful nanoparticle adhesion without compromising the structural integrity of the quartz.
A crucial advantage of the first coating is its controlled nanoparticle distribution, as confirmed by SEM analysis, which revealed a well-dispersed hydrophilic layer with minimal agglomeration. Unlike higher coatings, where excessive nanoparticle deposition led to clustering and pore blockage, the first coating maintains a balanced modification, ensuring high water flux while preserving selective oil rejection properties. The Raman spectrum of higher coatings (Coat 2, Coat 3, and Coat 4) exhibits increased spectral noise and peak distortions, confirming non-uniform surface modifications that reduce membrane efficiency due to excessive aggregation.
The Raman spectroscopy results, when analyzed alongside SEM, XRD, and elemental composition data, confirm that the first-coated quartz is the most effective filtration material. It achieves an optimal balance between structural integrity, surface hydrophilicity, and permeability, making it the best choice for oil–water separation applications. The controlled surface modification in the first coating maximizes filtration performance, preventing the issues seen in subsequent coatings, where agglomeration and structural inconsistencies hinder efficiency. This confirms that a single nanoparticle coating provides the most effective solution for scalable and sustainable water treatment applications.
The Raman spectroscopy spectrum of the second-coated quartz sample in
Figure 13 exhibits distinct changes compared to the first-coated quartz, highlighting the impact of additional nanoparticle deposition on the surface structure and chemical properties. The sharp peak at approximately 465 cm
−1, characteristic of Si-O-Si symmetric stretching, remains dominant, indicating that the quartz crystalline framework is still intact despite the second coating. However, compared to the first coating, the second coating introduces broader peaks and slight intensity variations, particularly in the low-wavenumber region (100–500 cm
−1), suggesting increased surface interactions due to nanoparticle accumulation. The presence of higher intensity fluctuations beyond 500 cm
−1 further indicates the onset of surface agglomeration, which is a critical factor in filtration performance.
The SEM results of the second-coated quartz confirm the non-uniform nanoparticle distribution, with visible localized clustering, which correlates with the peak broadening observed in this Raman spectrum [
34]. Unlike the first-coated quartz, which maintained a homogeneous dispersion of nanoparticles, the second coating introduces areas of excessive deposition, which can reduce permeability and increase resistance to water flux. This aligns with the elemental composition analysis (
Table 3,
Figure 8), where a decline in oxygen content (62.93%) compared to the first coating (82.54%) suggests reduced hydrophilicity due to excessive surface coverage. Additionally, the increased fluorine content (3.72%) in the second coating may have influenced the surface energy, further impacting the material’s wettability and filtration efficiency.
Comparing this with XRD data (
Table 4), the SiO
2 content in the second coating (82.6%) remains lower than in raw quartz (94.8%) but is higher than in the first-coated quartz (74.7%), indicating that nanoparticle integration is less controlled, leading to inconsistent surface modifications. This inconsistent deposition is reflected in the Raman spectrum, where the peak shifts and intensity variations suggest an increase in heterogeneity. Unlike the first coating, which provided a balance between hydrophilicity and structural integrity, the second coating shows a decline in spectral uniformity, confirming non-optimal surface modification for filtration applications.
In contrast, the first-coated quartz demonstrated the best filtration efficiency due to uniform nanoparticle distribution and controlled surface modifications, as seen in the SEM images and Raman spectra. The broader peaks and intensity fluctuations in the second coating indicate excessive nanoparticle accumulation, which could block filtration pores, increasing hydraulic resistance and ultimately reducing permeability. This confirms that the first coating remains the most effective in maintaining an optimal balance between hydrophilicity, permeability, and structural stability, making it the best choice for oil–water separation applications.
The Raman spectroscopy spectrum of the third-coated quartz sample in
Figure 13 exhibits further spectral broadening and intensity variations compared to both the first and second coatings, indicating progressive nanoparticle accumulation and surface modifications. The strong peak at approximately 465 cm
−1, corresponding to the Si-O-Si symmetric stretching mode, remains present but appears slightly broadened, suggesting increased surface interactions due to the denser nanoparticle layer. The additional coatings have introduced higher intensity fluctuations beyond 500 cm
−1, which correlates with the formation of agglomerates, as also observed in the SEM images of the third coating.
The increased surface agglomeration in the third coating reduces the uniformity of nanoparticle distribution, leading to localized clustering and potential pore blockage. This aligns with the elemental composition analysis (
Table 3,
Figure 8), where the oxygen percentage (64.07%) has slightly decreased compared to the first coating (82.54%), indicating that the hydrophilic functionality of the surface is becoming less effective. The fluorine content (4.06%) is at its highest concentration among all coatings, which suggests that the coating is beginning to exhibit excessive modifications that could alter surface energy in an unfavorable manner.
The XRD analysis (
Table 4) supports this observation, where the SiO
2 content in the third coating (81.5%) remains higher than the first coating (74.7%) but lower than the second coating (82.6%), implying inconsistent nanoparticle adhesion. The phosphorus pentoxide (P
2O₅) content (11.8%) is also at its highest, which may indicate over-modification of the quartz surface, leading to a reduction in efficiency. These factors collectively contribute to a less effective oil–water separation performance, as excess nanoparticles may hinder fluid permeability while providing diminishing improvements in hydrophilicity.
Compared to the first-coated quartz, which demonstrated controlled nanoparticle distribution, uniformity, and minimal surface resistance, the third coating introduces performance limitations due to excessive deposition. The broader peaks and higher intensity variations in the Raman spectrum confirm increased heterogeneity, making the third-coated quartz less efficient in filtration applications [
35]. In contrast, the first coating ensures the optimal balance of nanoparticle integration, hydrophilicity, and structural stability, making it the most effective choice for oil–water separation membranes.
The Raman spectroscopy spectrum of the fourth-coated quartz sample in
Figure 14 exhibits notable spectral broadening and reduced peak intensity compared to the previous coatings, confirming the adverse effects of excessive nanoparticle deposition. The characteristic Si-O-Si symmetric stretching peak at approximately 465 cm
−1 remains present but appears less sharp and more diffused, indicating progressive surface modifications that have altered the quartz structure. The increased background noise beyond 500 cm
−1 suggests that the coating process has reached a saturation point, where excessive nanoparticle accumulation is leading to surface heterogeneity and agglomeration. These spectral distortions correlate with SEM observations, where the fourth-coated quartz exhibited significant nanoparticle clustering, resulting in reduced porosity and increased hydraulic resistance.
The elemental composition analysis (
Table 3,
Figure 8) further supports this observation, as the oxygen content (65.81%) remains lower than that of the first coating (82.54%), confirming a decline in surface hydrophilicity due to excessive nanoparticle buildup. Additionally, the fluorine and phosphorus concentrations (3.85% and 5.8%, respectively) remain relatively high, reinforcing the idea that further coatings do not contribute to improved filtration efficiency but instead introduce unnecessary surface modifications that reduce permeability. The XRD results (
Table 4) reveal that SiO
2 content in the fourth coating (86.5%) has increased compared to the second and third coatings but remains lower than in raw quartz, suggesting inconsistent nanoparticle adherence and partial detachment from the surface.
The progressive decline in spectral clarity and structural uniformity in the fourth coating confirms that additional layers beyond the first coating do not enhance filtration performance. Instead, they introduce non-uniform nanoparticle deposition, which blocks pores, increases flow resistance, and reduces water flux efficiency [
36]. This contrasts sharply with the first-coated quartz, where controlled nanoparticle distribution ensured optimal permeability and oil–water separation efficiency. Unlike the first coating, which maintained a balance between structural stability and hydrophilicity, the fourth coating suffers from excessive modifications, making it less effective for sustainable water treatment applications.
The Raman spectrum, along with SEM, XRD, and elemental composition analysis, confirms that the first-coated quartz remains the most effective filtration material. The fourth coating introduces unnecessary complexities, reduces structural uniformity, and diminishes permeability, reinforcing that a single nanoparticle layer provides the best balance between surface hydrophilicity, structural integrity, and long-term filtration efficiency.